Abstract
Background
The prognosis for atrial fibrillation (AF) patients is based on data that is decades old. Given evolving standards of clinical practice, we sought to evaluate temporal trends in clinically important outcomes among patients with AF.
Methods and results
California's Department of Health Care Access and Information databases were used to identify adults aged ≥18 years with AF receiving hospital-based care in California. We compared three time-periods: 2005–2009, 2010–2014, and 2015–2019. International Classification of Diseases codes were used to identify chronic diseases and acute events. The outcomes were incident ischaemic stroke, intracranial haemorrhage, and overall mortality. We included 2 009 832 patients with AF (52.7% males, 70.7% Whites, and mean age of 75.0 years), divided in three cohorts: 2005–2009 (n = 738 954), 2010–2014 (n = 609 447), and 2015–2019 (n = 661 431). Each outcome became substantially less common with time: compared with 2005–2009, AF patients diagnosed in 2015–2019 experienced a 34% (adjusted hazard ratio [HR] 0.66, 95% confidence interval 0.64–0.69), 22% (HR 0.78, 0.75–0.82), and 24% (HR 0.76, 0.75–0.77) reduction in risk of incident ischaemic stroke, intracranial haemorrhage, and mortality, respectively. Between 2005–2009 and 2015–2019, patients aged ≥65 years experienced more reductions in each outcome compared with younger patients (P < 0.001 for all), and declines in each outcome were significantly lower for Hispanics and Blacks compared with white patients.
Conclusion
The risks of stroke, intracranial haemorrhage, and death have significantly declined among AF patients, although differences in the magnitude of improvement of these outcomes by demographic groups were observed. Commonly described estimates of the prognosis for AF patients should be updated to reflect contemporary care.
Keywords: Atrial fibrillation, Stroke, Mortality, Haemorrhage
Graphical Abstract
Graphical Abstract.
Introduction
Atrial fibrillation (AF) is an important contributor to the burden of disease in the United States and globally.1 Atrial fibrillation accounts for up to a third of all ischaemic strokes,2 and AF-related strokes are associated with higher morbidity and mortality compared with strokes from non-AF causes.3 Furthermore, AF is associated with an increased risk of mortality.4
The magnitude of adverse health effects of AF was determined mostly by studies that are now decades old and no longer reflect current practice patterns. Most occurred before guidelines clearly described the need for anticoagulation and prior to the broad use of risk prediction scores (such as the CHA2DS2-VASc score) to provide objective, uniform guidance regarding risks vs. benefits of anticoagulation therapies.5,6 When anticoagulation was given, warfarin was the mainstay, which carried a significantly higher risk of intracranial haemorrhage than currently available direct oral anticoagulants (DOACs). While the first DOAC was approved in 2010, prescriptions for those safer and more effective drugs began to exceed those for warfarin by 2013.7 Because of the convenience of DOACs, subsequent evidence also demonstrated that uptake of and compliance with these drugs are substantially higher than with warfarin.7–9 Also in recent years, catheter ablation has become safer, more effective, and more widespread, with growing evidence, the procedure may lead to improved outcomes in AF patients.10–12 Finally, observational studies and randomized trials have demonstrated that lifestyle factors may be modified to improve AF burden among those with the disease,13–17 with some evidence those lessons have penetrated clinical practice.17
Whether these changes have impacted outcomes among those with AF to a meaningfully degree that can be detected in the general population remains unknown. We therefore sought to characterize modern prognostication among patients with AF and compare more recent outcomes to those observed in the past.
Methods
Data source, study design, and population
This was a cohort study using the California's Department of Health Care Access and Information (HCAI). Death information was retrieved from both the healthcare databases (for in-hospital mortality) and the California Comprehensive Death File. We included individuals aged ≥18 years with AF who received care in an ambulatory surgery unit, emergency department, or inpatient hospital between 1 January 2005 and 31 December 2019. Patients were not included based on the presence of atrial flutter. Healthcare encounters with missing data (age, sex, race, and ethnicity) were excluded. We excluded AF coded within 30 days of an open cardiac surgery as post-operative AF likely represents a different mechanism and may resolve with lower risks of AF-associated outcomes.18 Patients were divided into three mutually-exclusive groups, each corresponding to a 5-year period according to the time of first AF diagnosis in the databases: 1st January 2005–31st December 2009, 1st January 2010–31st December 2014, and 1st January 2015–31st December 2019. Patients could belong to only one the three period cohorts.
Diagnosis ascertainment
Diagnoses were identified through the International Classification of Diseases, Ninth Revision (ICD-9) code from January 2005 to September 2015 and the International Classification of Diseases, Tenth Revision (ICD-10) codes from October 2015 to December 2019 (Supplementary material online, Table S1). In addition to ICD-9 and ICD-10 codes, we used the Clinical Classification Software (CCS) codes for diseases with specific CCS codes (Supplementary material online, Table S1). Cardiac surgical procedures were identified using ICD-9 (Supplementary material online, Table S2) and ICD-10 codes (Supplementary material online, Table S3).
Study variables and outcomes
We gathered information regarding patients’ demographics, including age, sex, race, and ethnicity. We used the normalized race group for a patient based on a combination of their reported race and ethnicity. If a patient's ethnicity was ‘Hispanic,’ then the normalized race and ethnicity group was coded as Hispanic. For others, the normalized race and ethnicity group was assigned the same value as the reported race. Hence, race and ethnicity were mutually exclusive and categorized as Asian/Pacific Islander, Black, Hispanic, Native American (American Indian/Alaska Native), White, and ‘Other’. Co-morbidities of interest included hypertension, diabetes mellitus, heart failure, dyslipidaemia, coronary artery disease, peripheral artery disease, and chronic kidney disease. Dichotomous medical comorbidity variables were accumulated at each healthcare encounter and carried forward over time. The outcomes were incident ischaemic stroke, intracranial haemorrhage (non-traumatic cerebral and sub-arachnoid haemorrhage), and death.
