Abstract
Background
A combination of FOLFOX and nivolumab is a first-line treatment for HER2-negative advanced gastric cancer, significantly improving survival. However, this regimen is associated with potential neurotoxicity. 5-Fluorouracil and oxaliplatin in FOLFOX have been linked to optic neuropathy, whereas nivolumab, an immune checkpoint inhibitor, may cause immune-related optic neuropathy. Although FOLFOX plus nivolumab provides considerable survival benefits, careful monitoring for ocular complications is essential. We report a case of bilateral optic neuropathy in a patient who received FOLFOX plus nivolumab. Despite discontinuation of chemotherapy and treated with high-dose corticosteroid pulse therapy, the patient’s symptoms did not improve.
Case presentation
A 48-year-old woman with a Krukenberg tumor developed progressive bilateral visual impairment during treatment with FOLFOX plus nivolumab. Her ophthalmologic history included branch retinal vein occlusion in the right eye but no systemic diseases. She presented with bilateral central scotomas and visual field defects. On examination, best-corrected visual acuity (BCVA) was 20/40 in the right eye and 20/25 in the left eye, with bilateral optic disc swelling and hemorrhage. Fluorescein angiography confirmed optic disc leakage, and a visual evoked potential (VEP) test indicated axonal loss. Brain magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT) ruled out metastatic disease, and autoimmune markers were negative. High-dose intravenous methylprednisolone was administered, and chemotherapy was discontinued. Despite initial stabilization, vision deteriorated, ultimately progressing to light perception in both eyes. Repeated steroid pulse therapy failed to improve outcomes.
Conclusions
This case highlights the potential for severe bilateral optic neuropathy associated with FOLFOX plus nivolumab, leading to irreversible vision loss. These findings suggest that close ophthalmologic monitoring is warranted in patients receiving FOLFOX plus nivolumab for early recognition of bilateral visual impairment. Further research is needed to elucidate the mechanisms and identify agents responsible for the development of optic neuropathy.
Keywords: FOLFOX, Nivolumab, Immune checkpoint inhibitor, Optic neuropathy, Krukenberg tumor, Immune-related adverse events
Background
Krukenberg tumors are metastatic ovarian tumors, most commonly originating from gastric cancer, accounting for approximately 70% of cases. They are histologically characterized by mucin-filled signet ring cells within a stromal tissue derived from the ovarian stroma [1]. As Krukenberg tumors are typically associated with a poor prognosis, appropriate systemic treatment is essential [1, 2].
The CheckMate 649 trial established the combination of nivolumab and FOLFOX as the recommended treatment for gastric origin Krukenberg tumor because it significantly prolonged overall and progression-free survival [3–5]. However, this regimen introduces a potential risk of optic neuropathy due to the neurotoxic properties of its components.
Anticancer drugs can cause various ocular adverse events, ranging from common ocular surface disorders such as dry eye, conjunctivitis, and superficial punctate keratitis to more severe complications like uveitis, optic neuritis, and retinal toxicity. While most mild cases are manageable with conservative medical treatments, severe adverse events may require discontinuation of the anticancer agent, high-dose systemic corticosteroids [6, 7].
The FOLFOX regimen includes 5-fluorouracil (5-FU) and cisplatin. 5-FU has been associated with optic neuropathy through mechanisms such as vascular toxicity and mitochondrial dysfunction, which can lead to visual impairment and color vision abnormalities [8, 9]. Similarly, platinum-based agents such as oxaliplatin rarely cause optic nerve damage via ischemic or cytotoxic effects [10–12]. Nivolumab is an immune checkpoint inhibitor that poses a risk of immune-related adverse effects, such as optic neuritis, which causes inflammatory damage to the optic nerve, leading to vision loss and visual field defects [13, 14].
We conducted an extensive literature search by using the PubMed/MEDLINE database covering time period between January 1997 and January 2025. The search was limited to articles published in English and used the following keywords: (“FOLFOX” OR “oxaliplatin” OR “fluorouracil” OR “nivolumab”) AND (“optic neuropathy” OR “optic neuritis”). No reports of bilateral optic neuropathy associated with combination therapy involving FOLFOX and nivolumab were identified. We therefore report a case of bilateral optic neuropathy in a patient who received this combination. Despite discontinuation of chemotherapy and administration of high-dose corticosteroid pulse therapy, no improvement was observed in visual symptoms.
