Abstract
Rationale
Postpartum haemorrhage (PPH) is common and potentially life‐threatening. The antifibrinolytic drug tranexamic acid (TXA) is thought to be effective for treating PPH. There is growing interest in whether TXA is effective for preventing PPH after vaginal birth. In randomised controlled trials (RCTs), TXA has been associated with increased risk of seizures and unexplained increased mortality when given more than three hours after traumatic bleeding. Reliable evidence on the effects, cost‐effectiveness and safety of prophylactic TXA is required before considering widespread use. This review updates one published in 2015.
Objectives
To assess the effects of TXA for preventing PPH compared to placebo or no treatment (with or without uterotonic co‐treatment) in women following vaginal birth.
Search methods
We searched MEDLINE, Embase, CENTRAL, and WHO ICTRP (to 6 September 2024). We also searched reference lists of retrieved studies.
Eligibility criteria
We included RCTs evaluating TXA alone or in addition to standard care (uterotonics) for preventing PPH following vaginal birth. For this update, we required trials to be prospectively registered (before participant recruitment), and we applied a trustworthiness checklist.
Outcomes
Critical outcomes were blood loss ≥ 500 mL and blood loss ≥ 1000 mL.
Important outcomes included maternal death, severe morbidity, blood transfusion, receipt of additional surgical interventions to control PPH, thromboembolic events, receipt of additional uterotonics, hysterectomy, and maternal satisfaction.
Risk of bias
We used the Cochrane risk of bias tool (RoB 1) to assess the risk of bias in the studies.
Synthesis methods
Two review authors independently selected trials, extracted data, assessed risk of bias, and assessed trial trustworthiness. We used random‐effects meta‐analysis to combine data. We assessed the certainty of the evidence using GRADE.
Included studies
We included three RCTs with 18,974 participants in total. The trials were conducted in both high‐ and low‐resource settings and involved participants at both low and high risk of PPH. The trials compared intravenous TXA (1 g) and standard care versus placebo (saline) and standard care.
After applying our trustworthiness checklist, we did not include any of the 12 trials in the previous version of this review.
Synthesis of results
Prophylactic tranexamic acid in addition to standard care compared to placebo in addition to standard care
TXA results in little to no difference in blood loss ≥ 500 mL (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.81 to 1.06; 2 studies, 18,897 participants; 5 fewer per 1000, 95% CI 15 fewer to 5 more; high‐certainty evidence).
TXA likely results in little to no difference in blood loss ≥ 1000 mL (RR 0.86, 95% CI 0.69 to 1.07; 2 studies, 18,897 participants; 3 fewer per 1000, 95% CI 6 fewer to 1 more; moderate‐certainty evidence).
TXA likely results in little to no difference in severe morbidity (RR 0.88, 95% CI 0.69 to 1.12; 1 study, 15,066 participants; 2 fewer per 1000, 95% CI 6 fewer to 2 more; moderate‐certainty evidence).
TXA results in little to no difference in receipt of blood transfusion (RR 1.00, 95% CI 0.95 to 1.06; 3 studies, 18,972 participants; 0 fewer per 1000, 95% CI 10 fewer to 12 more; high‐certainty evidence).
TXA may result in little to no difference in receipt of additional surgical interventions to control PPH (RR 0.63, 95% CI 0.32 to 1.23; 2 studies, 18,972 participants; 1 fewer per 1000, 95% CI 2 fewer to 1 more; low‐certainty evidence).
In women with anaemia, TXA results in little to no difference in receipt of additional uterotonics (RR 1.02, 95% CI 0.94 to 1.10; 1 study, 15,066 participants; 3 more women per 1000, 95% CI 8 fewer to 24 more; high‐certainty evidence).
In women with no anaemia, TXA results in a slight reduction in receipt of additional uterotonics (RR 0.75, 95% CI 0.61 to 0.92; 1 study, 3891 participants; 24 fewer women per 1000, 95% CI 38 fewer to 8 fewer; high‐certainty evidence).
TXA likely results in little to no difference in maternal satisfaction.
The evidence is very uncertain about the effect of TXA on maternal death, thromboembolic events, and hysterectomy (very low‐certainty evidence): maternal death (RR 0.99, 95% CI 0.39 to 2.49; 2 studies, 15,081 participants; 0 fewer per 1000, 95% CI 1 fewer to 2 more); thromboembolic events (RR 0.25, 95% CI 0.03 to 2.24; 3 studies, 18,774 participants; 3 fewer women per 10,000, 95% CI 4 fewer to 5 more); hysterectomy (RR 0.89, 95% CI 0.36 to 2.19; 1 study, 15,066 participants; 1 fewer women per 10,000, 95% CI 9 fewer to 16 more).
Authors' conclusions
Adding prophylactic TXA to standard care of women during vaginal birth makes little to no difference to blood loss ≥ 500 mL and likely makes little to no difference to blood loss ≥ 1000 mL or the risk of severe morbidity, compared to placebo and standard care.
TXA may result in little to no difference in additional surgical interventions to control PPH and results in little to no difference in blood transfusions. One trial found that TXA reduced the use of additional uterotonics in women without anaemia, whereas the largest trial found little to no difference in the use of additional uterotonics in women with anaemia.
Although there were very few serious adverse events reported, the evidence is insufficient to draw conclusions about the effect of TXA on maternal death, thromboembolic events, hysterectomy, or seizures.
TXA likely results in little to no difference in maternal satisfaction.
These findings are based mainly on two large trials. In the smaller of these, less than 30% of study participants were at high risk of PPH. In the largest trial, all participants had moderate to severe anaemia.
Those making decisions about routine administration of prophylactic TXA for all women having vaginal births should consider that current evidence does not show a benefit of TXA for blood loss outcomes and related morbidity, and the evidence is very uncertain about serious adverse events.
Funding
This review was partially funded by the World Health Organization (WHO).
Registration
Protocol (2009) DOI: 10.1002/14651858.CD007872 Original review (2010) DOI: 10.1002/14651858.CD007872.pub2 Review update (2015) DOI: 10.1002/14651858.CD007872.pub3
Plain language summary
What are the benefits and risks of tranexamic acid for preventing heavy bleeding after vaginal birth?
Key messages
Tranexamic acid given as a preventive treatment makes little to no difference to heavy bleeding after vaginal birth.
We are uncertain about whether there are any harmful effects from tranexamic acid.
What is the issue?
Postpartum haemorrhage, which is heavy bleeding after giving birth, is a common and potentially life‐threatening complication of birthing a child. Most women receive drugs (called uterotonics) that stimulate the womb to contract after a normal (vaginal) delivery to prevent postpartum haemorrhage. Tranexamic acid (TXA) is a medication that is used to decrease blood loss in surgery and health conditions associated with increased bleeding. It works by helping to prevent the breakdown of blood clots. If a woman is bleeding heavily after birth, it decreases blood loss. We do not know if TXA can help prevent heavy bleeding after vaginal birth.
What did we want to find out?
We wanted to know whether fewer women have heavy bleeding after a vaginal birth if they receive TXA, with or without additional uterotonics, during vaginal birth. We also wanted to find out if taking TXA during vaginal birth was associated with any harmful effects.
What did we do?
We searched for and selected all the studies that addressed our question. We used a checklist to make sure we only included studies with information we could verify. We made judgements about the quality of the studies before comparing and summarising the results of the studies. Lastly, we rated our confidence in the findings.
Why is this important?
It is important to determine whether TXA is effective in preventing heavy bleeding after birth when given to women during vaginal birth. If there is a benefit, it could help women around the world and even play a role in reducing the number of women who die after giving birth.
How up to date is this evidence?
We searched for all available evidence up to 6 September 2024.
What evidence did we find?
We identified three studies that investigated the effects of preventive TXA. The three studies involved a total of 18,974 participants at low or high risk of heavy bleeding. Participants were given either intravenous (into a vein) TXA plus standard care or placebo (saline) injection plus standard care.
We found that preventive TXA results in little to no difference in heavy bleeding after birth.
We are very uncertain about the effect of TXA on maternal death. TXA likely makes no difference to the risk of women developing serious illness.
We found that TXA has no effect on the likelihood of receiving a blood transfusion. TXA may result in no difference in the need for further surgical intervention after giving birth. We are very uncertain about the effect of TXA on blood clots. In women with anaemia, TXA makes no difference to the need for additional drugs to help the womb contract, but in women with no anaemia, there was a slight reduction in this outcome. We are very uncertain about the effect of TXA on hysterectomy (an operation to remove the womb). There does not seem to be a difference in maternal satisfaction.
What are the limitations of the evidence?
The studies included women in both high‐ and low‐resource settings. Few women experienced harmful effects. However, we cannot be certain that this is indeed the case in the real world.
What does this mean?
We found no difference in the number of women experiencing heavy bleeding after birth after they were given TXA preventatively during vaginal birth, and we are very uncertain about the effect of TXA on blood clots and other serious side effects. As these are harmful effects, clinicians should take into account the lack of benefit and the potential harms when considering giving routine TXA to women during vaginal birth.
Further research should focus on other interventions that may help to prevent heavy bleeding after vaginal birth.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Prophylactic tranexamic acid in addition to standard care compared to placebo in addition to standard care in women having vaginal births.
| Prophylactic tranexamic acid in addition to standard care compared to placebo in addition to standard care in women having vaginal births | ||||||
| Patient or population: women having vaginal births Setting: hospitals Intervention: prophylactic tranexamic acid in addition to standard care Comparison: placebo in addition to standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo in addition to standard care | Risk with prophylactic tranexamic acid in addition to standard care | |||||
| Estimated blood loss ≥ 500 mL ‐ total | 77 per 1000 | 72 per 1000 (62 to 82) | RR 0.93 (0.81 to 1.06) | 18897 (3 RCTs) | ⊕⊕⊕⊕ High | Prophylactic tranexamic acid in addition to standard care results in little to no difference in blood loss ≥ 500 ml. |
| Estimated blood loss ≥ 1000 mL | 18 per 1000 | 16 per 1000 (13 to 19) | RR 0.86 (0.69 to 1.07) | 18897 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | Prophylactic tranexamic acid in addition to standard care likely results in little to no difference in blood loss ≥ 1000 ml. |
| Maternal death | 1 per 1000 | 1 per 1000 (0 to 3) | RR 0.99 (0.39 to 2.49) | 15081 (2 RCTs) | ⊕⊝⊝⊝ Very lowb | The evidence is very uncertain about the effect of prophylactic tranexamic acid in addition to standard care on maternal death. There were 9 events in each group. |
| Severe morbidity | 18 per 1000 | 16 per 1000 (13 to 20) | RR 0.88 (0.69 to 1.12) | 15066 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Prophylactic tranexamic acid in addition to standard care likely results in little to no difference in severe morbidity. |
| Receipt of blood transfusion | 202 per 1000 | 202 per 1000 (192 to 214) | RR 1.00 (0.95 to 1.06) | 18972 (3 RCTs) | ⊕⊕⊕⊕ High | Prophylactic tranexamic acid in addition to standard care results in little to no difference in receipt of blood transfusion. |
| Receipt of additional surgical interventions to control postpartum haemorrhage | 2 per 1000 | 1 per 1000 (1 to 3) | RR 0.63 (0.32 to 1.23) | 18972 (3 RCTs) | ⊕⊕⊝⊝ Lowc | Prophylactic tranexamic acid in addition to standard care may result in little to no difference in receipt of additional surgical interventions to control postpartum haemorrhage. |
| Thromboembolic events | 4 per 10,000 | 1 per 10,000 (0 to 10) | RR 0.25 (0.03 to 2.24) | 18774 (3 RCTs) | ⊕⊝⊝⊝ Very lowb | The evidence is very uncertain about the effect of prophylactic tranexamic acid in addition to standard care on thromboembolic events. This was a rare event with only 1 event in the TXA group and 4 in the placebo group. |
| Receipt of additional uterotonics ‐ Women with anaemia | 138 per 1000 | 141 per 1000 (130 to 152) | RR 1.02 (0.94 to 1.10) | 15066 (1 RCT) | ⊕⊕⊕⊕ High | Prophylactic tranexamic acid in addition to standard care results in little to no difference in receipt of additional uterotonics in women with anaemia. |
| Receipt of additional uterotonics ‐ Women with no anaemia | 97 per 1000 | 73 per 1000 (59 to 89) | RR 0.75 (0.61 to 0.92) | 3891 (1 RCT) | ⊕⊕⊕⊕ High | Prophylactic tranexamic acid in addition to standard care results in a slight reduction in receipt of additional uterotonics in women with no anaemia. |
| Hysterectomy | 13 per 10,000 | 12 per 10,000 (5 to 29) | RR 0.89 (0.36 to 2.19) | 15066 (1 RCT) | ⊕⊝⊝⊝ Very lowb | The evidence is very uncertain about the effect of prophylactic tranexamic acid in addition to standard care on hysterectomy. This was a rare event with 9 events in the TXA group and 10 in the placebo group. |
| Maternal satisfaction | One study measured this outcome using a Likert scale questionnaire. The study found no difference between groups. | 3066 (1 RCT) | ⊕⊕⊕⊝ Moderated | Prophylactic tranexamic acid in addition to standard care likely results in little to no difference in maternal satisfaction. | ||
| Clinical diagnosis of postpartum haemorrhage ‐ Women with anaemia | 66 per 1000 | 70 per 1000 (62 to 79) | RR 1.05 (0.94 to 1.19) | 15066 (1 RCT) | ⊕⊕⊕⊕ High | Prophylactic tranexamic acid in addition to standard care results in little to no difference in clinical diagnosis of postpartum haemorrhage compared to placebo. |
| Clinical diagnosis of postpartum haemorrhage ‐ Women with no anaemia | 104 per 1000 | 77 per 1000 (63 to 95) | RR 0.74 (0.61 to 0.91) | 3906 (2 RCTs) | ⊕⊕⊕⊕ High | Prophylactic tranexamic acid in addition to standard care results in a slight reduction in clinical diagnosis of postpartum haemorrhage compared to placebo. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_451248226426734429. | ||||||
a Serious concerns about imprecision: the 95% CI includes both appreciable harm and benefit. Downgraded by one level b Extremely serious concerns about imprecision: the 95% CI includes both appreciable harm and benefit and the 95% CI is very wide. Events were rare. Downgraded by three levels c Very serious concerns about imprecision: the 95% CI includes both appreciable harm and benefit and the 95% CVI is very wide. Downgraded by two levels d Serious concerns about risk of bias: more than 20% attrition in both arms
Background
Description of the condition
Postpartum haemorrhage is one of the most common obstetric emergencies, occurring in up to 12% of all births. It is a leading cause of maternal mortality, particularly in low‐resource settings where 99% of deaths due to postpartum haemorrhage occur [1, 2, 3, 4]. For those who survive, many require costly, urgent care, and a prolonged hospital stay. Some women undergo a hysterectomy to control the bleeding, thereby denying them the chance to bear more children. The trauma of severe postpartum bleeding can also have a significant psychological impact and adversely affect a mother’s ability to breastfeed and bond with her baby [5].
Primary postpartum haemorrhage is often defined as blood loss greater than or equal to 500 mL after a vaginal birth, or greater than or equal to 1000 mL after a caesarean birth, within 24 hours of birth [6]. However, many patients, such as those with anaemia or with existing cardio‐respiratory or hepatic disease, are less able to compensate for blood loss and even a small amount of blood loss can be harmful.
Most cases of postpartum haemorrhage are attributed to uterine atony (when the uterus fails to contract after giving birth), although bleeding due to trauma to the genital tract, surgical bleeding after caesarean birth, retention of placental tissue, and failure of the coagulation system are other common causes [2]. The implementation of evidence‐based third stage of labour care [7], in particular the use of prophylactic oxytocin, has been the focus of efforts to reduce postpartum haemorrhage. However, the number of patients suffering postpartum haemorrhage remains high, and, indeed, in some populations, is increasing. There is a need for other effective interventions beyond the use of uterotonic drugs, which are medications that increase uterine muscle contractions.
Description of the intervention and how it might work
Tranexamic acid (TXA) is an anti‐fibrinolytic drug that inhibits fibrinolysis by blocking the interaction between plasmin and fibrin. Fibrinolysis is a physiological process that breaks down blood clots and prevents them from growing and causing problems. Since it was first identified in the early 1960s, TXA has primarily been used for heavy menstrual bleeding and for reducing bleeding during surgical procedures in patients at high risk of severe bleeding. More recently, early treatment with TXA has been shown to be effective in patients with life‐threatening haemorrhage caused by trauma and childbirth [8, 9].
The WOMAN trial assessed the effects of intravenous TXA in 20,060 participants with a clinical diagnosis of postpartum haemorrhage. The results showed that TXA reduces the risk of bleeding to death after postpartum haemorrhage by a third (RR 0.69, 95% CI 0.52 to 0.91; P = 0.008) when given within three hours of birth, with no apparent increase in adverse events [10]. As a result, the World Health Organization (WHO) recommends early intravenous TXA as a treatment for established postpartum haemorrhage [11]. However, there was no reduction in overall deaths or hysterectomy, which was the primary outcome of the trial.
Many deaths from postpartum haemorrhage occur on the day of birth, most within the first few hours after birth. Thus, for many patients who suffer postpartum haemorrhage, treatment is too late. This, coupled with the knowledge that TXA is most effective when given shortly after the onset of bleeding, provides reason to hope that prophylactic TXA could reduce the risk of PPH. The growing interest in prophylactic TXA has led to a growth in the number of randomised trials exploring its effects in the obstetric population. However, the quality of many of these trials has been questioned and uncertainties around their effects remain unresolved [12].
Why it is important to do this review
This is an update of a Cochrane review first published in 2010 [13] and previously updated in 2015 [14].
Authors of the 2015 update concluded that "TXA (in addition to uterotonic medications) decreases postpartum blood loss and prevents PPH and blood transfusions following vaginal birth and caesarean birth in women at low risk of PPH based on studies of mixed quality"[14]. Several new trials have been conducted in recent years and there is a need to update this review using updated methodology.
The WHO recommends intravenous TXA as a treatment for severe bleeding after childbirth [11]. Increasing TXA use by giving it to patients shortly before vaginal birth or immediately after cord clamping, could confer substantial additional health benefits, but requires robust evidence of its effects, including its safety. Because of the antifibrinolytic action of TXA, concerns persist regarding an increased risk of thrombosis associated with the use of TXA, although no such increased risk has been observed in most randomised trials [15]. Nevertheless, further reassurance based on evidence from the obstetric population may be prudent before widespread prophylactic use is considered.
In general, randomised trials are designed and powered to detect specific potential benefits of an intervention. Known potential adverse effects can be measured, but most trials are underpowered to detect uncommon adverse effects even if these are measured, and are unable to detect unknown adverse effects that are not measured. Given the inevitable uncertainty about possible unknown adverse effects, robust evidence of meaningful clinical benefit is needed to justify the use of an intervention. This is particularly so for prophylactic interventions, where large numbers of individuals will receive an intervention, most of whom would not have developed the condition and therefore do not stand to benefit at all from the intervention but are exposed to possible, as yet undiscovered, risks.
In the case of tranexamic acid, potential adverse effects need to be considered. In the CRASH 2 trial [16], participants who received TXA up to 1 hour and 1 to 3 hours after injury had reduced mortality (32% and 21% reduction, respectively), but those who received TXA after three hours had a 44% increased risk of mortality (144/3272 (4.4%) versus 103/3362 (3.1%); RR 1.44; 95% CI 1.12 to 1.84; P = 0.004). To our knowledge, the mechanism of this effect is not yet understood. In addition, pharmacovigilance data suggest an increased risk of renal ischaemic adverse drug events for women of childbearing age who receive tranexamic acid [17, 18]. Tranexamic acid has also been associated with an increased risk of seizures [19, 20].
For these reasons, it is important to ensure that decisions regarding the prophylactic use of TXA are based on the most rigorous evaluation of the evidence, taking into account potential but as yet poorly understood adverse effects.
This review is an update of a previous Cochrane review [14]. The original review has been split into two: one review of TXA for caesarean birth [21] and this one of TXA for vaginal birth. In addition to updated searches, this review includes revisions made to comply with changes in methodological approaches introduced since the previous version was prepared. Furthermore, in light of quality and integrity concerns affecting trials on this topic, we have implemented additional measures to help ensure that only the most reliable evidence is included.
Objectives
To assess the effects of tranexamic acid for preventing postpartum haemorrhage in comparison to placebo or no treatment (with or without uterotonic co‐treatment) in women following vaginal birth.
Methods
We followed the Methodological Expectations of Cochrane Intervention Reviews (MECIR) when conducting the review and PRISMA 2020 for the reporting. Several amendments have been made to this review since the protocol and the previous updates were published. Details are available in Supplementary material 8. The title of the review has changed since the protocol was published. The protocol is publicly available [22]. This update of the review has been conducted as two separate reviews: a review of studies involving participants with caesarean births has been recently published [21]; this review includes studies involving participants with vaginal births.
Criteria for considering studies for this review
Types of studies
We searched for all published, unpublished, and ongoing randomised controlled trials (RCTs) comparing the use of tranexamic acid (TXA) alone or in addition to uterotonics during vaginal birth to prevent postpartum haemorrhage. We excluded quasi‐RCTs (e.g. randomisation by date of birth, hospital number, or alternation). No cluster‐randomised controlled trials were identified in our search.
Since the first version of the review was published, concerns regarding the integrity of several trials in obstetrics, including those assessing the effects of TXA for preventing PPH, have been raised [7, 12]. For this reason, we added the inclusion criterion that trials must be prospectively registered (i.e. registered before the first participant was enroled) in a trial registry. We also applied current Cochrane trustworthiness criteria.
Types of participants
Women undergoing vaginal birth at any age or within any care setting. Women at low risk of postpartum haemorrhage or with specific risk factors for postpartum haemorrhage as reported by trial authors were included. We considered studies that only included a subset of relevant participants for inclusion. For example, in studies that included both caesarean and vaginal births, we extracted only relevant data that were stratified according to type of birth. If this information was not clear, we contacted trial authors for additional information.
Types of interventions
We included studies that compared TXA used during vaginal birth to prevent postpartum haemorrhage, alone or in combination with standard treatment (e.g. other uterotonics) versus placebo or standard treatment, or both, or no intervention. We included any dose of TXA given at any time point shortly before or after the birth. We considered intravenous, intramuscular, and oral routes of administration, but we excluded topical application of TXA as this is the subject of an existing Cochrane review [23].
Outcome measures
Critical outcomes
Blood loss ≥ 500 mL
Blood loss ≥ 1000 mL
For both outcomes, blood loss that was measured up to 48 hours after vaginal birth was considered.
We included the following measurements of blood loss:
estimated blood loss: gravimetrically estimated measures of blood loss (using surgical drapes or sponges that collect blood to measure blood loss) and provider‐estimated measures of blood loss (subjective visual estimation of how much blood was lost);
calculated blood loss (calculations based on haematocrit or haemoglobin levels pre‐ and post‐vaginal birth, height, and weight).
Important outcomes
Maternal death before hospital discharge or at time points reported by study authors
Severe morbidity (defined as maternal deaths or severe morbidity events including major surgery (laparotomy, uterine artery ligation, internal iliac artery ligation, B‐Lynch suture, hysterectomy, extensive vaginal repair), admission to the intensive care unit, or vital organ failure (temporary or permanent)) before hospital discharge or at time points reported by study authors
Receipt of blood transfusion before hospital discharge or at time points as reported by study authors
Receipt of additional surgical interventions to control postpartum haemorrhage (including laparotomy, compression suture of uterus, devascularisation of the uterus and hysterectomy) before hospital discharge or at time points reported by study authors
Thromboembolic events including pulmonary embolism and deep vein thrombosis before hospital discharge or at time points reported by study authors
Receipt of additional uterotonics including oxytocin, misoprostol, ergometrine, carbetocin, or others reported by authors, before hospital discharge or at time points reported by study authors
Hysterectomy before hospital discharge or at time points reported by study authors
Maternal satisfaction before hospital discharge or at time points reported by study authors
Breastfeeding at discharge or at time points reported by study authors
-
Mean blood loss (mL):
