Abstract
Taste aversion learning has sometimes been considered a specialized form of learning. In several other conditioning preparations, after a conditioned stimulus (CS) is conditioned and extinguished, re-exposure to the unconditioned stimulus (US) by itself can reinstate the extinguished conditioned response. Reinstatement has been widely studied in fear and appetitive Pavlovian conditioning, as well as operant conditioning, but its status in taste aversion learning is more controversial. Six taste-aversion experiments with rats therefore sought to discover conditions that might encourage it there. The results often yielded little to no evidence of reinstatement, and we also found no evidence of concurrent recovery, a related phenomenon in which responding to a CS that has been conditioned and extinguished is restored if a second CS is separately conditioned. However, a key result was that reinstatement occurred when the conditioning procedure involved multiple closely-spaced conditioning trials that could have allowed the animal to learn that a US presentation signaled or set the occasion for another trial with a US. Such a mechanism is precluded in many taste aversion experiments because they often use very few conditioning trials. Overall, the results suggest that taste aversion learning is experimentally unique, though not necessarily biologically or evolutionarily unique.
Keywords: Taste aversion learning, extinction, reinstatement, concurrent recovery
Taste aversion learning has sometimes been considered a special form of learning. One reason is that a taste conditioned stimulus (CS) and an illness unconditioned stimulus (US) can be separated by several hours and still yield taste aversion learning (e.g., Garcia et al., 1966; Smith & Roll, 1967; Revusky, 1971). A second reason is that rats appear to be “prepared” to associate illness with taste but not an audiovisual cue, whereas footshock could be readily associated with an audiovisual cue but not taste (Garcia & Koelling, 1966; Domjan & Wilson, 1972). Furthermore, a strong taste aversion can develop after as little as one trial (“one-trial learning”) (Garcia et al., 1974; Riley & Clarke, 1977; Rosas & Bouton, 1996). In the 1960s and 1970s, there were many discussions about whether taste aversions were special (e.g., Rozin & Kalat, 1971; Seligman, 1970; Shettleworth, 1972). Rozin and Kalat (1971), for example, suggested that the unique way in which the taste of a poisonous food and its gastrointestinal consequences might occur in nature might encourage the evolution of a unique and biologically adaptive learning system.
Over the years, however, additional experimental analysis suggested that taste aversion learning could be accommodated by general learning principles (Domjan, 1983; Domjan & Galef, 1983). As one example, extinction, one of many critical and well-known conditioning phenomena, occurs in taste aversion learning. That is, a taste aversion created by a taste-illness pairing becomes weaker if the animal receives repeated exposures to the taste without illness. Some extinction phenomena demonstrated in taste aversion learning further suggest that it follows rules that apply to other conditioning preparations. For example, extinction of a taste aversion is sensitive to context. An extinguished taste aversion recovers when it is returned to this original context after extinction in another context (i.e., the renewal effect, e.g., Rosas & Bouton, 1997, 1998; Rosas et al., 2007). Additionally, if a period of time passes after extinction of a taste aversion, spontaneous recovery can occur (e.g., Brooks et al., 1999; Rosas & Bouton, 1996, 1998). And in a recent study, the partial reinforcement extinction effect was obtained in taste aversion learning: A taste that was paired with illness on only some trials (“partially reinforced”) acquired an aversion that extinguished more slowly than that to a taste that was paired with illness on every trial (Bouton & Michaud, 2022). Although these phenomena are consistent with the idea that general laws apply to taste aversion learning, two other extinction phenomena, reinstatement and rapid reacquisition (defined below), appear to be more difficult to produce (Revusky & Coombes, 1979; Bouton, 1982; Danguir & Nicolaidis, 1977; Hart, Bourne, & Schachtman, 1995).
In several Pavlovian learning preparations, after a CS is conditioned and then the conditioned response is extinguished, presenting the US again by itself may partially restore the response to the CS (e.g., Pavlov, 1927; Rescorla & Heth, 1975). This reinstatement phenomenon also occurs in operant conditioning, where a response that is first reinforced with a food pellet (for example) and then extinguished similarly recovers after a few presentations of the food pellet alone (Reid, 1958; see also Skinner, 1938; Konorski, 1948; Hoffman, 1965; Rescorla & Skucy, 1969). At least three explanations of reinstatement have been entertained. First, the US or reinforcer may acquire discriminative stimulus (or “contextual”) properties during conditioning that allow it to set the occasion for the response again after extinction. This is easy to imagine in operant conditioning, where training often involves the animal earning a reinforcer for responding soon after earning a previous one (e.g., Reid, 1958). But reinforcers have also been shown to exert discriminative control of responding in Pavlovian preparations (e.g., Bouton, Rosengard, Achenbach, Peck, & Brooks, 1993; see also Goddard, 1999) as well as other operant preparations (e.g., Bouton & Trask, 2015; Ostlund & Balleine, 2007), and this account of reinstatement is especially viable if there is an amount of acquisition training sufficient for the animal to learn that the receipt of reinforcers sets the occasion for additional CS-reinforcer or response-reinforcer pairings. A second account is based on Rescorla’s “event-memory model” of conditioning (e.g., Rescorla, 1973), which proposed that conditioned responding depends on both the strength of CS-US association as well as the strength of the US representation that the CS activates. In this approach, extinction might occur because the memory of the US is weakened. When the US is presented again, the US memory is restrengthened and responding to the CS therefore returns (Rescorla & Cunningham, 1977, 1978; Rescorla & Heth, 1975). A third account of reinstatement claims that US presentations after extinction condition the context and recreate conditions that were originally associated with conditioning, so that contextual conditioning renews the response when the CS is presented again (Bouton, 1984; Bouton & Bolles, 1979b; Bouton & King, 1983), potentially again by setting the occasion for additional CS-US trials. This view is consistent with the findings that US presentations cause reinstatement only when the US is presented in the context where testing is to occur (Bouton, 1984; Bouton & Bolles, 1979; Bouton & King, 1983; Bouton & Peck, 1989; see also Baker et al., 1990), that individual differences in independently-measured contextual conditioning can predict individual differences in the strength of reinstatement (e.g., Bouton, 1984; Bouton & King, 1983), and that contextual conditioning can be extinguished after US presentation and abolish the reinstatement effect (Bouton & Bolles, 1979b). The related idea that contextual conditioning created by the US presentations merely summates with residual associative strength to the CS is inconsistent with the fact that similarly-created contextual conditioning does not affect responding to a CS that is not under the influence of extinction (Bouton, 1984; Bouton & King, 1986).
In Pavlovian learning, reinstatement has been well-documented in fear conditioning (e.g. Rescorla & Heth, 1975; Bouton & King, 1983; Bouton, 1984) and in appetitive conditioning (e.g. Bouton & Peck, 1989). There is also evidence of it in human preparations, including human fear conditioning (Dirikx et al., 2004; Norrholm et al., 2006) and human causal learning (Garcia-Gutierrez & Rosas, 2003). However, in at least two other Pavlovian conditioning preparations, reinstatement has been more difficult to find. For example, it has not been produced in eyeblink conditioning in rats (Thanellou & Green, 2011) or in nictating membrane response (NMR) conditioning in rabbits (e.g., Macrae & Kehoe, 1999). In NMR conditioning, however, the conditioning of a second CS after extinction of the first can restore extinguished responding in a related phenomenon known as concurrent recovery (e.g., Macrae & Kehoe, 1999; Wiedemann & Kehoe, 2004).
Another preparation where reinstatement is controversial is taste aversion learning. In an early report, Bouton (1982) found no evidence of reinstatement in a conditioned taste aversion preparation. In three experiments with rats, Bouton paired saccharin with lithium chloride (LiCl) injections to develop an aversion to the saccharin flavor. Then, after extinction, the US was presented again. Despite administering incomplete extinction and delivering one or multiple reinstating LiCl injections, no reinstatement was observed. Bouton (1982) also manipulated the context: Conditioning always occurred in one context (physical room), with extinction then occurring in a different context that was later also used for testing. Following contemporary work on reinstatement of extinguished fear (e.g., Bouton & Bolles, 1979; Bouton & King, 1983), the reinstating LiCl injection was delivered in either the conditioning context or in the test context. Despite deliberate manipulation of the context and the potential presence of contextual conditioning during testing, no evidence of reinstatement was found (Bouton, 1982).
In a subsequent study, Schachtman, Brown, and Miller (1985) suggested that according to the event-memory model (Rescorla, 1973; 1974), Bouton’s length of extinction and conditioning-to-reinstatement interval may have been too long. In Schachtman et al.’s interpretation of the model, extinction and the interval between conditioning and reinstatement should be short, so that extinction does not fully degrade the US representation. They reported a single experiment in which they conditioned an aversion to saccharin in rats, reduced the number of extinction trials (from five in Bouton’s method to three) and therefore the conditioning-to-reinstatement interval, and found a significant reinstatement effect. Given the prior work indicating the importance of conducting US exposure and testing in the same context, an unexpected additional feature of the experiment was that Schachtman et al.’s reinstating injection of LiCl took place in a novel context different from that used during conditioning, extinction, and testing. Despite this, reinstatement of the extinguished taste aversion was obtained.
One more study of reinstatement in taste aversion learning involved experiments with infant rats (Revillo, Castello, Paglini, & Arias, 2013). Here, after two conditioning and two extinction trials with a saccharin flavor, preweanling rats were given a reinstating half dose of LiCl (compared to the full dose they received during conditioning) or saline and were tested for flavor consumption ten min later. The authors noted that this half dose was selected based on a pilot study in which it was shown to have no effect on consumption after 10 minutes in naïve subjects. In the preweanling rats that received a reinstating injection of LiCl 10 min before testing, there was a decrease in extinguished saccharin consumption compared to that in rats that received saline or other controls that received LiCl but no prior saccharin conditioning, thereby suggesting a reinstatement effect.
Of the two existing experiments suggesting reinstatement in taste aversion, one featured a method in infant rats in which testing was conducted extremely soon after LiCl administration, and the other used a method that differed radically from that necessary to produce reinstatement in other forms of conditioning. At least as important, the literature on reacquisition in taste aversion learning seems to conflict with the positive results. Related to reinstatement, in some learning preparations, when an extinguished CS is again paired with the US, it may reacquire its ability to evoke the CR at a rate that is faster than the rate of original acquisition (e.g. Ricker & Bouton, 1996). This is known as rapid reacquisition. Several studies of reacquisition in taste aversion learning have only yielded slow reacquisition—that is, a reacquisition of the aversion that is slower (not faster) than that observed in original conditioning (Danguir & Nicolaidis, 1977; Revusky & Coombes, 1979; Hart, Bourne, & Schachtman, 1995). The fact that reacquisition is slow in taste aversion learning contrasts with the reinstatement effects observed by Schachtman et al. (1985) and Revillo et al. (2013). If a single US can produce a reinstatement effect, then why wouldn’t multiple USs cause rapid reacquisition in these failures to produce it?
