Abstract
Background
It is challenging to identify Papillary Thyroid Cancer (PTC) which shows atypia of undetermined significance (AUS) by Fine-needle Aspiration (FNA). This study aims to seek the meaningful quantitative biomarkers of the microvasculature and construct a classification model for PTC with AUS based on these new biomarkers and Thyroid Imaging Reporting and Data System (TI-RADS).
Methods
This prospective study enrolled 281 patients with 300 thyroid nodules showing AUS. These cases were divided into two groups with the largest dimension (LD) of 10 mm, A (< 10 mm) and B (≥ 10 mm). Firstly, an open-source artifact suppression algorithm, which combined a multi-scale Frangi filter and TOPHAT operation, was proposed for the segmentation of micro-vessels in Ultra Micro-Angiography (UMA) images. Then, 18 quantitative biomarkers were calculated and analyzed through Mann-Whitney test (U-test), while LASSO regression was utilized to remove collinear features. Finally, two different classification models were built using logistic regression through the selected biomarkers combined with Chinese TI-RADS (C TI-RADS) or American College of Radiology TI-RADS (ACR TI-RADS). The performances were evaluated using the mean Area Under the Curve (AUC) value and the DeLong test, through a 5-fold cross-validation experiment.
Results
Group A comprised 58 benign nodules and 104 PTCs, while Group B consisted of 60 benign nodules and 78 PTCs. Four biomarkers were selected in Group A. The 5-fold cross-validation experiment showed that the mean Area Under Curve (AUC) improved from 0.725 with ACR TI-RADS to 0.851 (P < 0.05), while the mean AUC improved from 0.809 with C TI-RADS to 0.882 (P < 0.05). In Group B, four different biomarkers were selected, and the classification models showed improvements from 0.841 with ACR TI-RADS to 0.874 and from 0.894 with C TI-RADS to 0.936.
Conclusions
This study demonstrated the potential value of microvasculature in the prediction of PTC in AUS Cases and improved the performance of ultrasound examination. Moreover, the morphology of microvasculature showed different changes at different LD groups.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14197-7.
Keywords: Papillary thyroid carcinoma, Atypia of undetermined significance, Ultra micro-angiography, Microvasculature
Background
Papillary Thyroid Carcinoma (PTC) is one of the most prevalent subtype of thyroid carcinomas [1]. Different from other malignant cancers, PTC shows the contradictory characteristics of high incidence rate and low progression risk level [2, 3]. Due to these two characteristics, ultrasound examination, which is cheap, convenient and non-invasive, is of great significance in early diagnosis of PTC [4]. There have been plenty of clinical guidelines published, such as American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS) [5], American Thyroid Association Guidelines (ATA) [6], Kwak Thyroid Imaging Reporting and Data System (KWAK TI-RADS) [7] and Chinese Thyroid Imaging Reporting and Data System (C TI-RADS) [8], to assist doctors in giving diagnostic results and provide principles for subsequent strategies. Usually, thyroid nodules are evaluated in five aspects, including margin, orientation, echogenicity, composition and calcification, which can be evaluated by B-model ultrasound images. According to the guidelines, thyroid nodules are assigned risk levels, and Fine Needle Aspiration (FNA) is implemented. But the cytological pathology may show atypia of undetermined significance (AUS) [9]. Currently, there are several effective examinations for identifying this kind of PTC. Genetic testing with Braf v600E [10] has higher sensitivity than cytological pathology. Sometimes, further tests will carried out such as histologic examination [11] by Core-needle biopsy (CNB) or FNA again. However, these methods are expensive and invasive. Thus, there is a contradiction between the low risk level of PTC and further examination methods.
The biomarkers based on medical imaging [12, 13] have demonstrated outstanding value in the clinical diagnosis of tumors. With the development of ultrasound imaging technology, multi-mode ultrasound diagnosis showed potential in finding malignant features with high specificity and enhance the performance of ultrasound examination [14–16]. The growth of tumors is usually relevant with the alterations of microvasculature [17]. Color Doppler Flow Imaging and Contrast Enhanced Ultrasound (CEUS) are two main ultrasound technologies to observe vessel information. However, Color Doppler Flow Imaging is limited by the angular and signal-to-noise ratio. The small vessels (< 1 mm) with low speed (3–5 cm/s) are usually unavailable [18] These factors have somewhat led to the restricted incorporation of blood flow in existing clinical guidelines [5, 7, 8]. Although CEUS could show micro vessels with 100 μm, this imaging mode exhibited a time-dependent and layered stacking pattern, leading to a confusion of the microvasculature structure. In addition, it was reported that nearly half of PTCs (43.6%) showed lack of blood supply in CEUS videos [19–21], indicating that PTC has produced vascular changes compared to normal thyroid gland tissue.
Ultra Micro-angiography (UMA) [22] technology, which is similar with Superb microvasculature imaging (SMI) [23] and High-Definition Micro-vessel Imaging (HDMI) [15], enhances the microvasculature through gathering ultrasound data with high frequency, and the tinny vessels with diameter over 300 μm can be obtained. There have been some researches [16, 24, 25] evaluating the value of microvasculature in the early detection of thyroid nodules, showing an exciting diagnostic value. Shi et al. [16] compared the quantitative factor, the density of vessels, with a qualitative evaluation and found that the sensitivity and accuracy reached 93.8% and 79.8% with the help of microvascular imaging. Kurti et al. [15] made a quantitative analysis between malignant and benign nodules, and the biomarkers with significant difference were found, indicating that PTC produced vascular changes compared to normal thyroid gland tissue.
