Abstract
Anti-amyloid monoclonal antibodies (MABs) have introduced a new era of Alzheimer's disease (AD) therapeutics with disease-targeted drugs. Three agents --- aducanumab, lecanemab, donanemab --- have been approved and others are in development. These agents are administered intravenously, once in the brain they activate microglia to engulf amyloid-beta protein fibrillar plaques. Each approved agent has a specific profile of administration, titration, amyloid target, and adverse events. In 18 month trials of participants with early AD (defined as mild cognitive impairment due to AD or mild AD dementia), anti-amyloid MABs slow cognitive and functional decline by approximately 30 %. Amyloid positron emission tomography reveals marked reductions in amyloid plaque burden; reductions below a threshold of 15–25 Centiloids are associated with clinical benefit. The magnitude, scope, and trajectory of clinical end points provide the basis for interpretations of clinical meaningfulness. Occurrence of amyloid-related imaging abnormalities with intracerebral edema, microhemorrhages, or superficial siderosis must be monitored and managed to prevent serious or rare catastrophic consequences. Infusion reactions occur and anticipatory management is required. Development of subcutaneous formulations and use of blood-based biomarkers for diagnosis and monitoring promises to increase the accessibility and decrease the demands on health care systems associated with these agents. Anti-amyloid MABs provide the foundation for further advances in developing a repertoire of disease-targeted therapies for AD.
Keywords: Alzheimer’s disease, Monoclonal antibodies, Amyloid, Donanemab, Lecanemab
Anti-amyloid monoclonal antibodies (MABs) are the first agents approved by the US Food and Drug Administration (FDA) as disease-targeted therapies (DTTs) for the treatment of early Alzheimer's disease (AD). They change one of the defining pathophysiological features of the disease: the presence of amyloid plaques. Anti-amyloid MABs represent a new chapter in the treatment of AD And provide a foundation for developing more efficacious, safer, and more convenient MABs as well as other types of DTTs for AD and other neurodegenerative disorders [1]. Aducanumab (Aduhelm®), lecanemab (Leqembi®), and donanemab (Kisunla®) have been approved by the FDA and some MABS have been approved in other global regions. The anti-amyloid MAB landscape is evolving; aducanumab has been discontinued and new anti-amyloid MABS are being developed [2]. Anti-amyloid MABS do not improve symptoms of arrest progression of AD; they slow clinical decline and offer patients with AD the opportunity to function at a higher level for a longer time. Use of anti-amyloid MABs requires the early detection of AD, the use of biomarkers to diagnose AD, and adjustments of healthcare systems to ensure safe delivery of these therapies. With expanded application, anti-amyloid MABs can change many aspects of human aging [3]. Use of anti-amyloid MABs in patient care makes new demands on clinicians as well as imposing new requirements on healthcare delivery systems. Successful implementation of anti-amyloid MAB therapy and extension of this treatment to appropriately defined patients depend on developing strategies for integrating advances of scientific understanding into patient care systems.
The success of anti-amyloid MABs builds on an extensive foundation of studies devoted to understanding the biology of amyloid in AD [4]. Amyloid plaques and neurofibrillary tangles have been recognized as defining pathophysiologic factors of AD since the disease was first described [5]. Neuropathological, genetic, and molecular biological studies led to the “amyloid hypothesis” suggesting that amyloid is toxic to brain cells and induces the other pathological changes observed in the AD brain including neurofibrillary tangles, brain inflammation, and synaptic dysfunction [6]. Interrogation of the dynamics of amyloid production and assembly in the brain revealed the multiple aggregation states of amyloid from low molecular weight monomers, dimers, and trimers to higher molecular weight ensembles including oligomers, protofibrils, fibrils, and plaques [7]. Many clinical trials targeted amyloid production and aggregation and all failed until MABs directed at advanced aggregates including protofibrils and plaques demonstrated reproducible relationships between plaque lowering and slowing of disease progression [8]. The anti-amyloid MAB trials have validated plaque lowering as a therapeutic target; they do not exclude other viable targets in the complex biology of AD.
Biomarkers have had a major role in the successful development of the anti-amyloid MABs. Amyloid positron emission tomography (PET) allows detection and accurate diagnosis of patients with AD and is used to document plaque removal by MABs in clinical trials. Diminished plaque burden demonstrated on amyloid PET was the basis for accelerated approval of aducanumab and lecanemab. With additional trial information, the latter received standard approval based on demonstrated clinical benefit. Cerebrospinal fluid (CSF) amyloid measures have diagnostic value and respond to therapeutic intervention with plaque lowering drugs. Emerging plasma biomarkers such as phosphorylated tau-217 (p-tau 217) may provide an alternative to amyloid PET and CSF measures for the diagnosis of AD and may be used for monitoring the pharmacodynamic impact of anti-amyloid MABs [9,10]. Advances in biomarker research will further enhance their application in clinical trials, will accelerate drug development, and can transform clinical practice [11].
Anti-amyloid MABs have adverse events including infusion reactions and amyloid-related imaging abnormalities (ARIA). The latter are usually asymptomatic but can have serious consequences and occasionally result in death [12]. Optimal use of anti-amyloid MABs involves knowledge of risk factors for ARIA, vigilance for evidence of ARIA in patients receiving treatment, and active management of ARIA to avoid serious outcomes.
This narrative review emphasizes the biological features of the anti-amyloid MABs, the use of anti-amyloid MABs in clinical practice, and the appropriate management of anti-amyloid MABs to optimize effectiveness and safety. The biology and management of ARIA are discussed. Phase 3 trials and endpoints are emphasized since these are most influential in regulatory and clinical decision-making. Phase 2 observations and drugs previously in development are cited when they provide insights into anti-amyloid MAB biology, clinical trials, or potential clinical use. The relationship between amyloid reduction and slowing of clinical decline is described. The meaningfulness of the drug-placebo difference observed in clinical trials of anti-amyloid MABs is discussed, and approaches to including patients who have received anti-amyloid MABs into clinical trials of novel agents are presented.
