Abstract
Abstract
Red clover isoflavones, particularly biochanin A and formononetin, are known for their benefits in enhancing feed efficiency and nitrogen utilization in ruminants. However, their specific effects on rumen fermentation and microbial diversity remain insufficiently explored. This study investigated the impacts of red clover isoflavones on rumen function and bacterial diversity in dairy cows, utilizing both in vivo and in vitro methodologies. In the in vivo study, 40 Holstein dairy cows were allocated to four groups, each receiving red clover isoflavones at doses of 0, 0.4, 0.8, and 1.6 g/kg. Rumen fluid was collected for analysis of fermentation parameters, enzyme activity, and microbial composition through shotgun metagenomic sequencing. Concurrently, an in vitro rumen fermentation trial was conducted to evaluate the effects of biochanin A and formononetin on urea hydrolysis. Results from the in vivo experiments showed that red clover isoflavones significantly decreased ammonia nitrogen (NH₃-N) concentrations and urease activity in the rumen (P < 0.05). Species level metagenomic analysis indicated a reduced abundance of proteolytic and ureolytic bacteria, such as Prevotella sp002317355 and Treponema_D bryantii_C, with a corresponding increase in cellulolytic bacteria, including Ruminococcus_D sp900319075 and Ruminococcus_C sp000433635 (P < 0.05). The in vitro trial further demonstrated that biochanin A and formononetin significantly reduced urea decomposition rates (P < 0.05), with biochanin A exerting a more pronounced effect. These findings align with the observed reduction in ureolytic and proteolytic bacteria, along with an increase in cellulolytic bacteria across both trials. In conclusion, biochanin A emerged as the primary active component of red clover isoflavones, modulating urea nitrogen hydrolysis and rumen fermentation. This study substantiates previous findings and highlights the potential of red clover isoflavones for enhancing rumen microbial fermentation, offering a promising strategy for future dairy industry applications.
Key points
• Red clover isoflavones inhibit urease activity to decrease the abundance of urealytic bacteria.
• Biochanin A reduces ammonia nitrogen and urease activity, promoting protein efficiency.
• Red clover isoflavones may improve dairy cow rumen health and nitrogen utilization.
Keywords: Red clover isoflavones, Rumen microbiome, Fermentation, Dairy cows, Microbial composition
Introduction
Red clover (Trifolium pratense), a leguminous plant, is notably rich in isoflavones, a class of phytoestrogens structurally and functionally similar to mammalian estrogens such as 17β-estradiol (Woclawek-Potocka et al. 2013; Li et al. 2022). Compared to soybeans, which contain 1.2 to 4.2 mg of isoflavones per gram of dry matter, red clover has a significantly higher isoflavone concentration, ranging from 10 to 25 mg/g (Kurzer and Xu 1997). The primary isoflavones in red clover are biochanin A and formononetin, with formononetin present at levels between 0.8 and 11 mg/g dry matter, while biochanin A is found at 10.30 mg/g in leaves and 1.62 mg/g in buds (Saloniemi et al. 1995). Isoflavone content can vary based on factors such as geographic location, growth stage, cultivar, and storage conditions (Kim et al. 2020). As secondary metabolites, these isoflavones confer numerous biological benefits, including bone development support (Kenny et al. 2009), osteoporosis prevention, immune system enhancement (Jeminiwa et al. 2020), and improved stress resilience in animals (Ma et al. 2021).
Rumen microorganisms are crucial for digestion, particularly in the breakdown of proteins, cellulose, and hemicellulose (Mizrahi et al. 2021). Through urease activity, these microorganisms convert proteins to amino acids and hydrolyze urea to ammonia (Wang et al. 2017). Key proteolytic bacteria in the rumen include Ruminobacter amylophilus, Butyrivibro fibrisolvens, Clostridium sp., Selenomonas ruminantium, and Prevotella (Shen et al. 2018). As a non-protein nitrogen source, urea is metabolized by rumen microbial urease, releasing ammonia. Studies have identified Succinivibrio sp., Treponema sp., and S. ruminantium as ureolytic bacteria capable of this activity (Liu et al. 2020, 2023; Jin et al. 2016). Supplementation with red clover isoflavones, particularly biochanin A, has been shown to reduce urease activity by 50% and lower amino acid degradation rates in vitro (Liu et al. 2020). Additionally, red clover isoflavones decrease the abundance of hyper-ammonia-producing bacteria (HAB), resulting in reduced ammonia production. Biochanin A’s effect in lowering NH₃-N concentration is linked to its reduction of proteolytic bacteria populations, including Prevotella and HAB (Flythe and Kagan 2010).
For optimal digestion, cattle require diets high in structural carbohydrates like cellulose and hemicellulose, which cellulolytic bacteria utilize to produce microbial protein, drawing on substrates such as -ketoglutaric acid and ATP derived from carbohydrates (Palombo et al. 2021; Russel et al. 2009). Biochanin A, a potent component of red clover isoflavones, has been reported to stimulate fiber breakdown by enhancing cellulolytic bacterial populations (Harlow et al. 2018).
Extensive research supports the use of red clover isoflavones as a feed additive to improve ruminant productivity and rumen health. For instance, Harlow et al. (2018) observed a significant 29% increase in average daily gain in steers when dried distiller grains were supplemented with purified biochanin A, compared to distiller grains alone. Other studies have shown that low-level (15%) red clover hay supplementation is more effective for average daily gain in growing steers than high-level (30%) supplementation (Harlow et al. 2020). At the same time, red clover can modify the rumen microbiota by inhibiting ammonia-producing bacteria in the rumen (Harlow et al. 2020). Xu et al. (2023) reported significant increases in the abundance of cellulolytic bacteria, including Prevotella sp., Treponema sp., in goats with biochanin A supplementation. A study found that red clover significantly reduced high ammonia-producing bacteria and increased lactate-utilizing and fiber-degrading bacteria in the rumen (Weinert-Nelson et al. 2023). Harlow et al. (2021) found biochanin A plays an important role in modulating the composition of the rumen microbiota, particularly suppressing lactic acid-producing bacteria, enhancing the growth of fiber-degrading microbes. Thus, red clover isoflavones hold promise for enhancing ruminant productivity and health.
While the beneficial effects of red clover isoflavones on rumen health are well-documented, the specific influence on the rumen microbiome particularly formononetin remains less understood. This study aims to address this knowledge gap by evaluating the impact of formononetin, with or without biochanin A, on rumen fermentation and microbial composition through an in vitro trial. By investigating these compounds in both in vivo and in vitro settings, we sought to determine whether red clover isoflavones could serve as effective urease inhibitors, thereby optimizing nitrogen utilization, enhancing rumen fermentation, and ultimately improving ruminant performance.
Materials and methods
Animal experimental design and diets
All experimental procedures were approved by the Animal Care and Use Committee of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (Approval No. IAS2022 - 107).