Inspired by these previous works, the retrospective study was conducted, which focused on the thyroid nodules in AUS, aiming to develop a classification model to classify PTC and benign nodule with the combination of UMA and B-mode images The microvascular morphology of PTC as visualized by UMA imaging was hypothesized to be different in PTC and benign nodules. This study included 300 AUS thyroid nodules. The nodules were categorized based on the largest diameter (LD) into two groups: Group A (< 10 mm) and Group B (≥ 10 mm), following the World Health Organization’s definition of Papillary Thyroid Microcarcinoma (PTMC) [26]. Firstly, an algorithm of image processing was employed in order to weaken the artifact of UMA images and extract microvasculature. Then 18 quantitative biomarkers of the microvasculature were calculated from four perspectives, including the richness of vessels, vascular distribution, each vessel and each branch point. The biomarkers were selected as independent risk factors through single variable logistic regression and LASSO analysis. A logistic model was utilized to combine two kinds of TI-RADS with the selected biomarkers. In order to evaluate the performance, a 5-fold cross-validation experiment was implemented.
Methods
Patients
This retrospective study was approved by the institutional ethics committee of the participating institutions, Ethics Committee of Tianjin Medical University General Hospital (NO. IRB2024-YX-226-01). All patients provided written informed consent.
As shown in Fig. 1, patients who took FNA from September 2023 to March 2024 were considered for inclusion when the cytological pathology showed AUS. The FNA procedures were carried out by a doctor with over 20 years of experience in ultrasound intervention examination. Due to the unclear results of FNA biopsy, further examinations consisting of CNB surgery and Braf v600E genetic test were conducted.
Fig. 1.
The flowchart of the participants in this retrospective study. AUS: atypia of undetermined significance; PTC: Papillary Thyroid Carcinoma
In summary, the identification of PTC was based on CNB pathology and Braf v600E mutation, while the identification of benign nodules relied on surgical pathology or CNB pathology. The baseline features, including age and gender, were collected. The baseline features of these patients were listed in Table 1. The pathological types of the collected benign nodules were listed in Table S1.
Table 1.
Characteristics of patients and thyroid nodules
| Characteristic | Group A (< 10 mm) | Group B (≥ 10 mm) | ||
|---|---|---|---|---|
| Benign | PTC | Benign | PTC | |
| Baseline characteristics (independent sample: each nodule) | ||||
| Sex (male / female) | 10/48 | 32/72 | 18/42 | 20/58 |
| Age (year) | 48 ± 13 | 39 ± 10 | 45 ± 13 | 48 ± 13 |
| Number of nodules | 58 | 104 | 60 | 78 |
| ACR TI-RADS* | ||||
| 0–2 | 0/58 | 0/104 | 8/60 | 0/78 |
| 3 | 2/58 | 0/104 | 2/60 | 0/78 |
| 4–6 | 28/58 | 6/104 | 36/60 | 8/78 |
| > 6 | 28/58 | 98/104 | 14/60 | 70/78 |
| C TI-RADS** | ||||
| < 4a | 2/58 | 0/104 | 8/60 | 0/78 |
| 4a | 10/58 | 0/104 | 18/60 | 2/78 |
| 4b | 38/58 | 32/104 | 28/60 | 12/78 |
| 4c | 8/58 | 62/104 | 6/60 | 50/78 |
| > 4c | 0/58 | 10/104 | 0/60 | 14/78 |
| C TI-RADS characteristics | ||||
| Margin (irregular) | 2/58 | 30/104 | 2/60 | 38/78 |
| Orientation (taller-than-wide) | 14/58 | 72/104 | 6/60 | 54/78 |
| Echogenicity (markedly hypoechoic) | 16/58 | 46/104 | 6/60 | 42/78 |
| Composition (solid) | 52/58 | 104/104 | 40/60 | 74/78 |
| Calcification (microcalcifications) | 26/58 | 60/104 | 42/60 | 70/78 |
| ACR TI-RADS characteristics | ||||
| Margin (0/2/3) | 56/2/0 | 74/30/0 | 58/2/0 | 40/36/2 |
| Orientation (0/3) | 44/14 | 32/72 | 54/6 | 24/54 |
| Echogenicity (0/1/2/3) | 0/0/42/16 | 0/0/58/46 | 0/10/44/6 | 0/0/36/42 |
| Composition (0/1/2) | 0/6/52 | 0/0/104 | 2/18/40 | 0/4/74 |
| Calcification (0/1/2/3) | 32/10/0/16 | 44/4/0/56 | 18/28/0/14 | 8/16/0/54 |
Note. *: represent American College of Radiology Thyroid Imaging Reporting and Data System; **: represent Chinese Thyroid Imaging Reporting and Data System. Numerical data was represented as, number (total number)
Ultrasound image collection
Ultrasound examination was led by a doctor with over 20 years of clinical diagnostic experience. The images were collected from the ultrasound workstation (Mindray RESONA R9Q) and a high frequency probe (L15-3WU). The B-model and UMA-model ultrasound images were obtained in the same time to ensure consistency of surfaces. The UMA-model parameters were the Frequency (F, 8.3), the Gain value (G, 40–50). During the examination process, patients were required to lie on their back and hold breath.
Then the ultrasound video was transferred into frames, from which one image was selected based on the following principles. Firstly, the image must be in longitudinal section because of the location of the trachea and carotid artery. Secondly, the image must be close to the maximum longitudinal section of the whole nodule. Due to the incomplete two-dimensional microvasculature information, the UMA images with the maximum longitudinal section were collected to enhance the reproducibility and interpretability of the results. Thirdly, the nodules with coarse calcification or ring calcification inside the lesion were discarded since these features would reduce UMA rendering [22]. These calcified structures would hinder microvascular imaging.
Finally, two ultrasound doctors with 7 years of experience were required to assign scores to these nodules based on C TI-RADS and ACR TI-RADS by the images, respectively. When the scores by two doctors were different, the results were checked by one ultrasound doctor with over 20 years of experience. The boundaries of thyroid nodules were drawn by the two young doctors with the help of Software called Labelme (5.3.0) separately, and the boundary of a nodule was defined as the intersection of these two independent labels.
Image processing and quantitative biomarker calculation
Due to the current limitation of the non-invasive microvascular imaging technology, the vascular artifacts usually occurred accompanied by the real blood vessels. Frangi filtering [27] was an image enhancement technique used in medical imaging that primarily enhanced the features of blood vessels and other linear structures for precise detection and recognition of subtle cellular structures. The previous studies have shown the value of Frangi filtering in enhancing the micro-vessels in UMA images. However, Frangi filtering was prone to causing isolated background points. Additionally, the range of tumor vascular diameters was diverse. Therefore, a multi-scale Frangi filter [28] was used, and the kernel size was scaled from 1 × 1 to 20 × 20 to accommodate the diverse vascular diameters. The details were listed in Table 2. Additionally, the TOPHAT operation was applied after Frangi filtering in order to remove isolated background points.
