Abstract

Transition-metal-free transformations are recognized as green and sustainable methods for constructing carbon–carbon bonds in organic synthesis. This review describes the application of six organic peroxides, including tert-butyl hydroperoxide (TBHP), di-tert-butyl peroxide (DTBP), tert-butyl peroxybenzoate (TBPB), benzoyl peroxide (BPO), dialauroyl peroxide (DLP), and diguyl peroxide (DCP), in C–C bond construction, highlighting selected examples and mechanisms of challenging transformations. Each section concludes with a detailed overview of suitable reagents for various coupling reactions and strengths and weaknesses of the reported works. This work aims to inspire further innovations in transition-metal-free oxidative transformations, promoting sustainable and eco-friendly chemical processes and paving the way for new peroxide-based organic synthesis methods.
1. Introduction
Since Wöhler’s first synthesis of an organic compound, synthesis paradigms have evolved significantly. The development of straightforward and practical methods for creating heterocycles has garnered considerable interest due to their extensive applications in various fields.1 Heterocyclic compounds are crucial and impactful in synthesizing biological and pharmaceutical compounds.2 The formation of carbon–carbon (C–C) bonds from two different carbon–hydrogen (C–H) bonds, without using transition metals, has intrigued synthetic chemists during the last years, generating evolutionary progress in the synthesis of valuable organic compounds. The use of transition metal catalysts in oxidative reactions is limited by their high cost, sensitivity to oxygen and moisture, and potential toxicity.3−6 Researchers have thus explored metal-free radical reactions, where radical species are typically generated by an oxidant under mild reaction conditions. These reactions exhibit high activity and excellent tolerance toward various functional groups.7,8 In direct oxidative transformations, terminal oxidants play an incisive role in promoting the reaction.9 Over the years, a diverse array of organic and inorganic oxidants have been employed in oxidative processes, each contributing uniquely to the advancement of synthetic methodologies.10 Oxidants have been utilized in hundreds of published studies for the oxidation of various organic compounds.11−14 Among them, organic peroxides have emerged as an significant option, gaining widespread popularity due to their versatility and efficacy in both metal-catalyzed and metal-free oxidative transformations.15 Peroxides exhibit versatile reactivity, enabling reactions to occur at elevated temperatures as well as serving as effective primary oxidants under room temperature when subjected to UV or visible light irradiation.16 Both heat and photoirradiation can readily break the O–O bonds in peroxides due to steric repulsion between the two oxygen atoms. The resulting reactive species have a short lifetime and readily oxidize other organic compounds in the reaction medium. These reactions typically proceed through radical pathways, which can be investigated using radical trappers like (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) and butylated hydroxytoluene (BHT), which trap free radicals in the medium and inhibit the progression of the reaction.17 In the past decade, significant strides have been made in transition-metal-free oxidative transformations using peroxides as compatible oxidants.18,19 Despite their extensive application and notable progress achieved in this domain, a conspicuous gap remains in the literature, and there has not been a comprehensive published review paper covering the full scope of organic peroxide-assisted oxidative transformations, including the latest advancements in this area. To fill this gap, this review aims to provide a detailed and systematic exploration of the role of organic peroxides in facilitating a wide range of oxidative transformations, thereby underscoring their pivotal role in the evolution of green and sustainable chemistry. Our primary focus is on the application of organic peroxides, including di-tert-butyl peroxide (DTBP), tert-butyl peroxybenzoate (TBPB), tert-butyl hydroperoxide (TBHP), benzoyl peroxide (BPO), dialauroyl peroxide (DLP), and dicumyl peroxide (DCP), in transition-metal-free cyclization and coupling reactions. These peroxides have been successfully employed in the synthesis of various pharmacologically active compounds such as indoles, azoles, coumarins, quinazolines, and quinolones. We have focused herein on the application of the eight peroxides used in carbon–carbon (C–C) bond reactions. The content is organized based on the types of peroxides used in the reactions. Due to the extensive volume of literature, only selected examples are highlighted. The features of these reactions are discussed, and the mechanisms of particularly challenging reactions are emphasized. Each section concludes with a detailed overview of suitable reagents for various coupling reactions. This review tries to inspire organic chemists to further investigate and innovate the realm of transition-metal-free oxidative transformations. By embracing these green methodologies, the scientific community can contribute to the development of more sustainable and eco-friendly chemical processes, ultimately benefiting both industry and the environment. It is hoped that this review will pave the way for the design of new transformations using peroxides in organic synthesis, especially for metal-free oxidative reactions.
2. Oxidative Transformation by Peroxides
2.1. Coupling Reactions with TBHP
Direct C–H functionalization reactions utilize metal catalysts and have notable advantages, but they are also accompanied by drawbacks,20 including the employment of expensive and hazardous transition-metal catalysts such as Pd, Ru, Pt, Rh, Cu, Co, Fe, or Ni complexes, as well as the requirement for strong bases in most cases. Consequently, there is a strong desire to develop simple, cost-effective, and environmentally friendly metal-free methods for these transformations. In this regard, researchers have extensively explored application of photocatalysts, nonmetal catalysts, and organic or inorganic oxidants.21 C–C bond formation will positively influence the next generation of chemical syntheses, both economically and ecologically. One notable oxidant among them is tert-butyl hydroperoxide (TBHP), which is a colorless liquid when dissolved in a 70% aqueous solution.15 It exhibits a half-life of 1 h at 193 °C and 10 h at 169 °C, making it a commonly used reagent in organic synthesis. Furthermore, the combination of TBHP with tetrabutyl ammonium iodide (TBAI), potassium iodide (KI), molecular iodine (I2), or potassium tert-butoxide (tBuOK) has shown compatibility and effectiveness as an oxidative system for various cross-coupling transformations. In 2011, the Wang group reported an efficient metal-free approach for the direct C2-alkylation of azoles 1a using alcohols and ethers 1b through oxidative C–H activation. This process, referred to as cross-dehydrogenative coupling (CDC), involves the activation of C(sp3)–H bonds in alcohols and ethers, with C(sp2)–H bonds in azoles in the presence of TBHP under solvent-free conditions (Scheme 1).22 The reaction yielded moderate product yields when other oxidants, such as TBPB, DCP, and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), were used. However, when DTBP, dibenzoyl peroxide, and cyclohexanone peroxide were employed, the product yields were significantly lower.
Scheme 1. Cross-Coupling Reaction of Azoles with Alcohols and Ethers.
Reddy and colleagues reported the utilization of KI and TBHP in the C–C coupling of amines 2a and 3a together with nitroalkanes 2b (Scheme 2).23 This reaction enabled the synthesis of various biologically significant N-heterocycles, including tetrahydroisoquinolines 2c and 3,4-dihydroquinazoline 3c. Excellent yields were obtained for all isolated products. The same methodology was also applied to the synthesis of dialkyl 2-(2,3-diphenyl-3,4-dihydroquinazolin-4-yl) malonate derivatives 4b, using dialkylmalonates 4a as nucleophiles instead of nitroalkanes.
Scheme 2. CDC Coupling between Amines and Nitroalkanes/Dialkylmalonates Catalyzed by Potassium Iodide.
Sun et al. utilized the TBAI/TBHP oxidative system to synthesize α-ketoamides 5c by activating multiple inert C(sp3)–H bonds in ethylarenes 5a and their subsequent reaction with N,N-dialkylformamide 5b, as shown in Scheme 3.24 This transformation involved the cleavage of the C–N bond in N,N-dialkylformamide by this oxidative system, which was confirmed by a 13C-isotope labeling experiment. Moreover, the product’s two oxygen atoms were produced through the oxidation of the compound by TBHP.
Scheme 3. TBHP/TBAI Oxidative System for the Synthesis of α-Ketoamides.
The Li group recently introduced a novel method for the oxidation-induced coupling of inactivated terminal alkenes (6a) with amides (6b and 6d) as shown in Scheme 4.25 This process involves the selective modification of C(sp3)–H bonds next to the nitrogen atom using the TBHP/tBuOK system, resulting in the formation of β-amino ketones (6c). However, when FeCl3 is incorporated in the presence of DTBP and DABCO, the chemoselectivity changes, leading to the production of α,β-unsaturated amides (6e). It is suggested that FeCl3 reduces the reactivity of alkenes (6a) toward the C(sp3)–H bonds of amines by consuming OH radicals and the formation of Fen+(OH) species. Two proposed radical pathways are illustrated in Scheme 4. In the first pathway, alkyl radical B is generated by abstracting the α-H atom of A with tBuOO·, derived from TBHP upon heating with tBuOK. Alkyl radical C is then formed by adding B to alkene G. The reaction of C with tBuOO· produces intermediate D, whose O–O bond is cleaved by tBuOK, followed by oxidation with TBHP to yield compound E. In the second pathway, Fe2+ ions convert DTBP to Fe3+(OtBu), generating tBuO radicals. These radicals detach a hydrogen atom from A, forming radical carbonyl F. Subsequent addition across alkene G produces alkyl radical H. A single electron transfer (SET) between H and Fe3+(OtBu) generates alkyl cation I, Fe2+ species, and tBuO–. Finally, I undergoes β-H elimination with tBuO– in the presence of DABCO, yielding the desired product J.
Scheme 4. Oxidation-Induced Coupling of Unactivated Terminal Alkenes Together with Amides by TBHP/tBuOK Oxidation and Suggested Mechanisms.
Li and colleagues have devised a metal-free approach for TBHP-mediated oxidative alkynylation of carbonyl C(sp2)–H bonds in aldehydes (7b) using (triisopropylsilyl)ethynyl benziodoxolones (TIPS-EBX) (7a), leading to the synthesis of ynones (7c) as illustrated in Scheme 5.26 This protocol utilizes an oxidative radical coupling process for carbonyl C(sp2)–H bonds, providing a versatile approach to construct ynone derivatives flexible to a diverse range of substrates and boasting excellent functional group compatibility.
Scheme 5. Cross-Coupling of Aldehydes with Ethynyl Benziodoxolones.

Shah and Kumar developed a method for the oxidative coupling of styrenes 8a or benzyl alcohols 8b with arenes 8c, leading to the formation of biaryls 8d (Scheme 6).27 The reaction process involves the formation of an aldehyde intermediate through the oxidative C–C bond cleavage of styrene and the oxidation of benzyl alcohols. This intermediate then undergoes decarbonylation and arylation steps to produce the desired biaryl compounds. The reaction efficiently works across a broad spectrum of substrates and shows supreme tolerance for diverse functional groups. However, using 2-vinylpyridine as a styrene derivative reduced the yield.
Scheme 6. TBHP-Promoted Cross-Coupling Reaction of Styrenes and Benzyl Alcohols with Arenes.
Wang and colleagues introduced a metal-free approach for the functionalization of inactivated C(sp3)–H bonds in alkyl nitriles 9b with terminal vinyl arenes 9a (Scheme 7).28 The reaction is advantageous due to its simplicity, broad substrate range, and atom economy. In the presence of Cu(OTf)2/1,10-phenanthroline/DBU, azodiisobutyronitrile (AIBN) 10a and its analogs react with terminal vinyl arenes 9a to produce γ-ketonitriles 10b through a radical process. The involvement of a free-radical pathway was certified by catching an alkyl radical with a radical scavenger and through density functional theory (DFT) calculations. Initially, tBuO· and HO· radicals, generated from the decomposition of TBHP under the reaction conditions, abstracted the α-H atom of acetonitrile A, forming a primary alkyl radical ·CH2CN. This ·CH2CN radical then adds to the double bond of vinyl arene B, creating a radical intermediate C, which reacts with tBuOO· to form intermediate D. Finally, peroxide intermediate D is converted to final product E in the presence of DBU.
Scheme 7. Functionalization of Alkyl Nitriles and AIBN Analogues with Terminal Vinyl Arenes and the Associated Mechanistic Pathway.
3-Aroyl coumarins have demonstrated diverse biological activities, including α-glucosidase inhibitory, DPPH scavenging, antibacterial, and antioxidant activities, and have also been used as fluorescent chemosensors.29−32 Jafarpour and Abbasnia devised a metal-free TBHP-mediated technique for the regioselective C–H functionalization of coumarins 11a, resulting in the synthesis of 3-acyl coumarins 11e (Scheme 8).33 This approach involves reacting coumarins or coumarin-3-carboxylic acids with aromatic aldehydes 11b. Additionally, styrenes 11c and benzyl alcohols 11d were used as acylating agents and demonstrated good reactivity. This protocol enables the efficient regioselective carbonylation of coumarins with aromatic aldehydes through metal-free coupling, effectively utilizing benzyl alcohol and styrene derivatives. In situ decarboxylation extends its applicability to coumarin-3-carboxylic acids, showing good functional group tolerance and efficiency.
Scheme 8. Synthesis of 3-Acyl Coumarins with Various Acylating Reagents.
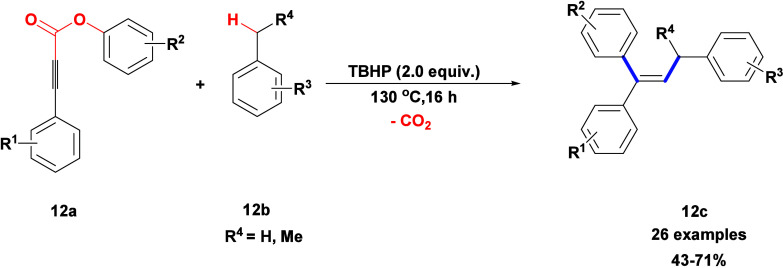
Wang and their team described a technique for the oxidative difunctionalization of aryl alkynoate 12a, which includes intramolecular 1,4-aryl migration followed by a decarboxylation tandem process. The reaction utilized TBHP as the sole oxidant (Scheme 9).34 Comparable yields were achieved using cumene hydroperoxide (CHP) instead of TBHP, whereas other peroxides such as DTBP, TBPB, DCP, and BPO led to reduced yields. Oxidants such as H2O2, O2, m-CPBA, and PhI(OAc)2 and inorganic oxidants like K2S2O8 and Ag2O failed to produce the desired product. This technique provides a straightforward and selective route for synthesizing three-substituent alkenes 12c from basic toluene derivatives 12b and aryl alkynoates 12a.
Scheme 9. Synthesis of Three-Substituent Alkenes from Aryl Alkynoates and Toluene Derivatives.
Jiao and colleagues described a highly regioselective CDC reaction of boron dipyrromethene (BODIPY) dyes 13a with allylic alkenes 13b and ethers 14a utilizing TBHP as the oxidant (Scheme 10).35 Alternative oxidants, including O2, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), K2S2O8, and DTBP, were determined to be unsuccessful in facilitating this reaction. The reaction followed a radical route and provided a convenient method to synthesize diverse α-functionalized BODIPYs 13c and 13d, which were challenging to access using previous methods.
Scheme 10. Synthesis of α-Functionalized BODIPYs Using Allylic Alkenes and Ethers.
Traditionally, the synthesis of 2-substituted benzothiazole derivatives involves the condensation of 2-aminothiophenol with aldehydes, esters, and carboxylic acids36 or utilizes transition metals as catalysts in the cross-coupling reactions between aryl halides and benzothiazole.37−40 However, in 2017, Ma and his team introduced a one-pot metal-free method for synthesizing 2-heteroaryl benzothiazoles 15c using N-iodosuccinimide (NIS) and TBHP as an efficient oxidative system (Scheme 11).41 This method enables the oxidative condensation of benzothiazoles 15b with quinoline derivatives 15a, leading to the creation of the desired coupling products.
Scheme 11. Synthesis of 2-Heteroarylbenzothiazoles Using NIS and TBHP.
In the same year, Jiang and colleagues introduced a cost-effective, transition-metal-free method for the trifluoromethylation of enamines 16a, utilizing trifluoromethanesulfinate 16b (commonly known as Langlois’ reagent) as the CF3 group source (Scheme 12).42 A diverse array of β-trifluoromethyl-substituted enamines 16c with E-configurations were synthesized by selectively attacking the electron-deficient CF3 radical to the electronegative β-carbon. Screening other peroxides, such as DTBP, H2O2, and benzoquinone (BQ), was unsuccessful; however, K2S2O8 worked with moderate efficacy. The method could be employed for efficient preparation of (E)-β-CF3 enamines via direct C–H trifluoromethylation using Langlois’ reagent and TBHP. The reaction benefits are mild to excellent yield, air- and water-tolerant conditions, and available β-amino acid precursors.
Scheme 12. TBHP-Mediated Trifluoromethylation of Enamines with Trifluoromethanesulfinate.
Hajra developed a method for the trifluoromethylation of indazoles 17a using CF3 radicals generated from Langlois’ reagent 17b under the same reaction conditions. This approach achieved the trifluoromethylation of a series of substituted 2H-indazoles, resulting in products 17c using the inexpensive Langlois’ reagent as the CF3 source (Scheme 13).43 This method provides a straightforward regioselective trifluoromethylation of 2H-indazoles under mild, metal-free conditions. It demonstrated high functional group tolerance, regioselectivity, and scalability. This approach shows potential in the fields of organic synthesis, medicinal chemistry, and material sciences.
Scheme 13. Direct Trifluoromethylation of Indazoles with Sodium Trifluoromethanesulfinate.
Lei et al. developed a visible-light-mediated direct cross-coupling reaction involving N-heterocycles 18a and aldehydes 18b (Scheme 14).44 They successfully synthesized acylated N-heterocycles 18c using either aliphatic or aromatic aldehydes, achieving moderate to high yields. The reaction proceeded with the formation of complex A in the presence of blue LED and TBHP. Then, through a HAT process, acyl radical formation, and subsequent SET process, the target product was formed. This method could efficiently and mildly acylate various N-heterocycles with diverse aldehydes, showing high functional group tolerance and scalability.
Scheme 14. Acylation of N-Heterocycles with Aldehydes.
Regioselective C3-alkylation of coumarins 19a and coumarin-3-carboxylic acids 20a with inactive ethers and alkanes 19b and 20b was achieved by TBHP as a commercially available oxidant (Scheme 15).45 The cross-coupling reaction was successfully performed under conditions free of metals, bases, and solvents, demonstrating efficacy across a wide range of coumarins. This practical and innovative approach, with its wide substrate scope, presents a valuable substitute for traditional transition-metal-catalyzed cross-coupling methods. It offers significant potential for expanding the library of coumarins, which are particularly prominent in the pharmaceutical industry.
Scheme 15. TBHP-Mediated C3-Alkylation of Coumarins and Coumarin-3-carboxylic Acids.
Liu and colleagues have devised a metal-free method using TBHP to synthesize a series of dichloroaryl compounds 21c from terminal alkenes 21a (Scheme 16).46 The reaction involves oxidative radical addition/chlorination with simple chloro reagents, eliminating the need for inert gas protection. This efficient, regioselective one-pot method is compatible with a wide range of functional groups. Preliminary mechanistic studies suggest that an oxidative radical process is involved, with triethylamine functioning as a base to suppress ATRA and facilitate proton transfer. This transformation showcases considerable potential for novel organic reactions. This strategy demonstrates high efficiency across a wide range of substrates and exhibits excellent tolerance for various functional groups, offering a new metal-free approach for the synthesis of biaryls. The advantages include metal-free conditions, avoiding metal catalysts, and being greener and more sustainable. It also offers excellent functional group tolerance, allowing a wide range of substrates to achieve appropriate yields of the products. However, the disadvantage is a reduction in yield with certain derivatives, such as using 2-vinylpyridine, which decreases the reaction yield.
Scheme 16. Oxidative Radical Addition–Chlorination of Alkenes.
In 2019, the Liang research team expanded the radical difluoroalkylation/trifluoromethylation and alkynylation of unactivated alkenes 22a under mild conditions (Scheme 17).47 This method involved synthesizing CF2/CF3-substituted linear alkynyl ketones 22c via 1,2-alkynyl radical migration. The authors suggested a radical pathway for this transformation, where CF2CO2Et and ·CF3 radical species were generated from ICF2CO2Et 22b and CF3SO2Na 23a in the presence of N-methylpiperidine and TBHP. The addition of ·CF2CO2Et or ·CF3 radicals to the inactivated double bond of the 1,4-enyne formed the radical intermediate A, which then underwent 3-exo-dig cyclization subsequently to 1,2-alkynyl migration to produce the hydroxyalkyl radical C. Intermediate C was oxidized by ·CF2CO2Et or tBuO· radicals to yield intermediate D, which was later transformed into target product E via deprotonation (Scheme 17).
Scheme 17. Radical Difluoroalkylation/Trifluoromethylation and Alkynylation of Unactivated Alkenes, Along with the Proposed Mechanism.
Triazoles, identifiable by their five-membered unsaturated ring with three nitrogen atoms, have been recognized as biologically and pharmaceutically active molecules (Scheme 18).35,48−53 In 2020, a general procedure for synthesizing 1,3,5-trisubstituted 1,2,4-triazoles 24c from 1,3-disubstituted 1,2,4-triazoles 24a in the presence of TBAI/TBHP via decarbonylation of aromatic aldehydes 24b was reported. Abebe Agisho and colleagues developed a straightforward, efficient, and environmentally friendly method for synthesizing 3,5-disubstituted 1,2,4-triazoles and 1,3,5-trisubstituted 1,2,4-triazoles from 3-monosubstituted 1,2,4-triazoles and 1,3-disubstituted 1,2,4-triazoles, to utilize potassium iodide as a catalyst and TBHP as an oxidizing agent under gentle reaction conditions. This approach provided structurally varied diverse 3,5-disubstituted and 1,3,5-trisubstituted 1,2,4-triazole compounds in good to outstanding yields.
Scheme 18. TBAI/TBHP-Mediated Synthesis of 1,3,5-Trisubstituted/3,5-Disubstituted 1H-1,2,4-Triazoles.