Using a randomized complete block design, 40 healthy Holstein dairy cows (average daily milk yield: 33.93 ± 3.81 kg, parity: 2.3 ± 1.21, lactation days: 165 ± 21) were selected and divided into four blocks (n = 8, 12, 16, 4) solely based on milk yield to maintain the similar physiological state of lactation within each group, and to avoid unbalanced block sizes that could compromise statistical analysis. Sample size and power analyses determined that a minimum of 10 replicates per treatment was required (St-Pierre 2007). Within each block, cows were randomly assigned to one of four treatment groups, receiving red clover isoflavones at 0, 0.4, 0.8, or 1.6 g/kg, respectively, based on dietary dry matter. Red clover isoflavones (Feituowei, Hunan, China) were provided as red clover extract containing 22.91% isoflavones (6.47% biochanin A and 16.44% formononetin), 51.54% carbohydrates, 9.09% of protein.
The experiment lasted 84 days, with samples collected on the final day. Cows were fed their respective diets three times daily (at 0800, 1500, and 1700 h) to allow for ad libitum intake, with isoflavones sourced from red clover extract. Diets were offered with a 10% surplus to facilitate unrestricted intake. The composition and chemical properties of the total mixed ration (TMR) are outlined in Table 1, aligning with the National Research Council (NRC 2001) dairy cow nutrient requirements. Cows were milked thrice daily (at 0730, 1400, and 2130 h) in a milking parlor, with unrestricted access to water.
Table 1.
Ingredients and chemical composition of total mixed diet fed to dairy cows
| Items | % of DM1 |
|---|---|
| Ingredients | |
| Corn silage | 30.01 |
| Alfalfa hay | 13.50 |
| Cottonseed meal | 5.68 |
| Corn bran | 7.32 |
| Soybean meal | 10.87 |
| Wheat shorts | 3.72 |
| Urea | 0.54 |
| Steam-flaked corn | 19.35 |
| Extruded soybean | 3.01 |
| Fatty powder | 0.82 |
| Potassium bicarbonate | 0.32 |
| Sodium bicarbonate | 0.33 |
| Rumen-protected lysine | 0.17 |
| Rumen-protected methionine | 0.08 |
| Molasses | 0.99 |
| Premix2 | 3.25 |
| Chemical composition | |
| NEL3 (Mcal/kg) | 1.68 |
| DM | 54.03 |
| ADF4 | 16.31 |
| NDF5 | 26.75 |
| CP6 | 17.58 |
| Starch | 27.73 |
1 DM, dry matter
2 The premix supplied the following amount per kilogram of diets: 650,000 international units (IU) of vitamin A, 350,000 IU of vitamin D, 4,000 IU of vitamin E, 2,500 mg of n, 200 mg of Cu, 1,200 mg of Mn. The supplemented with different red clover isoflavones are 0.4 g, 0.8 g and 1.6 g, respectively
3 NEL, or lactation net energy, is determined based on the nutrient requirements of dairy cattle as outlined in the Nutrient Requirements of Cows (NRC) (2001)
4 ADF, acid detergent fiber
5 NDF, neutral detergent fiber
6 CP, crude protein
Rumen fluid sample collection and analysis
Rumen fluid samples were collected using a gastric tube collector before morning feeding at the end of the trial to minimize stress on the cows and ensure microbial stabilization (Larsen et al. 2020; Bach et al. 2021). Approximately 100 mL of rumen fluid from each cow was filtered through eight layers of cheesecloth, and pH was measured immediately (Leici, Shanghai, China). To measure volatile fatty acids (VFAs), urea nitrogen (Urea-N), and ammonia nitrogen (NH₃-N) content, 1 mL of 25% (m/v) metaphosphate was added to 5 mL of rumen fluid. A separate 10 mL of rumen fluid was collected for microbial enzyme analysis and DNA extraction, with all samples stored at − 80 °C.
Volatile fatty acids (VFAs) were analyzed using gas chromatography (Agilent 7890 A, Santa Clara, CA, USA), NH₃-N concentration was determined by a phenol-hypochlorite assay, and Urea-N was measured using a kit (Jiancheng, Nanjing, China). Microbial cells were sonicated on ice (300 W for 5 min, with 15-s intervals) and centrifuged at 12,000 g for 10 min at 4 °C. Urease activity was assessed via ammonia production using a phenol/hypochlorite assay, with absorbance measured at 625 nm (Weatherburn 1967). Protein concentration was measured with the Bradford Protein Assay Kit (Takara, Dalian, China), and cellulase activity was quantified by the dinitrosalicylic (DNS) colorimetric method (Li and Mckee 2023).
Shotgun sequencing of microbial DNA
Total DNA was extracted from rumen fluid using the hexadecyltrimethylammonium bromide method (Minas et al. 2011). DNA quality and quantity were verified with a Nanodrop One spectrophotometer (Thermo Scientific, Waltham, MA, USA). Sequencing libraries were prepared using the MGIEasy kit (BGI, Shenzhen, China), and sequencing was conducted on the BGISEQ- 500 platform with 2 × 100 bp read lengths. Quality control was performed with TrimGalore (Mehdipour et al. 2020), filtering out reads shorter than 50 bp and those with Phred scores below 20. Host contamination (cows, corn, soybean, and alfalfa) was removed using BM Tagger (Uritskiy et al. 2018). Taxonomic classification utilized Kraken 2 (Kopylova et al. 2012) with the GTDB-r89 database (Waschulin et al. 2022), and the relative abundance and diversity of rumen microbiota were analyzed on MicrobiomeAnalyst (Chong et al. 2020). Taxa with a minimum count below four and prevalence under 20% were excluded. Linear discriminant analysis effect size (LEfSe) identified significant species-level differences, and abundance of functional bacteria was normalized by logarithmic transformation, with significance set at P < 0.05.
In vitro rumen fermentation
Before morning feeding, rumen fluid was manually collected from three lactating cannulated Holstein cows (average weight: 550 ± 23 kg) fed a total mixed ration (TMR) diet composed of 68.09% corn silage, 7.66% alfalfa hay, 4.24% corn meal, 17.87% soybean meal, and 2.13% premix (17.60% acid detergent fiber (ADF), 34.3% neutral detergent fiber (NDF), 14.90% crude protein, 2.58% ether extract). Filtered rumen fluid was transported to the lab in pre-warmed thermoses. Four treatment groups were created: 8.33 mg/100 mL biochanin A (TCI, Beijing, China), 25 mg/100 mL formononetin (Macklin, Shanghai, China), 33.33 mg/100 mL mixed isoflavones (8.33 mg biochanin A and 25 mg formononetin), and a control group, with dosages based on dietary dry matter (Dadáková et al. 2020).
Nylon bags containing 1.8 g of ration were placed in 500 mL bottles with 100 mL McDougall buffer (Mcdougall 1948). Each bottle received 50 mL rumen fluid, flushed with CO₂ to maintain anaerobic conditions. After 15 min, urea solution (0.5 g/L) was added, initiating incubation to assess urease activity. Bottles were incubated in a 39 °C shaker water bath, with samples collected at intervals (0, 1, 2, 4, 8, 12, and 24 h). For each timepoint, 1 mL rumen fluid was treated with 500 μL 25% metaphosphoric acid, centrifuged (12,000 g, 10 min), and supernatant stored at − 20 °C for VFA, NH₃-N, and Urea-N analysis. Microbial protein concentration (MCP) was measured at 12 h using the BCA kit (Welab, Beijing, China).