Table 2.
The details of the image processing algorithm and the calculation of biomarkers
| Image Processing Algorithm | |
|---|---|
| Frangi Filter |
Scales: 1 × 1 to 20 × 20, step = 1 α = 0.5, β = 15 |
| Calculation |
NV: the number of vessels (longer than 3 pixels). NB: the number of branch points, which were defined as the points where over three vessels passed. NVR: the ratio between NV and the area of the lesions. NBR: the ratio between NB and the area of the lesions. |
The whole algorithm was shown in Fig. 2(A). Firstly, the original UMA images were enhanced by the multi-scale Frangi filter. Secondly, an adaptive binarization algorithm, Otsu, was used to preliminarily distinguish vessel structures from background regions. Thirdly, the TOPHAT operation was utilized to reduce background points. Then, the segmented ROI was transformed into the Hue, Saturation, and Value (HSV) space. Finally, the eight-neighborhood connectivity algorithm was employed to fill holes within segmented vessels. The entire algorithm is open-sourced on GitHub (https://github.com/wanna0122/uma_extraction.git).
Fig. 2.
The algorithm instruction for enhancing vessels in UMA images (A) and the process of analysis (B)
As listed in Tables 3 and 18 biomarkers were calculated for quantitative analysis of microvasculature morphology from four aspects, including the richness of vessels, vascular distribution, each vessel and each branch point. In total, there were 13 biomarkers which have been evaluated by other research [29, 30] and 5 biomarkers innovatively.
Table 3.
Summary of biomarkers definition
| Biomarkers | Definition |
|---|---|
| Richness of vessel | |
| VD | vascular density. Ratio of vascular area to lesion area. |
| NV | number of vessels. After removing the bifurcation point. the number of vascular segments. |
| NB | number of branches. Intersection of three or more blood vessels. |
| NVR | density of NV. Ratio of NV and lesion area. |
| NBR | density of NB. Ratio of NB and lesion area |
| Vascular distribution | |
| mvFD | microvessel fractal dimension. |
| VDR | ratio of central and surrounding area. Calculate the ratio between the central VD and the surrounding VD. |
| SVP | vascularity pattern (= 1: VDR > = 1; = 0: VDR < 1). |
| Each vessel | |
| Dmax* | maximum of diameter. |
| Dave* | average of diameter. |
| Tmax | maximum of tortuosity. Tortuosity was defined as the ratio of the path distance between two vertices of blood vessels to the Euclidean distance. |
| Tave | average of tortuosity. |
| Each branch point | |
| MDmax | maximum of Murray’s deviation. For vessels connected to one branch point, the vessel with the largest diameter was defined as the mother branch (V-mother), and the other branches were child branches (V-child) **. |
| MDave | average of Murray’s deviation |
| MDmin | minimum of Murray’s deviation |
| BAmax+ | maximum of bifurcation angle. The bifurcation angle was defined as the angle between two V-childs. If the number of V-childs is over 2, select the largest bifurcation angle. |
| BAave+ | average of bifurcation angle |
| BAmin+ | minimum of bifurcation angle |
Note. *: the unit is centimeters (cm). **: Dmon is the diameter of V-mother; Dchild is the diameter of V-child
+: the unit is angle (°). max: maximum. ave: average. min: minimum. V-: Vessel-
For the richness of vessel, five biomarkers were calculated, including the vascular density (VD), the number of vessels (NV), the number of branches (NB), the density of NV (NVR) and the density of NB (NBR). The calculating details were listed in Table 2.
In the vascular distribution, three biomarkers were calculated. The ratio of central and surrounding area (VDR) and the vascularity pattern (SVP) represent the relationship between the central vessels and the surrounding vessels in the lesion. The equation was,
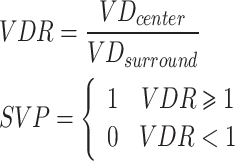
The biomarker, microvessel fractal dimension (mvFD) [30], was used to evaluate the complexity of vascular distribution. The calculation process was shown in Fig. 3. The Set 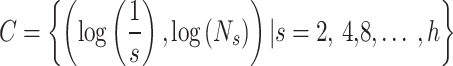 was a set of coordinate points, and Ns was the number required to cover the whole vascular area by a kernel with s×s size. h was the size of the image. Then these points were drawn into a coordinate and was synthesized as a straight line through the least square method. The slope of the line was the biomarker, mvFD.
was a set of coordinate points, and Ns was the number required to cover the whole vascular area by a kernel with s×s size. h was the size of the image. Then these points were drawn into a coordinate and was synthesized as a straight line through the least square method. The slope of the line was the biomarker, mvFD.
Fig. 3.
The calculation of the biomarker, microvessel fractal dimension (mvFD)
For each vessel, the diameter each vessel branch was calculated. Then the maximum of diameters (Dmax) and the average of diameters (Dave) of all branches were chosen for further analysis. The maximum and average of tortuosity (Tmax, Tave) were also counted, while the tortuosity was defined as,
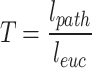
where lpath was the distance between two extreme points of one vessel; leuc was the euclidean distance.
There were six biomarkers in the aspect of each branch point. The Murray’s deviation (MD) [31, 32] was calculated, representing the relationship of diameters between the mother vessel and the sub-vessels in one branch point. The maximum, average and minimum of MD (MDmax, MDave, MDmin) as three biomarkers were calculated. The bifurcation angle (BA) of each branch point represented the maximum angle between two sub-vessels, ranging from 0° to 180°. For each lesion, three biomarkers were analyzed, including the maximum, average and minimum of BA (BAmax, BAave, BAmin).