estimated blood loss: gravimetrically estimated measures of blood loss (using surgical drapes or sponges that collect blood to measure blood loss) and provider‐estimated measures of blood loss (subjective visual estimation of how much blood was lost);
calculated blood loss: calculations based on haematocrit or haemoglobin levels pre‐ and post‐vaginal birth, and weight.
Myocardial infarction before hospital discharge or at time points reported by study authors
Stroke before hospital discharge or at time points reported by study authors
Seizures before hospital discharge or at time points reported by study authors
Organ failure/dysfunction before hospital discharge or at time points reported by study authors
Intensive care unit (ICU) admission before hospital discharge or at time points reported by study authors
Nausea before hospital discharge or at time points reported by study authors
Vomiting before hospital discharge or at time points reported by study authors
Headache before hospital discharge or at time points reported by study authors
Maternal well‐being before hospital discharge or at time points reported by study authors
Postpartum anaemia (Hb (haemoglobin) < 9 g/dL) before hospital discharge or at time points reported by study authors
Skin reactions before hospital discharge or at time points reported by study authors
Search methods for identification of studies
Electronic searches
For this update, the Information Specialists of the Cochrane Pregnancy and Childbirth Group searched the Cochrane Pregnancy and Childbirth Group's Trials Register for records available up to 20 June 2021. On 6 September 2024, one review author (KK) carried out further updated searches of MEDLINE (OvidSP 1946 to 6 September 2024), Embase (OvidSP 1980 to 2024 week 36), CENTRAL (2024, Issue 9) and WHO ICTRP (trialsearch.who.int/). These searches were limited to evidence added since 20 June 2021. The search strategies for each database are provided in Supplementary material 1.
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
For the methods used in the previous version of this review, see [13 ]and [14]. For this update, we used the following methods to assess the reports that we identified as a result of the updated search.
Selection of studies
To determine which new studies should be included in this update, two review authors (CR and KK or CR and AR) independently assessed all the titles, abstracts and full texts of potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, consultation with all authors. We created a study flow diagram to map out the number of records identified, included, excluded, ongoing, or awaiting classification.
All studies meeting our inclusion criteria were evaluated by two review authors (KK and CR or CR and AR) against predefined criteria to select studies that, based on available information, were deemed to be sufficiently trustworthy to be included in the analysis. We resolved discrepancies through discussion and consultation with all authors.
The trustworthiness criteria of the Cochrane Pregnancy and Childbirth Checklist were as follows [24].
Research governance
Are there any retraction notices or expressions of concern listed on the Retraction Watch Database relating to this study?
Was the study prospectively registered (for those studies published after 2010)? If not, was there a plausible reason?
When requested, did the trial authors provide/share the protocol and/or ethics approval letter?
Did the trial authors engage in communication with the Cochrane review authors within the agreed timelines?
Did the trial authors provide individual patient data upon request? If not, was there a plausible reason?
Baseline characteristics
Is the study free from characteristics of the study participants that appear too similar (e.g. distribution of the mean (standard deviation) excessively narrow or excessively wide, as noted by [25])?
Feasibility
Is the study free from characteristics that could be implausible (e.g. large numbers of participants with a rare condition (such as severe cholestasis in pregnancy) recruited within 12 months)?
In cases with (close to) zero losses to follow‐up, is there a plausible explanation?
Results
Is the study free from results that could be implausible (e.g. massive risk reduction for main outcomes with small sample size)?
Do the numbers randomised to each group suggest that adequate randomisation methods were used (e.g. is the study free from issues such as unexpectedly even numbers of participants ‘randomised’ or a mismatch between the numbers and the methods)? For example, if the authors say ‘no blocking was used’ but still end up with equal numbers of participants in each group, or if the authors say they used ‘blocks of four’ but the final numbers differ by six, this could raise concerns.
Where a study was classified as being at ‘high risk’ of untrustworthiness, we attempted to contact the study authors to address any possible lack of information or concerns. In cases where we could not obtain contact details for the study authors, or where adequate information remained unavailable, we put the study in the ‘awaiting classification’ category and described the reasons and communications with the trial author (or lack thereof).
Abstracts
We included data from abstracts if the study passed the trustworthiness assessment, and the study authors confirmed in writing that the data to be included in the review are from the final analysis and will not change. If such information was not available or provided, we put the study in the ‘awaiting classification’ category (as above). See Figure 1 for details of how we applied the trustworthiness screening criteria.
1.
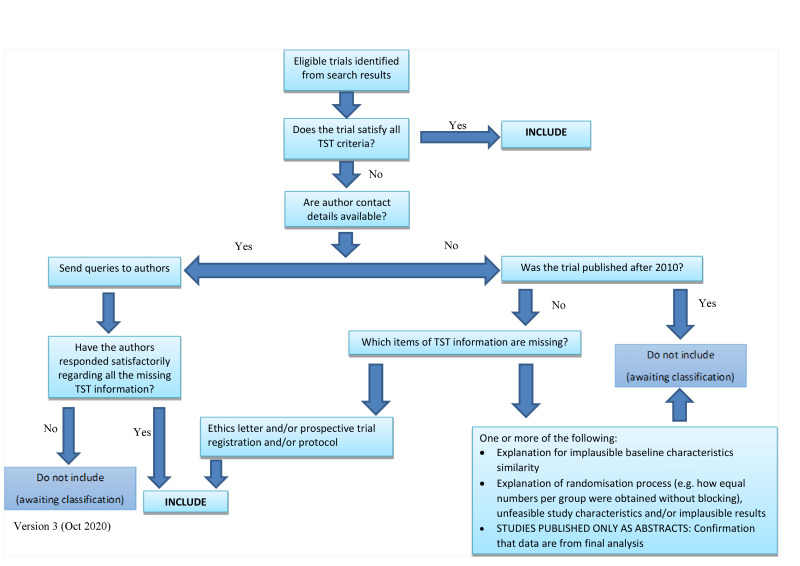
Applying the trustworthiness screening tool criteria No copyright, reproduced with permission from author Zarko Alfirevic [24]
Data extraction and management
We designed a form to extract data using Covidence [26]. Two review authors (CR and KK, or CR and AR) extracted data using this form. We resolved any discrepancies through discussion. When information regarding any of the above was unclear, we attempted to contact the authors of the original reports to provide further details.
In addition to the main outcomes and details of trial design, we systematically extracted the following data for each trial.
Characteristics of participants, including risk of postpartum haemorrhage
Inclusion and exclusion criteria
Characteristics of the intervention, including dose, timing, route of administration
Co‐interventions (e.g. use of other uterotonics)
Duration and technique of assessment of blood loss
Loss to follow‐up
If review authors were authors of studies that could potentially be included in the review, they were not involved in making decisions about the eligibility of these studies.
Risk of bias assessment in included studies
Two review authors (CC and KK, or CR and AR) independently assessed the risk of bias in each study using the Cochrane RoB 1 tool and the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions [27]. We resolved any disagreement by discussion. Review authors who were authors of included studies were not involved in making decisions about the risk of bias in their own studies.
Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the method as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
Blinding of participants, personnel and outcome assessors (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel;
low, high, or unclear risk of bias for outcome assessors.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data)
For each included study, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where these were reported, and whether missing data were balanced across groups or were related to outcomes. Where the study authors conducted sensitivity analysis of missing data for specific outcomes, we considered this when making judgements about attrition bias.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
Other bias (checking for bias due to problems not covered by domains above)
For each included study, we described any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias and judged it to be:
low risk of other bias;
high risk of other bias; or
unclear risk of other bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we used the mean difference (MD) with 95% CI because the outcomes were measured in the same way across trials. In future updates of this review, we will use the standardised mean difference to combine data from trials that use different methods to measure the same outcome.
Unit of analysis issues
Cluster‐randomised trials
If, in future updates of this review, we identify cluster‐randomised trials for inclusion, we will include them in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine results from both if there is little heterogeneity between the study designs and interaction between the effect of intervention and the choice of randomisation unit is considered unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not included as they are irrelevant for this intervention.
Multi‐arm trials
If, in future updates of this review, we identify multi‐arm trials for inclusion, we will combine all relevant experimental intervention groups in the trial into a single group and all relevant control intervention groups into a single control group for relevant outcomes. We will combine both the sample sizes and the number of people with events from all groups for dichotomous outcomes. For continuous outcomes, we will combine means and standard deviations as per the Cochrane Handbook for Systematic Reviews of Interventions. Where we consider one of the arms irrelevant, we will exclude it from the analysis [28].
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and to analyse all participants in the group to which they were allocated, regardless of whether they received the allocated intervention.
Where data were missing because the outcome was not measured in all participants, the reason for missing data was unrelated to the outcome, and missing data were balanced between groups, the denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing (modified intention‐to‐treat analysis). Where no explanation was given for missing outcome data, missing data were not balanced between groups, and we suspected that missing data were related to the outcome, we contacted the study authors and planned to conduct sensitivity analyses.
Reporting bias assessment
We planned to assess reporting biases if we included 10 or more studies in meta‐analysis; however, in this update (2024), no meta‐analysis included more than 10 studies. In future updates, if more studies are included, we will investigate reporting biases (such as publication bias) using funnel plots. We will visually assess funnel plot asymmetry.
Synthesis methods
We carried out statistical analysis using Review Manager (RevMan) [29]. We used random‐effects meta‐analysis for combining data as we expected clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials. The random‐effects summary is treated as the average of the range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If we did not consider the average treatment effect to be clinically meaningful, we planned not to combine trials. When we used random‐effects analyses, we presented the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
We used I² and Chi² statistics to measure heterogeneity amongst the trials in each analysis. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Investigation of heterogeneity and subgroup analysis
For analyses with substantial heterogeneity, we conducted subgroup analyses. We were able to perform subgroup analyses for participants with and without anaemia, and at high, low, and mixed risk for postpartum haemorrhage. We included secondary outcomes (e.g. thromboembolic events) in our subgroup analysis because of their potential association with the use of TXA. We used the interaction tests available within Review Manager [29] and reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
We plan to assess the following subgroups for all outcomes in future updates of this review if there are sufficient data:
different doses of TXA;
different routes of administering TXA;
different ways of estimating blood loss.
Equity‐related assessment
Although we did not carry out an equity‐related assessment, the topic of this review is of great importance for addressing health inequalities because of the high prevalence and disproportionally high impact of postpartum haemorrhage in low‐income countries.
Sensitivity analysis
We planned to perform sensitivity analyses for aspects of the review that might have affected the results, for example, where there was a risk of bias associated with the quality of some of the included trials or where there were high levels of missing data. We planned to perform sensitivity analysis for the critical outcomes (blood loss ≥ 500ml and blood loss ≥ 1000 mL). As only one study contributed to the critical outcomes, we did not conduct sensitivity analysis.
Certainty of the evidence assessment
For this update, we assessed the certainty of the evidence using the GRADE approach [30] in order to assess the certainty of the body of evidence relating to all outcomes. To create summary of findings tables, we used GRADEpro GDT [31]. We produced a summary of the intervention effect and a measure of certainty for each of the above outcomes using the GRADE approach, which has five considerations: study design limitations, consistency of effect, imprecision, indirectness and publication bias. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, inconsistency, imprecision of effect estimates, or potential publication bias.
We performed the certainty of evidence assessments on 10 outcomes, namely blood loss ≥ 500 mL, blood loss ≥ 1000 mL, maternal death, severe morbidity, receipt of blood transfusions, receipt of additional surgical interventions to control postpartum haemorrhage, thromboembolic events, receipt of additional uterotonics, hysterectomy, and maternal satisfaction.
GRADE judgements were informed by the Standard Operating Procedures for grading evidence for guidelines put forward by the WHO. We judged imprecision by considering the range of the confidence interval of the relative effect as well as the total cumulative study population and the total number of events per outcome. We downgraded for imprecision if there were very few events (less than 30) and if the confidence interval was very wide. The threshold for suggested appreciable benefit for the relative effect was 0.75 and the threshold for suggested appreciable harm was 1.25. Where the confidence interval crossed these thresholds, we downgraded the certainty of the evidence by one, two, or three levels depending on the width of the confidence interval.
Review authors who were also authors of included studies were not involved in making decisions about the certainty of the evidence.
Consumer involvement
We did not involve consumers in the review due to the pressure of the timeline for production and limited resources. However, our outcomes were informed by core outcome sets that had been developed with involvement from consumers [32].
Results
Description of studies
Results of the search
The PRISMA study flow diagram shows the results of the search and study selection process (Figure 2). Since the last version of the review was published, the search has been updated four times, with the most recent search being conducted on 6 September 2024. The combined searches yielded 405 records for title and abstract screening. We identified 143 records for full‐text review. Of these, we excluded 90 studies (109 records), mostly due to participants having caesarean births (see Excluded studies). We included three RCTs (reported in 12 records), placed 15 studies (16 records) in the 'awaiting classification' category, and six studies are ongoing.
2.
PRISMA flow diagram.
We excluded 10 studies that had been included in earlier versions of this review: two because their trial registration was retrospective (Abdel‐Aleem 2013 [33, 34]; Gungorduk 2013 [35]) and eight because they included women having caesarean deliveries (Gai 2004 [36]; Goswami 2013 [37]; Gungorduk 2011 [38]; Movafegh 2011 [39, 40]; Senturk 2013 [41]; Shahid 2013 [42]; Xu 2013 [43]; Yehia 2014 [44]). These latter studies were assessed for eligibility in the sister review we conducted, which focused on women having caesarean deliveries who received prophylactic tranexamic acid to prevent postpartum haemorrhage [21].
We placed two previously included studies in the awaiting classification category as no full text was available, and we were unable to obtain contact addresses for study authors (Mirghafourvand 2013 [45, 46]; Yang 2001 [47]).
The previous review had two studies awaiting classification. In this update we excluded both of these studies: one was retrospectively registered (Ahmed 2015 [48, 49, 50]) and the other included participants having a caesarean delivery (Bhavana 2013 [51, 52]).
We applied an assessment of trustworthiness to each study with the potential to be included in this update; studies without prospective registration were excluded. In instances where information about registration or other information was required, we contacted study authors via email. We placed studies in the awaiting classification category until an adequate response was received, and we could make a decision about eligibility. We judged 15 studies to have potential concerns regarding trustworthiness. Details are available in Supplementary material 4.
We identified six ongoing studies (CTRI 057612 2023 [53]; CTRI 066348 2024 [54]; CTRI 067790 2024 [55]; I’M WOMAN 2023 [56]; PACTR202306670143734 [57]; TAAPP‐V 2021 [58]). Details are available in Supplementary material 5.
In summary, this updated review comprises 3 included studies, 90 excluded studies, 6 ongoing studies, and 15 studies awaiting classification.
Included studies
We summarise the characteristics of the included studies in Table 2 and Supplementary material 2.
1. Overview of included studies and synthesis table.
| Study ID | Trial registration number | Country | Participants | Intervention | Control | Additional interventions | Outcomes |
| Alam 2023 | NCT03069859 | Canada | Women giving birth vaginally or via CB (elective or urgent), gestational age ≥ 32 weeks. Women at low risk of PPH; N = 27 total (15 VB and 12 CB) |
|
Placebo |
|
|
| Sentilhes 2018 | NCT02302456 | France | Women 18 years or older, with or without risk factors for PPH giving birth vaginally to a single live foetus, gestational age ≥ 35 weeks; N = 3891 |
|
Placebo |
|
|
| WOMAN‐2 2024 | ISRCTN62396133; NCT03475342; PACTR201909735842379 | Nigeria, Pakistan, Tanzania, and Zambia | Women of any age with moderate or severe anaemia (haemoglobin < 100 g/L) giving vaginal birth; N = 15,068 |
|
Placebo |
|
|
CB: caesarean birth; ICU: intensive care unit; IVI: intravenous injection; N: number of participants; PPH: postpartum haemorrhage; VB: vaginal birth
Participants
Three trials involving a combined total of 18,974 randomised participants investigated the efficacy of tranexamic acid (TXA) for preventing postpartum haemorrhage in participants giving birth vaginally. The largest trial, WOMAN‐2 2024 [59, 60, 61, 62] (n = 15,068), contributed over 79% of the total number of participants included in this review, whilst Sentilhes 2018 [63, 64, 65, 66, 67, 68] (n = 3891) contributed almost 21%. The third trial, Alam 2023 [69, 70], included 27 women giving birth vaginally and by caesarean section, with stratified data for the 15 women giving birth vaginally. The combined number of participants in the trials was 18974.
The largest trial, WOMAN‐2 2024, included women with moderate or severe anaemia. Sentilhes 2018, the other large trial, included women with or without risk factors for postpartum haemorrhage. More than 70% of the participants in this trial were deemed to be at low risk of postpartum haemorrhage and less than 30% had at least one risk factor for postpartum haemorrhage. Alam 2023 included women with no risk factors for postpartum haemorrhage.
Interventions
All three studies assessed the effect of tranexamic acid in addition to standard care compared to placebo in addition to standard care, and all trials administered oxytocin to all participants on delivery of the baby (Alam 2023; Sentilhes 2018; WOMAN‐2 2024).
Timing of TXA
In Sentilhes 2018, the trial regimen was administered by slow intravenous injection (over a period of 30 to 60 seconds) during the two minutes after delivery, after the routine prophylactic intravenous injection of oxytocin at delivery of the anterior shoulder and clamping of the umbilical cord.
In WOMAN‐2 2024, the women received the trial regimen by slow intravenous injection (about 1 mL per minute) as soon as possible but no later than 15 minutes after the umbilical cord was cut or clamped. In Alam 2023, it was administered at the time of shoulder delivery.
Route and dose of TXA
Intravenous TXA (1 g) was administered as a fixed dose in all trials (Alam 2023; Sentilhes 2018; WOMAN‐2 2024).
Comparisons
TXA was compared to placebo (normal saline) in all trials (Alam 2023; Sentilhes 2018; WOMAN‐2 2024).
Measurement of blood loss ≥ 500 mL
In the largest trial (WOMAN‐2 2024), blood loss was measured by provider estimation. The research teams were trained to estimate blood loss from the point of delivery until 24 hours after birth, or until earlier discharge, by monitoring and documenting the number of blood‐soaked pads used by the women during this time. Pictograms were provided to help with estimating.
Sentilhes 2018 measured blood loss using gravimetrical measurements. Blood loss was measured using a graduated collector bag that was placed just after delivery and remained in place for at least 15 minutes and until the birth attendant considered that the bleeding had stopped.
Alam 2023 did not measure this outcome.
Measurement of blood loss ≥ 1000 mL
This outcome was measured in the same way as the outcome of blood loss ≥ 500 mL.
Contact with trial authors
We emailed the corresponding authors of all trials to request additional information about outcomes. We received information from the authors of Alam 2023 and WOMAN‐2 2024.
The Alam 2023 trial measured the incidence of clinically diagnosed postpartum haemorrhage, blood transfusions, receipt of additional surgical interventions, intensive care unit (ICU) admission, organ failure, thromboembolic events, and seizures. However, none of the 15 participants relevant to this review experienced any of these events.
Excluded studies
Details of the excluded studies are available in Supplementary material 3. We excluded 90 studies for the reasons listed below.
Caesarean births (Bangsah 2023 [71]; Bhatia 2015 [72]; Bhavana 2013; Bose 2017 [73]; Chandak 2017 [74]; Dawoud 2023 [75]; Ducloy‐Bouthors 2018 [76, 77, 78]; ETAPPH 2023 [79]; Gai 2004; Ghosh 2014 [80]; Gobbur 2011 [81]; Gobbur 2014 [82]; Gohel 2007 [83]; Goswami 2013; Gungorduk 2011; Gwanzura 2018 [84]; Gwanzura 2022 [85]; Halder 2013 [86]; Hemapriya 2020 [87]; Ifunanya 2019 [88]; Jafarbegloo 2021 [89]; Kafayat 2019 [90]; Lakshmi 2016 [91]; Lee 2023 [92]; Maged 2015 [93, 94]; Malathi 2016 [95]; Markley 2015 [96]; Masood 2023 [97]; Milani 2019 [98]; Moradan 2018 [99]; Movafegh 2011; Naeiji 2020 [100]; Nandal 2022 [101]; Nargis 2020 [102]; NCT06060327 [103]; NCT02350179 [104]; NCT02688127 [105]; NCT03463993 [106]; Obi 2019 [107]; Ogunkua 2022 [108]; Ortuanya 2024 [109]; Pacheco 2023 [110, 111]; Pakniat 2019 [112]; Poonia 2012 [113]; Ramani 2014 [114]; Ramesh 2015 [115]; Rashid 2024 [116]; Rashmi 2012 [117]; Ray 2016 [118]; Sahu 2019 [119]; Salas 2017 [120]; Sallam 2018 [121]; Sekhavat 2009 [122]; Senturk 2013; Shah 2019 [123, 124]; Shahid 2013; Shalaby 2022 [125]; Sharma 2011 [126]; Shinde 2022 [127]; Singh 2014 [128]; Singh 2023 [129]; Sujata 2016 [130, 131]; TA TEG [132]; Taj 2014 [133]; TAPPAS [134, 135]; Tarabrin 2012a [136, 137]; Tarabrin 2012b [138, 139]; Torky 2020 [140]; TRAAP‐2 2021 [141, 142, 143, 144, 145]; TRAAPrevia [146]; Vishal 2023 [147]; WOMAN‐PharmacoTXA 2023 [148]; Xu 2013; Yehia 2014)
Retrospectively registered (Abdel‐Aleem 2013; Ahmed 2015; Gungorduk 2013; Igboke 2022 [149]; Ismail 2017 [150]; Samimi 2013 [151]; Shirazi 2012 [152]; Sujita 2018 [153, 154])
Not registered (Abbas 2019 [155])
Women with established postpartum haemorrhage at the time of randomisation (Hunt 2013 [156]; Javadi 2015 [157]; Sahaf 2014 [158]; Zargar 2018 [159])
Retracted (Arya 2023 [160])
Ineligible comparator (Ragusa 2024 [161])
Studies awaiting classification
We placed 15 studies in the 'awaiting classification' category as the information on methods that was presented or available from study authors was inadequate to permit inclusion. We contacted authors via email but did not receive any response (Al‐Nasrawi 2019 [162]; Cetin 2023 [163]; Diab 2020 [164]; Farhadifar 2021 [165]; Hinchigeri 2024 [166]; Mei 2019 [167]; Mirghafourvand 2013; Roy 2016 [168]; Shady 2017 [169]; Shady 2019 [170]; Shah 2024 [171]; Tali 2016 [172]; Yang 2001; Zhang 2024 [173]; Zheng 2000 [174]). Details are available in Supplementary material 4.
Risk of bias in included studies
We present the risk of bias in the included trials in Figure 3 and Figure 4. The details of our bias assessment are described in the Supplementary material 2 tables.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
4.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
We deemed all three trials to be at low risk of bias (Alam 2023; Sentilhes 2018; WOMAN‐2 2024).
Two trials used computer‐generated randomisation and a method of central randomisation (Alam 2023; Sentilhes 2018). In one trial (WOMAN‐2 2024), randomisation codes were generated by an information technology expert and a statistician who were not involved in the conduct of the trial. All trials had adequate allocation concealment as identical study kits were prepared. Therefore, we deemed the trials at low risk of selection bias.
All trials reported that participants, clinicians and investigators were blinded to allocation, and we deemed them at low risk of performance or detection bias.
All trials had minimal missing outcome data and presented outcome data on an intention‐to‐treat basis. Thus, we judged them to be at low risk of attrition bias. In Sentilhes 2018, data on the primary outcome were missing for 24 women in the tranexamic acid group and for 28 in the placebo group because no collector bag was available. An analysis done by trial authors using imputed data for missing values showed similar results.
All trials were prospectively registered, which was a criterion for inclusion in this review update, and data on all prespecified outcomes were presented. All trials were therefore rated as being at low risk of selective reporting bias.
We had no concerns about a risk of bias in any other aspect of the trials.
Synthesis of results
Details of our analyses are available in Supplementary material 6.
Prophylactic tranexamic acid in addition to standard care compared to placebo in addition to standard care
Critical outcomes
1.1 Estimated blood loss ≥ 500 mL
Prophylactic tranexamic acid in addition to standard care results in little to no difference in blood loss ≥ 500 mL compared to placebo (RR 0.93, 95% CI 0.81 to 1.06; P = 0.26, I2 = 23%; 2 RCTs, 18,897 participants; high‐certainty evidence), resulting in 5 fewer women per 1000 experiencing blood loss ≥ 500 mL (from 15 fewer to 5 more) (Analysis 1.1). Two RCTs contributed to the pooled effect (Sentilhes 2018; WOMAN‐2 2024). A forest plot of this analysis is available in Figure 5.
5.