Bacterial composition by amplicon sequencing
DNA extraction followed the same protocol as published (Minas et al. 2011), and quality was assessed using a Nanodrop One spectrophotometer (Thermo Scientific, Waltham, MA, USA). The V3 + V4 regions of the 16S rRNA gene were amplified with primers (341 F: CCTACGGGNGGCWGCAG; 806R: GGACTACHVGGGTATCTAAT). Sequencing was conducted by Gene Denovo Biotechnology Co., Ltd. (Guangzhou, China) on the Illumina HiseqTM 2500 platform (Illumina, San Diego, CA, USA). FASTP software (Chen et al. 2018) ensured data quality by removing low-quality reads (< 50 bp, base mass < 20). Taxonomic analysis used Usearch software (Edgar 2010), and beta diversity was calculated with Bray–Curtis indices (Bray and Curtis 1957). Principal component analysis (PCA) visualized operational taxonomic unit (OTU) based diversity (Wagner 2010), and Tax4 Fun (version 2.1.4) provided KEGG pathway predictions (Langille et al. 2013).
Statistical analysis
Rumen fermentation parameters (VFAs, Urea-N, NH₃-N, pH) and enzyme activity data from in vivo trials were analyzed using the MIXED model in SAS software(version 9.4, SAS Institute Inc., Cary, NC, USA) (SAS Institute Inc., 2021a): Yijk = μ + Ti + Bj + Cowk + eijk where Yijk is the dependent variable; μ is the overall mean; Ti is the fixed effect of treatment; Bj is the random effect of block; and eijk is the error term.
In vitro parameters were analyzed using the general linear model (GLM) model in SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA) (SAS Institute Inc., 2021b): Yi = μ + Ti + ei where Yi is the dependent variable; μ is the mean; Ti is the treatment effect; and ei is the error.
For in vitro Urea-N and NH₃-N, the MIXED model was: Yij = μ + Ti + Dj + TDij + eij where Ti is treatment effect; Dj is time effect; TDij is the treatment-time interaction. Tukey–Kramer was used for pairwise comparisons, with orthogonal polynomial contrasts assessing linear and quadratic effects (P < 0.05). Rumen microbiota comparisons were conducted with the Kruskal–Wallis test, with significance at P < 0.05.
Results
Rumen fermentation of dairy cows
Table 2 presents the rumen fermentation parameters and enzyme activities. Supplementation with red clover isoflavones did not significantly affect rumen pH (P > 0.05). However, at a dose of 0.4 g/kg, red clover isoflavones significantly decreased the acetate concentration (P < 0.05). The butyrate concentration with a linear increase was observed as the dose increased (P < 0.05). The NH₃-N concentration in the rumen was significantly reduced with red clover isoflavone supplementation (P < 0.05), exhibiting both linear and quadratic decreases as dosages increased (P < 0.05). Cellulase activity significantly increased at a dose of 0.8 g/kg compared with the control (P < 0.05), whereas urease activity was significantly reduced at all dose levels (P < 0.05), showing both linear and quadratic declines with higher doses (P < 0.05).
Table 2.
Effect of red clover isoflavones on rumen fermentation and enzyme activity in dairy cows
| Items | Red clover isoflavones (g/kg) | SEM1 | P value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.4 | 0.8 | 1.6 | Treatment | Linear | Quadratic | ||
| pH | 7.04 | 7.22 | 7.15 | 7.04 | 0.048 | 0.440 | 0.693 | 0.185 |
| Total VFA (mmol/L) | 68.70 | 54.10 | 58.90 | 72.30 | 2.300 | 0.085 | 0.210 | 0.005 |
| VFA2(mol %) | ||||||||
| Acetate | 65.80a | 62.50b | 64.30a | 63.90a | 1.200 | 0.032 | 0.210 | 0.105 |
| Propionate | 19.40a | 20.70a | 18.20b | 17.80b | 0.800 | 0.018 | 0.005 | 0.450 |
| Butyrate | 10.50b | 10.90b | 12.50a | 14.20a | 0.600 | <.001 | <.001 | 0.080 |
| Isobutyrate | 1.15 | 1.12 | 0.98 | 0.95 | 0.030 | 0.250 | 0.120 | 0.700 |
| Valerate | 1.41 | 1.49 | 1.43 | 1.62 | 0.070 | 0.041 | 0.300 | 0.850 |
| Isovalerate | 1.73 | 1.84 | 1.61 | 1.45 | 0.090 | 0.180 | 0.090 | 0.600 |
| NH3-N, mg/dL | 10.97a | 6.87b | 6.48bc | 5.93bc | 0.489 | 0.001 | 0.001 | 0.007 |
| Enzyme activity | ||||||||
| Urease, nmol/min/mg | 11.18a | 8.56b | 6.35bc | 5.52c | 1.552 | <.001 | <.001 | 0.033 |
| Cellulase, mol/min/mg | 3.89b | 1.77c | 6.67a | 5.14ab | 0.571 | <.001 | 0.001 | 0.526 |
1 SEM, Standard Error of the Mean
2 VFA proportions (mol%) were calculated as individual VFA molar concentration divided by total VFA molar concentration × 100
a-c.Different lowercase letters within a row indicate statistically significant differences (P < 0.05)
Rumen bacterial composition
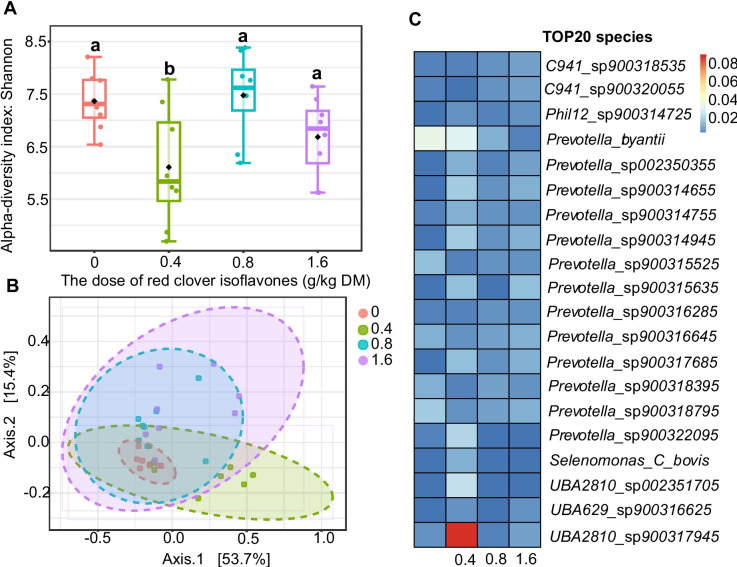
Shotgun sequencing of microbial DNA from the rumen yielded a total of 1.36 billion reads, averaging 42.59 million reads per sample. Good’s coverage values (> 0.99) confirmed sufficient sequencing depth to accurately represent the microbial community. Changes in alpha diversity, assessed using the Shannon index, showed decreased values in the 0.4 g/kg treatment group compared with the 0 g/kg group (Fig. 1A). Beta diversity, calculated via the Bray–Curtis dissimilarity index and visualized by principal coordinates analysis (PCoA), demonstrated significant differences in microbial structure at doses of 0.8 g/kg and 1.6 g/kg compared with 0 g/kg group (P < 0.05, Fig. 1B).
Fig. 1.