These considerations suggested that it was appropriate to revisit reinstatement after extinction of a conditioned taste aversion. To understand the conditions that might encourage it, we first turned to the methods used by Schachtman et al. (1985) to see if we could produce reinstatement of an extinguished taste aversion in the present laboratory. We then focused on the idea that recent USs can set the occasion for upcoming reinforced trials (cf. Reid, 1958) and thus generate reinstatement (or rapid reacquisition; see Ricker & Bouton, 1996) when they are reintroduced after extinction. Normally, this mechanism is precluded by standard taste aversion methods, because those methods usually involve very few conditioning trials that give little opportunity for the animal to learn that a US signals another upcoming reinforced trial. Consistent with this idea, the results suggest that in order to observe reinstatement in a taste aversion paradigm, it may be helpful to use multiple conditioning trials in a way that increases the potential for occasion setting by the US to be learned.
Experiment 1
Experiment 1 was designed to replicate aspects of the method of Schachtman et al. (1985) while combining them with methods we have used (e.g., Bouton & Michaud, 2022) that provide good control over the rats’ motivation to drink over a series of extinction and test trials. The experimental design is illustrated in Table 1. As in the Schachtman et al. experiment, a Paired group received a saccharin drink paired with a LiCl injection, while an Unpaired group received the saccharin drink and LiCl unpaired. The Paired groups then received either a reinstating LiCl injection or a control injection of saline. All phases of the experiment took place in the home cage.
Table 1.
Design of Experiments 1 and 3.
| Group | Acquisition | Extinction | Reinstatement | Test |
|---|---|---|---|---|
| Paired | S+ | S− | R (LiCl) | S? |
| NR (Sal) | ||||
| Unpaired | S / + | S− | R | S? |
Note. S = saccharin, + = reinforced (paired with LiCl), − = nonreinforced. In Experiment 1, all phases occurred in the home cage. In Experiment 3, all phases also occurred in the home cage except for the reinstatement treatment, which occurred in a novel context.
Method
Subjects and apparatus
Twenty-four female Wistar rats were purchased from Charles River (Raleigh, NC). They were approximately 75–90 days old at the start of the experiment. The rats were individually housed on sawdust in plastic home cages (31.75 × 19.05 × 19.05 cm), and all of the experimental procedures were conducted in those cages. Experimental solutions were delivered through a standard metal spout that extended from a plastic water bottle affixed to the top of the cage. The saccharin drink CS was a 0.1% w/v saccharin solution (Acros Organics). Injections were given ip with a 20 ml/kg dose of an (isotonic) 0.15 M lithium chloride (LiCl) solution (ThermoFisher Scientific). Saline injections were administered the same way, with a .9% w/v (isotonic) saline solution (ThermoFisher Scientific). Methods used in all experiments were approved by the University of Vermont Institutional Animal Care and Use Committee.
Transparency and Openness
All data used in the analyses throughout this article are available upon request to the first author. Sample sizes were based on our laboratory’s experimental standards and to counterbalance flavors where necessary. The experiments were not preregistered.
Procedure
Drink Training
For the first five days of the experiment, the rats were water deprived except for a 10-min drink of tap water in the home cage at 7:00 am and a second 10-min drink approximately two hrs later. Food was continuously available, but was removed immediately prior to the first drink and returned immediately following the second. The animals were weighed each day prior to the first drink. Water bottles were weighed before and after each drink throughout the experiment. The twice-daily drink schedule was maintained throughout the experiment, and the second drink was always water.
Conditioning
Following the five days of drink training, the animals were randomly assigned to three groups: Paired-R, Paired-NR, and Unpaired-R. On day one of conditioning, the animals in the Unpaired group received saccharin for their first 10-min drink instead of water. Animals in the other two groups received water. On day 2, both Paired groups received saccharin for their first drink, immediately followed by LiCl injection. The Unpaired group was not given saccharin on day 2, but also received the LiCl injection immediately following their first 10-min drink of water. On day 3, all animals received water for both of their drinks to recover from any illness that occurred the day prior.
Extinction
Extinction consisted of three daily extinction trials for all groups. On each of these days, all rats received saccharin for their first drink without any injection. As usual, the second drink (always water) allowed the rats to compensate if consumption had been suppressed during the first drink.
Reinstatement
On the day following the last extinction trial, the Paired-R and Unpaired-R groups received water for their first drink. Immediately following the removal of the water bottles, they received an ip injection of LiCI. The Paired-NR group received water followed by a 20 ml/kg injection of saline. In order to allow subjects to fully recover from illness, only water was administered for both drinks the following day. Following the reinstatement injection and the recovery day, all subjects received a 10-min drink of saccharin to test for reinstatement, as well as a second test the following day.
Results
Conditioning
The results are summarized in Figure 1. We first conducted a 3 (all Groups) × 2 (Trial) ANOVA from the conditioning trial to the first day of extinction, which revealed significant effects of trial, F(1,21) = 99.61, MSE = 178.25, p < .001, group, F(2,21) = 15.48, MSE = 78.19, p < .001, and a significant group × trial interaction, F(2,21) = 92.29, MSE = 165.15, p < .001. To confirm that Paired groups acquired a taste aversion, we ran a 2 (Group: Paired R, Paired NR) X 2 (Trial) ANOVA from the conditioning trial to the first day of extinction, which revealed a significant effect of trial, F(1,14) = 252.32, MSE = 450.0, p < .001 and no group difference (F < 1), indicating successful aversion conditioning. There was a significant group × trial interaction, F(1,14) = 6.33, MSE = 11.28, p = .025, likely due to different baseline consumption means between the two paired groups (Paired R = 10.13ml, Paired NR = 7.63ml). The Unpaired group increased consumption from the first saccharin consumption day to the first day of extinction, t(7) = 5.12, p = .001. Finally, a one-way analysis of the three groups on the first day of extinction revealed a significant effect, F(2,21) = 103.20, MSE = 222.07, p < .001, demonstrating significantly less consumption in the two paired groups than the unpaired group.
Figure 1.
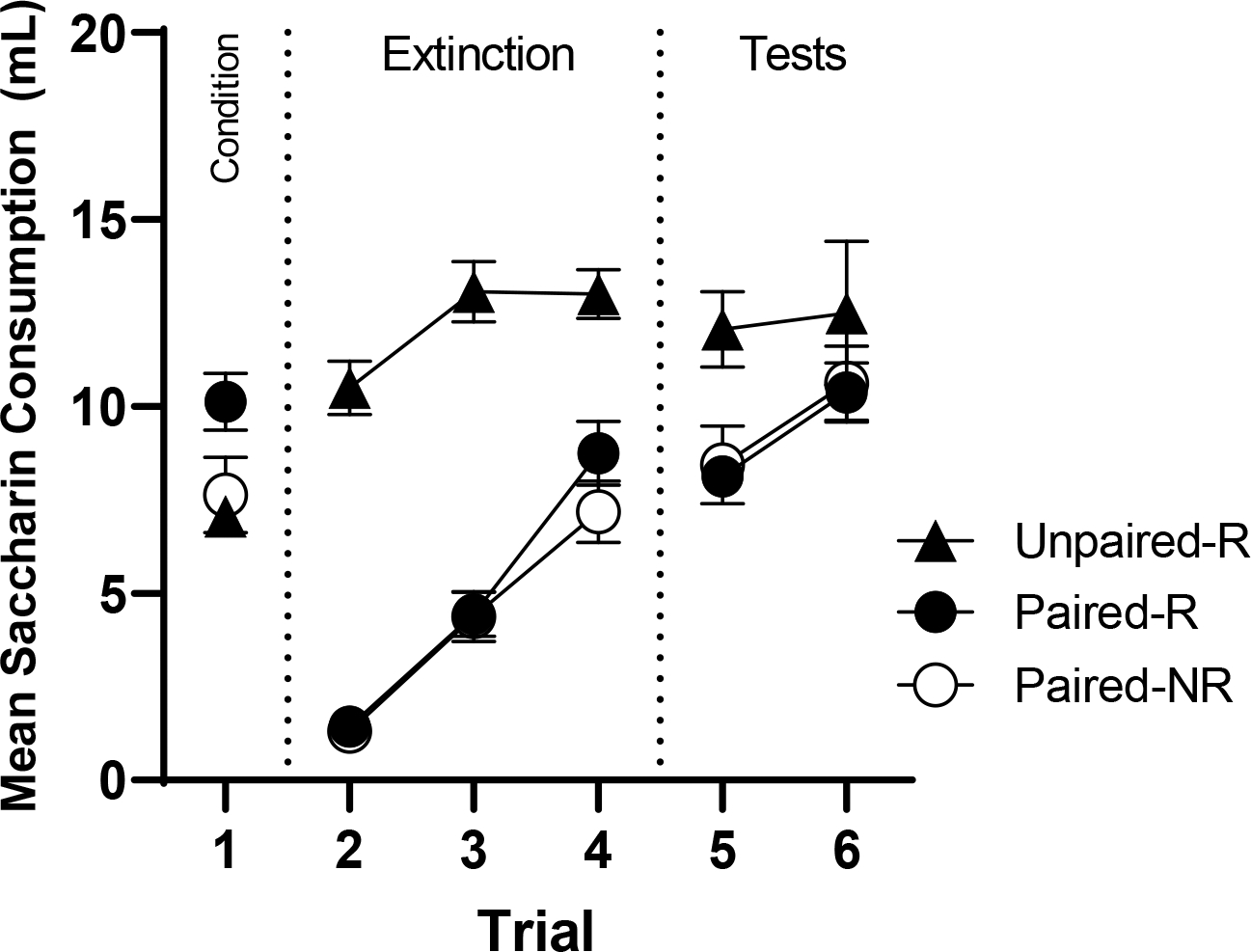
Mean saccharin consumption (ml) during acquisition, extinction, and reinstatement tests of Experiment 1. Error bars represent the standard errors of the means.
Extinction
Across the three trials of extinction, a 3 (Group) X 3 (Trial) ANOVA revealed significant effects of trial, F(2,42) = 119.59, MSE = 164.61, p < .001, group, F(2,21) = 56.84, MSE = 464.63, p < .001, and a group × trial interaction, F(4,42) = 10.77, MSE = 14.88, p < .001. The interaction was due to the Paired groups increasing consumption at a faster rate than the Unpaired group.
Reinstatement Test
The reinstatement test trials are shown at the right of Figure 1. As in previous work on reinstatement, the main focus was the difference between the two conditioned and extinguished groups that did and did not receive the reinstating US (i.e., Paired-R vs Paired-NR) on the first test trial. We first ran a one-way analysis over all three groups on the first test trial, which revealed a significant effect between groups, F(1,21) = 6.27, MSE = 38.32, p = .007. Extinction of the aversion was not complete. A comparison on Test 1 between Paired-R and Paired-NR revealed no significant effect (F < 1).
Discussion
The results of Experiment 1 suggested little evidence of reinstatement when we followed aspects of the method used by Schachtman et al. (1985), but keeping the physical context consistent throughout. The results are consistent with those of Bouton (1982).