Analysis 1.1 Blood loss ≥ 500 ml
There was no evidence of a subgroup difference between women at low risk versus women at high risk for PPH (test for subgroup differences: Chi² = 1.51, df = 1 (P = 0.22), I² = 33.6%). A forest plot of this subgroup analysis is available in Figure 5.
1.2 Calculated blood loss ≥ 500 mL
No trials measured this outcome.
1.3. Estimated blood loss ≥ 1000 mL
Prophylactic tranexamic acid in addition to standard care likely results in little to no difference in blood loss ≥ 1000 mL compared to placebo (RR 0.86, 95% CI 0.69 to 1.07; P = 0.19, I2 = 0%; 2 RCTs, 18,897 participants; moderate‐certainty evidence), resulting in 3 fewer women per 1000 experiencing blood loss ≥ 1000 mL (from 6 fewer to 1 more) (Analysis 1.3). Two RCTs contributed to the pooled effect (Sentilhes 2018; WOMAN‐2 2024). A forest plot of this analysis is available in Figure 6.
6.

Analysis 1.2 Blood loss ≥ 1000 ml
1.4 Calculated blood loss ≥ 1000 mL
No trials measured this outcome.
Important outcomes
1.5 Maternal death
The evidence is very uncertain about the effect of prophylactic tranexamic acid in addition to standard care on maternal death compared to placebo (RR 0.99, 95% CI 0.39 to 2.49; P = 0.98, I2 = not applicable; 2 RCTs, 15,081 participants; very low‐certainty evidence), resulting in 0 fewer women per 1000 dying (from 1 fewer to 2 more) (Analysis 1.5). This was a rare outcome with 9 events in each group. Two RCTs contributed to the pooled effect (Alam 2023; WOMAN‐2 2024). A forest plot of this analysis is available in Figure 7.
7.