Effect of isoflavones on the ruminal bacterial diversity in vivo. Numbers (0, 0.4, 0.8, 1.6) indicate the dose of red clover isoflavones (g/kg DM). Alpha diversity of rumen microflora across the four treatment groups (A). Beta diversity as assessed by principal coordinates analysis (PCA) (B). The relative abundance of the top 20 bacterial species in the rumen at the species level (C). DM = dry matter
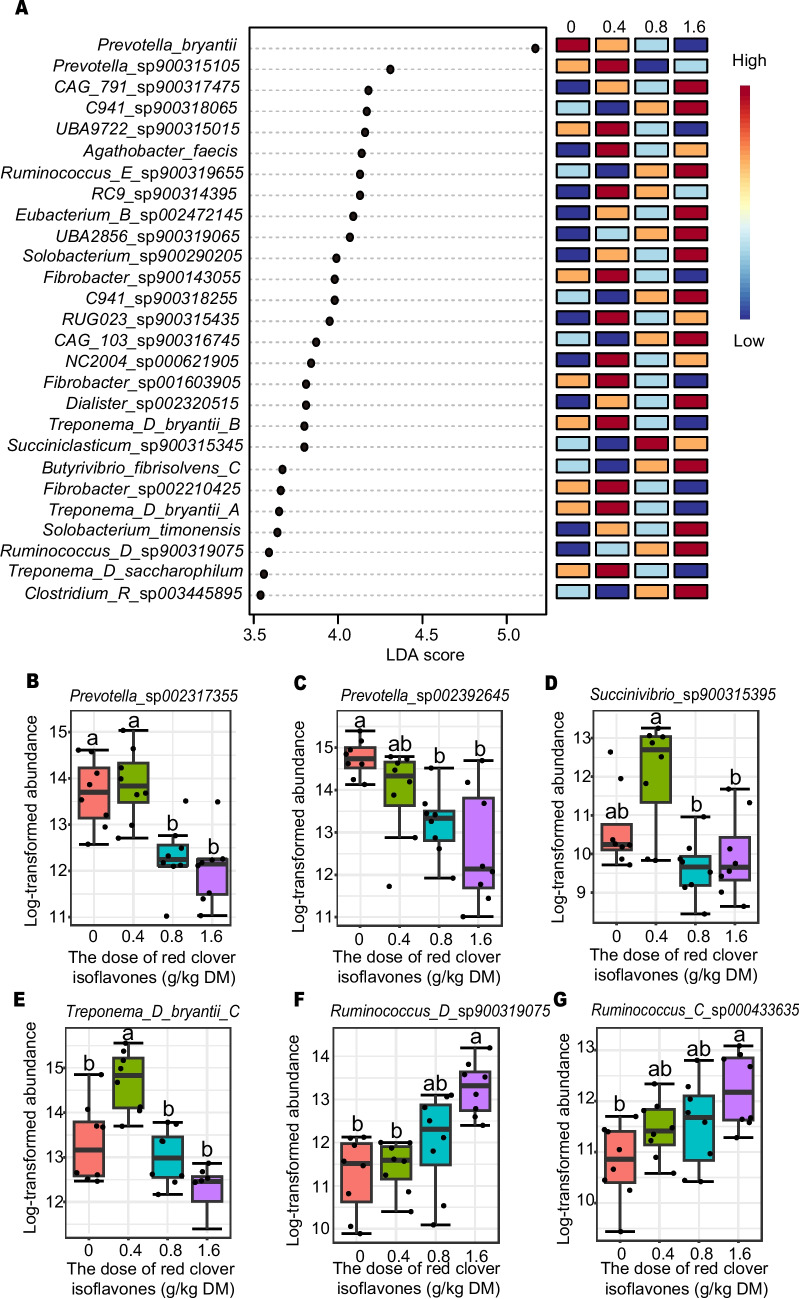
The 20 most abundant bacterial species are shown in Fig. 1C, with Prevotella species as the dominant taxa. LEfSe analysis identified significant changes in bacterial composition across groups (Fig. 2A), specifically among proteolytic, ureolytic, and cellulolytic bacteria. Red clover isoflavones at 0.8 g/kg and 1.6 g/kg significantly decreased the abundance of proteolytic bacteria Prevotella sp002317355 and Prevotella sp002392645 (P < 0.05, Fig. 2B, 2 C) and increased cellulolytic bacteria such as Ruminococcus_D sp900319075 and Ruminococcus_C sp000433635 at 1.6 g/kg (P < 0.05, Fig. 2F, 2G).
Fig. 2.
Effect of isoflavones on the ruminal bacterial abundance in vivo. LEfSe analysis of the four treatment groups (A). Relative abundance of the representive bacterial species (B-G). DM = dry matter
In vitro rumen fermentation
The effects of red clover isoflavones, specifically biochanin A and formononetin, on rumen fermentation in vitro are shown in Fig. 3 and Table 3. Biochanin A significantly reduced urea decomposition compared to formononetin (P < 0.05, Fig. 3A) and increased urea concentration more effectively than formononetin (P < 0.05). After 4 h of incubation, NH₃-N concentration decreased significantly in both the biochanin A and formononetin groups compared to the control, with a stronger effect observed for biochanin A (P < 0.05, Fig. 3B). Microbial protein concentration (MCP) was also significantly higher in the biochanin A group at 12 h (P < 0.05, Table 3). Compared with the control, biochanin A significantly increased acetate, butyrate, and the acetate-to-propionate ratio (P < 0.05).
Fig. 3.
Effects of biochanin A and formononetin on in vitro rumen fermentation. Urea-N concentration (A), NH₃-N concentration (B). Different letters indicate significant differences (P < 0.05), and error bars represent the standard error of the mean (SEM)
Table 3.
