Abstract
Cyclic GMP-AMP (cGAMP) synthase (cGAS) is a key component of the evolutionary conserved immune response pathway, acting upstream stimulator of interferon genes (STING). It is implicated in various human diseases, including Alzheimer's Disease (AD) and other neurodegenerative disorders. Recent studies have shown that pharmacological inhibition of cGAS in tauopathy mice reduces cytokine expression and ameliorates memory and cognition function. This review summarizes the development and application of high-throughput screening (HTS) strategies for identifying cGAS inhibitor hits and transitioning from hits to leads. Such efforts have provided diverse array of potent cGAS inhibitors that may be beneficial in treating central nervous system (CNS) disorders, such as AD and other neurodegenerative diseases. We describe three HTS strategies: the classical HTS using a chemical library of drug like compounds by cell-free or cell-based assays and the fragment-based screening, where the activity of potential inhibitors was determined by measuring the levels of unreacted ATP or assessing the production of cGAMP or pyrophosphate (PPi). These methods were instrumental in discovering cGAS inhibitor hits and subsequent modifications produced potent leads. Finally, we discuss various post-translational modifications of cGAS and consider whether some of these modifications may serve as useful targets for inhibiting cGAS activity or for promoting protein degradation.
Keywords: Alzheimer's disease, cGAS inhibitors, Drug development, High-throughput screening (HTS), Innate immunity
Introduction
Alzheimer's disease (AD) is the most common form of dementia in the elderly and a leading cause of death [[1], [2], [3]]. It is characterized by the buildup of pathological amyloid β (Aβ) plaques and neurofibrillary tangles [[4], [5], [6]]. Recent research suggests that Aβ pathology acts as a “trigger,” accelerating the spread and toxicity of tau in early AD [7], while toxic tau species acting as the ”bullets” are the primary contributors to AD pathology [8]. For many years, evidence has indicated that tau pathology plays a significant role on memory decline associated with both aging and AD [9,10], a finding further solidified in recent imaging studies [11]. Tau imaging studies reveal strong regional associations with clinical and anatomical variations in AD [[12], [13], [14]], showing a close correlation with the degree of cognitive decline compared to Aβ [15]. Despite these insights, there are no small molecule drugs available to specifically target Aβ pathology or tauopathy in AD. Recent research has revealed that innate immune genes are enriched as risk alleles in post-mortem brain tissues from AD patients, highlighting a potential new therapeutic avenue for treatment [[16], [17], [18], [19]]. While tau is primarily expressed in neurons, studies indicate that microglia–the brain's immune cells–play a crucial role in the development of tau pathology, as demonstrated in mouse models of tauopathy [[20], [21], [22], [23]]. In AD, microglia enter an inflammatory state characterized by increased levels of inflammatory signaling molecules, the clearance of toxic protein aggregates, and modification of neuronal synapses [24,25]. Antimicrobial and antiviral defense pathways have emerged as key regulators of microglial responses in AD [[26], [27], [28], [29]]. Notably, the IFN response, a major antiviral pathway, has been shown to promote synaptic dysfunction and neuronal death in AD mouse models associated with amyloid deposition [30,31]. Furthermore, elevating levels of IFNβ1a has been found to reverse memory impairment and mitigate microglia activation and the upregulation of pro-inflammatory cytokines (such as IL-6 and IL-1β) in the hippocampus of rats injected with Aβ(1–42) [32,33].
Cyclic GMP-AMP (cGAMP) synthase (cGAS) is a key component of the evolutionary conserved immune response mechanism directed by the stimulator of interferon genes (STING). It has been implicated in various neurodegenerative diseases, including AD. cGAS is a protein consisting of 522 amino acid residues and serves as a primary sensor of cytosolic double-stranded DNA (ds-DNA) originating from pathogens or the mislocalization of nuclear or mitochondrial self-dsDNA. In the absence of DNA, cGAS exists as a monomer [34] and interacts with dsDNA to form a 2:2 cGAS-DsDNA heterodimer, which catalyzes a reaction between GTP and ATP to produce cGAMP. As a potent nanomolar natural agonist of STING, which is a trans-membrane protein and exists as a constitutive dimer, it binds STING in its ligand-binding domain (LBD). Activated STING undergoes a conformational change, forming an oligomeric structure that stimulates downstream signaling pathways. This activation leads to the phosphorylation of IRF3 protein by TBK1, resulting in the expression of pro-inflammatory genes and cytokine in affected cells (Fig. 1) [35,36].
Fig. 1.
Activation of the cGAS-STING pathway increases interferon (IFN) expression.
In the brain, cGAS is predominantly expressed in microglia and, to some extent, in astrocytes [37]. Numerous studies have linked cGAS-STING signaling to inflammatory diseases and CNS disorders, including AD. Modulating the cGAS-STING pathway presents a therapeutic opportunity for treating these conditions [[38], [39], [40]]. Our studies suggest that inhibiting cGAS activity could decrease pro-inflammatory gene expression and cellular senescence, potentially offering therapeutic benefits for AD patients [39], [41]. However, it is noteworthy that, to date, no cGAS inhibitors have progressed to clinical trials to evaluate their effects on AD.
Inactive cGAS monomer interacts with dsDNA and forms 2:2 cGAS-dsDNA dimer as an active enzyme catalyzing conversion of ATP and GTP into the STING agonist 2′3′-cGAMP. Upon cGAMP is bound to STING, STING dimerizes and recruits TBK1. TBK1 then catalyzes the phosphorylation of IRF3, leading to an increase in IFN expression. Inhibitors targeting either cGAS or STING can disrupt the cGAS-STING pathway by blocking: (1) the conversion of ATP and GTP to produce cGAMP (indicated by ‘1’), or the binding of cGAMP to STING (indicated by ‘2’).
Human and mouse cGAS (h-cGAS and m-cGAS) proteins display less than <60 % similarity, with up to 116 different amino acid residues present in their enzymatic domains 42. The activity of h-cGAS is ∼20 times lower compared to m-cGAS [42]. Mutation of both amino acids present in human, K187 and L195 to N and R, respectively (as found in mcGAS) increased the activity comparable to m-cGAS [42]. Moreover, the catalytic activity of cGAS depends on the length of dsDNA; dsDNA longer than 20 base pairs (bp) is sufficient to activate m-cGAS, while dsDNA over 45 bp activates h-cGAS [42,43,44]. Typically, dsDNA of 100 bp or HT-DNA, which may contain 3000–10,000 bp, has been used in activity and cellular experiments involving h-cGAS to achieve robust and consistent results.
Detailed mechanistic study by Hall et al. [45] and others [46,47] have shown that cGAS produces 2′3′-cGAMP along with linear hetero-dinucleotides and about 10 % of the homo-linear AMP-3′-ATP and GMP-2′-GTP as side products (Scheme 1) under physiological concentrations of ATP and GTP (2 mmol and 0.5 mmol, respectively) [48]. However, the actual concentration of cGAMP produced in vivo is likely much lower than in these closed-vessel experiments because most of the AMP-2′-GTP hetero-dinucleotide intermediate, which is a weak binder (KM ∼ 25 μM) of cGAS, would be released, leaving only a portion that could reorient within the cGAS active site to form 2′,3′-cGAMP [45]. At least three methods have been used to measure cGAS activity or determine its inhibition. These include: 1) measuring consumption of ATP using Kinase-Glo® reagent, 2) production of cGAMP using mass spectral analysis or an ELISA, fluorescence polarization (FP) or Förster resonance energy transfer (FRET) experiment that uses cGAMP derivatives to compete with cGAMP produced in the reaction, and 3) using inorganic phosphatase to detect inorganic diphosphate (PPi) levels. All three methods, described above, have been successfully used to perform HTS. Based on our experience, measuring ATP depletion using Kinase-Glo® is the most efficient and user-friendly method for conducting both primary HTS and confirmatory assays.
Scheme 1.
cGAS catalyzed reaction of GTP and ATP produces 2′3′-cGAMP.
The discovery of cGAS inhibitors gained momentum in both academia and industry after Chen and colleagues identified the cGAS protein in 2013 as a cytosolic DNA sensor that activates the type I interferon pathway, with cGAMP as a key second messenger in innate immune signaling [49,50]. Since then, numerous cGAS inhibitors [38] have been identified and developed, including antimalarials, synthetic compounds from high-throughput screening (HTS) and medicinal chemistry [[51], [52], [53]], natural products [54], and anti-sense oligonucleotides that reduce cGAS expression [55,56]. This review article, summarizes the screening methods and results that have led to the identification and development of various cGAS inhibitors and discusses different classes of potent leads described in original research papers and patent applications. While numerous STING inhibitors exist, they are beyond this review's scope, and readers can refer to other published reviews on these topics [56], [57], [58]. The article also provides insights into selecting suitable cGAS inhibitors based on molecular structure and properties for potential AD therapy [39].