Analysis 1.5 Maternal death
1.6 Severe morbidity
Prophylactic tranexamic acid in addition to standard care likely results in little to no difference in severe morbidity compared to placebo (RR 0.88, 95% CI 0.69 to 1.12; P = 0.30, I2 = not applicable; 1 RCT, 15,066 participants; moderate‐certainty evidence), resulting in 2 fewer women per 1000 experiencing severe morbidity (from 6 fewer to 2 more) (Analysis 1.6). One RCT contributed to the effect (WOMAN‐2 2024). A forest plot of this analysis is available in Figure 8.
8.

Analysis 1.6 Severe morbidity
1.7 Receipt of blood transfusion
Prophylactic tranexamic acid in addition to standard care results in little to no difference on receipt of blood transfusion compared to placebo (RR 1.00, 95% CI 0.95 to 1.06; P = 0.92, I2 = 0%; 3 RCTs, 18,972 participants; high‐certainty evidence), resulting in 0 fewer women per 1000 receiving a blood transfusion (from 10 fewer to 12 more) (Analysis 1.7). Three RCTs contributed to the pooled effect (Alam 2023; Sentilhes 2018; WOMAN‐2 2024). A forest plot of this analysis is available in Figure 9.
9.

Analysis 1.5 Receipt of blood transfusion
1.8 Receipt of additional surgical interventions to control postpartum haemorrhage
Prophylactic tranexamic acid in addition to standard care may result in little to no difference in receipt of additional surgical interventions to control postpartum haemorrhage compared to placebo (RR 0.63, 95% CI 0.32 to 1.23; P = 0.18, I2 = 0%; 2 RCTs, 18,972 participants; low‐certainty evidence), resulting in 1 fewer women per 1000 receiving surgical intervention to control postpartum haemorrhage (from 2 fewer to 1 more) (Analysis 1.8). Three RCTs contributed to the pooled effect (Alam 2023; Sentilhes 2018; WOMAN‐2 2024). A forest plot of this analysis is available in Figure 10.
10.

Analysis 1.6 Receipt of additional surgical interventions to control postpartum haemorrhage
1.9 Thromboembolic events
The evidence is very uncertain about the effect of prophylactic tranexamic acid in addition to standard care on thromboembolic events compared to placebo (RR 0.25, 95% CI 0.03 to 2.24; P = 0.22, I2 = not applicable; 3 RCTs, 18,774 participants; very low‐certainty evidence), resulting in 3 fewer women per 10,000 experiencing a thromboembolic event (from 4 fewer to 5 more) (Analysis 1.9). This was a rare outcome with 1 event in the TXA group and 4 events in the placebo group. Three RCTs contributed to the pooled effect (Alam 2023; Sentilhes 2018; WOMAN‐2 2024). A forest plot of this analysis is available in Figure 11.
11.

Analysis 1.7 Thromboembolic events
1.10 Receipt of additional uterotonics
There was high heterogeneity (I2 = 87%; no overlap in 95% CIs) when we pooled the two trials that had data for this outcome. As the test for subgroup differences was significant (Chi2 = 7.52; df = 1(P = 0.006); I2 = 86.7%), we present the subgroups separately for this outcome.
In women with anaemia
In women with anaemia, prophylactic tranexamic acid in addition to standard care results in little to no difference in receipt of additional uterotonics compared to placebo (RR 1.02, 95% CI 0.94 to 1.10; P = 0.62, I2 = not applicable; 1 RCT, 15,066 participants; high‐certainty evidence), resulting in 3 more women per 1000 receiving additional uterotonics (from 8 fewer to 24 more) (Analysis 1.10). One RCT contributed to the effect (WOMAN‐2 2024). Forest plot available at Figure 12.
12.

Analysis 1.8 Receipt of additional uterotonics
In women with no anaemia
In women with no anaemia, prophylactic tranexamic acid in addition to standard care results in a slight reduction in receipt of additional uterotonics compared to placebo (RR 0.75, 95% CI 0.61 to 0.92; P = 0.006, I2 = not applicable; 1 RCT, 3891 participants; high‐certainty evidence) resulting in 24 fewer women per 1000 receiving additional uterotonics (from 38 fewer to 8 fewer) (Analysis 1.10). One RCT contributed to the effect (Sentilhes 2018). A forest plot of this analysis is available in Figure 12.
1.11 Hysterectomy
The evidence is very uncertain about the effect of prophylactic tranexamic acid in addition to standard care on hysterectomy compared to placebo (RR 0.89, 95% CI 0.36 to 2.19; P = 0.80, I2 = not applicable; 1 RCT, 15,066 participants; very low‐certainty evidence), resulting in 1 fewer women per 10,000 having a hysterectomy (from 9 fewer to 16 more) (Analysis 1.11). This was a rare outcome with 9 events in the TXA group and 10 events in the placebo group. One RCT contributed to the effect (WOMAN‐2 2024). A forest plot of this analysis is available in Figure 13.
13.

Analysis 1.11 Hysterectomy
1.12 Maternal satisfaction
Prophylactic tranexamic acid in addition to standard care likely results in little to no difference in maternal satisfaction. The Sentilhes 2018 trial measured maternal satisfaction (3066 participants) on day two using a Likert‐scale questionnaire and reported that this did not differ significantly between the two groups (Analysis 1.12).
1.13 Breastfeeding at discharge
Prophylactic tranexamic acid in addition to standard care results in little to no difference in breastfeeding compared to placebo (RR 1.00, 95% CI 0.99 to 1.01; P = 0.82, I2 = not applicable; 1 RCT, 14,644 participants; high‐certainty evidence) resulting in 0 fewer women per 1000 breastfeeding (from 9 fewer to 13 more) (Analysis 1.1). One RCT contributed to the effect (WOMAN‐2 2024). A forest plot of this analysis is available in Figure 14.
14.

Analysis 1.13 Breastfeeding
1.14 Clinical diagnosis of PPH
There was high heterogeneity (I2 = 88%; no overlap in 95% CIs) when we pooled the two trials that had data for this outcome. As the test for subgroup differences was significant (Chi2 = 8.53; df = 1 (P = 0.003); I2 = 88.3%), we present the subgroups separately for this outcome.
In women with anaemia
Prophylactic tranexamic acid in addition to standard care results in little to no difference in clinical diagnosis of PPH compared to placebo (RR 1.05, 95% CI 0.94 to 1.19; P = 0.39, I2 = not applicable; 1 RCT, 15,066 participants; high‐certainty evidence) resulting in 3 more women per 1000 receiving a clinical diagnosis of PPH (from 4 fewer to 13 more) (Analysis 1.1). One RCT contributed to the effect (WOMAN‐2 2024). A forest plot of this analysis is available in Figure 15.
15.

Analysi 1.14 Clinical diagnosis of postpartum haemorrhage
In women with no anaemia
Prophylactic tranexamic acid in addition to standard care results in a slight reduction in the clinical diagnosis of PPH compared to placebo (RR 0.74, 95% CI 0.61 to 0.91; P = 0.04, I2 = not applicable; 2 RCTs, 3906 participants; high‐certainty evidence), resulting in 27 fewer women per 1000 receiving a clinical diagnosis of PPH (from 41 fewer to 9 fewer) (Analysis 1.1). Two RCTs contributed to the pooled effect (Alam 2023; Sentilhes 2018). A forest plot of this analysis is available in Figure 15.
1.15 Mean estimated blood loss
Prophylactic tranexamic acid in addition to standard care makes little to no difference in mean blood loss (mL) compared to placebo (mean difference (MD) ‐6.42, 95% CI ‐20.98 to 8.14; P = 0.39, I2 = 61%; 2 RCTs, 18,957 participants; high‐certainty evidence; Analysis 1.15). Two RCTs contributed to the pooled effect (Sentilhes 2018; WOMAN‐2 2024). A forest plot of this analysis is available in Figure 16.
16.

Analysis 1.12 Mean blood loss
1.16 Calculated mean blood loss
No trials measured this outcome.
1.17 Myocardial infarction
Two trials (Sentilhes 2018; WOMAN‐2 2024) (18,957 participants) measured this outcome and reported no events in either group (Analysis 1.17).
1.18 Stroke
Two trials (Sentilhes 2018; WOMAN‐2 2024) (18,957 participants) measured this outcome and reported no events in either group (Analysis 1.18).
1.19 Seizures
The evidence is very uncertain about the effect of tranexamic acid on seizures compared to placebo (RR 2.97, 95% CI 0.89 to 9.95; P = 0.08, I2 = 0%; 3 RCTs, 18,774 participants; very low‐certainty evidence). This was a rare outcome with 10 events in the TXA group and 3 events in the placebo group (Analysis 1.19). Two RCTs contributed to the pooled effect (Alam 2023; Sentilhes 2018). A forest plot of this analysis is available in Figure 17.
17.

Analysis 1.15 Seizures
One small trial reported no seizures in either study arm (Alam 2023). One trial stated that one participant in the tranexamic acid group reported seizures by day 30 postpartum during telephonic follow‐up in the context of sleep deprivation and acute alcohol intake (Sentilhes 2018). The clinical examination, CT (computed tomography) scan of the head, and electroencephalogram were normal, and she received no additional treatment. The WOMAN‐2 2024 trial reported 9 seizures in the TXA group and 3 in the placebo group.
1.20 Organ failure
All trials (Alam 2023; Sentilhes 2018; WOMAN‐2 2024) reported no organ failure in either group (Analysis 1.20).
1.21 ICU admission
One small trial (Alam 2023) reported no ICU admissions in either group (Analysis 1.21).
1.22 Nausea
The pooled result yielded heterogeneity of 92%. We did not pool results because the two studies showed different effects (test for subgroup differences Chi2 = 13.13; df = 1 (P < 0.001); I2 = 92.4%).
When nausea was measured in the delivery room in Sentilhes 2018, the effect of prophylactic tranexamic acid compared to placebo was RR 2.10 (95% CI 1.5 to 2.94; P < 0.001, I2 = not applicable; 1 RCT, 3891 participants; Analysis 1.22). A forest plot of this analysis is available in Figure 18.
18.

Analysis 1.18 Nausea
When nausea was measured within 24 hours or before hospital discharge in WOMAN‐2 2024, the effect of prophylactic tranexamic acid compared to placebo was RR 1.00 (95% CI 0.80 to 1.25; P = 0.99, I2 = not applicable; 1 RCT, 15,066 participants; Analysis 1.22). A forest plot of this analysis is available in Figure 18.
1.23 Vomiting
The pooled result yielded heterogeneity of 88%. We did not pool results because the two studies showed different effects (test for subgroup differences Chi2 = 8.55; df = 1 (P = 0.003); I2 = 88.3%).
When vomiting was measured in the delivery room in Sentilhes 2018, the effect of prophylactic tranexamic acid compared to placebo was RR 2.21 (95% CI 1.47 to 3.32; P < 0.001, I2 = not applicable; 1 RCT, 3891 participants; Analysis 1.23). A forest plot of this analysis is available in Figure 19.
19.