Experiment 2
The difficulty in producing reinstatement in Experiment 1 (see also Bouton, 1982) is reminiscent of similar failures to observe it in NMR and eyeblink conditioning (Thanellou & Green, 2011; Macrae & Kehoe, 1999). As noted earlier, however, concurrent recovery is a related effect that does occur in NMR conditioning (Macrae & Kehoe, 1999; Weidemann & Kehoe, 2004). In that phenomenon, a conditioned response to a CS (CS “A”) is conditioned and then extinguished. During (or after) extinction, a novel CS (CS “B”) is introduced and conditioned. When later tested with CS A, recovery of the extinguished response to CS A may occur. It is important to note that in these experiments, subjects do not exhibit any reinstatement of extinguished responding when the US is presented alone (Weidemann & Kehoe, 2004; see also Macrae & Kehoe, 1999; Weidemann & Kehoe, 2003; 2005). Thus, the effect is produced in preparations that do not yield reinstatement by exposure to the US alone.
In Experiment 2, we therefore asked whether concurrent recovery might occur in taste aversion learning. The design is sketched in Table 2. There were two flavor CSs, sucrose and a weak citric acid solution, that were counterbalanced as Flavors A and B. Pilot work indicated no detectable generalization between these flavors, a finding that was further confirmed here. Following extinction of a taste aversion conditioned to Flavor A, instead of merely exposing the rat to another dose of LiCl as in reinstatement, a new Concurrent Recovery group received a paring of the alternate flavor (Flavor B) with the US (LiCl). Following this, we tested the consumption of the original CS to test for concurrent recovery, as well as tested the consumption of the new CS to ensure there was a significant aversion conditioned to it. Another group was tested for reinstatement (which controlled for any potential US-only effects that could have happened when the concurrent recovery group had the US paired with flavor B). There was also a group that received unpaired A and US exposures during acquisition, as well as a group that did not receive any conditioning until the new acquisition phase. (Both of these groups controlled for any aversion to flavor A detected during the test that was created by generalization between B and A.) If a concurrent recovery effect occurs, consumption of only the original conditioned-and-extinguished CS (A) would decrease after conditioning with the other CS (B). A concurrent recovery effect in this experiment would add to evidence that concurrent recovery can occur in settings where reinstatement does not (Weidemann & Kehoe, 2004).
Table 2.
Design of Experiment 2.
| Group | Acquisition | Extinction | New Acquisition | Test |
|---|---|---|---|---|
| Concurrent Recovery | A+ | A− | B+ | A? |
| Reinstatement | A+ | A− | + | A? |
| Unpaired | A / + | A− | B+ | A? |
| Rest | A− | A− | B+ | A? |
Note. A = conditioned flavor, B = novel flavor, + = reinforced, − = nonreinforced. A / + = flavor A and LiCl presented on separate days. A and B are sucrose and citric acid solutions, counterbalanced.
Method
Subjects and apparatus
Thirty-two female Wistar rats purchased from Charles River (Raleigh, NC) were used and were approximately 75–90 days old at the start of the experiment. The apparatus and LiCl solution used were the same as in Experiment 1. The citric acid CS was a 0.005 M solution of citric acid (Fisher Scientific) mixed in tap water. The sucrose CS was a 0.1 M sucrose (Domino Foods, Inc.) solution mixed in tap water. As noted above, pilot work indicated essentially no generalization between these flavors (see also the current results).
Procedure
Drink Training
For the first seven days of the experiment, the rats were water deprived except for a 10-min drink of tap water in the home cage at 12:00 pm and a second 10-min drink approximately three hours later. Food was consistently available, but was removed immediately prior to the first drink and returned immediately following the second. The animals were weighed each day prior to the first drink. As before, the twice-daily drink schedule was maintained throughout the experiment, and the second drink was always water.
Acquisition with Flavor A
Following the seven days of drink training, the animals were randomly assigned to four groups: Concurrent Recovery (n = 8), Reinstatement (n = 8), Unpaired (n = 8), or Rest (n = 8) (see Table 2). On day 8, the Unpaired group received a 10-min exposure to flavor A (sucrose or citric acid, counterbalanced), while the other groups received water. On day 9, all groups received their conditioning treatment. The Concurrent Recovery, Reinstatement, and Rest groups received flavor A (sucrose or citric acid, counterbalanced), and the Unpaired group received water. This was followed by an ip injection of LiCl in all groups except Group Rest, which received saline. The second daily drink (always water) allowed the rats to compensate if consumption had been suppressed during the first drink. All animals were given a recovery day on day 10 where they received water for their first and second drinks.
Extinction
On days 11, 12, and 13, all rats received extinction trials in which they were given a 10-min drink of flavor A. Extinction itself followed the procedure used in conditioning except that LiCl was never administered.
Acquisition with Flavor B
Following extinction of flavor A, groups Concurrent Recovery, Unpaired, and Rest received a conditioning trial with flavor B (sucrose or citric acid, the opposite of what they received for flavor A) on day 14. The method was the same as that with the previous conditioning trial with flavor A. Group Reinstatement received water instead of the flavor. All rats’ first 10-min drink was followed immediately by LiCl injection. The animals were given a recovery day on day 15 in which they were given water for their first and second drinks.
Tests
On days 16 and 17, all rats received a 10-min drink of flavor A to test for concurrent recovery. On days 18 and 19, all rats received a 10 min drink of flavor B to confirm a significant taste aversion to that flavor. As usual, all of these drinks were followed by a second 10-min drink of water 3 hrs later.
Data Analysis
In all of the present experiments that use counterbalanced flavors of sucrose and citric acid (i.e., the present experiment as well as Experiments 5 and 6), we present the data as means collapsed over flavor provided there were no interactions between flavor and other variables during the test. Flavor was always factored into each analysis, and it was expected that main effects of flavor would occur since sucrose consumption is generally higher than citric acid consumption.
Results
Acquisition with flavor A
One rat in the Reinstatement group was excluded from the experiment due to incorrect flavor administration on one of the extinction trials.
The results are summarized in Figure 2. To analyze the aversion conditioned to Flavor A, we first conducted a 2 (Trial) × 2 (Flavor) × 4 (Group) repeated measures ANOVA from the conditioning trial to the first extinction trial, which revealed significant effects of trial, F(1,23) = 14.57, MSE = 30.60, p = .001, group, F(3,23) = 27.59, MSE = 214.18, p < .001, and flavor (mean consumption for sucrose = 11.3 ml, citric acid = 7.75 ml), F(1,23) = 24.45, MSE = 289.00, p = .001, as well as a trial by group interaction, F(3,23) = 49.46, MSE = 103.86, p < .001. There were no other significant effects. To confirm that the interaction was due to the Concurrent Recovery and Reinstatement groups decreasing in consumption and acquiring a significant taste aversion, we then ran a 2 (Trial) × 2 (Flavor) × 2 (Group) repeated measures ANOVA on those two groups from the conditioning trial to the first extinction trial, which demonstrated a significant effect of trial, F(1,11) = 126.78, MSE = 253.08, p < .001, and flavor, F(1,11) = 8.18, MSE = 20.51, p = .016, as well as a trial by flavor interaction, F(1,11) = 6.78, MSE = 13.54, p = .024, and a three-way interaction approaching significance, F(1,11) = 4.51, MSE = 9.00, p = .057, with no other significant effects (largest F = 2.70). Overall, the analyses indicate successful and group-equivalent aversion conditioning. Groups Unpaired and Rest also demonstrated a significant increase from their flavor exposure trials to the first day of extinction with a significant effect of trial, F(1,12) = 33.48, MSE = 73.51, p < .001. As expected, there was also a significant effect of flavor, F(1,12) = 9.70, MSE = 122.07, p = .009, with no other significant effects (largest F = 2.99). Finally, an analysis of all groups on the first extinction trial revealed a significant trial effect demonstrating an aversion in the two paired groups and significantly higher consumption in the Unpaired and Rest groups, F(3,32) = 65.24, MSE = 305.46, p < .001 (see Figure 2). Here, we also found a significant effect of flavor, F(1,12) = 7.19, MSE = 33.67, p = .013, with no interaction (F = 1.89).
Figure 2.

Mean consumption data (ml) on critical trials of Experiment 2. Error bars represent standard errors of the means.
Extinction
Across the three trials of extinction, a 2 (Flavor) × 3 (Trial) × 4 (Group) ANOVA revealed a significant effect of trial, F(2,46) = 117.95, MSE = 221.06, p < .001. There was also a significant group effect, F(3,23) = 33.99, MSE = 401.76, p < .001, as well as a flavor effect (mean sucrose consumption = 12.67 ml, citric acid = 8.92 ml), F(1,23) = 24.45, MSE = 289.00, p < .001, a significant group × trial interaction, F(6,46) = 17.48, MSE = 32.75, p < .001 (Paired groups increasing consumption at a faster rate than Unpaired and Rest groups), a flavor × trial interaction, F(2,46) = 6.51, MSE = 12.21, p = .003, and a three-way interaction, F(6,46) = 3.64, MSE = 6.97, p = .005.
Tests
To examine concurrent recovery, we first analyzed consumption across all groups on the first test trial of Flavor A in a 2 (Flavor) × 4 (Group) ANOVA. The ANOVA revealed a significant effect between groups, F(3,23) = 4.32, MSE = 38.27, p = .015. Extinction in the paired groups was not complete at this time (and their consumption thus still differed from that of the Unpaired and Rest groups). As expected due to continued consumption differences between sucrose and citric acid (sucrose mean = 14.03 ml, citric acid mean = 9.25 ml), there was also an effect of flavor, F(1,23) = 19.14, MSE = 1369.50, p < .001, but there was no interaction (F < 1). When comparing the Reinstatement and Concurrent recovery groups alone in a 2 (Flavor) × 2 (Group) ANOVA on Test 1, there was no difference between them, F(1,11) = .143, MSE = 1.26, p = .712. In these analyses we consistently saw significant effects of flavor, as expected, but importantly no flavor × group interactions (Fs < 1). Overall, the results suggest that conditioning a novel flavor after extinction of the target flavor did not produce concurrent recovery of Flavor A.
To confirm that pairing flavor B had successfully conditioned an aversion to it, we lastly ran a 3 (Group) × 2 (Flavor) ANOVA from the Conditioning B trial to the first Test B, on the groups that received conditioning to Flavor B (i.e., all groups except Reinstatement). The ANOVA revealed significant aversion conditioning: There was an effect of trial, F(1,18) = 91.82, MSE = 336.02, p < .001, as well as flavor F(1,18) = 22.29, MSE = 136.69, p < .001, a trial by flavor interaction, F(1,18) = 13.67, MSE = 50.02, p = .002, and no other significant effects (largest F = 2.78).
Discussion
The results of this experiment suggest little evidence of concurrent recovery after the extinction of a taste aversion: Substantial conditioning of Flavor B had no apparent effect on consumption of the conditioned and extinguished Flavor A. The result contrasts with analogous research in NMR conditioning (e.g., Weidemann & Kehoe, 2004). It is worth noting that the lack of an effect of conditioning Flavor B on the consumption of Flavor A also further confirms that there is little generalization between the sucrose and citric acid flavors. And it may be worth noting that Group Reinstatement showed little evidence that exposure to LiCl after extinction caused reinstatement of the aversion over what might be expected due to spontaneous recovery (e.g., Brooks et al., 1999; Rosas & Bouton, 1996, 1998), although the experiment lacked a reinstatement control group that would force that conclusion.