Oxidative coupling of quinoxalin-2(1H)-ones 25c with coumarin derivatives 25a, 4-hydroxy-6-methyl-2-pyrone 25b, or 2-hydroxy-1,4-naphthoquinone 25d can be efficiently promoted by TBHP (Scheme 19).54 Various 3-substituted quinoxalin-2(1H)-one derivatives of 25c were compatible with this method. The reaction also successfully proceeded using K2S2O8, resulting in the coupling products with good yields. Under the reaction conditions, other oxidants such as Na2S2O8, (NH4)2S2O8, Mn (OAc)3, CAN, TBPB, and DTBP were ineffective for this transformation. Simple operation and using no toxic reagents were the advantages of this protocol.
Scheme 19. CDC Reaction between Quinoxalin-2(1H)-ones and 4-Hydroxycoumarins, 4-Hydroxy-6-methyl-2-pyrone, or 2-Hydroxy-1,4-naphthoquinone.

Acylation of the benzothiazoles 26a using aryl ketones 26b as an acylating agent can be achieved by I2 and TBHP as ring-opening reagents with a short reaction time and at low temperature (Scheme 20).55 According to the reported mechanism for the reaction, aryl ketone is transformed into arylglyoxal A during exposure to I2 in DMSO with Kornblum oxidation. HI is oxidized by DMSO or TBHP to reproduce I2 during exposure to I2, TBHP, and H2O, and the thiazole ring undergoes a ring-opening reaction to form 2-aminobenzenethiol. The reaction of intermediate B with intermediate C produces imine intermediate D, which is then converted to intermediate E through an intramolecular addition reaction. Next, the substitution reaction between intermediate E and I2 produces intermediate F, which ends with the final product G via the elimination of HI (Scheme 20).
Scheme 20. I2/TBHP as an Oxidative System for Acylation of the Benzothiazoles Using Aryl Ketones and Plausible Mechanism.
Arylation of benzothiazoles 27a can be carried out by using aryl aldehydes 27b with TBHP and N-chlorosuccinimide (NCS) (Scheme 21).56 Acylated products 27e were obtained amid the reaction conditions when 5-acetalbenzothiazole and aliphatic aldehydes 27c were used as the substrates. Also, it has been found that DMSO can act as a strong Lewis base, facilitating the cleavage of C–H acidic bonds. The inexpensive organic catalyst NCS serves as a radical initiator with TBHP as the oxidant. This reaction accommodates a broad substrate scope, yielding arylated products in 12–94% yield for 28 examples. Additionally, the method produces acylated benzothiazoles from aryl aldehydes and aliphatic aldehydes with four examples. Nevertheless, the reaction’s disadvantages consist of requiring high temperatures and extended reaction durations as well as low isolate yield for some of the products.
Scheme 21. NCS/TBHP-Promoted Arylation of the Benzothiazoles Using Aryl Aldehydes.
Amides constitute vital structural elements in biologically relevant molecules, such as proteins, natural products, pharmaceuticals, and synthetic intermediates.57−59 In 2021, Yao et al. synthesized a series of α-etherized glycine derivatives 28c, consisting of α-amino esters, α-amino ketones, or α-amino amides, through α-alkylation of glycines 28a with open-chain and cyclic ethers 28b (Scheme 22).60 This visible-light-induced oxidative α-alkylation of glycine derivatives uses catalytic eosin Y and a 3 W blue LED, attaining good to supreme yields and functional group tolerance using TBHP at room temperature. It delivers a budget-friendly, metal-free, and mild synthesis solution.
Scheme 22. α-Alkylation of Glycine Derivatives via Ether Compounds.
In one study, one-pot metal-free cross-coupling reaction between indole-2-thiones 29a and arylsulfonyl hydrazides 29b afforded achiral axial 3,3′-biindole-2,2′-dibenzenesulfonothioate derivatives 29c (Scheme 23).61 The reaction was performed in the presence of a NaI/TBHP catalytic system. Applying TBPB and DTBP was not useful in this oxidative reaction. The product was also produced in the presence of I2, TBAI, NIS, or KI as catalysts, but in lower yields. The radical pathway mechanism reported by the authors is seen in the scheme. In the proposed mechanism, the reaction of TBHP with I– generates tBuOO· and tBuO· radicals. Next, these radicals react with indole-2-thione A to obtain radical B, which gives compound C via a homocoupling process. Oxidation of the thiol enol tautomer of intermediate C affords radical D. The homocoupling of radical D leads to disulfide E. On the other hand, under oxidative conditions, sulfonyl radicals are formed from sulfonyl hydrazides, which then produce arenesulfonyl iodides by I2. Finally, arenesulfonyl iodides react with disulfide E to create product I through the radical propagation (Scheme 23).
Scheme 23. Cross-Coupling Rection between Indole-2-thiones and Arylsulfonyl Hydrazides and Proposed Mechanism Reported.
Just recently, in 2022, Bai and associates described an environmentally benign approach to produce diaryldiketone derivatives 30b applying TBAI and TBHP in an aqueous medium (Scheme 24).62 Both electron-donating groups (−CH3 and −OCH3) and electron-withdrawing groups (−F, −Cl, −Br, and −NO2) on the aromatic rings were compatible in the present reaction. Heteroaromatic substrates, including furan, thiophene, and naphthalene, also successfully generated the desired compounds in achieving yields between 44% and 85%. In the next step, a series of tetrasubstituted pyrroles 30c and furans 30d were smoothly prepared from α-methylene ketones.
Scheme 24. TBAI-Catalyzed Homocoupling of Benzyl Ketones.
2.1.1. Innovative Approaches to Organic Synthesis Using TBHP
The methodologies presented in this study demonstrate innovative approaches to organic synthesis (Figure 1). The use of substrates containing an active α carbon, such as glycines, for carbon–carbon coupling with open-chain and cyclic ethers showcases a robust process enhanced by eosin Y and TBHP. This method improves product yield, making it a valuable addition to synthetic chemistry.60 The use of light in photochemistry is energy-efficient and eco-friendly. Eosin Y, a nontoxic, cost-effective dye, effectively promotes chemical transformations and competes with metal-based processes. It remains a promising green photocatalyst, especially for stereoselective photoredox transformations.63 Furthermore, the reaction between thiazole derivatives and ketones using iodine (I2) and TBHP highlights a significant improvement in the carbon–carbon bond formation through Kornblum oxidation and subsequent intramolecular reactions. The efficiency of this method underscores the importance of I2 and TBHP in facilitating complex transformations.55 The I2/TBHP system has significantly advanced synthetic conversions for industrial and pharmacological products. It offers easy access to various starting materials and enables efficient, eco-friendly carbon–carbon, carbon–nitrogen, and carbon–sulfur linkage formation and ring-closure reactions. This system is also effective in developing ring-fused systems, including incorporating bioactive fused heterocycles, using accessible materials, making it a vital tool in organic synthesis.64,65 The addition of CF3 groups of enamine derivatives with sodium trifluoromethanesulfinate (Langlois reagent) exemplifies a highly selective and effective approach, outperforming other peroxide-based methods. The reagent’s ability to target the electronegative β-carbon of enamines illustrates its potential as a specialized and economical tool in synthetic processes.43 The Langlois reagent is an efficient trifluoromethylating and fluoroalkylating agent with diverse functionality, a broad substrate scope, and easy handling. It is widely used in CF3-incorporated compounds with various applications. However, its preparation involves fluoroalkyl halides, posing an environmental threat and necessitating an alternative route.66,67 Finally, the trifluoromethylation of indazole derivatives using the Langlois reagent and TBHP further demonstrates the reagent’s utility and effectiveness in synthesizing valuable trifluoromethylated compounds.42 Langlois’ reagent is widely used for trifluoromethylation, serving as an excellent CF3 source for difunctionalizing carbon–carbon double and triple bonds. It enables the formation of various functionalities, such as alkenyl, alkyl, carbonyl, and cyano groups, due to its versatile electrophilic, nucleophilic, and radical character.68 These methodologies collectively highlight significant advancements in organic synthesis, offering efficient, economical, and specialized solutions for various synthetic challenges (Figure 1).
Figure 1.

Suitable reagents for various types of coupling reactions with TBHP.
2.2. Coupling Reactions with DTBP
In recent years, efforts to replace transition metals in cross-coupling reactions have led scientists to choose organic peroxides as potential promoters in these syntheses.69 DTBP, owing to the bulky tert-butyl groups, is one of the most stable peroxides commonly used as a radical initiator in a variety of reactions such as intramolecular addition reactions, fragmentation reactions, and homolytic substitution reactions.70 DTBP is a colorless liquid with a half-life of approximately 3 h at 140 °C and 24 h at 120 °C. Heating, as well as UV irradiation, can dissociate DTBP to tert-butoxy radicals that can abstract the H atom of the molecules or attack the molecules.71 In 2014, Yi published a direct attachment of aryl groups of an unreactive aromatic C–H bond 31b by applying DTBP as an oxidant and tBuOK as a base under mild reaction conditions (Scheme 25).72 The transformation occurred through radical anion intermediates, and no coupling outcome was achieved without adding oxidants. In this reaction condition, bromobenzene afforded a slightly lower yield, and chlorobenzene yielded no product. The reason seems to be that the homolysis of the aryl carbon–chlorine bond is more challenging than that of the aryl carbon–iodine bond. A possible mechanism described by the authors involves an SET pathway. Initially, radical anion B is generated from aryl halide A through a single electron transfer process assisted by DTBP and tBuOK. Then, B is converted into radical C and attached to benzene to afford cyclohexadienyl radical E. Subsequently, tBuOK abstracts a proton from E to generate the radical anion F. Then, product G is achieved via a radical chain transfer between F and A and also the regeneration of the radical anion B.
Scheme 25. Carbon–Hydrogen Arylation of Inactivated Benzene Derivatives via Aryl Halides and the Proposed Mechanism Reported.
The chromanone framework is found in various bioactive natural substances and medicinal compounds, which exhibit a diverse array of biological actions, featuring antitumor, anticancer, antioxidant, antimicrobial, and antibacterial attributes.73−76 Transition-metal-catalyzed alkylation of chromanone derivatives through conjugate addition reactions has been published by various research teams.73,77−79 The Han group described oxidative C(sp3)–H bond activation mediated by DTBP of nonreactive alkanes 32b and conjugate addition involving chromone derivatives 32a (Scheme 26).80 The product 32c was obtained in 51% yield when 2.0 equiv of DTBP was used. The effectiveness increased by raising the loading of DTBP to 4.0 equiv (72% yield). Other peroxides like H2O2, TBHP, TBPB, BPO, and DCP were less effective, and there was no product when oxidants such as K2S2O8, BQ, DDQ, NaClO, and O2 (1 atm) were used. In their procedure, 2-alkylchromanones were provided in satisfactory to high yields.
Scheme 26. DTBP-Mediated Oxidative Activation of sp3 C–H Bonds of Nonreactive Alkanes and Addition Reaction to Chromones.
Cheng’s team developed a cross-dehydrogenative coupling reaction of α-amino carbonyl derivatives 33a with basic alkanes 33b followed by α-alkylation of amino compounds 33c (Scheme 27).81 Cyclohexane, cyclopentane, cycloheptane, cyclooctane, adamantine, and glycine esters demonstrated good reactivity in the reaction. The authors conducted some validation experiments to understand the reaction pathway. During the kinetic isotope effect investigation, the ratio of kH/kD for cyclohexane 34b was found to be 8.3, and the incorporation of TEMPO notably diminished the yield of the product. These results indicated the bond cleavage of the C(sp3)–H was the rate-limiting step, and a radical pathway was implicated in the reaction. Hence, the scientists put forward a radical mechanism in which the dissociation of DTBP under heating resulted in the formation of tert-butoxy radicals. Then, hydrogen atom abstraction by a tert-butoxy radical resulted in a cyclohexyl radical, which was identified as the rate-determining step. On the other hand, α-amino carbonyl produced imine intermediate B. Subsequently, a cyclohexyl radical was attached to B to create radical intermediate C. Finally, the capture of a hydrogen atom by intermediate C led to the creation of the final product D (Scheme 28).
Scheme 27. DTBP-Mediated α-Alkylation of α-Amino Carbonyl Derivatives by Basic Alkanes.
Scheme 28. Control Experiments for α-Alkylation of Amino Compounds and the Proposed Mechanism Reported.

Han and colleagues reported the direct synthesis of α-aryl-β-alkylated carbonyl ketones 35c through radical alkylation of symmetrical and unsymmetrical α,α-diaryl allylic alcohols 35a with basic alkanes 35b (Scheme 29).82 Direct C(sp3)–H activation and 1,2-aryl shift in α,α-diaryl allylic alcohol derivatives resulted in the creation of C(Ar)–C(sp3) and C(sp3)–C(sp3) in a single step. It is noteworthy that selective transfer of the two distinct aryl groups occurred in this transformation. They developed a DTBP-promoted radical addition reaction of olefins with alcohols and ether compounds, forming new C(sp3)–C(sp3) bonds without metal catalysts or light. This process, which functionalizes C(sp3)–H bonds, produces α,ω-amino alcohols directly from olefins and exhibits a wide range of substrates and high yields, enriching metal-free radical addition reactions.
Scheme 29. Direct Preparation of an α-Aryl-β-alkylated Carbonyl Ketone through a Radical 1,2-Aryl Shift in α,α-Diaryl Allylic Alcohol.
Cai and colleagues documented the formation of C(sp3)–C(sp2) bonds between isochroman compound 36a and indole derivatives 36b. Several cyclic ethers were achieved in reasonable yields, utilizing DTBP as the only oxidant in the absence of solvents (Scheme 30). Indoles with electron-withdrawing and electron-neutral groups exhibited good yields. However, electron-donor groups showed somewhat reduced yields relative to the electron-poor indoles. Under the conditions, no substituents on the 1- and 2-positions of indoles had a remarkable effect on the reaction. By contrast, the electronic properties of substituents situated at the 6- and 7-positions played a minor role in yields (Scheme 31).83
Scheme 30. DTBP-Promoted Direct Coupling of Isochroman and indole.
Scheme 31. Scope of Some Indoles with Different Electronic Groups.
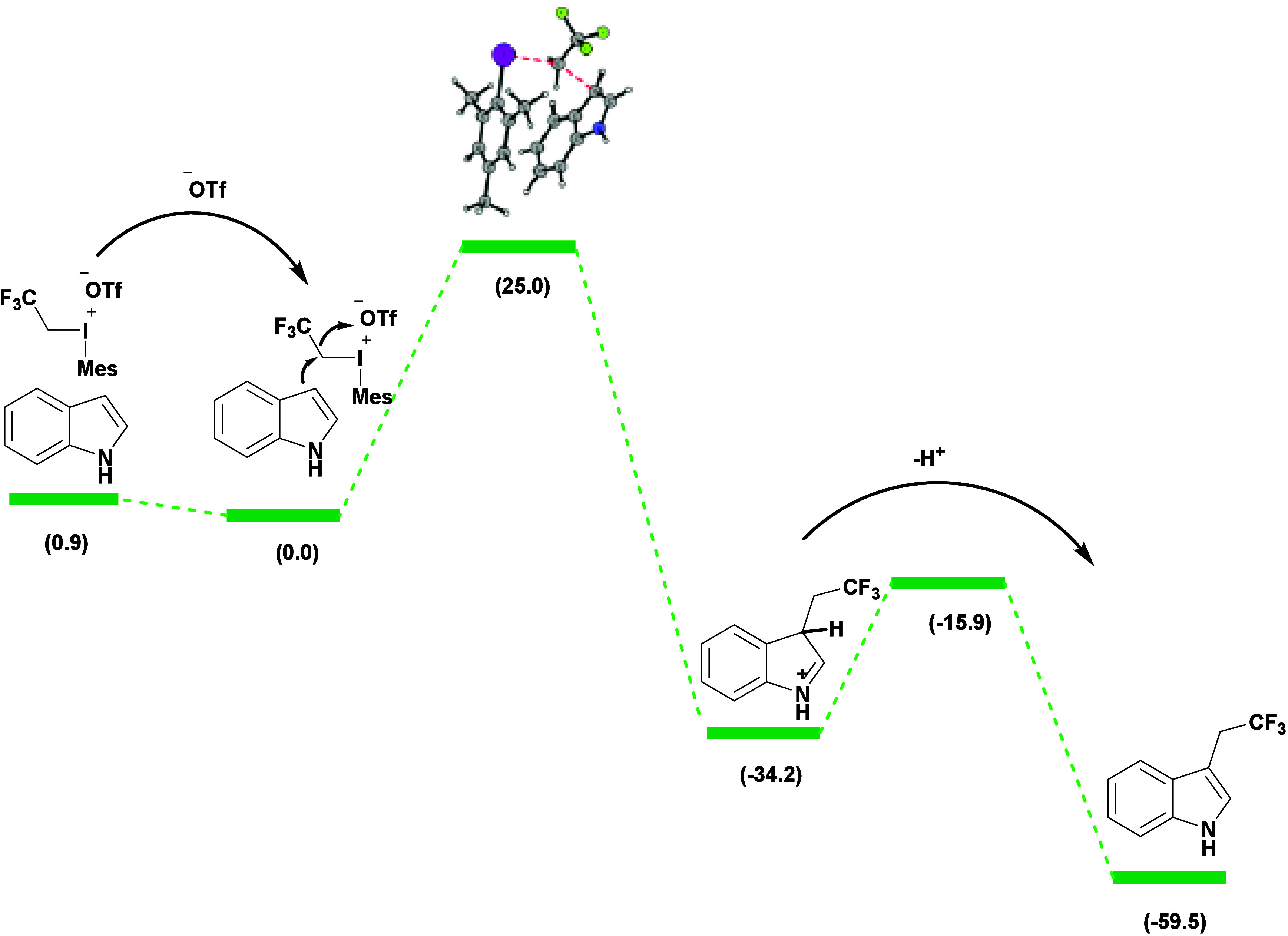
Novák and co-workers reported specific trifluoroethylation at the C3 position of unprotected indoles 38c using 2,2,2-trifluoroethyl(mesityl)-iodonium triflate 38b in the presence of 2,6-di-tert-butylpyridine or DTBPy at room temperature (Scheme 32).84 Electron-rich alkyl and alkoxy indoles 38a exhibited good to excellent yields, while electron-deficient ones afforded a lower efficiency. The authors used DFT calculations to study the mechanism and selectivity. The separation of the triflate ion was demonstrated to be energy-releasing (−0.9 kcal·mol–1). Hence, the slowest step was the attachment of a trifluoromethyl group to the indole nucleus. In the subsequent step, deprotonation of the σ-complex by the base occurred with a barrier of 18.3 kcal·mol–1. Both steps were highly energy-releasing, indicating one-way changes. Activation energy levels for all of the reactants were computed. The reaction pathway was excluded based on the prohibitively high barrier (52 kcal·mol–1) for constructing the key intermediate from 1 and 2 (Scheme 33).
Scheme 32. Trifluoroethylation of Indoles Promoted bt DTBPy.
Scheme 33. Energy Diagram in kcal·mol–1 of the Transformation Based on Density Functional Theory Calculation.
The same research group described another type of alkylation and migration in the decarboxylative arylation reaction on alkynoates (Scheme 34).85 A radical cascade difunctionalization reaction of aromatic alkynoates 39a proceeded through a consecutive reaction comprising breaking of the C(sp3)–H bond of cyclic alkanes 39b, alkylation of the C–C triple bond in 39a, 1,4-shift of the aryl group, and removal of a carboxyl group. According to the proposed mechanism by the authors, tert-butoxy radical intermediate B is formed through homolysis of DTBP under heating. H atom removal of cyclohexane compound C by a tert-butoxy radical forms cyclohexane radical species D, which reacts with the C–C triple bond of alkyne esters to afford species F. Then, F undergoes ipso ring closure to form spiro compound G. The following shift of the aryl group on the ester moiety forms carboxyl radical species H, which undergoes the decarboxylation reaction with the release of the CO2 to furnish species I. Finally, H atom removal of J by I creates the desired products K and D.
Scheme 34. Reaction of Aryl Alkynoates with Cycloalkanes Using DTBP and the Proposed Mechanism Reported.
Yadav et al. reported DTBP-promoted cross-coupling of aromatic aldehyde compounds 40a with aryldiazonium tetrafluoroborates 40b to access diaryl ketones 40c under metal-free conditions (Scheme 35).86 They developed an efficient one-pot method for synthesizing diaryl ketone compounds via a metal-free coupling reaction of aromatic aldehyde compounds and aryldiazonium tetrafluoroborates, using DTBP as a radical starter. This represents the first example of creating diaryl ketone compounds from aromatic aldehyde compounds through a radical–radical coupling reaction, offering new applications for DTBP in metal-free reactions and offering a substitute to the Friedel–Crafts acylation.
Scheme 35. Preparation of Diaryl Ketone Compounds.
The Han group described metal-free radical addition of primary and secondary hydroxyl compounds 41b or ethers 42b with N-allylbenzamides 41a/42a employing DTBP as the sole oxidant in the reaction (Scheme 36).82 Creation of a new carbon–carbon bond from functionalization of a C(sp3)–H bond using a radical addition cascade process resulted in straightforward synthesis of α,ω-amino alcohols 41c/42c from easily available olefins. It provides a wide range of substrates and favorable yields, enhancing the scope of radical addition under metal-free conditions.
Scheme 36. Direct Synthesis of α,ω-Amino Alcohols Promoted by DTBP.
Direct functionalization of indoles often occurs at the 2- or 3-position.87−89 In 2016, Yi reported C4-regioselective oxidative alkylation of unprotected indoles 43a with cycloalkanes 43b using DTBP as the sole oxidant in the reaction for the first time.90 Various indoles bearing substituents at different positions underwent this functionalization at 2-, 4-, and 7-positions with moderate to high regioselectivity (Scheme 37).