Statistical analysis
Categorical variables are expressed as frequencies and percentages, while continuous variables are expressed as means with standard deviations. We assessed differences between groups using the Student's t-test for continuous variables and the Pearson Chi square test for categorical variables. Time to incident ischaemic stroke, intracranial haemorrhage, and death were assessed using survival analysis. Crude incidence rates of ischaemic stroke, intracranial haemorrhage, and all-cause mortality were calculated as the number with each outcome divided by the number of person-years at risk for each outcome. To compare change in incidence rates between demographic groups, we calculated the incidence rate ratio (IRR) with 95% confidence interval (CI) as the incidence rate in the period 2015–2019 divided by the incidence rate in the period 2005–2009. An IRR < 1 indicates a decline in incidence rate, and the lower the IRR, the higher the decline. The IRR were then compared by age (<65 years vs. ≥ 65 years), sex, and race and ethnicity, using the Mantel Haenszel test of homogeneity. Furthermore, we used multivariable Cox regression analysis to explore whether, compared with 2005–2009, the periods 2010–2014 and 2015–2019 were associated with a significant change in the outcomes of interest after adjustment for demographics and cardiovascular co-morbidities (hypertension, diabetes mellitus, dyslipidaemia, heart failure, chronic kidney disease, coronary artery disease, and peripheral artery disease). Covariates for inclusion in multivariable models were selected a priori as potential mediators or confounders of the outcomes based on biological plausibility and the medical literature. We performed a sensitivity analysis including the population with possible post-operative AF. We also investigated differences in the amplitude of change in the rates of adverse outcomes between 2005–2009 and 2015–2019 across demographic groups. Follow-up time was constrained to the duration of the individual cohort (e.g. patients in the 2005–2009 cohort were only followed for outcomes until the end of 2009). Risk estimates were reported as adjusted hazard ratios (HR) with 95% CI. A two-tailed P-value of ≤0.05 was considered statistically significant. All analyses were performed using Stata 17 statistical package (Statacorp, College Station, TX, USA).
Ethical considerations
This study received ethical approval from the Institutional Review Board of the University of California-San Francisco.
Results
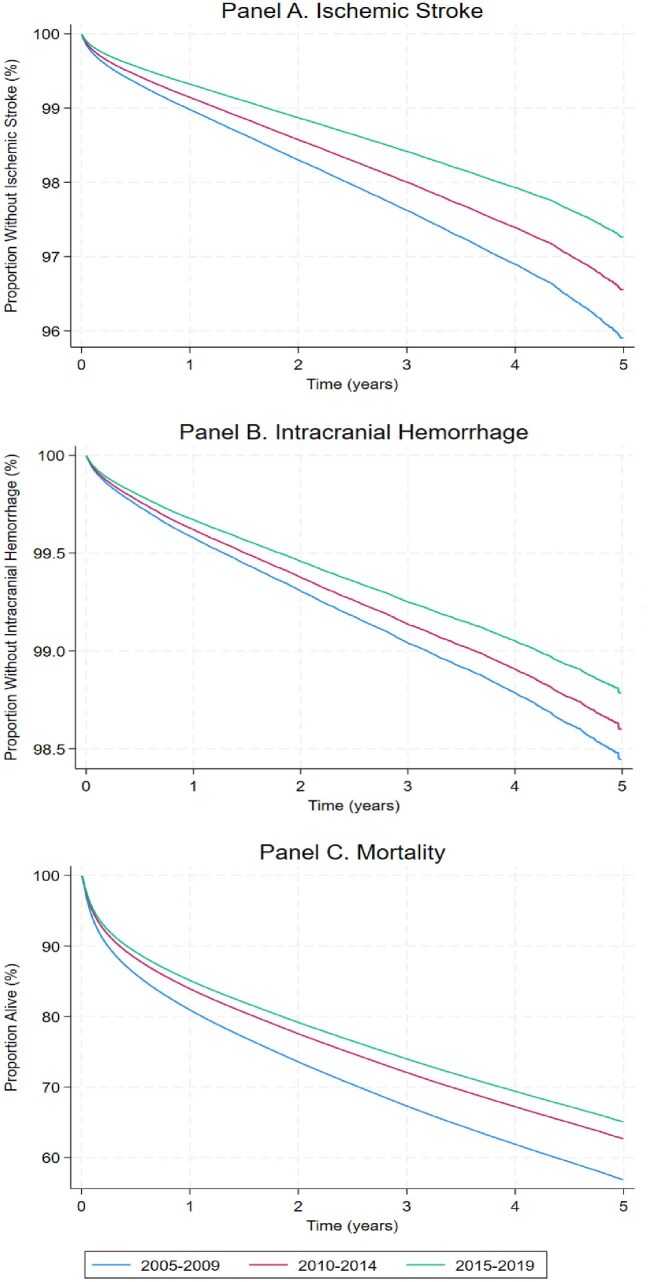
We included 2 009 832 patients with AF. They were mostly males (52.7%) and Whites (70.7%), with a mean age was 75.0 ± 13.6 years. Compared with more recent years, patients in the 2005–2009 cohort were older, were less likely to be males, more likely to be white, and exhibited variable differences in cardiovascular comorbidities (Table 1). Throughout the 15 years of study, the incidence rates per 1000 person-years were 7.83 (95% CI 7.73–7.92) for ischaemic stroke, 3.17 (3.11–3.23) for intracranial haemorrhage, and 137.81 (95% CI 137.44–138.18) for all-cause mortality (Table 2). The incidence of ischaemic stroke, intracranial haemorrhage, and all-cause mortality each consistently diminished over time (Figure 1 and Table 3). After adjusting for age, sex, race and ethnicity, and co-morbidities, compared with 2005–2009, the periods 2010–2014 and 2015–2019 were associated with lower risks of each outcome (Table 3 and Supplementary material online, Table S4). A sensitivity analysis including patients with post-operative AF showed consistent results (Supplementary material online, Table S5).
Table 1.