Analysis 1.19 Vomiting
When vomiting was measured within 24 hours or before hospital discharge in WOMAN‐2 2024, the effect of prophylactic tranexamic acid compared to placebo was RR 1.00 (95% CI 0.71 to 1.41; P = 0.99, I2 = not applicable; 1 RCT, 15,066 participants; Analysis 1.23). A forest plot of this analysis is available in Figure 19.
1.24 Headache
No trials measured this outcome.
1.25 Maternal well‐being
The Sentilhes 2018 trial measured maternal psychological status using the Edinburgh Postnatal Depression Scale (EPDS) at two months and reported that this did not differ significantly between the two groups (Analysis 1.25).
1.26 Anaemia
No trials measured this outcome.
1.27 Skin reactions
No trials measured this outcome.
Discussion
Summary of main results
This review is a substantive update to the previous version and includes the application of a quality assessment checklist, which led to the exclusion of the studies previously included. This update includes three randomised controlled trials (RCTs) that involved a combined total of 18,974 participants who had a vaginal delivery (Alam 2023; Sentilhes 2018; WOMAN‐2 2024). Sentilhes 2018 included women with and without risk factors for postpartum haemorrhage (29% and 71% of participants, respectively), and Alam 2023 included women without risk factors. WOMAN‐2 2024 included only women with moderate and severe anaemia, which is a risk factor for postpartum haemorrhage. The risk of bias was low in all three trials (Alam 2023; Sentilhes 2018; WOMAN‐2 2024).
Adding prophylactic tranexamic acid (TXA) to standard care of women during vaginal birth, compared to placebo and standard care, results in little to no difference in blood loss ≥ 500 mL and likely results in little to no difference in blood loss ≥ 1000 mL.
The evidence is very uncertain about the effect of prophylactic TXA on maternal death.
Prophylactic TXA likely results in little to no difference in severe morbidity, results in little to no difference in the likelihood of receiving a blood transfusion, and may result in little to no difference in the likelihood of receiving additional surgical interventions to control PPH.
The evidence is very uncertain about the effect of TXA on thromboembolic events.
TXA in addition to standard care, compared to placebo and standard care, results in little to no difference in the likelihood of receiving additional uterotonics in women with anaemia, but results in a slight reduction in the likelihood of receiving additional uterotonics in women with no anaemia.
The evidence is very uncertain about the effect of TXA in addition to standard care compared to placebo and standard care during vaginal birth on hysterectomy.
Prophylactic TXA in addition to standard care compared to placebo and standard care likely results in little to no difference in maternal satisfaction.
Although there were very few serious adverse events reported, the evidence is insufficient to draw conclusions about the effect of TXA on maternal death (9 events in each group), thromboembolic events (1 versus 4 events), hysterectomy (9 versus 10 events) and seizures (10 versus 3 events).
Details of these findings are available in the Table 1.
Limitations of the evidence included in the review
There are different ways of measuring blood loss after vaginal birth. The largest trial (WOMAN‐2 2024)(15,068 participants), which contributed 63% to the meta‐analysis, measured blood loss using provider‐estimated methods. The research team at each hospital were trained to estimate blood loss from the point of delivery until 24 hours after birth, or until earlier discharge, by monitoring and documenting the number of blood‐soaked pads used by the women during this time. Pictograms were provided to help estimation. The other large trial, Sentilhes 2018, which contributed 37% (3891 participants) to the meta‐analysis, measured blood loss objectively using a graduated collector bag (gravimetrical estimation). A Cochrane review concluded that the evidence is insufficient to support the use of one method over another for blood loss estimation after vaginal birth [175].
As part of our assessment of the certainty of evidence, we made judgements about imprecision based on the range of the confidence interval of the relative effect as well as the total number of participants and events per outcome. We downgraded for imprecision if there were very few events (less than 30) and if the confidence interval around the RR was very wide. The threshold for suggested appreciable benefit for the relative effect was 0.75 and the threshold for suggested appreciable harm was 1.25. Where the confidence interval crossed these thresholds, we downgraded by one, two, or three levels depending on the width of the confidence interval. Reasons for imprecision judgements are included in the footnotes of the Table 1.
The findings of our review are based mainly on data from two trials (Sentilhes 2018; WOMAN‐2 2024). One trial was conducted in a high‐resource setting (France; Sentilhes 2018), while the largest trial was conducted in lower‐resource settings (Nigeria, Pakistan, Tanzania, and Zambia; WOMAN‐2 2024).
Both trials assessed the effect of TXA in addition to standard care, which comprised routine administration of oxytocin after delivery. One trial included both women at low and at high risk for PPH, although 70% of included participants were at low risk of PPH (Sentilhes 2018). Study authors conducted a subgroup analysis of the outcome blood loss ≥ 500 mL and found no difference between groups. Stratified results were not available for other outcomes. The largest trial included women with moderate or severe anaemia (WOMAN‐2 2024). All trials administered TXA intravenously after vaginal birth. We therefore cannot make inferences about the effect of giving TXA earlier, before the woman has given birth. The ongoing I’M WOMAN 2023 trial is evaluating the effect of giving intravenous or intramuscular TXA just before birth in women with at least one risk factor for postpartum haemorrhage having either a vaginal or caesarean birth.
Limitations of the review processes
We have updated the outcomes in this review. For example, we added blood transfusion to secondary outcomes and removed haemoglobin below 6 g/dL. This is because blood transfusion is important as an outcome when evaluating an intervention for preventing haemorrhage. Blood transfusions are used in the treatment of severe haemorrhages, but they are costly, associated with significant adverse reactions, and may not be available in low‐resource settings. Blood transfusion is an outcome assessed in other Cochrane reviews on postpartum haemorrhage [176] and TXA [23; 177].
After applying the Cochrane Pregnancy and Childbirth trustworthiness criteria, we included three studies in this review [24]. There are 15 studies in the awaiting classification category as information required from trial authors has not yet been received. The review author team decided to strictly apply this trustworthiness tool due to known concerns about data integrity in this field of study [12; 7].
There were zero or very few events for some of the outcomes, namely, maternal mortality, blood transfusion, surgical interventions to control postpartum haemorrhage, thromboembolic events, myocardial infarction, stroke, seizures, organ failure, and ICU admissions. Rare events often lead to wide 95% confidence intervals around the relative effect. The sample size for these outcomes was large, and thus it is likely that randomisation has achieved prognostic balance [28]. Where events are rare and sample sizes are large, it is more reliable to use the absolute effect for clinical decision‐making [28; 178].
We planned to conduct subgroup analysis according to risk of postpartum haemorrhage, presence of anaemia, dose of TXA, and route of administration of TXA. We only conducted subgroup analysis for risk of postpartum haemorrhage and presence of anaemia. Sentilhes 2018 reported the subgroup analyses for the primary outcome (blood loss > 500 mL) according to risk of postpartum haemorrhage, and we included this in the review for completeness. We conducted subgroup analyses for the receipt of additional uterotonics and clinical diagnosis of postpartum haemorrhage according to anaemia status as this was the most distinct difference between the participants. We also conducted subgroup analyses on nausea and vomiting according to the time point where these outcomes were measured.
Agreements and disagreements with other studies or reviews
We are aware of six other systematic reviews of varying quality that have been published on this topic since 2020 [179; 180; 181; 182; 183; 184]. The large number of reviews in such a short period of time is indicative of the importance and relevance of this clinical question. Most of these reviews found a larger effect on the reduction of postpartum haemorrhage when compared to our findings. However, these reviews did not apply the trustworthiness tool and thus included the studies excluded or awaiting classification in our review. The previous version of this Cochrane review also found a larger effect in the reduction of postpartum haemorrhage. None of the trials included in that version were included in the current review. Two systematic reviews reported that the lack of high‐quality evidence made it difficult to establish conclusions with high certainty [181; 182].
A recent individual patient data (IPD) meta‐analysis including 54,404 participants from five trials assessed the effect of TXA in women giving vaginal or caesarean birth. The included trials administered TXA prophylactically and as treatment for postpartum haemorrhage. Authors found that TXA compared to placebo reduced life‐threatening bleeding (defined as death or surgery for bleeding within 24 hours of birth) when it was administered as treatment for postpartum haemorrhage, but there was little to no difference when TXA was administered prophylactically [185].
The other review undertaken as part of this update, which assessed prophylactic TXA in caesarean births, found that prophylactic TXA in addition to standard care results in little to no difference in estimated blood loss ≥ 1000 mL compared to placebo or standard care alone (high‐certainty evidence) [21]. Similarly to this review, the evidence on severe adverse events was also very uncertain in the caesarean birth review (very low certainty evidence).
Although the association of TXA with thromboembolic events has been hypothesised, it has not been proven to date. [16; 186; 187; 23; 188]. This review is in agreement with other publications suggesting that larger studies are required to assess this outcome and establish the safety of TXA. The IPD meta‐analysis found little to no difference between groups for thromboembolic events [185]. Events were rare (2/1000 in each group) and mostly occurred in women who received TXA as treatment. Mild side effects are more common in TXA compared to placebo or no intervention [189; 190]. Our findings are consistent with this, with this review showing increases in nausea and vomiting with TXA.
Authors' conclusions
Implications for practice
Adding prophylactic tranexamic acid (TXA) to standard care during vaginal birth, compared to placebo or standard care alone, results in little to no difference in blood loss ≥ 500 mL and likely results in little to no difference in blood loss ≥ 1000 mL, and severe morbidity.
Overall, event rates for interventions to control postpartum haemorrhage were small and balanced across groups, and the use of TXA in addition to standard care did not show a benefit. TXA may result in little to no difference in additional surgical interventions to control postpartum haemorrhage and results in little to no difference in receipt of blood transfusion. One trial found that TXA reduced the use of additional uterotonics, whereas the largest trial found little to no difference in the use of additional uterotonics in women with anaemia.
There were very few serious adverse events. The evidence is thus very uncertain about the effect of TXA on maternal death (9 events in each group), thromboembolic events (1 versus 4 events) and hysterectomy (9 versus 10 events).
TXA likely results in little to no difference in maternal satisfaction.
These findings are based on two large trials. In the smaller of these, less than 30% of study participants were at high risk of postpartum haemorrhage. In the larger trial, all women had moderate to severe anaemia.
Those making decisions about routine administration of prophylactic TXA to all women having vaginal births should consider that current evidence does not show a benefit of TXA for outcomes related to blood loss and related morbidity, and is very uncertain in terms of serious adverse events.
Equity‐related implications for practice
Although TXA is not a very expensive drug, in resource‐constrained settings any additional intervention used routinely at all vaginal births will impact expenditure on other services.
Implications for research
Randomised controlled trials (RCTs) on other interventions to prevent postpartum haemorrhage after vaginal birth are needed. Researchers should ensure that they use sound methods and the core outcome set for postpartum haemorrhage when conducting RCTs. There is also a need to assess the most reliable method for measuring blood loss and diagnosing postpartum haemorrhage. Future trials should use standardised methods to measure blood loss.
Equity‐related implications for research
Research should be relevant to the needs of those in low‐and middle‐income countries where the burden of mortality from haemorrhage is greatest.
Supporting Information
Supplementary materials are available with the online version of this article: 10.1002/14651858.CD007872.pub3.
Supplementary materials are published alongside the article and contain additional data and information that support or enhance the article. Supplementary materials may not be subject to the same editorial scrutiny as the content of the article and Cochrane has not copyedited, typeset or proofread these materials. The material in these sections has been supplied by the author(s) for publication under a Licence for Publication and the author(s) are solely responsible for the material. Cochrane accordingly gives no representations or warranties of any kind in relation to, and accepts no liability for any reliance on or use of, such material.
Supplementary material 1 Search strategies
Supplementary material 2 Characteristics of included studies
Supplementary material 3 Characteristics of excluded studies
Supplementary material 4 Characteristics of studies awaiting classification
Supplementary material 5 Characteristics of ongoing studies
Supplementary material 6 Analyses
Supplementary material 7 Data package
Supplementary material 8 Amendments to protocol and previous version of review
New search for studies and content updated (conclusions changed)
Additional information
Acknowledgements
We acknowledge the Cochrane Pregnancy and Childbirth Group team for technical support for previous versions of the review.
We acknowledge N Novikova for participating in the design of the original review, and in writing the protocol, review, and first update.
This Cochrane review is associated with the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
Editorial and peer reviewer contributions
Cochrane Pregnancy and Childbirth Group supported the authors in the development of this update.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): David Haas, Department of Obstetrics and Gynecology, Indiana University School of Medicine, USA
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Luisa M Fernandez Mauleffinch, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service
Copy Editor (copy editing of main article and production): Laura MacDonald, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Anne‐Sophie Bouthors, MD, PhD, Jeanne de Flandre Academic Women Hospital Lille 59‐France (clinical/content review); Sabaratnam Arulkumaran, Professor Emeritus in Obstetrics & Gynaecology, St George's University of London (clinical/content review); Marwa Ahmed (consumer review); Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review); and Jo Platt, Central Editorial Information Specialist (search review)
Contributions of authors
One author (N Novikova) involved in previous published versions of this review in 2010[13] and 2015[14] is no longer included on the author byline, which is now Rohwer C, Rohwer A, Cluver C, Ker K, Hofmeyr GJ. Some of the content retained in this review reflects her contributions.
In the original review, N Novikova participated in the design of the review, and in writing the protocol and review. She undertook the initial data analysis. GJ Hofmeyr conceived the review, and provided guidance in designing the review. He also provided a clinical perspective and performed duplicate data extraction.
In the first update, N Novikova assessed the new studies for eligibility, extracted the data and prepared the review. GJ Hofmeyr reviewed the drafts of the update. CC performed the duplicate data extraction and reviewed the draft of the update.
In this latest version, CR and KK or CR and AR performed study selection, data extraction, risk of bias assessment and trustworthiness assessments. CC and JH provided a clinical perspective on the review. CR and AR conducted the analyses, prepared the GRADE summary of findings table and drafted the review. All authors contributed to the final draft of the review and have approved it for submission.
Declarations of interest
Christa Rohwer: received financial support from the World Health Organization (WHO) to complete this review.
Anke Rohwer: is an editor with Cochrane Infectious Diseases but was not involved in the editorial process for this review.
Catherine Cluver: none known
Katharine Ker: is an author of the WOMAN‐2 trial (WOMAN‐2 2024) and did not participate in evaluating this trial in the review process.
G Justus Hofmeyr: has consulted for Equalize Health, a not‐for‐profit health technology company, and has done ad hoc consultation on the 'Maternawell Tray' blood loss monitoring device and integrated suction tube uterine tamponade device, for which he received consulting fees. The Maternawell tray is not mentioned in this review. GJH has published academic articles on tranexamic acid in medical journals.
Sources of support
Internal sources
-
Effective Care Research Unit, University of Witwatersrand and Walter Sisulu University, South Africa, South Africa
JH supported by the Effective Care Research Unit.
External sources
-
World Health Organization (WHO), Other
CR received financial support from the WHO to complete this review.
-
The Gates Foundation, Other
KK received financial support from the Gates Foundation to work on this update.
-
Foreign, Commonwealth and Development Office, UK
Project number 300342‐104
Registration and protocol
The published protocol and updates to the review can be accessed. Protocol (2009) DOI: 10.1002/14651858.CD007872 Original review (2010) DOI: 10.1002/14651858.CD007872.pub2 Review update (2015) DOI: 10.1002/14651858.CD007872.pub3
Data, code and other materials