Scheme 37. DTBP-Mediated Coupling Reaction of Indole Compounds with Cyclic Alkanes.
In 2016, Pan and Yu reported decarbonylative alkylation via 1,4-shift of the aryl group and removal of a carboxyl group of diaryl alkyne esters 44a with aliphatic aldehydes 44b as an inexpensive and plentiful alkyl radical source in the presence of DTBP (Scheme 38).91 A large number of trisubstituted alkenes 44c in reasonable to good yields were produced. They designed a metal-free approach for the oxidative decarbonylative alkylation of diaryl alkyne esters with aliphatic aldehydes, producing trisubstituted olefins in good to moderate yields. The process includes the removal of a carbonyl group, radical addition, 1,4-shift of the aryl group, and removal of a carboxyl group, all performed in a single pot. This approach efficiently and economically synthesizes 1,1-diaryl-2-alkyl ethylene compounds using aliphatic aldehyde compounds.
Scheme 38. DTBP-Mediated Decarbonylative Alkylation/Arylation of Alkyne Esters with Aldehyde Compounds.
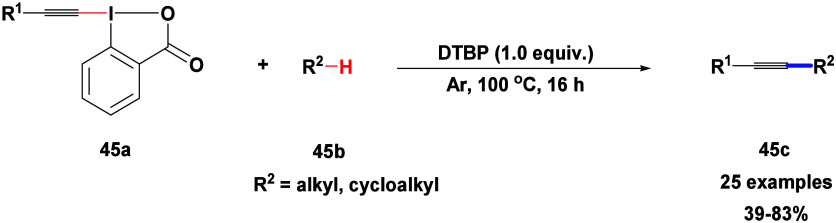
Xu and co-workers described direct radical formation of a C(sp3)–C(sp) bond between inactivated saturated alkanes 45b and hypervalent iodine alkynyl reagents like ethynylbenziodoxolones 45a using DTBP (Scheme 39).92 In a large-scale experiment using cyclohexane as the alkyl reagent, coupling product 45c was achieved with a good yield of 73%. This process was effective with various saturated hydrocarbons and hypervalent iodine alkynyl reagents, offering an efficient way to synthesize alkyl-substituted alkynes under gentle, metal-free conditions .
Scheme 39. DTBP-Mediated Alkynylation of Alkanes with Ethynylbenziodoxolones.
Kwong’s research team developed a transition-metal-free cross-dehydrogenative bond (CDC) formation. Using di-tert-butyl peroxide, they effectively coupled indoles with cyclic ethers and cycloalkanes, resulting in satisfactory yields. C2 and C3 functionalization of indoles has received significant attention from chemists in the organic synthesis field. Direct cross-coupling of unprotected indoles 46a with cyclic ether compounds 46b or cyclic alkanes using only DTBP as an oxidant in the reaction was performed (Scheme 40).93 The product 46c in the presence of 1.5 equiv of TBPB was also obtained in satisfactory yield (45%).
Scheme 40. Oxidative Cross-Coupling of Indole Compounds with Ether Compounds or Cycloalkanes.
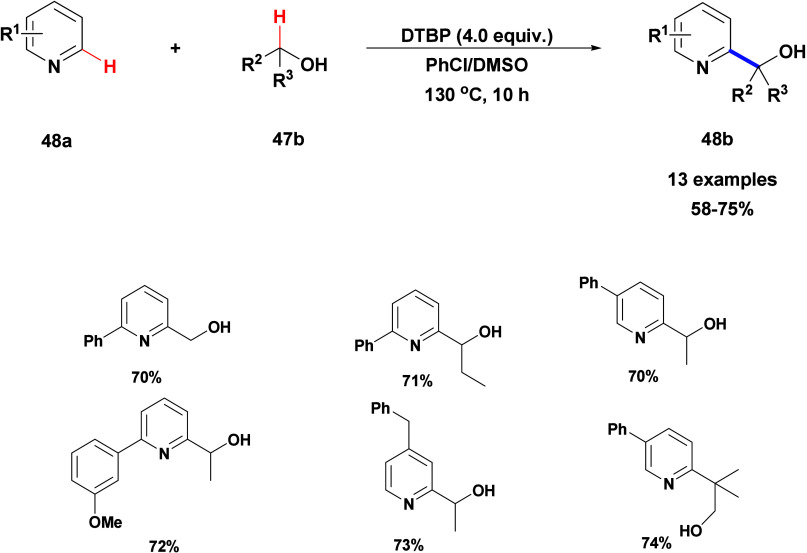
Kianmehr’s research team described a straightforward metal-free CDC reaction between neutral heterocycles 47a with alcohols and cyclic ether compounds 47b/47c (Scheme 41).94 In their protocol, heterocycles such as thiophene, furan, and pyridine 48a with first and secondary alcohol 47b and also cyclic ethers reacted with satisfactory efficiency (Scheme 42). They developed a simple and effective approach for the straightforward C-2 alkylation of nonbasic nitrogen-free and basic nitrogen-containing heterocycles employing different alcohols and cyclic ether compounds. This metal- and acid-free process, using DTBP, yields the desired products in moderate to high yields.
Scheme 41. DTBP-Promoted Hydroxyalkylation of Heterocycle Compounds with Alcohols and Cyclic Ether Compounds.
Scheme 42. DTBP-Promoted Hydroxyalkylation of Pyridines with Alcohols.
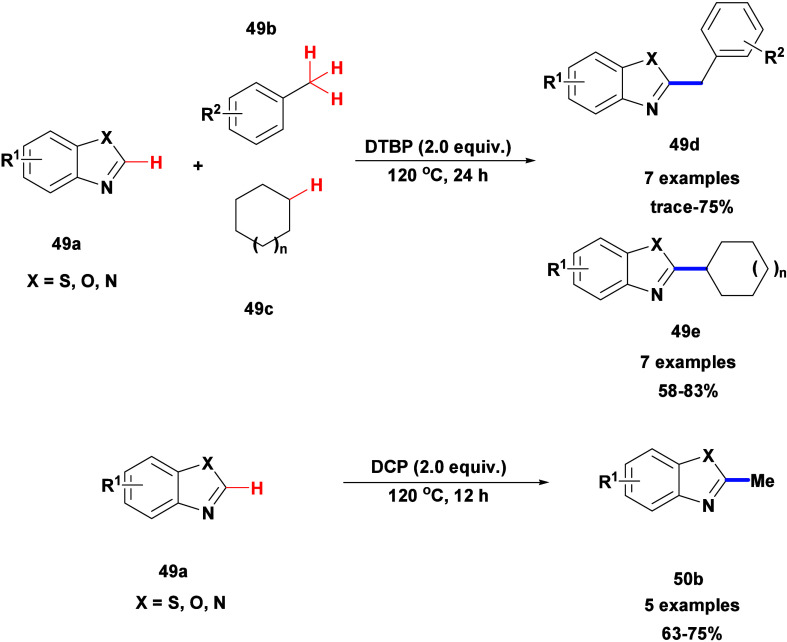
Cai’s research team described C–H alkylation and benzylation of azoles, like benzothiazoles, benzoxazoles, and benzimidazoles 49a, with cycloalkanes 49c or methylarenes 49b using DTBP as the oxidant and also methylation of these heteroarenes with dicumyl peroxide (DCP) as the methylation agent (Scheme 43).95 They developed an efficient, metal-free method for synthesizing 2-substituted azoles via C–H activation. The process involves reacting benzothiazole, benzoxazole, and benzimidazole compounds with dicumyl peroxide, methyl-substituted arenes, and cyclic alkanes using DTBP or DCP at 120 °C, resulting in good yields.
Scheme 43. C–H Alkylation of Azoles Using DTBP and DCP.
After a while, another C–H alkylation was reported by the Lei group. Their new protocol involved cross-dehydrogenative coupling between ketene dithioacetals 51a with simple alkanes, cycloalkanes, or cyclic ethers 51b under metal-free conditions with DTBP serving as the oxidant in acetic acid as the solvent (Scheme 44).96 Kinetic investigation of one of the derivatives showed kH/kD = 3.8, which demonstrated that the C–H bond breaking occurred in the rate-limiting step.
Scheme 44. Metal-Free C–H Alkylation of Ketene Dithioacetals and KIE Experiment.
Zhang and Fan introduced metal-free α-alkylation of ketone derivatives 52a with cycloalkanes 52bvia CDC reaction using DTBP under microwave irradiation for 1 h (Scheme 45).97 Under microwave heating, DTBP-initiated formation of α-ketone carbon 53a and cycloalkyl radical 52b results in the formation of coupling product 53b. However, the homocoupling products were also observed in lower yields.
Scheme 45. α-Alkylation of Ketones and 1,3-Diketones Using CDC Reaction.
Yang’s team introduced an unprecedented cascade three-component decarbonylative alkylation/aminoxidation of styrene 54a with different aliphatic aldehyde compounds 54b and N-hydroxyphthalimide (NHPI) 54c in the absence of metals (Scheme 46).98 The process advanced via the conversion of α-monosubstituted and α-disubstituted aliphatic aldehyde compounds into secondary and tertiary alkyl radicals for the sequential formation of C(sp3)–C(sp3) and C(sp3)–O bonds.
Scheme 46. Oxidative Decarbonylative Alkylation–Aminoxidation.
Straightforward functionalization of the carbon–hydrogen bond adjacent to the nitrogen atom in an amide has garnered notable attention in recent times.99 Yu’s research team introduced a method for selective direct benzylation of N-acyl-2-aminoacetophenones 55a with toluene derivatives 55b employing DTBP as the sole oxidant (Scheme 47).100 Different toluene derivatives with electron-donating and -withdrawing groups were effectively tolerated in this oxidative system. Amides with a methyl group on different positions of the benzoyl group did not show any steric hindrance with the benzylating agents.
Scheme 47. Selective Direct Benzylation of N-Acyl-2-aminoacetophenone Compounds with Toluene-Based Compounds.
The Liu group created a metal-free regioselective C2–H amidation of 8-amidoquinolines 56a to produce a series of C2-amidated 8-amidoquinoline derivatives 56c (Scheme 48).101 In their work, isocyanides 56b were employed as the amide source. The 18O labeling experiment proved that H2O served as an oxygen atom source for amide. This method offers a simple and straightforward method to C2-amidated 8-amidoquinoline compounds through selective metal-free C2–H amidation of 8-amidoquinoline compounds, employing isocyanides as the amide provider. This one-pot oxidation protocol yields a range of 8-quinoline-2-carboxamide compounds in fair to good yields, highlighting its practical utility.
Scheme 48. selective metal-free C2–H amidation of 8-amidoquinoline compounds.
Upon heating, DTBP can generate an acyl radical from aliphatic aldehydes 57b. Subsequently, through the removal of CO, the resulting alkyl radical attacks the C-2 position of chromones 57a, leading to the formation of alkylated products 57c (Scheme 49).102 In summary, this method represents an oxidative decarbonylation and alkylation of chromone compounds using aliphatic aldehyde compounds as alkylation agents through radical-based conjugate addition without metals. Tertiary, secondary, and primary alkyl aldehyde compounds have been shown to be effective alkyl radical donors, producing the respective 2-alkylated chromanones in fair to excellent yields. Additionally, preparation of the racemate of the natural product flindersiachromanone was effectively accomplished under the standard conditions. This approach serves as an efficient tool for the alkylation and late-stage modification of chromone-related bioactive compounds.
Scheme 49. DTBP-Promoted Alkylation of Chromone Compounds Employing Aliphatic Aldehyde Compounds.
At the same time, another approach was developed for C–H alkylation employing aliphatic aldehyde 58a as the alkylation agents. This alkylation was performed on the electron-poor alkene compounds 58b utilizing DTBP as an oxidant and radical starter (Scheme 50).103 The conversion of easily accessible α-unsubstituted, α-monosubstituted, and α-disubstituted aliphatic aldehydes 58a into primary, secondary, and tertiary alkyl radicals for the radical conjugate addition was observed in the transformation.
Scheme 50. DTBP-Mediated Alkylation of Electron-Deficient Alkenes Using Aliphatic Aldehydes.
The Cui team described a CDC reaction between the α-carbon of alcohols and C–H olefin of para-quinone methides using DTBP as a radical starter (Scheme 51).104 In the presence of DTBP, the alcohols were converted to α-oxy radicals, which then attached to para-quinone methide compounds to form phenol-containing alcohols and dihydroisocoumarins. Synthesis of two dihydroisocoumarin compounds 60c in optimized reaction conditions was also successful. This metal-free α-alkylation of alcohols with para-quinone methide compounds to obtain phenol-containing alcohols and dihydroisocoumarin compounds represents a straightforward C(sp3)–C(sp3) bond formation and extends the scope of radical addition reactions involving para-quinone methides.
Scheme 51. DTBP-Mediated α-Alkylation of Alcohols with Paraquinone Methide Compounds.
Various studies have investigated the coupling reactions of imidazo[1,2-a]pyridines 61a having a substituent on the C-3 position of the heterocycle ring. In 2019, Lin and Yan et al. introduced a new approach to C-5 regioselective hydroxyalkylation of imidazo[1,2-a]pyridines 61a with simple alcohols 61b using DTBP (Scheme 52).105 The procedure had good compatibility with electron-attracting and -releasing groups on the aromatic ring of the 2-phenylimidazo[1,2-a]pyridines.
Scheme 52. C-5 Hydroxylkylation of Imidazopyridines with Alcohols.
In 2020, Guo et al. described a metal-free oxidative cross-coupling of imidazopyridines 62a or 1-naphthylamines 63a with cycloalkanes 62b or cyclic ethers 63b using peroxides (Scheme 53).106 In their protocol, 3-alkyl-imidazopyridine 62c and 4-alkyl-1-naphthylamine derivatives 63c were isolated in moderate to good yields. This innovative, catalyst- and additive-free approach enables the synthesis of derivatives of 3-alkyl-imidazopyridine and 4-alkyl-1-naphthylamine under gentle conditions. The process demonstrates excellent functional group compatibility, offering an efficient method for synthesizing imidazopyridine and 1-naphthylamine compounds with significant usefulness in medicinal compounds and functional materials.
Scheme 53. Oxidative Cross-Coupling of Imidazopyridines and 1-Naphthylamines with Cycloalkanes and Cyclic Ethers.
Han and et al. described an additional example of an oxidative coupling process between biologically active quinolinone compounds 64a with ethers 64b or cycloalkanes 65b in the presence of peroxides (Scheme 54).107 3-Alkylquinolinones 64c/65c were prepared in this transformation. They developed an innovative DTBP-assisted radical CDC reaction of quinoline derivatives with ether compounds, enabling the preparation of various 3-alkylquinolinones under gentle conditions, without requiring transition metals. The approach demonstrates a diverse substrate range and great effectiveness, offering an appealing pathway for synthesizing quinolinone structures with significant utility in drugs and functional materials. Overall, the direct CDC method offers step-saving, atom-economical, and eco-friendly benefits.
Scheme 54. Oxidative Cross-Coupling of Imidazopyridines and 1-Naphthylamines with Cycloalkanes and Cyclic Ethers.
The Yao and Lin group created a method for the metal-free carbon removal and alkylation of quinoxaline-2(1H)-one compounds 66a with aliphatic aldehyde compounds 66b, utilizing di-tert-butyl peroxide (DTBP) as a promoter (Scheme 55).108 Other peroxides, including TBHP, tert-butyl hydroperoxide; H2O2, hydrogen peroxide; DCP, dicumyl peroxide; and CHP, cumene hydroperoxide, were less effective in the coupling process. The use of metal catalysts, such as Ni(CH3COO)2·4H2O, Mn(OAc)3·2H2O, Mn(OAc)2·4H2O, and Mn(OAc)2 resulted in lower yields. Compared with previous methods, this system demonstrates excellent compatibility with quinoxaline-2(1H)-one compounds, irrespective of whether the substituents on the nitrogen-containing ring and benzene ring are electron-accepting or electron-releasing groups. This procedure is expected to serve as a valuable approach for quinoxaline-2(1H)-one derivatization.
Scheme 55. DTBP-Promoted Direct Alkylation of Quinoxaline-2(1H)-ones.
Kianmehr and his team described C5-cyanoalkylation of 8-aminoquinolineamides/sulfonamides 67a with alkyl nitriles 67b using DTBP exclusively (Scheme 56).109 Various aryl, heteroaryl, and alkyl amides and sulfonamides as well as simple alkyl nitriles, especially acetonitrile as the simplest cyanomethylated reagent, were tolerated well in this kind of cross-coupling reaction. Transformation also resulted in the expected products with good efficiency in the presence of other H-active reagents such as acetone and nitromethane. Kinetic isotope effect experiments conducted on solvent revealed that the C(sp3)–H bond breaking of CH3CN was the slowest step. In the next step, the conversion of cyanoalkylated aminoquinolineamides 67c into other products was investigated. The aroyl moiety was easily removed through hydrolysis to produce 2-(8-aminoquinolin-5-yl) acetonitrile 68b (63% yield). Further basic hydrolysis converted the cyano group to a carboxylic acid group 69a (38% yield). Also, the 1,2,3,4-tetrahydroquinoline 70a scaffold was prepared from the reduction reaction of the pyridine ring in the presence of 10% Pd/C under H2 gas (89% yield) (Scheme 57).
Scheme 56. DTBP-Promoted C-5 Cyanoalkylation of 8-Aminoquinolineamides/Sulfonamides with Alkyl Nitriles.
Scheme 57. Conversion of Cyanoalkylated Aminoquinolineamides into Other Products.
Ouchi and his team showed a sunlight-induced, DTBP-mediated C–C bond formation between alcohols, ethers, or acetals (71b, 72a, 73a) and olefins 71a (Scheme 58).110 The reaction was efficiently completed within 3–4 h of sunlight irradiation at room temperature. The study also explored the addition of cyclic ethers or cyclic acetals to olefins under sunlight photolysis. The authors noted that yields of the products (71c, 71d, 72b, 73b) exceeded those achieved using a Xe lamp, which could be attributed to the intensity of sunlight. The reactions proceeded more rapidly and with comparable or better yields than those of many previously reported methods using sunlight and conventional lamp photolysis. Furthermore, gram-quantity experiments were conducted to test the practicality of this reaction in organic synthesis, demonstrating the method’s efficiency and scalability.
Scheme 58. Sunlight-Induced C–C Bond Formation between Alcohols, Ethers, Acetals, and Olefins.
In the difunctionalization of α-aryl α-alkynyl allylic alcohol compounds 74a with alkyl nitrile compounds 74b mediated by DTBP as the sole oxidant, two key transformations, including C(sp3)–H bond breaking of alkyl nitrile compounds and radical 3-exo-dig cyclization followed by 1,2-alkynyl radical migration occurred. These transformations allowed for the synthesis of numerous α-alkynyl γ-cyano functionalized ketones 74c (Scheme 59).111 When peroxides like TBHP, TBPB, DCP, and BPO were utilized instead of DTBP, the respective products were achieved in reduced yields
Scheme 59. Cyanoalkylation/Alkynylation of Allylic Alcohol.
Sun and his team described an approach for a cascade Wittig/hydroalkylation procedure using aldehydes 75a, ylides 75b, and ethyl 2-mercaptoesters 75c (Scheme 60).112 The saturated C3-homologation products 75d were obtained in the presence of DTBP as the alkoxyl radical mediator for hydrogen atom transfer (HAT) under visible-light irradiation. This innovative cascade Wittig/hydroalkylation process is initiated by visible light exposure, representing an eco-friendly and metal-free radical method that operates under gentle conditions and is suitable for different functional groups. This method provides direct access to saturated C3 homologation products from aldehydes or ketones via radical hydroalkylation to olefins.
Scheme 60. Visible-Light-Induced Nonmetal Sequential Wittig/Hydroalkylation Process.
Quinoxalins make up a significant group of N-heterocycles that act as c-met kinase inhibitors, histamine-4 receptor antagonists, angiotensin-II receptor antagonists, antitumors, and antidiabetic agents. Roy et al. developed transition nonmetal functionalization of quinoxalin-2(1H)-one derivatives 76a with cycloalkanes, cyclic ethers, or alkyl arenes 76b/77b as coupling partners (Scheme 61).113 Many substrates like quinolines, isoquinolines, quinazoline, benzothiazole, and phenylimidazo[1,2-a]pyridine 76c afforded coupling products in good yield. This protocol shows excellent compatibility and selectivity for various functional groups and allows selective functionalization of strong C–H bonds in adamantane. Its practical applications include accessing bioactive pharmaceuticals.
Scheme 61. Direct Functionalization of Heterocycles with Unactivated Alkanes and Direct Functionalization of Quinoxalin-2(1H)-one Compounds with Nonactivated Alkanes.
The Jin group synthesized 3-carbonyl-2-ene-indole compounds 78c from ketonization and olefin formation of indoles 78a by coupling reactions with 1,3-dicarbonyls 78b where DTBP worked as an oxidant and KF as a base (Scheme 62).114 The reaction proceeded through the creation of intermediate indol-3-one B with DTBP and O2, which had a significant role in this transformation. This innovative, metal-free oxidative ketonization/olefination method facilitates the creation of 3-carbonyl-2-ene-indole compounds. The reaction advances effectively without requiring metal catalysts or additives, providing quick access to a range of 3-carbonyl-2-ene-indole derivatives in medium to high yields.
Scheme 62. Oxidative Ketonization/Olefination of Indoles by Cross-Coupling Reactions with 1,3-Dicarbonyl Compounds.
Xiang et al. described oxidative alkene alkylation and alkynylation of 1,4-enyn-3-ols 79a with tertiary, secondary, and primary alkylaldehydes 79b through decarbonylation and 1,2-alkynyl migration using DTBP as an oxidant (Scheme 63).115 The reaction operated successfully by utilizing other peroxides such as TBPB and DCP, while H2O2 resulted in trace products. Various α-alkynyl ketones 79c were prepared through the construction of C(sp3)–C(sp3) and C(sp3)–C(sp) bonds via a radical pathway. This method is compatible with tertiary, secondary, and primary alkylaldehydes, demonstrating significant functional group tolerance and a wide range of substrates. It is particularly effective for synthesizing quaternary carbon-based but-3-yn-1-ones.