Strategies used to identify cGAS inhibitor hits
cGAS inhibitors fall into two main categories: those that inhibit cGAS activity by targeting the protein/dsDNA interaction and those that target the substrate binding site (Fig. 2). Compounds that block protein/dsDNA interaction include antimalarials, suramin and cyclopeptides [58], [59], [60], [61], which inhibit both h-cGAS and m-cGAS equally. In contrast, a large number of inhibitors target the substrate binding site, often showing a preference for inhibiting either h-cGAS or m-cGAS. The most notable h-cGAS inhibitors are G-140 and G-150, while TDI006570 is a prominent m-cGAS inhibitor. These compounds are among the most potent cGAS inhibitors described in the literature [62,63]. To identify and develop these inhibitors, a variety of cell-free and cell-based cGAS activity assays have been established. Many of these assays have been optimized for HTS, facilitating the discovery and optimization of effective cGAS inhibitors.
Fig. 2.
cGAS inhibitors targeting a. The protein/dsDNA interaction (PDB: 6CT9) [42], CPK representation for amino acids ARG376, SER434, and TYR436 to represent the catalytic pocket as yellow balls. b. those targets the substrate binding site (catalytic pocket, PDB: 6MJW) [62].
The classical HTS strategy
Three primary cell-free cGAS activity assays are employed in HTS to identify cGAS inhibitors. These assays leverage the measurement of ATP, cGAMP, and PPi. In each assay, a solution containing ATP and GTP, typically at concentrations of 50 μM or 100 μM, is incubated with either m-cGAS at concentrations of 10 nM–30 nM or h-cGAS at 30 nM–100 nM. This incubation occurs in the presence of dsDNA, 45 bp for m-cGAS and 100 bp for h-cGAS reactions. HT-DNA has also been used instead of synthetic dsDNA. After incubation, the remaining levels of ATP or the produced cGAMP or PPi were measured to evaluate cGAS activity.
Determining ATP levels
Kinase Glo® assay kits are widely used to measure remaining ATP levels in cGAS catalyzed reactions involving ATP and GTP. These kits employ a luminescence-based detection method that was initially developed for screening protein kinases [64]. Their availability and user-friendly design have made them a preferred choice for measuring ATP, facilitating both large compound library screening and hit-to-lead discovery. However, a key limitation of this method is that measuring residual ATP levels may not accurately reflect the extent of cGAMP production inhibition. To ensure reliable results, the reaction needs to progress significantly—typically around 50%—to minimize variability and enhance reproducibility. Initially, HTS assays using h-cGAS were conducted over 7 h to achieve 50 % ATP depletion. The protocol has since been optimized by lowering the NaCl concentration in the Tris buffer from 150 mM to 50 mM, successfully reducing incubation times to about 3 h.
Determining cGAMP levels
Determining cGAMP levels in the reaction product is arguably the most relevant strategy for identifying cGAS inhibitors that can block cGAS-STING activation. BellBrook Labs' Transcreener® cGAMP cGAS assay kits, which utilize Fluorescence Polarization (FP) and Time-Resolved Förster Resonance Energy Transfer (TR-FRET) technologies, enable high throughput measurement of cGAMP levels in cell-free conditions. Additionally, commercially available cGAMP ELISA kits can quantify cGAMP levels in both cell-free and cell-based assays. Both methods assess enzyme activity by using a tracer to displace the cGAMP generated in the reaction, and the displaced tracer is detected using a cGAMP-specific antibody. The advantages of BellBrook Labs’ Transcreener® cGAMP cGAS assay kits lies in their ease of use, one-step process capability, and efficiency; the process can be completed in about 1 h.
RapidFire mass spectrometry (RF-MS) is an effective method for accurately and quantitatively measuring cGAMP levels, and it eliminates potential false positives caused by assay interference [65]. However, RF-MS requires sample preparation, which reduces its throughput compared to other technologies. As a result, it is better suited for lower throughput scenarios, such as hit confirmation, rather than a primary HTS of large compound libraries [66].
PPi levels
The Inorganic Pyrophosphatase-coupled cGAS assay is a method that measures PPi, one of the products (Scheme 1) of cGAS catalysis involving ATP and GTP, and has been developed for HTS [67]. In this method, PPi generated in the reaction is catalytically hydrolyzed using the inorganic pyrophosphatase, and the amount of phosphoric acid produced is measured spectrophotometrically using the conventional phosphomolybdate-malachite green absorbance methodology [67]. The Pyrophosphatase-coupled cGAS assay enjoys a slow turnover of cGAS [42,68], but high enzymatic cleavage of PPi by Inorganic Pyrophosphatase.
Virtual HTS
Virtual HTS employs computational methods to screen large databases that encompass libraries of both existing compounds and those that can be conveniently synthesized on demand [69]. To achieve satisfactory results, robust modeling and crystallography data are essential prerequisites. This approach is faster, more cost-effective, and requires less manual labor compared to traditional screening methods; however, the outcomes can often be unpredictable [70]. Despite these challenges, the virtual HTS has been successfully used to identify potential cGAS inhibitors. The virtual hits identified are subsequently validated through experimental methods, typically involving the measurement of ATP or cGAMP levels.
DNA intercalator counter screen assay
DNA intercalators cause damages to DNA, leading to cell death by disrupting the structure through interactions with base pairs [71]. The DNA intercalator assay is designed to identify and eliminate cGAS inhibitor hits that interfere with the cGAS/dsDNA interaction by intercalating into the DNA. This assay operates as a displacement test and utilizes a fluorescent DNA intercalator dye, such as acridine orange. When a cGAS inhibitor binds to the DNA, it competes with the dye, reducing fluorescence. Mitoxantrone, a well-known DNA intercalator, is used as a reference in this assay [72].
Cell-based assays for identifying cGAS inhibitors
THP1-Dual™ cells, derived from the THP-1 monocyte cell line, stably express two inducible reporter constructs: secreted embryonic alkaline phosphatase (SEAP) and Lucia luciferase. These cells are utilized to study the NF-kB and IRF3 pathways, and have been employed to assess the activity of h-cGAS inhibitors in cells. Similarly, BV2 microglia cells stably expressing Lucia luciferase to analyze the IRF3 pathway have been developed to evaluate m-cGAS inhibitors [38].
Identification and development of various classes of cGAS inhibitors for the treatment of AD
In recent years, numerous small molecule cGAS inhibitors have been developed, many demonstrating high activity in cell-free assays and some in cellular assays. The previously described cell-free and cell-based assays, along with virtual HTS, in some cases, have facilitated the identification of these cGAS inhibitors. To effectively treats various human diseases associated with cGAS-STING pathway activation, it is crucial to develop potent inhibitors with drug-like properties, high stability and cell-permeability, low metabolism and efflux, low toxicity, and a high plasma half-life. However, only those inhibitors that can penetrate the blood-brain barrier (BBB) are suitable for treating the central nervous system, such as AD. The following paragraphs and figures describe the majority of known classes of cGAS inhibitors, their structures, and activities, including those known or predicted to cross BBB based on their molecular properties and in silico analyses.
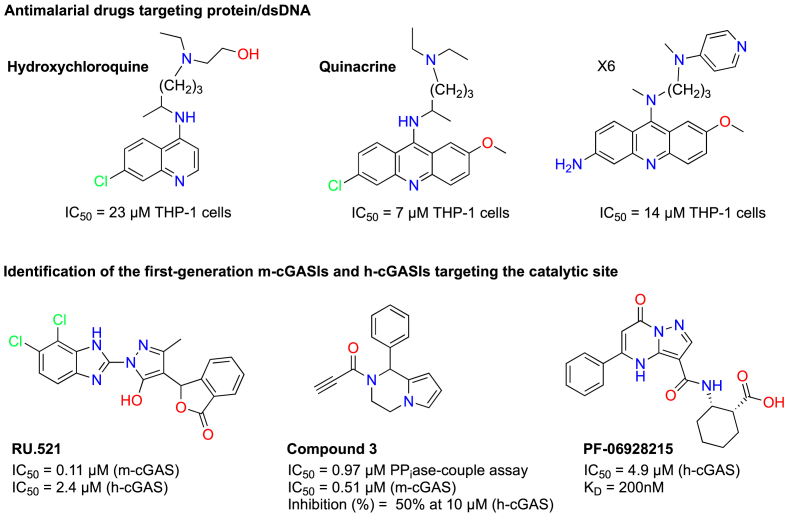
Antimalarial drugs targeting the protein/dsDNA interaction as cGAS inhibitors
Antimalarial drugs, such as hydroxychloroquine and quinacrine, have been identified as cGAS inhibitors with IC50 values in the low micromolar range (7–23 μM). These inhibitors bind to DNA, disrupting its interaction with cGAS [73]. An et al. [[61], [74]] have optimized the acridine backbone of quinacrine and developed a series of new analogs (Fig. 3). While all these compounds demonstrated strong cGAS inhibitory activity, many also exhibited cytotoxic effects.