Participant characteristics in three successive time periods
| Variables |
Total
(n = 2 009 832) |
2005–2009
(n = 738 954) |
2010–2014
(n = 609 447) |
2015–2019
(n = 661 431) |
P value |
|---|---|---|---|---|---|
| Mean age (years) | 75.0 ± 13.6 | 76.1 ± 13.2 | 74.6 ± 13.8 | 74.1 ± 13.7 | <0.001 |
| Male (%) | 1 058 493 (52.7) | 376 092 (50.9) | 321 741 (52.8) | 360 660(54.5) | <0.001 |
| Race and ethnicity (%) | <0.001 | ||||
| Asian | 163 356 (8.1) | 51 744 (7.0) | 50 686 (8.3) | 60 926 (9.2) | |
| Black | 106 411 (5.3) | 34 813 (4.7) | 32 340 (5.3) | 39 258 (5.9) | |
| Hispanic | 265 009 (13.2) | 80 860 (10.9) | 82 704 (13.6) | 101 445 (15.3) | |
| Native American | 5 067 (0.3) | 1 211 (0.2) | 1 757 (0.3) | 2 099 (0.3) | |
| White | 1 420 477 (70.7) | 553 506 (74.9) | 426 092 (69.9) | 440 879 (66.7) | |
| Other | 49 512 (2.5) | 16 820 (2.3) | 15 868(2.6) | 16 824 (2.5) | |
| Diabetes (%) | 542 448 (27.0) | 182 454 (24.7) | 165 980 (27.2) | 194 014 (29.3) | <0.001 |
| Hypertension (%) | 1 129 989 (57.9) | 365 191 (49.4) | 309 681 (50.8) | 455 117 (75.4) | <0.001 |
| Heart failure (%) | 558 259 (27.8) | 218 980(29.6) | 156 902 (25.7) | 182 377 (27.6) | <0.001 |
| Dyslipidaemia (%) | 729 306 (36.3) | 208 821 (28.3) | 234 796 (38.5) | 285 689 (43.2) | <0.001 |
| CKD (%) | 342 821 (17.1) | 79 539 (10.8) | 119 214 (19.6) | 144 068 (21.78) | <0.001 |
| CAD (%) | 562 050 (28.0) | 241 705 (32.7) | 180 857 (29.7) | 139 488(21.1) | <0.001 |
| PAD (%) | 130 233(6.5) | 36 897 (5.0) | 28 592 (4.7) | 64 744 (9.8) | <0.001 |
CAD, coronary artery disease; CKD, chronic kidney disease; PAD, peripheral artery disease.
Table 2.
Crude incidence rates (per 1000 person-years) of ischaemic stroke, intracranial haemorrhage, and all-cause mortality among patients with atrial fibrillation
| Variables |
Total
(n = 2 009 832) |
2005–2009
(n = 738 954) |
2010–2014
(n = 609 447) |
2015–2019
(n = 661 431) |
P value* |
|---|---|---|---|---|---|
| Ischaemic stroke | |||||
| Overall | 7.83 (7.73–7.92) | 9.20 (9.04–9.36) | 7.67 (7.50–7.83) | 6.41 (6.27–6.56) | <0.001 |
| Age (years) | |||||
| <65 | 2.92 (2.80–3.03) | 2.84 (2.65–3.04) | 2.84 (2.64–3.05) | 3.06 (2.87–3.27) | .17 |
| ≥65 | 9.31 (9.19–9.42) | 10.85 (10.66–11.05) | 9.25 (9.05–9.46) | 7.51 (7.33–7.69) | <0.001 |
| Sex | |||||
| Male | 6.15 (6.94–6.26) | 6.96 (6.77–7.16) | 5.96 (5.76–6.16) | 5.46 (5.29–6.65) | <0.001 |
| Female | 9.73 (9.59–9.88) | 11.58 (11.32–11.84) | 9.63 (9.36–9.90) | 7.58 (7.35–7.82) | <0.001 |
| Race and ethnicity | |||||
| Asian | 8.72 (8.38–9.07) | 9.55 (8.94–10.21) | 8.84 (8.23–9.49) | 7.91 (7.39–8.46) | <0.001 |
| Black | 9.29 (8.86–9.75) | 10.47 (9.68–11.34) | 8.92 (8.15–9.76) | 8.54 (7.86–9.28) | <0.001 |
| Hispanic | 8.23 (7.97–8.49) | 9.75 (9.26–10.27) | 7.91 (7.46–8.38) | 7.23 (6.85–7.64) | <0.001 |
| Native American | 6.02 (4.62–7.85) | 9.58 (6.31–14.56) | 4.21 (2.45–7.27) | 5.33 (3.44–8.26) | .03 |
| White | 7.59 (7.49–7.70) | 9.07 (8.88–9.25) | 7.42 (7.23–7.61) | 5.89 (5.73–6.06) | <0.001 |
| Others | 7.01 (6.49–7.57) | 7.54 (6.64–8.57) | 7.56 (6.61–8.66) | 6.04 (5.26–6.94) | .02 |
| Intracranial haemorrhage | |||||
| Overall | 3.17 (3.11–3.23) | 3.34 (3.25–3.44) | 3.18 (3.08–3.29) | 2.97 (2.87–3.06) | <0.001 |
| Age (years) | |||||
| <65 | 1.71 (1.62–1.80) | 1.61 (1.47–1.76) | 1.63 (1.49–1.79) | 1.87 (1.72–2.03) | .63 |
| ≥65 | 3.60 (3.53–3.67) | 3.79 (3.67–3.90) | 3.68 (3.55–3.81) | 3.32 (3.20–3.43) | <0.001 |
| Sex | |||||
| Male | 3.12 (3.04–3.20) | 3.27 (3.14–3.41) | 3.07 (2.93–3.21) | 3.00 (2.87–3.13) | <0.001 |
| Female | 3.23 (3.15–3.31) | 3.42 (3.28–3.56) | 3.31 (3.16–3.47) | 2.92 (2.75–3.07) | <0.001 |
| Race and ethnicity | |||||
| Asian | 5.59 (5.32–5.86) | 6.13 (5.66–6.65) | 5.56 (5.09–6.06) | 5.14 (4.73–5.58( | <0.001 |
| Black | 3.84 (3.57–4.13) | 3.64 (3.19–4.14) | 3.74 (3.27–4.28) | 4.09 (3.65–5.60) | .74 |
| Hispanic | 3.81 (3.64–3.99) | 3.85 (3.55–4.17) | 3.79 (3.50–4.12) | 3.79 (3.52–4.08) | .01 |
| Native American | 3.04 (2.11–4.37) | 3.35 (1.67–6.69) | 2.50 (1.25–5.00) | 3.29 (1.91–5.67) | .80 |
| White | 2.75 (2.68–2.81) | 3.00 (2.90–3.11) | 2.75 (2.63–2.87) | 2.42 (2.32–2.53) | <0.001 |
| Others | 3.15 (2.82–3.52) | 4.54 (2.95–4.25) | 3.40 (2.79–4.13) | 2.59 (2.10–3.18) | .06 |
| Mortality | |||||