Biological aspects of Amyloid and Anti-Amyloid monoclonal antibodies
Amyloid exists along a continuum of species of varying molecular weights and aggregation states (Fig. 1). Amyloid monomers are produced by enzymatic degradation of the amyloid precursor protein (APP). Beta-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE 1) is the first step in the sequential proteolytic cleavages leading to generation of amyloid beta protein (Aβ). Gamma-secretase removes the C-terminal fragment of soluble APPβ produced by BACE1 creating the Aβ monomer [4,[13], [14], [15]]. The 42 amino acid form of the Aβ is more aggregation prone than species with other amino acid compositions. Aggregation of monomers leads to dimers, trimers, and more complex species eventually forming oligomers, protofibrils, and fibrils that aggregate into amyloid plaques [6] (Fig. 1). In laboratory studies, the greatest neurotoxicity resides in the oligomeric species that disrupts synaptic plasticity and function, stimulates hyperphosphorylation of the tau protein to form neurofibrillary tangles, and activates a neuroinflammatory response. The amyloid cascade comprises the conceptual framework for understanding the role of Aβ in AD and provides the foundation for the development of anti-amyloid therapeutics [6].
Fig. 1.
Species of amyloid-beta protein (Aβ) beginning with monomers and aggregating to form amyloid plaques (© J Cummings, 2025; M de la Flor, PhD, Illustrator).
The putative mechanism of plaque-lowering anti-amyloid MABs is to produce amyloid clearance by labeling aggregated species expressing the target epitope and activating microglia to engulf and consume the amyloid, reducing the abundance of the targeted amyloid species [16] (Fig. 2). Microglia may degrade soluble Aβ primarily by secreting insulin degrading enzyme whereas their clearance of aggregated insoluble Aβ and plaques may result from phagocytosis [17]. Whether activated microglia engulf only the antibody-decorated pathologies or engulf other regional aggregates is uncertain. Peripherally originating monocytes/macrophages may play a role in central inflammatory responses when they enter the brain through a compromised blood brain barrier (BBB) and interact with brain-resident microglia to exaggerate inflammation or may have an indirect influence through peripheral inflammatory mechanisms [18,19].
Fig. 2.
Activation of microglia by labeling with anti-amyloid monoclonal antibodies to engulf and remove amyloid plaques (© J Cummings, 2025; M de la Flor, PhD, Illustrator). Resting microglia are engaged in environmental surveillance; some microglia may be activated by Aβ plaques prior to the administration of the antibody.
The available and emerging anti-amyloid MABs and those assessed in previous trials target different species of amyloid (Table 1). Solanezumab and ponezumab focused on the amyloid monomer; crenezumab targeted monomeric and oligomeric species; sabirnetug addresses oligomeric species of Aβ; bapineuzumab, gantenerumab, and aducanumab engage oligomers and aggregated amyloid fibrils; lecanemab targets protofibils and engages fibrillar forms of amyloid; and donanemab and remternetug address pyroglutamate Aß [16,20]. Thus far, anti-amyloid MABs that slow clinical decline have been associated with measurable plaque removal on amyloid PET. Anti-amyloid MABs targeting monomers or low molecular weight Aβ species have not shown benefit. The absence of consensus biomarkers measuring oligomers and protofibrils has limited progress in determining if targeting these species in the absence of an effect on plaques can slow progressive cognitive and functional decline. Donanemab and remturnetug specifically target the pyroglutamate Aβ unique to amyloid plaques, suggesting that plaque removal is sufficient to produce clinical benefit [21,22]. Plaque reducing anti-amyloid MABs have been associated with ARIA; MABs targeting lower molecular weight species have not produced ARIA (Table 1). Preliminary observations support the hypothesis that anti-amyloid MAB binding and mobilization of the amyloid of cerebral vessels are highly correlated with the occurrence of ARIA [23]. Amyloid-induced vessel inflammation may contribute to the vulnerability to ARIA [24].
Table 1.
Monoclonal antibodies, amyloid species targeted, and ARIA-E (amyloid-related imaging abnormality – effusion type) liability, and current development status. Agents in Phase 2 and Phase 3 included.
| Anti-Amyloid Antibody | Amyloid Species Targeted | ARIA-E Observed | Current Status |
|---|---|---|---|
| Solanezumab | Monomer | No | Discontinued |
| Ponezumab | Monomer | No | Discontinued |
| Crenezumab | Monomer and oligomer | No | Discontinued |
| Sabirnetug | Oligomer | Yes | Phase 2 |
| Bapineuzumab | Monomer, oligomer, fibrils | Yes | Discontinued |
| Aducanumab | Monomer, oligomer, fibrils, plaques | Yes | Accelerated approval; now discontinued |
| Gantenerumab | Oligomer, fibrils, plaques | Yes | Discontinued |
| Trontinemab | Oligomer, fibrils, plaques | Yes | Phase 2 |
| Lecanemab | Protofibril, fibrils, plaques | Yes | Approved |
| Donanemab | Pyroglutamate Aβ in plaques | Yes | Approved |
| Remternetug | Pyroglutamate Aβ in plaques | Yes | Phase 3 |
Several anti-amyloid MAB programs have been discontinued due to negative late-stage trials and will be discussed further only if they provide insight into a specific biological or clinical issue (Table 1). Solanezumab, ponezumab, crenezumab, and bapineuzumab development programs have been terminated. Gantenerumab is no longer being developed as an independent anti-amyloid MAB; it is being advanced in the trontinemab program where gantenerumab is attached to a transferrin-based brain shuttle that increases BBB penetration and brain exposure [25].
Clinical trials of Anti-Amyloid monoclonal antibodies
Administration of anti-amyloid monoclonal antibodies in clinical trials
Table 2 summarizes key aspects of the clinical pharmacology of anti-amyloid MABs currently in advanced stages of development. Aducanumab, sabirnetug, donanemab, and remternetug are administered intravenously every four weeks. Lecanemab is administered intravenously twice monthly for 18 months prior to transitioning to a once per month maintenance schedule. Aducanumab, lecanemab, and remternetug have subcutaneous formulations in development. Trontinemab is administered subcutaneously twice monthly. Trontinemab, donanemab, and remternetug are given at fixed doses without weight adjustments; other MABs are administered on a milligram per kilogram basis. Titration is required for all MABS except lecanemab. In trials, donanemab and remternetug were stopped when amyloid plaque levels were no longer detectable by amyloid PET; other anti-amyloid MABs are continued until the patient exits the therapeutic window for that agent or the clinician, patient, and family concur that stopping treatment is warranted (e.g. mild AD dementia).
