Abstract
Background
Neutrophils play a key role in sepsis-associated acute kidney injury (SAKI), a common and life-threatening complication of organ failure. High mobility group box 1 (HMGB1) modulates inflammatory responses and the formation of neutrophil extracellular traps (NETs). The present work aimed to explore whether HMGB1 lactylation promotes NET formation and exacerbates SAKI.
Methods
Venous blood samples were collected from healthy volunteers and SAKI patients. A SAKI mouse model was established using the cecal ligation and puncture method. A coculture system of macrophage-derived exosomes and neutrophils was established. Macrophage-derived exosomes were isolated and identified. ELISAs, immunofluorescence staining, coimmunoprecipitation, and Western blotting were utilized to determine protein levels.
Results
Elevated blood lactate levels were associated with increased HMGB1 levels in patients with SAKI. In mouse models, lactate increased HMGB1 expression, promoted NET formation, and exacerbated SAKI. Lactate stimulated M1 macrophages to secrete exosomes, leading to the accumulation and release of HMGB1 in the cytoplasm. Additionally, lactate promoted HMGB1 lactylation in macrophages, triggering the release of mitochondrial DNA from neutrophils and activating the cyclic GMP‒AMP synthase/stimulator of interferon genes pathway.
Conclusion
This study revealed that lactate-induced HMGB1 lactylation in macrophages plays a role in promoting NET formation in SAKI through the cGAS/STING pathway. These findings suggest that HMGB1 could be a potential target for therapeutic intervention in SAKI.
Graphical abstract
Lactate promotes the lactylation modification of HMGB1 in macrophages through H3K18lac, facilitating the secretion of HMGB1. After HMGB1 enters neutrophils, it induces the leakage of mitochondrial DNA (mtDNA) in neutrophils, activating the cGAS/STING pathway, promoting the formation of neutrophil extracellular traps (NETs), and exacerbating acute kidney injury in sepsis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10565-025-10026-6.
Keywords: Acute kidney injury, HMGB1, Lactylation, Macrophages, Neutrophil Extracellular Trap, Sepsis
Highlights
• Elevated blood lactate levels are associated with increased HMGB1 levels in SAKI
• Lactate increases HMGB1 expression, promotes NET formation, and exacerbates SAKI
• Lactate promotes HMGB1 lactylation in macrophages
• Lactate triggers the release of mitochondrial DNA from neutrophils
• Lactate activates the cyclic GMP-AMP synthase/stimulator of interferon genes pathway
Supplementary Information
The online version contains supplementary material available at 10.1007/s10565-025-10026-6.
Introduction
Sepsis is a life-threatening condition that arises from a dysregulated host response to infection, leading to widespread inflammation, tissue damage, and organ dysfunction (Jarczak et al. 2021). When left untreated, sepsis can lead to septic shock, a severe manifestation of sepsis characterized by persistent hypotension, despite adequate fluid resuscitation, and often results in multiorgan failure, including acute kidney injury (AKI) (Caraballo and Jaimes 2019). Septic shock is a leading cause of morbidity and mortality worldwide, affecting millions of people each year (Via et al. 2024). The incidence of AKI in sepsis patients is alarmingly high, with studies showing that sepsis-associated AKI (SAKI) affects up to 50% of septic patients (Zarbock et al. 2023). This condition substantially worsens patient outcomes, resulting in a greater need for renal replacement therapy and a higher risk of mortality. Research has demonstrated that SAKI strongly contributes to the global burden of kidney failure, and patients with septic shock who develop AKI have a significantly reduced chance of survival (Cao et al. 2023). Despite major advancements in early prediction and medical technology during the past several decades, the incidence and overall burden of SAKI remain persistently high (Poston and Koyner 2019). Most available therapies for SAKI are primarily palliative, focusing on symptom management, preventing further damage, and improving the patient’s overall condition to prolong survival (Manrique-Caballero et al. 2021). Currently, no treatments can completely reverse the progression of this complex syndrome (Gómez et al. 2023). Sepsis-induced immune dysfunction substantially contributes to SAKI by activating various immune cells of both innate and adaptive origins. Novel therapeutic strategies targeting immune regulation may offer sustained clinical benefits in treating this debilitating disease.
Neutrophils are key mediators of both acute and chronic inflammatory diseases (Herrero-Cervera et al. 2022). In response to proinflammatory cytokines, endotoxins, or other danger signals, neutrophils can form large, web-like structures decorated with antimicrobial proteins to effectively capture and kill pathogens (Demkow 2023). These neutrophil extracellular traps (NETs) are composed of decondensed chromatin, which includes modified histones such as citrullinated histone H3 (Cit-H3), along with granule proteins such as myeloperoxidase (MPO) and neutrophil elastase (Zou et al. 2018). Prolonged disruption of proinflammatory and anti-inflammatory homeostasis can cause NETs to become pathogenic, which is a characteristic of inflammatory responses (Wiersinga and Poll 2022). NET products, including Cit-H3, extracellular DNA, and granule enzymes, act as damage-associated molecular patterns (DAMPs) that initiate immunosuppression (Cicco et al. 2020). This process is closely related to the rapid progression of inflammation and tissue damage due to sepsis (Denning et al. 2019).
High mobility group box 1 (HMGB1) is a DAMP that plays an essential role in regulating inflammation (Mo et al. 2023). Under infectious and sepsis-related conditions, activated neutrophils release excessive NETs, leading to uncontrolled inflammatory responses and the development of multiple organ dysfunction (Shen et al. 2017). Previous data have suggested that HMGB1 mediates the recruitment of neutrophils to sites of necrosis and may trigger the formation of NETs (Zhang et al. 2022). Additionally, extracellular HMGB1 can bind to and activate various receptors on the surface of immune cells, such as receptors for advanced glycation end products and Toll-like receptors (Yue et al. 2022). The activation of these receptors triggers intracellular signaling pathways, resulting in the initiation of inflammatory responses. Another critical function of extracellular HMGB1 is its ability to enter the cytoplasm and form complexes with cytoplasmic DNA (Starkova et al. 2023). These HMGB1-DNA complexes activate the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway, increasing the capacity of cGAS to recognize and bind to DNA (Yang et al. 2022a). This process stimulates cGAS-mediated synthesis of cGAMP, activates STING and its downstream signaling pathways, and ultimately promotes nucleic acid-mediated innate immune responses (Wan et al. 2020). However, it remains unclear whether HMGB1 regulates NET formation in SAKI and whether this process involves the cGAS/STING pathway.
Lactate, the end product of glycolysis, is an independent predictor of poor prognosis in septic patients (Lee et al. 2021), with elevated lactate levels correlated with increased mortality and organ dysfunction (Lee and An 2016). In addition to its role in metabolic shifts during sepsis, lactylation has been shown to modulate cellular processes such as DNA repair and control the expression of related genes (Hu et al. 2024). Recent studies have suggested a link between lactate levels and HMGB1, a critical mediator of inflammation and the immune response during sepsis (Zhang et al. 2024; Andersson and Yang 2022). Specifically, lactate has been shown to promote HMGB1 lactylation in macrophages during polymicrobial sepsis (Yang et al. 2022b). Therefore, we hypothesized that lactate may induce HMGB1 lactylation in macrophages, which then promotes NET formation and exacerbates SAKI.