Statistical analysis
The univariable analysis and multivariable analysis were used to evaluate the effect of the above biomarkers on PTC diagnosis. Since the microvasculature was closely relevant with the size of thyroid nodules, the nodules were separated into two groups based on the LD. Group A included the small nodules with the LD smaller than 10 mm, while Group B included the relatively large nodules with the LD larger than 10 mm. The criterion for grouping based on a maximum diameter of 10 mm is that PTCs smaller than 10 mm are defined as Papillary thyroid microcarcinoma (PTMC) according to World Health Organization (WHO) [33].
The analyzed features included 18 biomarkers of microvasculature, age, gender and the LD. In the univariable analysis, the univariable Logistic Regression (LR) analysis were used to assess the differences of biomarkers between PTC and benign nodules. The biomarkers were selected with P value less than 0.05. The biomarker, SVP, was evaluated by Chi-squared (χ2) Test. The multivariable analysis was conducted through LASSO regression analysis. The alpha was set as -10 to 10, and the best alpha was chosen while the Mean Squared Error (MSE) was minimal.
Finally, a multi-variable LR model was trained and tested through a 5-fold cross validation experiment. Two kinds of TI-RADS were combined with the selected biomarkers of microvasculature. All the analysis was conducted by two authors in the computational environment of Python (3.8).
Results
Patient baseline features
As shown in Fig. 1, this study enrolled 281 patients with 300 thyroid nodules, all showing AUS after FNA biopsy. The further examination consisted of CNB pathology examination and genetic test. Because some patients decided to take thyroid nodule resection surgery, there were a few benign nodules identified according to the surgical pathology.
These cases were divided into two groups, A and B, based on whether the LD was over 10 mm or not. Table 1 listed the details of these participants. Group A included 58 benign nodules (mean age, 48 ± 13[SD]; gender, 48 of 58 (82.7%) females) and 104 PTCs (mean age, 39 ± 10[SD]; gender, 72 of 104 (69.2%) females). Group B had the lesions over than 10 mm, including 60 benign nodules (mean age, 45 ± 13[SD]; gender, 42 of 60 (70.0%) females) and 78 PTCs (mean age, 48 ± 13[SD]; gender, 58 of 78 (74.4%) females). The sizes of nodules in Group A ranged from 4 mm to 10 mm, while the sizes ranged from 10 mm to 30 mm in Group B.
The scores of C TI-RADS and ACR TI-RADS by two doctors and one senior doctor were also listed in Table 1. In Group A, the best decision boundary of C TI-RADS (AUC = 0.816) and ACR TI-RADS (AUC = 0.731) was level 4c or score 3. In Group B, the best decision boundary of C TI-RADS (AUC = 0.898) and ACR TI-RADS (AUC = 0.841) was also Level 4c or Score 3. The details were drawn in Fig. 4.
Fig. 4.
The Receiver Operating Characteristic (ROC) curves for C TI-RADS and ACR TI-RADS in the participants. A1 was in Group A, while B1 was in Group B
Visualization of image processing
There were two samples of PTCs and two samples of benign nodules in Fig. 5 randomly selected for visualization of intermediate results. Through this algorithm, the mask of microvasculature and the skeletonize was obtained.
Fig. 5.
Visualization of Image Processing Algorithm and the pathological images. A1 and B1 were two benign nodules; A2 and B2 were two PTCs
This step was important due to the phenomenon of artifacts in the current UMA technology. The artifacts were usually caused by the breathing of patients or the inappropriate Gain parameter, G. Although patients were asked to reduce their breathing during the collection process, it was still unavoidable to produce a certain degree of artifacts.
The algorithm were inspired by a few previous studies [29, 34]. The principle of Frangi filters was to find tubular structures in one frame, and the algorithm could weaken the unnecessary artifacts. In summary, not only the background information was filtered out, but also the phantom was reduced. After the image processing algorithm, one microvasculature mask image and the microvasculature skeleton diagram were obtained.
Statistical analysis results
The single-variable analysis was by the LR analysis and χ2 test. The analysis results were delineated in Table 4. Group A found 8 biomarkers with significant difference between PTCs and benign nodules, including NV (P = 0.002, 95%CI=(1.695, 10.412)), NVR (P = 0.004, 95%CI=(1.429, 6.986)), NB (P = 0.002, 95%CI=(1.692, 10.714)), NBR (P = 0.005, 95%CI=(1.394, 6.656)), Dave (P = 0.048, 95%CI=(0.351, 0.996)), MDmax (P = 0.047, 95%CI=(1.008, 2.985)), mvFD (P = 0.016, 95%CI=(1.124, 3.158)), BAmin (P = 0.003, 95%CI=(0.270, 0.772)). For Group B, there were 4 biomarkers that were selected, including NVR (P = 0.002, 95%CI=(1.583, 8.169)), NBR (P = 0.004, 95%CI=(1.397, 6.069)), MDmax (P = 0.044, 95%CI=(1.014, 2.890)) and mvFD (P = 0.003, 95%CI=(1.460, 6.344)).
Table 4.