Effect of red clover isoflavones on volatile fatty acids and microbial protein in vitro
| Items | Treatment | SEM1 | P | |||
|---|---|---|---|---|---|---|
| Control | Biochanin A | Formononetin | Biochanin A + Formononetin | Treatment | ||
| Total VFA (mmol/L) | 68.20 | 72.50 | 65.80 | 70.10 | 2.100 | 0.085 |
| VFA2(mol %) | ||||||
| Acetate | 62.30b | 65.10a | 60.80c | 63.50ab | 0.900 | 0.004 |
| Propionate | 20.50a | 18.20b | 21.00a | 19.30ab | 0.700 | 0.012 |
| Butyrate | 12.10b | 14.60a | 11.80b | 13.20ab | 0.500 | <.001 |
| Isobutyrate | 1.20 | 1.10 | 1.30 | 1.20 | 0.100 | 0.450 |
| Valerate | 1.50 | 1.60 | 1.40 | 1.50 | 0.100 | 0.620 |
| Isovalerate | 1.40 | 1.40 | 1.70 | 1.30 | 0.100 | 0.210 |
| A:P3 | 3.04b | 3.58a | 2.90b | 3.29ab | 0.120 | 0.003 |
| Microbial protein (mg/mL) | 0.63b | 1.07a | 0.69b | 0.78b | 0.063 | 0.001 |
1.SEM, Standard Error of the Mean
2 VFA proportions (mol%) were calculated as individual VFA molar concentration divided by total VFA molar concentration × 100
3 A:P means acetic acid divided by propionic acid
a-c.Different lowercase letters within a row indicate statistically significant differences (P < 0.05)
Changes in bacterial composition during in vitro fermentation
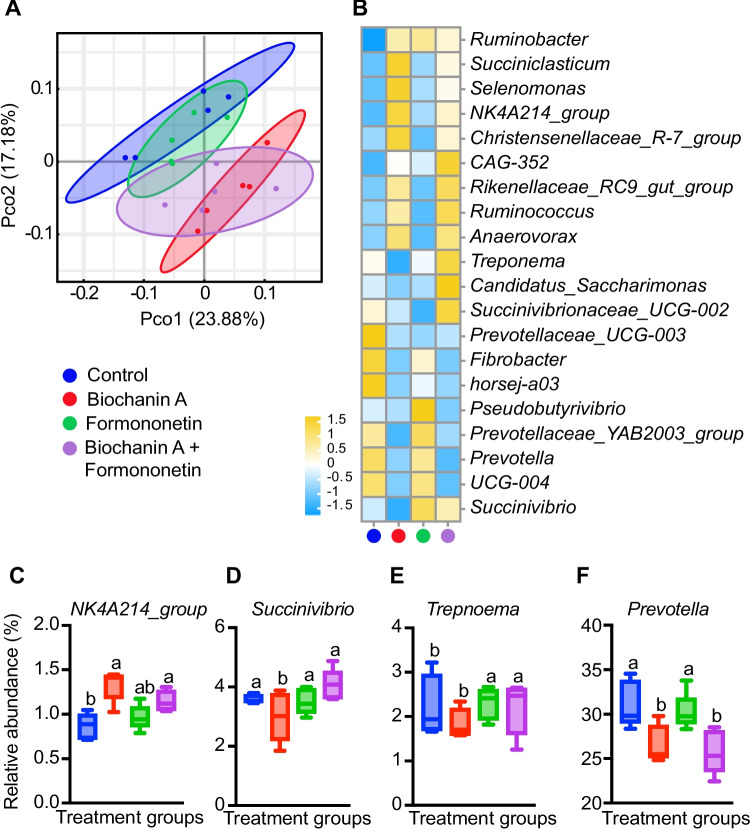
In vitro fermentation analysis categorized all 16S rRNA gene sequences into 41,021 OTUs with a 97% similarity cutoff. PCoA analysis showed significant changes in bacterial community structure (P < 0.05, Fig. 4A), with biochanin A producing a more pronounced effect than the biochanin A plus formononetin group. Bacterial taxa with relative abundances ≥ 0.1% are shown in Fig. 4B. At the genus level, Ruminobacter, Rikenellaceae_RC9_gut_group, and Prevotella were the most prevalent across treatments. Biochanin A significantly altered the abundance of Treponema, Succinivibrio, NK4 A214_group, and Prevotella (P < 0.05, Fig. 4B).
Fig. 4.
Effects of biochanin A and formononetin on in vitro rumen bacterial diversity and composition. Beta diversity based on principal coordinates analysis (PCoA) (A). The cluster analysis of abundance at genus-level (B). The changed bacteria at genus level (C-F). Different letters indicate significant differences (P < 0.05), with error bars representing SEM, (B) to (F) Treatment groups: color code as presented in subfigure A
The addition of biochanin A had the greatest impact on ureolytic bacteria Treponema and Succinivibrio, cellulolytic bacteria NK4 A214_group, and proteolytic bacteria Prevotella (P < 0.05, Fig. 4C-F). The abundance of ureolytic bacteria Succinivibrio and Treponema decreased significantly with biochanin A, while cellulolytic bacteria NK4 A214_group increased (P < 0.05, Fig. 4C-E). Similarly, biochanin A significantly reduced proteolytic bacteria Prevotella (P < 0.05, Fig. 4F). These findings corroborated the in vivo observations, where red clover isoflavones reduced the abundance of proteolytic and ureolytic bacteria, including Prevotella sp002317355, Prevotella sp002392645, Succinivibrio sp900315395, and Treponema D bryantii C.
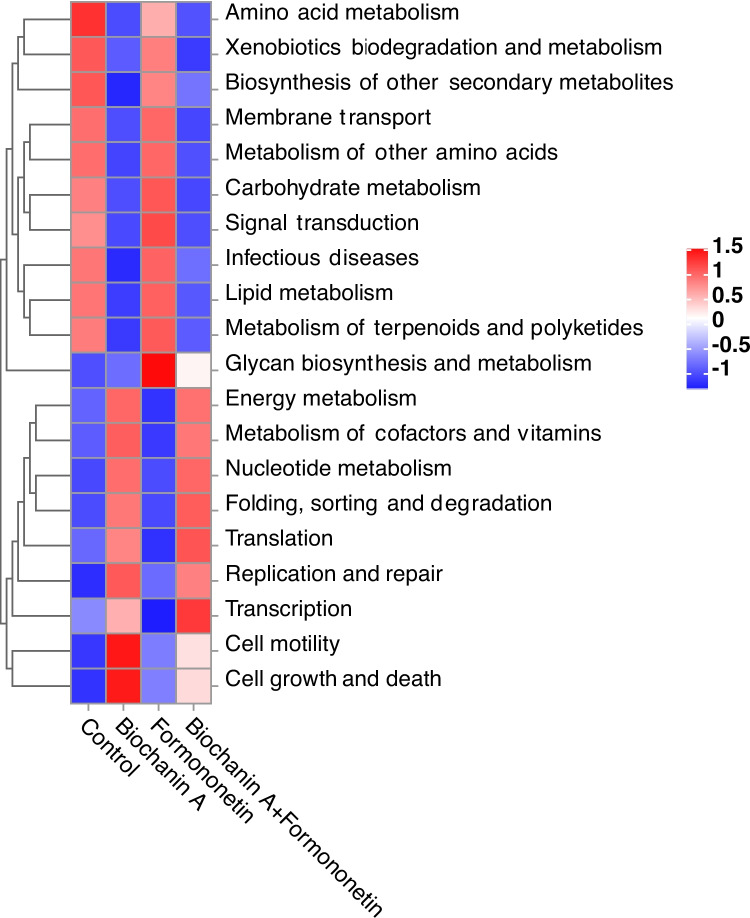
Functional prediction based on KEGG profiles identified 31 s-level metabolic pathways categorized into six primary functions. Among these, the pathways related to cofactors and vitamins metabolism, carbohydrate metabolism, and amino acid metabolism were highly represented in the rumen microbial community due to their importance in nutrient utilization. Notably, biochanin A inhibited amino acid metabolism in the rumen microbial community, as indicated by the functional predictions of the four treatment groups (Fig. 5).
Fig. 5.
Effects of biochanin A and formononetin on in vitro rumen bacterial function. Functional predictions across the four treatments, displayed through KEGG functional abundance cluster analysis and heatmaps. Color intensity indicates abundance
Discussion
Previous studies have demonstrated various benefits of red clover isoflavones for livestock (Zhang et al. 2023; Xiong et al. 2024). In an unpublished study from our laboratory, we observed that red clover isoflavone supplementation in dairy cows did not affect feed intake, supporting the non-disruptive nature of these compounds. Our research evaluated the effects of red clover isoflavones on rumen fermentation and microbial composition in dairy cows through both in vivo and in vitro trials. This integrated approach enhanced the reliability and scientific validity of our findings, providing a useful reference for future research in this field.