Materials and methods
Human blood samples
Venous blood (20 mL) treated with EDTA anticoagulant was collected from 30 patients who had been diagnosed with sepsis within 24 h of hospitalization. The diagnosis of sepsis was made according to the Third International Consensus Definitions for Sepsis and Septic Shock. AKI was assessed using the Kidney Disease: Improving Global Outcomes criteria, and only patients with SAKI were included in the study. Patients were excluded if they had a history of chronic kidney disease, were pregnant, had undergone recent major surgery, or had other severe comorbidities, such as chronic liver disease or heart failure, that might independently affect kidney function. Blood samples were collected during the early phase of sepsis (within 24 h) to capture data before any potential progression of organ dysfunction. However, as SAKI can develop in the days following sepsis diagnosis, patients who did not develop AKI within the first 72 h after sepsis onset were excluded from the study to avoid confounding factors related to delayed renal injury. Blood samples were also collected from 30 healthy, sex- and age-matched volunteers who had no history of hypertension, diabetes, or autoimmune diseases. Exclusion criteria for healthy controls included any notable past or present medical conditions, including hypertension, diabetes, and immune system disorders. The age and sex distributions of both patients and controls were matched, with controls aged 25–55 years and an equal distribution of sex, clinical information on AKI is presented in Table 1.
Table 1.
The clinical information of AKI patients
| Characteristic | Pre renal factors (N = 10) |
Renal factors (N = 12) |
Post renal factors (N = 8) |
P | |
|---|---|---|---|---|---|
| Hospital stays | 6 | 8 | 5 | 0.32 | |
| Gender | Male | 6 | 9 | 3 | 0.24 |
| Female | 4 | 3 | 5 | ||
| Age | 54 ± 5 | 64 ± 12 | 62 ± 10 | 0.06 | |
| Smoking | 10 | 4 | 6 | 0.15 | |
| Cardiopathy | 1 | 2 | 2 | 0.22 | |
| NYHA class | III | 5 | 7 | 6 | 0.24 |
| IV | 5 | 5 | 2 | ||
| Hypertension | 3 | 2 | 3 | 0.51 | |
| COPD | 4 | 5 | 4 | 0.36 | |
| CKD | 5 | 7 | 6 | 0.42 | |
| Cerebrovascular | 3 | 2 | 5 | 0.16 | |
| Disturbance of consciousness | 1 | 5 | 4 | 0.07 | |
| Body temperature | 36.9 | 36.7 | 36.7 | 0.21 | |
| Diabetes | 2 | 3 | 1 | 0.35 | |
| Sphygmus | 3 | 3 | 4 | 0.44 | |
| Breathing rate | 5 | 4 | 4 | 0.16 | |
| Systolic blood pressure | 2 | 3 | 3 | 0.28 | |
| Diastolic blood pressure | 1 | 2 | 4 | 0.43 | |
| hsCRP > 8 mg/mL | 6 | 7 | 5 | < 0.05 | |
| Lactate ≥ 2.2 mmol/L | 8 | 10 | 5 | < 0.05 | |
| PO2 ≤ 90% | 3 | 5 | 2 | 0.34 | |
All the samples were aliquoted, stored at –80 °C, and handled according to the Declaration of Helsinki. Protocol approval was obtained from the local ethics committee. Informed consent was obtained from all the participating individuals. Logistic regression analysis was performed to evaluate the relationships between HMGB1 expression and clinical outcomes, including survival and renal function recovery, adjusting for covariates such as age, sex, and comorbidities.
Cell culture and treatments
Macrophages (RAW 264.7) were obtained from the Cell Bank of the Chinese Academy of Sciences. Primary mouse macrophages were isolated as described below. The cells were cultured in DMEM supplemented with penicillin (100 U/mL), 10% fetal bovine serum, and streptomycin (100 ng/mL) (all from Sigma‒Aldrich, USA). For determination of the effect of lactate on the viability of macrophages, various concentrations of exogenous lactate (sodium lactate, Nala; #71718, Sigma‒Aldrich) (10–50 mM) were added to the macrophages. After incubation for 30 min, the MTT assay was utilized to evaluate cell viability as mentioned previously (Wang, et al. 2020).
For subsequent experiments, the macrophages were pretreated with 10 mM Nala for 24 h. The groups subjected to LPS stimulation were exposed to 500 ng/mL LPS for 6 h (Molagoda et al. 2021). Macrophages were preincubated with 20 mM oxamate for 2 h prior to LPS stimulation to suppress lactate production (Yang et al. 2022b). For overexpression or knockdown of HMGB1 in RAW 264.7 macrophages, plasmid vectors carrying the Hmgb1 gene sequence or short hairpin RNA against Hmgb1 (both purchased from GenePharma, China) were transfected into cells using Lipofectamine™ 3000 (Thermo Fisher Scientific, USA). After 48 h, the cells were subjected to LPS stimulation.
Exosome isolation and identification
The macrophages were cultured until they reached approximately 80% confluence and then maintained in serum-free medium overnight. The cell culture supernatant was harvested and subjected to a series of centrifugation steps: 2,000 × g to remove cell debris for 30 min, 10,000 × g to eliminate subcellular components for 30 min, and 100,000 × g to isolate exosomes for 1 h. The exosome pellet was resuspended in 1 mL of 0.01 M PBS and centrifuged again for 1 h at 100,000 × g. Transmission electron microscopy (JEOL, Japan) was used to determine the morphology of the exosomes as previously described (Jiao et al. 2023). Nanoparticle tracking analysis (NTA) was used to measure the size of the exosomes. The exosome samples were diluted appropriately and analyzed using an NTA system (Malvern Panalytical, UK), which tracks the Brownian motion of the nanoparticles in suspension to determine their size.
Treatment of neutrophils with exosomes
Mouse neutrophils (ATCC, USA) were incubated with macrophage-derived exosomes as previously reported (Zhang et al. 2019). In brief, neutrophils were seeded in 6-well plates and allowed to adhere overnight. The next day, macrophage-derived exosomes were added to the neutrophil culture. The coculture was maintained for 24 h for subsequent analyses. Confocal fluorescence microscopy was used to observe neutrophils incubated with macrophage-derived exosomes labeled with PKH67 (Sigma‒Aldrich).
Establishment of a SAKI mouse model
A SAKI mouse model was established in wild-type male C57BL/6 J mice (6–8 weeks old) using the cecal ligation and puncture (CLP) method. In brief, 8-week-old mice were obtained from a local animal facility and housed in a controlled environment. An intraperitoneal injection of pentobarbital was used for anesthesia at a dosage of 60 mg/kg (Ge et al. 2023). After the area was sterilized, a 1 cm midline incision was made in the abdomen. For induction of sepsis, the cecum was exposed, ligated beneath the cecal valve, and finally punctured with an 18-gauge needle. The cecum was subsequently returned to the abdominal cavity, and the incision was sutured. Sham-operated mice were subjected to identical surgical procedures except for cecum ligation and puncture. All the mice received a single dose of resuscitative fluid via subcutaneous injection following surgery and were then placed on a heated pad for recovery. For determination of whether elevated lactate exacerbates SAKI, the mice were given an intraperitoneal injection of NaLa (Sigma‒Aldrich) at 1 g/kg 30 min before CLP. For analysis of the effects of mtDNA depletion and NET inhibition, the mice were subcutaneously injected with 450 μg/kg RU320521 (RU.521; #SML2347, Sigma‒Aldrich) (Ding et al. 2022) and 30 mg/kg sivelestat (SIVE; #7198, Sigma‒Aldrich), respectively, after the CLP procedure (Kumagai et al. 2013). For examination of the effects of exosomal HMGB1, mice were injected with exosomes derived from RAW 264.7 macrophages overexpressing HMGB1 (exo-HMGB1) or with insufficient HMGB1 expression (exo-sh-HMGB1) at a concentration of 2 μg/kg body weight via intravenous injection 4 h after CLP. After 18 h of CLP, the mice were euthanized, and blood samples and kidney tissues were collected. Each group contained six animals.