Case presentation
A 48-year-old woman diagnosed with a Krukenberg tumor presented with recently developed visual symptoms. She was receiving chemotherapy with a combination of FOLFOX and nivolumab, with nivolumab introduced from the second cycle following initial FOLFOX regimen. After five cycles of the combined regimen, she was referred to the ophthalmology department for evaluation of decreased visual acuity. She had no history of systemic diseases such as diabetes or hypertension. However, her ophthalmic history was notable for superotemporal branch retinal vein occlusion (BRVO) in the right eye, which had been previously treated with laser therapy. During chemotherapy, she developed progressive bilateral visual impairment accompanied by scotoma and visual field defects. Her visual acuity gradually worsened, with a specific complaint of disturbances in the lower visual field.
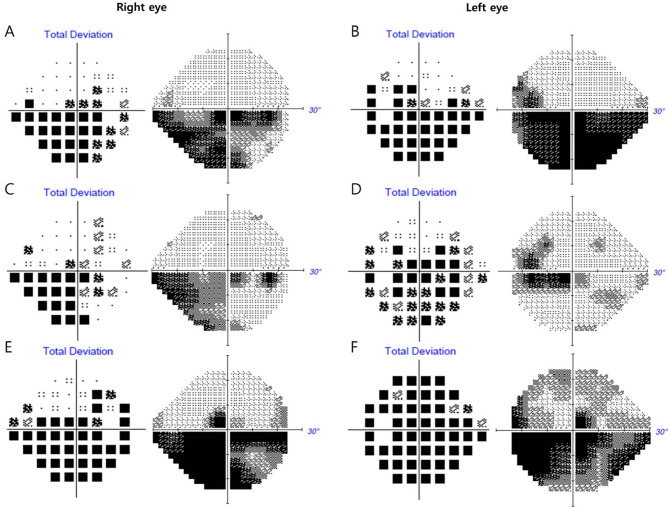
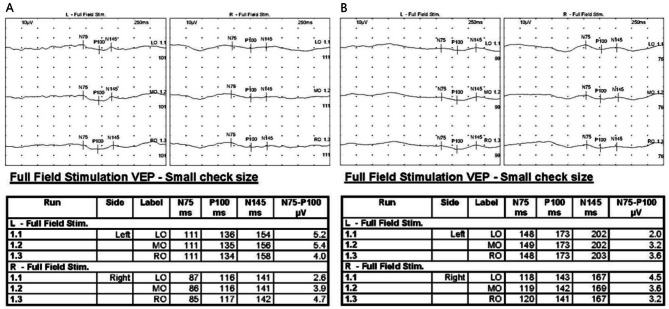
On initial presentation to the ophthalmology department, her best-corrected visual acuity (BCVA) was 20/40 in the right eye and 20/25 in the left eye. Pupillary light reflexes were isocoric, with no definite signs of a relative afferent pupillary defect. Fundus examination revealed bilateral optic disc swelling and hemorrhage (Fig. 1A and D). Fluorescein angiography (FA) showed leakage at the optic discs in both eyes (Fig. 2). The Humphrey visual field test revealed a field defect with central scotoma (Fig. 3A and B). Visual evoked potentials (VEP) demonstrated delayed P100 latencies and reduced amplitudes in both eyes (Fig. 4A), indicating delayed conduction and reduced signal strength. The delay was more pronounced in the left eye, as evidenced by a P100 latency of 135 ms in the left eye compared to 116 ms in the right eye. Additionally, the P100 amplitude was 5.4 µV in the left eye and 3.9 µV in the right eye, suggesting axonal injury in the left optic nerve. A systemic evaluation, including positron emission tomography-computed tomography (PET-CT) and brain magnetic resonance imaging (MRI), revealed stable disease with no evidence of metastases in the central nervous system, brain, or leptomeninges. No abnormalities in intracranial pressure were observed. All inflammatory markers, including anti-myelin oligodendrocyte glycoprotein (MOG), AQP-4, anti-ENA, anti-DNA, and paraneoplastic antibodies, tested negative. The patient was diagnosed with bilateral optic neuropathy associated with FOLFOX and nivolumab. Immediate treatment with intravenous methylprednisolone (MPD, 250 mg QID for 5 days) was initiated, followed by oral steroid tapering. Chemotherapy with FOLFOX plus nivolumab was temporarily discontinued.
Fig. 1.