Fig. 3.
Antimalarial drug and first-generation m- and h-cGAS inhibitors.
Identification of first-generation m-cGAS and h-cGAS inhibitors targeting the catalytic site
Vincent et al. [68] performed a HTS of a small molecule library using the RF-MS method to identify m-cGAS inhibitor hits and optimization of one of the hits, leading to the discovery of the first sub-μM potent m-cGAS inhibitor, compound RU.521, which has an IC50 of 0.11 μM and strongly suppresses downstream signaling. Recently, Song et al. [75] identified a compound, referred to as compound 3, which covalently binds to m-cGAS and exhibits better activity than RU.521. Compound 3 improved ISD-stimulated m-cGAS activation in Raw-Lucia ISG cells, with an IC50 of 0.51 μM compared to RU.521 with an IC50 of 2.41 μM in their study. The authors demonstrated through mass spectrometry and mutation analysis that compound 3 covalently binds to cysteine 419 of m-cGAS. Unfortunately, these compounds showed weak activity against h-cGAS, which the authors attribute to the relatively low amino acid identity (60 %) between m-cGAS and h-cGAS (see Fig. 3 for more details).
At the same time RU.521 was discovered, Hall et al. [76] determined the structure of h-cGAS and conducted fragment screening using NMR and FP assays. They further optimized one of the hits to develop a low μM potent h-cGAS inhibitor, with an IC50 of 4.9 μM. Crystallization of PF-06928215 with h-cGAS revealed that the inhibitor binds at the catalytic site. However, despite its potency, PF-06928215 (Fig. 3) exhibited negligible cellular activity in THP1 dual cells. Subsequently, Lama et al. [62] from Rockefeller University (RU), Memorial Sloan-Kettering Cancer Center (MSKCC) and the Tri-Institutional Therapeutics Discovery Institute (TDI) developed a new Kinase Glo® [64] based HTS method, and screened a library of 350,000 compounds. They identified several h-cGAS inhibitor hits, including G001 (IC50 = 2.08 μM) and J001 (IC50 = 1.04 μM). The RU/TDI team then synthetically optimized G001 and J001, affording low nM potent h-cGAS inhibitors (Fig. 4) [62]. Additionally, several other low μM hits identified in this HTS may have been further developed by other researchers (see later sections).
Fig. 4.
Development of new cGAS inhibitors by the TDI/RU group and analogs by Ventus Therapeutics.
Development of G001 analogs, the tetrahydro-γ-carboline derivatives, by the RU/TDI team and Ventus Therapeutics
The hit compound G001, which features a tetrahydro-γ-carboline pharmacophore linked to
2-methoxyacetic acid, underwent extensive medicinal chemistry optimization, resulting in several low nM potent h-cGAS inhibitors. Among the most potent inhibitors in this series are G140 (IC50 = 14 nM) and G150 (IC50 = 10.2 nM), but both show low activity against m-cGAS (WO2019/153002 A167). These inhibitors contain a glycolic acid linker instead of the methoxy-substituted analog, along with additional heterocycles or other substituents in the benzene ring at C-9. In contrast, a similar compound TDI006570, which lacks the heterocycle at C-9, exhibits weak activity against h-cGAS (IC50 = 138 nM) and demonstrates strong activity (IC50 = 12.8 nM against m-cGAS inhibitor (Fig. 4) [62]. Both G140 and G150 significantly inhibit STING activation and the downstream signaling in THP1-Dual cells, while TDI006570 shows similar effects in BV2 cells. Moreover, modifications of the tetrahydro-γ-carboline scaffold, such as introducing an additional nitrogen atom into the aromatic ring of the TDI compounds or converting it into an indazole (as seen in compound 67, WO 2020/186027 A1 [77]), have yielded highly potent h-cGAS inhibitors (Fig. 4).
Development of G001 and TDI006570 analogs by Ventus Therapeutics
Beveridge et al. from Ventus Therapeutics disclosed a significant finding regarding their new cGAS inhibitors in patent WO 2022/066851 A1. [78] They found that incorporating a methyl group with S-stereochemistry into the tetrahydropyridine ring, positioned between the nitrogen and the sp2 carbon, as seen in compound 102 (Fig. 4), dramatically enhanced activity against h-cGAS compared to the parent non-methylated analog (Fig. 4). In contrast, the R-stereoisomer of compound 102 exhibited low activity, with an IC50 of 4.325 μM. In another patent by the same group [79], the authors investigated new linkers for compound 102 and identified several low nM potent cGAS inhibitors, including compounds 45B, 51B, and 157 (Fig. 4). Again, the R-stereoisomers demonstrated weak activity [79].
Development of G140 analogs by the Shanghai/Beijing group
In a collaborative study involving multiple institutes in Shanghai and Beijing, Tan et al. [80] aimed to redesign G140 by modifying both its side chain and the central tricyclic core to produce new analogs. One such analog, compound 25 (IC50 = 1.38 ± 0.29 μM) (Fig. 5), demonstrated slightly better activity in THP1-Dual cells compared to the benchmark compound G140 (IC50 = 2.69 ± 1.15 μM) and exhibited in vivo anti-inflammatory effects in a lipopolysaccharide-induced mouse model. The authors also rationalized these results through docking studies based on the previously determined X-ray structure of h-cGAS (see latter). Recently, the same group described new analogs featuring spirocyclic scaffolds (Fig. 5) [81]. Compound 30d-S was identified as one of the potent and selective cGAS inhibitors, showing an IC50 of 0.006 μM in vitro. It also exhibited improved cellular effects in human and mouse cell lines, with IC50 values of 2.87 μM in THP1-dual cells and 1.02 μM in RAW cells, respectively [81]. Notably, many of these compounds possess stereoisomeric spirocyclic structures and inhibit cGAS in a stereochemically dependent manner.
Fig. 5.
Development of new cGAS inhibitors by the Shanghai/Beijing group (upper), Lilly (lower-left) and by the Gan and Sinha labs at Weill Cornell Medicine (lower-right).
Discovery of indole and indazole h-cGAS inhibitors by Lilly
Recently, Ahmed et al. [82] from the Lilly group (WO 2023/235809 A1) reported new cGAS inhibitors that appear to be inspired by the RU/TDI inhibitors described earlier but with extensive modifications. There is considerable variability among Lilly's h-cGAS inhibitors. The authors conducted in vitro assays by measuring cGAMP levels using the RF-MS. They determined the inhibition of h-cGAS activity in THP-1 macrophages through IFNβ analysis with the IFNβ AlphaLISA detection kit. Numerous potent compounds displayed excellent activity in both in vitro and cell-based assays. One notable compound 171 (Fig. 5, lower-left), exhibited an IC50 of 3.5 nM in a cell-free assay measured by cGAMP production using the RF-MS method and an IC50 of 68.9 nM in THP1-derived macrophages using the IFNβ AlphaLISA assay. Additionally, we prepared and evaluated compound 171 in THP1 monocyte cells, confirming similar activity as measured by cGAMP ELISA of the cell lysates.
Discovery of cGAS inhibitors by the Gan-Sinha groups
A collaborative research program between the Gan and Sinha laboratories at Weill Cornell Medicine (WCM) led to the discovery of three novel series of cGAS inhibitors, including dihydrobenzo-furo-pyridine, dihydrobenzo-thieno-pyridine (with nitrogen substitutions at the 2nd and 3rd positions), and tetrahydroimidazo-dipyridine derivatives, as disclosed in patent application (WO 2023/154962 A1) [83]. Among these scaffolds, the dihydrobenzo-furo-pyridine demonstrated the highest potency, followed by the dihydrobenzo-thieno-pyridine series, while the tetrahydroimidazo-dipyridine derivatives exhibited comparatively weaker activity. Notably, compound 24, a methyl pyrazole-substituted dihydrobenzo-furo-pyridine, showed strong inhibition of human cGAS, achieving 75–100 % inhibition at 10 μM and 50–74 % inhibition at 1 μM, comparable to TDI008246. Additionally, compound 24 (Fig. 5, lower-right) demonstrated good activity in THP-1 dual cells, with 50–74 % inhibition at 10 μM. In the dihydrobenzo-thieno-pyridine series, most compounds with nitrogen positioned at the 3rd position exhibited good inhibition, achieving 75–100 % at 10 μM against mouse cGAS. Importantly, these compounds showed very high brain permeability compared to similar indole cGAS inhibitors developed by RU/TDI. Ongoing improvements to these compounds yield more potent cGAS inhibitors, which are undergoing further evaluations in our laboratories.