The results of single-variable analysis for 18 UMA biomarkers and baseline features
| Factors | Group A | Group B | ||||
|---|---|---|---|---|---|---|
| OR+ | 95%CI++ | P | OR | 95%CI | P | |
| NV | 4.201 | (1.695, 10.412) | 0.002* | 1.741 | (0.896, 3.381) | 0.102 |
| NVR | 3.159 | (1.429, 6.986) | 0.004* | 3.596 | (1.583, 8.169) | 0.002* |
| NB | 4.257 | (1.692, 10.714) | 0.002* | 1.773 | (0.896, 3.510) | 0.100 |
| NBR | 3.047 | (1.394, 6.656) | 0.005* | 2.912 | (1.397, 6.069) | 0.004* |
| VD | 1.084 | (0.678, 1.733) | 0.735 | 1.736 | (0.983, 3.066) | 0.057 |
| VDR | 0.523 | (0.187, 1.460) | 0.216 | 1.032 | (0.638, 1.668) | 0.899 |
| D max | 0.881 | (0.564, 1.376) | 0.576 | 1.255 | (0.740, 2.126) | 0.399 |
| D ave | 0.591 | (0.351, 0.996) | 0.048* | 1.033 | (0.636, 1.677) | 0.897 |
| MD max | 1.735 | (1.008, 2.985) | 0.047* | 1.712 | (1.014, 2.890) | 0.044* |
| MD min | 0.781 | (0.493, 1.238) | 0.293 | 0.309 | (0.062, 1.545) | 0.153 |
| MD ave | 1.137 | (0.721, 1.792) | 0.581 | 1.027 | (0.635, 1.662) | 0.913 |
| mvFD | 1.884 | (1.124, 3.158) | 0.016* | 3.044 | (1.460, 6.344) | 0.003* |
| T max | 1.497 | (0.820, 2.733) | 0.189 | 0.760 | (0.444, 1.299) | 0.316 |
| T ave | 1.157 | (0.724, 1.849) | 0.543 | 0.647 | (0.390, 1.073) | 0.092 |
| BA max | 1.156 | (0.739, 1.809) | 0.526 | 1.558 | (0.840, 2.892) | 0.160 |
| BA min | 0.456 | (0.270, 0.772) | 0.003* | 0.672 | (0.402, 1.122) | 0.128 |
| BA ave | 0.910 | (0.573, 1.444) | 0.688 | 0.597 | (0.351, 1.016) | 0.057 |
| LD − | 0.538 | (0.035, 8.257) | 0.657 | 0.368 | (0.132, 1.031) | 0.057 |
| SVP | 0.452 | (0.161, 1.268) | 0.131 | 1.202 | (0.451, 3.198) | 0.713 |
| Gender | 2.133 | (0.690, 6.599) | 0.188 | 0.805 | (0.278, 2.325) | 0.688 |
Note. --- the qualitive variable, SVP, was summarized as number of 0 / number of 1. +: Odds Ratio; ++: 95% Confidence Interval; −: the largest diameter (cm) of nodules; *: variables showing significant difference (P < 0.05) in analysis
The selected biomarkers by the single-variable analysis were then input into the multi-variable analysis model, LASSO. The process of filtering variables was shown in Fig. 6. The variables with non-zero coefficients when the MSE was minimum were selected as the input variables for subsequent modeling. As shown in Fig. 6(A1) and Fig. 6(B1), Group A found 4 biomarkers, including NV, Dave, mvFD, BAmin. Figure 6(A2) and Fig. 6(B2) implemented that there were four biomarkers with significant difference in Group B, including NVR, NBR, MDmax and mvFD. It was obvious that the number of vessel branches and the number of branch points in PTCs was higher than the number in benign nodules. The average diameter of vessels in Group A showed a negative correlation with PTCs. The MDmax showed a positive correlation with PTCs in Group B. Moreover, the biomarker, mvFD, was a positive variable in both two groups.
Fig. 6.
The regression coefficient path diagrams, Mean Squared Error (MSE) chart and final coefficient histogram by LASSO regression analysis. (A1), (B1) and (C1) was in Group A, while (A2), (B2), (C2) was in Group B
Model performance evaluation
The performance of the proposed LR models was shown in Figs. 7 and 8. The 5-fold cross-validation experiment was conducted on the purpose of improving the results’ reliability. We compared the mean AUC values by C TI-RADS, ACR TI-RADS, UMA biomarkers, C TI-RADS + UMA biomarkers and ACR TI-RADS + UMA biomarkers. Group A showed increases from 0.809 with C TI-RADS to 0.882 (P = 0.037) and from 0.725 with ACR TI-RADS to 0.851 (P = 0.028) after adding UMA biomarkers. Group B also showed improvements from 0.841 with ACR TI-RADS to 0.874 (P = 0.057) and from 0.894 with C TI-RADS to 0.936 (P = 0.046). The results implemented that the UMA biomarkers had potential of improving the performance of the ultrasound diagnosis. The Decision curve analysis (DCA) was shown in Figs. 9, implementing the value of the selected UMA biomarkers.
Fig. 7.
The ROC curves of the 5-fold cross validation experiments in Group A. A, B, C, D, E was with C TI-RADS, ACR TI-RADS, UMA biomarkers, C TI-RADS + UMA biomarkers and ACR TI-RADS + UMA biomarkers, separately
Fig. 8.
The ROC curves of the 5-fold cross validation experiments in Group B. A, B, C, D, E was with C TI-RADS, ACR TI-RADS, UMA biomarkers, C TI-RADS + UMA biomarkers and ACR TI-RADS + UMA biomarkers, separately
Fig. 9.
The Decision curve analysis (DCA) of Group A and Group B in 4 different models. A1-A4 were for Group A, while B1-B4 were for Group B
Table 5 implemented the comparison of TI-RADS-based models and TI-RADS & UMA-based models. The mean AUC of the 5-fold cross-validation experiment was tested through Delong Test. Four groups of models were compared, including two different TI-RADS and two different subgroups. All groups showed statistically significant difference (P < 0.05), except the Group B when using ACR TI-RADS (P = 0.057). In Group A, when using C TI-RADS, the F1-Score, Accuracy, Recall and AUC all showed increase, while the precision decreased from 0.905 to 0.860. This phenomenon is related to the probability threshold of 0.5. It is noteworthy that when using ACR TI-RADS, the recall rate remains at 1.0. This is consistent with the fact that the number of samples with ACR TI-RADS scores below 3 recorded in Table 1 is zero. This aligned with the distribution of B-mode ultrasound imaging for patients with AUS targeted by this work. Two studies similar to our research are listed in Table 6. Compared to these previous studies, the proposed method demonstrates superior diagnostic performance (average AUC value of 0.909 and 0.8625 corresponding to models using C TI-RADS and ACR TI-RADS) by leveraging 18 biomarkers and a significantly larger dataset (182 malignant, 118 benign), particularly when utilizing C TI-RADS.
Table 5.