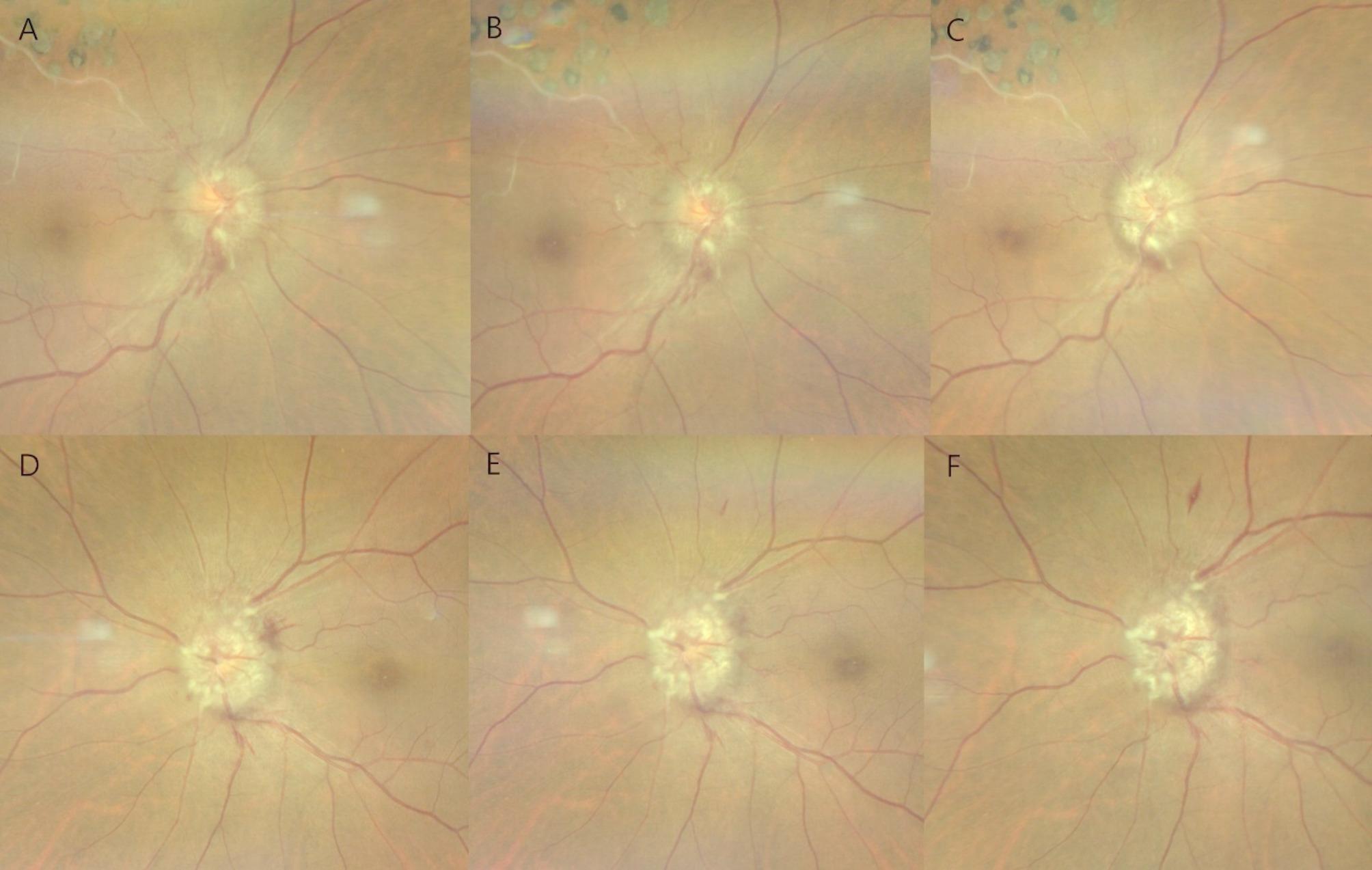
Color fundus photography (CLARUS 700, Carl Zeiss Meditec AG, Germany) of a patient with bilateral optic neuropathy. The upper row (A–C) represents the right eye, while the lower row (D–F) represents the left eye. (A, D) Initial fundus images reveal optic disc swelling accompanied by disc hemorrhage. The right eye (A) has a history of superotemporal branch retinal vein occlusion and prior scatter laser treatment. (B, E) Two weeks after the initial visit, partial resolution of disc hemorrhage is observed in both eyes; however, disc edema persists. (C, F) One month after the initial visit, fundus images show persistent disc edema and disc hemorrhage, indicating ongoing optic neuropathy
Fig. 2.