Experiment 3
Given the lack of evidence of reinstatement in Experiment 1, and no evidence of concurrent recovery in Experiment 2, we conducted another experiment using the methods and design of Experiment 1, but adding Schachtman et al.’s (1985) use of a novel context for the reinstating injection phase. Recall that this feature of their method seemed to ignore research indicating that US presentation and testing must occur in the same context to observe reinstatement in fear and appetitive conditioning (e.g., Bouton, 1984; Bouton & Bolles, 1979; Bouton & King, 1983; Bouton & Peck, 1989). If we obtained a significant reinstatement effect, then we might tentatively conclude that Schachtman et al.’s reinstatement effect was due to their unusual use of the novel reinstatement context. The design of Experiment 3 was the same as that of Experiment 1 (Table 1), except the groups received reinstatement injections of either LiCl or saline in a new and novel context that was very different from the home cage.
Method
Subjects and apparatus
Thirty-two female Wistar rats purchased from Charles River (Raleigh, NC) were used and were approximately 75–90 days old at the start of the experiment. The apparatus and experimental solutions were the same as in Experiment 1, except all groups were given their reinstatement injection in conditioning chambers located outside the colony room, in a laboratory suite across the hall. Each chamber (Model ENV-007-VP; Med Associates, St. Albans, VT) was housed in its own sound-attenuating chamber. All conditioning chambers measured 31.75 × 24.13 × 29.21 cm (length × width × height). The chambers were illuminated by 7.5-W incandescent bulbs mounted to the ceiling of the sound-attenuation chamber.
Procedure
Drink Training
For the first five days of the experiment, the rats were water deprived except for a 10-min drink of tap water in the home cage at 9:00 am and a second 10-min drink approximately two hours later. The method followed that of the preceding experiments.
Conditioning and Extinction
Following the five days of drink training, the animals were randomly assigned to three groups: Paired-R (n = 11), Paired-NR (n = 11), or Unpaired-R (n = 10). The groups received the same conditioning and extinction treatments described for the identically-named groups in Experiment 1.
Reinstatement and Tests
On the day following the last day of extinction, all rats received water for their first drink in the home cage. Then, immediately following removal of the water bottles, the rats were transported individually to a conditioning chamber in the suite across the hall and allowed to acclimate to it for 10 min. The Paired-R and Unpaired-R groups then received an ip injection of LiCI, while the Paired-NR group received an ip injection of saline. Rats were returned to the chamber for one hour before they were transported back to the home cage, consistent with Schachtman et al (1985). In order to allow subjects to fully recover from illness, only water was administered for both drinks the next day. Following the recovery day, all subjects were then tested for reinstatement by receiving a 10-min drink of saccharin (the first drink of the day). A second test drink was also given the next day.
Results
Conditioning
The results are summarized in Figure 3. We first conducted a 3 (Group) × 2 (Trial) ANOVA from the conditioning trial to the first extinction trial, which revealed significant effects of trial, F(1,29) = 39.39, MSE = 95.85, p < .001, group, F(2,29) = 19.55, MSE = 169.10, p < .001, and a significant group × trial interaction, F(2,29) = 49.87, MSE = 121.35, p < .001. To confirm that the Paired groups acquired a significant taste aversion, we ran a 2 (Group: Paired R, Paired NR) X 2 (Trial) ANOVA from the conditioning trial to the first day of extinction, which revealed a significant effect of trial, F(1,19) = 174.27, MSE = 303.19, p < .001 and no group effect or interaction, indicating successful and equivalent aversion conditioning. Group Unpaired also demonstrated a significant increase from their saccharin exposure trial to the first day of extinction, t(9) = 3.53, p = .006. Finally, an analysis of the three groups on the first day of extinction revealed a significant effect, F(2,29) = 46.10, MSE = 255.77, p < .001, demonstrating significant aversion in the two paired groups and significantly higher consumption in the unpaired group (see Figure 3).
Figure 3.
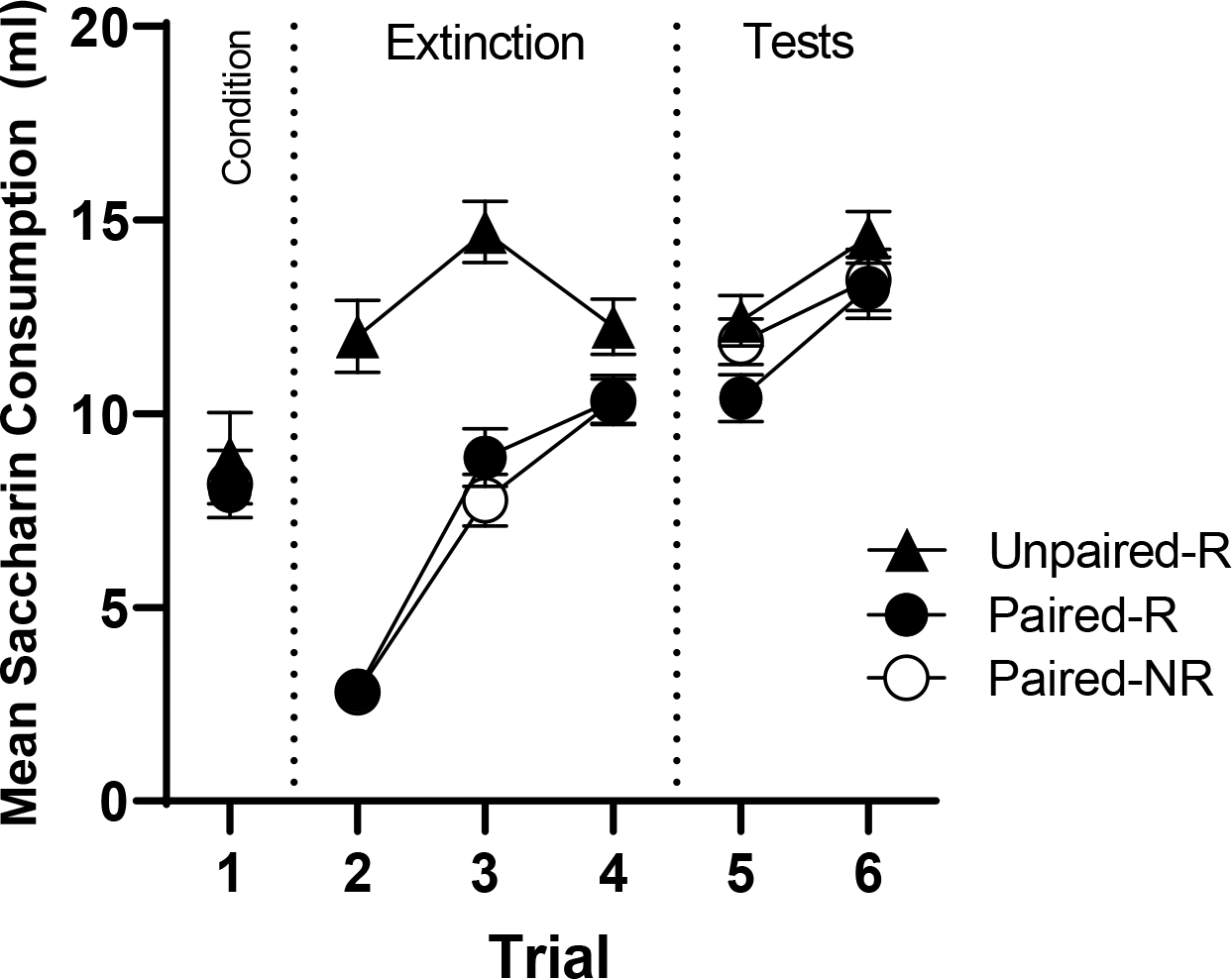
Mean consumption (ml) for acquisition, extinction, and reinstatement tests of Experiment 3. Error bars represent standard errors of the means.
Extinction
Across the three trials of extinction, a 3 (Group) X 3 (Trial) ANOVA revealed a significant effect of trial, F(2,58) = 98.05, MSE = 249.31, p < .001. There was also a significant group effect, F(2,29) = 41.65, MSE = 350.20, p < .001, and a significant group × trial interaction, F(4,58) = 18.48, MSE = 47.00, p < .001. The interaction was due to the Paired groups increasing consumption while the Unpaired group did not.
Reinstatement Test
The reinstatement test trials are shown at the right of Figure 3. First, we ran a one-way analysis across all three groups on the first test trial, which revealed a significant difference between groups, F(2,29) = 3.88, MSE = 16.96, p = .032. Extinction in the paired groups was not complete at this time (their consumption still differed from the unpaired group’s). Another univariate analysis isolating Paired-R and Paired-NR on Test 1 also revealed a reliable effect, F(1,21) = 5.75, MSE = 24.21, p = .027—suggesting a significant reinstatement effect. To characterize the effect further, we compared the last extinction trial and first test trial between the two Paired groups in a 2 (Group) × 2 (Trial) ANOVA, which did not reveal an effect of trial, F(1,20) = 1.57, MSE = 3.84, p = .224, or group, F(1,20) = 1.48, MSE = 9.09, p = .238. The interaction fell just short of significance, F(1,20) = 4.10, MSE = 10.02, p = .056. But to further clarify the reinstatement effect, we conducted paired t-tests for each Paired group from their last extinction trial to Test 1. Group Paired-R did not change consumption significantly between trials, t(10) = .531, p = .607, while Group Paired-NR increased consumption from extinction to the test, t(10) = 2.38, p = .038. The pattern is consistent with the idea that reinstatement took the form of the US preventing further extinction rather than causing an absolute decrease in consumption of the extinguished flavor.
Discussion
The results of Experiment 3 revealed a small, but statistically significant, reinstatement effect in taste aversion learning when adopting Schachtman et al.’s (1985) use of a novel context during the reinstatement phase. The findings thus suggest that the use of a novel context may enhance the reinstatement effect. Interestingly, LiCl exposure appeared to prevent further extinction rather than cause an absolute decrease in consumption (i.e., an actual increase in the conditioned response). Given that the reinstatement effect in the means was not numerically large, we further expanded the investigation with other methods that might better allow the rats to learn that a US exposure sets the occasion for upcoming conditioning trials.
Experiment 4
Experiment 4 differed from the previous ones in that it used eight acquisition trials rather than one. Eight trials were used to increase the rats’ exposure to the contingency that a trial containing a US was followed by another one. Such exposure is unusual when studying taste aversions because, in most experiments, only one or two conditioning trials are needed and used. To make multiple-trial conditioning possible, we used a weaker dose of LiCl to avoid consumption reaching a consumption floor. We in fact began by studying two weakened doses to ascertain which might be better to use in the experiments that followed. The experiment also used a new extinction-to-criterion technique that helped ensure reliable but incomplete extinction in every subject involved in the experiment.