Comparison of 5-Fold Cross-Validation experiments for four different diagnostic models and Delong test
| Metrics | C TI-RADS | C TI-RADS & UMA |
P | ACR TI-RADS | ACR TI-RAD & UMA |
P |
|---|---|---|---|---|---|---|
| Group A | ||||||
| F1-Score | 0.780 ± 0.102 | 0.867 ± 0.100 | - | 0.781 ± 0.018 | 0.796 ± 0.060 | - |
| Accuracy | 0.754 ± 0.110 | 0.829 ± 0.123 | - | 0.641 ± 0.024 | 0.728 ± 0.086 | - |
| Precision | 0.905 ± 0.086 | 0.860 ± 0.103 | - | 0.642 ± 0.024 | 0.777 ± 0.078 | - |
| Recall | 0.698 ± 0.140 | 0.887 ± 0.133 | - | 1.000 ± 0.000 | 0.829 ± 0.111 | - |
| AUC | 0.809 ± 0.108 | 0.882 ± 0.063 | 0.037* | 0.725 ± 0.121 | 0.851 ± 0.075 | 0.028* |
| Group B | ||||||
| F1-Score | 0.865 ± 0.057 | 0.853 ± 0.043 | - | 0.722 ± 0.011 | 0.856 ± 0.087 | - |
| Accuracy | 0.855 ± 0.064 | 0.840 ± 0.035 | - | 0.565 ± 0.013 | 0.838 ± 0.090 | - |
| Precision | 0.927 ± 0.093 | 0.877 ± 0.068 | - | 0.565 ± 0.013 | 0.852 ± 0.095 | - |
| Recall | 0.818 ± 0.071 | 0.843 ± 0.102 | - | 1.000 ± 0.000 | 0.868 ± 0.120 | - |
| AUC | 0.894 ± 0.052 | 0.936 ± 0.032 | 0.046* | 0.841 ± 0.058 | 0.874 ± 0.046 | 0.057 |
Note. --- the threshold when calculating F1-Score, Accuracy, Precision, Recall was 0.5 (C TI-RADS via UMA and ACR TI-RADS via UMA), Level 4c (C TI-RADS), Score 3 (ACR TI-RADS)
Table 6.
Comparison between the proposed model and the previous studies
| Studies | Biomarkers | TI-RADS | Dataset | AUC |
|---|---|---|---|---|
| Shi et al. [16] | VD | C TI-RADS |
81 malignant 28 benign |
0.666 95% CI: (0.569–0.753) |
| Kurti et al. [15] | 12 biomarkers | ACR TI-RADS |
38 malignant 57 benign |
0.9044 95% CI: (0.833–0.976) |
| Current study | 18 biomarkers | C TI-RADS |
182 malignant 118 benign- |
< 10 mm: 0.882 ± 0.063 ≥ 10 mm: 0.936 ± 0.032 |
|
Current study |
18 biomarkers | ACR TI-RADS |
182 malignant 118 benign |
< 10 mm: 0.851 ± 0.075 ≥ 10 mm: 0.874 ± 0.046 |
Note. --- Kurti’s study directly split the dataset into training and testing sets, and therefore the AUC values are reported with 95% confidence intervals. In contrast, our model used cross-validation, and the AUC values are reported as the mean ± standard deviation across folds
Discussion
Currently, thyroid nodules classified as AUS [35] require either a repeat fine-needle aspiration (FNA) or genetic testing [36], such as BRAF V600E analysis [10], which is invasive or expensive. Despite the established association between malignant tumor proliferation and the formation of new blood vessels, conventional Color Doppler Flow Imaging [37] is unable to detect these new vessels. The UMA technology enables imaging of low-flow and small-diameter blood vessels through ultra-fast ultrasound acquisition and spatiotemporal filtering. This work focused on the classification of PTC and benign thyroid nodules showing AUS. In order to avoid the statistical impact of the tumor size on vascular biomarkers [38], these nodules were divided into two groups, Group A (< 10 mm) and Group B (≥ 10 mm). With the addition of the selected four UMA biomarkers, Group A showed the mean Area Under Curve (AUC) improving from 0.725 ± 0.121 to 0.851 ± 0.075 (P < 0.05) when using ACR TI-RADS and from 0.809 ± 0.108 to 0.882 ± 0.063 (P < 0.05) with C TI-RADS. Group B achieved an increase in the mean AUC value from 0.841 ± 0.058 to 0.874 ± 0.046 when combined with ACR TI-RADS, and from 0.894 ± 0.052 (P < 0.05) to 0.936 ± 0.032 (P < 0.05) when combined with C TI-RADS. Additionally, an open-sourced UMA image post-processing algorithm was developed for enhancing micro-vessels and suppressing artifacts. The experiment also implemented that the microvasculature of PTC exhibited changes in different aspects at different LD groups. The proposed model showed the potential in accurate diagnosis of PTC without additional examinations and avoiding unnecessary repeat FNA and expensive genetic test.
Although our results showed favorable increase both in Group A and Group B, it was interesting that there was only one vascular biomarker that was the same between the two groups. In both groups, PTC had a higher mvFD value, meaning that the distribution of blood vessels was broader and more complex in tumor lesions. This finding was similar with Kuiti [15] (thyroid cancer) and Ternifi [29] (breast cancer). After U-test and LASSO regression, Group A retained 4 biomarkers, including Bamin (P = 0.003, 95%CI = (0.270,0.772)), mvFD (P = 0.016, 95%CI = (1.124,3.158)), Dave (P = 0.048, 95%CI = (0.351,0.996)) and NV (P = 0.002, 95%CI = (1.695,10.412)). The Bamin was the minimum angle of the vascular bifurcation. Malignant tumors are usually accompanied by the formation of new blood vessels, which will lead to a decrease in BAmin. The results of the LASSO regression also confirmed this theory. As shown in Fig. 6C1, the weight of Bamin was − 0.056, meaning that in PTCs smaller than 10 mm, the vascular branching angles were smaller than those of benign nodules. The weight of Dave was − 0.085, which meant that the average diameter of PTC (< 10 mm) was smaller than benign nodules. This phenomenon could also be explained by the increased formation of new blood vessels in tumors. While Dmax didn’t show significant difference between PTC and benign nodules (P = 0.576), the number of vessels showed increased from benign nodules to PTC (P = 0.002*). The LASSO regression also retained the biomarker, NV, the weight of which was 0.044. As for Group B, there were the other three biomarkers retained, including MDmax (P = 0.044, 95%CI = (1.014,2.890)), NBR (P = 0.004, 95%CI = (1.397,6.069)) and NVR (P = 0.002, 95%CI = (1.583,8.169)). The higher MDmax in PTC lesions meant that the thicker main blood vessels and thinner branch blood vessels. It was interesting to find that the NVR (weight = 0.665) and NBR (weight=-0.563) had opposite weight signs. This meant that in the PTC (≥ 10 mm), there were more vascular branching points with more than three blood vessels connected with each other.