| Overall | 137.81 (137.44–138.18) | 161.04 (160.39–161.70) | 132.55 (131.88–133.22) | 116.37 (115.78–116.97) | <0.001 |
| Age (years) | |||||
| <65 | 54.04 (53.56–54.53) | 58.29 (57.42–59.18) | 51.85 (51.01–52.71) | 52.01 (51.21–52.83) | <0.001 |
| ≥65 | 162.43 (161.97–162.89) | 187.06 (186.27–187.86) | 158.34 (157.50–159.18) | 136.91 (136.17–137.65) | <0.001 |
| Sex | |||||
| Male | 131.55 (131.05–132.05) | 154.94 (154.04–155.84) | 126.41 (125.52–127.32) | 111.50 (110.72–112.29) | <0.001 |
| Female | 144.86 (144.30–145.41) | 167.42 (166.46–168.38) | 139.51 (138.51–140.51) | 122.30 (121.40–123.21) | <0.001 |
| Race and ethnicity | |||||
| Asian | 133.05 (131.75–134.35) | 147.97 (145.57–150.40) | 129.82 (127.52–132.16) | 122.87 (120.85–124.93) | <0.001 |
| Black | 159.19 (157.41–160.99) | 183.14 (179.83–186.52) | 153.86 (150.67–157.11) | 139.47 (139.47–145.03) | <0.001 |
| Hispanic | 125.29 (124.31–126.28) | 142.55 (140.69–144.43) | 122.71 (120.97–124.48) | 113.23 (111.73–114.75) | <0.001 |
| Native American | 120.90 (114.15–128.05) | 138.79 (124.67–154.50) | 105.36 (94.75- 117.16) | 122.72 (112.32–134.10) | .11 |
| White | 139.86 (139.42–140.30) | 164.68 (163.92–165.45) | 133.97 (133.17- 134.77) | 114.12 (113.41–114.84) | <0.001 |
| Others | 119.27 (117.12–121.45) | 127.88 (124.09–131.79) | 115.36 (111.55–119.30) | 114.46 (110.99–118.04) | <0.001 |
*P value is for comparison of incidence rates between all three time periods.
Figure 1.
Multivariable adjusted survival curves for incident ischaemic stroke, intracranial haemorrhage, and all-cause mortality among patients with atrial fibrillation over 3 successive 5-year time periods. Adjusted for age, sex, race and ethnicity, and co-morbidities (hypertension, diabetes, dyslipidaemia, heart failure, chronic kidney disease, coronary artery disease, and peripheral artery disease).
Table 3.
Predictors of incident stroke, intracranial haemorrhage, and all-cause mortality among patients with atrial fibrillation
| Ischaemic stroke | Intracranial haemorrhage | Mortality | ||||
|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| Era | ||||||
| 2005–2009 | Ref | – | Ref | – | Ref | – |
| 2010–2014 | 0.84 (0.82–0.86) | <0.001 | 0.90 (0.86–0.94) | <0.001 | 0.83 (0.82–0.84) | <0.001 |
| 2015–2019 | 0.66 (0.64–0.69) | <0.001 | 0.78 (0.75–0.82) | <0.001 | 0.76 (0.75–0.77) | <0.001 |
| Age | ||||||
| <65 | Ref | – | Ref | – | Ref | - |
| ≥65 | 2.69 (2.58–2.81) | <0.001 | 1.98 (1.87–2.10) | <0.001 | 2.62 (2.60–2.65) | <0.001 |
| Sex | ||||||
| Male | Ref | – | Ref | – | Ref | – |
| Female | 1.45 (1.41–1.48) | <0.001 | 0.96 (0.93–0.99) | .03 | 1.03 (1.02–1.04) | <0.001 |
| Race and ethnicity | ||||||
| White | Ref | – | Ref | – | Ref | – |
| Asian | 1.12 (1.08–1.17) | <0.001 | 1.94 (1.84–2.05) | <0.001 | 0.92 (0.91–0.93) | <0.001 |
| Black | 1.27 (1.21–1.34) | <0.001 | 1.42 (1.31–1.53) | <0.001 | 1.11 (1.09–1.12) | <0.001 |
| Hispanic | 1.13 (1.09–1.17) | <0.001 | 1.40(1.33–1.47) | <0.001 | 0.91 (0.90–0.91) | <0.001 |
| Native American | 0.87 (0.66–1.14) | .31 | 1.17(0.81–1.69) | .41 | 0.93 (0.87–0.98) | .009 |
| Others | 0.97 (0.90–1.05) | .44 | 1.19 (1.06–1.33) | .004 | 0.87 (0.86–0.89) | <0.001 |
| Co-morbidities | ||||||
| Diabetes | 1.10 (1.07–1.13) | <0.001 | 1.18(1.13–1.23) | <0.001 | 1.11 (1.10–1.12) | <0.001 |
| Hypertension | 1.20 (1.17–1.23) | <0.001 | 1.20 (1.16–1.25) | <0.001 | 0.92 (0.91–0.92) | <0.001 |
| Heart failure | 1.37 (1.34–1.41) | <0.001 | 1.14 (1.09–1.19) | <0.001 | 1.76 (1.75–1.78) | <0.001 |
| CKD | 1.44 (1.40–1.49) | <0.001 | 1.52 (1.45–1.60) | <0.001 | 1.65 (1.63–1.66) | <0.001 |
| Dyslipidaemia | 0.90 (0.87–0.92) | <0.001 | 0.95 (0.91–0.99) | .009 | 0.73 (0.73–0.74) | <0.001 |
| CAD | 1.08 (1.05–1.11) | <0.001 | 0.98 (0.94–1.02) | .40 | 1.14 (1.13–1.15) | <0.001 |
| PAD | 1.33 (1.27–1.138) | <0.001 | 1.14 (1.06–1.22) | <0.001 | 1.33 (1.32–1.35) | <0.001 |
CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; PAD, peripheral artery disease; Ref, reference category age, sex, race and ethnicity, cardiovascular co-morbidities (hypertension, diabetes, dyslipidaemia, heart failure, chronic kidney disease, coronary artery disease, and peripheral artery disease), and era were all included in the multivariable Cox regression analysis.