Small animal in vivo imaging was performed using near-infrared (NIR)-labeled exosomes to track their organ distribution after intravenous injection. Exosomes were labeled with an NIR fluorescent dye (Lumiprobe, USA) and injected into mice via the tail vein. An in vivo dynamic imaging system (Berthold Technologies, Germany) was used to assess exosome accumulation in major organs. For assessment of the 7-day prognosis of the mice, survival analysis was performed. The mice were monitored for 7 days following the experimental treatments, and survival data were recorded daily. The Kaplan‒Meier method was used to calculate survival curves, and significant differences between groups were evaluated using the log-rank test. The study protocol was performed following the rules approved by the local ethics committee.
Isolation of primary mouse macrophages
Primary bone marrow-derived mouse macrophages were harvested as previously described (Assouvie et al. 2018). In brief, after a mouse was sacrificed, the epiphyses of the bones were cut. The marrow was then flushed into a centrifugation tube using a 23G needle and a 5-mL syringe. The cell suspension was centrifuged at 250 × g at 4 °C for 5 min and then filtered. The purity of the macrophages was verified by flow cytometry (BD Biosciences, USA) using anti-F4/80 and anti-CD11b antibodies (Abcam, UK).
Histological examination
For observation of morphological alterations in the kidney, renal tissues were harvested and fixed in 4% paraformaldehyde for hematoxylin and eosin (H&E) staining (Sigma‒Aldrich). The stained tissue sections were assessed by an experienced pathologist blinded to the experimental design via a scoring system based on tubular damage. Each field was scored on a scale of 0–4, where 0 indicated no damage (normal tubules), and 1 indicated mild damage, characterized by brush border loss in ≤ 10% of tubules with minimal tubular dilation. A score of 2 represented moderate damage, with 10–25% of tubules affected, moderate brush border loss, occasional cast formation, and mild tubular necrosis. Severe damage was assigned a score of 3, indicating that 25–50% of the tubules were affected, and widespread brush border loss, frequent cast formation, and moderate necrosis occurred. A score of 4 represented extensive damage, with more than 50% of tubules affected, severe necrosis, extensive cast formation, and substantial tubular dilation. The final histological score for each kidney was determined by averaging the scores from five randomly selected fields per mouse.
Assessment of human and mouse blood samples
The levels of lactate and HMGB1 in human serum samples were measured using the L-Lactate Assay Kit (Colorimetric) (#ab65331, Abcam) and the Human HMGB1 ELISA Kit (#E-EL-H1554, Elabscience, USA), respectively, following the manufacturer’s protocols. The serum content of NETs was assessed via a Neutrophil Extracellular Traps ELISA Kit (#KBH4548, Eagle Biosciences, USA).
For determination of the levels of circulating free DNA (cfDNA) in mouse and human serum samples, the MagMAX Cell-Free DNA Isolation Kit (#A29319, Thermo Fisher Scientific) was used for cfDNA isolation. Then, quantitative PCR (qPCR) was performed to determine their quantity.
A PicoGreen dsDNA Quantification Kit (Invitrogen, USA) and capture ELISA were used to quantify the content of circulating MPO-DNA in the mouse serum samples (Jiao et al. 2023). An anti-MPO monoclonal antibody (1 μg/mL; #ab208670, Abcam) was used as a capture antibody.
The levels of kidney injury molecule- 1 (KIM- 1) and serum creatinine (SCr) in mouse serum samples were measured with a Mouse KIM- 1 ELISA Kit (#80652, Crystal Chem, USA) and a Mouse Creatinine Assay Kit (#MBS763433, MyBioSource, Inc., USA). The levels of IL- 6 and IL- 8 in mouse serum were measured by ELISA kits purchased from R&D Systems (#M6000B, USA) and MyBioSource (#MBS286946), respectively.
Immunofluorescence (IF) staining
For assessment of NETs in mouse kidney tissues, kidney specimens were embedded in paraffin, sectioned, blocked with phosphate-buffered saline (PBS) containing 1% goat serum and 3% bovine serum albumin (Sigma‒Aldrich), and permeabilized with 0.01% Triton X- 100 diluted in PBS. These slides were then incubated with antibodies targeting MPO and citrullinated histone H3 (Cit-H3) (#ab5103, Abcam). After being washed with PBS, the slides were incubated with a secondary antibody (Abcam) for 1 h, counterstained with DAPI (Sigma‒Aldrich), and examined under a fluorescence microscope (Olympus, Japan).
For analysis of double-strand DNA (dsRNA) and mitochondria in the neutrophils cocultured with RAW 264.7 macrophages, the cells were incubated with an anti-dsDNA antibody (#ab27156, Abcam) at 4 °C for 8 h. After washes with PBS, the cells were incubated for 1 h with a secondary antibody (Abcam). and observed under a fluorescence microscope. The mitochondria were counterstained with DAPI.
Coimmunoprecipitation (co-IP)
The colocalization of lysine lactylation (Klac) and HMGB1 in the RAW 264.7 macrophage cytoplasm was detected by co-IP as previously described (Yang et al. 2022b). In brief, 200 µg of total protein isolated from macrophages was stained with an anti-lactyl-lysine antibody (#PTM- 1401, PTM Bio, China) overnight at 4 °C. Then, 20 µL of protein A/G-agarose beads (Santa Cruz Biotechnology, USA) was added to all the samples. The precipitates were washed with lysis buffer (Promega, USA) four times and boiled in SDS sample buffer (Sigma‒Aldrich). The supernatant was then assessed by immunoblotting with an anti-HMGB1 antibody (#ab190377, Abcam).
Quantification of mitochondrial DNA (mtDNA) in the neutrophil cytoplasm
The cytoplasmic mtDNA in neutrophils was detected as previously reported (Nakahira et al. 2011). In brief, neutrophils were centrifuged at 700 × g at 4 °C for 10 min. Cell pellets were then sequentially treated with Mitochondria Isolation Reagents A, B, and C, according to the instructions provided with the Mitochondrial DNA Isolation Kit (#ab65321, Abcam). The resulting supernatant was carefully collected after centrifugation at 1,000 × g for 10 min at 4 °C and transferred to a new tube. After another centrifugation at 12,000 × g for 15 min at 4 °C, the cytoplasmic mtDNA was collected and quantified using qPCR.
Quantitative measurement of protein and mRNA expression
Western blotting (WB) was conducted to quantify protein levels in cells, mouse kidney tissues, and human serum samples as previously reported (Mishra et al. 2017). Total protein (40 μg per sample) was used for analysis. The primary antibodies used in this study included anti-HMGB1 (#ab190377, Abcam), anti-laminB1 (#sc- 374015, Santa Cruz Biotechnology, USA), anti-CD63 (#ab217345, Abcam), anti-tumor susceptibility gene 101 (TSG101; #ab30871, Abcam), anti-CD9 (#ab223052, Abcam), anti-calnexin (#ab22595, Abcam), anti-cyclic GMP-AMP synthase (cGAS; #sc- 515777, Santa Cruz Biotechnology), anti-stimulator of interferon genes (STING; #ab288157, Abcam), anti-TANK-binding kinase 1 (TBK1; #3013, Cell Signaling Technology, USA), anti-phosphorylated TBK1 (p-TBK; #5483, Cell Signaling Technology), anti-interferon regulatory transcription factor 3 (IRF3; #4302, Cell Signaling Technology), and anti-phosphorylated IRF3 (p-IRF3; #4947, Cell Signaling Technology). The internal control used for protein expression analysis was β-actin.