Table 2.
Key aspects of the clinical pharmacology of anti-amyloid monoclonal antibodies currently in development.
| Anti-Amyloid Antibody | Formulation | Fixed Dose or Weight-Based Dosing | Administration Schedule | Titration Required | Stopped When Amyloid Plaque Levels are Not Detectable |
|---|---|---|---|---|---|
| Aducanumab | IV/SC | Weight-based | Q 4 weeks | Yes | No |
| Sabirnetug | IV | Weight-based | Q 4 weeks | Yes | No |
| Trontinemab | SC | Fixed | Q 2 weeks | Yes | No |
| Lecanemab | IV | Weight-based | Q 2 weeks | No | No |
| Donanemab | IV | Fixed | Q 4 weeks | Yes | Yes |
| Remternetug | IV/SC | Fixed | Q 4 weeks | Yes | Yes |
IV – intravenous; Q – every; SC – subcutaneous.
There is rapid evolution in the development of anti-amyloid MABs and their transition to clinical care. Less frequent administration of lecanemab after introduction of therapy has been approved. Maintenance of low amyloid levels may require less frequent administration compared to the initiation phase where the goal is amyloid plaque or protofibril reduction. Donanemab was stopped in clinical trials when low levels of amyloid were determined by amyloid PET. Prescribing Instructions from the FDA suggest considering stopping donanemab in patients with low plaque burden but do not require stopping the treatment. Continued administration may prevent re-accumulation of plaques.
Clinical endpoints of trials of anti-amyloid monoclonal antibodies
Aducaumab is approved for the treatment of early AD with a confirmed presence of Aβ pathology. Aducanumab received accelerated approval from the FDA based on trials showing marked reduction of Aβ plaque burden on amyloid PET that was considered reasonably likely to predict clinical benefit. A follow-up trial confirming the clinical effects of aducanumab was stipulated as a requirement for standard approval and continued availability on the market [26,27]. Aducanumab availability has been suspended by Biogen and Eisai. Further development of the subcutaneous formulation is ongoing by Neurimmune.
A Phase 1B trial of aducanumab demonstrated marked reduction of Aβ plaque after 12 months of exposure in the participants receiving monthly infusions at a dose of 10 mg/kg [28]. There was preliminary evidence of associated slowing of clinical decline. The Phase 1B (PRIME) trial was followed by two Phase 3 trials, EMERGE and ENGAGE. The Phase 3 program, including both trials, was halted based on a futility analysis of pooled data from the first 50 % of enrolled patients. Follow-up efficacy analyses based on a larger data set collected up to the time of futility declaration revealed that EMERGE met the trial's primary outcome and ENGAGE did not. The final data set included 65.2 % more data than the data set used for the futility analysis [29].
The EMERGE and ENGAGE trials were identically designed and included patients with early AD defined as mild cognitive impairment (MCI) due to AD or early AD dementia with amyloid abnormalities confirmed by amyloid PET and Mini-Mental State Examination (MMSE) scores in the range of 24–30 (Table 3). As measured by the Clinical Dementia Rating - sum of boxes (CDR-sb), the EMERGE high dose aducanumab population declined at a rate 22 % less than that observed in the placebo group. There was a −0.39 difference between the drug and placebo groups in the high dose arms at week 78 from a baseline score of 2.47 (±1) in the placebo group and a baseline score of 2.51 (±1.05). Secondary endpoints supported the primary endpoint observations with 18 % slowing on the Mini-Mental State Examination (MMSE), 27 % slowing on the Alzheimer's Disease Assessment Scale - cognitive subscale (ADAS-cog), and 40 % slowing on the Alzheimer's Disease Cooperative Study Activities of Daily Living scale Mild Cognitive Impairment version (ADCS-ADL-MCI) at week 78. The Neuropsychiatric Inventory (NPI) was a tertiary endpoint and showed an 87 % drug placebo-difference in change from baseline [29]. The ENGAGE trial showed no drug-placebo difference; this trial began later and had fewer participants who were exposed to the high dose of aducanumab for periods equivalent to participants in the EMERGE study. This difference in exposure may have contributed to the observed differences in trial endpoints. The low dose did not produce clinical benefit in either study.
Table 3.
Percent slowing on endpoints of Phase 3 trials of approved anti-amyloid monoclonal antibodies (active therapy compared to placebo).
| Aducanumab (EMERGE; High Dose Group) | Lecanemab (Clarity) | Donanemab (Trailblazer-ALZ2; Low-Medium Tau Group) | |
|---|---|---|---|
| Primary outcome | |||
| CDR-sb | 22 % | 27 % | 37 %a |
| iADRS | NA | NA | 35.1 % |
| Secondary outcomes | |||
| ADAS-cog | 27 % | 26 % | 32.4 % |
| ADCS-ADL-MCI | 40 % | 36 % | 39.9 % |
| MMSE | 18 % | NA | 22.9 % |
ADAS-cog - Alzheimer's Disease Assessment Scale - cognitive subscale; ADCS-ADL-MCI - Alzheimer's Disease Collaborative Study Activities of Daily Living Mild Cognitive Impairment; iADRS - integrated Alzheimer's Disease Rating Scale; MMSE – Mini-Mental State Examination; NA - not available.
CDR-sb was a secondary outcome for the Trailblazer-ALZ2 donanemab study.
Lecanemab received accelerated approval by the FDA based on amyloid plaque lowering observed in a Phase 2 study and additional trial-derived data linking amyloid clearance to clinical benefit [30]. Lecanemab then received standard approval based on clinical benefits and biomarker changes observed in the Phase 3 Clarity AD study [31]. Patients included in the lecanemab Phase 3 trial had early AD and amyloid abnormalities demonstrated by amyloid PET or CSF measurement of Aβ1-42. Cognitively, participants had objective memory impairment indicated by a score at least one standard deviation below the age-adjusted mean on the Wechsler Memory Scale Logical Memory test. Allowable MMSE scores at baseline were 22–30(31). The short half-life of lecanemab (7 days) informs the need for dosing every other week [32].
The Clarity AD trial met its primary endpoint of significant slowing (27 %) of disease progression as measured by the CDR-sb in the active treatment group compared to placebo. There was a corresponding statistically significant 26 % slowing of the ADAS-cog and 36 % slowing on the ADCS-ADL-MCI scale [31].
