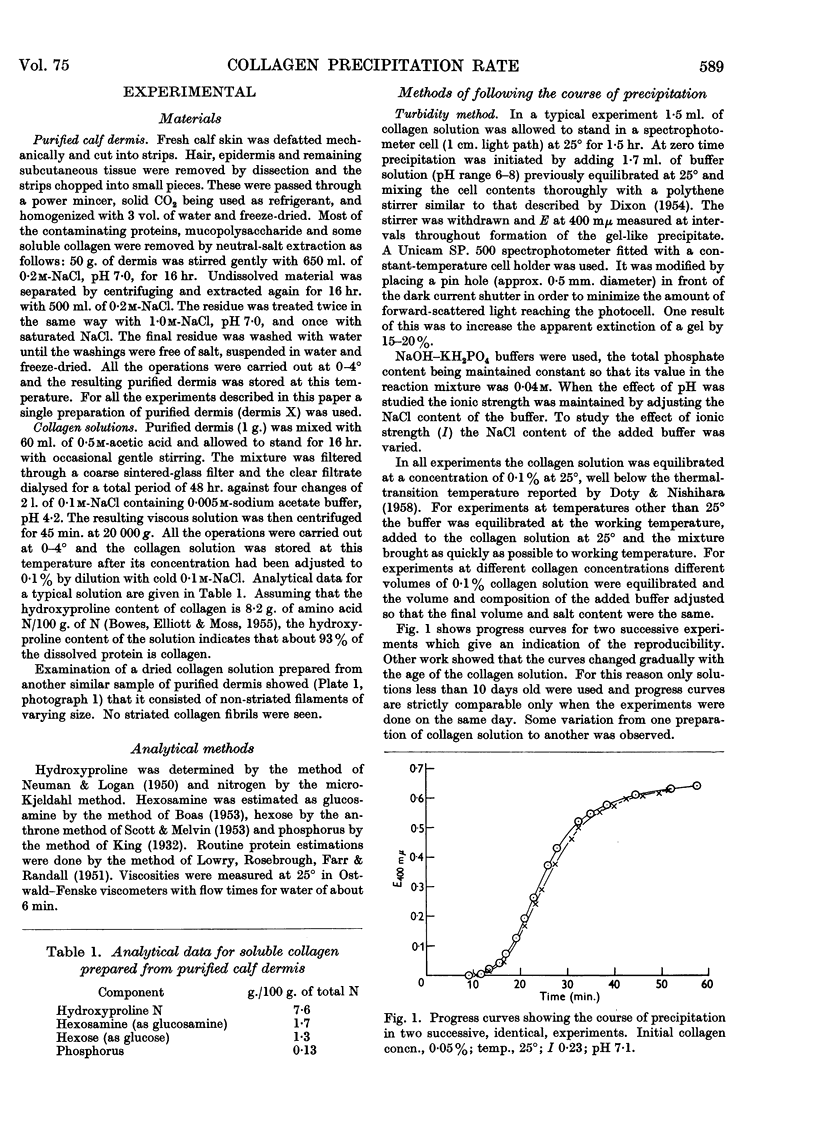
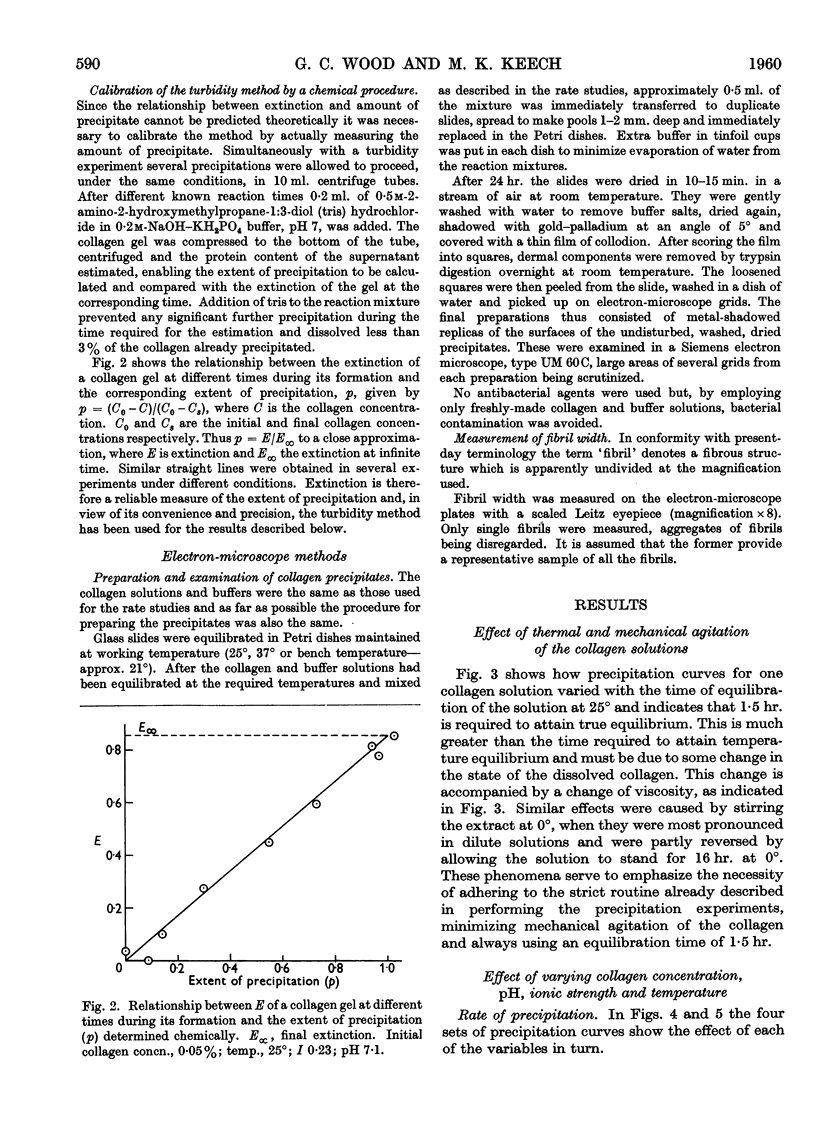
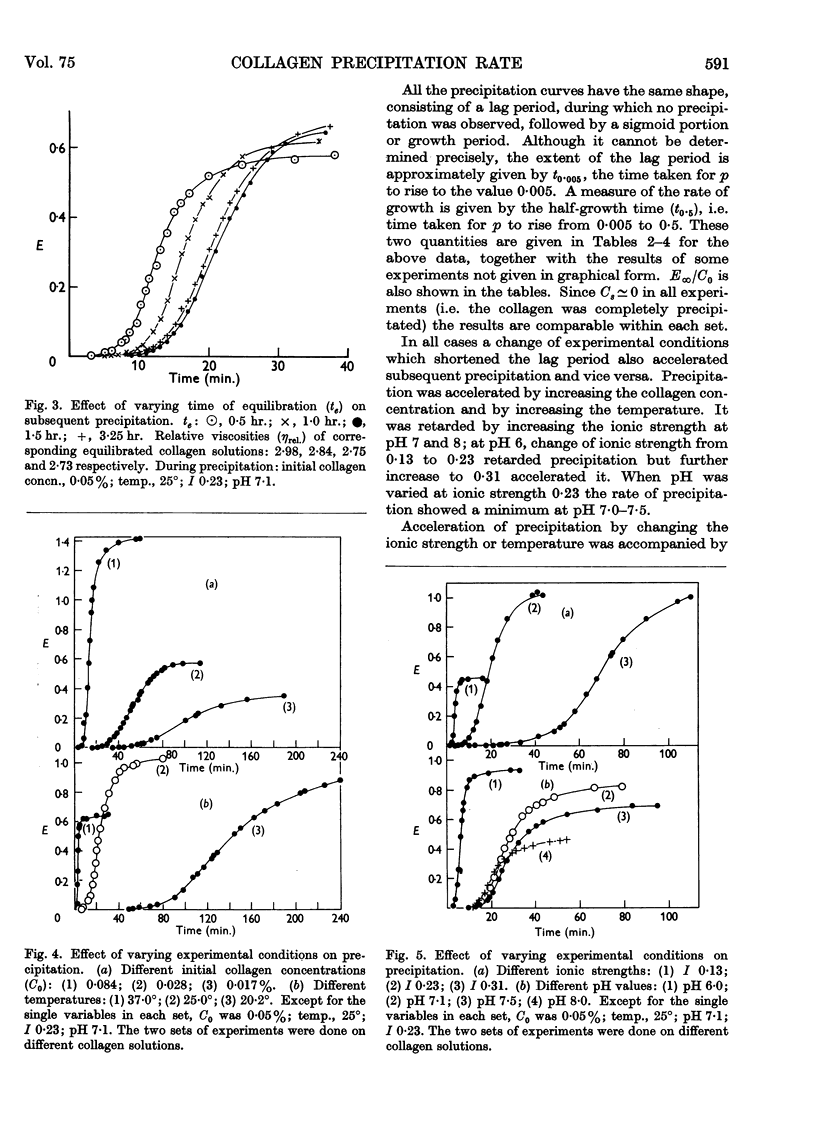
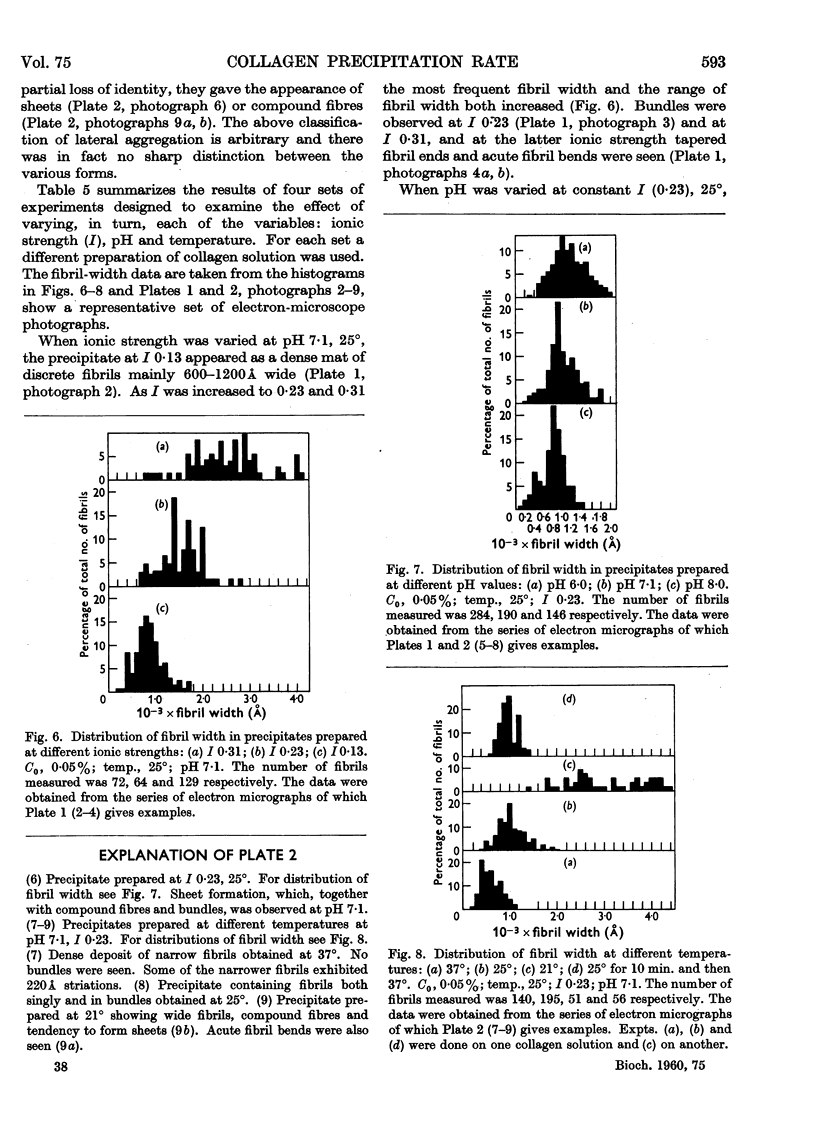
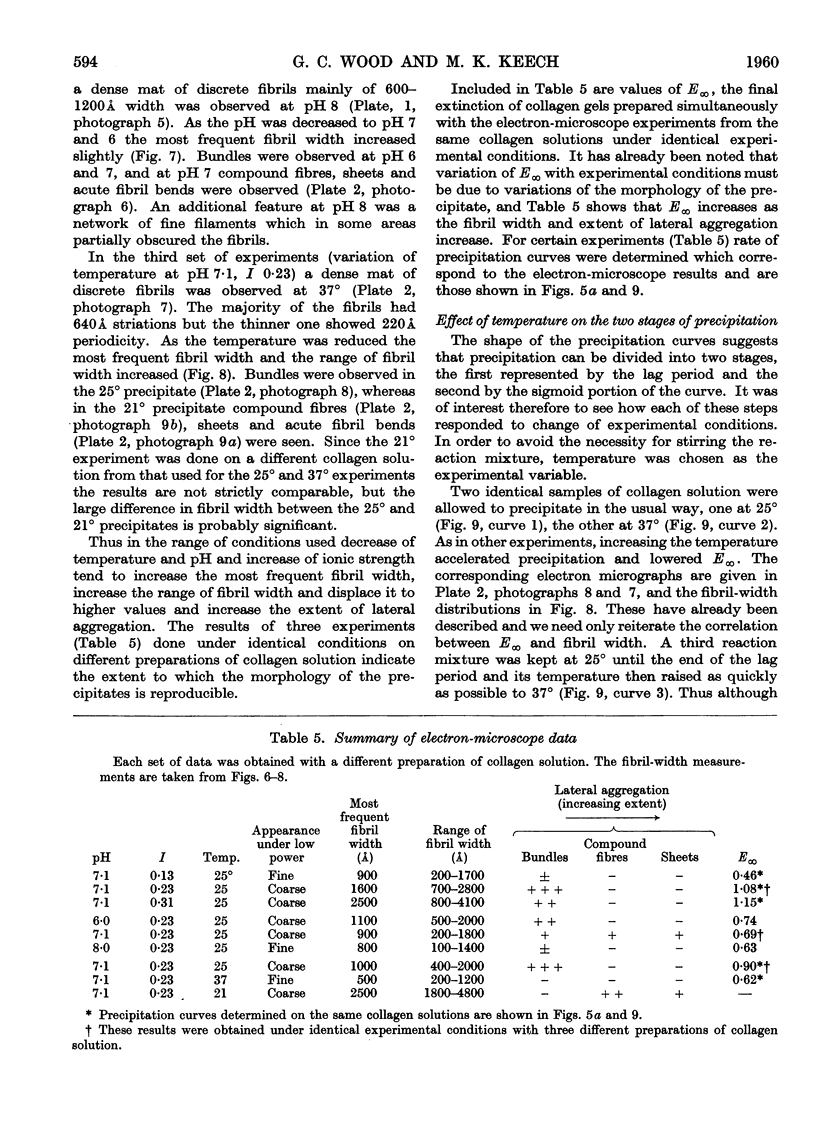
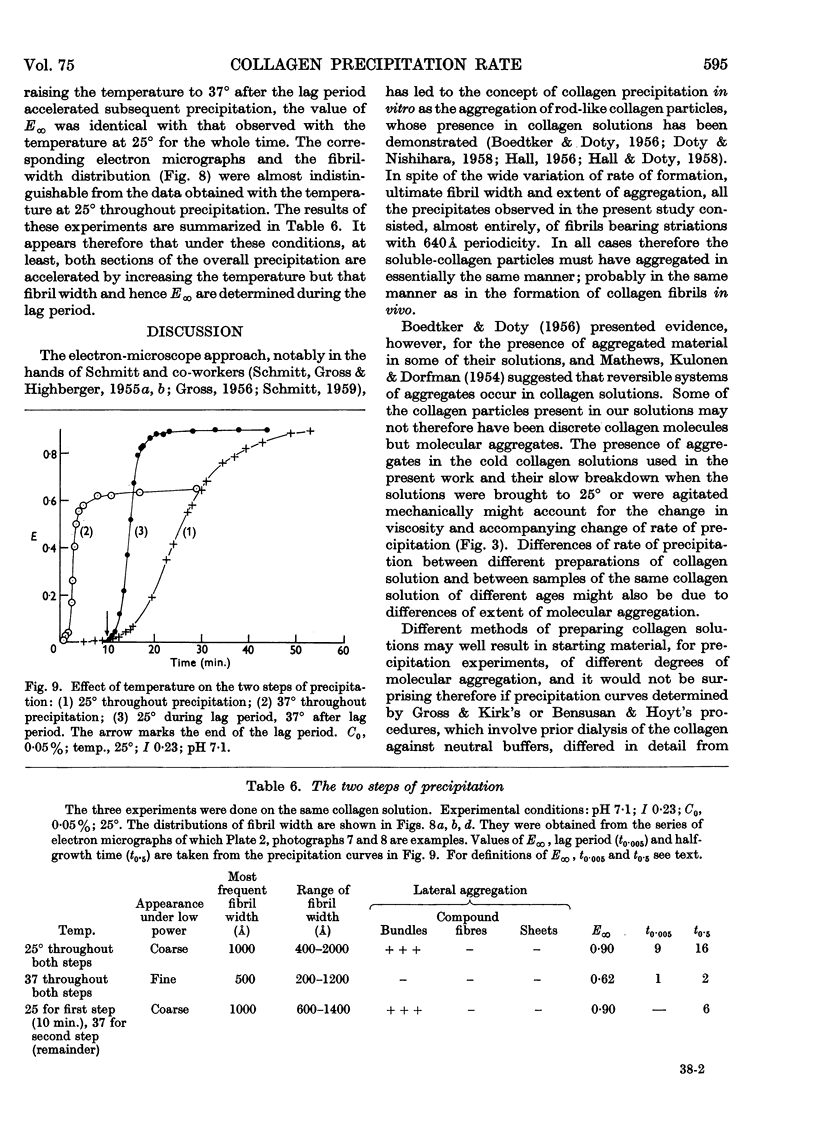
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- BOWES J. H., ELLIOTT R. G., MOSS J. A. The composition of collagen and acid-soluble collagen of bovine skin. Biochem J. 1955 Sep;61(1):143–150. doi: 10.1042/bj0610143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. A thermostat cell-holder for the Beckman spectrophotometer. Biochem J. 1954 Sep;58(1):1–3. doi: 10.1042/bj0580001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDSALL J. T., LEVER W. F. Effects of ions and neutral molecules on fibrin clotting. J Biol Chem. 1951 Aug;191(2):735–756. [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- GROSS J. The behavior of collagen units as a model in morphogenesis. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):261–274. doi: 10.1083/jcb.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J. [Studies on the formation of collagen. III. Time-dependent solubility changes of collagen in vitro]. J Exp Med. 1958 Aug 1;108(2):215–226. doi: 10.1084/jem.108.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON D. S., FESSLER J. H. Isolation and properties of a collagen soluble in salt solution at neutral pH. Nature. 1955 Jul 9;176(4471):69–70. doi: 10.1038/176069a0. [DOI] [PubMed] [Google Scholar]
- JACKSON D. S., NEUBERGER A. Observations on the isoionic and isoelectric point of acid-processed gelatin from insoluble and citrate-extracted collagen. Biochim Biophys Acta. 1957 Dec;26(3):638–639. doi: 10.1016/0006-3002(57)90112-9. [DOI] [PubMed] [Google Scholar]
- JACKSON S. F. Fibrogenesis in connective tissues. Nature. 1954 May 15;173(4411):950–951. doi: 10.1038/173950a0. [DOI] [PubMed] [Google Scholar]
- JACKSON S. F. The fine structure of developing bone in the embryonic fowl. Proc R Soc Lond B Biol Sci. 1956 Mar 26;146(923):270–280. doi: 10.1098/rspb.1957.0010. [DOI] [PubMed] [Google Scholar]