The Hmgb1 mRNA level was determined using qPCR as described previously (Gao et al. 2021). The primers (GenePharma, China) utilized in this experiment were as follows: Hmgb1 forward, 5ʹ-CTG TCC ATT GGT GAT GTT GC- 3ʹ; Hmgb1 reverse, 5ʹ-CTG ATA GCC TGC TCC AGG TC- 3ʹ. The results were normalized to the GAPDH levels and quantified using the 2−∆∆Ct method.
Statistical analysis
All experiments were performed in triplicate and repeated three times to ensure reproducibility, with the data presented as the means ± standard deviations. Data analyses were conducted using SPSS software (v 24.0). Data normality was assessed using the Shapiro‒Wilk test. For comparisons of two groups, one-way analysis of variance (ANOVA) followed by Tukey's honest significant difference test was employed. Comparisons between two groups were made using an independent samples t test. If the normality assumption was not met, the Mann‒Whitney U test was used. Correlations between normally distributed variables were identified using Pearson's correlation coefficient. A p value of less than 0.05 was considered statistically significant for all analyses.
Results
High lactate levels are associated with HMGB1 and NET marker levels in patients with SAKI
We first compared the serum levels of lactate and HMGB1 between SAKI patients (n = 30) and healthy controls (n = 30). ELISAs revealed a significant increase in both the lactate and HMGB1 levels in the SAKI group compared with those in the control group (Fig. 1A). Additionally, a positive correlation was found between lactate and HMGB1 in the SAKI patients (Fig. 1B). WB analysis confirmed that HMGB1 expression was significantly higher in the serum samples of the SAKI patients than in those of the healthy volunteers (Fig. 1C). The levels of cfDNA and NETs in the serum samples of both groups were subsequently measured. The ratio of cfDNA to NETs was markedly greater in the SAKI patients than in the control participants (Fig. 1D). The above findings suggest that the elevated blood lactate levels in SAKI patients are associated with HMGB1 levels and may be linked to NET markers.
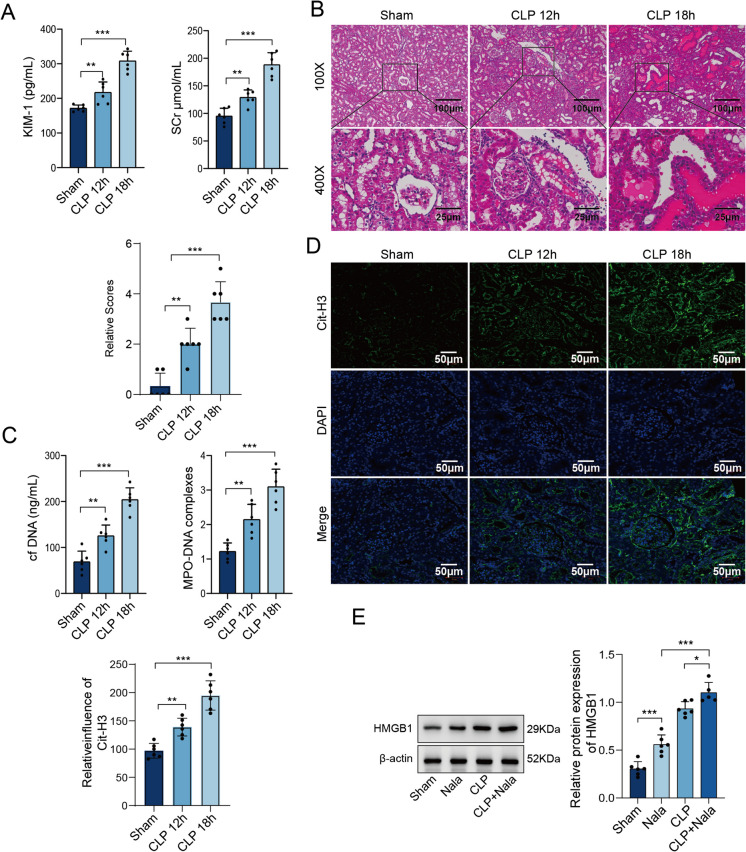
Fig. 1.
Levels of lactate, HMGB1, and NET markers in patients with SAKI versus healthy controls. Venous blood was collected from 30 patients with recently diagnosed sepsis and 30 healthy volunteers. A The levels of lactate and HMGB1 in the serum were measured by ELISAs. B Pearson's correlation coefficient was used to determine the correlation between serum HMGB1 and lactate. C The protein levels of HMGB1 were determined using WB in 4 randomly selected patients with SAKI and 4 healthy controls. D The ratio of cf-DNA to NETs in the serum samples was compared between the SAKI group and the control group using ELISAs. Multiple-group comparisons were performed with one-way ANOVA with Tukey's post hoc test; two-group comparisons were performed with independent t tests or Mann‒Whitney U tests
Elevated lactate levels promote HMGB1 expression and exacerbate SAKI
To explore how lactate levels affect SAKI and HMGB1 expression, we established a SAKI mouse model using the CLP method. The levels of KIM- 1 and SCr increased significantly after CLP surgery and continued to rise over time (Fig. 2A). H&E staining revealed substantial inflammatory cell infiltration near the glomeruli in the CLP-treated mice, along with swelling and degeneration of renal tubular epithelial cells and a disordered arrangement. Renal tissue damage progressively worsened over time (Fig. 2B). In addition, the serum levels of cfDNA and MPO-DNA substantially increased after the CLP procedure (Fig. 2C), accompanied by an increase in Cit-H3 expression (Fig. 2D). Both changes continued to increase over time. Furthermore, the levels of HMGB1 in the mouse serum samples were elevated after CLP surgery, and the addition of lactate further promoted the protein expression of HMGB1 (Fig. 2E). These data suggest that elevated lactate levels increase HMGB1 expression and NET formation, exacerbating SAKI in mice.
Fig. 2.
Effects of elevated lactate levels on HMGB1 expression and SAKI. A-C SAKI mouse models were established in wild-type male C57BL/6 J mice. After 12 or 18 h of CLP, the mice were euthanized. A The serum levels of KIM- 1 and SCr in the sham and CLP groups were measured using commercially available kits and compared. B Kidney tissues were collected for histopathological assessment using H&E staining. C The contents of cfDNA and MPO-DNA in the serum were measured using ELISAs. D IF staining was used to determine the expression of Cit-H3. E Mice were subjected to CLP (18 h) or sham operation. An intraperitoneal injection of NaLa (1 g/kg) was administered 30 min before CLP to examine the effect of elevated lactate levels. The protein expression of HMGB1 in the serum was determined by WB. N = 6 per group. Multiple-group comparisons were performed with one-way ANOVA with Tukey's post hoc test; two-group comparisons were performed with independent t tests or Mann‒Whitney U tests
Lactate promotes HMGB1 secretion from M1 macrophage-derived exosomes
To examine the impact of lactate on HMGB1 expression in vitro, we treated RAW 264.7 macrophages with various doses of Nala. The MTT assay results revealed that lactate concentrations above 30 mM significantly reduced macrophage viability (Fig. 3A). WB analysis revealed significantly increased expression of HMGB1 following LPS treatment, and the addition of Nala further upregulated HMGB1 expression in the cytoplasm (Fig. 3B). Upon incubation with oxamate, a glycolysis inhibitor, oxamate effectively reduced the elevated cytoplasmic HMGB1 levels induced by LPS and the combined treatment with LPS and Nala (Fig. 3C). Macrophage-derived exosomes exhibited a distinctive double-membrane structure and a cup-shaped morphology (Fig. 3D). NTA revealed that the size of the exosomes derived from M1 macrophages ranged primarily from 30 to 150 nm (Fig. 3E). Successful exosome isolation was confirmed by the presence of the exosomal marker proteins CD63, TSG101, and CD9 and the absence of calnexin via WB analysis (Fig. 3F). The qPCR results demonstrated that the exosomal expression of Hmgb1 increased following LPS induction and that this expression was further increased by the addition of Nala (Fig. 3G). These in vitro data indicate that lactate stimulates M1 macrophages to secrete exosomes, leading to increased accumulation and release of HMGB1 in the cytoplasm.