Scheme 63. Oxidative Alkylation/Alkynylation of Terminal Alkenes through Aliphatic Aldehyde Decarbonylation and 1,2-Alkynyl Shift.
2.2.1. DTBP for Selective Functionalization and Coupling Reactions in Organic Synthesis
This section of the study identifies suitable reagents for various types of reactions, including C3-selective trifluoromethylation of indoles, C–H alkylation by oxidative coupling, and the preparation of 3-carbonyl-2-ene-indole derivatives. The C3-selective trifluoromethylation of indole derivatives in the presence of 2,2,2-trifluoroethyl(mesityl)iodonium triflate in the presence of 2,6-di-tert-butylpyridine (DTBPy) as a specific reagent demonstrates exceptional efficiency. The high achieved yields and selectivity make this reagent a highly suitable option for the selective trifluoromethylation of indole-based compounds, establishing it as a valuable tool in organic synthesis.84 Selective trifluoromethylation could also be evaluated by similar substrates and heterocycles having similar reactivity profiles like indole, using DTBPy. This reaction can be performed under similar conditions to achieve high selectivity and yield.116 C–H alkylation of heteroaromatic compounds and cross-dehydrogenative coupling (CDC) reactions could also be examined on heteroaromatic compounds such as pyridines and pyrazoles using DTBPy as a catalyst. This method allows for the direct functionalization of C–H bonds, providing a straightforward approach to synthesizing alkylated heteroaromatic derivatives. These reactions highlight the versatility of DTBPy in various organic synthesis applications, making it a valuable tool for chemists working with indole-based and other heteroaromatic compounds.117 Additionally, the dehydrogenative cross-coupling of ketene dithioacetals with simple alkanes, cycloalkanes, or cyclic ethers is achieved effectively under metal-free conditions using diethyl peroxide (DTBP) as an oxidant in acetic acid. Acetic acid plays a crucial role by stabilizing reaction intermediates and creating a conducive environment, leading to a high efficiency and selectivity. The type of the employed solvent and oxidant has a great role in DTBP-assisted carbon–carbon bond formation.96 It could also be applied for other heterocyclic compounds with similar reactivity profiles. For instance, DTBP has been used for the cross-dehydrogenative coupling of 3-aryl benzofuran-2(3H)-ones and toluenes/phenols, generating all-carbon quaternary centers.118 Furthermore, for the synthesis of 3-carbonyl-2-ene-indole derivatives and other structurally related compounds, DTBP can be used as an oxidant in combination with KF as a base.114 This approach is especially effective for substrates with activated methylene groups and 1,3-dicarbonyl compounds. The metal-free oxidative ketonization/olefination method could also be extended to other indole derivatives or heterocyclic compounds with similar reactivity profiles, showcasing its versatility and effectiveness in organic synthesis119−121 (Figure 2).
Figure 2.

Suitable reagents for various types of coupling reactions with DTBP.
2.3. Coupling Reactions with TBPB
tert-Butyl peroxybenzoate (TBPB) is a light yellow liquid ester peroxide with a half-life of 10 h at 104 °C, 1 h at 124 °C, and 1 min at 165 °C.122 TBPB, due to the tert-butyl and benzoyl groups, has good solubility in organic solvents. Therefore, it has been used in various coupling reactions with transition metals or in the absence of metals as an efficient radical initiator.123 In 2011, Wang and co-workers reported a metal-free and without base method for C2-amidation of azole 80a derivatives in the use of TBPB as the sole oxidant in the reaction (Scheme 64).124 Screening of other organic oxidants such as (t-C4H9O)2, (C6H5COO)2, TBHP, and DCP showed that none of them yielded better results. When inorganic oxidants K2S2O8 and (NH4)2S2O8 were utilized, product 80c was achieved in a lower yield. Moreover, no product was isolated in the presence of cyclohexanone peroxide, I2, PhI(OAc)2, Ag2CO3, and Ag2O. The authors also investigated the reaction under transition metal conditions (FeCl3: 57% yield, FeCl2: 26% yield, Fe(OAc)2: 65% yield, Cu(OAc)2: 23% yield, CuBr2: 14% yield, CoCl2: 32% yield, Pd(OAc)2: 0% yield). The reaction exhibited a lower efficiency under such conditions. The findings showed that metals did not enhance the reaction speed but hindered it. The target product was achieved with a 75% yield when TBPB was used under metal-free conditions.
Scheme 64. Direct Amidation of Azoles Facilitated by TBPB in the Presence of Formamides.
In 2012, Wang et al. documented the C3-formylation of both N–H and N-substituted indoles 81a with N-methylaniline 81b. This reaction was catalyzed by nBu4NI and employed TBPB as the oxidant (Scheme 65).125 Notably, exploration of various amines, including Et3N, DMF, Et2NMe, Me2NH, Cy2NMe, Ph2NMe, and BnNHPh, as potential carbonyl sources for the formylation of indoles 81c did not yield any formylation products. However, PhNMe2 and BnNMe2 produced the corresponding formylation products with yields of 39% and 5%, respectively. This approach is especially beneficial because it can be used for both N–H and N-substituted indoles without requiring harmful phosphorus oxychloride or transition metals.
Scheme 65. nBu4NI-Catalyzed C3-Formylation of Indoles Using N-Methylaniline.
The Ji group introduced an innovative method for the functionalization of C(sp3)–H bonds in both cyclic and open-chain ethers 82b using α,α-diaryl allylic alcohols 82a, facilitated by TBPB, and notably without the use of any transition metals (Scheme 66).126 In the protocol, a variety of α-aryl-β-alkylated carbonyl ketones were produced through cascade oxidative addition and migration, achieving yields ranging from moderate to excellent. With the introduction of a radical scavenger (TEMPO) by the authors, the reaction was entirely halted. This finding indicated that the reaction followed a radical mechanism, with the decomposition of TBPB leading to the formation of tBuO· and benzoate radicals. Then, hydrogen abstraction of 1,4-dioxane with the tBuO· radical resulted in the formation of the alkyl radical B, which attached to allylic alcohol A, providing intermediate D. Subsequently, intramolecular 5-ipso cyclization produced spiro[2,5]octadienyl E. Simultaneously, 1,2-aryl migration formed the radical species F. Finally, the expected product G was isolated by further oxidation and deprotonation.
Scheme 66. TBPB-Facilitated Alkylation of α,α-Diaryl Allylic Alcohols with Basic Ethers, Along with the Proposed Mechanism.
Following a similar strategy, Ji and colleagues outlined a technique for the alkylation of α,α-diaryl allylic alcohols 84a with carbonyl compounds 84b, including ketones, esters, and amides, utilizing TBPB (Scheme 67).127 They synthesized 1,5-diketones 84c from simple ketones, 1,5-diketones and γ-acyloxy ketones from esters, and γ-amido ketones from amide substrates. This method showcases efficient C(sp3)–H functionalization alongside radical addition and 1,2-aryl migration using simple carbonyl derivatives. It demonstrates excellent functional group compatibility and selectivity, producing various diketones and ketones. Future work should be performed to delve into the reaction mechanism.
Scheme 67. Alkylation of α,α-Diaryl Allylic Alcohols with Carbonyl Compounds Facilitated by TBPB.
Nitriles serve as versatile intermediates for the preparation of amides, amidines, esters, primary amines, ketones, aldehydes, and carboxylic acids.128,129 Hence, the synthesis of these compounds via cross-coupling has been highly regarded as being useful by scientists in this field. In 2015, the same group investigated the same conditions in α,α-diaryl allylic alcohols with acetonitrile and simple alkanes (Scheme 68). Echoing their earlier research, this procedure also entailed cascade oxidative addition and migration in α,α-diaryl allylic alcohols, culminating in the production of satisfactory results.130
Scheme 68. TBPB-Mediated Cyanomethylation and Alkylation of α,α-Diaryl Allylic Alcohols.
Li and colleagues outlined a technique for synthesizing 5-oxo-pentanenitriles 87c through the oxidative radical 1,2-alkylarylation. This process involves the reaction between the C–C double bonds of inactive alkenes, such as α-aryl allylic alcohols 87a, and the α-C(sp3)–H bonds of acetonitriles 87b (Scheme 69).131 As outlined by the authors, the proposed mechanism suggests that the decomposition of TBPB leads to the generation of tBuO· and PhCOO· radicals. Subsequently, alkyl radical B was generated via SET and then added to the C–C double bond of substrate C, resulting in the formation of alkyl radical D. At the same time, alkyl radical intermediate D underwent a 1,2-migration of the aryl group through spiro[2,5]octadienyl radical E, leading to the formation of intermediate F. The further oxidation of intermediate F led to the formation of the final product G with the help of TBPB (Scheme 70).
Scheme 69. 1,2-Alkylarylation of Alkenes with α-C(sp3)–H Bonds from Acetonitriles and the Proposed Mechanism.
Scheme 70. Cross-Coupling of α,α-Diarylallylic Alcohols and Aromatic Aldehydes Facilitated by TBPB.
Wu’s group detailed the oxidative acylation of α,α-diarylallylic alcohol compounds 88a with aromatic aldehyde compounds 88b, utilizing TBPB without the use of heavy metals (Scheme 70).132 3.0 equiv of TBPB resulted in a 76% yield of the product, while 4.0 equiv of TBPB resulted in a yield of 94%. Utilization of other oxidants, including TBHP, DTBP, K2S2O8, H2O2, and O2, was partially effective in this reaction. They introduced an innovative acylation technique for α,α-diarylallylic alcohols, which utilizes aryl aldehydes and involves aryl rearrangement. This process results in the formation of new C(Ar)–C(sp3) and C(sp3)–C(CO) bonds, yielding various 1,2,4-triphenylbutane-1,4-diones with moderate to excellent efficiency. The reaction also demonstrated the chemoselective migration of diverse aryl groups.
Peng and Ding conducted an extensive study of the direct C-4 alkylation of quinazoline N-oxides with ethers through an oxidative cross-coupling reaction in the absence of metals. Their research emphasized cross-dehydrogenative coupling between quinazoline-3-oxide derivatives and ethers, utilizing TBPB as the oxidizing agent (Scheme 71).133 The direct alkylation of quinazoline N-oxides specifically targeted the C4-position of the substrates. Utilizing cyclic and open-chain ethers, including 1,4-dioxane, 1,3-dioxolane, 1,3-benzodioxole, 1,2-dimethoxyethane, diethoxymethane, and diethyl ether, resulted in moderate to good yields.
Scheme 71. TBPB-Initiated Cross-Dehydrogenative Coupling of Quinazoline-3-oxides with 1,4-Dioxane.
Yan et al. successfully developed a radical C3-functionalization method for coumarin derivatives using acetonitrile and acetone (91b and 92b) using tert-butyl peroxybenzoate (TBPB) as the oxidant, thereby eliminating the necessity for a metal catalyst (Scheme 72).134 Their study also explored the 3-cyanomethylation of α,β-unsaturated ketones under these conditions. In their experiments, inorganic bases such as Na2CO3 (61%), NaHCO3 (43%), and KF (67%) proved to be more effective than organic bases like NEt3 (23%) or DBU (trace) for synthesizing C3-cyanomethylated coumarin 91c derivatives. They reported an innovative dehydrogenation coupling reaction involving coumarins and acetonitrile through direct C(sp3)–H activation of acetonitrile, resulting in the production of 3-cyanomethyl-coumarins with commendable functional group tolerance and moderate to good yields. When acetonitrile was replaced by acetone, this led to the production of 3-acetomethyl coumarins in moderate yields. This approach offers a highly effective and novel method for synthesizing cyanomethyl- (or acetomethyl)-substituted coumarins. The proposed mechanism involves a radical pathway to explain the oxidative C–H activation of acetonitrile.
Scheme 72. Oxidative Cross-Coupling of Coumarins and α,β-Unsaturated Ketones with Acetonitrile and Acetone.
Zhang’s team devised a method for the direct C(sp3)–H cyanation of hydrocarbons, utilizing cyanobenziodoxolones 93c as both cyanating reagents and oxidants (Scheme 73).135 Tertiary amines 93a or simple alkanes/ethers 93b underwent this transformation, and a series of corresponding nitriles 93d/93e were obtained. The mechanistic investigations revealed that, based on the substrates used, alkanes and ethers followed a free radical pathway, while tertiary amines underwent an oxidative pathway. The mechanism suggested by the authors involved two types of peptides (I and II). A free radical mechanism was proposed for the cyanation of alkanes and ethers, while an oxidative cyanation pathway (pathway II) was suggested for tertiary amines. Both mechanisms initiated with the generation of the tBuO· radical from TBPB. In the radical pathway I, hydrogen atom abstraction from an R–H bond in the substrate by the tBuO· radical led to the formation of carbon radical B. The interaction between C and B resulted in the capture of B by C, producing nitrile 3(E) and an iodine-centered radical D. Subsequently, D underwent hydrogen atom abstraction from the substrate via a metal-free catalytic process. Concurrently, B was regenerated, leading to the formation of 2-iodobenzoic acid J. In tertiary amines, a single-electron transfer (SET) process occurred from nitrogen to the tert-butoxy radical, forming nitrogen-centered cation radical H and a tert-butoxy anion. The α-C(sp3) radical G was generated from H via deprotonation by a base, which then underwent SET oxidation by C to produce iminium cations F and released D alongside a cyanide anion. The nucleophilic addition of a cyanide anion to F formed cyanated product 5(E), while H was regenerated through SET oxidation of substrate 4 with D (Scheme 73).
Scheme 73. Cyanation of Tertiary Amines, Alkanes, and Ethers and the Tentative Mechanism.

Patel described a method for C3-functionalization of 4-arylcoumarins 94a using cycloalkanes 94b as the cycloalkylation reagents, formamides 95a as the amidation sources, and TBPB as the reaction oxidant (Scheme 74).136 They introduced a technique for C-3 cycloalkylation and amidation of 4-arylcoumarins. This method demonstrates excellent tolerance for a variety of substituted 4-arylcoumarins, cycloalkanes, and formamides, resulting in C-3 functionalized products. This streamlined protocol significantly enhances the efficiency of the C–H bond functionalization process, particularly for internal alkenes.
Scheme 74. TBPB-Facilitated C-3 Functionalization of Coumarins.
Zhao and his team developed an elegant method for the β-selective C(sp2)–H acylation of enamides 96a with aldehydes 96b. The reaction utilizes Na2-eosin Y as a photocatalyst and TBPB as the oxidant, under the irradiation of a 60 W blue LED at room temperature (Scheme 75).137 The yields of the products 96c were lower in the absence of irradiation, Na2-eosin Y, or TBPB. After screening some photocatalysts such as fac-Ir(ppy)3 (37%), Ru(bpy)3Cl2 (trace), and eosin Y (37%), various oxidants such as H2O2 (12%), PhI(OAc)2 (13%), K2S2O8 (15%), BPO (34%), and O2 (trace) and also a series of solvents including 1,2-dichloroethane (39%), dioxane (33%), toluene (43%), and acetonitrile (61%), optimal results were achieved using Na2-eosin Y and TBPB in ethyl acetate, yielding 69%. Various enamides bearing different para-, ortho-, or meta-substituents as well as sensitive ester and sulfone substituents with different aliphatic and aromatic aldehydes were investigated under reaction conditions. Some control experiments and KIE studies were conducted by the authors.
Scheme 75. Na2-eosin Y Catalyzed Acylation of Enamides with Aldehydes in the Presence of TBPB.
In a radical-trapping experiment, the use of TEMPO prevented the formation of the product, suggesting that the reaction proceeded through a radical pathway. During intermolecular competition between enamide and enamide-d2, a KH/KD value of 0.54 was observed, indicating that cleavage of the olefinic C–H bond was unlikely to be the rate-determining step. In parallel experiments, when enamide-d2 was used, the kinetic isotope effect (KIE) displayed a KH/KD value of 0.60, while a KH/KD value of 2.0 was obtained when aldehyde-d was used. This suggests that the rate-limiting step in the reaction may involve generation of the acyl radical via cleavage of the C–H bond in aldehydes (Scheme 76).
Scheme 76. Control Experiments and KIE Studies.
Consequently, the authors suggested a mechanism in which Na2-eosin Y transitions into its excited state, Na2-eosin Y*, upon absorbing a photon. This is followed by a single-electron transfer (SET) between the excited Na2-eosin Y* and TBPB, generating a radical cation Na2-eosin Y+ and a tBuO· radical. Afterward, the tBuO· radical interacted with aldehyde D, forming an acyl radical species, which was then intercepted by enamide E to create the radical intermediate A. Subsequently, the single-electron oxidation of A by Na2-eosin Y+ produced carbon cationic species B, which could be transformed into iminium ion C. Finally, the desired product F was obtained through the deprotonation of intermediate C or D. The analysis of iminium ion D to clarify stereospecificity in the reaction demonstrated that conformer 1 was sterically more favorable compared to conformer 2 due to reduced allylic strain between the newly introduced acyl group and the bulky group on the nitrogen atom, leading to the preferential formation of the E-acylated enamides following deprotonation (Scheme 77).
Scheme 77. Plausible Mechanism for Acylation of Enamides with Aldehydes.
Chaudhary and colleagues introduced a method for regioselective straightforward C-3 acylation and benzoylation of substituted 2H-indazoles 97a using a diverse variety of aldehydes 97c, benzyl alcohols 97b, and styrenes 97d to generate substituted 3-(acyl/benzoyl)-2H-indazoles 97e (Scheme 78).123 Aryl and heteroaryl 2H-indazoles 98a reacted well with 4-methylbenzaldehyde 98b, producing the corresponding products 98c with moderate to good yields, while 2-cyclohexyl-2H-indazole and 1H-indazole were unreactive under this reaction system.
Scheme 78. C-3 Acylation and Benzoylation of 2H-Indazoles Using TBPB.
The gram-scale synthesis and introduction of a simple metal-free method for the synthesis of anti-inflammatory agent 98d were other advantages of this work (Scheme 79).
Scheme 79. Synthesis of Anti-inflammatory Agent 98d.

2.3.1. Versatility and Efficiency of TBPB in Organic Synthesis
This section of the study categorizes suitable reagents for various types of reactions, highlighting the versatility and effectiveness of TBPB in facilitating diverse coupling reactions. TBPB could be employed as an oxidant in the C3 radical functionalization of coumarin derivatives with acetonitrile and acetone under metal-free conditions. Inorganic bases such as Na2CO3, NaHCO3, and KF exhibited higher efficiency compared to organic bases like NEt3 and DBU. To broaden the applicability of this reaction, it is promising to explore decarboxylative coupling reactions with aliphatic carboxylic acids to synthesize 3-alkyl coumarins under metal-free conditions.134 The reaction proceeds via the decarboxylation of aliphatic carboxylic acids, forming a carbon–carbon bond.138−140 Additionally, investigating the 3-cyanomethylation of other α,β-unsaturated ketones or similar substrates can further broaden the applicability of this method.13,141 Furthermore, similar metal-free conditions could be applied to functionalize other heterocyclic compounds, to create diverse bioactive molecules.142 These proposals leverage the efficiency of TBPB and metal-free conditions to develop innovative synthetic methodologies for a wide range of substrates. For β-selective C(sp2)–H acylation of enamides, Na2-eosin Y could serve as a photocatalyst and TBPB as an oxidant. Future proposals for C(sp2)–H acylation include exploring the acylation of different enamides with aldehydes or various ketones to broaden the reaction scope.137 Additionally, radical addition reactions involving other alkenes and benzaldehydes can be investigated for the synthesis of new carbonyl-containing compounds.123 Moreover, C(sp2)–H functionalization of other substrates, such as aromatic compounds, could be explored to create new derivatives with diverse functional groups143,144 (Figure 3).
Figure 3.

Suitable reagents for various types of coupling reactions with TBPB.
2.4. Coupling Reactions with BPO
Dibenzoyl peroxide (BPO) is a white granular solid peroxide with a half-life of 1 h at 91 °C and 10 h at 72 °C, which is mainly used in polymerization and also the medical industry.145 In 2014, Cheng and co-workers described phenanthridinylation of simple alkanes 99b with 2-aryl phenyl isonitrile 99a using BPO as the sole oxidant in a radical aromatic cyclization reaction (Scheme 80).146 Notably, the utilization of FeCl2, CuCl2, and CuI in the reaction did not improve the efficiency. Both alkanes and cycloalkanes, especially cyclohexane, were tolerated well in the transformation. Mechanistic investigations (KH/KD = 6.1–8.1) indicated that the rate-determining step involved the cleavage of the C(sp3)–H bond in alkanes.
Scheme 80. BPO-Promoted Phenanthridinylation of Simple Alkanes with 2-Aryl Phenyl Isonitrile.
Zhao and his team introduced a procedure for intramolecular aryl-aldehyde 100a C(sp2)–C(sp2) bond formation through CDC, mediated by PhI(OAc)2 to generate acridones 100b (Scheme 81).147 In previous studies, the construction of acridones was performed by using metals, whereas iodine(III) was used as a reagent in this process. A plausible radical mechanism for this transformation begins with the decomposition of BPO upon heating, producing benzoyloxy radical A. This radical then reacts with iodobenzene diacetate B, forming radical C. Radical C abstracts a hydrogen atom from the CHO group of aldehyde F, leading to the generation of the acyl radical D. Subsequently, the splitting of a π-bond results in the formation of cyclohexadienyl radical E. The second hydrogen atom abstraction by radical B from the C(sp3) position restores aromaticity, thereby forming desired product G. Alternatively, single-electron oxidation of radical E by either B or radical C produces cation F, which is then deprotonated by the acetate anion to yield final product G (Scheme 81).