As part of the published Cochrane review, the following is made available for download for users of the Cochrane Library: full search strategies for each database; full citations of each unique report for all studies included, ongoing, or awaiting classification, or excluded at the full‐text screen, in the final review; study data, including study information, study arms, and study results or test data; consensus risk of bias assessments; and analysis data, including overall estimates and settings, subgroup estimates, and individual data rows. Appropriate permissions have been obtained for such use. Analyses and data management were conducted within Cochrane’s authoring tool, RevMan, using the inbuilt computation methods. Template data extraction forms from Covidence are available from the authors on reasonable request.
All data available in Supplementary material 7.
What's new
| Date | Event | Description |
|---|---|---|
| 15 January 2025 | New search has been performed | The author team has changed since the last review. N Novikova is no longer an author, and authors C Rohwer and A Rohwer were added to the author team. We have split the original review into two separate reviews: this one for studies with participants having vaginal births and another for those having caesarean births. We updated the methodology, following the Methodological Expectations of Cochrane Intervention Reviews (MECIR) when conducting the review and PRISMA 2020 when reporting. We changed our eligibility criteria to include only participants who have vaginal births. We applied a trustworthiness checklist to all eligible studies to ensure that the evidence is based only on trustworthy studies. Studies were ineligible if the trial was not prospectively registered. As a result, the studies previously included in this review, were not included in this update. The search carried out in September 2024 was conducted by our review author team, as the Cochrane Pregnancy and Childbirth Group no longer exists and therefore could not assist in the search. We did not conduct the subgroup analysis that was planned in the protocol as it was no longer relevant. This is due to the fact that the review was split and thus no subgroup analysis between modes of delivery (vaginal birth and caesarean birth) was required. Nor did we perform subgroup analysis investigating comparisons with or without the use of routine oxytocics, or inconsistent use or not stated, as all studies routinely administered these. |
| 15 January 2025 | New citation required and conclusions have changed | This current update of the review found that prophylactic tranexamic acid (TXA) in addition to standard care, compared to placebo or standard care alone, results in little to no difference for the outcomes of postpartum haemorrhage (PPH) or severe PPH in women during vaginal birth. The evidence is very uncertain about the effect of TXA on maternal death, and TXA likely results in little to no difference in severe morbidity. Prophylactic TXA for vaginal birth results in little to no difference in the receipt of blood transfusion and may result in little to no difference in receipt of additional surgical interventions to control PPH. The evidence is very uncertain about the effect of TXA on thromboembolic events. In women with anaemia, TXA results in little to no difference in receipt of additional uterotonics. The evidence is very uncertain about the effect of TXA on hysterectomy and likely results in little to no difference in maternal satisfaction. These results are in contrast to the previous version of the review (2015), which included both vaginal births and caesarean sections, and reported that tranexamic acid (TXA) (in addition to uterotonic medications) decreased postpartum blood loss and prevented postpartum haemorrhage (PPH) and blood transfusions following vaginal birth and caesarean section in women at low risk of PPH based on studies of mixed quality. In that version, the review authors stated there was insufficient evidence to draw conclusions about serious side effects, but there was an increase in the incidence of minor side effects with the use of TXA, and they stated that the effects of TXA on thromboembolic events and mortality, as well as its use in high‐risk women, should be investigated further. |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 10 February 2015 | New citation required and conclusions have changed | Ten new trials incorporated. The review now includes a total of 12 trials. The review's conclusions have changed: the previous review concluded that TA decreases postpartum blood loss after vaginal birth and CS based on two RCTs of unclear quality which reported only a few outcomes. Following inclusion of ten studies the new conclusion has changed to "TA (in addition to uterotonic medications) decreases postpartum blood loss and prevents PPH following vaginal birth and CS in low‐risk women. TA is not associated with severe side effects. The evidence suggests that TA should be considered as part of routine management for prevention of PPH". We have added information in the methods section on how the trials with multiple groups are handled. We have updated the secondary outcomes (added blood transfusion, removed haemoglobin < 6 G%). We have added a description of blood loss measurement in methods / outcomes section. A 'Summary of findings' table has been incorporated for this update. |
| 10 February 2015 | New search has been performed | Search updated. Methods updated. We identified 20 reports of 19 trials. We have added ten studies: Abdel‐Aleem 2013; Goswami 2013; Gungorduk 2011; Gungorduk 2013; Mirghafourvand 2013; Movafegh 2011; Senturk 2013; Shahid 2013; Xu 2013; Yehia 2014 to the previously included two trials. Two trials are still ongoing TA TEG; Shirazi 2012. One trial is awaiting classification Bhavana 2013. Sentilhes 2014 is a protocol of ongoing trial, Ahmed 2014 was published in abstract form only and is lacking information for adequate assessment at this stage. We have excluded four newly identified trials (Gobbur 2011; Gohel 2007; Sekhavat 2009; Tarabrin 2012a). |
| 15 February 2011 | New search has been performed | Search updated. We identified and excluded three new trials Gobbur 2011; Gohel 2007; Sekhavat 2009 |
References
- 1.Calvert C, Thomas SL, Ronsmans C, Wagner KS, Adler AJ, Filippi V. Identifying regional variation in the prevalence of postpartum haemorrhage: a systematic review and meta-analysis. PLOS One 2012;7(7):e41114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Practice & Research. Clinical Obstetrics & Gynaecology 2008;22(6):999-1012. [DOI] [PubMed] [Google Scholar]
- 3.Feduniw S, Warzecha D, Szymusik I, Wielgos M. Epidemiology, prevention and management of early postpartum hemorrhage - a systematic review. Ginekologia Polska 2020;91(4):38-44. [DOI] [PubMed] [Google Scholar]
- 4.Say L, Pattinson R, Gulmezoglu A. WHO systematic review of maternal morbidity and mortality: the prevalence of severe acute maternal morbidity (near miss). BMC Reproductive Health 2004;1(3):1. [DOI: 10.1186/1742-4755-1-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson JF, Heal LJ, Roberts CL, Ellwood DA. Women's breastfeeding experiences following a significant primary postpartum haemorrhage: a multicentre cohort study. International Breastfeeding Journal 2010;5(5):1. [DOI: 10.1186/1746-4358-5-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO recommendations for the prevention and treatment of postpartum haemorrhage; January 2012. Available at https://www.who.int/publications/i/item/9789241548502 (accessed 1 September 2024). [PubMed]
- 7.Li W, Bordewijk EM, Mol BW. Assessing research misconduct in randomized controlled trials. Obstetrics & Gynecology 2021;138(3):338-47. [DOI: 10.1097/AOG.0000000000004513] [DOI] [PubMed] [Google Scholar]
- 8.Ker K, Roberts I, Shakur H, Coats TJ. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD004896. [DOI: 10.1002/14651858.CD004896.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shakur H, Beaumont D, Pavord S, Gayet‐Ageron A, Ker K, Mousa HA. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD012964. [DOI: 10.1002/14651858.CD012964] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woman Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389(10084):2105-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO recommendation on tranexamic acid for the treatment of postpartum haemorrhage; November 2017. Available at https://www.who.int/publications/i/item/9789241550154 (accessed 1 September 2024). [PubMed]
- 12.Ker K, Shakur H, Roberts I. Does tranexamic acid prevent postpartum haemorrhage? A systematic review of randomised controlled trials. BJOG 2016;123(11):1745-52. [DOI] [PubMed] [Google Scholar]
- 13.Novikova N, Hofmeyr GJ. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2010, Issue 7. Art. No: CD007872. [DOI: 10.1002/14651858.CD007872.pub2] [DOI] [PubMed] [Google Scholar]
- 14.Novikova N, Hofmeyr J, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD007872. [DOI: 10.1002/14651858.CD007872.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murao S, Nakata H, Roberts I, Yamakawa K. Effect of tranexamic acid on thrombotic events and seizures in bleeding patients: a systematic review and meta-analysis. Critical Care 2021;25(1):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23-32. [DOI: 10.1016/S0140-6736(10)60835-5] [DOI] [PubMed] [Google Scholar]
- 17.Agarwal S, Heesen M. Effects and side-effects of tranexamic acid: they both matter. Anaesthesia 2023;78(11):1320-2. [DOI] [PubMed] [Google Scholar]
- 18.Stämpfli D, Weiler S, Weiniger CF, Burden AM, Heesen M. Renal ischemic adverse drug events related to tranexamic acid in women of child-bearing age: an analysis of pharmacovigilance data. European Journal of Clinical Pharmacology 2021;77(6):913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HALT-IT Trial Collaborators. Effects of a high-dose 24-hour infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet 2020;395(10241):1927-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al; ATACAS Investigators of the ANZCA Clinical Trials Network. Tranexamic acid in patients undergoing coronary-artery surgery. New England Journal of Medicine 2017;376(2):136-48. [DOI] [PubMed] [Google Scholar]
- 21.Rohwer C, Rohwer A, Cluver C, Ker K, Hofmeyr GJ, Winer L. Tranexamic acid for preventing postpartum haemorrhage after caesarean section. Cochrane Database of Systematic Reviews 2024, Issue 11. Art. No: CD016278. [DOI: 10.1002/14651858.CD016278] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novikova N, Hofmeyr GJ. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007872. [DOI: 10.1002/14651858.CD007872] [DOI] [PubMed] [Google Scholar]
- 23.Ker K, Beecher D, Roberts I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD010562. [DOI: 10.1002/14651858.CD010562.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfirevic Z, Kellie FJ, Weeks J, Stewart F, Jones L, Hampson L, on behalf of the Cochrane Pregnancy and Childbirth Group Editorial Board. Identifying and handling potentially untrustworthy trials – Trustworthiness Screening Tool (TST) developed by the Cochrane Pregnancy and Childbirth Group. Available at https://documentation.cochrane.org/download/attachments/169771061/PGC%20TST%20FINAL.pdf?version=1&modificationDate=1694523757481&api=v2 (accessed prior to 5 January 2024).
- 25.Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trial in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. [DOI] [PubMed] [Google Scholar]
- 26.Covidence. Version accessed prior to 12 November 2024. Melbourne, Australia: Veritas Health Innovation, 2024. Available at https://www.covidence.org.
- 27.Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from https://training.cochrane.org/handbook/archive/v5.1/.
- 28.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated August 2022). Cochrane, 2022. Available from https://training.cochrane.org/handbook/archive/v6.3.
- 29.Review Manager (RevMan). Version 8.10.0. The Cochrane Collaboration, 2024. Available at https://revman.cochrane.org.
- 30.Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 31.GRADEpro GDT. Version accessed prior to 12 November 2024. Hamilton (ON): McMaster University (developed by Evidence Prime), 2024. Available at https://www.gradepro.org.
- 32.Meher S, Cuthbert A, Kirkham JJ, Williamson P, Abalos E, Aflaifel N, et al. Core outcome sets for prevention and treatment of postpartum haemorrhage: an international Delphi consensus study. British Journal of Obstetrics and Gynaecology 2019;126:83-93. [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Aleem H, Alhusaini TK, Abdel-Aleem MA, Menoufy M, Gulmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. Journal of Maternal-Fetal & Neonatal Medicine 2013;26(17):1705-9. [DOI] [PubMed] [Google Scholar]
- 34.ACTRN12612000313831. The use of tranexamic acid to reduce blood loss during and after cesarean section [Tranexamic acid for prevention of postpartum hemorrhage in women undergoing elective cesarean section]. https://anzctr.org.au/ACTRN12612000313831.aspx (first registered 20 March 2012).
- 35.Gungorduk K, Asicioglu O, Yildirim G, Ark C, Tekirdag AI, Besimoglu B. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? A randomized controlled study. American Journal of Perinatology 2013;30(5):407-13. [DOI] [PubMed] [Google Scholar]
- 36.Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi-center, randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;112:154-7. [DOI] [PubMed] [Google Scholar]
- 37.Goswami U, Sarangi S, Gupta S, Babbar S. Comparative evaluation of two doses of tranexamic acid used prophylactically in anemic parturients for lower segment cesarean section: a double-blind randomized case control prospective trial. Saudi Journal of Anaesthesia 2013;7(4):427-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gungorduk K, Yildirim G, Asicioglu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double-blind, placebo-controlled study. American Journal of Perinatology 2011;28(3):233-40. [DOI] [PubMed] [Google Scholar]
- 39.Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. International Journal of Gynecology and Obstetrics 2011;115(3):224-6. [DOI] [PubMed] [Google Scholar]
- 40.NCT01085006. The effect of tranexamic acid administration on postpartum hemorrhage during and after cesarean delivery. https://clinicaltrials.gov/study/NCT01085006 (first posted 11 March 2010). [CENTRAL: CN-01067257]
- 41.Senturk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double-blind, placebo-controlled, randomized clinical trial. Archives of Gynecology and Obstetrics 2013;287(4):641-5. [DOI] [PubMed] [Google Scholar]
- 42.Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. Journal of the College of Physicians and Surgeons--Pakistan: JCPSP 2013;23(7):459-62. [PubMed] [Google Scholar]
- 43.Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double-blind randomization trial. Archives of Gynecology and Obstetrics 2013;287(3):463-8. [DOI] [PubMed] [Google Scholar]
- 44.Yehia AH, Koleib MH, Abdelazim IA, Atik A. Tranexamic acid reduces blood loss during and after cesarean section: a double blinded, randomized, controlled trial. Asian Pacific Journal of Reproduction 2014;3(1):53-6. [Google Scholar]
- 45.Mirghafourvand M, Alizadeh SM, Abasalizadeh F, Shirdel M. The effect of intravenous tranexamic acid on hemoglobin and hematocrit levels after vaginal delivery: a randomized controlled trial. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;16(60):1-8. [Google Scholar]
- 46.Mirghafourvand M, Mohammad-Alizadeh S, Abasalizadeh F, Shirdel M. The effect of prophylactic intravenous tranexamic acid on blood loss after vaginal delivery in low risk of postpartum haemorrhage women: a double-blind randomized controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2015;55(1):53-8. [DOI: 10.1111/ajo.12262] [DOI] [PubMed]
- 47.Yang H, Zheng S, Shi C. [Clinical study on the efficacy of tranexamic acid in reducing postpartum blood loss: a randomized comparative, multicenter trial] [Chinese]. Chung-Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics and Gynaecology] 2001;36(10):590-2. [PubMed] [Google Scholar]
- 48.Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. Journal of Maternal-Fetal & Neonatal Medicine 2015;28(9):1014-8. [CENTRAL: CN-01086300] [EMBASE: 605117379] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 49.Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. Journal of Maternal-Fetal & Neonatal Medicine 2014 Jul 28 [Epub ahead of print]. [DOI] [PubMed]
- 50.Maged AM, Helal OM, Elsherbini MM, Eid MM, Elkomy RO, Dahab S, et al. A randomized placebo-controlled trial of preoperative tranexamic acid among women undergoing elective cesarean delivery. Internatonal Journal of Gynecology and Obstetrics 2015;131(3):265-8. [DOI] [PubMed] [Google Scholar]
- 51.Bhavana G, Abhishek MV, Suneeta M. Efficacy of prophylactic tranexamic acid in reducing blood loss during and after caesarean section. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2016;5(6):2011-6. [Google Scholar]
- 52.Bhavana G, Mittal S. Evaluation of efficacy of prophylactic injection tranexamic acid in decreasing blood loss before and after caesarean section. BJOG: an International Journal of Obstetrics and Gynaecology 2013;120(Suppl s1):32. [Google Scholar]
- 53.CTRI/2023/09/057612. A clinical trial to study the effect of drug tranexamic acid in addition to oxytocin in reducing blood loss after vaginal delivery [A randomized double blind placebo control trial to assess whether tranexamic acid as prophylaxis lowers the risk of PPH in women delivering vaginally]. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=91328 (first registered 14 September 2023).
- 54.CTRI/2024/04/066348. Effect of prophylactic tranexamic acid in reducing postpartum blood loss in vaginal deliveries at tertiary care centre; placebo controlled randomized clinical trial. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=102242 (first registered 25 April 2024).
- 55.CTRI/2024/05/067790. Efficacy and safety of IV tranexamic acid in the prevention of postpartum haemorrhage: a randomized controlled study. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=104552 (first registered 21 May 2024).
- 56.Brenner A, Shakur-Still H, Chaudhri R, Muganyizi P, Olayemi O, Arribas M, et al. Tranexamic acid by the intramuscular or intravenous route for the prevention of postpartum haemorrhage in women at increased risk: a randomised placebo-controlled trial (I'M WOMAN). Trials 2023;24(1):7822023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.PACTR202306670143734. The effect of varying doses of intravenous tranexamic acid on blood loss after vaginal delivery at term in University of Benin Teaching Hospital [The effect of two varying doses of intravenous tranexamic acid on blood loss after vaginal delivery at term in University of Benin Teaching Hospital: a double-blinded randomized placebo-controlled trial]. https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=25659 (first approved 28 June 2023).
- 58.CTRI/2021/05/033500. Tranexamic acid along with AMTSL for preventing PPH in vaginal delivery: TAAPP-V [Effect of intravenous tranexamic acid (TXA) in addition to active management of third stage of labour on postpartum blood loss in vaginal delivery: a double blinded, randomized controlled trial]. https://ctri.nic.in/Clinicaltrials/login.php (first registered 10 May 2021).
- 59.WOMAN-2 Trial Collaborators. The effect of tranexamic acid on postpartum bleeding in women with moderate and severe anaemia (WOMAN-2): an international, randomised, double-blind, placebo-controlled trial. Lancet 2024;404(10463):1645-56. [DOI: 10.1016/S0140-6736(24)01749-5] [DOI] [PubMed] [Google Scholar]