Discovery of cGAS inhibitors at Bellbrook and Boehringer Ingelheim laboratories
The Bellbrook laboratory has identified numerous cGAS inhibitors, described in two patent applications (WO 2020/142735A1 [84] and WO 2024/035622 A1 [85]). Examples of these inhibitors include compounds 10 and 25, both featuring the pharmacophore “1-(2-methylbenzofuro [3,2-d]pyrimidin-4-yl)pyrrolidine-2-carboxylic acid” with substituents in position 4, as well as the quinoline derivative compound 14 (Fig. 6). All these compounds demonstrated promising activity in cell free assay. Interestingly, Boehringer Ingelheim has also reported novel cGAS inhibitors, such as compounds 1.12 and 2.04 WO 2022/238327A1) (Fig. 6). These compounds possess a major pharmacophore similar to those developed by BellBrook (compounds 10 and 25) but exhibit exceptional potent in both cell-free and cell-based assays [86].
Fig. 6.
Discovery of cGAS inhibitors at the Bellbrook and Boehringer Ingelheim laboratories.
Discovery of thiadiazol-amide derivatives as novel cGAS inhibitors at Ventus Therapeutics
In recent years, Ventus Therapeutics has described two series of 1,3,4-thiadiazol-amide derivatives in two patent applications (WO 2023/081441 A1 [87], WO 2024/137607 A1 [88]), focusing on modifications to both sides of the mentioned pharmacophore (Fig. 7). One series includes compounds cpd2 and cpd4, both of which demonstrated sub-μM IC50 activity in vitro. The second series features stereoisomeric compounds 7a and 7b, which incorporate aliphatic linkers. The IC50 values for several compounds in this series ranged from 0.005 μM to 0.02 μM. Notably, the compounds disclosed in WO 2024/137607 A1 exhibited a superior in vitro profile compared to those reported in WO 2023/081441 A1 [87].
Fig. 7.
Additional class cGAS inhibitors identified by Immune sensor, Ventus, and Roche.
Discovery of cGAS inhibitors at immune sensor, LLC and Roche
Immune sensor disclosed novel cGAS inhibitors, featuring a (Z)-(benzylidene)-3-methylthiazolidine-2,4-dione pharmacophore in a patent back in 2017 (WO 2017/176812 A1) [89]. Among these compounds, Ex28 (Fig. 7), exhibited sub-μM IC50 values in cell-free assays and low micromolar IC50 in THP1 cells.
Several years later, in 2021, Roche unveiled multiple series of cGAS inhibitors (Fig. 7) across five different patent applications. These include sulfamoylamides (WO 2021/209475 A1) [90], various complementary series of biphenylcarboxylic acid derivatives detailed in three patent applications (WO 2021/209481 A1 [91], WO 2021/209473 A1 [92], and WO2021/209484 A1 [93]), and malononitrile derivatives (WO 2021/233854 A1) [94]. All these classes of cGAS inhibitors, except for the malononitrile derivatives, demonstrated sub-μM or low μM activity against cGAS, with the malononitrile derivatives being the most active among them.
cGAS inhibitors developed from hits obtained by virtual HTS
Padilla-Salinas et al. [95] utilized computational chemistry and performed docking studies and virtual HTS to identify compounds targeting the cGAS protein-protein interface when complexed with dsDNA. They employed the Maybridge and Enamine drug databases and subsequently rationally designed the identified hits, resulting in the development of potent cGAS inhibitors, including compound CU-76 (Fig. 8). This compound selectively inhibited the DNA pathway in human cells and showed no effects on the RIG-I-MAVS or Toll-loke receptor pathways. In a separate virtual HTS study, Zhao and collaborators [96] identified another potent cGAS inhibitor, compound S3 (Fig. 8). Additionally, Li et al. [97] performed virtual screening supplemented by X-ray crystallography, focusing on flavonoids and identified Baicalin and baicalein as the low μM cGAS inhibitors.
Fig. 8.
cGAS inhibitor developed from Virtual HTS hits.
Structural information
Numerous Xray structures of h-cGAS have been determined both as apo enzyme or with its inhibitors, ATP or cGAMP. RU/TDI group determined the structure of h-cGAS bound to the cGAS inhibitor G150 as well as with its several analogs [62]. The X-ray structure of h-cGAS bound to G150 (PBD ID: 6MJW) clearly showed that the compound occupied the h-cGAS catalytic site as expected. Recently, a new structure (PBD ID: 7FUR) of h-cGAS with G150 and ATP has been reported, showing both compounds bound to the catalytic site (Fig. 9) [98]. This new structure significantly differs from the original X-ray structure, highlighting a shift in the position of G150, and ATP occupying the previously defined inhibitor position. We performed docking studies using the new structure (PBD ID: 7FUR) with compound 25 (Fig. 5) from the Shanghai/Beijing group, as well as of our benzofuran compound 24 (Fig. 5) and ATP. This was compared to the docking of compound 25 or 24 alone docked into the original structure (PBD ID: 7MJW) to identify similar modes of interactions as observed with G150 (docking structure for compound 24 not shown). This advancement opens new opportunities for identifying and designing novel cGAS inhibitors.
Fig. 9.
Docking analysis of G150 (yellow) and compound 25 (cyan) performed into two different crystal structures. a. G150 docked into PDB-6MJW; b. Compound 25 docked into PDB-6MJW; c. G150, ATP and MgCl2 docked into PDB-7FUR; d. Compound 25 docked into PDB-7FUR with ATP and MgCl2 (docking studies performed by us in house using Schrödinger docking program).
Modulating cGAS by targeting specific post-translational modifications and protein-protein interactions
Studies have demonstrated that cGAS undergoes numerous post-translational modifications that increase or decrease the cGAS activity. cGAS acetylation is one such modification that inhibit its activity. One notable modification is acetylation, which inhibits cGAS activity and can be induced by treatment with aspirin [99,100]. Other post-translational modifications that can upregulate or downregulate cGAS activity, expression or degradation include phosphorylation-dephosphorylation [[101], [102], [103], [104], [105], [106]], ubiquitination-deubiquitination [[107], [108], [109], [110], [111], [112]], sumoylation-desumoylation [113,114], monomethylation [115], palmitoylation-depalmitoylation [116,117], glutamylation [118,119], poly-ADP-ribosylation, acylation [99,100], deamidation of certain ‘Asn’ [[120], [121], [122]], proteolytic cleavage [123,124], and selective autophagy [107,125]. These modifications represent new therapeutic opportunities for regulating cGAS activity and its pathways. Additionally, protein-protein interactions, such as cGAS-cGAS dimerization which enhances cGAS activity, along with other interactions that activate cGAS-STING signaling in both cGAMP-dependent and independent manners, merit further investigation to identify potential modulators of the cGAS-STING pathway [126].
The uses of cGAS inhibitors for the treatment of AD and other neurodegenerative diseases
Numerous reports have established a connection between cGAS-STING activation–where cGAS plays a significant role–and AD along with other neurodegenerative diseases at the molecular level. These links have been demonstrated in mice bearing amyloid pathology or tauopathy [38,41,127,128]. In various studies, pharmacological interventions using cGAS or STING inhibitors have shown efficacy in AD models [41,127,128]. Udeochu et al. [38] demonstrated the therapeutic potential of the small molecule m-cGAS inhibitor TDI-6570 and h-cGAS inhibitor TDI-8246 in both in vitro and in vivo studies. These inhibitors produced effects resembling the cGAS knockout (cGAS−/−) phenotype in cultured cells and the PS19 tauopathy mice [38].
In human induced pluripotent cell (iPSC)-derived microglia, h-cGAS inhibitor TDI-8246 completely reversed or significantly reduced the tau-fibril-induced increases in phosphorylated STING (p-STING), CXCL10, and CCL5. The study found that tau-fibril treatment led to the release of mitochondrial DNA (mtDNA) into the cytosol, activating cGAS and the interferon genes. Treatment with TDI-8246 significantly reversed the expression of tau-inducible genes and pathways, including interferon signaling and cytokine signaling in the immune system.