The diagnostic value of microvasculature in detecting PTC has been proved by a few previous studies [15, 16]. Shi et al. [16] focused on the comparison of quantitative and qualitative evaluation of microvasculature, concluding that there were no differences between these two evaluation ways. But the quantitative biomarker only included the VD, which was relevant with the vascular richness, lacking the information on distribution and vascular connectivity. Kurti et al. [15] firstly made a relatively comprehensive analysis through 13 biomarkers and single-variable analysis. Our work was their continuation. 18 biomarkers were analyzed through single-variable LR analysis and multi-variable LASSO analysis. In order to avoid the relationship between the nodule size and vascular richness, the participants were divided into two groups based on the LD. However, the study of Kurti et al. used a smaller and unevenly distributed dataset, and their malignant nodule group included all types of malignant nodules. In contrast, our study focused solely on nodules classified as AUS, which increased the diagnostic difficulty. This difference in inclusion criteria may explain why their AUC value appears higher than that of our model. There were plenty of patients showing low risk level by original B-mode ultrasound examination, and the FNA biopsy was unnecessary. This study didn’t include these patients due to the missing pathological result. With the help of the clinical data, the proposed method was proved to be useful for the early diagnosis of PTCs when showing AUS without any further examination.
However, there were a few limitations. The maximum limitation was lacking 3-dimension (3D) structural information. In this study, the imaging section was set on the maximum longitudinal section on the purpose of diluting this limitation. But the 3D view would provide a more comprehensive information. Therefore, the following step of our work was the construction of a 3D scanning platform and data evaluation. What’s more, the conclusions would be verified in multiple centers in the future. Finally, to analyze the role of micro-vessels in PTC, particularly in slowly developing cancers like PTMC, further animal experiments will be conducted to observe the morphological changes of micro-vessels during the progression of PTC.
Conclusions
In conclusion, this work made an analysis of the tumor microvasculature in four aspects, which was inspired by clinical ultrasound guidelines, and successfully found a multi-mode diagnostic method for detecting PTC non-invasively. It was a successful exploration for the early diagnosis of PTC with AUS without any further examination. However, the selected biomarkers were different in two Groups. The morphology of microvasculature changed differently at different LD groups. The further works would be conducted in 3D scanning and analysis as well as verifying our findings in multiple centers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank for the machine provided by Mindray as well as the help of doctors from the Pathology Department of Tianjin Medical University General Hospital.
Abbreviations
- UMA
Ultra Micro-angiography
- PTC
Papillary Thyroid Carcinoma
- TI-RADS
Thyroid Imaging Reporting and Data System
- LD
Largest dimension
- AUC
Area Under the Curve
- PTMC
Papillary Thyroid Microcarcinoma
- AUS
Atypia of undetermined significance
Author contributions
QW and ZL: software & writing-original draft; HY: visualization; SZ and JL: data annotation & validation; LW, SW and JS: writing-review; WZ: data analysis; JZ and HY: funding & project permission. All authors read and approved the final manuscript.
Funding
This research was funded by Tianjin Major Special Projects and Projects, China (23YFZCSN00070); Ultrasound guided microwave ablation of thyroid nodules (NO. 22TSC023- 303078200323) from Tianjin Medical University General Hospital; Tianjin Health Technology Project (NO.20188).
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study was approved by the institutional ethics committee of the participating institutions, Ethics Committee of Tianjin Medical University General Hospital (NO. IRB2024-YX-226-01). The participants were included followed with the Helsinki Declaration. All person information were excluded.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qingsong Wang, Zhewei Li and Hongjian Yao share first authorship.
Contributor Information
Jie Zhang, Email: wxq_bf@163.com.
Hui Yu, Email: yuhui@tju.edu.cn.