While declines in incident stroke and mortality were consistent across demographic subgroups, the magnitude of observed differences differed by age, sex, and race and ethnicity; differences in changes in incident intracranial haemorrhage were heterogenous (Figure 2). After adjustment for potential confounders, each racial and ethnic group except Native Americans experienced less declines in ischaemic strokes compared with white individuals; each group except Asians and Native Americans exhibited less declines in haemorrhagic stroke compared with white individuals; and white individuals had more declines in mortality compared with all other racial and ethnic groups (Table 4).
Figure 2.

Differences in temporal changes of crude incidence of ischaemic stroke, intracranial haemorrhage, and all-cause mortality by individual demographic characteristics. 95% CI, 95% confidence interval. IRR, incidence rate ratio comparing incidence rate between 2005–2009 and 2015–2019; an IRR < 1 indicates a decline in incidence rate; the lower the IRR, the higher the decline. P values are for groups comparison.
Table 4.
Differences in risk of ischaemic stroke, intracranial haemorrhage, and all-cause mortality between 2005–2009 and 2015–2019 by individual demographic characteristics
| Ischaemic stroke | Intracranial haemorrhage | All-cause mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables |
HR
(95% CI) |
P value | P value for group difference |
HR
(95% CI) |
P value | P value for group difference |
HR
(95% CI) |
P value | P value for group difference |
| Age | |||||||||
| <65 | 0.93 (0.84–1.03) | .18 | 0.97 (0.85–1.11) | .67 | 0.89 (0.87–0.91) | <0.001 | |||
| ≥65 | 0.65 (0.62–0.67) | <0.001 | <0.001 | 0.77 (0.73–0.81) | <0.001 | <0.001 | 0.75 (0.74–0.76) | <0.001 | <0.001 |
| Sex | |||||||||
| Male | 0.72 (0.68–0.75) | <0.001 | 0.79 (0.74–0.84) | <0.001 | 0.75 (0.74–0.76) | <0.001 | |||
| Female | 0.63 (0.60–0.66) | <0.001 | <0.001 | 0.79 (0.74–0.85) | <0.001 | 0.19 | 0.77 (0.76–0.78) | <0.001 | <0.001 |
| Race and ethnicity | |||||||||
| White | 0.63 (0.61–0.66) | <0.001 | – | 0.76 (0.72–0.81) | <0.001 | – | 0.73 (0.72–0.74) | <0.001 | – |
| Asian | 0.77 (0.69–0.86) | <0.001 | .001 | 0.73 (0.64–0.83) | <0.001 | .82 | 0.87 (0.84–0.89) | <0.001 | <0.001 |
| Black | 0.78 (0.68–0.89) | <0.001 | .001 | 1.01 (0.83–1.23) | 0.95 | 0.001 | 0.83 (0.80–0.85) | <0.001 | <0.001 |
| Hispanic | 0.71 (0.65–0.77) | <0.001 | .008 | 0.86 (0.76–0.98) | .02 | .003 | 0.84 (0.83–0.86) | <0.001 | <0.001 |
| Native American | 0.46 (0.23–0.91) | .03 | .34 | 1.12 (0.43–2.92) | .82 | .72 | 0.88 (0.75–1.03) | .10 | .007 |
| Others | 0.79 (0.64–0.97) | .03 | .03 | 0.76 (0.56–1.03) | .08 | .58 | 0.92 (0.88–0.97) | .001 | <0.001 |
CI, confidence interval; HR, hazard ratio.
Hazard ratios represents the risks for each outcome in 2015–2019 compared with 2005–2009 (baseline and reference category) in different demographic groups. A hazard ratio <1 denotes a decline in risk in 2015–2019 compared with 2005–2009.
Age, sex, race and ethnicity (each category compared with Whites), cardiovascular co-morbidities (hypertension, diabetes, dyslipidaemia, heart failure, chronic kidney disease, coronary artery disease, and peripheral artery disease), and era (2005–2009 and 2015–2019 time periods) were included in the multivariable Cox regression analysis.
For race and ethnicity, each group is compared with Whites.
Discussion
AF patients have experienced substantial declines in stroke, intracerebral haemorrhage, and death over time. However, differences by demographic categories were observed, with older patients experiencing the largest gains in avoiding these outcomes and Hispanics and Blacks consistently experiencing less improvements compared with their white counterparts.