In PS19 tauopathy mice, chronic treatment with TDI-6570, which inhibits cGAS activity, protected against synapse loss and cognitive deficits. This protection was associated with enhanced MEF2C activity and a supportive transcriptional network, contributing to cognitive resilience [38]. In a separate study, Naguib et al. [129] investigated the effects of m-cGAS inhibitor TDI-6570 in PS19 tauopathy mice carrying wild type human APOE3. They found that the inhibitor protected against tau-induced synaptic loss and induced similar transcriptomic changes to those caused by the Christchurch mutation (R136S) across various brain cell types, suggesting that cGAS-STING-IFN inhibition mimics the protective effects of the R136S mutation against tauopathy [129]. In another study, Gao et al. [130] demonstrated that a tetrahydroxy stilbene glycoside, a natural product, blocks the cGAS-STING pathway by reducing the expression of cGAS, p-STING and p-TBK1. This intervention ameliorated the inflammatory response both in vitro and in vivo, ultimately improving cognitive impairment in APP/PS1 mice.
Future Directions
The identification and development of human cGAS inhibitors for treating AD and other neurodegenerative diseases depend on finding inhibitors that are both potent and capable of penetrating the BBB. Most studies mentioned above, except for those involving benzofuran and benzothiophene derivatives [83] by Gan and Sinha labs, and of new G140 analogs by the Shanghai/Beijing group [80,81], have primarily reported only the in vitro and cellular activities. The properties of active inhibitors from various published articles and patents in this review can be estimated using in silico analyses.
Among all cGAS inhibitor classes, the tetrahydro-γ-carboline derivatives identified by the RU/TDI/MSKCC group [62] became the main focus for many researchers. Ventus Therapeutics, actively modifying RU/TDI/MSKCC compounds, has advanced a cGAS inhibitor, Vent-03 (structure not known), which possess excellent PK properties in plasma, into clinical trials for treating inflammatory and cardiometabolic diseases, with promising plasma PK properties, though its brain permeability hasn't been disclosed. Our work has led to the development of dihydrobenzo-furo-pyridine and dihydrobenzo-thieno-pyridine derivatives, which exhibit high brain permeability. The G140 analogs developed by the Shanghai/Beijing team and numerous unique homologs by Lilly, incorporate the full or partial structure of RU/TDI inhibitor G150, are likely to cross the BBB and are potentially suitable for testing in AD models.
Author Contributions
J. Alarcon-Esposito performed the literature review and wrote the draft of the manuscript.
R. Kumar Nagiri performed the literature review and wrote the draft of the manuscript.
Li Gan provided suggestions and reviewed.
S. C. Sinha edited and provided comments and revisions.
All authors reviewed and agreed with the final version.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Subhash Sinha reports a relationship with Aeton Therapeutics that includes: equity or stocks. Li Gan reports a relationship with Aeton Therapeutics that includes: equity or stocks. Li Gan has patent pending to Weill Cornell Medicine. Subhash Sinha has patent pending to Weill Cornell Medicine. Ravi Kumar Nigiri has patent pending to Weill Cornell Medicine. Guest Editor, Neurotherapeutics, Gan. Guest Editor, Neurotherapeutics, Sinha. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We extend our gratitude to the NIH (R01AG074541 to L.G. and S.C.S), the Cure Alzheimer's fund (to L.G. and S.C.S.), the Daedalus fund (to L.G. and S.C.S.), and the Rainwater charitable foundation (to L.G.) for their funding support.
Footnotes
This article is part of a special issue on Alzheimer's Disease published in Neurotherapeutics.
References
- 1.Ashrafian H., Zadeh E.H., Khan R.H. Review on Alzheimer's disease: inhibition of amyloid beta and tau tangle formation. Int J Biol Macromol. 2021;167:382–394. doi: 10.1016/j.ijbiomac.2020.11.192. [DOI] [PubMed] [Google Scholar]
- 2.Korczyn A.D., Grinberg L.T. Is Alzheimer disease a disease? Nat Rev Neurol. 2024;20:245–251. doi: 10.1038/s41582-024-00940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abyadeh M., Gupta V., Paulo J.A., Mahmoudabad A.G., Shadfar S., Mirshahvaladi S., et al. Amyloid-beta and tau protein beyond Alzheimer’s disease. Neural Regeneration Research. 2024;19:1262–1276. doi: 10.4103/1673-5374.386406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouras G.K., Almeida C.G., Takahashi R.H. Intraneuronal Aβ accumulation and origin of plaques in Alzheimer's disease. Neurobiol Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Gouras G.K., Tampellini D., Takahashi R.H., Capetillo-Zarate E. Intraneuronal β-amyloid accumulation and synapse pathology in Alzheimer's disease. Acta Neuropathol. 2010;119:523–541. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy M.P., LeVine Iii H. Alzheimer's disease and the amyloid-β peptide. J Alzheim Dis. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pooler A.M., Polydoro M., Maury E.A., Nicholls S.B., Reddy S.M., Wegmann S., et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol Commun. 2015;3:14. doi: 10.1186/s40478-015-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloom G.S. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 9.Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 10.Ghoshal N., Garcia-Sierra F., Wuu J., Leurgans S., Bennett D.A., Berry R.W., et al. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol. 2002;177:475–493. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 11.Frontzkowski L., Ewers M., Brendel M., Biel D., Ossenkoppele R., Hager P., et al. Earlier Alzheimer’s disease onset is associated with tau pathology in brain hub regions and facilitated tau spreading. Nat Commun. 2022;13:4899. doi: 10.1038/s41467-022-32592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ossenkoppele R., Schonhaut D.R., Schöll M., Lockhart S.N., Ayakta N., Baker S.L., et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholl M., Lockhart S.N., Schonhaut D.R., O’Neil J.P., Janabi M., Ossenkoppele R., et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison T.M., La Joie R., Maass A., Baker S.L., Swinnerton K., Fenton L., et al. Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann Neurol. 2019;85:229–240. doi: 10.1002/ana.25406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaz M., Silvestre S. Alzheimer's disease: recent treatment strategies. Eur J Pharmacol. 2020;887 doi: 10.1016/j.ejphar.2020.173554. [DOI] [PubMed] [Google Scholar]
- 16.Jorfi M., Maaser-Hecker A., Tanzi R.E. The neuroimmune axis of Alzheimer's disease. Genome Med. 2023;15:6. doi: 10.1186/s13073-023-01155-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jian X., Kunkle B.W., Chen Y., Hamilton-Nelson K.L., Bush W.S., et al. Whole exome sequencing study identifies novel rare and common Alzheimer’s-Associated variants involved in immune response and transcriptional regulation. Mol Psychiatr. 2018 doi: 10.1038/s41380-018-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sims R., van der Lee S.J., Naj A.C., Bellenguez C., Badarinarayan N., Jakobsdottir J., et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet. 2017;49:1373–1384. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J.C., Carrasquillo M.M., et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y., Manis M., Long J., Wang K., Sullivan P.M., Remolina Serrano J., et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J Exp Med. 2019;216:2546–2561. doi: 10.1084/jem.20190980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyns C.E.G., Ulrich J.D., Finn M.B., Stewart F.R., Koscal L.J., Remolina Serrano J., et al. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci U S A. 2017;114:11524–11529. doi: 10.1073/pnas.1710311114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho S.H., Chen J.A., Sayed F., Ward M.E., Gao F., Nguyen T.A., et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1beta. J Neurosci. 2015;35:807–818. doi: 10.1523/jneurosci.2939-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., Lamb B.T., et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. S0896-6273(10)00633-1 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heneka M.T., McManus R.M., Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018;19:610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 25.Ising C., Venegas C., Zhang S., Scheiblich H., Schmidt S.V., Vieira-Saecker A., et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575:669–673. doi: 10.1038/s41586-019-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnaider L., Arnon Z.A., Gazit E. Reevaluating the microbial infection link to Alzheimer's disease. J Alzheim Dis : JAD. 2020;73:59–62. doi: 10.3233/jad-190765. [DOI] [PubMed] [Google Scholar]