References
- 1.Chen DW, Lang BHH, McLeod DSA, Newbold K, Haymart MR. Thyroid cancer. Lancet. 2023;401:1531–44. [DOI] [PubMed] [Google Scholar]
- 2.Boucai L, Zafereo M, Cabanillas ME. Thyroid Cancer: Rev JAMA. 2024;331:425–35. [DOI] [PubMed]
- 3.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid Cancer incidence and mortality in the united States, 1974–2013. JAMA. 2017;317:1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The diagnosis and management of thyroid nodules: A review. JAMA. 2018;319:914–24. [DOI] [PubMed] [Google Scholar]
- 5.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587–95. [DOI] [PubMed] [Google Scholar]
- 6.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315–89. [DOI] [PubMed] [Google Scholar]
- 7.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: A step in Establishing better stratification of Cancer risk. Radiology. 2011;260:892–9. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine. 2020;70:256–79. [DOI] [PubMed] [Google Scholar]
- 9.Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid®. 2023;:thy.2023.0141. [DOI] [PubMed]
- 10.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu X-Y, Song X-Y, Huang Z-L, Hu Q-H, Chen S-X, Fan X-M. Role of Core-Needle biopsy in thyroid nodules with initially nondiagnostic cytologic results. Radiology. 2014;270:629–30. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor JPB, Aboagye EO, Adams JE, Aerts HJWL, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14:169–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pai S, Bontempi D, Hadzic I, Prudente V, Sokač M, Chaunzwa TL, et al. Foundation model for cancer imaging biomarkers. Nat Mach Intell. 2024;6:354–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan S, Sun P-F, Xue H, Fu S, Zhang Z-P, Mei F, et al. Evaluation of thyroid micro-carcinoma using shear wave elastography: initial experience with qualitative and quantitative analysis. Eur J Radiol. 2021;137:109571. [DOI] [PubMed] [Google Scholar]
- 15.Kurti M, Sabeti S, Robinson KA, Scalise L, Larson NB, Fatemi M, et al. Quantitative biomarkers derived from a novel Contrast-Free ultrasound High-Definition microvessel imaging for distinguishing thyroid nodules. Cancers. 2023;15:1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi X, Liu R, Xia Y, Gao L, Da W, Li X, et al. Qualitative and quantitative Superb vascular imaging in the diagnosis of thyroid nodules ≤ 10 mm based on the Chinese thyroid imaging reporting and data system 4 (C-TIRADS 4). Quant Imaging Med Surg. 2023;13:3213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamma R, Ingravallo G, Annese T, d’Amati A, Lorusso L, Ribatti D. Tumor microenvironment and microvascular density in human glioblastoma. Cells. 2022;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder R-J, Bostanjoglo M, Rademaker J, Maeurer J, Felix R. Role of power doppler techniques and ultrasound contrast enhancement in the differential diagnosis of focal breast lesions. Eur Radiol. 2003;13:68–79. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Wu H, Zhou Q, Gou J, Xu J, Liu Y, et al. Diagnostic value of conventional ultrasonography combined with Contrast-Enhanced ultrasonography in thyroid imaging reporting and data system (TI-RADS) 3 and 4 thyroid micronodules. Med Sci Monit. 2016;22:3086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du J, Wang L, Wan C-F, Hua J, Fang H, Chen J, et al. Differentiating benign from malignant solid breast lesions: combined utility of conventional ultrasound and contrast-enhanced ultrasound in comparison with magnetic resonance imaging. Eur J Radiol. 2012;81:3890–9. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Li Y, Long M, Li J, Ma J, Luo Y. Vascularity depicted by contrast-enhanced ultrasound predicts recurrence of papillary thyroid cancer. Eur J Radiol. 2023;159:110667. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Wang Q, Wang M, Tao X, Liu S, Qi Z, et al. Ultra-microangiography in evaluating the disease activity of rheumatoid arthritis and enhancing the efficacy of ultrasonography: A preliminary study. Eur J Radiol. 2021;137:109567. [DOI] [PubMed] [Google Scholar]
- 23.Lu R, Meng Y, Zhang Y, Zhao W, Wang X, Jin M, et al. Superb microvascular imaging (SMI) compared with conventional ultrasound for evaluating thyroid nodules. BMC Med Imaging. 2017;17:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn HS, Lee JB, Seo M, Park SH, Choi BI. Distinguishing benign from malignant thyroid nodules using thyroid ultrasonography: utility of adding Superb microvascular imaging and elastography. Radiol Med. 2018;123:260–70. [DOI] [PubMed] [Google Scholar]
- 25.Kong J, Li J, Wang H, Wang Y, Zhao R, Zhang Y, et al. Role of Superb Micro-Vascular imaging in the preoperative evaluation of thyroid nodules: comparison with power doppler flow imaging: Superb Micro-Vascular imaging of thyroid nodules. J Ultrasound Med. 2017;36:1329–37. [DOI] [PubMed] [Google Scholar]
- 26.Seifert G, Brocheriou C, Cardesa A, Eveson JW. WHO international histological classification of tumours tentative histological classification of salivary gland tumours. Pathol - Res Pract. 1990;186:555–81. [DOI] [PubMed] [Google Scholar]
- 27.Ghavami S, Bayat M, Fatemi M, Alizad A. Quantification of morphological features in Non-Contrast-Enhanced ultrasound microvasculature imaging. IEEE Access. 2020;8:18925–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frangi A, Niessen WJ, Vincken K, Viergever M. Multiscale vessel enhancement filtering. Med Image Comput Comput Assist Interv. 2000;1496.
- 29.Ternifi R, Wang Y, Gu J, Polley EC, Carter JM, Pruthi S, et al. Ultrasound high-definition microvasculature imaging with novel quantitative biomarkers improves breast cancer detection accuracy. Eur Radiol. 2022;32:7448–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ternifi R, Wang Y, Polley EC, Fazzio RT, Fatemi M, Alizad A. Quantitative biomarkers for Cancer detection using Contrast-Free ultrasound High-Definition microvessel imaging: fractal dimension, Murray’s deviation, bifurcation angle & Spatial vascularity pattern. IEEE Trans Med Imaging. 2021;40:3891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman TF. On connecting large vessels to small. The meaning of Murray’s law. J Gen Physiol. 1981;78:431–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taber LA, Ng S, Quesnel AM, Whatman J, Carmen CJ. Investigating Murray’s law in the chick embryo. J Biomech. 2001;34:121–4. [DOI] [PubMed] [Google Scholar]
- 33.Gao M, Ge M, Ji Q, Cheng R, Lu H, Guan H, et al. 2016 Chinese expert consensus and guidelines for the diagnosis and treatment of papillary thyroid microcarcinoma. Cancer Biol Med. 2017;14:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayat M, Fatemi M, Alizad A. Background removal and vessel filtering of Noncontrast ultrasound images of microvasculature. IEEE Trans Biomed Eng. 2019;66:831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steward DL, Carty SE, Sippel RS, Yang SP, Sosa JA, Sipos JA, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology. JAMA Oncol. 2019;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaprak Bayrak B, Eruyar AT. Malignancy rates for Bethesda III and IV thyroid nodules: a retrospective study of the correlation between fine-needle aspiration cytology and histopathology. BMC Endocr Disord. 2020;20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leng X, Liu J, Zou Q, Wang C, Yang S. Application of color doppler ultrasound and US shear wave elastography with connective tissue growth factor in the risk assessment of papillary thyroid carcinoma. BMC Med Imaging. 2024;24:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forster JC, Harriss-Phillips WM, Douglass MJ, Bezak E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia (Auckl). 2017;5:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.