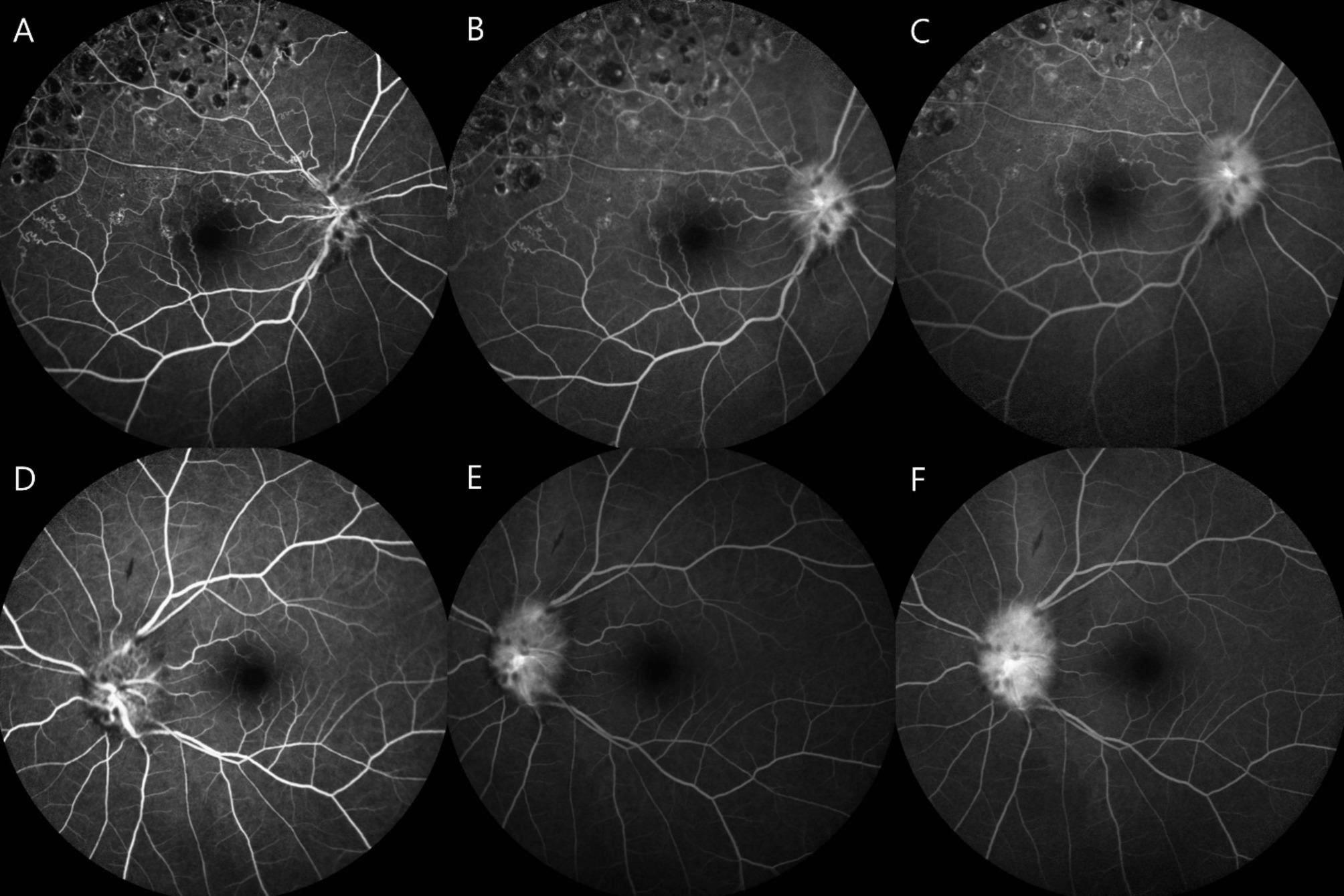
Fluorescein angiography (Spectralis, Heidelberg Engineering GmbH, Germany) findings in a patient with bilateral optic neuropathy. The upper row (A–C) represents the right eye, while the lower row (D–F) represents the left eye. Early-phase (A, D) images demonstrate well-perfused retinal vasculature with initial disc hyperfluorescence and mild leakage. Mid-phase (B, E) images show increasing disc leakage with pronounced disc edema. Late-phase (C, F) images reveal significant disc staining with diffuse leakage, consistent with optic disc edema
Fig. 3.
Humphrey visual field test (Humphrey Field Analyzer 3, Carl Zeiss Meditec Inc., USA) results of a patient with bilateral optic neuropathy. Initial visual field testing shows a significant central scotoma with inferior altitudinal visual field defects in both eyes. (B, D) Two weeks after the initial visit, the central scotoma persists, with slight improvement in the visual field defect. (E, F) One month after the initial visit, progressive worsening of the visual field defects is observed, with further central scotoma progression and inferior visual field defect in both eyes
Fig. 4.