Scheme 81. Proposed Mechanism and Intramolecular Oxidative Aryl-aldehyde C(sp2)–C(sp2) Bond Formation Facilitated by PhI(OAc)2.
Carbocycle frameworks such as indinones are widely abundant in natural compounds.148−151 The construction of an indenone skeleton is conventionally achieved in the presence of transition metals such as Pd, Co, Rh, and Mn or MeOTf.152−160 Pan and Yu reported a protocol for preparation of 2-alkyl-3-aryl indenones 101c through a radically oxidative carboannulation of ynones 101a with simple alkanes 101b smoothly promoted by benzoyl peroxide (BPO) under metal- and solvent-free conditions (Scheme 82).161 This BPO-promoted method tolerates various functional groups, producing indenone 101c efficiently.
Scheme 82. BPO-Mediated Construction of 3-Aryl-2-cycloalkyl Indenones.
Isoquinolines are important building blocks of natural products, serving as excellent scaffolds in drug discovery.162−165 Traditionally, isoquinoline scaffolds are synthesized via Pomeranz–Fritsch, Bischler–Napieralski, and Pictet–Spengler reactions.166−169 Due to the harsh conditions often associated with such reactions, the functionalization of the C–H bond to form the C–C bond through oxidative cross-coupling has gained much interest in recent years. In 2018, Shao and his team reported the synthesis of 1-alkylisoquinolines 102c via a radical cyclization of vinyl isocyanides 102a with simple alkanes 102b (Scheme 83).170 Different aliphatic alkanes, cycloalkanes, toluene, 2-ethylpyridine, 4-ethylpyridine, 3-ethylpyridine, and phenylethane exhibited moderate to good yields in this cyclization reaction.
Scheme 83. BPO-Promoted Preparation of 1-Alkylisoquinolines.
In 2019, Sun and Liu devised a method for synthesizing a range of 6-alkyl 6H-benzo[c]chromene derivatives 103c. This was achieved through a cascade reaction involving biaryl vinyl ethers 103a and inactivated alkanes 103b, mediated by BPO in a transition-metal-free environment (Scheme 84).171 Benzoyloxy radicals abstract an H atom from alkanes, which results in the formation of alkane radicals. The addition of these alkane radicals to the C=C double bond of biaryl vinyl ethers leads to the cyclization products. This method efficiently accesses 6-alkyl-6H-benzo[c]chromenes, avoiding costly catalysts and bases. It also enables the synthesis of 6-alkyl-6H-benzo[c]thiochromenes and holds promise for medicinal chemists in constructing oxygen- or sulfur-containing heterocyclic compounds.
Scheme 84. Direct Oxidative C(sp3)–H Functionalization of Unactivated Alkanes Facilitated by BPO.
Klussmann and Liu devised a technique for the difunctionalization of styrenes 104a through the sequential addition of a radical and a nucleophile (Scheme 85).172 The combination of BPO with HPF6 as a strong Brønsted acid facilitated the radical initiation to transfer both the radical and nucleophile to thioxanthene 105b and xanthene 104c. The study demonstrated that BPO remained unchanged, even in the acidic environment created by HPF6. However, when thioxanthene was present, benzoic acid was formed, highlighting thioxanthene’s significant role in peroxide decomposition and HPF6’s influence on the reduction potential of BPO. In the reaction, nitriles, alcohols, and thiophenols 106b also served as nucleophiles. The product was not formed without BPO or in the presence of other peroxides. In the presence of N,N-dimethylaniline derivatives 107a, two molecules of CH3CN were added to styrene, creating the target product 107b.
Scheme 85. Difunctionalization of Styrenes with Stabilized Radicals and (N,O)-Nucleophiles Facilitated by HPF6/BPO.
Sun and colleagues presented a metal-free approach for synthesizing structurally diverse benzimidazo[2,1-a]isoquinolin-6(5H)-ones 108c. This was achieved through a radical carbocyclization reaction involving 2-arylbenzoimidazoles 108a and cyclic ethers 108b, with BPO acting as the sole oxidant in the process (Scheme 86).173 Peroxides, including TBPB (75%), TBHP (58%), DTBP (33%), and DCP (41%), resulted in the formation of the desired products in lower yields compared with BPO (76%). During the process, the only byproducts of the reaction were nontoxic benzoic acids.
Scheme 86. Metal-Free Preparation of Benzimidazo[2,1-a]isoquinolin-6(5H)-ones.
There are different methods for functionalization of terminal alkynes such as radical vicinal difunctionalization,174−176 radical–addition–translocation–cyclization,177−184 nonradical geminal difunctionalization,185−188 nonradical reductive geminal difunctionalization,189−192 and 1,1,2-trifunctionalization.193 Studer and his team unveiled a radical addition–translocation–cyclization–trapping process initiated by BPO/AIBN, utilizing readily accessible alkynyl triflones as trifluoromethyl radical precursors and trapping agents (Scheme 87).194 A series of (1-trifluoromethyl)propargyl cyclopentane diastereoisomers 109c was successfully synthesized through the addition of the trifluoromethyl 109b radical to a terminal alkyne 109a, followed by 1,5-hydrogen atom transfer and 5-exocyclization. DLP, TBHP, DTBP, TBPB, and ACBN also served as initiators in the reaction. Among them, DLP showed better efficiency, while the others provided lower yields. A mixture of two diastereoisomers was isolated in the transformation. The authors suggested a radical mechanism involving the formation of a ·CF3 radical by thermal decomposition of BPO or AIBN and its reaction with triflone via an addition, β-elimination, SO2-fragmentation sequence. Then, the chemoselective addition of the trifluoromethyl radical at the terminal position of the alkyne resulted in the formation of the vinyl radical B. Radical B then underwent 1,5-HAT to form radical C. Subsequently, 5-exocyclization resulted in radical D, which was trapped by the triflone reagent F to produce vinyl radical G. β-Fragmentation furnished target product H along with further fragmentation of the trifluoromethylsulfonyl radical to produce SO2 and the CF3 radical (Scheme 87).
Scheme 87. BPO/AIBN-Promoted 1,1,2-Trifunctionalization of Terminal Alkynes and the Suggested Mechanism Reported.
2.4.1. Versatility and Effectiveness of BPO in Coupling Reactions
The studies highlight the versatility and effectiveness of BPO in facilitating diverse coupling reactions. In the difunctionalization of styrenes, benzoyl peroxide (BPO) and hexafluorophosphoric acid (HPF6) play crucial roles. BPO, as an oxidant, generates benzoyloxyl radicals that abstract hydrogen atoms from thioxanthene, forming new radicals that add to styrenes to produce benzylic cations. These cations are then oxidized to yield the final products. HPF6, acting as a strong Brønsted acid, facilitates the radical initiation by protonating BPO, enhancing its reduction potential and promoting overall reaction efficiency.172 Given the significant role of thioxanthene in peroxide decomposition, other derivatives of thioxanthene and similar substrates could also be explored for similar reactions. Additionally, nitriles, alcohols, thiophenols, and other nucleophiles used in the reaction can be tested with different substrates to expand the reaction scope. BPO and HPF6 could be employed to develop innovative synthetic methodologies for a wide range of substrates, potentially leading to the synthesis of new compounds with diverse functional groups.195,196 In the functionalization of terminal alkynes, BPO and AIBN act as initiators, generating radicals. These radicals undergo a series of reactions, including addition, displacement, cyclization, and trapping, to form final products. The crucial steps include generating a trifluoromethyl radical, which reacts with the terminal alkyne. This is followed by hydrogen atom transfer and cyclization, resulting in the formation of the target cyclopentane derivatives.194 Suggestions for similar substrates include using alkynes with electron-withdrawing groups such as cyano, nitro, or ester groups, which can undergo comparable radical reactions. Additionally, alkenes with halogen substituents such as bromine or iodine are suitable for similar radical addition processes. Furthermore, unsaturated carbonyl compounds such as halogen-containing malonic acid/esters can be employed to generate radicals under visible light irradiation197−199 (Figure 4).
Figure 4.

Suitable reagents for coupling reactions with BPO.
2.5. Coupling Reactions with DLP
Lauroyl peroxide or dilauroyl peroxide (DLP) is a white solid diacylperoxide with a fast half-life of 1 h at 79 °C and 10 h at 61 °C. DLP is a readily biodegradable peroxide commonly used for polymerization processes. However, there are some reports of the use of this peroxide as a radical initiator in cross-coupling reactions. Construction of the coumarin framework has attracted significant attention because of its valuable pharmacological activities.200−204 Pan and Yu described a radical addition/cyclization method to access 3-(β-carbonyl)coumarins 110c via a DLP-promoted cascade reaction using aryl alkynoates 110a and xanthates 110b (Scheme 88).205 Yield of the products was low in the presence of other organic oxidants such as BPO, DTBP, and TBHP. The utilization of K2S2O8, PhI(OAc)2, and H2O2 was not effective in this reaction.
Scheme 88. DLP-Promoted Radical Addition/Cyclization of Alkynoates with Xanthates.
Miranda and colleagues devised a method employing xanthate-based radical chemistry for the regioselective direct alkylation of caffeine, uracil, and imidazo[1,2-a]pyridine systems. This technique utilizes dilauroyl peroxide (DLP) as both the initiator and the oxidant under microwave irradiation. It enables the formation of Csp2–Csp3 bonds via C–H functionalization, starting from readily available materials. Electrophilic radicals (positioned alpha to a carbonyl function, like ketones, amides, esters, malonates, and cyano groups) can be regioselectively added to imidazo[1,2-a]pyridines, caffeine, and 1,3-dimethyluracil in the presence of DLP as a radical initiator under microwave irradiation (Scheme 89).206 Cross-coupling reaction results in low products under convectional heating; however, the yield increases under microwave heating.
Scheme 89. Oxidative Radical Alkylation of Imidazo[1,2-a]pyridines, Caffeine, and 1,3-Dimethyluracil.
In general, due to the widespread presence of heteroarenes in many biologically active molecules and functional organic compounds, the synthesis and functionalization of these structures have garnered significant importance.207 In 2018, Zard introduced a strategy for the synthesis of methylated and alkylated heteroarenes 114c using an excess amount of xanthates 114b in the presence of stoichiometric DLP, added to the reaction in an amount of 20 mol % per 1 h (Scheme 90).208 Then, decarboxylation of the carboxymethyl adduct was carried out using MW irradiation at 180 °C for 10 min, resulting in the formation of alkylated product 114d. In this transformation, prevalent heteroarenes in the pharmaceutical field, such as caffeine, pyrazine, quinoxaline, purine, and also imidazopyridine, imidazopyrimidine, and imidazopyridazine exhibited a satisfactory reactivity.
Scheme 90. DLP-Mediated Methylation and Related Alkylations of Heteroarenes and Decarboxylation by MW Irradiation.
Sun and colleagues detailed a radical cascade addition/cyclization reaction between N-arylacrylamides 115a and trialkylsilanes 115b using DLP under metal-free conditions (Scheme 91).209 Mechanistic studies indicated that the reaction commenced with the addition of a silyl radical to the electron-deficient C=C bond in N-arylacrylamides, followed by intramolecular cyclization involving the CN group. This sequence culminated in the formation of the desired product via hydrogen abstraction. They devised a rapid cascade cyclization method to synthesize silyl-functionalized pyrido[4,3,2-gh]phenanthridin derivatives, using easily accessible hydrosilanes as sources of silyl radicals, all under metal-free conditions. This reaction is both efficient and atom-economical, exhibiting good tolerance for functional groups, thus proving valuable for organic synthesis and pharmaceutical research.
Scheme 91. DLP-Mediated Synthesis of Silyl-Functionalized Pyrido[4,3,2-gh]phenanthridin-5(6H)-one Derivatives.
Zard and Chen reported a surprising vinylation reaction in which the radical addition of β-phthalimido-α-xanthyl propionic acid, whether as the free carboxylic acid or its ethyl ester, resulted in the formation of various protected β2-amino acids and β-heteroarylethylamines (Scheme 92).210 The reaction was effectively performed in stoichiometric quantities of peroxide. Key practical advantages of this method include the low cost and ready availability of the reagents, the mildness of the experimental conditions, and the compatibility with various functionalities, particularly polar groups.
Scheme 92. Flexible Route to β2-Amino Acids and β-Heteroarylethylamines in the Presence of DLP.
DLP-promoted regioselective β-C(sp2)–H alkylation of cyclic and acyclic enamides 116a by xanthates 116b leads to the formation of γ-amino-β,γ-unsaturated acyl scaffold 116c/116d (Scheme 93).211 MeOH, EtOH, and PhOH are used as the related nucleophiles. The process involves a radical and polar reaction. This innovative regioselective reaction offers a wide substrate scope and exceptional functional group tolerance, demonstrating considerable potential for synthesizing a diverse range of N-containing building blocks. The extensive availability of xanthates further enhances the reaction’s versatility, effectively merging a radical process with a polar reaction.
Scheme 93. DLP-Promoted β-C(sp2)–H Alkylation of Enamides and DLP-Promoted Difunctionalization of Enamides.
2.6. Coupling Reactions with DCP
Dicumyl peroxide (DCP) is a white solid organic peroxide with a half-life of 1 h at 135 °C and 10 h at 114 °C. It recently showed good potential as a radical initiator and also a methylating reagent in cross-coupling reactions.212 Zhu and colleagues outlined a protocol for a cascade reaction involving N-phenyl-N-(phenyl sulfonyl)-methacrylamides 119a, which includes C(sp3)–H activation, SO2 elimination, and C–C bond formation. This process is conducted with simple alkanes 119b using DCP as the only oxidant (Scheme 94).213 In this metal-free process, amides with an electron-donating group on the aromatic ring afforded high yields of products 119c. Moreover, N-phenyl-N-(phenylsulfonyl)-methacrylamide provided a good yield. When an aromatic ring with an electron-withdrawing group was employed, the yields of the products were moderate. Additionally, no expected product was observed when using amides with groups such as NO2 or Br on the N-phenyl (SO2).
Scheme 94. Cascade Reaction between N-Phenyl-N-(phenylsulfonyl)-methacrylamides and Cyclohexane.

Guo’s team described the formation of benzylated quinoline N-oxides 120d, isoquinoline N-oxides 120e, and pyridine N-oxides 120a, and 120b with toluene derivatives 120c was mediated by DCP as the sole oxidant (Scheme 95).214 A significant decrease in the yield was observed when the reaction was performed at a higher temperature (120–130 °C) or in the presence of 3.0 equiv of the oxidant. Benzoylated products emerged under these conditions. The substitutes on the phenyl ring or pyridyl ring of the N-oxide compounds had no obvious effects in this cross-coupling reaction, whereas electron-rich toluenes showed higher reactivity compared to electron-poor ones due to the higher nucleophilicity of the generated benzylic carbon radical.
Scheme 95. Possible Mechanism for Selenation of Imidazoheterocycles with Alkanes or Ethers.
3. Conclusion and Perspective
In summary, the use of organic peroxides in transition-metal-free cyclization and coupling reactions marks a significant advancement in green and sustainable chemistry. This comprehensive review underscores the versatility and effectiveness of organic peroxides, such as TBHP, DTBP, TBPB, BPO, DLP, and DCP, in facilitating a wide range of oxidative transformations. These methodologies present a promising alternative to traditional transition-metal-catalyzed processes, thereby reducing the environmental impact and potential toxicity associated with heavy metals. The ability to achieve multibond cleavage and formation in a single step, driven by free radical mechanisms, highlights the efficiency of these processes. Despite the progress made, challenges remain in fully elucidating the mechanistic pathways of peroxide-mediated reactions, particularly when combined with nonmetal catalysts. Further research is needed to explore the exact roles of nonmetal catalysts and additives in these transformations. Additionally, developing novel, low-cost, and environmentally friendly methods for drug synthesis and other applications under metal-free conditions is crucial to the continued advancement of this field. The future of organic synthesis lies in the development and implementation of green and sustainable methodologies. The use of organic peroxides in transition-metal-free cyclization and coupling reactions represents a pivotal step toward achieving this goal. Moving forward, several avenues of research can be pursued to further enhance the application of organic peroxides in transition-metal-free reactions. First, a deeper understanding of the mechanistic pathways involved in peroxide-mediated transformations is essential. Advanced spectroscopic and computational techniques can be employed to elucidate the roles of free radicals, nonmetal catalysts, and additives in these reactions. Such insights will enable the rational design of more efficient and selective synthetic protocols. Second, the exploration of novel organic peroxides with tailored reactivity profiles can expand the scope of transition-metal-free oxidative transformations. By designing peroxides with specific functional groups or steric properties, chemists can achieve greater control over reaction outcomes, thereby broadening the range of accessible chemical structures with excellent regioselectivity and stereoselectivity. Third, the integration of organic peroxides with other green chemistry principles, such as solvent-free conditions, microwave-assisted synthesis, and flow chemistry, holds promise for the development of more sustainable and scalable synthetic processes. These approaches can reduce the environmental footprint of chemical manufacturing and enhance the overall efficiency of synthetic routes. Furthermore, the application of organic peroxide-mediated reactions in the synthesis of complex natural products, pharmaceuticals, and advanced materials presents a significant opportunity for innovation. By leveraging the unique reactivity of organic peroxides, chemists can develop new strategies for the construction of intricate molecular architectures, thereby addressing the current challenges in drug discovery and materials science. In conclusion, the continued exploration and optimization of organic peroxide-mediated transition-metal-free reactions will play a crucial role in advancing the field of green chemistry. By embracing these methodologies, the scientific community can contribute to the development of more sustainable and eco-friendly chemical processes, ultimately benefiting both industry and the environment. The insights and perspectives provided in this Review are expected to inspire further research and innovation in this exciting and rapidly evolving area of organic synthesis.
Acknowledgments
We gratefully acknowledge the financial support of Tehran University of Medical Sciences.
Glossary
ABBREVIATIONS
- CDC
Cross-dehydrogenative coupling
- DPPH
1,1-Diphenyl-2-picrylhydrazyl
- NCS
N-Chlorosuccinimide
- NIS
N-Iodosuccinimide
- KIE
Kinetic isotope effect
- DABCO
1,4-Diazabicyclo[2.2.2]octane
- TEMPO
2,2,6,6-Tetramethylpiperidinyloxy
The authors declare no competing financial interest.