- 60.NCT03475342. World Maternal Antifibrinolytic Trial_2 (WOMAN-2) [Tranexamic acid for the prevention of postpartum bleeding in women with anaemia: an international, randomised, double-blind, placebo controlled trial]. https://clinicaltrials.gov/ct2/show/NCT03475342 (first posted 23 March 2018).
- 61.Ker K, Roberts I, Chaudhri R, Fawole B, Beaumont D, Balogun E, et al. Tranexamic acid for the prevention of postpartum bleeding in women with anaemia: study protocol for an international, randomised, double-blind, placebo-controlled trial. Trials 2018;19(1):712. [CENTRAL: CN-01792461] [DOI: 10.1186/s13063-018-3081-x] [EMBASE: 625692661] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ISRCTN62396133. World maternal antifibrinolytic trial-2 [Tranexamic acid for the prevention of postpartum bleeding in women with anaemia: an international, randomised, double-blind, placebo controlled trial]. https://www.isrctn.com/ISRCTN62396133 (first registered 7 December 2017). [CENTRAL: CN-01885095]
- 63.Sentilhes L, Winer N, Azria E, Senat MV, Le Ray C, Vardon D, et al. Tranexamic acid for the prevention of blood loss after vaginal delivery. New England Journal of Medicine 2018;379(8):731-42. [CENTRAL: CN-01626893] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 64.Sentilhes L, Winer N, Azria E, Senat M-V, Le Ray C, Vardon D, et al. Tranexamic acid for the prevention of postpartum hemorrhage after vaginal delivery: the TRAAP trial. American Journal of Obstetrics and Gynecology 2018;218(1 Supplement 1):S2-3. [CENTRAL: CN-01450841] [EMBASE: 620309567] [Google Scholar]
- 65.Durand-Zaleski I, Deneux-Tharaux C, Seco A, Malki M, Frenkiel J, Sentilhes L. An economic evaluation of tranexamic acid to prevent postpartum haemorrhage in women with vaginal delivery: the randomised controlled TRAAP trial. BJOG: an International Journal of Obstetrics & Gynaecology 2021;128(1):114-20. [CENTRAL: CN-02178159] [EMBASE: 2006051932] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 66.EUCTR2014-001748-39-FR. [Impact de l'injection d'acide tranexamique en prevention de l'hemorragie du post partum apres un accouchement par voie bases] [TRAnexamic Acid for Preventing postpartum hemorrhage following a vaginal delivery: a multicenter randomised, double blind placebo controlled trial - TRAAP]. https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-001748-39/FR (first posted 3 December 2015). [CENTRAL: CN-01864814]
- 67.NCT02302456. Tranexamic acid for preventing postpartum haemorrhage following a vaginal delivery (TRAAP). https://clinicaltrials.gov/study/NCT02302456 (first received 17 November 2014).
- 68.Sentilhes L, Daniel V, Darsonval A, Deruelle P, Vardon D, Perrotin F, et al. Study protocol. TRAAP - TRAnexamic Acid for Preventing postpartum hemorrhage after vaginal delivery: a multicenter randomized, double-blind, placebo-controlled trial. BMC Pregnancy and Childbirth 2015;15(1):135. [CENTRAL: CN-01257586] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alam AQ, Barrett J, Callum J, Kaustov L, Au S, Fleet A, et al. Tranexamic acid for the prevention of postpartum haemorrhage: the TAPPH‑1 pilot randomized trial and lessons learned for trials in Canadian obstetrics. Scientific Reports 2023;13(1):4512. [DOI: 10.1038/s41598-023-30947-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.NCT03069859. Use of TXA to prevent postpartum hemorrhage (TAPPH-1). https://clinicaltrials.gov/study/NCT03069859 (first posted 3 March 2017).
- 71.Bangsah AG, Riaz S, Akhtar Z, Naz T, Naib JM. Tranexamic acid plus oxytocin prophylaxis in reducing blood loss and preventing postpartum hemorrhage during cesarean section. Journal of Medical Sciences (Peshawar) 2023;31(3):203-7. [Google Scholar]
- 72.Bhatia SK, Deshpande H. Role of tranexamic acid in reducing blood loss during and after caesarean section. Medical Journal of Dr DY Patil University 2015;8(1):21-5. [Google Scholar]
- 73.Bose D, Beegum R. Sublingual misoprostol vs intravenous tranexamic acid in reducing blood loss during cesarean section: a prospective randomized study. Journal of SAFOG 2017;9(1):9-13. [CENTRAL: CN-01430856] [EMBASE: 619028533] [Google Scholar]
- 74.Chandak AV, Gupta I, Sreemayi C. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randomized case controlled prospective study. International Journal of Science and Research 2017;6(1):1321-3. [Google Scholar]
- 75.Dawoud M, Al-Husseiny M, Helal O, Elsherbini M, Abdel-Rasheed M, Sediek M. Intravenous tranexamic acid vs sublingual misoprostol in high-risk women for postpartum haemorrhage following cesarean delivery; a randomised clinical trial. BMC Pregnancy and Childbirth 2023;23(1):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ducloy-Bouthors A-S, Jeanpierre E, Saidi I, Baptiste A-S, Simon E, Lannoy D, et al. TRAnexamic acid in hemorrhagic CESarean section (TRACES) randomized placebo controlled dose-ranging pharmacobiological ancillary trial: study protocol for a randomized controlled trial. Trials 2018;19(1):149. [CENTRAL: CN-01464518] [EMBASE: 620904152] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouthors A-S, Hennart B, Jeanpierre E, Baptiste A-S, Saidi I, Simon E, et al. Therapeutic and pharmaco-biological, dose-ranging multicentre trial to determine the optimal dose of TRAnexamic acid to reduce blood loss in haemorrhagic CESarean delivery (TRACES): study protocol for a randomised, double-blind, placebo-controlled trial. Trials 2018;19(1):148. [CENTRAL: CN-01464283] [EMBASE: 620904151] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ducloy-Bouthors A-S, Jeanpierre E, Hennart B, Baptiste A-S, Giovannoni S, Saidi I, et al. TRAnexamic acid to reduce blood loss in haemorrhagic CESarean delivery: therapeutic and pharmaco-biological dose-ranging multicentre randomised double-blind placebo-controlled study: TRACES trial methodology [Tranexamic acid to reduce blood loss in haemorrhagic caesarean delivery: a multicentre randomised double blind placebo controlled therapeutic and pharmaco-biological dose ranging study (TRACES) for its optimal benefit/risk]. Transfusion Medicine (Oxford, England) 2017;27:61-2, Abstract no: P95. [CENTRAL: CN-01558936] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.NCT04733157. The Efficacy of Tranexamic Acid in Preventing Postpartum Haemorrhage after caesarean section (ETAPPH). https://clinicaltrials.gov/ct2/show/study/NCT04733157 (first posted 1 February 2021).
- 80.Ghosh A, Chaudhurib P, Muhuri B. Efficacy of intravenous tranexamic acid before cesarean section in preventing post partum hemorrhagea prospective randomised double blind placebo controlled study. International Journal of Biological & Medical Research 2014;5(4):4461-4. [Google Scholar]
- 81.Gobbur VR, Reddy SV, Bijapur UJ. Efficacy of tranexamic acid in reducing blood loss during lower segment caesarean section. In: 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5-9; Hyderabad, Andhra Pradesh, India. 2011:92.
- 82.Gobbur VR, Shiragur SS, Jhanwar UR, Tehalia MJ. Efficacy of tranexamic acid in reducing blood loss during lower segment caesarean section. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2014;3(2):414-7. [CENTRAL: CN-02272414] [Google Scholar]
- 83.Gohel M, Patel P, Gupta A, Desai P. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randomized case controlled prospective study. Journal of Obstetrics and Gynaecology of India 2007;57(3):228-30. [Google Scholar]
- 84.Gwanzura C. Efficacy of tranexamic acid in preventing postpartum haemorrhage after elective caesarean section. Data on file.
- 85.NCT03463993. Efficacy of tranexamic acid in preventing postpartum haemorrhage after elective caesarean section. https://clinicaltrials.gov/study/NCT03463993?term=NCT03463993&rank=1 (first posted 13 March 2018).
- 86.Halder S, Samanta B, Sardar R, Chattopadhyay S. Tranexamic acid used before caesarean section reduces blood loss based on pre- and postoperative hemoglobin level: a case-control study. Journal of the Indian Medical Association 2013;111(3):184-6. [PubMed] [Google Scholar]
- 87.Hemapriya L, More G, Kumar A. Efficacy of tranexamic acid in reducing blood loss in lower segment caesarean section: a randomised controlled study. Journal of Obstetrics and Gynaecology of India 2020;70(6):479-84. [CENTRAL: CN-02178207] [EMBASE: 2006073040] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ifunanya NJ, Chukwu IC, Nobert OC, Blessing O, Chibuzor U, Uchenna O. Tranexamic acid versus placebo for prevention of primary postpartum haemorrhage among high risk women undergoing caesarean section in Abakaliki: a randomized controlled trial. Open Journal of Obstetrics and Gynecology 2019;9(6):914-22. [Google Scholar]
- 89.Jafarbegloo E, Faridnyia F, Ahangari R, Mohammadbeigi A. Prophylactic use of tranexamic acid on blood loss in cesarean delivery: a randomized controlled- clinical trial. Trauma Monthly 2021;26(1):19-24. [Google Scholar]
- 90.Kafayat H, Janjua M, Naheed I, Iqbal T. To assess the prophylactic role of tranexamic acid in reducing blood loss during and after two hours of caesarean section. Pakistan Journal of Medical and Health Sciences 2019;12(4):1662-5. [CENTRAL: CN-01914956] [EMBASE: 2001674035] [Google Scholar]
- 91.Lakshmi SJ, Abraham R. Role of prophylactic tranexamic acid in reducing blood loss during elective caesarean section: a randomized controlled study. Journal of Clinical and Diagnostic Research 2016;10(12):OC17-21. [CENTRAL: CN-01286685] [EMBASE: 613547126] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee SH, Kwek ME, Tagore S, Wright A, Ku CW, Teong AC, et al. Tranexamic acid, as an adjunct to oxytocin prophylaxis, in the prevention of postpartum haemorrhage in women undergoing elective caesarean section: a single-centre double-blind randomised controlled trial. BJOG 2023;130(9):1007-15. [DOI] [PubMed] [Google Scholar]
- 93.Maged AM, Helal OM, Elsherbini MM, Eid MM, Elkomy RO, Dahab S, et al. A randomized placebo-controlled trial of preoperative tranexamic acid among women undergoing elective cesarean delivery. International Journal of Gynecology and Obstetrics 2015;131(3):265-8. [CENTRAL: CN-01140746] [EMBASE: 2015345374] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 94.ACTRN12615000312549. Efficiency and safety of preoperative tranexamic acid in reducing perioperative blood loss in elective caesarean section. https://anzctr.org.au/ACTRN12615000312549.aspx (first registered 2 April 2015).
- 95.Malathi P, Anupama D, Habitha P. Effect of injection tranexamic acid on peri-operative blood loss during cesarean section. International Archives of Integrated Medicine 2016;3(10):280-9. [Google Scholar]
- 96.Markley JC, Tsen LC, Varelmann DJ, Farber MK. The influence of prophylactic tranexamic acid on thromboelastography during cesarean delivery: a randomized, double-blind, placebo-controlled trial. In: Society for Obstetric Anesthesia and Perinatology (SOAP) 47th Annual Meeting; 2015 May 13-17; Colorado, USA. 2015:T-36.
- 97.Masood J, Hayat Z, Ahmed N, Jabeen R, Shifa N, Masud F. Comparison of estimated blood loss between tranexamic acid and control in women undergoing elective cesarean section. Pakistan Journal of Medical and Health Sciences 2023;17(4):288-90. [Google Scholar]
- 98.Milani F, Sharami SH, Farzadi S, Haryalchi K, Atrkarroshan Z. Prophylactic effect of tranexamic acid on hemorrhage during and after the cesarean section. International Journal of Women's Health and Reproduction Sciences 2019;7(1):74-8. [CENTRAL: CN-01793985] [EMBASE: 625996781] [Google Scholar]
- 99.Moradan S, Anaraki RM, Mirmohammadkhani M. Prophylactic effect of misoprostol versus tranexamic acid in conjunction with oxytocin in reduction of post-partum hemorrhage after cesarean sectionin: a randomized clinical trial. Koomesh 2018;20(4):620-5. [CENTRAL: CN-01651839] [EMBASE: 624107599] [Google Scholar]
- 100.Naeiji Z, Delshadiyan N, Saleh S, Moridi A, Rahmati N, Fathi M. Prophylactic use of tranexamic acid for decreasing the blood loss in elective cesarean section: a placebo-controlled randomized clinical trial. Journal of Gynecology Obstetrics and Human Reproduction 2020;50(1):101973. [CENTRAL: CN-02210140] [EMBASE: 2010158550] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 101.Nandal I, Kochar SP, Dahiya A, Kaur R. Role of intravenous tranexamic acid in reducing blood loss during caesarean delivery. International Medical Journal 2022;29(1):23-5. [Google Scholar]
- 102.Nargis N, Dewan F. Prophylactic use of tranexamic acid during caesarean section in preventing postpartum haemorrhage-a prospective randomised double blind placebo controlled study. Bangladesh Journal of Obstetrics and Gynecology 2020;33(2):125-30. [CENTRAL: CN-02193474] [EMBASE: 2007609862] [Google Scholar]
- 103.NCT06060327. Comparing tranexamic acid versus ecbolics in preventing hemorrhage during and after cesarean section. https://clinicaltrials.gov/ct2/show/NCT06060327 (first posted 29 September 2023).
- 104.NCT02350179. Efficacy of tranexamic acid in reducing blood loss during and after caesarean section. https://clinicaltrials.gov/show/NCT02350179 (first received 1 January 2015). [CENTRAL: CN-01551809]
- 105.NCT02688127. Efficacy of tranexamic acid in reducing blood loss during cesarean section because of placenta previa [Efficacy of tranexamic acid in reducing blood loss among pregnant women during cesarean section because of placenta previa]. https://clinicaltrials.gov/show/NCT02688127 (first received 23 February 2016). [CENTRAL: CN-01555966]
- 106.NCT03463993. Efficacy of tranexamic acid in preventing postpartum haemorrhage after elective caesarean section. https://clinicaltrials.gov/study/NCT03463993 (last updated 1 June 2022).
- 107.Obi VO, Umeora OU, Dimejesi IB, Asiegbu O, Mgbafulu CC, Ifemelumma CC, et al. Efficacy of intravenous tranexamic acid at reducing blood loss during elective caesarean section in Abakaliki: a double blind randomized placebo controlled trial. African Journal of Medical and Health Sciences 2019;18(2):10-7. [Google Scholar]
- 108.Ogunkua OT, Duryea EL, Nelson DB, Eddins MM, Klucsarits SE, McIntire DD, et al. Tranexamic acid for prevention of hemorrhage in elective repeat cesarean delivery—a randomized study. American Journal of Obstetrics and Gynecology 2022;4(2):100573. [DOI] [PubMed] [Google Scholar]
- 109.Ortuanya KE, Eleje GU, Ezugwu FO, Odugu BU, Ikechebelu JI, Ugwu EO, et al. Prophylactic tranexamic acid for reducing intraoperative blood loss during cesarean section in women at high risk of postpartum hemorrhage: a double-blind placebo randomized controlled trial. Womens Health (London England) 2024;20(1):17455057231225311. [DOI: 10.1177/17455057231225311. ] [PMCID:: PMC10822094] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pacheco LD, Clifton RG, Saade GR, Weiner SJ, Parry S, Thorp JM, et al for the Eunice Kennedy Shriver National Institute of Child Healthand Human Development Maternal–Fetal Medicine Units Network. Tranexamic acid to prevent obstetrical hemorrhage after cesarean delivery. New England Journal of Medicine 2023;388(15):1365-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pacheco LD. Tranexamic acid for the prevention of obstetrical hemorrhage after cesarean delivery: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2022;226(1):S779‐80. [Google Scholar]
- 112.Pakniat H, Chegini V, Shojaei A, Khezri MB, Ansari I. Comparison of the effect of intravenous tranexamic acid and sublingual misoprostol on reducing bleeding after cesarean section: a double-blind randomized clinical trial. Journal of Obstetrics and Gynaecology of India 2019;69(3):239-45. [CENTRAL: CN-02004087] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poonia M, Bansal A, Bhardwaj N, Kumari S. Role of tranexamic acid in reducing blood loss after caesarean section: a randomized case control prospective study. Journal of Medical Science and Research 2012;3(2):44-6. [Google Scholar]
- 114.Ramani B. Intravenous 1 gram tranexamic acid for prevention of blood loss and blood transfusion during caesarean section: a randomized case control study. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2014;3(2):366-9. [Google Scholar]
- 115.Ramesh AC, Rajni S, Deka N. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randomized case controlled prospective study. Indian Journal of Public Health Research and Development 2015;6(2):12-5. [CENTRAL: CN-01076660] [EMBASE: 604818004] [Google Scholar]
- 116.Rashid M, Banerjeee D, Sharma R, Roy D, Hansda J. Effectivity of intravenous tranexamic acid in addition to oxytocin on blood loss during and after caesarean delivery - a [sic] RCT. Journal of Cardiovascular Disease Research 2024;15(1):71-82. [Google Scholar]
- 117.Rashmi PS, Sudha TR, Prabhudev P, Patil R, Vijayanath V. Role of tranexamic acid in reducing blood loss during and after cesarean section. A randomized case control prospective study. Journal of Medical Research and Practice 2012;1(2):40-3. [Google Scholar]
- 118.Ray I, Bhattacharya R, Chakraborty S, Bagchi C, Mukhopadhyay S. Role of intravenous tranexamic acid on caesarean blood loss: a prospective randomised study. Journal of Obstetrics and Gynaecology of India 2016;66(Suppl 1):S347-52. [CENTRAL: CN-01761234] [EMBASE: 610988100] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sahu J, Mishra N. Role of intravenous tranexamic acid in reducing blood loss during caesarean section: study at tribal-dominated area hospital in Chhattisgarh, India. Journal of Obstetrics and Gynaecology Research 2019;45(4):841-8. [CENTRAL: CN-01932139] [EMBASE: 626068178] [DOI] [PubMed] [Google Scholar]
- 120.Salas MT. A single-blinded randomized controlled trial on the effect of prophylactic intravenous administration of tranexamic acid on the reduction of blood loss during and after primary cesarean section. Journal of Obstetrics and Gynaecology Research 2017;43:26-7, Abstract no: 0086. [CENTRAL: CN-01760536] [EMBASE: 616813053] [Google Scholar]
- 121.NCT03778242. Oxytocin and tranexamic acid in pregnant women with twin pregnancy undergoing cesarean section [Effects of co-administered oxytocin and intravenous tranexamic acid on prevention of postpartum hemorrhage in pregnant women with twin pregnancy undergoing elective cesarean section: a double-blind randomized clinical trial]. https://clinicaltrials.gov/show/nct03778242 (first received 18 December 2018). [CENTRAL: CN-01918664]
- 122.Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducing blood loss after cesarean section. Journal of Maternal-Fetal & Neonatal Medicine 2009;22(1):72-5. [DOI] [PubMed] [Google Scholar]
- 123.Shah P, Agrawal A, Chhetri S, Rijal P, Bhatta NK. Tranexamic acid in prevention of postpartum hemorrhage in elective cesarean section. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2019;8(2):372-6. [DOI: 10.18203/2320-1770.ijrcog20190017] [DOI] [Google Scholar]
- 124.Agrawal A. Tranexamic acid (TA) in prevention of postpartum haemorrhage in elective cesarean section. International Journal of Gynecology and Obstetrics 2018;143(Supplement 3):294-5. [CENTRAL: CN-01654075] [EMBASE: 624606757] [Google Scholar]
- 125.Shalaby MA, Maged AM, Al-Asmar A, El Mahy M, Al-Mohamady M, Mohamed Ali Rund N. Safety and efficacy of preoperative tranexamic acid in reducing intraoperative and postoperative blood loss in high-risk women undergoing cesarean delivery: a randomized controlled trial. BMC Pregnancy and Childbirth 2022;22(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharma R, Najam R, Misra MK. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section. Biomedical and Pharmacology Journal 2011;4(1):231-5. [CENTRAL: CN-00893909] [EMBASE: 362564557] [Google Scholar]