Serial visual evoked potential (Nicolet 2015 Visual Stimulator, Natus Medical Incorporated, USA) recordings demonstrating progressive deterioration in visual conduction in both eyes. (A) Initial VEP shows a marked latency delay and reduced amplitude, more pronounced in the left eye. (B) Follow-up VEP after one month reveals further deterioration, with increased latency and greater amplitude reduction
Two weeks after the initial presentation, the patient returned for a follow-up at the ophthalmology clinic. BCVA was 20/63 in the right eye and 20/30 in the left eye. The Humphrey visual field test revealed persistent central scotomas; however, an improvement was noted in the mean deviation (MD) of the 24 − 2 test, with the right eye improving from − 12.42 dB to − 9.31 dB and the left eye from − 17.95 dB to − 8.47 dB, along with subjective improvement in visual field disturbances (Fig. 3C and D). However, bilateral optic disc edema persisted, similar to the findings of a previous study (Fig. 1B and E). Steroid pulse therapy was tapered with oral prednisolone.
One month after the initial presentation, the patient experienced a recurrence of symptoms, reporting distorted visual fields and difficulty focusing. BCVA decreased to 20/125 in the right eye and 20/63 in the left eye. Bilateral pupillary light reflexes were diminished. Fundus examination revealed persistent optic disc swelling (Fig. 1C and F). Humphrey visual field perimetry demonstrated worsening visual field constriction, with a decline in MD values on the 24 − 2 test from − 9.31 dB to − 18.75 dB in the right eye and from − 8.47 dB to − 18.88 dB in the left eye. The combination of FOLFOX and nivolumab was still discontinued, and the patient was maintained on dexamethasone 4 mg QID based on systemic status. Due to deterioration of symptoms, MPD 250 mg QID pulse therapy was reinitiated. Repeat VEP testing demonstrated further deterioration in both eyes compared with the previous VEP test. P100 latency increased to 173 ms in the left eye and 142 ms in the right eye, while P100 amplitude decreased to 3.2 µV in the left eye and 3.6 µV in the right eye (Fig. 4B). Despite high-dose steroid pulse therapy, visual acuity did not improve. Two weeks later, BCVA in both eyes deteriorated to counting fingers, with persistent optic disc edema. Despite the discontinuation of chemotherapy and reinitiation of steroid pulse therapy, the patient’s symptoms did not improve. Pupillary light reflexes were diminished, and visual acuity deteriorated to light perception in both eyes.
Discussion
The combination of FOLFOX and nivolumab provides a synergistic approach to cancer treatment. FOLFOX reduces the tumor burden through cytotoxic chemotherapy, whereas nivolumab, a PD-1 inhibitor, enhances T cell-mediated immune responses against residual cancer cells. The CheckMate 649 trial demonstrated notable improvements in overall and progression-free survival with FOLFOX plus nivolumab compared with FOLFOX alone [3]. Based on these findings, this combination has been established as the first-line treatment for Krukenberg tumor. Despite its efficacy, potential adverse effects, including immune-related adverse events and chemotherapy-associated toxicities, must be carefully considered.
Platinum-based agents are associated with ophthalmic adverse effects, which are speculated to result from vascular dysfunction and ischemic injury affecting the optic nerve and retinal vasculature [10]. Shihadeh et al. reported a case of bilateral optic neuropathy induced by carboplatin arterial ischemia, a mechanism thought to cause irreversible vision loss [11]. Similarly, Maleki et al. described carboplatin-induced bilateral optic neuropathy. Fundus examination in this case revealed bilateral disc swelling with cotton wool spots and retinal vessel dilation around the optic nerve head in the left eye [12]. Likewise, Beaumont et al. reported oxaliplatin-induced bilateral optic disc swelling with visual loss, which improved after discontinuation of oxaliplatin [15].
5-FU is metabolized by DPD, the rate-limiting enzyme in the breakdown of fluoropyrimidines. Patients with reduced or absent DPD activity are at an increased risk for enhanced toxicity, including ophthalmic adverse effects [16]. In patients with complete DPD deficiency, 5-FU is contraindicated due to the risk of severe toxicity [17]. Transient ischemic episodes of the optic nerve or toxicity due to dihydropyrimidine dehydrogenase deficiency may contribute to optic neuropathy development [18]. In clinical settings, it is important to consider DPD deficiency screening in patients with 5-FU administration or when adverse effects of chemotherapy are suspected. Optic neuropathy associated with 5-FU often resolves within weeks to months after discontinuing the drug and administering corticosteroids [8, 9, 19]. Recent cases have also described unilateral optic neuritis without demyelinating lesions on MRI [9]. Liam et al. reported bilateral optic disc edema and accompanying optic neuropathy characterized by transient bilateral inferior altitudinal field defects during continuous FOLFOX chemotherapy infusion. These episodes were hypothesized to result from arterial vasospasm of the short posterior ciliary arteries induced by 5-FU [18].