Clarity AD included health related quality of life measures. After 18 months of exposure to treatment with lecanemab, there was a 49 % slowing of decline on the European Quality of Life - 5 Dimensions (EQ-5D) scale and a 56 % slowing of decline in Quality of Life - Alzheimer's Disease (QoL-AD) as rated by the participant. There was a 23 % reduction in decline on the QoL-AD rated by the care partner as a participant proxy. The Zarit Burden Inventory demonstrated 38 % less increase in caregiver burden in the active treatment group [33].
The donanemab Phase 3 (Trailblazer-ALZ2) trial included 1736 randomized participants with early AD of whom 1320 completed the trial. Amyloid PET and tau PET (demonstrating the brain neurofibrillary tangle burden) were used to characterize the trial population. Participants also had abnormal amyloid imaging with baseline amyloid levels of ≥37 centiloids (CL) assessed by amyloid PET. Allowable MMSE scores at baseline were 22–28. Tau PET revealed that 996 participants had low/medium tau pathology and 552 had high tau pathology. The primary outcome was the change on the integrated Alzheimer's Disease Rating Scale (iADRS) at week 76 in the low/medium tau group or the combined low/medium and high tau groups.
Trailblazer-ALZ2 met its primary outcome [34]. The low/medium tau group exhibited a 35.1 % slowing of disease progression on the iADRS and the combined population evidenced a 22.3 % slowing. Both outcomes were statistically significant in favor of donanemab (p < 0.001). Secondary outcomes were consistent with the primary outcome observations. There was a 36.0 % slowing on the CDR-sb, 39.9 % slowing on the ADCS-ADL-MCI scale, and 32.4 % slowing on the ADAS-cog in the low/medium tau group. The combined tau group exhibited 28.9 % slowing on the CDR-sb, 27.8 % slowing on the ADCS ADL MCI scale, and 19.5 % slowing on the ADAS-cog. Prespecified exploratory analyses demonstrated a 38.6 % lower risk of disease progression in the low/medium tau population and a 37.4 % lower risk of progression in the combined population. Progression was defined for these analyses as a change in score on the global CDR at consecutive visits. At week 76, disease progression in the low/medium tau group treated with donanemab was delayed 4.36 months on the iADRS and 7.53 months on the CDR-sb compared to placebo [34]. No significant slowing was observed in the high tau group when analyzed separately. The absence of a therapeutic benefit in the high tau group suggests that patients with more advanced disease and more extensive tau pathology may not respond to donanemab; if confirmed, this observation might also apply to other anti-plaque antibodies. This finding of possible treatment resistance in those with high tau levels has important implications for the use of these agents in more advanced stages of AD and requires further study.
Biomarker endpoints of trials of anti-amyloid monoclonal antibodies
The EMERGE and ENGAGE studies of aducanumab showed a marked reduction in amyloid PET standard uptake value ratio (SUVR) in both studies [29]. Plasma p-tau181 increased in the placebo arm of the EMERGE and ENGAGE trials and showed a marked decrease in the active treatment high dose arms at week 78. CSF studies on a subset of patients revealed a dose-dependent increase in CSF Aβ1-42 and a dose-dependent decrease in CSF p-tau and total tau (t-tau) levels in EMERGE with more modest changes in ENGAGE. Increases in Aβ1-42 and Aß42/40 ratio are expected as brain plaques are disassembled by MAB treatment and plaque formation from monomers or other amyloid species is prevented. Pooled results from EMERGE and ENGAGE trials demonstrated a reduction in the neurofibrillary tangle burden signal on tau PET in the medial temporal and frontal lobes in the high dose aducanumab treatment group. Ventricular volume as measured by magnetic resonance imaging (MRI) increased in the active treatment groups of the EMERGE and ENGAGE studies. There were no drug-placebo differences on hippocampal or whole brain volumetric measures [29].
Following 18 month's treatment with lecanemab, the brain amyloid plaque burden as measured by amyloid PET declined 55.4 CL from a baseline of 77.9 CL in the active treatment group compared to an increase of 3.64 CL in the placebo group (P < 0.001) [31]. There were nominally significant increases in CSF Aβ1-42 and Aβ42/40 ratio in CSF and plasma respectively. There were nominal decreases in CSF t-tau, p-tau181 and neurogranin. There were decreases in plasma p-tau 181, and glial fibrillary acidic protein (GFAP). No drug-placebo differences were observed in CSF or plasma measures of neurofilament light (NfL) [31].
In the Trailblazer-ALZ2 trial, treatment with donanemab resulted in an 88 CL decrease in brain amyloid plaque level at week 76 in the low-medium tau group compared to an increase of 0.2 CL in the placebo group. In the combined population, amyloid plaque level decreased by 87 CL following donanemab treatment and decreased by 0.76 CL in the placebo group. No drug-placebo difference was observed on tau PET in the low/medium tau or combined populations. Volumetric MRI showed a greater decrease in whole brain volume, less change in hippocampal volume, and greater increase in ventricular volume in the donanemab group compared to the placebo group. Plasma p-tau 217 was significantly reduced with donanemab treatment compared with placebo in both the low/medium tau and combined populations.
Amyloid-related imaging abnormalities (ARIA) in trials of anti-amyloid monoclonal antibodies
ARIA has been observed to a variable extent in clinical trials of all plaque lowering anti-amyloid MABs. Conversely, ARIA has not been observed in trials of MABs directed at lower molecular weight amyloid targets such as monomers; these agents also lacked efficacy. ARIA includes edema (ARIA-E) and hemorrhage (ARIA-H), the latter is comprised of microhemorrhages and superficial siderosis [35]. ARIA-E and ARIA-H are more common in individuals with the apolipoprotein E 4 (APOE4) genotype, and there is a gene-dose relationship, with ARIA occurring more commonly in APOE4 homozygotes than APOE4 heterozygotes. If ARIA occurs, it tends to be observed within the first few months of treatment initiation. Symptoms of ARIA that alert patients and clinicians to its occurrence include headache, confusion, dizziness, gait changes, and nausea.