In taste aversion experiments that use only one or two conditioning trials, a 20 ml/kg dose of .15 M lithium chloride is common. As noted above, in order to lengthen our conditioning procedure to eight trials, we needed to use a smaller dose. Previous experiments (Bouton & Michaud, 2022) successfully used a 5 ml/kg dose of .15 M lithium chloride. However, to further prevent possible consumption floor effects, we also tested an even smaller dose of .15 M LiCl at 3.75 ml/kg. In addition, a factor that might have made it difficult to produce reinstatement in earlier studies is that the rat might habituate to the effects of LiCl during conditioning. To test such habituation, which might become an even bigger possible issue with multiple conditioning trials, we scored the rats’ behavior for visible signs of illness (lying-on-belly, e.g., Parker, Hills, & Jensen, 1984) after the injections given on trials 2, 5, and 8. After the acquisition and extinction phases, rats were assigned to reinstatement groups that either received a reinstating injection of LiCl (the US) or an injection of saline before being tested with the CS the following day. The design is illustrated in Table 3.
Table 3.
Design of Experiment 4.
| Group | Acquisition | Extinction | Reinstatement | Test |
|---|---|---|---|---|
| 5 ml/kg | S+ | S− | R (LiCl) | S? |
| NR (Sal) | ||||
| 3.75 ml/kg | S+ | S− | R (LiCl) | S? |
| NR (Sal) |
Note. S = saccharin, + = reinforced, − = nonreinforced.
Method
Subjects and apparatus
Thirty-two female Wistar rats purchased from Charles River (Raleigh, NC) were used and were housed as in Experiments 1 – 3. The saccharin drink CS was again a 0.1% w/v solution and injections were given ip with either a 5ml/kg or 3.75ml/kg dose of a 0.15 M lithium chloride (LiCl, Fisher Scientific) solution.
Procedure
Drink training
For the first five days of the experiment, rats were given the two-drinks-a-day procedure used in the preceding experiments, with the first drink occurring at 10 am and the second drink occurring approximately two hours later.
Conditioning
Following drink training, the animals were randomly assigned to two groups: 5 ml/kg (n = 16) or 3.75 ml/kg (n = 16). Conditioning was then conducted over the next eight days, with all rats receiving eight daily conditioning trials. Trials consisted of a 10-min saccharin drink followed immediately by LiCl injection, the dose being dependent on group. As usual, the second daily drink (always water) allowed the rats to compensate if consumption had been suppressed during the first drink. On days 2, 5, and 8 of conditioning we also scored each rat for “lying on belly” as an indication of illness (Parker, Hills, & Jensen, 1984). Each rat was scored as either on its belly or not immediately after all injections were administered, then again 5 minutes after the first scoring, and again 5 minutes after the second scoring.
Extinction
All rats initially received five extinction trials. However, at that point we instituted an extinction criterion designed to prevent a rat’s aversion from becoming over-extinguished before it received the reinstatement treatment. Once a rat reached 5 ml or more of saccharin consumption on an extinction trial, it was scheduled for reinstatement the next day. On each of the daily extinction trials, rats received a 10-min drink of saccharin without a LiCl injection during the first daily drink.
Reinstatement and Tests
Two rats from Group 3.75 ml/kg were excluded from the study for not reaching extinction criterion after 13 extinction trials. For the rest of the animals, we used a randomized block method, such that one of every pair of rats that successively reached extinction criterion was randomly assigned to each of the two reinstatement groups (Groups R and NR). For reinstatement, rats received a 10-min drink of water, and immediately following removal of the bottle were given an ip injection of LiCI (group R) or saline (group NR). For each rat, the LiCl dose was the same as that used in conditioning. Twenty-four hours after reinstatement, all subjects were tested for reinstatement effects with a 10-min drink of saccharin. This test was repeated the following two days (48 and 72 hours after the reinstatement injection). All experimental events occurred around the time of the first drink; as usual, the second drink each day was water.
Results
Conditioning
The results of the conditioning phase are summarized in Figure 4. A 2 (Group) × 8 (Trial) ANOVA revealed a significant effect of trial, F(7,210) = 120.77, MSE = 221.29, p < .001, and no other significant effects (largest F = 1.35), indicating successful and apparently similar aversion conditioning in both groups. Additionally, as summarized in Figure 5, lying on the belly was evident and did not appear to habituate over trials. We analyzed these data in a 2 (Dose) × 3 (within-trial block) × 3 (Trial) ANOVA, which only revealed a significant effect of within-trial block, F(2,180) = 19.94, MSE = 3.38, p < .001. There was no reliable trial effect, F(2,90) = 1.54, MSE = .545, p = .220, nor any other effects (largest F = 1.19).
Figure 4.

Mean consumption (ml) of saccharin during acquisition. Error bars represent standard errors of the means.
Figure 5.

Percent sick behavior in rats after receiving LiCl injections during acquisition, in five-minute intervals during acquisition trials 2, 5, and 8.
Extinction
Saccharin consumption during extinction trials for each individual rat is depicted in Figure 6. As noted above, data from two rats were eliminated from the 3.75ml/kg group for not reaching the extinction criteria (at least 5 ml of consumption). To confirm that the remaining rats’ taste aversions extinguished, we conducted a 2 (Group) × 2 (Trial) ANOVA from the first extinction trial to each rat’s last extinction trial, which revealed a significant effect of trial, F(1,28) = 272.66, MSE = 393.60, p < .001, as well as a significant trial by group interaction, F(1,28) = 5.96, MSE = 8.60, p = .021, but no significant group effect (F < 1). This confirms that the aversion indeed extinguished, and the interaction effect is likely due to 3.75 ml/kg rats extinguishing in fewer trials than the 5ml/kg rats (see Figure 6). The mean number of extinction trials received by groups 5ml/kg-R and NR, and 3.75ml/kg-R and NR were 8.67, 8.14, 6.86, and 6.86, respectively.
Figure 6.

Consumption of saccharin for individual subjects during Extinction in Experiment 4.
Reinstatement and Tests
Saccharin consumption on the crucial reinstatement test trials (as well as selected trials of conditioning and extinction) are summarized in Figure 7. A 2 (Dose) × 2 (Paired-R, Paired-NR) ANOVA on Test 1 did not reveal a significant reinstatement effect (F = 1.34). This suggests that with this particular procedure, an increased number of acquisition trials did not produce a reinstatement effect with either LiCl dose.
Figure 7.

Mean consumption data (ml) on critical trials of Experiment 4. Reinstatement tests are shown at right. Error bars represent standard errors of the means. C1 = first conditioning trial, E1 = first extinction trial, E(L-1) = second-to-last extinction trial, E(L) = last extinction trial, T1 and T2 = tests 1 and 2.
Discussion
The results of Experiment 4 first indicated that there was not a significant difference between the 3.75 and 5 ml/kg two doses of LiCl in their effects on saccharin conditioning. There was also little evidence that the lying-on-belly index of sickness created by LiCl habituated over the eight conditioning trials. And perhaps most important, the results suggest that enhancing the potential for a US to signal (or set the occasion for) upcoming conditioning trials by increasing the number of conditioning trials was not enough to produce reinstatement of an extinguished taste aversion.
Experiment 5
In Experiment 5, we used a method like that in Experiment 4, but now shortened the interval between the reinstating injection (US) and the test of flavor consumption (CS). Historically, reinstatement procedures in CTA experiments deliver the reinstating US on one day, allow a day or two for recovery, and then test with the flavor CS on a following day (but see Revillo et al., 2013). In Experiment 4, there was a 24 h period between the US and the first test trial. However, reinstatement procedures in other learning paradigms often administer the reinstating US and the CS test in the same day (although it is typically a 24 h interval in fear conditioning). In Experiment 5, by shortening the interval between the reinstating US and test CS to only three hours, we took advantage of the possibility that a more recent US might enhance any reinstatement effect. Observations of sickness behavior in Experiment 4 suggested that the rats would not be sick three hours after the LiCl injection.
The design of the experiment is presented in Table 4. After conditioning and extinguishing an aversion to a flavor, rats received either a reinstating injection of LiCl (R) or saline (NR) as in Experiment 4. Since the shorter reinstatement-test interval had the potential of unconditionally suppressing consumption (from lingering effects of LiCl injection), rats were either tested with the flavor they were conditioned and extinguished with (flavor A; groups Extinguished-R and Extinguished-NR) or with a novel flavor (flavor B; groups Novel-R and Novel-NR). We knew from prior work that the first exposure to the flavor would be modestly suppressed (e.g., by neophobia), and the idea was to extinguish the aversion in the Extinguished groups to an incomplete point where consumption would be equal to that in the controls. If we find suppressed consumption in the group that is reinstated with LiCl and tested with the novel flavor, then this would indicate an unconditioned effect of the recent LiCl exposure on consumption of a novel flavor that is at a similar level of consumption as the extinguished CS. As in Experiment 2, flavors A and B were provided by sucrose and citric acid solutions (counterbalanced).
Table 4.
Design of Experiments 5 and 6.
| Group | Acquisition | Extinction | Reinstatement | Test |
|---|---|---|---|---|
| Extinguished | A+ | A− | R (LiCl) | A? |
| NR (Sal) | ||||
| Novel | A+ | A− | R (LiCl) | B? |
| NR (Sal) |
Note. A = conditioned flavor, B = novel flavor, + = reinforced, − = nonreinforced. A and B are sucrose and citric acid, counterbalanced. In Experiment 5, the eight conditioning trials were separated by 24 hrs and the interval between reinstatement injections and the test was 3 hrs. In Experiment 6, there was a shorter 5-hr gap between acquisition trials 1-2, 3-4, 5-6, 7-8, and the interval between reinstatement and test was also 5 hrs.
Method
Subjects and apparatus
Thirty-two female Wistar rats purchased from Charles River (Raleigh, NC) were used and were approximately 75–90 days old at the start of the experiment. The apparatus and solutions used were the same as in Experiment 3, but all doses were 5 ml/kg based on the results of Experiment 4. The injected saline solution used was .9% w/v. As in Experiment 2, the citric acid CS was a 0.005 M solution of citric acid (Fisher Scientific) and the sucrose CS was a 0.1 M sucrose (Domino Foods, Inc.). Both solutions were mixed in tap water.
6.1.2. Procedure
Drink Training
For the first five days of the experiment, the rats were water deprived except for a 10 min drink of tap water in the home cage at 10:00 am and a second 10 min drink approximately three hours later. Food was consistently available, but was removed immediately prior to the first drink and returned immediately following the second. The animals were weighed each day three hrs prior to the first drink. The twice-daily drink schedule was maintained throughout the experiment, and the second drink was always water.
Conditioning
Following the five days of drink training, animals were randomly assigned to the two flavors that served as Flavor A (sucrose or citric acid). Starting on day 6, groups received eight daily conditioning trials, each of which consisted of a 10 min exposure to flavor A. This was followed by an ip injection of LiCl in both groups. The second daily drink was always water.
Extinction
Using the method developed in Experiment 4, each rat was then extinguished to a consumption criterion that was based on the mean flavor consumption on day 1 of conditioning (10 ml for sucrose, 5 ml for citric acid). Unlike Experiment 4, the criterion was in effect as soon as extinction started. On each of the daily extinction trials, rats received a 10-min drink of flavor A without a LiCl injection.