References
- Sheikh A. R.; Arif A.; Khan M. M. Aryl glyoxal: a prime synthetic equivalent for multicomponent reactions in the designing of oxygen heterocycles. RSC adv. 2023, 13, 11652–11684. 10.1039/D2RA08315A. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mohammadi Ziarani G.; Moradi R.; Ahmadi T.; Gholamzadeh P. The molecular diversity scope of 4-hydroxycoumarin in the synthesis of heterocyclic compounds via multicomponent reactions. Mol. Divers. 2019, 23, 1029–1064. 10.1007/s11030-019-09918-7. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Hong J.; Sun B.; Bai G.; Li F.; Liu G.; Yang Y.; Mo F. Transition-metal-free borylation of alkyl iodides via a radical mechanism. Org. Lett. 2019, 21, 6597–6602. 10.1021/acs.orglett.9b01951. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Wang T.; Zhang Y.; You J.; Hu F.; Zhang H. Recent progress of transition metal compounds as electrocatalysts for electrocatalytic water splitting. Chem. Rec. 2023, 23, e202300109 10.1002/tcr.202300109. [DOI] [PubMed] [Google Scholar]
- Lu L.; Cheng D.; Zhan Y.; Shi R.; Chiang C.-W.; Lei A. Metal-free radical oxidative alkoxycarbonylation and imidation of alkanes. Chem. Commun. 2017, 53, 6852–6855. 10.1039/C7CC03671J. [DOI] [PubMed] [Google Scholar]
- Sun K.; Chen X.-L.; Zhang Y.-L.; Li K.; Huang X.-Q.; Peng Y.-Y.; Qu L.-B.; Yu B. Metal-free sulfonyl radical-initiated cascade cyclization to access sulfonated indolo [1, 2-a] quinolines. ChemComm. 2019, 55, 12615–12618. 10.1039/C9CC06924K. [DOI] [PubMed] [Google Scholar]
- Chen J.-R.; Hu X.-Q.; Lu L.-Q.; Xiao W.-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016, 45, 2044–2056. 10.1039/C5CS00655D. [DOI] [PubMed] [Google Scholar]
- Yi H.; Zhang G.; Wang H.; Huang Z.; Wang J.; Singh A. K.; Lei A. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 2017, 117, 9016–9085. 10.1021/acs.chemrev.6b00620. [DOI] [PubMed] [Google Scholar]
- Mandal S.; Bera T.; Dubey G.; Saha J.; Laha J. K. Uses of K2S2O8 in metal-catalyzed and metal-free oxidative transformations. ACS Catal. 2018, 8, 5085–5144. 10.1021/acscatal.8b00743. [DOI] [Google Scholar]
- Miller J. L.; Lawrence J.-M. I.; Del Rey F. O. R.; Floreancig P. E. Synthetic applications of hydride abstraction reactions by organic oxidants. Chem. Soc. Rev. 2022, 51, 5660–5690. 10.1039/D1CS01169C. [DOI] [PubMed] [Google Scholar]
- Qiu L.; Cooks R. G. Spontaneous oxidation in aqueous microdroplets: water radical cation as primary oxidizing agent. Angew. Chem., Int. Ed. 2024, 136, e202400118 10.1002/ange.202400118. [DOI] [PubMed] [Google Scholar]
- Wu T.; Müller T.; Wang N.; Byron J.; Langer S.; Williams J.; Licina D. Indoor Emission, Oxidation, and New Particle Formation of Personal Care Product Related Volatile Organic Compounds. Environ. Sci. Technol. Lett. 2024, 11, 1053–1061. 10.1021/acs.estlett.4c00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyoti; Devi S.; Manisha; Wadhwa D.; Sindhu J.; Kumar V. TBHP: A Sustainable Alternative For Carbon-Oxygen Bond Formation. Eur. J. Org. Chem. 2024, 27, e202301030 10.1002/ejoc.202301030. [DOI] [Google Scholar]
- Dixit V.; Kumar G.; Kumar P.; Soni A.; Nemiwal M. Emerging Strategies for Synthesis of Heterocyclic Compounds Enabled by Titanium Oxide Nanoparticles as Heterogeneous Catalyst. Tetrahedron 2024, 160, 134039 10.1016/j.tet.2024.134039. [DOI] [Google Scholar]
- Loch-Caruso R.; Korte C. S.; Hogan K. A.; Liao S.; Harris C. Tert-butyl hydroperoxide stimulated apoptosis independent of prostaglandin E2 and IL-6 in the HTR-8/SVneo human placental cell line. Reprod. Sci. 2020, 27, 2104–2114. 10.1007/s43032-020-00231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S.; Mukherjee S.; Sodeoka M. Recent advances in reactions using diacyl peroxides as sources of O-and C-functional groups. Org. Biomol. Chem. 2021, 19, 2096–2109. 10.1039/D0OB02349C. [DOI] [PubMed] [Google Scholar]
- Ono T. Electrochemical Analysis Using Organic Nitroxyl Radicals. Yakugaku Zasshi: J. Pharm. Soc. Jpn. 2023, 143, 95–100. 10.1248/yakushi.22-00143. [DOI] [PubMed] [Google Scholar]
- Shi L.; Li T.; Mei G.-J. Recent advances in transition-metal-free C–H functionalization of imidazo [1, 2-a] pyridines. Green Synth. Catal. Lett. 2022, 3, 227–242. 10.1016/j.gresc.2022.03.007. [DOI] [Google Scholar]
- Kumar S.; Padala K. The recent advances in K2S2O8-mediated cyclization/coupling reactions via an oxidative transformation. Chem. Commun. 2020, 56, 15101–15117. 10.1039/D0CC06036D. [DOI] [PubMed] [Google Scholar]
- Das A.; Patil N. T. Enantioselective C- H functionalization reactions under gold catalysis. Chem. Eur. J. 2022, 28, e202104371 10.1002/chem.202104371. [DOI] [PubMed] [Google Scholar]
- Tsiotsias A. I.; Charisiou N. D.; Yentekakis I. V.; Goula M. A. Bimetallic Ni-based catalysts for CO2 methanation: a review. Nanomaterials 2021, 11, 28. 10.3390/nano11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T.; Yu L.; Zhang L.; Wang L.; Wang M. Direct C2-alkylation of azoles with alcohols and ethers through dehydrogenative cross-coupling under metal-free conditions. Org. Lett. 2011, 13, 5016–5019. 10.1021/ol201779n. [DOI] [PubMed] [Google Scholar]
- Kumar R. A.; Saidulu G.; Prasad K. R.; Kumar G. S.; Sridhar B.; Reddy K. R. Transition Metal-Free α-C (sp3)-H Bond Functionalization of Amines by Oxidative Cross Dehydrogenative Coupling Reaction: Simple and Direct Access to C-4-Alkylated 3, 4-Dihydroquinazoline Derivatives. Adv. Synth. Catal. 2012, 354, 2985–2991. 10.1002/adsc.201200679. [DOI] [Google Scholar]
- Du B.; Jin B.; Sun P. The syntheses of α-ketoamides via nBu4NI-catalyzed multiple sp 3 C–H bond oxidation of ethylarenes and sequential coupling with dialkylformamides. Org. Biomol. Chem. 2014, 12, 4586–4589. 10.1039/C4OB00520A. [DOI] [PubMed] [Google Scholar]
- Yang X.-H.; Wei W.-T.; Li H.-B.; Song R.-J.; Li J.-H. Oxidative coupling of alkenes with amides using peroxides: selective amide C (sp3)–H versus C (sp2)–H functionalization. Chem. Commun. Lett. 2014, 50, 12867–12869. 10.1039/C4CC05051G. [DOI] [PubMed] [Google Scholar]
- Ouyang X.-H.; Song R.-J.; Wang C.-Y.; Yang Y.; Li J.-H. Metal-free carbonyl C (sp2)–H oxidative alkynylation of aldehydes using hypervalent iodine reagents leading to ynones. Chem. Commun. 2015, 51, 14497–14500. 10.1039/C5CC03362D. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Shah B. A. Synthesis of biaryls via benzylic C–C bond cleavage of styrenes and benzyl alcohols. Organic lett. 2015, 17, 5232–5235. 10.1021/acs.orglett.5b02578. [DOI] [PubMed] [Google Scholar]
- Lan X.-W.; Wang N.-X.; Bai C.-B.; Lan C.-L.; Zhang T.; Chen S.-L.; Xing Y. Unactivated C (sp3)–H Bond Functionalization of Alkyl Nitriles with Vinylarenes and Mechanistic Studies. Organic lett. 2016, 18, 5986–5989. 10.1021/acs.orglett.6b02692. [DOI] [PubMed] [Google Scholar]
- Chen P.; Niu Z.; Wang E. 3-Aroylbenzocoumarin-Based Luminogens: Bright Solid-State Emission, AIE Properties and Cell Imaging Application. J. Fluoresc. 2024, 1–8. 10.1007/s10895-024-03667-z. [DOI] [PubMed] [Google Scholar]
- Raju B. C.; Tiwari A. K.; Kumar J. A.; Ali A. Z.; Agawane S. B.; Saidachary G.; Madhusudana K. α-Glucosidase inhibitory antihyperglycemic activity of substituted chromenone derivatives. Bioorg. Med. Chem. 2010, 18, 358–365. 10.1016/j.bmc.2009.10.047. [DOI] [PubMed] [Google Scholar]
- Vazquez-Rodriguez S.; Figueroa-Guinez R.; Matos M. J.; Santana L.; Uriarte E.; Lapier M.; Maya J. D.; Olea-Azar C. Synthesis of coumarin–chalcone hybrids and evaluation of their antioxidant and trypanocidal properties. MedChemComm. 2013, 4, 993–1000. 10.1039/c3md00025g. [DOI] [Google Scholar]
- Vazquez-Rodriguez S.; Lama Lopez R.; Matos M. J.; Armesto-Quintas G.; Serra S.; Uriarte E.; Santana L.; Borges F.; Munoz Crego A.; Santos Y. Design, synthesis and antibacterial study of new potent and selective coumarin–chalcone derivatives for the treatment of tenacibaculosis. Bioorg. Med. Chem. 2015, 23, 7045–7052. 10.1016/j.bmc.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Jafarpour F.; Abbasnia M. A regioselective metal-free construction of 3-aroyl coumarins by Csp2-H functionalization. J. Org. Chem. 2016, 81, 11982–11986. 10.1021/acs.joc.6b02051. [DOI] [PubMed] [Google Scholar]
- Miao T.; Xia D.; Li Y.; Li P.; Wang L. Direct difunctionalization of activated alkynes via domino oxidative benzylation/1, 4-aryl migration/decarboxylation reactions under metal-free conditions. Chem. Commun. 2016, 52, 3175–3178. 10.1039/C5CC10084D. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Jiao L.; Wang J.; Wang H.; Yu C.; Hao E.; Boens N. Bu4 NI/tBuOOH catalyzed, α-regioselective cross-dehydrogenative coupling of BODIPY with allylic alkenes and ethers. Chem.Comm. 2017, 53, 581–584. 10.1039/C6CC08098G. [DOI] [PubMed] [Google Scholar]
- Teli S.; Sethiya A.; Agarwal S. Pioneering Synthetic Strategies of 2-Substituted Benzothiazoles Using 2-Aminothiophenol. Chemistry 2024, 6, 165–206. 10.3390/chemistry6010009. [DOI] [Google Scholar]
- Yokooji A.; Okazawa T.; Satoh T.; Miura M.; Nomura M. Palladium-catalyzed direct arylation of thiazoles with aryl bromides. Tetrahedron 2003, 59, 5685–5689. 10.1016/S0040-4020(03)00879-2. [DOI] [Google Scholar]
- Huang J.; Chan J.; Chen Y.; Borths C. J.; Baucom K. D.; Larsen R. D.; Faul M. M. A highly efficient palladium/copper cocatalytic system for direct arylation of heteroarenes: an unexpected effect of Cu (Xantphos) I. JACS. 2010, 132, 3674–3675. 10.1021/ja100354j. [DOI] [PubMed] [Google Scholar]
- Xie K.; Yang Z.; Zhou X.; Li X.; Wang S.; Tan Z.; An X.; Guo C.-C. Pd-catalyzed decarboxylative arylation of thiazole, benzoxazole, and polyfluorobenzene with substituted benzoic acids. Org. Lett. 2010, 12, 1564–1567. 10.1021/ol100296b. [DOI] [PubMed] [Google Scholar]
- Yu X.; Tian R.; Shi C.; Song R.; Wei Z. Commercial Nickel-catalyzed Cross-coupling of Organolithium Reagents with 2-arylbenzothiazoles. Catal. Lett. 2024, 154, 5069–5074. 10.1007/s10562-024-04707-w. [DOI] [Google Scholar]
- Chu X.; Duan T.; Liu X.; Feng L.; Jia J.; Ma C. N-Iodosuccinimide involved one-pot metal-free synthesis of 2-heteroaromatic benzothiazole compounds. Org. Biomol. Chem. 2017, 15, 1606–1611. 10.1039/C6OB02731H. [DOI] [PubMed] [Google Scholar]
- Jiang H.; Huang W.; Yu Y.; Yi S.; Li J.; Wu W. Transition-metal-free synthesis of ß-trifluoromethylated enamines with trifluoromethanesulfinate. Chem. Commun. 2017, 53, 7473–7476. 10.1039/C7CC03125D. [DOI] [PubMed] [Google Scholar]
- Ghosh P.; Mondal S.; Hajra A. Metal-free trifluoromethylation of indazoles. JOC 2018, 83, 13618–13623. 10.1021/acs.joc.8b02312. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhang G.; Li Y.; Wang S.; Lei A. The synergistic effect of self-assembly and visible-light induced the oxidative C–H acylation of N-heterocyclic aromatic compounds with aldehydes. Chem. Commun. 2018, 54, 5744–5747. 10.1039/C8CC02342E. [DOI] [PubMed] [Google Scholar]
- Jafarpour F.; Darvishmolla M. Peroxy mediated Csp2–Csp3 dehydrogenative coupling: regioselective functionalization of coumarins and coumarin-3-carboxylic acids. Org. Biomol. Chem. 2018, 16, 3396–3401. 10.1039/C7OB02771K. [DOI] [PubMed] [Google Scholar]
- Chen C.; Li Y.; Pan Y.; Duan L.; Liu W. Oxidative radical addition–chlorination of alkenes to access 1, 1-dichloroalkanes from simple reagents. Org. Chem. Front. 2019, 6, 2032–2036. 10.1039/C9QO00400A. [DOI] [Google Scholar]
- Li M.; Zhu X. Y.; Qiu Y. F.; Han Y. P.; Xia Y.; Wang C. T.; Li X. S.; Wei W. X.; Liang Y. M. Metal-Free Promoted CF2/CF3-Difunctionalization of Unactivated Alkenes. Adv. Synth. Catal. 2019, 361, 2945–2950. 10.1002/adsc.201900231. [DOI] [Google Scholar]
- Naim M.; Gestetner B.; Zilkah S.; Birk Y.; Bondi A. Soybean isoflavones. Characterization, determination, and antifungal activity. JAFC 1974, 22, 806–810. 10.1021/jf60195a031. [DOI] [PubMed] [Google Scholar]
- Parmar N. S.; Tariq M.; Ageel A. M. Effect of thromboxane A2 and leukotriene C4 inhibitors on the experimentally induced gastric lesions in the rat. Research communications in chemical pathology and pharmacology 1987, 58, 15–25. [PubMed] [Google Scholar]
- Marder M.; Viola H.; Bacigaluppo J. A.; Colombo M. a. I.; Wasowski C.; Wolfman C.; Medina J. H.; Rúveda E. A.; Paladini A. C. Detection of benzodiazepine receptor ligands in small libraries of flavone derivatives synthesized by solution phase combinatorial chemistry. Biochem. Biophys. Res. Commun. 1998, 249, 481–485. 10.1006/bbrc.1998.9146. [DOI] [PubMed] [Google Scholar]
- Chohan Z. H.; Shaikh A. U.; Rauf A.; Supuran C. T. Antibacterial, antifungal and cytotoxic properties of novel N-substituted sulfonamides from 4-hydroxycoumarin. J. Enzyme Inhib. Med. Chem. 2006, 21, 741–748. 10.1080/14756360600810340. [DOI] [PubMed] [Google Scholar]
- Zhou T.; Shi Q.; Lee K. H. Anti-AIDS agents 83. Efficient microwave-assisted one-pot preparation of angular 2, 2-dimethyl-2H-chromone containing compounds. Tetrahedron lett. 2010, 51, 4382–4386. 10.1016/j.tetlet.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agisho H. A.; Esatu H.; Hairat S.; Zaki M. TBHP/TBAI–Mediated simple and efficient synthesis of 3, 5-disubstituted and 1, 3, 5-trisubstituted 1H-1, 2, 4-triazoles via oxidative decarbonylation of aromatic aldehydes and testing for antibacterial activities. Tetrahedron Lett. 2020, 61, 151989 10.1016/j.tetlet.2020.151989. [DOI] [Google Scholar]
- Sharma S.; Dutta N. B.; Bhuyan M.; Das B.; Baishya G. tert-Butylhydroperoxide (TBHP) mediated oxidative cross-dehydrogenative coupling of quinoxalin-2 (1H)-ones with 4-hydroxycoumarins, 4-hydroxy-6-methyl-2-pyrone and 2-hydroxy-1, 4-naphthoquinone under metal-free conditions. Org. Biomol. Chem. 2020, 18, 6537–6548. 10.1039/D0OB01304H. [DOI] [PubMed] [Google Scholar]
- Wang B.; Zhang Q.; Guo Z.; Ablajan K. Iodine-and TBHP-promoted acylation of benzothiazoles under metal-free conditions. Synthesis 2020, 52, 3058–3064. 10.1055/s-0040-1707204. [DOI] [Google Scholar]
- Xu W.-X.; Ye F.-X.; Liu X.-H.; Weng J.-Q. NCS/TBHP promoted C2 arylation of benzothiazoles with aldehydes in DMSO. Tetrahedron Lett. 2020, 61, 151807 10.1016/j.tetlet.2020.151807. [DOI] [Google Scholar]
- Yuan Y.; Li M.; Apostolopoulos V.; Matsoukas J.; Wolf W. M.; Blaskovich M. A.; Bojarska J.; Ziora Z. M. Tetrazoles: A multi-potent motif in drug design. Eur. J. Med. Chem. 2024, 279, 116870 10.1016/j.ejmech.2024.116870. [DOI] [PubMed] [Google Scholar]
- Carey J. S.; Laffan D.; Thomson C.; Williams M. T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 2006, 4, 2337–2347. 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]
- Allen C. L.; Williams J. M. Metal-catalysed approaches to amide bond formation. Chem. Soc. Rev. 2011, 40, 3405–3415. 10.1039/c0cs00196a. [DOI] [PubMed] [Google Scholar]
- Song Y.; Zhang H.; Guo J.; Shao Y.; Ding Y.; Zhu L.; Yao X. Visible-Light-Induced Oxidative a-Alkylation of Glycine Derivatives with Ethers under Metal-Free Conditions. Eur. J. Org. Chem. 2021, 2021, 5914–5921. 10.1002/ejoc.202101242. [DOI] [Google Scholar]
- Xu L.; Xu P.; Zhu Y.-M.; Rao W.; Wang S.-Y. NaI/TBHP-promoted reaction of indole-2-thiones with arylsulfonyl hydrazides: construction of achiral axial 3, 3′-biindole-2, 2′-dibenzenesulfonothioate derivatives. Org. Chem. Front. 2021, 8, 5383–5388. 10.1039/D1QO00972A. [DOI] [Google Scholar]
- Kong L.; Hu X.; Bai L.-P. TBAI-catalyzed oxidative coupling of benzyl ketones to synthesize 2, 3-diaryl-1, 4-diketones in Water. ACS omega 2022, 7, 2337–2343. 10.1021/acsomega.1c06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbrik F.; Camarero González P.; Krstic M.; Puglisi A.; Benaglia M.; Sanz M. A.; Rossi S. Eosin Y: Homogeneous photocatalytic in-flow reactions and solid-supported catalysts for in-batch synthetic transformations. Appl. Sci. 2020, 10, 5596. 10.3390/app10165596. [DOI] [Google Scholar]
- Slathia N.; Gupta A.; Kapoor K. K. I2/TBHP Reagent System: A Modern Paradigm for Organic Transformations. Eur. J. Org. Chem. 2022, 2022, e202200460 10.1002/ejoc.202200460. [DOI] [Google Scholar]
- Gao X.; Yang H.; Cheng C.; Jia Q.; Gao F.; Chen H.; Cai Q.; Wang C. Iodide reagent controlled reaction pathway of iodoperoxidation of alkenes: a high regioselectivity synthesis of α-and β-iodoperoxidates under solvent-free conditions. Green Chem. 2018, 20, 2225–2230. 10.1039/C8GC00209F. [DOI] [Google Scholar]
- Anusha K.; Mahesh M.; Shikha S.; Rangarajan T. M.; Pasricha S. Langlois Reagent: An Efficient Trifluoromethylation Reagent. SynOpen 2023, 7, 65–68. 10.1055/a-2024-1382. [DOI] [Google Scholar]
- Uozumi Y.; Niimi R. Graphene Oxide-Catalyzed Trifluoromethylation of Alkynes with Quinoxalinones. Synfacts 2022, 18, 0413. 10.1055/s-0041-1737375. [DOI] [Google Scholar]
- Aradi K.; Kiss L. Carbotrifluoromethylations of C- C Multiple Bonds (Excluding Aryl-and Alkynyltrifluoromethylations). Chem. Eur. J. 2023, 29, e202203499 10.1002/chem.202203499. [DOI] [PubMed] [Google Scholar]
- Zhu N.; Yao H.; Zhang X.; Bao H. Metal-catalyzed asymmetric reactions enabled by organic peroxides. Chem. Soc. Rev. 2024, 53, 2326. 10.1039/D3CS00735A. [DOI] [PubMed] [Google Scholar]
- Li J.-Z.; Mei L.; Yu X.-C.; Wang L.-T.; Cai X.-E.; Li T.; Wei W.-T. C-centered radical-initiated cyclization by directed C (sp3)–H oxidative functionalization. Org. Chem. Front. 2022, 9, 5726–5757. 10.1039/D2QO01128J. [DOI] [Google Scholar]
- Ryckaert B.; Demeyere E.; Degroote F.; Janssens H.; Winne J. M. 1, 4-Dithianes: attractive C2-building blocks for the synthesis of complex molecular architectures. Beilstein J. Org. Chem. 2023, 19, 115–132. 10.3762/bjoc.19.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. W.; Yi W. B.; Qian J. L.; Cai C. Di-tert-butyl Peroxide Promoted Direct C-H Arylation of Unactivated Arenes with Aryl Halides. ChemCatChem. 2014, 6, 733–735. 10.1002/cctc.201301058. [DOI] [Google Scholar]