Our results showed that the butyrate concentration with a linear increase was observed as the dose increased in vivo. Biochanin A supplementation significantly increased acetate, butyrate, and the acetate-to-propionate ratio compared to the control, the total VFAs show no significant influence. Previous research suggested that red clover isoflavones influence total VFA concentrations (Melchior et al. 2018), it not aligns with our results, variations between our findings and those of Melchior et al. (2018) could stem from differences in experimental conditions, animal models, or red clover isoflavone composition. Acetic, butyric, and propionic acids, as rumen carbohydrate metabolites, serve essential roles: propionic acid provides energy for rumen microorganisms, acetic acid acts as a precursor for fat synthesis, and butyric acid aids in gut health (Matthews et al. 2019). Acetic acid is one of the main products of ruminal fermentation and is produced by the decomposition of cellulose and hemicellulose by fibrinolytic bacteria (Houtert 1993). In addition, acetic acid promotes the health of the rumen epithelial cells and maintains the normal function of the rumen (Gastelen et al. 2015). Biochanin A increases acetate concentration, it can enhance fiber degradation by cellulolytic bacteria (Harlow et al. 2021). Butyrate, which increased linearly with dosage in our experiment, has two functions: it promotes villus growth and enhances nutrient absorption while also supporting energy metabolism and rumen epithelial health (Gastelen et al. 2015). In line with biochanin A’s known suppression of amylolytic microorganisms, the simultaneous decrease in propionate indicates decreased activity of starch-fermenting bacteria such as S. ruminantium (Liu et al. 2020). The isoflavone biochanin A may provide additional energy for microbial protein synthesis and promote gut health by increasing acetate and butyrate.
A marked reduction in NH₃-N concentration was observed with red clover isoflavone supplementation in vivo, with consistent decreases across treatment groups in vitro. Biochanin A was particularly effective in enhancing microbial protein (MCP) concentration, as similarly reported by Harlow et al. (2018), who highlighted biochanin A's role in reducing ammonia production and increasing MCP in the rumen. Liu et al. (2020) also observed that biochanin A suppressed NH₃-N production by at least 26.4% in vitro. Although our study did not show a direct effect on hyper-ammonia-producing bacteria (HAB), Flythe and Kagan (2010) found that red clover isoflavones significantly diminished HAB, reducing ammonia production.
Ureolytic bacteria play a crucial role in rumen urea metabolism, affecting amino acid levels and urease activity (Hailemariam et al. 2021). Functional predictions indicated that biochanin A suppressed amino acid metabolism pathways, reducing the abundance of ureolytic bacteria. Ammonia generated through urea hydrolysis serves as a precursor for microbial protein synthesis, contributing to the overall protein pool available to the host (Prahl et al. 2022; Zhou et al. 2017). Thus, by inhibiting urease activity and lowering ureolytic bacterial populations, red clover isoflavones enhance microbial protein synthesis efficiency, increase the amount of bypass protein, and improve overall dietary protein and urea utilization. A parallel study from our laboratory using the same cows reported that red clover isoflavones reduced urea nitrogen excretion and enhanced nitrogen utilization efficiency (Zhang et al. 2023), these results are consistent with our findings. Another study from the laboratory revealed that red clover isoflavones supplementation significantly decreased total urinary nitrogen to further inhibit rumen-protein degradation and significantly increased total milk nitrogen to improve the rate of microbial protein synthesis in the rumen (Xiong et al. unpublished). This moderation of ammonia production leads to increased metabolizable protein, reducing excess ammonia and improving nitrogen efficiency (Wang et al. 2018).
Supplementation with red clover isoflavones also enhanced cellulase activity and significantly increased cellulolytic bacteria, particularly the NK4 A214_group. Increases in cellulolytic bacteria, such as Ruminococcus_D sp002391065 and Ruminococcus_C sp000433635, further underscored the isoflavones'positive effects on cellulose degradation in the rumen. Biochanin A, as the primary active ingredient, effectively prevented the decline of cellulolytic bacteria and reduced bacteria involved in peptide and amino acid metabolism, thus promoting fiber degradation (Harlow et al. 2021). These findings align with previous research by Xu et al. (2023), who observed significant increases in cellulolytic bacteria, including Ruminococcus flavefaciens and R. amylophilus, with biochanin A supplementation. Through modulation of the rumen microbial community, red clover isoflavones promote cellulose utilization and fiber degradation efficiency, highlighting their potential for optimizing rumen function.
Our study found that Ruminobacter, Rikenellaceae_RC9_gut_group, and Prevotella were the dominant genera, consistent with findings by Melchior et al. (2018). Red clover isoflavones and biochanin A did not affect the alpha diversity of rumen microflora in our study, which may be due to the stable microbial environment maintained over the 84-day period. However, significant decreases in proteolytic bacteria, particularly Prevotella, were observed at the species level. Previous studies reported that biochanin A suppressed Acetoanaerobium sticklandii, a hyper-ammonia-producing bacterium (Lee et al. 2018), although no specific data for Prevotella were provided. Liu et al. (2020) found that biochanin A significantly reduced Prevotella abundance. Given Prevotella's role in protein degradation and ammonia production, the antimicrobial properties of biochanin A likely contribute to reduced Prevotella abundance and urease activity, enhancing rumen microbial health (Stevenson et al. 2007).
Our in vitro experiments revealed that microbial community structure changed with increasing doses of red clover isoflavones, with biochanin A exerting a more pronounced effect than the combination of biochanin A and formononetin. This suggests complex interactions between these isoflavones. While biochanin A alone elicits specific microbial responses, the combination with formononetin may result in synergistic or antagonistic effects, creating a unique microbial environment distinct from biochanin A alone.
In conclusion, our study demonstrated that red clover isoflavones reduce NH₃-N concentration, enhance cellulase activity, and decrease urease activity. These isoflavones effectively reduce the abundance of proteolytic and ureolytic bacteria, while increasing cellulolytic bacteria populations. Furthermore, biochanin A, the primary effective ingredient, was shown to inhibit amino acid metabolism. Collectively, these findings indicate that red clover isoflavones offer significant benefits for rumen health in dairy cows, promoting efficient protein utilization, and a balanced rumen microbiota.
Acknowledgements
Thank for Yangzhou University Farm to provide the dairy cows and facilities.
Author contributions
YB: investigation, writing – original draft, software, formal analysis. XYZ: conceptualization. ZBX: methodology. KXL: formal analysis. SQZ: methodology. SGZ: design, methodology, writing – review and editing, resources. GQZ: provided the dairy cows. ML: provided the facilities. JQW: provided laboratory facilities for analysing and financial support. NZ: resources. All authors have read and agreed to the submit version of the manuscript.
Funding
This study’s finance was provided by National Key R&D Program of China (2022YFD1301000), the Agricultural Science and Technology Innovation Program (CAAS-ZDRW202308), and State Key Laboratory of Animal Nutrition and Feeding (2004DA125184G2108).
Data availability
The raw data of metagenomic sequencing was submitted to China National Microbiology Data Center (http://nmdc.cn/en) under the No. NMDC10018507.
Declarations
Ethical approval
All experimental procedures were approved by the Animal Care and Use Committee of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (Approval No. IAS2022 - 107).