Reinstatement and Tests
Once each rat’s consumption reached extinction criterion, we used a randomized block method, analogous to the one used in Experiment 4, such that one of every four successive rats reaching criterion were randomly assigned to each of the two reinstatement groups (Groups R and NR) as well as each of the two test groups (Groups Extinguished and Novel). On the next day, the reinstatement injection was given three hrs prior to the first scheduled drink. This consisted of an ip injection of LiCI (group R) or saline (group NR). Three hours after reinstatement, all subjects were then tested for reinstatement effects with a 10-min drink of flavor A (Group Extinguished) or flavor B (Group Novel). The test was repeated the following two days. As usual, all experimental events occurred around the time of the first drink and the second drink was always water.
Results
Conditioning
The results of the conditioning phase are summarized in Figure 8. A 2 (Group) × 8 (Trial) ANOVA revealed a significant effect of trial, F(7,210) = 110.13, MSE = 192.87, p < .001, flavor (sucrose mean = 11.28 ml, citric acid mean = 6.59 ml), F(1,30) = 20.03, MSE = 158.60, p < .001, as well as a significant trial by flavor interaction, F(7,210) = 11.56, MSE = 20.24, p < .001. These results indicate successful aversion conditioning in both groups, and reflect the consumption differences seen during the first two acquisition trials.
Figure 8.

Mean consumption (ml) of sucrose and citric acid during acquisition. Error bars represent standard errors of the means, but are difficult to detect because variability was low.
Extinction
Flavor consumption during the extinction trials for each rat is depicted in Figure 9. To confirm that the rats’ taste aversions extinguished, we conducted a 2 (Flavor) × 2 (Trial) ANOVA from the first extinction trial to each rat’s last extinction trial, which revealed a significant effect of trial, F(1,30) = 410.80, MSE = 1143.29, p < .001, and flavor, F(1,30) = 50.60, MSE = 106.35, p < .001, as well as a significant interaction, F(1,30) = 54.47, MSE = 151.60, p < .001. The pattern confirms that the aversion indeed extinguished, and the interaction effect is likely due to sucrose and citric acid differences in extinction criteria (10 ml for sucrose, 5 ml for citric acid; see Figure 9). Mean number of extinction trials for the groups Extinguished-R and NR, and Novel-R and NR were 5.00, 5.13, 5.00, and 4.88, respectively.
Figure 9.

Consumption during extinction trials for individual subjects in Experiment 5.
Reinstatement and Tests
Results of the test trials are summarized in Figure 10 (along with selected conditioning and extinction trials). We first ran a 2 (Flavor) × 2 (Extinguished, Novel) × 2 (R, NR) ANOVA on the first test trial, which only demonstrated a significant effect of flavor (sucrose mean = 14.13 ml, citric acid mean = 8.00 ml), F(1,24) = 19.56, MSE = 300.13, p < .001. The ANOVA did not reveal any other significant effects (largest F = 1.49). Once again, there was no suggestion of reinstatement, despite using both multiple acquisition trials and a shorter reinstatement-to-test interval (see Figure 11).
Figure 10.

Mean consumption data (ml) on critical trials of Experiment 5. Reinstatement tests are shown at right. Error bars represent standard errors of the means. C1 = first conditioning trial, E1 = first extinction trial, E(L-1) = second-to-last extinction trial, E(L) = last extinction trial, T1 and T2 = tests 1 and 2.
Figure 11.
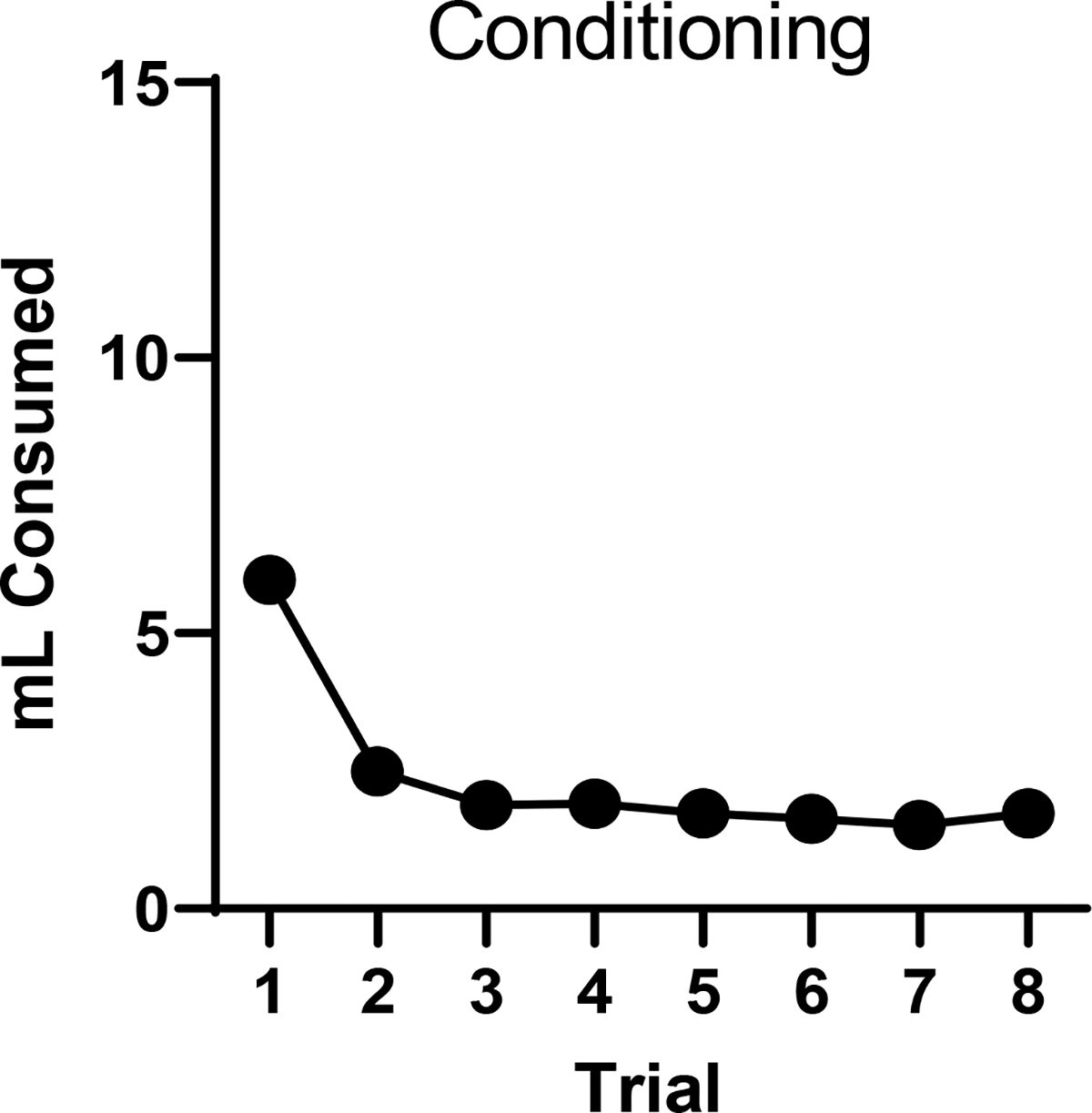
Mean flavor consumption (ml) during acquisition in Experiment 6. Error bars represent standard errors of the means, but are difficult to detect because of low variability.
Discussion
The results of Experiment 5 suggest that utilizing eight conditioning trials (as in Experiment 4), as well as shortening the reinstatement-to-test interval, was not enough to produce a reliable reinstatement effect. The experiment’s method also successfully matched consumption levels at test between a novel and the conditioned-and-extinguished flavors. It is important to note that the reinstating injection did not suppress consumption of either the novel flavor or the conditioned-and-extinguished flavor.
Experiment 6
In Experiment 6, we looked further into the idea that a US might cause reinstatement in taste aversion learning if it could signal or set the occasion for more conditioning trials. Here we used the same design as in Experiment 5 (Table 4), except that rats now received two acquisition trials on each of four days. The daily trials were separated by five hrs. In this method, a reinforced trial (at the time of Drink 1) might more readily signal another reinforced trial scheduled a few hours later (at the time of Drink 2). Recall that consecutive trials were spaced by 24 hrs in Experiments 4 and 5. Having two reinforced trials per day should thus strengthen a US’s prediction of another CS-US pairing. And in the reinstatement test, the US preceded the test trial by the same 5-hr interval. If we obtain reinstatement in the experimental group here, the result would be consistent with the idea that reinstatement occurs when animals learn that USs signal other reinforced trials. The same novel controls were used as in Experiment 5.
Method
Subjects and apparatus
To provide good experimental power, 64 female Wistar rats purchased from Charles River (Raleigh, NC) were used and were approximately 75–90 days old. For practical reasons (space in the colony room), the experiment was run in two replications of 32 rats, with each group represented equally in each replication. The apparatus and solutions were the same as in Experiments 3 and 5.
Procedure
Drink Training
For the first seven days of the experiment, the rats were water deprived except for a 5-min drink of tap water in the home cage at 10:00 am, a second 5-min drink approximately five hours later, and a third 10-min drink approximately three hours after the second drink. Food was consistently available, but was removed immediately prior to the first drink and returned immediately following the third. The animals were weighed each day prior to the first drink. This schedule was maintained throughout the experiment.
Conditioning
Following the five days of drink training, animals were randomly assigned to the two flavors: sucrose (n = 32), or citric acid (n = 32). Both groups received eight conditioning trials (two per day, four days total), which each consisted of a 5-min exposure to flavor A (sucrose or citric acid, counterbalanced) followed by an ip injection of LiCl. The third daily 10-min drink (always water) allowed the rats to compensate if consumption had been suppressed during the first two drinks.
Extinction
Using the method developed in Experiment 4, consumption of each rat was then extinguished to a criterion that was based on the mean flavor consumption on day 1 of conditioning (7 and 5 ml of sucrose for the first and second experimental replications, respectively; always 4 ml for citric acid). The criterion was in place from the start of extinction. As in conditioning, rats received two extinction trials daily. If a rat reached the extinction criterion on the first extinction trial of the day, it still received the second extinction trial of that day. On each of the extinction trials, rats received a 5-min drink of flavor A without a LiCl injection.
Reinstatement and Tests
Once each rat reached its extinction criterion, we used the randomized block method of Experiment 5, such that one of every four successive rats reaching criterion were randomly assigned to each of the two reinstatement phase groups (Groups R and NR) as well as each of the two test groups (Groups Extinguished and Novel). As just noted, in keeping with the two-trials-a-day procedure, all animals received two extinction trials on their final extinction day regardless of whether criterion was reached on the first or second trial. On the reinstatement day that followed, the rats received an ip injection of LiCI (group R) or saline (group NR) after water given for the first drink. The LiCl dose was the same as that used in conditioning. Five hours after the injection, all rats were tested for reinstatement with a 5 min drink of flavor A (groups Extinguished) or flavor B (groups Novel). The test was repeated the following day during the first two drinks. As usual, the third 10-min drink each day was water.
Results
One rat in the sucrose conditioning group was eliminated from the experiment due to not reaching criterion in the Extinction phase. The results reported below are averaged over the two replications of the experiment, which produced similar patterns of data.