- Kamboj S.; Singh R. Chromanone-A prerogative therapeutic scaffold: an overview. Arab. J. Sci. Eng. 2022, 47, 75–111. 10.1007/s13369-021-05858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbulenko N.; Shokol T.; Khilya V. Chromones Modified with 7-Membered Heterocycles: Synthesis and Biological Activity. Fr.-Ukr. J. Chem. 2023, 11, 95–116. 10.17721/fujcV11I2P95-116. [DOI] [Google Scholar]
- Chakraborty K.; Francis P. Apoptotic effect of chromanone derivative, hyrtiosone A from marine demosponge Hyrtios erectus in hepatocellular carcinoma HepG2 cells. Bioorg. Chem. 2021, 114, 105119 10.1016/j.bioorg.2021.105119. [DOI] [PubMed] [Google Scholar]
- Bakherad M.; Keivanloo A.; Bajestani M. A.; Rezaeifard A. Green synthesis of chromeno [2, 3-c] pyrazoles and 4, 4′-(arylmethylene) bis (1H-pyrazole-5-ols) via catalyst-free multicomponent reaction in magnetized distilled water. S. Afr. J. Chem. 2023, 77, 171–177. 10.17159/0379-4350/2023/v77a22. [DOI] [Google Scholar]
- Wang X.-H.; Liu X.; Xue Y.-W.; Wei X.-H.; Wang Y.-B.; Su Q. Acid-Promoted Multicomponent Reaction To Synthesize 4-Phosphorylated 4 H-Chromenes. J. Org. Chem. 2024, 89, 8531–8536. 10.1021/acs.joc.4c00454. [DOI] [PubMed] [Google Scholar]
- Narayan R.; Antonchick A. P. Hypervalent Iodine-Mediated Selective Oxidative Functionalization of (Thio) chromones with Alkanes. Chem. Eur. J. 2014, 20, 4568–4572. 10.1002/chem.201400186. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. R.; Manpadi M.; Spatney R. Tin-free radical reactions under minimal solvent conditions for the synthesis of substituted chromones and coumarins. Green Chem. 2011, 13, 3103–3106. 10.1039/c1gc15775b. [DOI] [Google Scholar]
- Zhao J.; Fang H.; Qian P.; Han J.; Pan Y. Metal-free oxidative C (sp3)–H bond functionalization of alkanes and conjugate addition to chromones. Organic lett. 2014, 16, 5342–5345. 10.1021/ol502524d. [DOI] [PubMed] [Google Scholar]
- Peng H.; Yu J.-T.; Jiang Y.; Yang H.; Cheng J. Di-tert-butyl peroxide-promoted α-alkylation of α-amino carbonyl compounds by simple alkanes. J. Org. Chem. 2014, 79, 9847–9853. 10.1021/jo5017426. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Qian P.; Zhao J.; Fang H.; Han J.; Pan Y. Metal-free oxidative functionalization of C (sp3)–H bond adjacent to oxygen and radical addition to olefins. Org. lett. 2015, 17, 1160–1163. 10.1021/acs.orglett.5b00088. [DOI] [PubMed] [Google Scholar]
- Jin L.; Feng J.; Lu G.; Cai C. Di-tert-butyl Peroxide (DTBP)-Mediated Oxidative Cross-Coupling of Isochroman and Indole Derivatives. Adv. Synth. Catal. 2015, 357, 2105–2110. 10.1002/adsc.201500048. [DOI] [Google Scholar]
- Tolnai G. L.; Székely A.; Makó Z.; Gáti T.; Daru J.; Bihari T.; Stirling A.; Novák Z. Efficient direct 2, 2, 2-trifluoroethylation of indoles via C–H functionalization. Chem. Commun. 2015, 51, 4488–4491. 10.1039/C5CC00519A. [DOI] [PubMed] [Google Scholar]
- Ni S.; Zhang Y.; Xie C.; Mei H.; Han J.; Pan Y. Oxidative difunctionalization of alkynoates through alkylation and migration decarboxylative arylation. Org. lett. 2015, 17, 5524–5527. 10.1021/acs.orglett.5b02356. [DOI] [PubMed] [Google Scholar]
- Tripathi S.; Singh S. N.; Yadav L. D. S. Metal-free efficient cross coupling of aromatic aldehydes with aryldiazonium tetrafluoroborates using DTBP as a radical initiator. Tetrahedron lett. 2015, 56, 4211–4214. 10.1016/j.tetlet.2015.05.055. [DOI] [Google Scholar]
- Cano R.; Yus M.; Ramon D. J. Environmentally friendly and regioselective C3-alkylation of indoles with alcohols through a hydrogen autotransfer strategy. Tetrahedron Lett. 2013, 54, 3394–3397. 10.1016/j.tetlet.2013.04.062. [DOI] [Google Scholar]
- Cao L.-L.; Wang D.-S.; Jiang G.-F.; Zhou Y.-G. An efficient route to 2, 3-disubstituted indoles via reductive alkylation using H2 as reductant. Tetrahedron lett. 2011, 52, 2837–2839. 10.1016/j.tetlet.2011.03.099. [DOI] [Google Scholar]
- Fukuda T.; Mine Y.; Iwao M. Selective C-3 and C-2 lithiation of 1-(2, 2-diethylbutanoyl) indole. Tetrahedron 2001, 57, 975–979. 10.1016/S0040-4020(00)01063-2. [DOI] [Google Scholar]
- Xiu J.; Yi W. Radical-based regioselective cross-coupling of indoles and cycloalkanes. Catal. Sci. Technol. 2016, 6, 998–1002. 10.1039/C5CY01907A. [DOI] [Google Scholar]
- Pan C.; Chen Y.; Song S.; Li L.; Yu J.-T. Metal-Free Cascade Oxidative Decarbonylative Alkylation/Arylation of Alkynoates with Alphatic Aldehydes. J. Org. Chem. 2016, 81, 12065–12069. 10.1021/acs.joc.6b02451. [DOI] [PubMed] [Google Scholar]
- Cheng Z.-F.; Feng Y.-S.; Rong C.; Xu T.; Wang P.-F.; Xu J.; Dai J.-J.; Xu H.-J. Directed alkynylation of unactivated C (sp3)–H bonds with ethynylbenziodoxolones mediated by DTBP. Green Chem. 2016, 18, 4185–4188. 10.1039/C6GC01336H. [DOI] [Google Scholar]
- Yang Q.; Choy P. Y.; Wu Y.; Fan B.; Kwong F. Y. Oxidative coupling between C (sp2)–H and C (sp3)–H bonds of indoles and cyclic ethers/cycloalkanes. Org. Biomol. Chem. 2016, 14, 2608–2612. 10.1039/C6OB00076B. [DOI] [PubMed] [Google Scholar]
- Kianmehr E.; Fardpour M.; Khan K. M. Direct Regioselective Alkylation of Non-Basic Heterocycles with Alcohols and Cyclic Ethers through a Dehydrogenative Cross-Coupling Reaction under Metal-Free Conditions. Eur. J. Org. Chem. 2017, 2017, 2661–2668. 10.1002/ejoc.201700030. [DOI] [Google Scholar]
- Li Z.-l.; Jin L.-k.; Cai C. Efficient synthesis of 2-substituted azoles: radical C–H alkylation of azoles with dicumyl peroxide, methylarenes and cycloalkanes under metal-free condition. Org. Chem. Front. 2017, 4, 2039–2043. 10.1039/C7QO00396J. [DOI] [Google Scholar]
- Wen J.; Zhang F.; Shi W.; Lei A. Metal-Free Direct Alkylation of Ketene Dithioacetals by Oxidative C (sp2)- H/C (sp3)- H Cross-Coupling. Chem. Eur. J. 2017, 23, 8814–8817. 10.1002/chem.201701664. [DOI] [PubMed] [Google Scholar]
- Wang Q. Q.; Wang Z. X.; Zhang X. Y.; Fan X. S. Microwave-promoted metal-free α-alkylation of ketones with cycloalkanes through cross-coupling of C (sp3)- H bonds. Asian J. Org. Chem. 2017, 6, 1445–1450. 10.1002/ajoc.201700256. [DOI] [Google Scholar]
- Li Y.-X.; Wang Q.-Q.; Yang L. Metal-free decarbonylative alkylation–aminoxidation of styrene derivatives with aliphatic aldehydes and N-hydroxyphthalimide. Org. Biomol. Chem. 2017, 15, 1338–1342. 10.1039/C7OB00030H. [DOI] [PubMed] [Google Scholar]
- Mou Q.; Zhao R.; Sun B. Recent Advances in Transition-Metal-Catalyzed C- H Functionalization of Ferrocene Amides. Chem. Asian J. 2022, 17, e202200818 10.1002/asia.202200818. [DOI] [PubMed] [Google Scholar]
- Yu H.; Xu Y.; Dong R.; Fang Y. Direct Oxidative Cross-Coupling of Toluene Derivatives and N-Acyl-2-aminoacetophenones. Adv. Synth. Catal. 2017, 359, 39–43. 10.1002/adsc.201600712. [DOI] [Google Scholar]
- Hao W.; Wang Y.; Miao J.; Liu Y. Metal-Free Selective C2-H Amidation of 8-Amidoquinolines by Using Isocyanide as the Source of Amide. ChemistrySelect 2018, 3, 5194–5197. 10.1002/slct.201800647. [DOI] [Google Scholar]
- Chen R.; Yu J.-T.; Cheng J. Metal-free oxidative decarbonylative alkylation of chromones using aliphatic aldehydes. Org. Biomol. Chem. 2018, 16, 3568–3571. 10.1039/C8OB00720A. [DOI] [PubMed] [Google Scholar]
- Li Y.-X.; Li W.-Y.; Jiang Y.-Y.; Yang L. Decarbonylative radical conjugate addition of aliphatic aldehydes for alkylation of electron-deficient alkenes. Tetrahedron Lett. 2018, 59, 2934–2937. 10.1016/j.tetlet.2018.06.042. [DOI] [Google Scholar]
- Qi J.; Deng Z.; Huang B.; Cui S. Metal-free α-alkylation of alcohols with para-quinone methides. Org. Biomol. Chem. 2018, 16, 2762–2767. 10.1039/C8OB00568K. [DOI] [PubMed] [Google Scholar]
- Jin S.; Xie B.; Lin S.; Min C.; Deng R.; Yan Z. Metal-free site-specific hydroxyalkylation of imidazo [1, 2-a] pyridines with alcohols through radical reaction. Org. lett. 2019, 21, 3436–3440. 10.1021/acs.orglett.9b01212. [DOI] [PubMed] [Google Scholar]
- Dong Z.; Liu Y.; Zhou C.-W.; Huang J.-J.; Guo T.; Wang Y. Direct cross-dehydrogenative coupling reactions of imidazopyridines and 1-naphthylamines with ethers under metal-free conditions. Synth. Commun. 2020, 50, 1972–1981. 10.1080/00397911.2020.1761394. [DOI] [Google Scholar]
- Wang G.; Wang J.-T.; Zhao B.; Chen H.; Zhang P.-K.; Han S.-L. Transition-metal-free direct cross-dehydrogenative coupling reactions of quinolinones with ethers. Tetrahedron Lett. 2020, 61, 151426 10.1016/j.tetlet.2019.151426. [DOI] [Google Scholar]
- Wang B.; Yao H.; Zhong X.; Yan Z.; Lin S. DTBP-promoted decarbonylative alkylation of quinoxaline-2 (1H)-ones with aldehydes. Tetrahedron Lett. 2021, 64, 152720 10.1016/j.tetlet.2020.152720. [DOI] [Google Scholar]
- Doraghi F.; Kianmehr E.; Foroumadi A. Metal-free regioselective C5-cyanoalkylation of the 8-aminoquinolineamides/sulfonamides via oxidative cross-dehydrogenative coupling with alkylnitriles. Org. Chem. Front. 2021, 8, 5424–5431. 10.1039/D1QO00570G. [DOI] [Google Scholar]
- Hayakawa M.; Shirota H.; Hirayama S.; Yamada R.; Aoyama T.; Ouchi A. Sunlight-induced CC bond formation reaction: Radical addition of alcohols/ethers/acetals to olefins. J. Photochem. Photobiol., A 2021, 413, 113263 10.1016/j.jphotochem.2021.113263. [DOI] [Google Scholar]
- Jin S.; Chen F.; Qian P.; Cheng J. Cyanoalkylation/alkynylation of allylic alcohol through intramolecular radical 1, 2-alkynyl migration. Org. Biomol. Chem. 2021, 19, 2416–2419. 10.1039/D1OB00192B. [DOI] [PubMed] [Google Scholar]
- Miao P.; Li R.; Lin X.; Rao L.; Sun Z. Visible-light induced metal-free cascade Wittig/hydroalkylation reactions. Green Chem. 2021, 23, 1638–1641. 10.1039/D1GC00091H. [DOI] [Google Scholar]
- Singh S.; Dagar N.; Raha Roy S. Direct functionalization of quinoxalin-2 (1 H)-one with alkanes: C(sp2)–H/C(sp3)–H cross coupling in transition metal-free mode. Org. Biomol. Chem. 2021, 19, 5383–5394. 10.1039/D1OB00665G. [DOI] [PubMed] [Google Scholar]
- Tan X.; Zhang J.; Liu X.; Jin Y. Metal-free oxidative ketonization–olefination of indoles by cross-coupling with 1, 3-dicarbonyl substrate. Tetrahedron Lett. 2021, 80, 153322 10.1016/j.tetlet.2021.153322. [DOI] [Google Scholar]
- Zhang J.-J.; Chen D.; Qin Y.-Q.; Deng W.; Luo Y.-Y.; Xiang J.-N. Oxidative alkylation/alkynylation of terminal alkenes via alkylaldehyde decarbonylation and 1, 2-alkynyl migration. Org. Biomol. Chem. 2021, 19, 3154–3158. 10.1039/D1OB00212K. [DOI] [PubMed] [Google Scholar]
- Kuninobu Y.; Torigoe T. Regioselective CH trifluoromethylation of heteroaromatic compounds. Bull. Chem. Soc. Jpn. 2021, 94, 532–541. 10.1246/bcsj.20200302. [DOI] [Google Scholar]
- Budhwan R.; Yadav S.; Murarka S. Late stage functionalization of heterocycles using hypervalent iodine (III) reagents. Org. Biomol. Chem. 2019, 17, 6326–6341. 10.1039/C9OB00694J. [DOI] [PubMed] [Google Scholar]
- Tong Z.; Peng X.; Tang Z.; Yang W.; Deng W.; Yin S.-F.; Kambe N.; Qiu R. DTBP-mediated cross-dehydrogenative coupling of 3-aryl benzofuran-2 (3H)-ones with toluenes/phenols for all-carbon quaternary centers. RSC adv. 2022, 12, 35215–35220. 10.1039/D2RA06231C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangai S.; Fernandes R.; Mhaske K.; Narayan R. Cu (ii)-catalyzed aerobic oxidative coupling of furans with indoles enables expeditious synthesis of indolyl–furans with blue fluorescence. RSC adv. 2024, 14, 1239–1249. 10.1039/D3RA08226A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Y.; Wang N. X.; Lucan D.; Cheung W.; Xing Y. Recent Advances in Aerobic Oxidative of C- H Bond by Molecular Oxygen Focus on Heterocycles. Chem. Eur. J. 2023, 29, e202301700 10.1002/chem.202301700. [DOI] [PubMed] [Google Scholar]
- Duh Y.-S.; Kao C.-S.; Lee W.-L. W. Chemical kinetics on thermal decompositions of di-tert-butyl peroxide studied by calorimetry: An overview. J. Therm. Anal. Calorim. 2017, 127, 1071–1087. 10.1007/s10973-016-5859-y. [DOI] [Google Scholar]
- Dahiya A.; Patel B. K. The Rich Legacy and Bright Future of Transition-Metal Catalyzed Peroxide Based Radical Reactions. Chem. Rec. 2021, 21, 3589–3612. 10.1002/tcr.202100115. [DOI] [PubMed] [Google Scholar]
- Sharma R.; Yadav L.; Yadav R. K.; Chaudhary S. Oxidative cross-dehydrogenative coupling (CDC) via C(sp2)–H bond functionalization: tert-butyl peroxybenzoate (TBPB)-promoted regioselective direct C-3 acylation/benzoylation of 2H-indazoles with aldehydes/benzyl alcohols/styrenes. RSC adv. 2021, 11, 14178–14192. 10.1039/D1RA02225C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T.; Li H.; Li P.; Wang L. Direct amidation of azoles with formamides via metal-free C–H activation in the presence of tert-butyl perbenzoate. Chem. Commun. 2011, 47, 8946–8948. 10.1039/c1cc13086b. [DOI] [PubMed] [Google Scholar]
- Li L.-T.; Huang J.; Li H.-Y.; Wen L.-J.; Wang P.; Wang B. nBu4NI-catalyzed C3-formylation of indoles with N-methylaniline. Chem. Commun. 2012, 48, 5187–5189. 10.1039/c2cc31578e. [DOI] [PubMed] [Google Scholar]
- Chu X.-Q.; Meng H.; Zi Y.; Xu X.-P.; Ji S.-J. Metal-free oxidative direct C(sp3)–H bond functionalization of ethers with α,α-diaryl allylic alcohols. Chem. Commun. 2014, 50, 9718–9721. 10.1039/C4CC04282D. [DOI] [PubMed] [Google Scholar]
- Chu X. Q.; Meng H.; Zi Y.; Xu X. P.; Ji S. J. Metal-Free Oxidative Radical Addition of Carbonyl Compounds to α,α-Diaryl Allylic Alcohols: Synthesis of Highly Functionalized Ketones. Chem. Eur. J. 2014, 20, 17198–17206. 10.1002/chem.201404463. [DOI] [PubMed] [Google Scholar]
- Cai X.; Wei X.; Huang M. Recent Developments of Transition-Metal-Catalyzed Cross-Coupling of Nitriles and Alcohols. ChemistrySelect 2024, 9, e202402955 10.1002/slct.202402955. [DOI] [Google Scholar]
- Song P.; Cui Z.; Meng T.; Rong H.; Mao M.; Yang C. A Simple Conversion of Aldoximes to Nitriles Using Thiourea Dioxide. Asian J. Org. Chem. 2024, 13, e202400291 10.1002/ajoc.202400291. [DOI] [Google Scholar]
- Chu X.-Q.; Meng H.; Zi Y.; Xu X.-P.; Ji S.-J. Oxidative C(sp3)–H functionalization of acetonitrile and alkanes with allylic alcohols under metal-free conditions. Org. Chem. Front. 2015, 2, 216–220. 10.1039/C4QO00314D. [DOI] [Google Scholar]
- Li Y.; Liu B.; Li H.-B.; Wang Q.; Li J.-H. Oxidative radical 1, 2-alkylarylation of alkenes with α-C(sp3)–H bonds of acetonitriles involving 1, 2-aryl migration. Chem. Commun. 2015, 51, 1024–1026. 10.1039/C4CC08902B. [DOI] [PubMed] [Google Scholar]
- Li Y.; Leng Y.; Wang S.; Gao Y.; Lv H.; Chang J.; Wu Y.; Wu Y. Oxidative acylation of α,α-diarylallylic alcohols: Synthesis of 1, 2, 4-triarylbutane-1, 4-diones. Appl. Organomet. Chem. 2018, 32, e4407 10.1002/aoc.4407. [DOI] [Google Scholar]
- Yang Q.; Lou M.; Yin Z.; Deng Z.; Ding Q.; Peng Y. Direct C-4 alkylation of quinazoline N-oxides with ethers via an oxidative cross-coupling reaction under metal-free conditions. Org. Biomol. Chem. 2018, 16, 8724–8731. 10.1039/C8OB01429A. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Jin S.; Liu Q.; Lin S.; Yan Z. Transition metal-free cross-dehydrogenative coupling reaction of coumarins with acetonitrile or acetone. J. Org. Chem. 2018, 83, 13030–13035. 10.1021/acs.joc.8b01508. [DOI] [PubMed] [Google Scholar]
- Sun M.-X.; Wang Y.-F.; Xu B.-H.; Ma X.-Q.; Zhang S.-J. A metal-free direct C(sp3)–H cyanation reaction with cyanobenziodoxolones. Org. Biomol. Chem. 2018, 16, 1971–1975. 10.1039/C8OB00173A. [DOI] [PubMed] [Google Scholar]
- Joychandra Singh S.; Ahmad Mir B.; Patel B. K. A TBPB-Mediated C-3 Cycloalkylation and Formamidation of 4-Arylcoumarin. Eur. J. Org. Chem. 2018, 2018, 1026–1033. 10.1002/ejoc.201701677. [DOI] [Google Scholar]
- Zhao K.; Zhang X.-C.; Tao J.-Y.; Wu X.-D.; Wu J.-X.; Li W.-M.; Zhu T.-H.; Loh T.-P. Regio-and stereoselective C(sp2)–H acylation of enamides with aldehydes via transition-metal-free photoredox catalysis. Green Chem. 2020, 22, 5497–5503. 10.1039/D0GC01947J. [DOI] [Google Scholar]
- Hooshmand S. E.; Alavioon S. I.; Saeb M.; Brahmachari G.; Shiri M. Decarboxylation and cross-coupling reactions of coumarin-3-carboxylic acid: a comprehensive review. Tetrahedron 2024, 167, 134238 10.1016/j.tet.2024.134238. [DOI] [Google Scholar]
- Rahaman A.; Chauhan S. S.; Bhadra S. Recent advances in catalytic decarboxylative transformations of carboxylic acid groups attached to a non-aromatic sp2 or sp carbon. Org. Biomol. Chem. 2023, 21, 5691–5724. 10.1039/D3OB00682D. [DOI] [PubMed] [Google Scholar]
- Patel D. J.; Patel P. P.; Chikhalia K. H. Recent Advances in the Decarboxylative Strategy of Coumarin-3-carboxylic Acid. ChemistrySelect 2023, 8, e202301755 10.1002/slct.202301755. [DOI] [Google Scholar]
- Yu Y.; Zhuang S.; Liu P.; Sun P. Cyanomethylation and cyclization of aryl alkynoates with acetonitrile under transition-metal-free conditions: Synthesis of 3-cyanomethylated coumarins. J. Org. Chem. 2016, 81, 11489–11495. 10.1021/acs.joc.6b02155. [DOI] [PubMed] [Google Scholar]
- Batra A.; Singh K. N. Recent Developments in Transition Metal-Free Cross-Dehydrogenative Coupling Reactions for C–C Bond Formation. Eur. J. Org. Chem. 2020, 2020, 6676–6703. 10.1002/ejoc.202000785. [DOI] [Google Scholar]
- Fang H.; Zhao J.; Ni S.; Mei H.; Han J.; Pan Y. Metal-free oxidative functionalization of a C(sp3)–H bond adjacent to nitrogen and intramolecular aromatic cyclization for the preparation of 6-amidophenanthridines. J. Org. Chem. 2015, 80, 3151–3158. 10.1021/acs.joc.5b00058. [DOI] [PubMed] [Google Scholar]
- Wang K.; Meng L.-G.; Wang L. Visible-light-initiated Na2-Eosin Y catalyzed highly regio-and stereoselective difunctionalization of alkynes with alkyl bromides. J. Org. Chem. 2016, 81, 7080–7087. 10.1021/acs.joc.6b00973. [DOI] [PubMed] [Google Scholar]