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bach A, Joulie I, Chevaux E, Elcoso G, Ragués J (2021) Milk performance and rumen microbiome of dairy cows as affected by the inclusion of corn silage or corn shredlage in a total mixed ration. Animal 15(1):100014. 10.1016/j.animal.2020.100014 [DOI] [PubMed] [Google Scholar]
- Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349. 10.2307/1942268 [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Liu P, Zhou G, Xia J (2020) Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc 15:799–821. 10.1038/s41596-019-0264-1 [DOI] [PubMed] [Google Scholar]
- Dadáková K, Trnková A, Kašparovská J, Křížová L, Lochman J, Kašparovský T (2020) In vitro metabolism of red clover isoflavones in rumen fluid. J Anim Physiol Anim Nutr (Berl) 104:1647–1654. 10.1111/jpn.13402 [DOI] [PubMed] [Google Scholar]
- Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Flythe M, Kagan I (2010) Antimicrobial effect of red clover (Trifolium pratense) phenolic extract on the ruminal hyper ammonia-producing bacterium, Clostridium sticklandii. Curr Microbiol 61:125–131. 10.1007/s00284-010-9586-5 [DOI] [PubMed] [Google Scholar]
- Hailemariam S, Zhao S, He Y, Wang J (2021) Urea transport and hydrolysis in the rumen: A review. Anim Nutr 7:989–996. 10.1016/j.aninu.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow BE, Flythe MD, Aiken GE (2018) Biochanin A improves fibre fermentation by cellulolytic bacteria. J Appl Microbiol 124:58–66. 10.1111/jam.13632 [DOI] [PubMed] [Google Scholar]
- Harlow BE, Flythe MD, Kagan IA, Goodman JP, Klotz JL, Aiken GE (2020) Isoflavone supplementation, via red clover hay, alters the rumen microbial community and promotes weight gain of steers grazing mixed grass pastures. PLoS ONE 15:e0229200. 10.1371/journal.pone.0229200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow BE, Flythe MD, Klotz JL, Harmon DL, Aiken GE (2021) Effect of biochanin A on the rumen microbial community of Holstein steers consuming a high fiber diet and subjected to a subacute acidosis challenge. PLoS ONE 16:e0253754. 10.1371/journal.pone.0253754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeminiwa BO, Knight RM, Braden TD, Cruz-Espindola C, Boothe DM, Akingbemi BT (2020) Regulation of the neuroendocrine axis in male rats by soy-based diets is independent of age and due specifically to isoflavone action. Biol Reprod 103:892–906. 10.1093/biolre/ioaa101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Zhao S, Wang P, Zheng N, Bu D, Beckers Y, Wang J (2016) Insights into abundant rumen ureolytic bacterial community using rumen simulation system. Front Microbiol 7:1006. 10.3389/fmicb.2016.01006 [DOI] [PMC free article] [PubMed]
- Kenny AM, Mangano KM, Abourizk RH, Bruno RS, Anamani DE, Kleppinger A, Walsh SJ, Prestwood KM, Kerstetter JE (2009) Soy proteins and isoflavones affect bone mineral density in older women: a randomized controlled trial. Am J Clin Nutr 90:234–242. 10.3945/ajcn.2009.27600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MR, Kim HJ, Yu SH, Lee BS, Jeon SY, Lee JJ, Lee YC (2020) Combination of red clover and hops extract improved menopause symptoms in an ovariectomized rat model. Evid Based Complement Alternat Med 2020:7941391. 10.1155/2020/7941391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopylova E, Noé L, Touzet H (2012) SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28:3211–3217. 10.1093/bioinformatics/bts611 [DOI] [PubMed] [Google Scholar]
- Kurzer MS, Xu X (1997) Dietary phytoestrogens. Annu Rev Nutr 17:353–381. 10.1146/annurev.nutr.17.1.353 [DOI] [PubMed] [Google Scholar]
- Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Hansen NP, Weisbjerg MR, Lund P (2020) Technical note: Evaluation of the ororuminal FLORA sampling device for rumen fluid sampling in intact cattle. J Dairy Sci 103:447–450. 10.3168/jds.2019-16972 [DOI] [PubMed] [Google Scholar]
- Lee KT, Toushik SH, Baek JY, Kim JE, Lee JS, Kim KS (2018) Metagenomic mining and functional characterization of a novel KG51 bifunctional cellulase/hemicellulase from black goat rumen. J Agri Food Chem 66:9034–9041. 10.1021/acs.jafc.8b01449 [DOI] [PubMed] [Google Scholar]
- Li H, McKee LS (2023) Measuring enzyme kinetics of glycoside hydrolases using the 3,5-Dinitrosalicylic acid assay. Methods Mol Biol 2657:15–25. 10.1007/978-1-0716-3151-5_2 [DOI] [PubMed] [Google Scholar]
- Li M, Cai Q, Gao YT, Franke AA, Zhang X, Zhao Y, Wen W, Lan Q, Rothman N, Shyr Y, Shu XO, Zheng W, Yang G (2022) Phytoestrogens and lung cancer risk: a nested case-control study in never-smoking Chinese women. Am J Clin Nutr 115:643–651. 10.1093/ajcn/nqab358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang Z, Hailemariam S, Zheng N, Wang M, Zhao S, Wang J (2020) Biochanin A inhibits ruminal nitrogen-metabolizing bacteria and alleviates the decomposition of amino acids and urea in vitro. Animals 10:368. 10.3390/ani10030368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Yu Z, Zhong H, Zheng N, Huws S, Wang J, Zhao S (2023) Functional gene-guided enrichment plus in situ microsphere cultivation enables isolation of new crucial ureolytic bacteria from the rumen of cattle. Microbiome 11:76. 10.1186/s40168-023-01510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Li X, Ma L, Chen Y, He S (2021) Soy isoflavones alleviate polycystic ovary syndrome in rats by regulating NF- κB signaling pathway. Bioengineered 12:7215–7223. 10.1080/21655979.2021.1979864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C, Crispie F, Lewis E, Reid M, O’Toole PW, Cotter PD (2019) The rumen microbiome: a crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 10:115–132. 10.1080/19490976.2018.1505176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdougall EI (1948) Studies on ruminant saliva. I. The composition and output of sheep’s saliva. Biochem J 43:99–109 [PMC free article] [PubMed] [Google Scholar]
- Mehdipour P, Marhon SA, Ettayebi I, Chakravarthy A, Hosseini A, Wang Y, de Castro FA, Loo Yau H, Ishak C, Abelson S, O’Brien CA, De Carvalho DD (2020) Epigenetic therapy induces transcription of inverted SINEs and ADAR1 dependency. Nature 588:169–173. 10.1038/s41586-020-2844-1 [DOI] [PubMed] [Google Scholar]