Acquisition
The results of the conditioning phase are summarized in Figure 11. A 2 (Flavor) × 8 (Trial) ANOVA revealed a significant effect of trial, F(7,427) = 158.07, MSE = 136.62, p < .001, no group main effect (F < 1), but a significant trial by flavor interaction, F(7,427) = 8.54, MSE = 7.38, p < .001. The interaction effect likely reflects the consumption differences on the first trial; as usual, there was less consumption of citric acid than sucrose (sucrose mean = 6.81 ml, citric acid mean = 5.13 ml).
Extinction
Flavor consumption during extinction trials for each rat is depicted in Figure 12. To confirm that subjects’ taste aversions extinguished, we also conducted a 2 (Flavor) × 2 (Trial) ANOVA from the first extinction trial to each rat’s last extinction trial, which revealed a significant effect of trial, F(1,61) = 366.48, MSE = 1134.07, p < .001, and no other significant effects (F’s < 1). This confirms successful and similar aversion conditioning between flavors. Mean number of extinction trials received by groups Extinguished R and NR, and Novel-R and NR, were 8.13, 8.44, 8.38, and 7.86, respectively.
Figure 12.

Consumption during extinction trials for individual subjects in Experiment 6.
Reinstatement and Tests
Test results, as well as selected conditioning and extinction trial results, are depicted in Figure 13. First, a 2 (group: Extinguished, Novel) × 2 (R, NR) × 2 (Flavor) ANOVA on the first test trial revealed a significant effect of group, F(1,55) = 4.11, MSE = 26.71, p = .047, as well as flavor, F(1,55) = 10.52, MSE = 68.32, p = .002, with no other significant effects (largest F = 1.95). To test for a reinstatement effect in a manner like that in the earlier experiments, we ran a 2 (R, NR) × 2 (Flavor) ANOVA in Extinguished subjects on the first test trial, which revealed a significant reinstatement effect represented by a comparison of R and NR subjects, F(1,28) = 4.39, MSE = 33.01, p = .045, as well as a significant effect of flavor (as expected, given the established consumption differences between sucrose and citric acid), F(1,28) = 8.22, MSE = 61.88, p = .008. There was no interaction (F = 1.42). As in Experiment 3, reinstatement did not take the form of a decrease in flavor consumption from the last extinction trial to the test in the extinguished R subjects, t (15) = .780, p = .447. To examine whether there were unconditioned effects from the reinstating injections, we ran the same (R, NR) × 2 (Flavor) ANOVA in the Novel subjects on the first test trial, which did not reveal a difference between R and NR subjects, F(1,27) = .472, MSE = 2.56, p = .498, or any other effects (largest F = 2.75).
Figure 13.

Mean flavor consumption (ml) on critical trials of Experiment 6. Reinstatement tests are shown at right. Error bars represent standard errors of the means. C1 = first conditioning trial, E1 = first extinction trial, E(L-1) = second-to-last extinction trial, E(L) = last extinction trial, T1 and T2 = tests 1 and 2.
Discussion
Experiment 6 used the multiple conditioning trial method established in Experiments 4 and 5. However, it further improved upon it by massing the conditioning trials to two per day and then using the same interval (5 hrs) between the reinstatement injections and the first reinstatement test. The method resulted in a significant reinstatement effect in the Extinguished condition. Importantly, there was no such effect of LiCl injection evident in the Novel condition, suggesting true reinstatement rather than an unconditional suppression of fluid consumption. Reinstatement may thus be enhanced if specific (and historically unusual) methods are used.
General Discussion
The present experiments were aimed at understanding conditions that might allow reinstatement to occur after the extinction of a conditioned taste aversion. As discussed in the Introduction, the field has observed other post-extinction “relapse” effects in conditioned taste aversion, including renewal and spontaneous recovery (e.g. Rosas & Bouton, 1996; 1997; 1998), and although these other results suggest that extinction in taste aversion shares principles with extinction in other paradigms, reinstatement has been more controversial and difficult to find. Experiments 1 and 3 revisited the methods of Schachtman et al. (1985), who reported a sizable reinstatement effect in a single experiment. Experiment 1 failed to produce reinstatement, while Experiment 3 (which used a novel context at the time of the reinstatement injection) created a modest but significant effect. Experiment 2 also investigated the possibility of concurrent recovery of an aversion to an extinguished flavor when a second flavor is then conditioned, using the Experiment 1 method in which reinstatement had not been obtained. Here, there was little evidence of concurrent recovery, in contrast to an analogous effect observed in the NMR preparation (e.g. Weidemann & Kehoe, 2004).
Experiments 4–6 then aimed to increase the ability to observe reinstatement by increasing the opportunity for the animals to learn that a US signals or sets the occasion for an upcoming CS-US (conditioning) trial (cf. Reid, 1958; cf. Ricker & Bouton, 1996). Experiment 4 increased the number of conditioning trials from one to eight, but failed to produce a reinstatement effect. Observations of the animals after LiCl injections suggested that the sickness-inducing effect of LiCl did not habituate during conditioning. Experiment 5 additionally introduced a shortened (3-hr) reinstatement-injection-to-test interval, but also failed to produce reinstatement. Finally, Experiment 6 further arranged conditioning so that there were two trials per day (as opposed to one trial per day). The twice-daily trials, which were separated by only 5 hrs, along with use of the same 5-hr interval between reinstatement injection and testing, should have maximized the opportunity to learn and use the US as a signal for another conditioning trial, and it did enable a significant reinstatement effect.
The results are consistent with the view that USs can act as discriminative cues or contexts, which is consistent with work in other paradigms supporting the idea that US or reinforcer presentations can signal upcoming events or set the occasion for responding. The work of Goddard has clearly established that USs can signal other USs in appetitive conditioning (e.g., Goddard, 1996; 1997; see Goddard, 1999, for review). Also in appetitive conditioning, the US can clearly act as a discriminative stimulus, either setting the occasion for extinction performance when when extra USs are featured in the intertrial intervals of extinction or conditioning performance when they are similarly featured in the intertrial intervals of conditioning (e.g., Bouton, Rosengard, Achenbach, Peck, & Brooks, 1993). There is also evidence for occasion setting in reinstatement of instrumental responding; If two different responses are each trained with a specific reinforcer, and each extinguished, noncontingent reinforcer delivery reinstates the response that the outcome had consistently preceded (Delamater, LoLordo, & Sosa, 2003; Ostlund & Balleine, 2007). Further evidence has been found in the instrumental resurgence paradigm. Resurgence occurs when an extinguished response (R1) returns after conditioning and extinction of a different response (R2). In one relevant study, R1 was followed by one reinforcer, and R2 was reinforced with a different one while R1 was being extinguished. Noncontingent presentations of the second reinforcer, but not the first reinforcer, during resurgence tests prevented resurgence of R1, providing evidence that the reinforcer for R2 had set the occasion for not performing R1 (Bouton & Trask, 2015). Note that there is little possibility of learning about such a reinforcer context in typical taste aversion methods, which often use very few conditioning trials. Therefore, in Experiment 6, eight twice-daily conditioning trials allowed for US-containing trials to predict the occurrence of other US-containing trials and a similarly short reinstatement-to-test interval could have enhanced the effect. These experimental features may need to be present in order for reinstatement to occur in taste aversion learning. To date, the idea that USs may signal or set the occasion for conditioning trials has rarely been discussed in the taste aversion setting, but it is in general agreement with contextual conditioning theories of reinstatement (e.g. Bouton & Bolles, 1979; see Introduction). The present results are also consistent with other taste aversion research in this laboratory which found evidence that multiple training trials enabled a partial reinforcement extinction effect, potentially by allowing the animals to learn signaling relationships between nonreinforced and reinforced trials (Bouton & Michaud, 2022; i.e., “trial signaling,” Ricker & Bouton, 1996; see also Capaldi, 1994).
There are at least two unresolved issues. We have no clear explanation of why the Schachtman et al. (1985) method of using a novel and unique context for reinstatement injection caused a statistically-significant reinstatement effect in Experiment 3. We note again that a substantial amount of work on reinstatement indicates that using a different physical context for the reinstatement phase discourages a reinstatement effect (Bouton, 1984; Bouton & Bolles, 1979; Bouton & King, 1983; Bouton & Peck, 1989). One possibility is that neophobia and/or fear or stress created by the unexpected exposure to a new and different context during injection made the reinstating injection especially impactful or salient, although it is still not necessarily clear how this would affect flavor consumption 48 hrs later. Another unresolved issue is why concurrent recovery did not occur in Experiment 2. In NMR conditioning, the concurrent recovery effect appears robust (e.g. Weidemann & Kehoe, 2003; 2004). Concurrent recovery has been explained as a type of “learning to learn” effect (Kehoe, 1988; Kehoe & Macrae, 1997). Perhaps learning to learn effects are more difficult to produce in taste aversion learning. The question requires more research.
As noted previously, although features of taste aversion learning have historically suggested that it is a unique form of learning, many have been reconciled with a general-process view of learning. For example, although taste aversions can be learned in as little as one trial (e.g., Experiments 1–3; Rosas & Bouton, 1996) and with a long delay between the CS and US (e.g., Garcia et al., 1966; Smith & Roll, 1967; Revusky, 1971), fear learning can also occur with one trial (e.g. Mahoney & Ayres, 1976) and long delay learning arguably occurs in taste aversion because there are no other relevant stimuli (i.e. flavors) present during the delay to cause interference (Revusky, 1971). The fact that reinstatement can occur in taste aversion learning is also consistent with the idea that taste aversion learning follows rules that may be similar to those of other forms of learning. From an evolutionary perspective, it has been argued that a distinct learning system only evolves when there is “functional incompatibility” between the features of an existing system and a new environmental learning problem (Sherry & Schacter, 1987). We should not assume the existence of a new or different learning system unless we are sure that the proposed different system performs a function that cannot be performed by an existing learning system (Sherry & Schacter, 1987). Given the present results (along with others from our laboratory, see Bouton & Michaud, 2022), the rules of operation required to learn how to identify and react to poisonous foods in nature might not be functionally incompatible with those required for other forms of associative learning. In other words, it might make sense for an animal to suppress consumption of a food that was previously poisoned when once again experiencing the symptoms of the poison, in the same way that it makes sense to resume an extinguished food-seeking response when exposed to that food once more.
The results of the present experiments are thus consistent with the idea that taste aversions may sometimes seem special because they often involve unusual methods (such as use of a very small number of conditioning trials). This may make them experimentally unique, rather than biologically or evolutionarily unique. The present experiments suggest that reinstatement in taste aversion learning can be encouraged by using methods that maximize the potential for animals to learn that USs signal (or set the occasion for) upcoming conditioning trials, and are therefore somewhat unusual for taste aversion learning.
Acknowledgments
This research was supported by Grant RO1 DA 033123 from the National Institutes of Health.