Fig. 3.
Lactate mediates HMGB1 secretion from M1 macrophage-derived exosomes. A RAW 264.7 macrophages were treated with Nala at 10–50 mM for 30 min. Cell viability was determined via the MTT assay. B RAW 264.7 macrophages were pretreated with 10 mM Nala for 24 h. The groups subjected to LPS stimulation were exposed to 500 ng/mL LPS for 6 h. The control group remained untreated. The protein levels of HMGB1 and LaminB1 were measured by WB. C To suppress lactate production, macrophages were preincubated with 20 mM oxamate for 2 h prior to LPS stimulation. The protein expression of HMGB1 and Lamin B1 was determined by WB. D The morphology of the exosomes was observed using transmission electron microscopy. E NTA was performed to determine the size of the exosomes derived from M1 macrophages. F WB was used to determine the expression of CD63, TSG101, CD9, and calnexin in the exosomes and cells. G The mRNA levels of Hmgb1 in the control, LPS, and LPS + Nala groups were measured by qPCR. Multiple-group comparisons were performed with one-way ANOVA with Tukey's post hoc test; two-group comparisons were performed with independent t tests or Mann‒Whitney U tests
Lactate promotes HMGB1 lactylation
We further examined the effects of lactate on HMGB1 lactylation. The co-IP assay demonstrated a predominant increase in Klac levels in the cells treated with Nala, LPS, or their combination. The highest Klac levels were observed in the group receiving both Nala and LPS, suggesting that both LPS and Nala can induce HMGB1 lactylation (Suppl. Figure 1 A). However, the glycolysis inhibitor oxamate significantly reduced the cellular level of Klac (Suppl. Figure 1B). The IF staining results indicated that lactate promoted both Klac and HMGB1 expression in the cytoplasm of macrophages (Suppl. Figure 1 C). These results confirm that lactate promotes HMGB1 lactylation in macrophages.
Macrophage-derived exosomal HMGB1 promotes NET formation via the cGAS/STING pathway
We further established a coculture system of macrophage-derived exosomes and neutrophils (Fig. 4A). Confocal fluorescence microscopy images revealed that the PKH67-labeled macrophage-derived exosomes successfully entered the neutrophils (Fig. 4B). LPS stimulation promoted HMGB1 expression, and the addition of exosomes derived from HMGB1-overexpressing macrophages (exo-HMGB1) further elevated the mRNA levels of Hmgb1. In contrast, exosomes derived from HMGB1-knockdown macrophages (exos-sh-HMGB1) presented lower Hmgb1 mRNA levels (Fig. 4C). The levels of Hmgb1 in neutrophils showed a similar trend (Fig. 4C). Additionally, the mtDNA content was highest in the cytoplasm of neutrophils exposed to exo-HMGB1, whereas it was significantly reduced in neutrophils treated with exo-sh-HMGB1 (Fig. 4D). IF staining revealed that LPS induced mitochondrial fragmentation and mtDNA release, and these effects were exacerbated in the exo-HMGB1-treated group. The presence of exos-sh-HMGB1, however, mitigated mitochondrial fragmentation and mtDNA release in neutrophils (Fig. 4E). WB analysis revealed increased protein expression of cGAS and STING in the LPS + exo-HMGB1 group, whereas these proteins were markedly downregulated by exos-sh-HMGB1 (Fig. 4F). The above data suggest that macrophage-derived exosomal HMGB1 induces mtDNA leakage in neutrophils, leading to cGAS/STING activation.
Fig. 4.
Macrophage-derived exosomal HMGB1 mediates NET formation via cGAS/STING. RAW 264.7 macrophages were transfected with plasmid vectors carrying the Hmgb1 gene sequence or short hairpin RNA against Hmgb1. A coculture system of macrophage-derived exosomes and neutrophils was established. A Confocal fluorescence microscopy images were obtained of neutrophils incubated with PKH67-labeled M1-exosomes (green). B Hmgb1 mRNA expression in exosomes and neutrophils was measured by qPCR. C Cytoplasmic mtDNA was quantified by qPCR. D IF staining was used to detect dsDNA (green) and mitochondria (red) in neutrophils. E WB was performed to determine the expression levels of cGAS and STING. Multiple-group comparisons were performed with one-way ANOVA with Tukey's post hoc test; two-group comparisons were performed with independent t tests or Mann‒Whitney U tests
HMGB1 mediates SAKI in mice by regulating NET formation through the cGAS/STING pathway
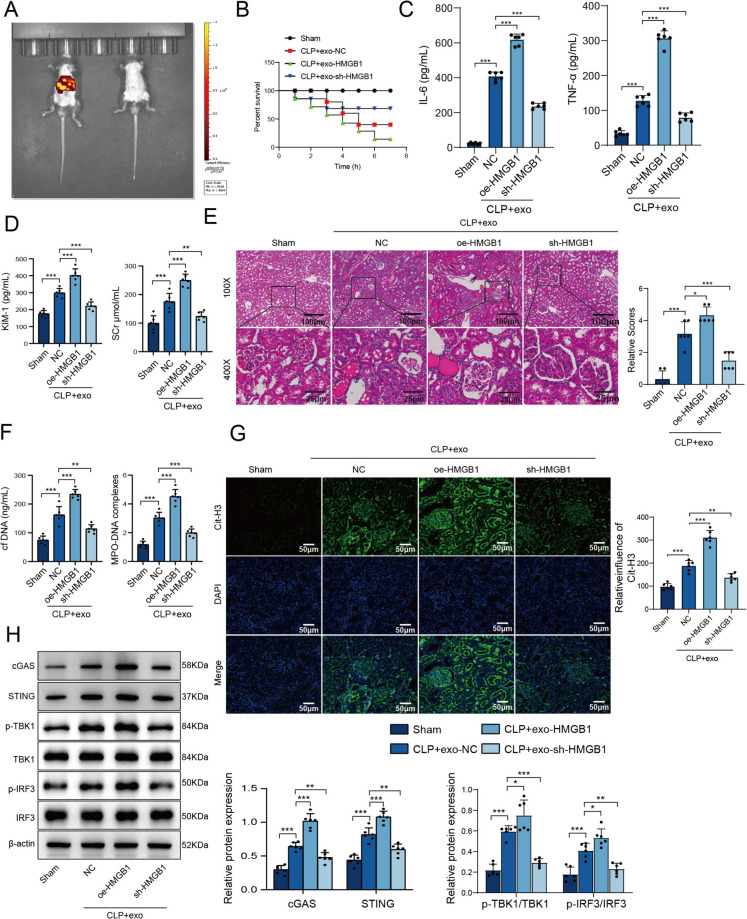
To validate these findings in vivo, we injected mice with exos-HMGB1 or exos-sh-HMGB1 following the CLP procedure. The distribution of exosomes was primarily concentrated in the liver, spleen (mononuclear phagocyte system), lungs, and kidneys (Fig. 5A). We analyzed the 7-day survival rate of mice in each group, the survival rate of Sham group was 100%, the survival rate of CLP group was decreased, the survival rate of CLP + exo-HMGB1 group was the lowest, and the survival rate of CLP + exo-sh-HMGB1 group was remission (Fig. 5B). The expression of IL- 6 and IL- 8 was elevated in the CLP group, and the addition of exosomal HMGB1 further exacerbated this effect. In contrast, the knockdown of HMGB1 in exosomes reversed this phenomenon (Fig. 5C). Exos-HMGB1 further amplified the LPS-induced increase in the SCr and KIM- 1 levels, whereas exosomes from HMGB1-knockdown macrophages alleviated this effect (Fig. 5D). Furthermore, the CLP group exhibited extensive inflammatory cell infiltration near the glomeruli, swelling and degeneration of renal tubular epithelial cells, disorganized cell arrangement, and the presence of renal casts. Injection of exos-HMGB1 exacerbated these injuries, whereas administration of exos-sh-HMGB1 alleviated the damage (Fig. 5E). In the CLP model, the serum levels of cfDNA and MPO-DNA were elevated, with exos-HMGB1 further exacerbating these increases. Conversely, exosomes from HMGB1-knockdown macrophages reduced these increases (Fig. 5F). IF staining revealed a notable increase in Cit-H3 expression in the model group compared with the sham-operated group. This increase was further amplified by exos-HMGB1 but was reduced by exos-sh-HMGB1 (Fig. 5G). Moreover, compared with those in the sham-operated group, the protein levels of cGAS, STING, p-TBK1/TBK1, and p-IRF3/IRF3 in the CLP group were elevated. Exosomes from HMGB1-overexpressing macrophages further increased these protein levels, whereas exos-sh-HMGB1 attenuated this effect (Fig. 5H). These data collectively confirm that macrophage-derived exosomal HMGB1 affects the formation of NETs and exacerbates SAKI in mice through the cGAS/STING pathway.