Nivolumab, a PD-1 immune checkpoint inhibitor, binds to the PD-1 receptor on T cells, thereby blocking its interaction with PD-L1 [14, 20]. Immune checkpoint inhibitors (ICIs) may cause immune-related adverse events (irAEs) in ophthalmology by interfering with immune regulatory processes. The eye is a site of immune privilege and possesses a unique microenvironment that protects visual function by limiting immune responses. This privilege is maintained through the expression of the Fas ligand and transforming growth factor-beta, which promote the conversion of T cells into regulatory T cells and induce the apoptosis of immune cells. These mechanisms suppress local inflammation and protect visual function [21]. However, ICIs block the function of regulatory T cells, causing excessive activation of the immune response. This disruption can reprogram immune cell death pathways, possibly triggering the development of ophthalmic irAEs. These adverse events can manifest as optic neuropathy, uveitis, keratitis, and other ocular complications from the loss of immune tolerance within the immune-privileged environment of the eye [22]. Omer et al. reported a case of bilateral optic neuritis in a pediatric patient with glioblastoma multiforme following the treatment with nivolumab monotherapy [14]. The patient’s visual acuity declined to counting fingers. However, after discontinuation of nivolumab and administration of intravenous corticosteroids (1 g/day) for 5 days, the patient’s visual acuity recovered to 20/20 within 1 week. Unlike the case reported by Omer et al., certain reports have described irreversible vision loss despite corticosteroid treatment, underscoring the variability in clinical outcomes and the potential for severe permanent ocular damage [23]. Francis et al. reported 11 cases of ICI-associated optic neuritis [13]. The visual field patterns were variable and not limited to central defects. Most cases showed a good response to systemic steroid therapy. However, one case showed no improvement following treatment, suggesting that ICI-associated inflammation may, in some instances, lead to ischemic damage.
The damage in the present case was unlikely to have resulted from infiltrative or metastatic optic neuropathy. Optic nerve metastasis from a Krukenberg tumor is extremely rare, and no evidence of such lesions has been observed in systemic imaging studies, including MRI or fundus examination. Transient symptom improvement suggests a different etiology. The patient simultaneously developed bilateral optic neuropathy during treatment with FOLFOX plus nivolumab, a pattern that did not align with the typical tumor-related involvement. Simultaneous effects on both optic nerves are indicative of drug-induced toxicity.
The present case suggests that bilateral optic neuropathy developed during treatment with a combination of FOLFOX and nivolumab. The partial improvement following drug discontinuation and steroid pulse therapy may suggest the involvement of an immune-mediated mechanism. However, the subsequent worsening of symptoms despite continued drug cessation and repeated steroid treatment raises the possibility that, in addition to an immune-related response, ischemic injury or direct drug toxicity may have also contributed. This feature distinguishes the present case from previously reported cases of optic neuropathy associated with FOLFOX or nivolumab monotherapy. (Table 1) Serial VEP findings suggested a progressive axonal injury, particularly in the left optic nerve. The first VEP test demonstrated a significant delay in P100 latency and reduced amplitude, particularly in the left eye, indicating impaired neural conduction consistent with optic neuropathy. On follow-up, the second VEP further worsened, with increased P100 latency, greater interocular latency difference, and progressive reduction in amplitude, suggesting ongoing axonal injury and demyelination.
Table 1.