Data regarding the incidence of ischaemic stroke in patients with AF is mostly limited to the era before 2010. Based on randomized controlled trial data, the use of warfarin for stroke prevention became part of standard recommendations for the management of patients with AF in the 1990s.19 Declines in strokes accompanied by rising use of warfarin were observed in studies focusing on trends from that time into the first decade of the millennium, although anticoagulation uptake remained suboptimal and diminishing stroke rates were not universally observed.20–23
Direct oral anticoagulants were first introduced in the USA in 2010, the same year as the CHA2DS2-VASc score,24 a risk score meant to identify appropriate candidates for anticoagulation that was more inclusive of the predecessor CHADS2 score.25 Since that year, DOAC use has increased in the US, and, as expected given the enhanced convenience of these drugs versus warfarin, evidence has shown increased uptake of appropriate anticoagulation in general.7–9,26 It was not until 2014 that the CHA2DS2-VASc score was recommended and that DOACs were recommended as first line ahead of warfarin in official US guidelines.6 Prior European studies have demonstrated declines in AF-related stroke with greater use of DOACs,27,28 but to our knowledge no study has re-examined contemporary trends in ischaemic stroke among US patients with AF. Indeed, we observed a 42% decline in the risk of stroke in these patients. Of note, the current study was not designed to determine the mechanisms of observed differences over time (e.g. the database utilized does not include medication prescriptions), but rather to describe changes in the pertinent epidemiology, and therefore other explanations may be operative. For example, prior to the year 2000, improved systolic blood pressure control had been described as a progressive phenomenon explaining reductions in ischaemic stroke in AF, a trend that may have continued.21 Similarly, although no randomized controlled trial has shown a reduction in stroke due to catheter ablation of AF, the procedure has both evolved and become substantially more common over the past two decades, with multiple observational studies suggesting that successful procedures may reduce the risk of stroke.12,29,30 Another potential explanation for the decline in the incidence of ischaemic stroke in patients with AF is the increasing detection of short-episodes AF by extended cardiac monitoring with an implantable loop recorder, pacemaker, defibrillator, or implantable cardiac monitor.31 Such subclinical AF may be associated with a lower risk of ischaemic stroke than AF detected by more conventional means.32
Lower rates of major bleeding, particularly intracranial haemorrhage, occur with DOACs compared with warfarin.33–35 Hence, in parallel to a decline in incident ischaemic stroke, a decrease in the incidence of intracranial haemorrhage would be expected with a transition from warfarin to DOACs. Similarly, strategies to reduce the burden or impact of AF, such as more successful catheter ablations and lifestyle modifications, might theoretically reduce the frequency of anticoagulant use, which itself might contribute to lower risks of intracranial bleeding. Prior to multivariable adjustment, the crude incidence rate of intracranial haemorrhage in patients with AF failed to reveal any significant changes over time. However, after multivariable adjustment, we observed a statistically significant 17% decline in intracranial haemorrhage among AF patients in the most contemporary 5-year period vs. the earliest 5-year period, suggesting that comorbidities likely mediated the relationship. A prior analysis utilizing the inpatient sample concluded that the 3-year incidence of intracranial haemorrhages increased in comparing 2004–2006 with 2016–2018, a finding that coincided with a higher prevalence of hypertension and anticoagulation use.36 However, the inpatient sample does not allow for a longitudinal assessment of the same patients (hence two intracranial haemorrhage events in the same patient would be considered the same as two independent patients) and is taken from only a sample of the patients experiencing inpatient hospitalization (whereas our current data enables tracking the same individual over time, includes multiple types of encounters, and is inclusive of every actual patient in three different healthcare settings in California).
Previous data on changes in overall mortality among patients with AF are especially limited. No significant change in AF-associated mortality in Olmsted County was observed between 1980 and 2000,37 an era prior to many of the advancements already discussed. In the current study, mortality decreased by nearly a third when comparing the most recent time period to 2005–2009. Observational studies and randomized trials suggest that overall mortality may be lower than with DOACs than warfarin,33–35 and once again the growing use of catheter ablation could potentially contribute to this observation.11,12,38 A growing recognition that lifestyle factors, such as obesity and alcohol use, contribute to AF13–17 might also motivate healthier behaviours that result in reduced recurrences and burden of AF, most recently shown to influence adverse outcomes in these patients.39–41 Furthermore, with the advent of patient-centric monitoring devices and increased ambulatory monitoring in general, it is possible that individuals with paroxysmal AF who would have never been previously diagnosed with AF now carry this diagnosis in more contemporary periods. Hence, the observed decline in adverse outcomes could be partly explained by higher proportion of ‘lower risk’ patients with AF in the most recent period compared with 2005–2009.
We observed that several fundamental demographic characteristics were associated with substantial differences in each outcome, suggesting important public health implications when considering equitable improvements in AF-related care. For each demographic variable of interest, it is important to consider multiple possible explanations for the disparities observed, ranging from biological differences to healthcare access issues to reluctance to receive therapies when offered.
Older individuals consistently enjoyed a greater decline in each outcome than those who were younger. This is likely related to the fact that, not surprisingly and precisely as observed, older individuals experienced a higher risk for each outcome. Given the low-risk nature of the younger group, there may simply be little improvements to be had (and certainly less opportunity for improvements compared with the older individuals who began at a higher risk). It is also interesting to consider the age 65-year-old cut-off inherent to anticoagulation recommendations.24 While that age threshold has persisted as conferring a higher-risk ‘point’ for both the CHADS2 and CHA2DS2-VASc and during the transition from warfarin to DOACs,6,24 some have speculated that the more convenient and lower risk DOACs might warrant consideration of broader inclusion among the younger and lower risk patients.42
Differences in ischaemic stroke, intracranial haemorrhage, and death by sex were statistically significant, but relatively small in magnitude and variable in direction. The higher incidence rates of ischaemic stroke and mortality in females have been previously documented,43,44 and yet, in contrast to the benefits of the higher risk among the older vs. younger, our study demonstrated that women also experienced less of a decline in stroke rates over time. Whereas females with AF have been less likely to receive vitamin K antagonist, more recent evidence suggests they are just as likely to receive DOACs than males,45 which should have resulted in larger gains in improvements in stroke and intracranial haemorrhage if universally true. It is worth noting that females receive less rhythm control therapy,46 which might ultimately translate into diminishing benefits in both stroke and intracranial haemorrhage.39 It is surprising that, despite less improvements in stroke for men compared with women, men experienced more improvements in mortality over time. There are several potential explanations for these apparently discordant findings. First, again differences by sex were small and the P values were not particularly low—in these secondary analyses, we need to acknowledge that multiple hypothesis testing may lead to type 1 errors. However, the particularly small P value (of <0.001) for mortality favouring men would likely withstand even the most conservative adjustment for multiple tests, which, in the context of reduced declines in stroke and intracranial haemorrhage, suggest that other factors important to longevity are at play in AF patients and that women may have benefitted in particular in more contemporary times.
Multiple studies have demonstrated less commonly prescribed rhythm control, anticoagulation receipt, and more strokes for minority populations compared with Whites.47–49 Once again, using the findings related to older vs. younger patients as an example, these racial disparities arguably provide more opportunity for improvements over time. To our knowledge, this is the first report to demonstrate that such disparities persist in considering improvements over time. Socioeconomic issues related to education and healthcare access for patients and potentially bias on the part of some providers may persist,50 requiring ongoing evaluation and remediation.