- 27.Moir R.D., Lathe R., Tanzi R.E. The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimers Dement. 2018;14:1602–1614. doi: 10.1016/j.jalz.2018.06.3040. [DOI] [PubMed] [Google Scholar]
- 28.Eimer W.A., Vijaya Kumar D.K., Navalpur Shanmugam N.K., Rodriguez A.S., Mitchell T., Washicosky K.J., et al. Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99:56–63.e53. doi: 10.1016/j.neuron.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar D.K., Choi S.H., Washicosky K.J., Eimer W.A., Tucker S., Ghofrani J., et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8:340ra372. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy E.R., Wang B., Wan Y.W., Chiu G., Cole A., Yin Z., et al. Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J Clin Invest. 2020;130:1912–1930. doi: 10.1172/jci133737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pluchino S., Willis C. Intrinsic antiviral immunity drives neurodegeneration in Alzheimer disease. J Clin Invest. 2020;130:1622–1624. doi: 10.1172/jci135906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mudò G., Frinchi M., Nuzzo D., Scaduto P., Plescia F., Massenti M.F., et al. Anti-inflammatory and cognitive effects of interferon-β1a (IFNβ1a) in a rat model of Alzheimer’s disease. J Neuroinflammation. 2019;16:44. doi: 10.1186/s12974-019-1417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavoshinezhad S., Mohseni Kouchesfahani H., Ahmadiani A., Dargahi L. Interferon beta ameliorates cognitive dysfunction in a rat model of Alzheimer's disease: modulation of hippocampal neurogenesis and apoptosis as underlying mechanism. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;94 doi: 10.1016/j.pnpbp.2019.109661. [DOI] [PubMed] [Google Scholar]
- 34.Tao J., Zhang X.W., Jin J., Du X.X., Lian T., Yang J., et al. Nonspecific DNA binding of cGAS N terminus promotes cGAS activation. J Immunol. 2017;198:3627–3636. doi: 10.4049/jimmunol.1601909. [DOI] [PubMed] [Google Scholar]
- 35.Shang G., Zhu D., Li N., Zhang J., Zhu C., Lu D., et al. Crystal structures of STING protein reveal basis for recognition of cyclic di-GMP. Nat Struct Mol Biol. 2012;19:725–727. doi: 10.1038/nsmb.2332. [DOI] [PubMed] [Google Scholar]
- 36.Hussain B., Xie Y., Jabeen U., Lu D., Yang B., Wu C., et al. Activation of STING based on its structural features. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.808607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffries A.M., Marriott I. Human microglia and astrocytes express cGAS-STING viral sensing components. Neurosci Lett. 2017;658:53–56. doi: 10.1016/j.neulet.2017.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udeochu J.C., Amin S., Huang Y., Fan L., Torres E.R., Carling G.K., et al. Tau activation of microglial cGAS–IFN reduces MEF2C-mediated cognitive resilience. Nat Neurosci. 2023;26:737–750. doi: 10.1038/s41593-023-01315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y., Liu B., Sinha S.C., Amin S., Gan L. Mechanism and therapeutic potential of targeting cGAS-STING signaling in neurological disorders. Mol Neurodegener. 2023;18:79. doi: 10.1186/s13024-023-00672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Govindarajulu M., Ramesh S., Beasley M., Lynn G., Wallace C., Labeau S., et al. Role of cGAS-sting signaling in Alzheimer’s disease. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24098151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carling, G. K. et al. Alzheimer's disease-linked risk alleles elevate microglial cGAS-associated senescence and neurodegeneration in a tauopathy model. Neuron, 10.1016/j.neuron.2024.09.006. [DOI] [PMC free article] [PubMed]
- 42.Zhou W., Whiteley A.T., de Oliveira Mann C.C., Morehouse B.R., Nowak R.P., Fischer E.S., et al. Structure of the human cGAS-DNA complex reveals enhanced control of immune surveillance. Cell. 2018;174:300–311.e311. doi: 10.1016/j.cell.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Shu C., Yi G., Chaton C.T., Shelton C.L., Diao J., et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. 2013;39:1019–1031. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shu C., Li X., Li P. The mechanism of double-stranded DNA sensing through the cGAS-STING pathway. Cytokine Growth Factor Rev. 2014;25:641–648. doi: 10.1016/j.cytogfr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall J., Ralph E.C., Shanker S., Wang H., Byrnes L.J., Horst R., et al. The catalytic mechanism of cyclic GMP-AMP synthase (cGAS) and implications for innate immunity and inhibition. Protein Sci. 2017;26:2367–2380. doi: 10.1002/pro.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao P., Ascano M., Wu Y., Barchet W., Gaffney B.L., Zillinger T., et al. Cyclic [G(2’,5’)pA(3’,5’)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kranzusch P.J., Lee A.S.Y., Wilson S.C., Solovykh M.S., Vance R.E., Berger J.M., et al. Structure-guided reprogramming of human cGAS dinucleotide linkage specificity. Cell. 2014;158:1011–1021. doi: 10.1016/j.cell.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traut T.W. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/bf00928361. [DOI] [PubMed] [Google Scholar]
- 49.Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science (New York, N.Y.) 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J., Sun L., Chen X., Du F., Shi H., Chen C., et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science (New York, N.Y.) 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian X., Xu F., Zhu Q., Feng Z., Dai W., Zhou Y., et al. Medicinal chemistry perspective on cGAS-STING signaling pathway with small molecule inhibitors. Eur J Med Chem. 2022;244:114791. doi: 10.1016/j.ejmech.2022.114791. [DOI] [PubMed] [Google Scholar]
- 52.Wu G., Zhao T., Kang D., Zhang J., Song Y., Namasivayam V., et al. Overview of recent strategic advances in medicinal chemistry. J Med Chem. 2019;62:9375–9414. doi: 10.1021/acs.jmedchem.9b00359. [DOI] [PubMed] [Google Scholar]
- 53.Das B., Baidya A.T.K., Mathew A.T., Yadav A.K., Kumar R. Structural modification aimed for improving solubility of lead compounds in early phase drug discovery. Bioorg Med Chem. 2022;56 doi: 10.1016/j.bmc.2022.116614. [DOI] [PubMed] [Google Scholar]
- 54.Chu L., Li C., Li Y., Yu Q., Yu H., Li C., et al. Perillaldehyde inhibition of cGAS reduces dsDNA-induced interferon response. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.655637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valentin R., Wong C., Alharbi A.S., Pradeloux S., Morros Makala P., Lennox Kim A., et al. Sequence-dependent inhibition of cGAS and TLR9 DNA sensing by 2′-O-methyl gapmer oligonucleotides. Nucleic Acids Res. 2021;49:6082–6099. doi: 10.1093/nar/gkab451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding C., Song Z., Shen A., Chen T., Zhang A. Small molecules targeting the innate immune cGAS‒STING‒TBK1 signaling pathway. Acta Pharm Sin B. 2020;10:2272–2298. doi: 10.1016/j.apsb.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen A., Chen M., Chen Q., Liu Z., Zhang A. Recent advances in the development of STING inhibitors: an updated patent review. Expert Opin Ther Pat. 2022;32:1131–1143. doi: 10.1080/13543776.2022.2144220. [DOI] [PubMed] [Google Scholar]