Clinical characteristics of previously reported cases and the present case of optic neuropathy associated with FOLFOX or nivolumab therapy
| Causative agent | Age/Sex Cancer diagnosis |
Clinical presentation | Ophthalmic treatment | Treatment outcome | Author |
|---|---|---|---|---|---|
| 5-FU |
72/F Breast cancer |
Decreased visual acuity (OU) Optic disc swelling (OU) |
Discontinuation of 5-FU IV methylprednisolone 40 mg tid |
Normal optic disc (OD) Reduced swelling of optic disc (OS) Moderately improved vision (OU) |
Delval L, et al. 2002 [16] |
| 5-FU |
57/M Colorectal cancer |
No optic disc swelling Visual field constriction (OD) |
Discontinuation of 5-FU IV methylprednisolone 1 g/day for 5 days |
Resolution of visual field constriction (OD) |
Raina AJ, et al. 2019 [9] |
| 5-FU |
53/M Malignent hemangioendothelioma |
Decreased visual acuity (OS) |
Discontinuation of 5-FU prednisone 40 mg/day for 10 days |
Almost complete resolution (OS) |
Langley JR, et al. 1978 [19] |
| Oxaliplatin |
72/M Metastatic urothelial cancer |
Decreased visual acuity (OU) Optic disc swelling (OU) Subretinal fluid (OS) |
Discontinuation of oxaliplatin | Resolution with persistent blind spot enlargement (OU) |
Beaumont W, et al. 2021 [15] |
| FOLFOX |
57/M Colorectal cancer |
Decreased visual acuity (OU) Optic disc swelling (OU) Inferior altitudinal field defect (OS) |
Discontinuation of FOLFOX Aspirin 100 mg/day Topical brimonidine |
Persistent inferior altitudinal defect (OS) Optic disc pallor (OS) |
Turner LD, et al. 2013 [18] |
| Nivolumab |
9/M Glioblastoma multiforme |
Decreased visual acuity (OU) Optic disc swelling (OU) |
Discontinuation of nivolumab IV methylprednisolone 1 g/day for 5 days |
Progressive VA improvement (OU) |
Kartal Ö, et al. 2018 [14] |
| Nivolumab |
59/F Small cell lung cancer |
Decreased visual acuity (OS) Optic disc swelling (OS) Diffuse reduction in mean deviation (OS) |
Oral prednisone 60 mg |
Improved vision (OS) Pallor of superior left disc (OS) |
Francis JH, et al. 2020 [13] |
| Nivolumab |
63/M Cutaneous melanoma |
Decreased visual acuity (OU) Optic disc swelling (OU) Inferior arcuate defect (OD) Superior altitudinal defect (OS) |
IV methylprednisone Topical difluprednate, timolol, dorzolamide, brimonidine |
Improved vision (OU) Optic disc pallor (OU) Improvement of inferior arcuate defect (OD) Improvement of superior altitudinal defect (OS) |
Francis JH, et al. 2020 [13] |
| FOLFOX + Nivolumab |
48/F Gastric cancer |
Decreased visual acuity (OU) Optic disc swelling (OU) Central scotoma with inferior altitudinal field defects (OU) |
Discontinuation of chemotherapy IV methylprednisolone 1 g/day for 5 days |
Persistent optic disc edema (OU) VA transiently improved but deteriorated to counting fingers (OU) |
This study |
BCVA, best-corrected visual acuity; DPD, dihydropyrimidine dehydrogenase; HVF, Humphrey visual field; IV, intravenous; MPD, methylprednisolone; NA, not available; RAPD, relative afferent pupillary defect
In cases of optic neuropathy, the standard intervention typically involves discontinuation of the causative agent and administration of steroid pulse therapy, which is generally effective in resolving symptoms [13]. However, in the present case, despite the discontinuation of chemotherapy and the administration of two cycles of steroid pulse therapy, the patient’s ophthalmic symptoms did not improve. Unlike previously reported cases, the patient’s visual symptoms continued to deteriorate even after the cessation of chemotherapy and repeated pulse steroid therapy.
This case report has several limitations. Firstly, it is difficult to determine the exact causative agent of the optic neuropathy due to the use of multiple drugs. Both FOLFOX and nivolumab were discontinued, and steroid therapy was initiated, which led to partial improvement of symptoms followed by subsequent worsening. As the therapeutic response was only partial and neither drug was reintroduced, identifying the causative agent with certainty was not possible. Secondly, there was a lack of quantitative evidence to evaluate the etiology. Due to the patient’s deteriorating general condition, cooperation during ophthalmic examinations was limited, and the degree of optic disc edema could not be assessed quantitatively.
Conclusion
To date, bilateral optic neuropathy has not been reported as an adverse effect of FOLFOX plus nivolumab therapy. This case describes bilateral optic neuropathy in a patient with a Krukenberg tumor undergoing treatment with this combination regimen. Despite the discontinuation of the chemotherapy regimen and repeated steroid pulse therapy, the patient’s symptoms did not improve. The combination of FOLFOX and nivolumab increases the risk of adverse ophthalmic effects, which can cause irreversible vision loss. Therefore, close monitoring of ophthalmic symptoms is essential for detecting and managing potential complications.
Acknowledgements
None.