- 127.Shinde S, Zende PP, Waikar S. Study of prevention of post-partum haemorrhage after caesarean section with use of tranexamic acid. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2022;13(5):157-62. [Google Scholar]
- 128.Singh T, Burute SB, Deshpande HG, Jethani S, Ratwani K. Efficacy of tranexamic acid in decreasing blood loss during and after caesarean section: a randomized case control prospective study. Journal of Evolution of Medical and Dental Sciences 2014;3:2780-8. [Google Scholar]
- 129.Singh KP, Singh HA. Tranexamic acid and blood loss during and after cesarean section. A randomized case control prospective study. Journal of Obstetrics and Gynaecology Research 2023;49:51‐2. [DOI: 10.1111/jog.15585] [DOI] [Google Scholar]
- 130.Sujata N, Tobin R, Kaur R, Aneja A, Khanna M, Hanjoora VM. Randomized controlled trial of tranexamic acid among parturients at increased risk for postpartum hemorrhage undergoing cesarean delivery. International Journal of Gynaecology and Obstetrics 2016;133(3):312-5. [CENTRAL: CN-01158811] [EMBASE: 20160186494] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 131.CTRI/2015/05/005752. Tranexamic acid to reduce bleeding after cesarean section in high risk patients [Role of tranexamic acid in reducing the use of uterotonic drugs in parturients at increased risk for obstetric haemorrhage undergoing cesarean section]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2015/05/005752 (first received 2015). [CENTRAL: CN-01847694]
- 132.NCT02026297. Tranexamic acid and thromboelastography during cesarean delivery (TA TEG). https://clinicaltrials.gov/study/NCT02026297?intr=Tranexamic%20acid%20and%20thromboelastography&rank=5 (first posted 1 January 2014).
- 133.Taj N, Firdous A, Akhtar N, Chaudhary MH, Sarah, Bajwa Z, et al. Efficacy of tranexamic acid in reducing blood loss during and after cesarean section. Rawal Medical Journal 2014;39(3):311-3. [CENTRAL: CN-01002077] [EMBASE: 373862877] [Google Scholar]
- 134.Kremer M, Cortez C. Perioperative administration of Tranexamic Acid for Placenta Previa and Accreta Study (TAPPAS): a randomized trial. Obstetrics and Gynecology 2019;133:57S. [CENTRAL: CN-02228782] [EMBASE: 633844323] [Google Scholar]
- 135.NCT02806024. Perioperative administration of Tranexamic Acid for Placenta Previa and Accreta Study [Perioperative administration of Tranexamic Acid for Placenta Previa and Accreta Study (TAPPAS): a randomized, placebo-controlled double blind trial]. https://clinicaltrials.gov/show/NCT02806024 (first received 20 June 2016). [CENTRAL: CN-01559173]
- 136.Tarabrin O, Kaminskiy V, Galich S, Tkachenko R, Gulyaev A, Shcherbakov S, et al. Efficacy of tranexamic acid in decreasing blood loss during cesarean section. Critical Care 2012;16 Suppl 1:S157. [Google Scholar]
- 137.Zaporozhan V, Tarabrin O, Gavrychenko D, Mazurenko G, Saleh O, Lyoshenko I. Efficacy of tranexamic acid in decreasing blood loss during cesarean section. Critical Care 2013;17 (Suppl 2):S135-6. [Google Scholar]
- 138.Tarabrin O, Galich S, Tkachenko R, Gulyaev A, Shcherbakov S, Gavrychenko D. Reduced blood loss during caesarean section under the action of tranexamic acid. European Journal of Anaesthesiology 2012;29:97. [CENTRAL: CN-01155631] [Google Scholar]
- 139.Tarabrin O, Kaminskiy V, Galich S, Tkachenko R, Gulyaev A, Shcherbakov S, et al. Efficacy of tranexamic acid in decreasing blood loss during cesarean section. Critical Care (London, England) 2012;16(Suppl 1):S157. [CENTRAL: CN-02272422] [EMBASE: 70735379] [Google Scholar]
- 140.Torky H, El-Desouky E-S, Abo-Elmagd I, Mohamed A, Abdalhamid A, El-Shahat A, et al. Pre-operative tranexemic acid vs. etamsylate in reducing blood loss during elective cesarean section: randomized controlled trial. Journal of Perinatal Medicine 2020;49(3):353-6. [CENTRAL: CN-02191237] [EMBASE: 633191392] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 141.Sentilhes L, Senat MV, Le Lous M, Winer N, Rozenberg P, Kayem G, et al. Tranexamic acid for the prevention of blood loss after cesarean delivery. New England Journal of Medicine 2021;384(17):1623-34. [CENTRAL: CN-02268529] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 142.Sentilhes L, Marie-Victoire senat, Le Lous M, Winer N, Rozenberg P, Kayem G, et al. 1 Tranexamic acid for the prevention of postpartum hemorrhage after cesarean delivery: the TRAAP2 trial. American Journal of Obstetrics and Gynecology 2021;224(2):S1. [CENTRAL: CN-02247791] [EMBASE: 2010867550] [Google Scholar]
- 143.EUCTR2017-001144-36-FR. TRAnexamic Acid for Preventing postpartum hemorrhage following a Cesarean Delivery [TRAnexamic Acid for Preventing postpartum hemorrhage following a Cesarean Delivery:a multicenter randomised, double blind placebo controlled trial (TRAAP2) - TRAAP2]. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2017-001144-36 (first received 2017). [CENTRAL: CN-02067652]
- 144.NCT03742947. Haemostasis and tranexamic acid in caesarean delivery [Study of peripartum haemostasis and effects of tranexamic acid in caesarean delivery: biologic ancillary study in TRAAP2 patients recruited at the Bordeaux University Hospital: BIO-TRAAP]. https://clinicaltrials.gov/show/nct03742947 (first received 15 November 2018). [CENTRAL: CN-01918391]
- 145.Sentilhes L, Daniel V, Deneux-Tharaux C. TRAAP2 - TRAnexamic Acid for Preventing postpartum hemorrhage after cesarean delivery: a multicenter randomized, doubleblind, placebo- controlled trial - a study protocol. BMC Pregnancy and Childbirth 2020;20(1):63. [CENTRAL: CN-02085587] [EMBASE: 630806397] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.NCT04304625. TRAnexamic Acid for Preventing blood loss following a cesarean delivery in women with placenta pREVIA (TRAAPrevia). https://www.clinicaltrials.gov/ct2/show/NCT04304625 (first posted 11 March 2020).
- 147.Vishal AK, Aggarwal MK, Sharma SK, Prasad GV, Yashi K. Safety and efficacy of prophylactic tranexamic acid in reducing blood loss during and after caesarean delivery: a comparative study. International Journal of Academic Medicine and Pharmacy 2023;5(3):407-13. [Google Scholar]
- 148.Shakur-Still H, Roberts I, Grassin-Delyle S, Chaudhri R, Geer A, Arribas M, et al. Alternative routes for tranexamic acid treatment in obstetric bleeding (WOMAN-PharmacoTXA trial): a randomised trial and pharmacological study in caesarean section births. British Journal of Ostetrics and Gynaecology 2023;130(10):1177-86. [DOI: doi: 10.1111/1471-0528.17455.] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 149.Igboke FN, Obi VO, Dimejesi BI, Lawani LO. Tranexamic acid for reducing blood loss following vaginal delivery: a double-blind randomized controlled trial. BMC Pregnancy and Childbirth 2022;22(178):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ismail AM, Abbas AM, Shahat MA, Ali MK. Evaluation of subendometrial and intramyometrial blood flow after intravenous tranexamic acid for prevention of postpartum hemorrhage in vaginal delivery: a randomized controlled study. Journal of Gynecological Research and Obstetrics 2017;3(2):46-52. [CENTRAL: CN-01609034] [Google Scholar]
- 151.Samimi M, Moravveji SA, Heidari-Shirazi F. The effect of tranexamic acid on pregnancy outcome and vaginal post-parturition hemodynamics. Feyz: Journal of Kashan University of Medical Sciences 2013;17(2):114-22. [CENTRAL: CN-02134955] [Google Scholar]
- 152.IRCT201204079399N1. A placebo-controlled clinical trial to assess efficacy of tranexamic acid in reducing hemorrhage after vaginal delivery. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201204079399N1 (first received 2012). [CENTRAL: CN-01864636]
- 153.Sujita A, Songthamwat S, Songthamwat M. Effectiveness of tranexamic acid for reducing postpartum blood loss in the first two hours after vaginal delivery: a randomised controlled trial. Journal of Clinical and Diagnostic Research 2018;12(3):QC01-4. [CENTRAL: CN-01572688] [EMBASE: 621390430] [Google Scholar]
- 154.Sujita A, Songthamwat M, Songthamwat S. Efficacy of tranexamic acid for reducing blood loss after vaginal delivery: a randomized control trial, double blind. Journal of Obstetrics and Gynaecology Research 2017;43:41. [CENTRAL: CN-01744328] [EMBASE: 616813318] [Google Scholar]
- 155.Abbas AM, Shady NW, Sallam HF. Bilateral uterine artery ligation plus intravenous tranexamic acid during cesarean delivery for placenta previa: a randomized double-blind controlled trial. Journal of Gynecology Obstetrics and Human Reproduction 2019;48(2):115-9. [CENTRAL: CN-01930043] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 156.Hunt BJ. Tranexamic acid for the treatment of postpartum haemorrhage-preliminary results of the Woman trial. Transfusion Medicine (Oxford, England) 2013;23(Suppl 1):7. [Google Scholar]
- 157.Javadi EH, Sadeghipour Z, Barikani A, Javadi M. Tranexamic acid in the control of uterine atony during labor. Biotechnology and Health Sciences 2015;2(2):e26898.
- 158.Sahhaf F, Abbasalizadeh S, Ghojazadeh M, Velayati A, Khandanloo R, Saleh P, et al. Comparison effect of intravenous tranexamic acid and misoprostol for postpartum haemorrhage. Nigerian Medical Journal 2014;55(4):348-53. [CENTRAL: CN-01076651] [PMCID:: PMC4124551] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zargar M, Nikbakht R, Ahmadi M. The effect of tranexamic acid on preventing post-partum hemorrhage due to uterine atony: a triple-blind randomized clinical trial. Current Clinical Pharmacology 2018;13(2):136-9. [CENTRAL: CN-01649688] [EMBASE: 624513891] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 160.Arya P, Yadav G, Singh P, Ghuman NK, Sharma C, Gothwal M, et al. WITHDRAWN: Tranexamic acid to reduce postpartum blood loss after a vaginal delivery: a double-blinded, randomized controlled trial. American Journal of Obstetrics and Gynecology 2023;25:S0002-9378(23)02192-0. [DOI: 10.1016/j.ajog.2023.12.033] [PMID:: 38151222] [DOI] [PubMed] [Google Scholar]
- 161.Ragusa A, Ficarola F, Ferrari A, Spirito N, Ardovino M, Giraldi D, et al. Tranexamic acid versus oxytocin prophylaxis in reducing post-partum blood loss, in low-risk pregnant women: TRANOXY STUDY, a phase III randomized clinical trial. eClinicalMedicine 2024;73:1. [DOI: 10.1016/j.eclinm.2024.102665] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Al-Nasrawi H. Evaluation of the role of tranexamic acid in prevention of postpartum bleeding: a case control study. Indian Journal of Forensic Medicine and Toxicology 2019;13(4):895-900. [CENTRAL: CN-02077566] [EMBASE: 2003259860] [Google Scholar]
- 163.Cetin C, Tanoglu FB, Hanligil E, Gokce A, Pasin O, Ozcan P. Carbetocin versus oxytocin with or without tranexamic acid for prophylactic prevention of postpartum hemorrhage after a vaginal delivery: a randomized clinical trial. Gynecologic and Obstetric Investigation 2023;88(6):366-74. [DOI: 10.1159/000534375] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 164.Diab KM, Mohamed RM, Abdelhay AG. The efficacy of intravenous tranexamic acid in reduction of blood loss in women at high risk of postpartum hemorrhage. QJM: An International Journal of Medicine 2020;113(Suppl 1):i163. [DOI: 10.1093/qjmed/hcaa056.014] [DOI] [Google Scholar]
- 165.Farhadifar F, Shahgheibi S, Zare S, Rezaie M, Seyedolshohadae F, Sharami SR, et al. Investigation of prophylactic effect of tranexamic acid in preventing postpartum hemorrhage in besat hospital in Sanandaj. Pakistan Journal of Medical and Health Sciences 2021;15(3):966-9. [CENTRAL: CN-02275027] [EMBASE: 2011993833] [Google Scholar]
- 166.Hinchigeri K, Patil KP, Patil A, Metgud MC. Injection tranexamic acid in preventing postpartum hemorrhage following vaginal delivery: a one-year hospital-based randomized placebo-controlled trial. Journal of South Asian Federation of Obstetrics and Gynaecology 2024;16(3):239-42. [Google Scholar]
- 167.Mei LL, Ruey S, Wen EP, Ghani NA, Wahab AV. Tranexamic acid usage in third stage labour in reducing post-partum haemorrhage in high risk mothers following a vaginal delivery: a randomised prospective, double-blinded clinical trial. Medical Journal of Malaysia 2019;74:2. [CENTRAL: CN-02205541] [EMBASE: 633276952] [Google Scholar]
- 168.Roy P, Sujatha MS, Bhandiwad A, Biswas B. Role of tranexamic acid in reducing blood loss in vaginal delivery. Journal of Obstetrics and Gynaecology of India 2016;66(Suppl 1):S246-50. [CENTRAL: CN-01730606] [EMBASE: 609598409] [PMID: ] [DOI] [PMC free article] [PubMed]
- 169.Shady NW, Sallam HF, Elsayed AH, Abdelkader AM, Ali SS, Alanwar A, et al. The effect of prophylactic oral tranexamic acid plus buccal misoprostol on blood loss after vaginal delivery: a randomized controlled trial. Journal of Maternal-fetal & Neonatal Medicine 2017;27:1-7. [CENTRAL: CN-01440910] [EMBASE: 619981098] [DOI] [PubMed] [Google Scholar]
- 170.Shady NW, Sallam HF, Elsayed AH, Abdelkader AM, Ali SS, Alanwar A, et al. The effect of prophylactic oral tranexamic acid plus buccal misoprostol on blood loss after vaginal delivery: a randomized controlled trial. Journal of Maternal-fetal & Neonatal Medicine 2019;32(11):1806-12. [CENTRAL: CN-01944542] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 171.Shah HN, Raghavan V, Shukla J, Sandeepti KP. Use of tranexamic acid in preventing postpartum hemorrhage following vaginal delivery. International Journal of Pharmaceutical and Clinical Research 2024;16(7):300-4. [Google Scholar]
- 172.Tali K, Ignacio Alensuela A. The effect of prophylactic intravenous tranexamic acid in reducing blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: a prospective, randomised, double-blind, placebo-controlled study. Australian and New Zealand Journal of Obstetrics and Gynaecology 2016;56(Suppl 1):61. [CENTRAL: CN-01252518] [EMBASE: 613101716] [Google Scholar]
- 173.Zhang P, Jia YJ, Lv Y, Fan YF, Geng H, Zhao Y, et al. Effects of tranexamic acid preconditioning on the incidence of postpartum haemorrhage in vaginal deliveries with identified risk factors in China: a prospective, randomized, open-label, blinded endpoint trial. Annals of Medicine 2024;56(1):238930. [DOI: 10.1080/07853890.2024.2389302] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zheng SR, Li MZ, Chi GZ. Effects of tranexamic acid on decreasing blood loss within two hours after delivery. A multicenter randomized comparative study. Blood 2000;96(11):846a. [CENTRAL: CN-00486589] [Google Scholar]
- 175.Diaz V, Abalos E, Carroli G. Methods for blood loss estimation after vaginal birth. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD010980. [ART.NO: CD010980] [DOI: 10.1002/14651858.CD010980.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mousa HA, Blum J, Abou El Senoun G, Shakur H, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD003249. [DOI: 10.1002/14651858.CD003249.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Perel P, Ker K, Morales Uribe CH, Roberts I. Tranexamic acid for reducing mortality in emergency and urgent surgery. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD010245. [DOI: 10.1002/14651858.CD010245.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI: 10.1016/j.jclinepi.2011.01.012] [DOI] [PubMed] [Google Scholar]
- 179.Al-Dardery NM, Abdelwahab OA, Abouzid M, Albakri K, Elkhadragy A, Katamesh BE, et al. Efficacy and safety of tranexamic acid in prevention of postpartum hemorrhage: a systematic review and meta-analysis of 18,649 patients. BMC Pregnancy and Childbirth 2023;23(1):817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Assis IC, Govêia CS, Miranda D, Ferreira RS, Riccio LG. Analysis of the efficacy of prophylactic tranexamic acid in preventing postpartum bleeding: systematic review with meta-analysis of randomized clinical trials. Brazilian Journal of Anesthesiology (Elsevier) 2023;73(4):467-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cheema HA, Ahmad AB, Ehsan M, Shahid A, Ayyan M, Azeem S, et al. Tranexamic acid for the prevention of blood loss after cesarean section: an updated systematic review and meta-analysis of randomized controlled trials. American Journal of Obstetrics & Gynecology 2023;5(8):101049. [DOI] [PubMed] [Google Scholar]
- 182.Ferrari FA, Garzon S, Raffaelli R, Cromi A, Casarin J, Ghezzi F, et al. Tranexamic acid for the prevention and the treatment of primary postpartum haemorrhage: a systematic review. Journal of Obstetrics and Gynaecology 2022;42(5):1-13. [DOI] [PubMed] [Google Scholar]
- 183.Lee AL, Wang M, Roy D, Wang J, Gokhale A, Miranda-Cacdac L, et al. Prophylactic tranexamic acid prevents postpartum hemorrhage and transfusions in cesarean deliveries: a systematic review and meta-analysis. American Journal of Perinatology 2024;41(S 01):e2254-68. [DOI] [PubMed] [Google Scholar]
- 184.Yang F, Wang H, Shen M. Effect of preoperative prophylactic intravenous tranexamic acid on perioperative blood loss control in patients undergoing cesarean delivery: a systematic review and meta-analysis. BMC Pregnancy and Childbirth 2023;23(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ker K, Sentilhes L, Shakur-Still H, Madar H, Deneux-Tharaux C, Saade G, et al; Anti-fibrinolytics Trialists Collaborators Obstetric Group. Tranexamic acid for postpartum bleeding: a systematic review and individual patient data meta-analysis of randomised controlled trials. Lancet 2024;404(10463):1657-67. [DOI: 10.1016/S0140-6736(24)02102-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gandhi R, Evans HM, Mahomed SR, Mahomed NN. Tranexamic acid and reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Research Notes 2013;6:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Poeran J, Rasul R, Suzuki S, Danninger T, Opperer, Boettner F, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 2014;349:4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ducloy-Bouthors AS, Jude B, Duhamel A, Broisin F, Huissoud C, Keita-Meyer, et al. High-dose tranexamic acid reduces blood loss in postpartum haemorrhage. Critical Care 2011;15:R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Peitsidis P, Kadir RA. Antifibrinolytic therapy with tranexamic acid in pregnancy and postpartum. Expert Opinion on Pharmacotherapy 2011;12(4):503-16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 Search strategies
Supplementary material 2 Characteristics of included studies
Supplementary material 3 Characteristics of excluded studies
Supplementary material 4 Characteristics of studies awaiting classification
Supplementary material 5 Characteristics of ongoing studies
Supplementary material 6 Analyses
Supplementary material 7 Data package
Supplementary material 8 Amendments to protocol and previous version of review
Data Availability Statement
As part of the published Cochrane review, the following is made available for download for users of the Cochrane Library: full search strategies for each database; full citations of each unique report for all studies included, ongoing, or awaiting classification, or excluded at the full‐text screen, in the final review; study data, including study information, study arms, and study results or test data; consensus risk of bias assessments; and analysis data, including overall estimates and settings, subgroup estimates, and individual data rows. Appropriate permissions have been obtained for such use. Analyses and data management were conducted within Cochrane’s authoring tool, RevMan, using the inbuilt computation methods. Template data extraction forms from Covidence are available from the authors on reasonable request.
All data available in Supplementary material 7.