ARIA-E occurred in 35 % of EMERGE and 36 % of ENGAGE participants who received high dose aducanumab [29]. Among APOE4 carriers, ARIA-E was observed in 43 % of EMERGE and 42 % of ENGAGE participants receiving high dose aducanumab. Across both trials, 65 % of individuals homozygous for APOE4 exhibited ARIA-E. In the aducanumab trials, approximately 25 % of individuals with evidence of ARIA reported symptoms. Serious ARIA events were uncommon, occurring in 1.5 % and 1.4 % of individuals receiving high dose aducanumab in the EMERGE and ENGAGE trials respectively. Of participants with ARIA-E in the aducanumab Phase 3 trials, 98.2 % resolved within 16 weeks [36]. Table 4 summarizes the rates of ARIA observed in Phase 3 trials of anti-amyloid MABs. Each trial had somewhat different populations, and entry criteria may have affected the ARIA rates; the figures are not directly comparable.
Table 4.
Rates of ARIA observed in Phase 3 trials of anti-amyloid monoclonal antibodies. Each trial had different entry criteria that may have affected the ARIA rates, and the figures are not directly comparable.
| Aducanumab (EMERGE) | Aducanumab (ENGAGE) | Lecanemab (Clarity) | Donanemab (Trailblazer-ALZ2) | |
|---|---|---|---|---|
| ARIA-E placebo | 2 | 3 | 1.7 | 1.9 |
| ARIA-E Tx; symptomatic | 20 | 29 | 2.8 | 6.1 |
| ARIA-E Tx; APOE non-carriers | 18 | 23 | 5.4 | 15.7 |
| ARIA-E Tx; APOE carriers | 43 | 42 | 15.8 | NA |
| ARIA-E Tx; APOE heterozygotes | NA | NA | 10.9 | 22.8 |
| ARIA-E Tx; APOE homozygotes | 65 | 65 | 32.6 | 40.6 |
| ARIA-H placebo; microhemorrhage | 7 | 6 | 7.6 | 12.5 |
| ARIA-H placebo; siderosis | 3 | 2 | 2.3 | 3.0 |
| ARIA-H Tx; microhemorrhage | 20 | 19 | 14.0 | 26.8 |
| ARIA-H Tx; siderosis | 13 | 16 | 5.6 | 15.7 |
∗Aducanumab percentages are from the high dose arms of the EMERGE and ENGAGE trials; NA - not available; Tx – active therapy.
In the Clarity AD trial, ARIA-E occurred in 12.6 % of those treated with lecanemab and 1.7 % of those on placebo [31]. Seventy-eight percent of those with ARIA had no symptoms, and 81 % resolved within four months of occurrence. Of those in the lecanemab group, 2.8 % had symptomatic ARIA-E. ARIA-H was noted in 17.3 % of those on lecanemab and 9 % of those on placebo. Most (71 %) of ARIA-E occurred within the first three months of treatment initiation. ARIA-E resolved within 16 weeks in 81 % of participants.
In the Trailblazer-ALZ2 trial, donanemab was associated with 24 % ARIA-E in the active treatment group compared to 1.9 % in the placebo group [34]. Noncarriers had a 15.7 % rate of ARIA-E; heterozygotes exhibited ARIA-E in 22.8 %; and 40.6 % of homozygotes had ARIA-E. Of the ARIA-E events, 6.1 % were symptomatic in the donanemab group. Microhemorrhages occurred in 26.8 % of those treated with donanemab and 12.5 % of those on placebo; superficial siderosis was observed in 15.7 % of those treated with donanemab compared to 3 % of those receiving placebo. ARIA-E resolved in 98 % of participants with a mean resolution time of 72.4 days.
The more frequent occurrence of ARIA in individuals with the APOE4 genotype, particularly homozygotes, requires that APOE genotyping be conducted to inform risk discussions with candidates for therapy. Interpreting APOE genotype information requires genetic counseling expertise either through genetic counselors or appropriate training of clinicians in practices administering anti-amyloid MABs.
ARIA associated with use of anti-amyloid MABs can have serious consequences including stroke-like episodes, status epilepticus, and, rarely, death. Physicians and practices choosing to administer anti-amyloid MABs must ensure the safety of patients having severe ARIA with preparedness protocols involving emergency departments and intensive care units [37,38].
Infusion and injection site reactions
Infusion reactions can occur with anti-amyloid MABs, and patients must be monitored carefully during the infusion and for up to 2 h following [38]. Most reactions occur in the first or second infusion and include flu-like symptoms (fever, chills, generalized aches, and joint pain), nausea, vomiting, hypotension, hypertension, and oxygen desaturation. Delayed reactions can occur. Acute reactions can be treated and prophylactic therapy can be administered with antihistamines, acetaminophen, or corticosteroids.
Infusion reactions occurred in 26.4 % of participants receiving lecanemab in the Phase 3 Clarity AD study and in 7.4 % of participants receiving placebo [31]. In the Phase 3 Trailblazer-ALZ2 study, infusion-related reactions occurred in 8.7 % participants receiving the donanemab and in 0.5 % of participants receiving placebo [34].
None of the currently approved anti-amyloid MABs are available in subcutaneous formulations, but several subcutaneous anti-amyloid MABs are in development, and injection site reactions must be anticipated. In trials with gantenerumab --- an anti-amyloid MAB that was delivered subcutaneously --- injection site reactions occurred in 16.8 % of participants in the active therapy group compared to 7.7 % of those on placebo. Reactions were typically mild [39].
Brain volume decrease observed on magnetic resonance imaging
Decrease in brain volume following therapy with anti-amyloid MABs is observed. Increased ventricular volume occurred in trials of aducanumab (EMERGE and ENGAGE); no effect on brain volume or hippocampal volume was observed [29]. In the Phase 3 trial of donanemab, patients in the active treatment group had greater whole brain volume loss, greater ventricular volume increase, and less hippocampal volume loss than those on placebo [34]. Treatment-related volume reduction has been observed with other amyloid-related interventions such as the beta-secretase inhibitor verubecestat [40], as well as non-amyloid interventions such as the GSK-3β inhibitor divalproex sodium [41]. In their meta-analysis, of anti-amyloid MAB trials, Alves and colleagues, found a relationship between the occurrence of ARIA and greater volume loss [42]. In their study of volume loss observed in verubecestat trials, Sur et al. noted greater regional volume loss in areas of high amyloid content [40].