References
- Bernstein IL (1978). Learned taste aversions in children receiving chemotherapy. Science, 200(4347), 1302–1303. [DOI] [PubMed] [Google Scholar]
- Bouton ME (1982). Lack of reinstatement of an extinguished taste aversion. Animal Learning & Behavior, 10(2), 233–241. [Google Scholar]
- Bouton ME (1984). Differential control by context in the inflation and reinstatement paradigms. Journal of Experimental Psychology: Animal Behavior Processes, 10(1), 56. [Google Scholar]
- Bouton ME (1988). Context and ambiguity in the extinction of emotional learning: Implications for exposure therapy. Behaviour Research and Therapy, 26(2), 137–149. [DOI] [PubMed] [Google Scholar]
- Bouton ME, & Bolles RC (1979). Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of Experimental Psychology: Animal Behavior Processes, 5(4), 368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, & King DA (1983). Contextual control of the extinction of conditioned fear: tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes, 9(3), 248. [PubMed] [Google Scholar]
- Bouton ME, & King DA (1986). Effect of context on performance to conditioned stimuli with mixed histories of reinforcement and nonreinforcement. Journal of Experimental Psychology: Animal Behavior Processes, 12, 4–15. [Google Scholar]
- Bouton ME, & Michaud NL (2022). Partial reinforcement effects on acquisition and extinction of a conditioned taste aversion. Learning & Behavior, 50(3), 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, & Peck CA (1989). Context effects on conditioning, extinction, and reinstatement in an appetitive conditioning preparation. Animal Learning & Behavior, 17(2), 188–198. [Google Scholar]
- Bouton ME, Rosengard C, Achenbach GG, Peck CA, & Brooks DC (1993). Effects of contextual conditioning and unconditional stimulus presentation on performance in appetitive conditioning. The Quarterly Journal of Experimental Psychology Section B, 46(1b), 63–95. [PubMed] [Google Scholar]
- Bouton ME, & Swartzentruber D (1991). Sources of relapse after extinction in Pavlovian and instrumental learning. Clinical Psychology Review, 11(2), 123–140. [Google Scholar]
- Bouton ME, & Trask S (2016). Role of the discriminative properties of the reinforcer in resurgence. Learning & Behavior, 44, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Woods AM, & Pineño O (2004). Occasional reinforced trials during extinction can slow the rate of rapid reacquisition. Learning and Motivation, 35(4), 371–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DC, Palmatier MI, Garcia EO, & Johnson JL (1999). An extinction cue reduces spontaneous recovery of a conditioned taste aversion. Animal Learning & Behavior, 27, 77–88. [Google Scholar]
- Capaldi EJ (1994). The sequential view: From rapidly fading timulus traces to the organization of memory and the abstract concept of number. Psychonomic Bulletin & Review, 1, 156–181. [DOI] [PubMed] [Google Scholar]
- Danguir J, & Nicolaidis S (1977). Lack of reacquisition in learned taste aversions. Animal Learning & Behavior, 5(4), 395–397. [Google Scholar]
- Delamater AD, LoLordo VM, & Sosa W (2003). Outcome-specific conditioned inhibition in Pavlovian backward conditioning. Animal Learning & Behavior, 31(4). 393–402. [DOI] [PubMed] [Google Scholar]
- Dirikx T, Hermans D, Vansteenwegen D, Baeyens F, & Eelen P (2004). Reinstatement of extinguished conditioned responses and negative stimulus valence as a pathway to return of fear in humans. Learning & Memory, 11(5), 549–554. [DOI] [PubMed] [Google Scholar]
- Domjan M (1983). Biological constraints on instrumental and classical conditioning: Implications for general process theory. Psychology of Learning and Motivation, 17, 215–277. [Google Scholar]
- Domjan M, & Galef BG (1983). Biological constraints on instrumental and classical conditioning: Retrospect and prospect. Animal Learning & Behavior, 11(2), 151–161. [Google Scholar]
- Domjan M, & Wilson NE (1972). Specificity of cue to consequence in aversion learning in the rat. Psychonomic Science, 26(3), 143–145. [Google Scholar]
- Garcia J, Ervin FR, & Kölling RA (1966). Learning with prolonged delay of reinforcement. Psychonomic Science, 5(3), 121–122. [Google Scholar]
- García-Gutiérrez A, & Rosas JM (2003). Context change as the mechanism of reinstatement in causal learning. Journal of Experimental Psychology: Animal Behavior Processes, 29(4), 292. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankins WG, & Rusiniak KW (1974). Behavioral regulation of the milieu interne in man and rat: Food preferences set by delayed visceral effects facilitate memory research and predator control. Science, 185(4154), 824–831. [DOI] [PubMed] [Google Scholar]
- Garcia J, & Koelling RA (1966). Relation of cue to consequence in avoidance learning. Psychonomic Science, 4, 123–124. [Google Scholar]
- Goddard MJ (1996). Effect of US signal value on blocking of a CS-US association. Journal of Experimental Psychology: Animal Behavior Processes, 22(3), 258. [DOI] [PubMed] [Google Scholar]
- Goddard MJ (1997). Spontaneous recovery in US extinction. Learning and Motivation, 28(1), 118–128. [Google Scholar]
- Goddard MJ (1999). The role of US signal value in contingency, drug conditioning, and learned helplessness. Psychonomic Bulletin & Review 6, 412–423. [DOI] [PubMed] [Google Scholar]
- Hart JA, Bourne MJ, & Schachtman TR (1995). Slow reacquisition of a conditioned taste aversion. Animal Learning & Behavior, 23(3), 297–303. [Google Scholar]
- Hoffman HS The stimulus generalization of conditioned suppression. In Mostofsky DI (Ed.), Stimulus Generalization. Stanford: Stanford University Press, 1965. [Google Scholar]
- Kehoe EJ (1988). A layered network model of associative learning: Learning to learn and configuration. Psychological review, 95(4), 411. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, & Macrae M (1997). Savings in animal learning: Implications for relapse and maintenance after therapy. Behavior Therapy, 28(1), 141–155. [Google Scholar]
- Konorski J Conditioned reflexes and neuron organization. New York: Cambridge University Press, 1948. [Google Scholar]
- Macrae M, & Kehoe EJ (1999). Savings after extinction in conditioning of the rabbit’s nictitating membrane response. Psychobiology, 27(1), 85–94. [Google Scholar]
- Mahoney WJ, & Ayres JJ (1976). One-trial simultaneous and backward fear conditioning as reflected in conditioned suppression of licking in rats. Animal Learning & Behavior, 4(4), 357–362. [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, & Duncan EJ (2006). Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning & Memory, 13(6), 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, & Balleine BW (2007). Selective reinstatement of instrumental performance depends on the discriminative stimulus properties of the mediating outcome. Animal Learning & Behavior, 35(1), 43–52. [DOI] [PubMed] [Google Scholar]
- Parker LA, Hills K, & Jensen K (1984). Behavioral CRs elicited by a lithium-or an amphetamine-paired contextual test chamber. Animal Learning & Behavior, 12(3), 307–315. [Google Scholar]
- Reid RL (1958). The role of the reinforcer as a stimulus. British Journal of Psychology, 49(3), 202–209. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1973). Effect of US habituation following conditioning. Journal of Comparative and Physiological Psychology, 1973, 82, 137–143. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1974). Effect of inflation of the unconditioned stimulus value following conditioning. Journal of Comparative and Physiological Psychology, 1974, 86, 101–106. [Google Scholar]
- Rescorla RA, & Cunningham CL (1977). The erasure of reinstated fear. Animal Learning & Behavior, 5(4), 386–394. [Google Scholar]
- Rescorla RA, & Cunningham CL (1978). Recovery of the US representation over time during extinction. Learning and Motivation, 9(4), 373–391. [Google Scholar]
- Rescorla RA, & Heth CD (1975). Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes, 1(1), 88. [PubMed] [Google Scholar]
- Revillo DA, Castello S, Paglini G, & Arias C (2014). Reacquisition, reinstatement, and renewal of a conditioned taste aversion in preweanling rats. Developmental Psychobiology, 56(4), 713–725. [DOI] [PubMed] [Google Scholar]
- Revusky S (1971). The role of interference in association over a delay. Animal Memory, 155–213. [Google Scholar]
- Revusky S, & Coombes S (1979). Reacquisition of learned taste aversions. Animal Learning & Behavior, 7(3), 377–382. [Google Scholar]
- Ricker ST, & Bouton ME (1996). Reacquisition following extinction in appetitive conditioning. Animal Learning & Behavior, 24(4), 423–436. [Google Scholar]
- Riley AL, & Clarke CM (1977). Learning Mechanisms in Food Selection.
- Rosas JM, & Bouton ME (1996). Spontaneous recovery after extinction of a conditioned taste aversion. Animal Learning & Behavior, 24(3), 341–348. [Google Scholar]
- Rosas JM, & Bouton ME (1997). Renewal of a conditioned taste aversion upon return to the conditioning context after extinction in another one. Learning and Motivation, 28(2), 216–229. [Google Scholar]
- Rosas JM, & Bouton ME (1998). Context change and retention interval can have additive, rather than interactive, effects after taste aversion extinction. Psychonomic Bulletin & Review, 5(1), 79–83. [Google Scholar]
- Rosas JM, García-Gutiérrez A, & Callejas-Aguilera JE (2007). AAB and ABA renewal as a function of the number of extinction trials in conditioned taste aversion. Psicológica, 28(2), 129–150. [Google Scholar]
- Rozin P, & Kalat JW (1971). Specific hungers and poison avoidance as adaptive specializations of learning. Psychological Review, 78(6), 459. [DOI] [PubMed] [Google Scholar]
- Schachtman TR, Brown AM, & Miller RR (1985). Reinstatement-induced recovery of a taste-LiCl association following extinction. Animal Learning & Behavior, 13(3), 223–227. [Google Scholar]
- Seligman ME (1970). On the generality of the laws of learning. Psychological Review, 77(5), 406. [Google Scholar]
- Sherry DF, & Schacter DL (1987). The evolution of multiple memory systems. Psychological Review, 94(4), 439. [Google Scholar]
- Shettleworth SJ (1972). Constraints on learning. In Advances in the Study of Behavior (Vol. 4, pp. 1–68). Academic Press. [Google Scholar]
- Skinner BF (1938). The behavior of organisms. New York; Appleton-Century-Crofts. [Google Scholar]
- Smith JC, & Roll DL (1967). Trace conditioning with X-rays as an aversive stimulus. Psychonomic Science, 9, 11–12. [Google Scholar]
- Thanellou A, & Green JT (2011). Spontaneous recovery but not reinstatement of the extinguished conditioned eyeblink response in the rat. Behavioral Neuroscience, 125(4), 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann G, & Kehoe EJ (2003). Savings in classical conditioning in the rabbit as a function of extended extinction. Learning & Behavior, 31(1), 49–68. [DOI] [PubMed] [Google Scholar]
- Weidemann G, & Kehoe EJ (2004). Recovery of the rabbit’s conditioned nictitating membrane response without direct reinforcement after extinction. Animal Learning & Behavior, 32(4), 409–426. [DOI] [PubMed] [Google Scholar]
- Weidemann G, & Kehoe EJ (2005). Stimulus specificity of concurrent recovery in the rabbit nictitating membrane response. Learning & Behavior, 33(3), 343–362. [DOI] [PubMed] [Google Scholar]