- 58.Yu X., Cai L., Yao J., Li C., Wang X. Agonists and inhibitors of the cGAS-STING pathway. Molecules. 2024;29 doi: 10.3390/molecules29133121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X., Wang Y., Cao A., Luo Q., Chen D., Zhao W., et al. Development of cyclopeptide inhibitors of cGAS targeting protein-DNA interaction and phase separation. Nat Commun. 2023;14:6132. doi: 10.1038/s41467-023-41892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M., Sooreshjani M.A., Mikek C., Opoku-Temeng C., Sintim H.O. Suramin potently inhibits cGAMP synthase, cGAS, in THP1 cells to modulate IFN-β levels. Future Med Chem. 2018;10:1301–1317. doi: 10.4155/fmc-2017-0322. [DOI] [PubMed] [Google Scholar]
- 61.An J., Woodward J.J., Sasaki T., Minie M., Elkon K.B. Cutting edge: antimalarial drugs inhibit IFN-β production through blockade of cyclic GMP-AMP synthase-DNA interaction. J Immunol. 2015;194:4089–4093. doi: 10.4049/jimmunol.1402793. [DOI] [PubMed] [Google Scholar]
- 62.Lama L., Adura C., Xie W., Tomita D., Kamei T., Kuryavyi V., et al. Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nat Commun. 2019;10:2261. doi: 10.1038/s41467-019-08620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lama L., Tuschl T., Tomita D., Patel D., Glickman J.K., Kamei T., et al. 2,3,4,5-TETRAHYDRO-1H-PYRIDO[4, 3-B]indole inhibitors of CGAS for treating autoinflammatory diseases. WO patent WO 2019/153002 A1. 2019 [Google Scholar]
- 64.Koresawa M., Okabe T. High-throughput screening with quantitation of ATP consumption: a universal non-radioisotope, homogeneous assay for protein kinase. Assay Drug Dev Technol. 2004;2:153–160. doi: 10.1089/154065804323056495. [DOI] [PubMed] [Google Scholar]
- 65.Dueñas M.E., Peltier-Heap R.E., Leveridge M., Annan R.S., Buttner F.H., Trost M., et al. Advances in high-throughput mass spectrometry in drug discovery. EMBO Mol Med. 2023;15 doi: 10.15252/emmm.202114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pu F., Elsen N.L., Williams J.D. Emerging chromatography-free high-throughput mass spectrometry technologies for generating hits and leads. ACS Med Chem Lett. 2020;11:2108–2113. doi: 10.1021/acsmedchemlett.0c00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hooy R., Sohn J. In: Sohn Jungsan., editor. vol. 625. Academic Press; 2019. pp. 77–86. (Methods in enzymology). [Google Scholar]
- 68.Vincent J., Adura C., Gao P., Luz A., Lama L., Asano Y., et al. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat Commun. 2017;8:750. doi: 10.1038/s41467-017-00833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan X.C., Sanders J.M., Gao Y.-D., Tudor M., Haidle A.M., Klein D.J., et al. Augmenting hit identification by virtual screening techniques in small molecule drug discovery. J Chem Inf Model. 2020;60:4144–4152. doi: 10.1021/acs.jcim.0c00113. [DOI] [PubMed] [Google Scholar]
- 70.Scior T., Bender A., Tresadern G., Medina-Franco J.L., Martinez-Mayorga K., Langer T., et al. Recognizing pitfalls in virtual screening: a critical review. J Chem Inf Model. 2012;52:867–881. doi: 10.1021/ci200528d. [DOI] [PubMed] [Google Scholar]
- 71.Venugopal S., Sharma V., Mehra A., Singh I., Singh G. DNA intercalators as anticancer agents. Chem Biol Drug Des. 2022;100:580–598. doi: 10.1111/cbdd.14116. [DOI] [PubMed] [Google Scholar]
- 72.Beauchemin C., Moerke N.J., Faloon P., Kaye K.M. Assay development and high-throughput screening for inhibitors of kaposi's sarcoma-associated herpesvirus N-Terminal latency-associated nuclear Antigen binding to nucleosomes. J Biomol Screen. 2014;19:947–958. doi: 10.1177/1087057114520973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.An J., Minie M., Sasaki T., Woodward J.J., Elkon K.B. Antimalarial drugs as immune modulators: new mechanisms for old drugs. Annu Rev Med. 2017;68:317–330. doi: 10.1146/annurev-med-043015-123453. [DOI] [PubMed] [Google Scholar]
- 74.An J., Woodward J.J., Lai W., Minie M., Sun X., Tanaka L., et al. Inhibition of cyclic GMP-AMP synthase using a novel antimalarial drug derivative in Trex1-Deficient mice. Arthritis Rheumatol. 2018;70:1807–1819. doi: 10.1002/art.40559. [DOI] [PubMed] [Google Scholar]
- 75.Song J., Yang R.-R., Chang J., Liu Y.-D., Lu C.-H., Chen L.-F., et al. Discovery and characterization of a novel cGAS covalent inhibitor for the treatment of inflammatory bowel disease. Acta Pharmacol Sin. 2023;44:791–800. doi: 10.1038/s41401-022-01002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hall J., Brault A., Vincent F., Weng S., Wang H., Dumlao D., et al. Discovery of PF-06928215 as a high affinity inhibitor of cGAS enabled by a novel fluorescence polarization assay. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomita D., Lama L., Tuschl T., Patel D., Glickman J.F., Kamei T., et al. Inhibitors of cGAS for treating autoinflammatory diseases and cancer metastasis. WO patent WO 2020/186027 A1. 2020 [Google Scholar]
- 78.Fader L.E.E., Burch J., St-Onge M., Dorich S. PYRIDO[4,3-b]INDOLE derivatives and their use as pharmaceuticals. WO patent WO 2022/066851 A1. 2022 [Google Scholar]
- 79.Beveridge R., Burch J., Cyr P. HEXAHYDROPYRIDO[4,3-B]INDOLYL ketone derivatives useful as CGAS modulators. WO patent WO 2023/183275 A1. 2023 [Google Scholar]
- 80.Tan J., Wu B., Chen T., Fan C., Zhao J., Xiong C., et al. Synthesis and pharmacological evaluation of tetrahydro-γ-carboline derivatives as potent anti-inflammatory agents targeting cyclic GMP–AMP synthase. J Med Chem. 2021;64:7667–7690. doi: 10.1021/acs.jmedchem.1c00398. [DOI] [PubMed] [Google Scholar]
- 81.Chen M., Lei S., Zhou Z., Wang M., Feng C., Gao X., et al. Design, synthesis, and pharmacological evaluation of spiro[carbazole-3,3′-pyrrolidine] derivatives as cGAS inhibitors for treatment of acute Lung Injury. J Med Chem. 2024;67:6268–6291. doi: 10.1021/acs.jmedchem.3c02229. [DOI] [PubMed] [Google Scholar]
- 82.Ahmed A., Martinez Brokaw C., Carson Cheryl A.N.N., Conner Scott E., Fortner Kevin C., Franciskovich Jeffry B., et al. CGAS Inhibitors. WO patent WO 2023/235809 A1. 2023 [Google Scholar]
- 83.Sinha S., Gan L.I., Nagiri Ravi K., Amin S., Huang Y. Cgas inhibitors and uses thereof. WO patent WO 2023/154962 A1. 2023 [Google Scholar]
- 84.Lowery Robert G., Kumar M., Boxer M., Maloney D., Boyd S. Inhibitors of CGAS activity as therapeutic agents. WO patent WO 2020/142735 A1. 2020 [Google Scholar]
- 85.Lowery R., Boxer M., Maloney D., Kumar M. Inhibitors of cGAS activity as therapeutic agents. WO patent WO 2024/035622 A1. 2024 [Google Scholar]
- 86.Heimann Annekatrin C., Gnamm C., Godbout C., Gross P., Handschuh Sandra R., Hoenke C., et al. Pyridine derivatives with N-linked cyclic substituents as CGAS inhibitors. WO patent WO 2022/238327 A1. 2022 [Google Scholar]
- 87.Beveridge R., Burch J., Fader L.E.E., Boily M.-O., St-Onge M., Dorich S., et al. Heterocyclic compounds and uses thereof. WO patent WO 2023/081441 A1. 2023 [Google Scholar]
- 88.Beveridge R., Burch J., Ciblat S., Cyr P. Thiadiazole derivatives as inhibitors of cyclic GMP-AMP synthase and uses thereof. WO patent WO 2024/137607 A1. 2024 [Google Scholar]
- 89.Zhong B., Sun L., Shi H., Li J., Chen C., Chen Z. cGAS antagonist compounds. WO patent WO 2017/176812 A1. 2017 [Google Scholar]
- 90.Berchtold S., Galley G., Groebke Zbinden K., Guba W., Krumm D. Sulfamoylamide derivatives. WO patent WO 2021/209475 A1. 2021 [Google Scholar]
- 91.Berchtold S., Galley S., Groebke Zbinden K., Guba W., Hunziker D., Krummenacher D. Indazole derivatives. WO patent WO 2021/209481 A1. 2021 [Google Scholar]
- 92.Berchtold S., Galley G., Groebke Zbinden K., Guba W., Hunziker D., Krumm D. Biphenyl derivatives. WO patent WO 2021/209473 A1. 2021 [Google Scholar]
- 93.Galley G., Groebke Zbinden K., Hunziker D., Guba W., Berchtold S., Krumm D., et al. Benzimidazole derivatives. WO patent WO 2021/209484 A1. 2021 [Google Scholar]
- 94.Décoret G., Galley G., Groebke Zbinden K., Grossmann N., Guba W., Hunziker D. New malonitrile derivatives. WO patent WO 2021/233854 A1. 2021 [Google Scholar]
- 95.Padilla-Salinas R., Sun L., Anderson R., Yang X., Zhang S., Chen Z.J., et al. Discovery of small-molecule cyclic GMP-AMP synthase inhibitors. J Org Chem. 2020;85:1579–1600. doi: 10.1021/acs.joc.9b02666. [DOI] [PubMed] [Google Scholar]
- 96.Zhao W., Xiong M., Yuan X., Li M., Sun H., Xu Y. In silico screening-based discovery of novel inhibitors of human cyclic GMP–AMP synthase: a cross-validation study of molecular docking and experimental testing. J Chem Inf Model. 2020;60:3265–3276. doi: 10.1021/acs.jcim.0c00171. [DOI] [PubMed] [Google Scholar]