Fig. 5.
HMGB1 mediates NET formation via the cGAS/STING pathway in SAKI model mice. A The mice were injected with exo-HMGB1 or exo-sh-HMGB1 (2 μg/kg body weight) via intravenous injection 4 h after CLP. A near-infrared-labeled exosome plus in vivo dynamic imaging system was used to assess the organ distribution of the exosomes after intravenous injection. B 7-day survival rate of mouse model. C The serum levels of IL- 6 and IL- 8 were determined by ELISAs. D The levels of KIM- 1 and SCr in the sham, CLP, CLP + exo-HMGB1, and CLP + exo-sh-HMGB1 groups were measured using commercially available kits and compared. E H&E staining was used to assess kidney injury. F The serum levels of cfDNA and MPO-DNA were measured using commercially available kits. G Cit-H3 expression was detected by IF staining. H The levels of cGAS, STING, p-IRF3/IRF3, and p-TBK1/TBK1 were measured by WB. N = 6 per group. Multiple-group comparisons were performed with one-way ANOVA with Tukey's post hoc test; two-group comparisons were performed with independent t tests or Mann‒Whitney U tests
Lactate-induced macrophage HMGB1 lactylation promotes NET formation in SAKI mice
Finally, we investigated whether lactate induces HMGB1 lactylation in macrophages. H&E staining revealed inflammatory cell infiltration near the glomeruli in the CLP-treated mice, along with swelling, degeneration, and disorganized arrangement of renal tubular epithelial cells, as well as the presence of renal casts. However, these pathological changes were ameliorated by treatment with RU.521, a cGAS inhibitor, or SIVE, a NET inhibitor (Fig. 6A). Treatment with Nala in CLP model mice led to marked increases in the levels of SCr and KIM- 1, and these increases were mitigated by treatment with RU.521 or SIVE (Fig. 6B). Furthermore, lactate treatment resulted in elevated levels of serum cfDNA and MPO-DNA (Fig. 6C) and promoted NET formation (Fig. 6D) in the CLP mouse model. RU.521 and SIVE, however, reversed these effects. In addition, WB analysis revealed elevated expression levels of cGAS, STING, p-TBK1/TBK1, and p-IRF3/IRF3 following lactate treatment. However, this increase was reduced by treatment with RU.521 or SIVE. Notably, HMGB1 protein levels were not significantly affected by RU.521 or SIVE treatments (Fig. 6E). Taken together, these findings indicate that lactate-induced HMGB1 lactylation in macrophages promotes NET formation in SAKI through the cGAS/STING pathway.
Fig. 6.
Lactate-induced HMGB1 lactylation promotes NET formation in mice with SAKI. In the CLP mouse model, mice subjected to Nala treatment were intraperitoneally administered 1 g/kg NaLa 30 min before CLP. Mice in the CLP + Nala + RU.521 and CLP + Nala + SIVE groups were subcutaneously injected with 450 μg/kg RU.521 or 30 mg/kg SIVE, respectively, after the CLP procedure. A Pathological changes in kidney tissues were examined by H&E staining. B The serum levels of KIM- 1 and SCr in all groups were measured by commercially available kits and compared. C The serum levels of cfDNA and MPO-DNA were measured using commercially available kits. D Cit-H3 expression was detected by IF staining. E The levels of cGAS, STING, p-IRF3/IRF3, and p-TBK1/TBK1 were measured by WB. N = 6 per group. Multiple-group comparisons were performed with one-way ANOVA with Tukey's post hoc test; two-group comparisons were performed with independent t tests or Mann‒Whitney U tests
Discussion
Lactate produced during sepsis has been shown to impair the functional roles of macrophages as well as other immune cells, leading to immune dysfunction—a hallmark of SAKI (Tao et al. 2023). Serum lactate is considered an important indicator of the severity, treatment response, and prognosis of sepsis (Suetrong and Walley 2016). In the present work, we showed that lactate induced NET formation and exacerbated SAKI by inducing HMGB1 lactylation in macrophages. These results underscore the importance of HMGB1 as a potential therapeutic target for SAKI treatment.
NETs, which are released from neutrophils, are crucial for pathogen clearance but can also induce tissue damage during sepsis (Papayannopoulos 2018). Abundant NET formation has been observed in several inflammatory disorders associated with sepsis, such as acute respiratory distress syndrome and acute lung injury (Lefrançais et al. 2018). Our results showed that lactate increased NETs both in SAKI model mice and in vitro. The main components of NETs, such as DNA, histones, and granule proteins, function as DAMPs that trigger inflammation, leading to cell death and organ failure, while also promoting further NET formation (Wang et al. 2024). A rapid and sustained elevation in serum MPO‒DNA complex levels was observed in critically ill patients, indicating early-stage NET formation in sepsis, and these levels were also correlated with the severity of organ dysfunction (Maruchi et al. 2018). Here, we found that both MPO-DNA and cfDNA levels in the serum increased following the CLP procedure. Additionally, macrophage-derived exosomes have been reported to affect neutrophil recruitment and NET formation in sepsis (Jiao et al. 2023). Our data revealed that changes following the CLP procedure were further exacerbated by exosomal HMGB1.