- JACKSON S. F. The formation of connective and skeletal tissues. Proc R Soc Lond B Biol Sci. 1954 Sep 27;142(909):536–548. doi: 10.1098/rspb.1954.0042. [DOI] [PubMed] [Google Scholar]
- JACKSON S. F. The morphogenesis of avian tendon. Proc R Soc Lond B Biol Sci. 1956 Mar 13;144(917):556–572. doi: 10.1098/rspb.1956.0011. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATHEWS M. B., KULONEN E., DORFMAN A. Studies in procollagen. II. Viscosity and molecular weight. Arch Biochem Biophys. 1954 Sep;52(1):247–260. doi: 10.1016/0003-9861(54)90107-1. [DOI] [PubMed] [Google Scholar]
- NEUMAN R. E., LOGAN M. A. The determination of hydroxyproline. J Biol Chem. 1950 May;184(1):299–306. [PubMed] [Google Scholar]
- VANAMEE P., PORTER K. R. Observations with the electron microscope on the solvation and reconstitution of collagen. J Exp Med. 1951 Sep;94(3):255–266. doi: 10.1084/jem.94.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSERMANN F. Fibrillogenesis in the regenerating rat tendon with special reference to growth and composition of the collagenous fibril. Am J Anat. 1954 May;94(3):399–437. doi: 10.1002/aja.1000940304. [DOI] [PubMed] [Google Scholar]
- WOOD G. C. The formation of fibrils from collagen solutions. 2. A mechanism of collagen-fibril formation. Biochem J. 1960 Jun;75:598–605. doi: 10.1042/bj0750598. [DOI] [PMC free article] [PubMed] [Google Scholar]