The causes and characteristics of the volume loss observed in trials of anti-amyloid MABs are undetermined. The greater slowing of cognitive decline in patients on active treatment compared to placebo suggests that atrophy and cell loss are not the cause of the volume changes. Reduction of amyloid and associated neuroinflammation is one candidate explanation for the volume differences in drug-treated compared to placebo-treated participants. Hydration-related fluid shifts that occur with alcohol abstinence and in dehydration states are known to have measurable volumetric effects on MRI, and fluid shift differences might contribute to volume changes observed with MAB therapy [43,44]. Recent reports suggest that the volume changes can be ascribed to amyloid-removal-related pseudoatrophy like the volume reductions occurring with anti-inflammatory therapy in patients with multiple sclerosis [45].
Threshold of amyloid plaque reduction associated with clinical benefit
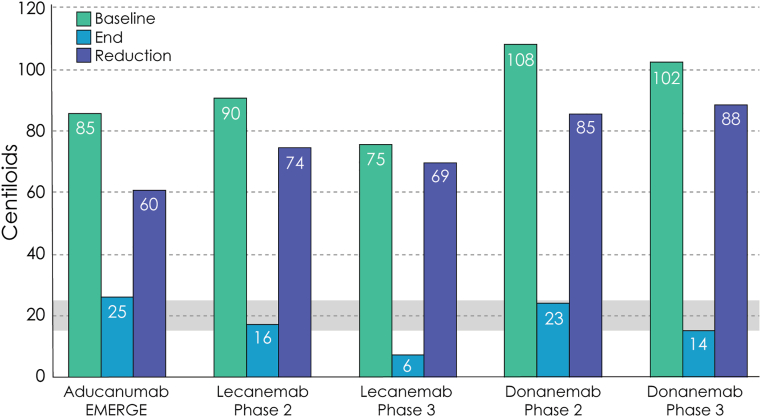
Data from plaque lowering anti-amyloid MAB trials suggest that there is a threshold which must be achieved for measurable clinical benefit to be observed. End-of-trial plaque reduction to the 15–25 CL level is associated with slowing of cognitive and functional decline. This relationship was observed in the aducanumab EMRGE trial [29], the lecanemab Phase 2 and Phase 3 trials [30,31], and the donanemab Phase 2 and Phase 3 trials [34,46]. Gantenerumab lowered plaque levels but did not, on average, reach this threshold, and no meaningful slowing was observed in the Phase 3 GRADUATE trials [39]. The data suggest that lowering plaque levels to below the 15 to 25 CL is more critical than the total amount of amyloid plaque reduction observed (Fig. 3) [16]. A CL value of greater than 26 corresponds to the threshold for a positive visual read of an amyloid PET(47). A CL value of 30 is a threshold for high certainty of amyloid plaque presence [47]. Clinicians with access to CL measures of amyloid PET can have confidence that at this level the amyloid target is present, and the individual could be considered for MAB therapy if other eligibility criteria are met.
Fig. 3.
The graphs demonstrate the beginning amyloid plaque level, end-of-trial amyloid plaque level, and reduction of plaque observed in Phase 3 trials. End of trial reductions below 15 to 25 Centiloids (gray shaded bar) were associated with slowing of clinical decline; less robust reductions did not have observed clinical benefit. Graph A shows positive trials associated with measurable clinical slowing; Graph B shows negative studies with no slowing of clinical decline (© J Cummings, 2025; M de la Flor, PhD, Illustrator).
Modeling based on the donanemab data suggests that without further treatment after reaching this threshold, amyloid plaque levels will reaccumulate to a level above 25 CL in 3.9 years (range 1.9–8.3 years) [48].
CSF and plasma Aβ 42/40 ratios and p-tau 217 are correlated with plaque reductions documented on amyloid PET, but no specific CSF or plasma levels of these measures has been established corresponding to a 15 to 25 CL threshold.
There is a convergence of information suggesting that the 15–25 CL amyloid level is a key inflection point in the biology of AD. Individuals with baseline measures of amyloid of 13.3 CL, progressed to become amyloid positive, whereas those who did not progress had baseline levels of 1.5 CL [49]. Amyloid negative individuals who reached levels of 15–18.5 CL progressed to amyloid positivity and cognitive decline whereas those who did not accumulate to this level did not progress [50]. When the rate of amyloid production is measured, the highest rate is found in individuals with amyloid levels in the 20–25 CL range [49]. Plasma p-tau 217 and p-tau 231 become abnormal when amyloid levels are in the range of 20–30 CL(52). A sharp rise in tau PET SUVR occurs when patients have reached the 25 CL level of amyloid deposition [51]. This is followed by a marked increase in tau PET positivity in patients in the 40–60 CL level of amyloid positivity [51]. Thus, the likelihood of further amyloid accumulation, cognitive decline, increase in plaque-associated p-tau (p-tau 217, p-tau 231), and production of amyloid and insoluble tau are triggered at the level of amyloid burden of 15–25 CL suggesting that a critical change in the dynamic biology of AD occurs at this level.
Meaningfulness of the magnitude of clinical slowing observed in Anti-Amyloid monoclonal antibody clinical trials
The range of slowing of clinical decline observed in Phase 3 trials of anti-amyloid MABs ranges from 20 to 40 % depending on the antibody studied, the characteristics of the trial population, and the clinical measures employed (described above). There is no single specific measure of meaningfulness and different stakeholders have varying approaches to interpreting the meaningfulness of trial outcomes. Global measures with composites of cognition and function have generally been seen as measures of clinical meaningfulness [52]. The CDR-sb and iADRS used as primary outcomes in anti-amyloid MAB trials are global composite measures and indicative of the meaningfulness of the interventions. Functional measures assessing activities of daily living are important trial outcomes for patients, care partners, clinicians, and other stakeholders. Functional measures are generally regarded as measures of meaningful benefit [53]. Anti-amyloid MAB trials show drug-placebo differences in favor of active therapy using the ADCS-ADL-MCI scale (Table 3). The FDA has emphasized that cognition is a key aspect of therapeutic benefit, and significant drug-placebo differences on the ADAS-cog --- a measure of cognition including memory, language, and praxis --- has been demonstrated in anti-amyloid MAB trials (Table 3) [54]. The lecanemab Phase 3 Clarity trial Included measures of QoL and caregiver burden (discussed above) and demonstrated reduced caregiver burden in the active treatment group compared to those on placebo and better preservation of QoL in patients receiving lecanemab compared to those receiving placebo [33]. Patient QoL and caregiver burden benefits support the meaningfulness of the observed outcomes.