Our study has several limitations. The coding of race and ethnicity was mutually exclusive, resulting in misclassification of multiracial individuals. Furthermore, race may be coded differently for the same individual and self-identified race may not fully represent genetic ancestry.51 Because outpatient data was limited only to procedures, our study population, inclusive of nearly every hospitalization, emergency department visit, and outpatient procedure in California, may not be fully representative of the entire AF population. However, we would expect stroke and intracranial haemorrhage to result in healthcare utilization captured by these databases. We were able to leverage the social security index to capture total mortality (even including deaths that occurred outside the three encounter types), which might also explain enhanced power for that particular outcome. We also acknowledge that some strokes and intracranial haemorrhages may present as death, potentially leading to misclassification in the current manuscript—however, given the severity of each condition and the nature of the datasets utilized, at least one of those outcomes was likely reliably ascertained. The use of administrative databases and diagnosis codes may lead to ascertainment bias from errors in coding and disease classification. However, prior research comparing use of these codes to chart review have revealed highly accurate test characteristics,52 and several of our observations within each time period (such as relationships between certain demographics and risk of each outcome) have been previously well established in other cohorts,43,44,47,48 providing a degree of validation. Although we were able to account for multiple cardiovascular comorbidities, data regarding medication use, such as oral anticoagulation, and the degree of control of cardiovascular risk factors, was not available. However, the purpose of the current study was to describe the changing epidemiology and prognosis of adverse outcomes among AF patients and those limitations are pertinent to the mechanistic pathways that might explain the observed differences. The majority of patients were non-Hispanic white, and the findings observed may represent phenomena specific to California, potentially limiting generalizability. Finally, as this is an observational study, we cannot make confident statements regarding causality; however, again, this is predominately a description of the changing epidemiology of the most severe complications among nearly two million AF patients in the general population without the intent of identifying or describing the underlying causes of observed changes.
Conclusion
The risks of ischaemic stroke, intracranial haemorrhage, and death among AF patients have declined substantially over a recent 15-year period. These observations should be reassuring to modern AF patients, while reinforcing the ongoing importance of original research and implementation strategies to optimize evidence-based care. Differential improvements by demographic characteristics, most prominently less consistent improvements among Hispanic and Black AF patients, reveals subgroups most worthy of attention to enhance the quality of care.
Supplementary Material
Contributor Information
Jean Jacques Noubiap, From the Division of Cardiology, Department of Medicine, University of California-San Francisco, San Francisco, CA, USA.
Janet J Tang, From the Division of Cardiology, Department of Medicine, University of California-San Francisco, San Francisco, CA, USA.
Thomas A Dewland, From the Division of Cardiology, Department of Medicine, University of California-San Francisco, San Francisco, CA, USA.
Gregory M Marcus, From the Division of Cardiology, Department of Medicine, University of California-San Francisco, San Francisco, CA, USA.
Funding
NIH/NHLBI R01 HL158825-01 to Dr Marcus. Dr Marcus reports receiving funding from the NIH, PCORI, and the California Department of Cannabis Control; he is a consultant for and equity holder in InCarda.
Data Availability
The data analyzed are available with permission from the Department of Health Care Access and Information (HCAI) at https://hcai.ca.gov/data/request-data/.
Authors’ contributions
Conception and design: G.M.M. and J.J.N. Access to data: G.M.M. Data analysis and interpretation: J.J.N., G.M.M., T.A.D., and J.J.T. Manuscript drafting: J.J.N. Manuscript revision: J.J.N., T.A.D., J.J.T., and G.M.M. Supervision: G.M.M. Approval of the final manuscript: J.J.N., G.M.M., T.A.D., and J.J.T.
References
- 1. Dai H, Zhang Q, Much AA, Maor E, Segev A, Beinart R et al. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes 2021;7:574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J et al. Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN International collaboration. Circulation 2019;140:1834–1850. [DOI] [PubMed] [Google Scholar]
- 3. Jørgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke 1996;27:1765–1769. [DOI] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 5. Habboushe J, Altman C, Lip GYH. Time trends in use of the CHADS(2) and CHA(2) DS(2) VASc scores, and the geographical and specialty uptake of these scores from a popular online clinical decision tool and medical reference. Int J Clin Pract 2019;73:e13280. [DOI] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010–2017. Pharmacotherapy 2018;38:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banerjee A, Benedetto V, Gichuru P, Burnell J, Antoniou S, Schilling RJ et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: a population-based study. Heart 2020;106:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Navar AM, Kolkailah AA, Overton R, Shah NP, Rousseau JF, Flaker GC et al. Trends in oral anticoagulant use among 436 864 patients with atrial fibrillation in community practice, 2011–2020. J Am Heart Assoc 2022;11:e026723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. J Am Med Assoc 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 12. Yang PS, Sung JH, Jang E, Yu HT, Kim TH, Uhm JS et al. Catheter ablation improves mortality and other outcomes in real-world patients with atrial fibrillation. J Am Heart Assoc 2020;9:e015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. J Am Med Assoc 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 14. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 15. Marcus GM, Modrow MF, Schmid CH, Sigona K, Nah G, Yang J et al. Individualized studies of triggers of paroxysmal atrial fibrillation: the I-STOP-AFib randomized clinical trial. JAMA Cardiol 2022;7:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dixit S, Alonso A, Vittinghoff E, Soliman EZ, Chen LY, Marcus GM. Past alcohol consumption and incident atrial fibrillation: the Atherosclerosis risk in Communities (ARIC) study. PLoS One 2017;12:e0185228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation 2020;141:e750–e772. [DOI] [PubMed] [Google Scholar]
- 18. Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. J Am Med Assoc 2014;312:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laupacis A, Albers G, Dalen J, Dunn M, Feinberg W, Jacobson A. Antithrombotic therapy in atrial fibrillation. Chest 1995;108:352S–359S. [DOI] [PubMed] [Google Scholar]
- 20. Shroff GR, Solid CA, Herzog CA. Temporal trends in ischemic stroke and anticoagulation therapy among Medicare patients with atrial fibrillation: a 15-year perspective (1992–2007). JAMA Intern Med 2013;173:159–160. [DOI] [PubMed] [Google Scholar]