- Melchior EA, Smith JK, Schneider LG, Mulliniks JT, Bates GE, McFarlane ZD, Flythe MD, Klotz JL, Goodman JP, Ji H, Myer PR (2018) Effects of red clover isoflavones on tall fescue seed fermentation and microbial populations in vitro. PLoS ONE 13:e0201866. 10.1371/journal.pone.0201866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minas K, McEwan NR, Newbold CJ, Scott KP (2011) Optimization of a high-throughput CTAB-based protocol for the extraction of qPCR-grade DNA from rumen fluid, plant and bacterial pure cultures. FEMS Microbiol Lett 325:162–169. 10.1111/j.1574-6968.2011.02424.x [DOI] [PubMed] [Google Scholar]
- Mizrahi I, Wallace RJ, Moraïs S (2021) The rumen microbiome: balancing food security and environmental impacts. Nat Rev Microbiol 19:553–566. 10.1038/s41579-021-00543-6 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) (2001) Nutrient Requirements of Dairy Cattle, 7th edn. National Academy Press, Washington, DC [Google Scholar]
- Palombo V, Alharthi A, Batistel F, Parys C, Guyader J, Trevisi E, D’Andrea M, Loor JJ (2021) Unique adaptations in neonatal hepatic transcriptome, nutrient signaling, and one-carbon metabolism in response to feeding ethyl cellulose rumen-protected methionine during late-gestation in Holstein cows. BMC Genomics 22:280. 10.1186/s12864-021-07538-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl MC, Müller CBM, Albrecht D, Koch F, Wimmers K, Kuhla B (2022) Hepatic urea, creatinine and uric acid metabolism in dairy cows with divergent milk urea concentrations. Sci Rep 12:17593. 10.1038/s41598-022-22536-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JB, Muck RE, Weimer PJ (2009) Quantitative analysis of cellulose degradation and growth of cellulolytic bacteria in the rumen. FEMS Microbiol Ecol 67:183–197. 10.1111/j.1574-6941.2008.00633.x [DOI] [PubMed] [Google Scholar]
- Saloniemi H, Wähälä K, Nykänen-Kurki P, Kallela K, Saastamoinen I (1995) Phytoestrogen content and estrogenic effect of legume fodder. Proc Soc Exp Biol Med 208:13–17. 10.3181/00379727-208-43825 [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. (2021a) SAS/STAT® 15.2 User's Guide: The GLM Procedure. SAS Institute Inc., Cary, NC. Available at: https://documentation.sas.com/doc/en/pgmsascdc/9.4_3.3/statug/statug_glm_syntax.htm. Accessed 11 Jan 2024
- SAS Institute Inc. (2021b) SAS/STAT® 15.2 User's Guide: The MIXED Procedure. SAS Institute Inc., Cary, NC. Available at: https://documentation.sas.com/doc/en/pgmsascdc/9.4_3.3/statug/statug_mixed_syntax.htm. Accessed 11 Jan 2024
- Shen J, Yu Z, Zhu W (2018) Insights into the populations of proteolytic and amino acid-fermenting bacteria from microbiota analysis using in vitro enrichment cultures. Curr Microbiol 75:1543–1550. 10.1007/s00284-018-1558-1 [DOI] [PubMed] [Google Scholar]
- Stevenson DM, Weimer PJ (2007) Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol 75(1):165–174. 10.1007/s00253-006-0802-y [DOI] [PubMed] [Google Scholar]
- St-Pierre NR (2007) Design and analysis of pen studies in the animal sciences. J Dairy Sci 90:87–99. 10.3168/jds.2006-612 [DOI] [PubMed] [Google Scholar]
- Uritskiy GV, DiRuggiero J, Taylor J (2018) MetaWRAP-a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 6:158. 10.1186/s40168-018-0541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gastelen S, Antunes-Fernandes EC, Hettinga KA, Klop G, Alferink SJ, Hendriks WH, Dijkstra J (2015) Enteric methane production, rumen volatile fatty acid concentrations, and milk fatty acid composition in lactating Holstein-Friesian cows fed grass silage- or corn silage-based diets. J Dairy Sci 98(3):1915–1927. 10.3168/jds.2014-8552 [DOI] [PubMed] [Google Scholar]
- Van HMFJ (1993) The production and metabolism of volatile fatty acids by ruminants fed roughages: A review. Anim Feed Sci Tech 43(3–4):189–225. 10.1016/0377-8401(93)90078-X [Google Scholar]
- Wagner H (2010) Vegan: Community ecology package. R package version 1.17–4. https://cran.r-project.org/web/packages/vegan/index.html. Accessed 26 January 2024
- Wang YJ, Xiao JX, Li S, Liu JJ, Alugongo GM, Cao ZJ, Yang HJ, Wang SX, Swanson KC (2017) Protein metabolism and signal pathway regulation in rumen and mammary gland. Curr Protein Pept Sci 18:636–651. 10.2174/1389203717666160627075021 [DOI] [PubMed] [Google Scholar]
- Wang R, Wang M, Ungerfeld EM, Zhang XM, Long DL, Mao HX, Deng JP, Bannink A, Tan ZL (2018) Nitrate improves ammonia incorporation into rumen microbial protein in lactating dairy cows fed a low-protein diet. J Dairy Sci 101:9789–9799. 10.3168/jds.2018-14904 [DOI] [PubMed] [Google Scholar]
- Waschulin V, Borsetto C, James R, Newsham KK, Donadio S, Corre C, Wellington E (2022) Biosynthetic potential of uncultured Antarctic soil bacteria revealed through long-read metagenomic sequencing. ISME J 16:101–111. 10.1038/s41396-021-01052-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974. 10.1021/ac60252a045 [Google Scholar]
- Weinert-Nelson JR, Ely DG, Flythe MD, Hamilton TA, May JB, Ferrell JL, Hamilton MC, LeeAnn Jacks W, Davis BE (2023) Red clover supplementation modifies rumen fermentation and promotes feed efficiency in ram lambs. J Anim Sci 101:skad036. 10.1093/jas/skad036 [DOI] [PMC free article] [PubMed]
- Wocławek-Potocka I, Mannelli C, Boruszewska D, Kowalczyk-Zieba I, Waśniewski T, Skarżyński DJ (2013) Diverse effects of phytoestrogens on the reproductive performance: cow as a model. Int J Endocrinol 2013:650984. 10.1155/2013/650984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z, Li Y, Zhang X, Zhang S, Li K, Zheng N, Zhao S, Wang J (2024) Effects of biochanin A on lactational performance, nitrogen metabolism, and blood metabolites in dairy cows. Anim Nutr 18:441–449. 10.1016/j.aninu2024.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Li Y, Du W, Zheng N, Wang J, Zhao S (2023) Effect of dietary biochanin A on lactation performance, antioxidant capacity, rumen fermentation and rumen microbiome of dairy goat. Front Microbiol 14:1101849. 10.3389/fmicb.2023.1101849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang X, Xiong Z, Li K, Gao Y, Bu Y, Zheng N, Zhao S, Wang J (2023) Effect of red clover isoflavones on hormone, immune, inflammatory, and plasma biochemistry in lactating dairy cows. Anim Nutr 16:306–312. 10.1016/janinu.2023.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JW, Zhong CL, Liu H, Degen AA, Titgemeyer EC, Ding LM, Shang ZH, Guo XS, Qiu Q, Li ZP, Yang G, Long RJ (2017) Comparison of nitrogen utilization and urea kinetics between yaks (Bos grunniens) and indigenous cattle (Bos taurus). J Anim Sci 95:4600–4612. 10.2527/jas2017.1428 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data of metagenomic sequencing was submitted to China National Microbiology Data Center (http://nmdc.cn/en) under the No. NMDC10018507.