Abbreviations
- 5-FU
5-fluorouracil
- BCVA
Best-corrected visual acuity
- BRVO
Branch retinal vein occlusion
- FA
Fluorescein angiography
- IgG
Immunoglobulin G
- MD
Mean deviation
- MO
Middle occipital
- MPD
Methylprednisolone
- VEP
Visual evoked potential
Author contributions
Study concept and design: All authors Drafting of the manuscript: J.L., J.C., and K.K.Critical revision of the manuscript: J.L., M.S.K., and S.Y.Y.Reviewed the manuscript: All authors.
Funding
None.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the tenets of the Declaration of Helsinki. Ethics Committee approval was not required for this case report.
Consent for publication
We obtained written informed consent to publish from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med. 2006;130(11):1725–30. [DOI] [PubMed] [Google Scholar]
- 2.Yasufuku I, Tsuchiya H, Fujibayashi S, et al. Oligometastasis Gastric Cancer: Rev Cancers (Basel). 2024;16(3):673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021. Gastric Cancer. 2023;26(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah MA, Kennedy EB, Alarcon-Rozas AE, et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J Clin Oncol. 2023;41(7):1470–91. [DOI] [PubMed] [Google Scholar]
- 6.Lixi F, Giannaccare G, Salerno G, et al. Side Effects of Novel Anticancer Drugs on the Posterior Segment of the Eye: A Review of the Literature. J Pers Med. 2024;14(12):1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitiello L, Lixi F, Coco G, Giannaccare G. Ocular Surface Side Effects of Novel Anticancer Drugs. Cancers (Basel). 2024;16(2):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bygrave H, Geh J, Jani Y, Glynne-Jones R. Neurological complications of 5-fluorouracil chemotherapy: case report and review of the literature. Clin Oncol. 1998;10(5):334–6. [DOI] [PubMed] [Google Scholar]
- 9.Raina AJ, Gilbar PJ, Grewal GD, Holcombe DJ. Optic neuritis induced by 5-fluorouracil chemotherapy: Case report and review of the literature. J Oncol Pharm Pract. 2020;26(2):511–6. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Li J, Pi G, Tan W. Paclitaxel-and/or cisplatin-induced ocular neurotoxicity: a case report and literature review. OncoTargets therapy 2014:1361–6. [DOI] [PMC free article] [PubMed]
- 11.Shihadeh S, Patrick MM, Postma G et al. Blinding Optic Neuropathy Associated With Carboplatin Therapy: A Case Report and Literature Review. Cureus 2024;16(1). [DOI] [PMC free article] [PubMed]
- 12.Maleki A, Lagrew MK, Slaney ED. Bilateral Optic Neuropathy Secondary to Intravenous Carboplatin Therapy. J Ophthalmic Vis Res. 2024;19(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis JH, Jaben K, Santomasso BD, et al. Immune checkpoint inhibitor-associated optic neuritis. Ophthalmology. 2020;127(11):1585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kartal Ö, Ataş E. Bilateral optic neuritis secondary to nivolumab therapy: a case report. Medicina. 2018;54(5):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaumont W, Sustronck P, Souied EH. A case of oxaliplatin-related toxic optic neuropathy. J Fr Ophtalmol. 2021;44(7):e393–5. [DOI] [PubMed] [Google Scholar]
- 16.Delval L, Klastersky J. Optic neuropathy in cancer patients. Report of a case possibly related to 5 fluorouracil toxicity and review of the literature. J Neurooncol. 2002;60(2):165–9. [DOI] [PubMed] [Google Scholar]
- 17.Dean L, Kane M. Fluorouracil therapy and DPYD genotype. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries. Bethesda (MD): National Center for Biotechnology Information (US); 2012 [cited 2025 Apr 10]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK395610/ [PubMed]
- 18.Turner LD, Harrison JD. Bilateral optic disc oedema and associated optic neuropathy in the setting of FOLFOX chemotherapy. BMC Ophthalmol. 2013;13:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langley JR, Rosato FE, El-Mahdi A. Primary malignant hemangioendothelioma of the liver: survival following nonoperative treatment. J Surg Oncol. 1978;10(6):533–41. [DOI] [PubMed] [Google Scholar]
- 20.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2(5):662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunturi A, McDermott DF. Nivolumab for the treatment of cancer. Expert Opin Investig Drugs. 2015;24(2):253–60. [DOI] [PubMed] [Google Scholar]
- 22.Zhou R, Caspi RR. Ocular immune privilege. F1000 biology reports 2010, 2. [DOI] [PMC free article] [PubMed]
- 23.Thomas M, Armenti ST, Ayres MB, Demirci H. Uveal effusion after immune checkpoint inhibitor therapy. JAMA Ophthalmol. 2018;136(5):553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.