- 21. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Seward JB, Bailey KR et al. Time trends of ischemic stroke incidence and mortality in patients diagnosed with first atrial fibrillation in 1980–2000: report of a community-based study. Stroke 2005;36:2362–2366. [DOI] [PubMed] [Google Scholar]
- 22. Chamberlain AM, Brown RD Jr., Alonso A, Gersh BJ, Killian JM, Weston SA et al. No decline in the risk of stroke following incident atrial fibrillation since 2000 in the community: a concerning trend. J Am Heart Assoc 2016;5:e003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yiin GS, Howard DP, Paul NL, Li L, Mehta Z, Rothwell PM. Recent time trends in incidence, outcome and premorbid treatment of atrial fibrillation-related stroke and other embolic vascular events: a population-based study. J Neurol Neurosurg Psychiatry 2017;88:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 25. Orchard JJ, Giskes K, Orchard JW, La Gerche A, Neubeck L, Hespe C et al. In a large primary care dataset, the CHA₂DS₂-VASc score leads to almost universal recommendation for anticoagulation treatment in those aged ≥65 years with atrial fibrillation. Eur J Cardiovasc Nurs 2023;22:769–772. [DOI] [PubMed] [Google Scholar]
- 26. Chen Q, Toorop MMA, Tops LF, Lijfering WM, Cannegieter SC. Time trends in patient characteristics, anticoagulation treatment, and prognosis of incident nonvalvular atrial fibrillation in the Netherlands. JAMA Netw Open 2023;6:e239973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding M, Ebeling M, Ziegler L, Wennberg A, Modig K. Time trends in atrial fibrillation-related stroke during 2001–2020 in Sweden: a nationwide, observational study. Lancet Reg Health Eur 2023;28:100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cowan JC, Wu J, Hall M, Orlowski A, West RM, Gale CP. A 10 year study of hospitalized atrial fibrillation-related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J 2018;39:2975–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scott M, Baykaner T, Bunch TJ, Piccini JP, Russo AM, Tzou WS et al. Contemporary trends in cardiac electrophysiology procedures in the United States, and impact of a global pandemic. Heart Rhythm O2 2023;4:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barra S, Narayanan K, Boveda S, Primo J, Gonçalves H, Baran J et al. Atrial fibrillation ablation and reduction of stroke events: understanding the paradoxical lack of evidence. Stroke 2019;50:2970–2976. [DOI] [PubMed] [Google Scholar]
- 31. Noseworthy PA, Kaufman ES, Chen LY, Chung MK, Elkind MSV, Joglar JA et al. Subclinical and device-detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the American Heart Association. Circulation 2019;140:e944–e963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camm AJ, Simantirakis E, Goette A, Lip GY, Vardas P, Calvert M et al. Atrial high-rate episodes and stroke prevention. Europace 2017;19:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carnicelli AP, Hong H, Connolly SJ, Eikelboom J, Giugliano RP, Morrow DA et al. Direct oral anticoagulants versus Warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation 2022;145:242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan YH, Lee HF, Chao TF, Wu CT, Chang SH, Yeh YH et al. Real-world comparisons of direct oral anticoagulants for stroke prevention in Asian patients with non-valvular atrial fibrillation: a systematic review and meta-analysis. Cardiovasc Drugs Ther 2019;33:701–710. [DOI] [PubMed] [Google Scholar]
- 35. Ntaios G, Papavasileiou V, Makaritsis K, Vemmos K, Michel P, Lip GYH. Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke 2017;48:2494–2503. [DOI] [PubMed] [Google Scholar]
- 36. Bako AT, Pan A, Potter T, Tannous J, Johnson C, Baig E et al. Contemporary trends in the nationwide incidence of primary intracerebral hemorrhage. Stroke 2022;53:e70–e74. [DOI] [PubMed] [Google Scholar]
- 37. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007;49:986–992. [DOI] [PubMed] [Google Scholar]
- 38. Sohns C, Fox H, Marrouche NF, Crijns H, Costard-Jaeckle A, Bergau L et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med 2023;389:1380–1389. [DOI] [PubMed] [Google Scholar]
- 39. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 40. Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P et al. Atrial fibrillation burden: moving beyond Atrial fibrillation as a binary entity: a scientific statement from the. Am Heart Assoc Circ 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chew DS, Li Z, Steinberg BA, O'Brien EC, Pritchard J, Bunch TJ et al. Arrhythmic burden and the risk of cardiovascular outcomes in patients with paroxysmal atrial fibrillation and cardiac implanted electronic devices. Circ Arrhythm Electrophysiol 2022;15:e010304. [DOI] [PubMed] [Google Scholar]
- 42. Fong KY, Chan YH, Yeo C, Lip GYH, Tan VH. Systematic review and meta-analysis of direct oral anticoagulants versus Warfarin in atrial fibrillation with low stroke risk. Am J Cardiol 2023;204:366–376. [DOI] [PubMed] [Google Scholar]
- 43. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. Br Med J 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Noubiap JJ, Feteh VF, Middeldorp ME, Fitzgerald JL, Thomas G, Kleinig T et al. A meta-analysis of clinical risk factors for stroke in anticoagulant-naïve patients with atrial fibrillation. Europace 2021;23:1528–1538. [DOI] [PubMed] [Google Scholar]
- 45. Lee KK, Doudesis D, Bing R, Astengo F, Perez JR, Anand A et al. Sex differences in oral anticoagulation therapy in patients hospitalized with atrial fibrillation: a nationwide cohort study. J Am Heart Assoc 2023;12:e027211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noubiap JJ, Thomas G, Agbaedeng TA, Fitzgerald JL, Gallagher C, Middeldorp ME et al. Sex differences in clinical profile, management, and outcomes of patients hospitalized for atrial fibrillation in the United States. Eur Heart J Qual Care Clin Outcomes 2022;8:852–860. [DOI] [PubMed] [Google Scholar]
- 47. Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR et al. Racial differences in atrial fibrillation-related cardiovascular disease and mortality: the atherosclerosis risk in communities (ARIC) study. JAMA Cardiol 2016;1:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Norby Faye L, Benjamin Emelia J, Alonso A, Chugh Sumeet S. Racial and ethnic considerations in patients with atrial fibrillation. J Am Coll Cardiol 2021;78:2563–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mital R, Bayne J, Rodriguez F, Ovbiagele B, Bhatt DL, Albert MA. Race and ethnicity considerations in patients with coronary artery disease and stroke: JACC Focus Seminar 3/9. J Am Coll Cardiol 2021;78:2483–2492. [DOI] [PubMed] [Google Scholar]
- 50. Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol 2016;35:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics 2015;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alhajji M, Kawsara A, Alkhouli M. Validation of acute ischemic stroke codes using the International Classification of Diseases tenth revision. Am J Cardiol 2020;125:1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed are available with permission from the Department of Health Care Access and Information (HCAI) at https://hcai.ca.gov/data/request-data/.