Mean drug-placebo differences used as endpoints in clinical trials may be difficult to interpret at the individual patient level. Analyses such as the Minimum Clinically Important Difference (MCID) or Within Person Meaningful Change (WPMC) can be applied at the individual patient level to assess patient benefit [55]. Preliminary studies suggest that an MCID for the CDR-sb will be in the range of 1–2 points and patients who decline by this amount can be regarded as having meaningfully increased disability and require reassessment. In trials, the MCID can be used as a responder analysis comparing the number or percent of participants receiving active therapy experiencing this degree of decline compared to those on placebo. The increasing drug-placebo difference observed in trials of anti-amyloid MABs and other DTTs indicates that the number of patients reaching the MCID will be time-dependent [56].
“Time saved” analyses convert drug-placebo differences into a time metric that is more easily interpreted than scale score differences and can be communicated more consistently to patients, care partners, and clinicians. Based on data from the Trailblazer-ALZ study of donanemab, disease progression was delayed by 5.3 months on the iADRS and 5.2 months on the CDR-sb during the 18-month treatment period [57]. Studies with lecanemab indicate the treatment for 2.9 years would extend independence in instrumental activities of daily living by 11 months [58]. Longer periods of treatment are anticipated to be associated with greater time saved [59].
The duration of clinical benefit is an important aspect of determining meaningfulness. The 18-month exposure used for anti-amyloid MAB Phase 2 and Phase 3 trials artificially truncates the potential long-term effects of therapy possible in real world settings. Modeling based on the lecanemab Phase 2 data suggests that development of mild, moderate, and severe AD dementia for patients with early AD will be delayed 2.51, 3.13, and 2.34 years, respectively. The model also predicted a lower lifetime probability of admission to institutional care for those on therapy (25 % versus 31 %) [60].
Meaningful benefit must be balanced against meaningful harm which can be produced by ARIA. Expert care with avoidance and management of ARIA are key to benefit/harm ratio considerations for anti-amyloid MAB therapy.
Considerations of clinical meaningfulness of MAB treatment include the magnitude of the drug-placebo difference, the effect across multiple domains (clinical and biomarker), and the trajectory of assessments over time. Conclusions that the effects observed with anti-amyloid MABs represent meaningful benefit are based on the consistency of these observations across trials, agents, and measures.
Discussion
Anti-amyloid MABs are approved for patients with MCI due to AD and mild AD dementia who have been shown to be amyloid positive, have four or fewer microhemorrhages and no extensive white matter changes on MRI, and have no medical or psychiatric disorders that render them inappropriate for treatment. These relatively stringent treatment eligibility requirements reflect the inclusion and exclusion criteria applied for clinical trial participation and echo the Prescribing Information from the FDA as well as the Appropriate Use Recommendations developed by the Alzheimer's Disease and Related Disorders Therapeutic Working Group [38]. A conservative approach to use of the MABs has been adopted until additional data on ARIA management and other aspects of MAB use in real world circumstances are available. Depending on the specifics of the populations served in clinics reporting experience with anti-amyloid MABs, from approximately 20 % to as few as 5 % of early AD patients met all eligibility criteria for treatment [[61], [62], [63], [64]].
The presence of multiple anti-amyloid MABs on the market requires that clinicians have discussions with patients about treatment alternatives. Current data suggest that the efficacy of these agents is in the range of 20–40 % slowing of cognitive and functional decline. The available trials were performed in somewhat different populations of early AD and no direct head-to-head comparisons are available. More efficacy data may become available that will suggest differential efficacy or greater or less efficacy in specific populations. In the Phase 3 trials, donanemab had a higher rate of ARIA and a lower rate of infusion reactions than lecanemab. Donanemab is given monthly and may be stopped after amyloid clearance is achieved; lecanemab is given twice monthly for 18 months and monthly thereafter until the patient, family, and clinician decide that treatment is ineffective or unwarranted. In the Phase 3 trial, donanemab was titrated from 700 mg per infusion for the first three infusions followed by 1400 mg thereafter. Observations from a recent titration trial suggests that a titration beginning with 350 mg, progressing to 700 mg, 1050, mg and then 1400 mg may be associated with a lower rate of ARIA(67). This approach represents an alternative titration schedule. Lecanemab has weight-dependent dosing of 10 mg/kg and is not titrated. MRIs are obtained at baseline and before the 2nd, 3rd, 4th, and 7th infusions of donanemab. MRIs are required at baseline and before the 5th, 7th, and 14th infusions of lecanemab. Monthly maintenance dosing of lecanemab after the first 18 months of twice monthly dosing is approved, and subcutaneous administration is being studied. Both agents have black box warning regarding the higher risk of ARIA in APOE4 homozygotes. Discussion of these features of anti-amyloid MABs with patients and family members will be key to ensuring treatment adherence with optimal benefit and minimal risk for the patient.
Populations of individuals participating in clinical trials are highly selected and not representative of older individuals in the US or the world. Clinical trial participants tend to be younger, healthier, take fewer concomitant medications, and are more well educated than the general population [65,66]. White participants predominate in most trials [67,68]. Generalizability of clinical trial results to US or global populations depends on engaging trial populations with more diverse socioeconomic, geographic, and ethnoracial diversity. Systematic collection of real-world data after drug approval may provide important insights into effectiveness and safety of the agents in more representative populations (Galvin, 2024).
Great strides are being made in development of blood-based biomarkers (BBBM). In clinical trials, BBBMs will have an increasingly important roles in diagnosis, prognosis, population enrichment, participant or analytic stratification, longitudinal monitoring, pharmacodynamic assessment, and safety. As their performance is better understood, BBBM's will be implemented in clinical care to improve the detection and management of AD in clinical practice [69,70].