- Reyes P.; D’hooge D. R.; Cardon L.; Cornillie P. From identifying polymeric resins to corrosion casting applications. J. Appl. Polym. Sci. 2022, 139, 52284 10.1002/app.52284. [DOI] [Google Scholar]
- Sha W.; Yu J.-T.; Jiang Y.; Yang H.; Cheng J. The benzoyl peroxide-promoted functionalization of simple alkanes with 2-aryl phenyl isonitrile. Chem. Commun. 2014, 50, 9179–9181. 10.1039/C4CC03304C. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Dian L.; Yuan Y.; Zhang-Negrerie D.; Du Y.; Zhao K. PhI (OAc)2-Mediated Intramolecular Oxidative Aryl-Aldehyde C Sp2–C Sp2 Bond Formation: Metal-Free Synthesis of Acridone Derivatives. J. Org. Chem. 2014, 79, 7451–7458. 10.1021/jo5011697. [DOI] [PubMed] [Google Scholar]
- Han C.; Dang Y.; Mo L.; Zhao R.; Wang Z.; Zhao J. Approach to Seven-Membered Carbocycles via a Lewis Acid Catalyzed Intramolecular Michael Addition of Allenone. Org. Lett. 2023, 25, 4923–4927. 10.1021/acs.orglett.3c01732. [DOI] [PubMed] [Google Scholar]
- Ahn J. H.; Shin M. S.; Jung S. H.; Kang S. K.; Kim K. R.; Rhee S. D.; Jung W. H.; Yang S. D.; Kim S. J.; Woo J. R.; et al. Indenone derivatives: a novel template for peroxisome proliferator-activated receptor γ (PPARγ) agonists. J. Med. Chem. 2006, 49, 4781–4784. 10.1021/jm060389m. [DOI] [PubMed] [Google Scholar]
- Anstead G. M.; Wilson S. R.; Katzenellenbogen J. A. 2-Arylindenes and 2-arylindenones: molecular structures and considerations in the binding orientation of unsymmetrical nonsteroidal ligands to the estrogen receptor. J. Med. Chem. 1989, 32, 2163–2171. 10.1021/jm00129a024. [DOI] [PubMed] [Google Scholar]
- Morrell A.; Placzek M.; Parmley S.; Grella B.; Antony S.; Pommier Y.; Cushman M. Optimization of the indenone ring of indenoisoquinoline topoisomerase I inhibitors. J. Med. Chem. 2007, 50, 4388–4404. 10.1021/jm070307+. [DOI] [PubMed] [Google Scholar]
- Jafarpour F.; Azizzade M.; Golpazir-Sorkheh Y.; Navid H.; Rajai-Daryasarei S. Divergent synthesis of α-aroyloxy ketones and indenones: a controlled domino radical reaction for di-and trifunctionalization of alkynes. J. Org. Chem. 2020, 85, 8287–8294. 10.1021/acs.joc.0c00967. [DOI] [PubMed] [Google Scholar]
- Chen S.; Yu J.; Jiang Y.; Chen F.; Cheng J. Rhodium-catalyzed direct annulation of aldehydes with alkynes leading to indenones: proceeding through in situ directing group formation and removal. Org. lett. 2013, 15, 4754–4757. 10.1021/ol4021145. [DOI] [PubMed] [Google Scholar]
- Fatma S.; Ahmad F.; Pankhade Y. A.; Ranga P. K.; Vijaya Anand R. A HMPA–H2O mediated oxygenative carbocyclization of 2-alkynylphenyl-substituted p-quinone methides to indenones. Org. Biomol. Chem. 2024, 22, 5891–5896. 10.1039/D4OB00966E. [DOI] [PubMed] [Google Scholar]
- Li B. J.; Wang H. Y.; Zhu Q. L.; Shi Z. J. Rhodium/copper-catalyzed annulation of benzimides with internal alkynes: indenone synthesis through sequential CH and CN cleavage. Angew. Chem. (International ed. in English) 2012, 51, 3948–3952. 10.1002/anie.201200271. [DOI] [PubMed] [Google Scholar]
- Liu C. C.; Korivi R. P.; Cheng C. H. Cobalt-Catalyzed Regioselective Synthesis of Indenamine from o-Iodobenzaldimine and Alkyne: Intriguing Difference to the Nickel-Catalyzed Reaction. Chem. Eur. J. 2008, 14, 9503–9506. 10.1002/chem.200801457. [DOI] [PubMed] [Google Scholar]
- Miura T.; Murakami M. Rhodium-catalyzed annulation reactions of 2-cyanophenylboronic acid with alkynes and strained alkenes. Org. Lett. 2005, 7, 3339–3341. 10.1021/ol051249u. [DOI] [PubMed] [Google Scholar]
- Pletnev A. A.; Tian Q.; Larock R. C. Carbopalladation of nitriles: synthesis of 2, 3-diarylindenones and polycyclic aromatic ketones by the Pd-catalyzed annulation of alkynes and bicyclic alkenes by 2-iodoarenenitriles. J. Org. Chem. 2002, 67, 9276–9287. 10.1021/jo026178g. [DOI] [PubMed] [Google Scholar]
- Qi Z.; Wang M.; Li X. Access to indenones by rhodium (III)-catalyzed C–H annulation of arylnitrones with internal alkynes. Org. lett. 2013, 15, 5440–5443. 10.1021/ol4025309. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H.; Kondo Y. Palladium (II)-catalyzed annulation of alkynes with ortho-ester-containing phenylboronic acids. Org. lett. 2007, 9, 4227–4230. 10.1021/ol701776m. [DOI] [PubMed] [Google Scholar]
- Pan C.; Huang B.; Hu W.; Feng X.; Yu J.-T. Metal-free radical oxidative annulation of ynones with alkanes to access indenones. J. Org. Chem. 2016, 81, 2087–2093. 10.1021/acs.joc.6b00072. [DOI] [PubMed] [Google Scholar]
- Peterson K. E.; Cinelli M. A.; Morrell A. E.; Mehta A.; Dexheimer T. S.; Agama K.; Antony S.; Pommier Y.; Cushman M. Alcohol-, diol-, and carbohydrate-substituted indenoisoquinolines as topoisomerase I inhibitors: investigating the relationships involving stereochemistry, hydrogen bonding, and biological activity. J. Med. Chem. 2011, 54, 4937–4953. 10.1021/jm101338z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter B. W.; Nanda K. K.; Kett N. R.; Regan C. P.; Lynch J. J.; Stump G. L.; Kiss L.; Wang J.; Spencer R. H.; Kane S. A.; et al. Design and synthesis of novel isoquinoline-3-nitriles as orally bioavailable Kv1. 5 antagonists for the treatment of atrial fibrillation. J. Med. Chem. 2006, 49, 6954–6957. 10.1021/jm060927v. [DOI] [PubMed] [Google Scholar]
- Ukita T.; Nakamura Y.; Kubo A.; Yamamoto Y.; Moritani Y.; Saruta K.; Higashijima T.; Kotera J.; Takagi M.; Kikkawa K.; et al. Novel, potent, and selective phosphodiesterase 5 inhibitors: synthesis and biological activities of a series of 4-aryl-1-isoquinolinone derivatives. J. Med. Chem. 2001, 44, 2204–2218. 10.1021/jm000558h. [DOI] [PubMed] [Google Scholar]
- Garcia Maza L. J.; Salgado A. M.; Kouznetsov V. V.; Melendez C. M. Pyrrolo [2, 1-a] isoquinoline scaffolds for developing anti-cancer agents. RSC Adv. 2024, 14 (3), 1710–1728. 10.1039/D3RA07047F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R.; Xu X.; Dang Q.; Bai X. Synthesis of Novel Tricyclic Pyrimido [4, 5-b][1, 4] benzothiazepines via Bischler- Napieralski-Type Reactions. J. Org. Chem. 2005, 70, 10810–10816. 10.1021/jo051873k. [DOI] [PubMed] [Google Scholar]
- Kotha S.; Deodhar D.; Khedkar P. Diversity-oriented synthesis of medicinally important 1, 2, 3, 4-tetrahydroisoquinoline-3-carboxylic acid (Tic) derivatives and higher analogs. Org. Biomol. Chem. 2014, 12, 9054–9091. 10.1039/C4OB01446D. [DOI] [PubMed] [Google Scholar]
- Zein A. L.; Valluru G.; Georghiou P. E. Recent asymmetric syntheses of tetrahydroisoquinolines using “named” and some other newer methods. Stud. Nat. Prod. Chem. 2012, 38, 53–80. 10.1016/B978-0-444-59530-0.00003-4. [DOI] [Google Scholar]
- Yang X.; Miao X.; Dai L.; Guo X.; Jenis J.; Zhang J.; Shang X. Isolation, biological activity, and synthesis of isoquinoline alkaloids. Nat. Prod. Rep. 2024, 41, 1652–1722. 10.1039/D4NP00023D. [DOI] [PubMed] [Google Scholar]
- Xue D.; Xue Y.; Yu H.; Shao L. Metal-Free Radical Cyclization of Vinyl Isocyanides with Alkanes: Synthesis of 1-Alkylisoquinolines. Synthesis 2019, 51, 874–884. 10.1055/s-0037-1610661. [DOI] [Google Scholar]
- Ji L.; Deng Q.; Liu P.; Sun P. BPO-promoted direct oxidative C–H functionalization of unactivated alkanes into 6-alkyl-6H-benzo [c] chromenes under transition-metal-free conditions. Org. Biomol. Chem. 2019, 17, 7715–7722. 10.1039/C9OB01396B. [DOI] [PubMed] [Google Scholar]
- Liu S.; Klussmann M. Acid promoted radical-chain difunctionalization of styrenes with stabilized radicals and (N, O)-nucleophiles. Chem. Commun. 2020, 56, 1557–1560. 10.1039/C9CC09369A. [DOI] [PubMed] [Google Scholar]
- Sun K.; Li G.; Li Y.; Yu J.; Zhao Q.; Zhang Z.; Zhang G. Oxidative Radical Relay Functionalization for the Synthesis of Benzimidazo [2, 1-a] iso-quinolin-6 (5H)-ones. Adv. Synth. Catal. 2020, 362, 1947–1954. 10.1002/adsc.202000040. [DOI] [Google Scholar]
- Wang J.; Wu X.; Cao Z.; Zhang X.; Wang X.; Li J.; Zhu C. E-Selective Radical Difunctionalization of Unactivated Alkynes: Preparation of Functionalized Allyl Alcohols from Aliphatic Alkynes. Adv. Sci. 2024, 11, 2309022 10.1002/advs.202309022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.-H.; Hao W.-J.; Li G.; Tu S.-J.; Jiang B. Recent advances in radical transformations of internal alkynes. Chem. Commun. 2018, 54, 10791–10811. 10.1039/C8CC04618B. [DOI] [PubMed] [Google Scholar]
- Xuan J.; Studer A. Radical cascade cyclization of 1, n-enynes and diynes for the synthesis of carbocycles and heterocycles. Chem. Soc. Rev. 2017, 46, 4329–4346. 10.1039/C6CS00912C. [DOI] [PubMed] [Google Scholar]
- Beaufils F.; Dénès F.; Becattini B.; Renaud P.; Schenk K. Thiophenol-Mediated 1, 5-Hydrogen Atom Abstraction: Easy Access to Mono-and Bicyclic Compounds. Adv. Synth. Catal. 2005, 347, 1587–1594. 10.1002/adsc.200505211. [DOI] [Google Scholar]
- Beaufils F.; Dénès F.; Renaud P. Thiophenol-mediated hydrogen atom abstraction: an efficient tin-free procedure for the preparation of cyclopentane derivatives. Org. lett. 2004, 6, 2563–2566. 10.1021/ol049162g. [DOI] [PubMed] [Google Scholar]
- Denes F.; Beaufils F.; Renaud P. Preparation of five-membered rings via the translocation-cyclization of vinyl radicals. Synlett. 2008, 2008, 2389–2399. 10.1055/s-2008-1078016. [DOI] [Google Scholar]
- Dénès F.; Beaufils F.; Renaud P. Thiophenol-mediated 1, 5-hydrogen transfer for the preparation of pyrrolizidines, indolizidines, and related compounds. Org. lett. 2007, 9, 4375–4378. 10.1021/ol702017t. [DOI] [PubMed] [Google Scholar]
- Gloor C. S.; Dénès F.; Renaud P. Hydrosulfonylation reaction with arenesulfonyl chlorides and tetrahydrofuran: conversion of terminal alkynes into cyclopentylmethyl sulfones. Angew. Chem., Int. Ed. 2017, 129, 13514–13517. 10.1002/ange.201707791. [DOI] [PubMed] [Google Scholar]
- Huang L.; Ye L.; Li X.-H.; Li Z.-L.; Lin J.-S.; Liu X.-Y. Stereoselective radical cyclization cascades triggered by addition of diverse radicals to alkynes to construct 6 (5)–6–5 fused rings. Org. lett. 2016, 18, 5284–5287. 10.1021/acs.orglett.6b02599. [DOI] [PubMed] [Google Scholar]
- Lachia M.; Dénès F.; Beaufils F.; Renaud P. Highly Diastereoselective Formation of Spirocyclic Compounds via 1, 5-Hydrogen Transfer: A Total Synthesis of (−)-Erythrodiene. Org. lett. 2005, 7, 4103–4106. 10.1021/ol051426r. [DOI] [PubMed] [Google Scholar]
- Renaud P.; Beaufils F.; Feray L.; Schenk K.. Diastereoselective radical mediated hydrogen atom abstraction. Recent Developments in Radical-Mediated Hydrogen Atom Transfer Reaction 2003, 43. [DOI] [PubMed] [Google Scholar]
- Kehr G.; Erker G. 1, 1-Carboboration. Chem. Commun. 2012, 48, 1839–1850. 10.1039/C1CC15628D. [DOI] [PubMed] [Google Scholar]
- Krautwald S.; Bezdek M. J.; Chirik P. J. Cobalt-catalyzed 1, 1-diboration of terminal alkynes: scope, mechanism, and synthetic applications. J. Am. Chem. Soc. 2017, 139, 3868–3875. 10.1021/jacs.7b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga A.; Nagao K.; Ohmiya H.; Sawamura M. Synthesis of 1, 1-Diborylalkenes through a Brønsted Base Catalyzed Reaction between Terminal Alkynes and Bis (pinacolato) diboron. Angew. Chem., Int. Ed. 2015, 127, 16085–16088. 10.1002/ange.201509218. [DOI] [PubMed] [Google Scholar]
- Sun Q.; Li L.; Liu L.; Yang Y.; Zha Z.; Wang Z. Copper-catalyzed 1, 1-difunctionalization of terminal alkynes: a three-component reaction for the construction of vinyl sulfones. Sci. China Chem. 2019, 62, 904–908. 10.1007/s11426-019-9454-8. [DOI] [Google Scholar]
- Armstrong M. K.; Lalic G. Differential dihydrofunctionalization of terminal alkynes: synthesis of benzylic alkyl boronates through reductive three-component coupling. JACS. 2019, 141, 6173–6179. 10.1021/jacs.9b02372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D.-W.; Gao Y.; Shao H.; Qiao T.-Z.; Wang X.; Sanchez B. B.; Chen J. S.; Liu P.; Engle K. M. Cascade CuH-catalysed conversion of alkynes into enantioenriched 1, 1-disubstituted products. Nat. Catal. 2020, 3, 23–29. 10.1038/s41929-019-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.; Wang H.; Xing S.; Hong X.; Lu Z. Cobalt-catalyzed asymmetric synthesis of gem-bis (silyl) alkanes by double hydrosilylation of aliphatic terminal alkynes. Chem. 2019, 5, 881–895. 10.1016/j.chempr.2019.02.001. [DOI] [Google Scholar]
- Nishino S.; Hirano K.; Miura M. Cu-Catalyzed Reductive gem-Difunctionalization of Terminal Alkynes via Hydrosilylation/Hydroamination Cascade: Concise Synthesis of α-Aminosilanes. Chem. Eur. J. 2020, 26, 8725–8728. 10.1002/chem.202001799. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Chen J.; Lu H. X.; Li X.; Gao Y.; Coombs J. R.; Goldfogel M. J.; Engle K. M. Palladium (0)-Catalyzed Directed syn-1, 2-Carboboration and-Silylation: Alkene Scope, Applications in Dearomatization, and Stereocontrol by a Chiral Auxiliary. Angew. Chem. Int. Ed 2019, 58, 17068–17073. 10.1002/anie.201910304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Daniliuc C. G.; Studer A. 1, 1, 2-Trifunctionalization of terminal alkynes by radical addition–translocation–cyclization–trapping for the construction of highly substituted cyclopentanes. Angew. Chem. 2021, 133, 2173–2176. 10.1002/ange.202011785. [DOI] [PubMed] [Google Scholar]
- Yao H.; Hu W.; Zhang W. Difunctionalization of alkenes and alkynes via intermolecular radical and nucleophilic additions. Molecules 2021, 26, 105. 10.3390/molecules26010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Difunctionalization of Olefins by Radicals and Nucleophiles; Universität zu Köln, 2021. [Google Scholar]
- Yu X.-Y.; Chen J.-R.; Xiao W.-J. Visible light-driven radical-mediated C–C bond cleavage/functionalization in organic synthesis. Chem. Rev. 2021, 121, 506–561. 10.1021/acs.chemrev.0c00030. [DOI] [PubMed] [Google Scholar]
- Varala R.; Seema V.; Hussein M.; Alam M. M.; Beda D. P. tert-Butyl Hydroperoxide (TBHP): Recent Progress in C-H Functionalization and Heteroatom-Heteroatom Bond Formations. ChemistrySelect 2024, 9, e202401937 10.1002/slct.202401937. [DOI] [Google Scholar]
- Ramani A.; Desai B.; Patel M.; Naveen T. Recent advances in the functionalization of terminal and internal alkynes. Asian J. Org. Chem. 2022, 11, e202200047 10.1002/ajoc.202200047. [DOI] [Google Scholar]
- Chimenti F.; Bizzarri B.; Bolasco A.; Secci D.; Chimenti P.; Granese A.; Carradori S.; Rivanera D.; Zicari A.; Scaltrito M. M.; et al. Synthesis, selective anti-Helicobacter pylori activity, and cytotoxicity of novel N-substituted-2-oxo-2H-1-benzopyran-3-carboxamides. Bioorg. Med. Chem. Lett. 2010, 20, 4922–4926. 10.1016/j.bmcl.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Lyu H.-W.; Liang H.-M.; Zhu M.; Wang H.; Li X. Research progress in biosynthesis-related enzymes of coumarin compounds and their bioactivities. China Journal of Chinese Materia Medica 2024, 49, 3693–3705. 10.19540/j.cnki.cjcmm.20240412.601. [DOI] [PubMed] [Google Scholar]
- Medina F. G.; Marrero J. G.; Macias-Alonso M.; Gonzalez M. C.; Cordova-Guerrero I.; Teissier Garcia A. G.; Osegueda-Robles S. Coumarin heterocyclic derivatives: chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. 10.1039/C4NP00162A. [DOI] [PubMed] [Google Scholar]
- Srikrishna D.; Godugu C.; Dubey P. K. A review on pharmacological properties of coumarins. Mini reviews in Med. Chem. 2018, 18, 113–141. 10.2174/1389557516666160801094919. [DOI] [PubMed] [Google Scholar]
- Kostova I.; Bhatia S.; Grigorov P.; Balkansky S.; Parmar V.; Prasad A.; Saso L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. 10.2174/092986711803414395. [DOI] [PubMed] [Google Scholar]
- Pan C.; Chen R.; Shao W.; Yu J.-T. Metal-free radical addition/cyclization of alkynoates with xanthates towards 3-(ß-carbonyl) coumarins. Org. Biomol. Chem. 2016, 14, 9033–9039. 10.1039/C6OB01732K. [DOI] [PubMed] [Google Scholar]
- Pérez V. M.; Fregoso-López D.; Miranda L. D. Xanthate-based microwave-assisted CH radical functionalization of caffeine, 1, 3-dimethyluracil, and imidazo [1, 2-a] pyridines. Tetrahedron Lett. 2017, 58, 1326–1329. 10.1016/j.tetlet.2017.02.050. [DOI] [Google Scholar]
- Bromer W.; Egge H.; Eiter K.; Eyjólfsson R.; Gross D.; Hikino H.; Hikino Y.; Jackson B.; Morin R.; Pike J.. Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer Science & Business Media: 2012; Vol. 28. [Google Scholar]
- Huang Q.; Zard S. Z. Inexpensive radical methylation and related alkylations of heteroarenes. Org. lett. 2018, 20, 1413–1416. 10.1021/acs.orglett.8b00190. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Pi J.; Wang L.; Liu P.; Sun P. Silyl radical initiated radical cascade addition/cyclization: synthesis of silyl functionalized 4 H-pyrido [4, 3, 2-gh] phenanthridin-5 (6H)-ones. Org. Biomol. Chem. 2018, 16, 9223–9229. 10.1039/C8OB02670J. [DOI] [PubMed] [Google Scholar]
- Chen X.; Zard S. Z. Convergent Route to ß-Amino Acids and to ß-Heteroarylethylamines: An Unexpected Vinylation Reaction. Org. let. 2020, 22, 3628–3632. 10.1021/acs.orglett.0c01087. [DOI] [PubMed] [Google Scholar]
- Bertho S.; Dondasse I.; Retailleau P.; Nicolas C.; Gillaizeau I. ß-C (sp2)–H alkylation of enamides using xanthate chemistry. New J. Chem. 2020, 44, 7129–7141. 10.1039/D0NJ01209B. [DOI] [Google Scholar]
- Kubo T.; Chatani N. Dicumyl peroxide as a methylating reagent in the Ni-catalyzed methylation of ortho C–H bonds in aromatic amides. Org. lett. 2016, 18, 1698–1701. 10.1021/acs.orglett.6b00658. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Pan C.; Jin N.; Gu Z.; Hu H.; Zhu C. Metal-free cascade construction of C–C bonds by activation of inert C(sp3)–H bonds. Chem. Commun. 2015, 51, 1320–1322. 10.1039/C4CC08629E. [DOI] [PubMed] [Google Scholar]
- Wan L.; Qiao K.; Sun X.; Di Z.; Fang Z.; Li Z.; Guo K. Benzylation of heterocyclic N-oxides via direct oxidative cross-dehydrogenative coupling with toluene derivatives. New J. Chem. 2016, 40, 10227–10232. 10.1039/C6NJ02560A. [DOI] [Google Scholar]