Neutrophils may be the primary recipient cells affected by exosome-targeted cells due to their high abundance, accounting for 50–70% of circulating immune cells, and their greater likelihood of encountering exosomes (Rubenich et al. 2021). In addition to their abundance, exosomes interact with neutrophils through specific molecular mechanisms. Exosomal integrins, such as Mac- 1, bind to CD11b/CD18 on neutrophil surfaces, facilitating uptake and potential intracellular signaling (Pang et al. 2023). However, neutrophils are not the only recipients of exosomes; monocytes, endothelial cells, and hepatocytes may also internalize these extracellular vesicles, contributing to systemic inflammation and immune modulation (Zhou et al. 2022). Our in vivo imaging data demonstrated that intravenously injected exosomes predominantly accumulated in the liver, spleen, lungs, and kidneys. These findings suggest that macrophage-derived exosomes carrying HMGB1 may exert systemic effects beyond the local renal environment, potentially affecting neutrophil activity in multiple organs, as observed in other inflammatory diseases (Ge et al. 2021). Moreover, IL- 6 and IL- 8 levels were markedly increased in the CLP group, and this effect was further exacerbated by exosomal HMGB1, whereas HMGB1 knockdown in exosomes reversed these changes. These findings further support the role of exosomal HMGB1 in amplifying systemic inflammation and promoting NET formation. Given the complex composition of exosomes, other molecules in addition to HMGB1 may also regulate NET formation and warrant further investigation.
HMGB1 is a highly conserved, ubiquitous protein released by activated macrophages to induce innate immune responses and amplify proinflammatory signaling pathways in sepsis (Wang et al. 2014). Circulating HMGB1 levels are significantly elevated and positively correlated with mortality and disease severity in septic patients (Li and Lu 2020). Post-translational modification of HMGB1 often leads to subsequent HMGB1 release during inflammatory responses (Tang et al. 2016). A novel effect of lactate in promoting HMGB1 lactylation and acetylation, as well as triggering its release through exosome secretion from macrophages, has been identified (Yang et al. 2022b). However, reducing lactylation affects the expression of damage repair genes, mediating the transformation of inflammatory macrophages to repair macrophages (Liu et al. 2024). In this work, we demonstrated that elevated lactate levels increased HMGB1 expression, induced its lactylation, and facilitated its secretion via exosomes derived from M1 macrophages, thereby promoting the progression of SAKI.
The cGAS/STING pathway is highly implicated in sepsis and its complications, and blocking this signaling pathway effectively protects mice against sepsis-associated acute liver injury (Li et al. 2022). Recent evidence has shown that NETs promote neuroinflammation and worsen neurological impairment following surgical brain injury by activating cGAS-STING signaling (Li et al. 2024). Similarly, NETs also activate the cGAS/STING pathway in acute lung injury, thereby exacerbating inflammatory damage (Zhao, et al. 2023). The modulatory effect of lactate on the cGAS/STING pathway has been reported in immune disorders. For example, lactate induces mtDNA accumulation in Sjögren's syndrome, resulting in cGAS/STING signaling activation and a subsequent inflammatory response (Xu et al. 2023). Moreover, Wang et al. reported that HMGB1 mediated dsDNA shuttling for cGAS/STING activation in dendritic cells, suggesting the regulatory effect of HMGB1 on this signaling pathway (Wang et al. 2022).
Although our data revealed that lactate-induced HMGB1 lactylation in macrophages promoted NET formation and exacerbated SAKI through the cGAS/STING pathway, several limitations remain. First, the sample sizes of both the SAKI patients and the animal models were relatively small, which may limit the generalizability of these findings. Larger, multicenter studies with diverse patient populations and more extensive animal experiments are necessary to validate our results. Second, while our study effectively demonstrated the role of lactate-induced HMGB1 lactylation in promoting NET formation, the underlying mechanisms warrant further exploration. Future studies should investigate the potential interacting partners and downstream signaling pathways of HMGB1 that contribute to NET formation in SAKI. Additionally, this study focused primarily on a mouse model of SAKI, which may not fully replicate the complexity of human sepsis and SAKI. Future research should incorporate ex vivo tissue cultures or large animal models to better mimic clinical scenarios. Finally, other factors influencing NET formation and HMGB1 function were not investigated, potentially limiting the comprehensive understanding of this pathological process. Investigating these factors and their potential crosstalk with the cGAS/STING pathway will provide a more comprehensive understanding of SAKI pathogenesis.
In conclusion, this study demonstrated that lactate induces HMGB1 lactylation in macrophages, which drives NET formation through the cGAS/STING pathway and contributes to the progression of SAKI. These findings support future investigations of HMGB1 and its potential application as a therapeutic target for this disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Suppl. Fig. 1 Lactate promotes HMGB1 lactylation. The colocalization of Klac and HMGB1 in the cytoplasm of (A) RAW 264.7 macrophages. (B) To suppress lactate production, macrophages were preincubated with 20 mM oxamate for 2 hours prior to LPS stimulation. The colocalization of Klac and HMGB1 was assessed by co-IP. (C) IF staining was used to confirm the colocalization of Klac and HMGB1 in the cytoplasm of RAW 264.7 macrophages. Multiple-group comparisons were performed with one-way ANOVA with Tukey's post hoc test; two-group comparisons were performed with independent t tests or Mann‒Whitney U tests
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Authors'contributions
Conceptualization: Siwei Wei, Zhen Du Data Curation: Zijuan Dai, Lei Wu Formal analysis: Zijuan Dai, Lei Wu Investigation: Siwei Wei, Zhen Xiang Methodology: Zijuan Dai, Lei Wu Project administration: Zhen Du Resources: Zhen Xiang, Liubing Jiang Software: Zhen Xiang, Xiaoxiao Yang Supervision: Zhen Du, Siwei Wei Validation: Siwei Wei, Zhen Xiang, Xiaoxiao Yang, Liubing Jiang Visualization: Xiaoxiao Yang, Liubing Jiang Writing—Original Draft: Siwei Wei Writing—Review & Editing: Siwei Wei All authors read and approved the final manuscript.
Funding
This study was supported by Hunan Province Natural Science Foundation of China (Grant No. 2023 JJ40348).
Data availability
All data generated or analysed during this study are included in this article.