- 97.Li J., Xiong M., Liu J., Zhang F., Li M., Zhao W., et al. Discovery of novel cGAS inhibitors based on natural flavonoids. Bioorg Chem. 2023;140:106802. doi: 10.1016/j.bioorg.2023.106802. [DOI] [PubMed] [Google Scholar]
- 98.Leibrock L., Benz J., Groebke-Zbinden K., Rudolph M.G., editors. Rscb PDB. 2024. [DOI] [Google Scholar]
- 99.Dai J., Huang Y.J., He X., Zhao M., Wang X., Liu Z.S., et al. Acetylation blocks cGAS activity and inhibits self-DNA-induced autoimmunity. Cell. 2019;176:1447–1460.e1414. doi: 10.1016/j.cell.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song Z.M., Lin H., Yi X.M., Guo W., Hu M.M., Shu H.B. KAT5 acetylates cGAS to promote innate immune response to DNA virus. Proc Natl Acad Sci U S A. 2020;117:21568–21575. doi: 10.1073/pnas.1922330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu H., Zhang H., Wu X., Ma D., Wu J., Wang L., et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature. 2018;563:131–136. doi: 10.1038/s41586-018-0629-6. [DOI] [PubMed] [Google Scholar]
- 102.Li T., Huang T., Du M., Chen X., Du F., Ren J., et al. Phosphorylation and chromatin tethering prevent cGAS activation during mitosis. Science (New York, N.Y.) 2021:371. doi: 10.1126/science.abc5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li M., Shu H.B. Dephosphorylation of cGAS by PPP6C impairs its substrate binding activity and innate antiviral response. Protein Cell. 2020;11:584–599. doi: 10.1007/s13238-020-00729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun X., Liu T., Zhao J., Xia H., Xie J., Guo Y., et al. DNA-PK deficiency potentiates cGAS-mediated antiviral innate immunity. Nat Commun. 2020;11:6182. doi: 10.1038/s41467-020-19941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seo G.J., Yang A., Tan B., Kim S., Liang Q., Choi Y., et al. Akt kinase-mediated checkpoint of cGAS DNA sensing pathway. Cell Rep. 2015;13:440–449. doi: 10.1016/j.celrep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhong L., Hu M.M., Bian L.J., Liu Y., Chen Q., Shu H.B. Phosphorylation of cGAS by CDK1 impairs self-DNA sensing in mitosis. Cell Discov. 2020;6:26. doi: 10.1038/s41421-020-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen M., Meng Q., Qin Y., Liang P., Tan P., He L., et al. TRIM14 inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote innate immune responses. Mol Cell. 2016;64:105–119. doi: 10.1016/j.molcel.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 108.Wang Q., Huang L., Hong Z., Lv Z., Mao Z., Tang Y., et al. The E3 ubiquitin ligase RNF185 facilitates the cGAS-mediated innate immune response. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seo G.J., Kim C., Shin W.J., Sklan E.H., Eoh H., Jung J.U. TRIM56-mediated monoubiquitination of cGAS for cytosolic DNA sensing. Nat Commun. 2018;9:613. doi: 10.1038/s41467-018-02936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Z.S., Zhang Z.Y., Cai H., Zhao M., Mao J., Dai J., et al. RINCK-mediated monoubiquitination of cGAS promotes antiviral innate immune responses. Cell Biosci. 2018;8:35. doi: 10.1186/s13578-018-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo Y., Jiang F., Kong L., Li B., Yang Y., Zhang L., et al. Cutting edge: USP27X deubiquitinates and stabilizes the DNA sensor cGAS to regulate cytosolic DNA-mediated signaling. J Immunol. 2019;203:2049–2054. doi: 10.4049/jimmunol.1900514. [DOI] [PubMed] [Google Scholar]
- 112.Yang X., Shi C., Li H., Shen S., Su C., Yin H. MARCH8 attenuates cGAS-mediated innate immune responses through ubiquitylation. Sci Signal. 2022;15:eabk3067. doi: 10.1126/scisignal.abk3067. [DOI] [PubMed] [Google Scholar]
- 113.Hu M.-M., Yang Q., Xie X.-Q., Liao C.-Y., Lin H., Liu T.-T., et al. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity. 2016;45:555–569. doi: 10.1016/j.immuni.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 114.Cui Y., Yu H., Zheng X., Peng R., Wang Q., Zhou Y., et al. SENP7 potentiates cGAS activation by relieving SUMO-mediated inhibition of cytosolic DNA sensing. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiao Y., Li J., Liao X., He Y., He T., Yang C., et al. RIOX1-demethylated cGAS regulates ionizing radiation-elicited DNA repair. Bone Res. 2022;10:19. doi: 10.1038/s41413-022-00194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi C., Yang X., Liu Y., Li H., Chu H., Li G., et al. ZDHHC18 negatively regulates cGAS-mediated innate immunity through palmitoylation. EMBO J. 2022;41 doi: 10.15252/embj.2021109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fan Y., Gao Y., Nie L., Hou T., Dan W., Wang Z., et al. Targeting LYPLAL1-mediated cGAS depalmitoylation enhances the response to anti-tumor immunotherapy. Mol Cell. 2023;83:3520–3532.e3527. doi: 10.1016/j.molcel.2023.09.007. [DOI] [PubMed] [Google Scholar]
- 118.Xia P., Ye B., Wang S., Zhu X., Du Y., Xiong Z., et al. Glutamylation of the DNA sensor cGAS regulates its binding and synthase activity in antiviral immunity. Nat Immunol. 2016;17:369–378. doi: 10.1038/ni.3356. [DOI] [PubMed] [Google Scholar]
- 119.Wu Y., Li S. Role of post-translational modifications of cGAS in innate immunity. Int J Mol Sci. 2020;21:7842. doi: 10.3390/ijms21217842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu Y., Liu J., Liu C., Liu R., Liu L., Yu Z., et al. Post-translational modifications of cGAS-STING: a critical switch for immune regulation. Cells. 2022;11:3043. doi: 10.3390/cells11193043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang J., Zhao J., Xu S., Li J., He S., Zeng Y., et al. Species-specific deamidation of cGAS by herpes simplex virus UL37 protein facilitates viral replication. Cell Host Microbe. 2018;24:234–248.e235. doi: 10.1016/j.chom.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song B., Liu D., Greco T.M., Cristea I.M. In: Gerold Gisa., editor. vol. 109. Academic Press; 2021. pp. 163–199. (Advances in virus research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ning X., Wang Y., Jing M., Sha M., Lv M., Gao P., et al. Apoptotic caspases suppress type I interferon production via the cleavage of cGAS, MAVS, and IRF3. Mol Cell. 2019;74:19–31.e17. doi: 10.1016/j.molcel.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y., Ning X., Gao P., Wu S., Sha M., Lv M., et al. Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity. 2017;46:393–404. doi: 10.1016/j.immuni.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 125.Jena K.K., Mehto S., Nath P., Chauhan N.R., Sahu R., Dhar K., et al. Autoimmunity gene IRGM suppresses cGAS-STING and RIG-I-MAVS signaling to control interferon response. EMBO Rep. 2020;21 doi: 10.15252/embr.202050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang S.D., Li H., Zhou Y.L., Liu X.C., Li D.C., Hao C.F., et al. Protein-protein interactions in cGAS-STING pathway: a medicinal chemistry perspective. Future Med Chem. 2024;16:1801–1820. doi: 10.1080/17568919.2024.2383164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xie X., Ma G., Li X., Zhao J., Zhao Z., Zeng J., et al. Activation of innate immune cGAS-STING pathway contributes to Alzheimer’s pathogenesis in 5×FAD mice. Nat Aging. 2023;3:202–212. doi: 10.1038/s43587-022-00337-2. [DOI] [PubMed] [Google Scholar]
- 128.Chung S., Jeong J.-H., Park J.-C., Han J.W., Lee Y., Kim J.-I., et al. Blockade of STING activation alleviates microglial dysfunction and a broad spectrum of Alzheimer’s disease pathologies. Exp Mol Med. 2024;56:1936–1951. doi: 10.1038/s12276-024-01295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Naguib S., Torres E.R., Lopez-Lee C., Fan L., Bhagwat M., Norman K., et al. APOE3-R136S mutation confers resilience against tau pathology via cGAS-STING-IFN inhibition. bioRxiv. 2024 doi: 10.1101/2024.04.25.591140. [DOI] [Google Scholar]
- 130.Gao D., Hao J.-p, Li B.-y., Zheng C.-c, Miao B.-b., Zhang L., et al. Tetrahydroxy stilbene glycoside ameliorates neuroinflammation for Alzheimer’s disease via cGAS-STING. Eur J Pharmacol. 2023;953:175809. doi: 10.1016/j.ejphar.2023.175809. [DOI] [PubMed] [Google Scholar]