Declarations
Ethics approval
This study was approved by the Ethics Committee of The Affiliated Children's Hospital Of Xiangya School of Medicine, Central South University (Hunan Children's Hospital).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andersson U, Yang H. HMGB1 is a critical molecule in the pathogenesis of Gram-negative sepsis. J Intensive Med. 2022;2(3):156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assouvie A, Daley-Bauer LP, Rousselet G. Growing murine bone marrow-derived macrophages. Methods Mol Biol. 2018;1784:29–33. [DOI] [PubMed] [Google Scholar]
- Cao M, Wang G, Xie J. Immune dysregulation in sepsis: experiences, lessons and perspectives. Cell Death Discov. 2023;9(1):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo C, Jaimes F. Organ dysfunction in sepsis: An ominous trajectory from infection to death. Yale J Biol Med. 2019;92(4):629–40. [PMC free article] [PubMed] [Google Scholar]
- Cicco S, et al. Neutrophil extracellular traps (NETs) and damage-associated molecular patterns (DAMPs): Two potential targets for COVID-19 treatment. Mediators Inflamm. 2020;2020:7527953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demkow U. Molecular mechanisms of neutrophil extracellular trap (NETs) degradation. Int J Mol Sci. 2023;24(5):4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning NL, et al. DAMPs and NETs in sepsis. Front Immunol. 2019;10:2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, et al. Activating cGAS-STING axis contributes to neuroinflammation in CVST mouse model and induces inflammasome activation and microglia pyroptosis. J Neuroinflammation. 2022;19(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, et al. Exosomal HMGB1 derived from hypoxia-conditioned bone marrow mesenchymal stem cells increases angiogenesis via the JNK/HIF-1α pathway. FEBS Open Bio. 2021;11(5):1364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Huang M, Yao YM. The effect and regulatory mechanism of high mobility group box-1 protein on immune cells in inflammatory diseases. Cells. 2021;10(5):1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge CL, et al. Hippocampus-prefrontal cortex inputs modulate spatial learning and memory in a mouse model of sepsis induced by cecal ligation puncture. CNS Neurosci Ther. 2023;29(1):390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez H, et al. Feasibility assessment of a biomarker-guided kidney-sparing sepsis bundle: The limiting acute kidney injury progression in sepsis trial. Crit Care Explor. 2023;5(8):e0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19(2):177–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, et al. Lactylation: the novel histone modification influence on gene expression, protein function, and disease. Clin Epigenetics. 2024;16(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarczak D, Kluge S, Nierhaus A. Sepsis-pathophysiology and therapeutic concepts. Front Med (Lausanne). 2021;8:628302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, et al. Exosomal PGE2 from M2 macrophages inhibits neutrophil recruitment and NET formation through lipid mediator class switching in sepsis. J Biomed Sci. 2023;30(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K, et al. The neutrophil elastase inhibitor sivelestat suppresses accelerated gastrointestinal tumor growth via peritonitis after cecal ligation and puncture. Anticancer Res. 2013;33(9):3653–9. [PubMed] [Google Scholar]
- La Via L, et al. The global burden of sepsis and septic shock. Epidemiologia (Basel). 2024;5(3):456–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, An WS. New clinical criteria for septic shock: serum lactate level as new emerging vital sign. J Thorac Dis. 2016;8(7):1388–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, et al. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactatemia: A retrospective cohort study according to the Sepsis-3 definitions. Medicine (Baltimore). 2021;100(7):e24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançais E, et al. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3(3):e98178. [DOI] [PMC free article] [PubMed]
- Li L, Lu YQ. The regulatory role of high-mobility group protein 1 in sepsis-related immunity. Front Immunol. 2020;11:601815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lu Y, Lin G. Blocking cGAS/STING signaling protects against sepsis-associated acute liver injury. Int Immunopharmacol. 2022;113(Pt A):109276. [DOI] [PubMed] [Google Scholar]
- Li B, et al. Neutrophil extracellular traps regulate surgical brain injury by activating the cGAS-STING pathway. Cell Mol Neurobiol. 2024;44(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, et al. Lactate and lactylation in sepsis: A comprehensive review. J Inflamm Res. 2024;17:4405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique-Caballero CL, Del Rio-Pertuz G, Gomez H. Sepsis-associated acute kidney injury. Crit Care Clin. 2021;37(2):279–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruchi Y, et al. Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit Care. 2018;22(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Tiwari S, Gomes AV. Protein purification and analysis: next generation Western blotting techniques. Expert Rev Proteomics. 2017;14(11):1037–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J, Hu J, Cheng X. The role of high mobility group box 1 in neuroinflammatory related diseases. Biomed Pharmacother. 2023;161:114541. [DOI] [PubMed] [Google Scholar]
- Molagoda IMN, et al. Fisetin inhibits lipopolysaccharide-induced inflammatory response by activating β-catenin, leading to a decrease in endotoxic shock. Sci Rep. 2021;11(1):8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. 2023;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–47. [DOI] [PubMed] [Google Scholar]
- Poston JT, Koyner JL. Sepsis associated acute kidney injury. Bmj. 2019;364:k4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenich DS, et al. Small extracellular vesicle-mediated bidirectional crosstalk between neutrophils and tumor cells. Cytokine Growth Factor Rev. 2021;61:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XF, et al. Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med. 2017;21(9):1687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkova TY, et al. Structural characteristics of high-mobility group proteins HMGB1 and HMGB2 and their interaction with DNA. Int J Mol Sci. 2023;24(4):3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetrong B, Walley KR. Lactic acidosis in sepsis: It’s not all anaerobic: Implications for diagnosis and management. Chest. 2016;149(1):252–61. [DOI] [PubMed] [Google Scholar]
- Tang Y, et al. Regulation of posttranslational modifications of HMGB1 during immune responses. Antioxid Redox Signal. 2016;24(12):620–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, et al. Unveiling the veil of lactate in tumor-associated macrophages: a successful strategy for immunometabolic therapy. Front Immunol. 2023;14:1208870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan D, Jiang W, Hao J. Research advances in how the cGAS-STING pathway controls the cellular inflammatory response. Front Immunol. 2020;11:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis. Expert Opin Ther Targets. 2014;18(3):257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, et al. Anti-inflammatory activity of 3-cinnamoyltribuloside and its metabolomic analysis in LPS-activated RAW 264.7 cells. BMC Complement Med Ther. 2020;20(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. A protein-based cGAS-STING nanoagonist enhances T cell-mediated anti-tumor immune responses. Nat Commun. 2022;13(1):5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Composition and function of neutrophil extracellular traps. Biomolecules. 2024;14(4):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga WJ, van der Poll T. Immunopathophysiology of human sepsis. Ebiomedicine. 2022;86:104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, et al. Lactate-induced mtDNA Accumulation Activates cGAS-STING signaling and the inflammatory response in Sjögren’s syndrome. Int J Med Sci. 2023;20(10):1256–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Huang Y, Zeng Z. Advances in cGAS-STING signaling pathway and diseases. Front Cell Dev Biol. 2022a;10:800393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022b;29(1):133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, et al. Receptor for advanced glycation end products (RAGE): A pivotal hub in immune diseases. Molecules. 2022;27(15):4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, et al. Sepsis-associated acute kidney injury: consensus report of the 28th acute disease quality Initiative workgroup. Nat Rev Nephrol. 2023;19(6):401–17. [DOI] [PubMed] [Google Scholar]
- Zhang YG, et al. Exosomes derived from oxLDL-stimulated macrophages induce neutrophil extracellular traps to drive atherosclerosis. Cell Cycle. 2019;18(20):2674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, et al. HMGB1-promoted neutrophil extracellular traps contribute to cardiac diastolic dysfunction in mice. J Am Heart Assoc. 2022;11(4):e023800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, et al. Lactate’s impact on immune cells in sepsis: unraveling the complex interplay. Front Immunol. 2024;15:1483400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, et al. NETs promote inflammatory injury by activating cGAS-STING pathway in acute lung injury. Int J Mol Sci. 2023;24(6):5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, et al. Pathogenic and potential therapeutic roles of exosomes derived from immune cells in liver diseases. Front Immunol. 2022;13:810300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, et al. Neutrophil extracellular traps promote lipopolysaccharide-induced airway inflammation and mucus hypersecretion in mice. Oncotarget. 2018;9(17):13276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1 Lactate promotes HMGB1 lactylation. The colocalization of Klac and HMGB1 in the cytoplasm of (A) RAW 264.7 macrophages. (B) To suppress lactate production, macrophages were preincubated with 20 mM oxamate for 2 hours prior to LPS stimulation. The colocalization of Klac and HMGB1 was assessed by co-IP. (C) IF staining was used to confirm the colocalization of Klac and HMGB1 in the cytoplasm of RAW 264.7 macrophages. Multiple-group comparisons were performed with one-way ANOVA with Tukey's post hoc test; two-group comparisons were performed with independent t tests or Mann‒Whitney U tests
Data Availability Statement
All data generated or analysed during this study are included in this article.