Patients with full or partial treatment-related amyloid clearance (TRAC) may be eligible for participation in clinical trials of novel therapeutic agents. This provides the opportunity to develop add-on combination therapies with anti-amyloid MABs targeting amyloid plaques and novel agents targeting other aspects of AD pathophysiology such as different amyloid species, tau, or inflammation [71]. Sequential combinations with agents such as gamma secretase modulators that decrease amyloid production could be used following amyloid clearance by MABs [71,72]. Interpretation of biomarkers in TRAC patients participating in trials is complex since amyloid PET as well as CSF and plasma amyloid, p-tau, and GFAP measures are altered by the MAB therapy [9,10].
A significant challenge for anti-amyloid MABs is penetration of the blood brain barrier (BBB). It is estimated that 0.1–0.2 % of antibodies cross the BBB and enter the brain where therapeutic activity can be achieved [73]. An alternative being explored to overcome MAB exclusion by the BBB is development of trontinemab comprised of a bispecific protein composed of gantenerumab, an anti-amyloid MAB, fused with a transferrin receptor-1 directed Brainshuttle® module. In non-human primates, this resulted in a 4-to-18-fold increase in exposure of the brain to gantenerumab [25]. Another alternative to enhancing brain entrance is the use of focused ultrasound to disrupt the BBB and temporarily allow antibody access to the brain. This approach improved the BBB penetration of aducanumab in a preliminary study [74].
All trials with anti-amyloid MABs that have been completed so far are in populations of patients with early AD defined as MCI due to AD or mild AD dementia. Prevention trials are in progress for both lecanemab and donanemab. Current prevention trials focus on individuals who are cognitively normal and have biomarker evidence of AD pathology in the brain. The goal of therapy is to delay the onset of cognitive decline. Prevention beginning earlier in the disease cycle can be envisioned involving cognitively normal at-risk individuals without evidence of AD pathology with the goal of therapy being delay of onset of biomarkers followed by confirmation of delay of onset of clinical symptoms. The delay in biomarker appearance might provide the basis for accelerated approval [75,76]. Earlier intervention may interrupt disease progression prior to the occurrence of extensive brain injury leading to better outcomes, and treating with MABs before deposition of vascular amyloid may reduce ARIA and increase the safety of treatment. Further pursuit of prevention strategies will depend in part on the outcome of the ongoing prevention trials.
Summary
The approval of anti-amyloid MABs has changed the therapeutic landscape and ushered in a new era of DTTs for treatment of AD. Eligible patients can anticipate an approximately 30 % reduction in the rate of loss of cognition and function. In 18 month clinical trials, 30 % slowing translated into an approximately 5 month delay in disease-milestones. Longer periods of treatment are projected to produce greater clinical benefits. ARIA represents the principle adverse event observed with anti-amyloid MABs and must be carefully managed to avoid serious or catastrophic consequences. Patient selection is critical to the successful use of anti-amyloid MABs including baseline MRI, determination of amyloid status, and identification of patients who can adhere to the treatment regimen. Anti-amyloid MABs are administered once or twice monthly, and infusion resources are necessary for their administration. The magnitude and scope of effects, consistency of effects across outcomes, and trajectory of therapeutic impact support the clinical meaningfulness of anti-amyloid MAB therapy. A greater role for BBBM's and development of subcutaneous formulations of anti-amyloid MABs will lead to greater accessibility of AD therapies. Anti-amyloid MABs do not arrest or reverse AD and alternative or combination approaches are needed to further ameliorate decline of cognitive function in AD patients. The restricted eligibility and relatively limited access to anti-amyloid MABs points to the need for therapies with greater safety and convenience. Anti-amyloid MABs are important therapies addressing a key pathology of AD and are a new therapeutic alternative for patients with AD. They provide the foundation for the next steps in advances in DTTs for AD.
Author contributions
As sole author, Jeffrey Cummings contributed to all portions of the manuscript including interpretation of data, drafting and revising the manuscript, and final approval of the manuscript.
Disclosures
JC has provided consultation to Acadia, Actinogen, Acumen, AlphaCognition, ALZpath, Aprinoia, AriBio, Artery, Axsome, Biogen, Biohaven, BioVie, BioXcel, Bristol-Myers Squib, Cassava, Cerecin, Diadem, Eisai, GAP Foundation, GemVax, Hummingbird Diagnostics, Janssen, Jocasta, Karuna, Lighthouse, Lilly, Lundbeck, LSP/eqt, Merck, NervGen, New Amsterdam, Novo Nordisk, Oligomerix, ONO, Optoceutics, Otsuka, Oxford Brain Diagnostics, Prothena, ReMYND, Roche, Sage Therapeutics, Signant Health, Simcere, sinaptica, Suven, T-Neuro, TrueBinding, Vaxxinity, and Wren pharmaceutical, assessment, and investment companies. JC owns the copyright of the Neuropsychiatric Inventory. JC has stocks/options in Artery, Vaxxinity, Behrens, Alzheon, MedAvante-Prophase, Acumen.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jeffrey Cummings reports financial support was provided by Acadia, Actinogen, Acumen, AlphaCognition, ALZpath, Aprinoia, AriBio, Artery, Biogen, Biohaven, BioVie, BioXcel, Bristol-Myers Squib, Cassava, Cerecin, Jeffrey Cummings reports financial support was provided by NervGen, New Amsterdam, Novo Nordisk, Oligomerix, ONO, Optoceutics, Otsuka, Oxford Brain Diagnostics, Prothena, ReMYND, Roche. Jeffrey Cummings reports financial support was provided by Sage Therapeutics, Signant Health, Simcere, sinaptica, Suven, TrueBinding, Vaxxinity, and Wren pharmaceutical, assessment, and investment companies. Jeffrey Cummings reports financial support was provided by Diadem, Eisai, GAP Foundation, GemVax, Janssen, Jocasta, Karuna, Lighthouse, Lilly, Lundbeck, LSP, Merck. Jeffrey Cummings reports a relationship with Artery, Vaxxinity, Behrens, Alzheon, MedAvante-Prophase, Acumen that includes: equity or stocks. Jeffrey Cummings reports a relationship with National Institute on Aging that includes: funding grants. Jeffrey Cummings reports a relationship with National Institute on General Medical Science that includes: funding grants. Jeffrey Cummings reports a relationship with Alzheimer's Drug Discovery Foundation that includes: funding grants. Jeffrey Cummings reports a relationship with Ted and Maria Quirk Endowment that includes: funding grants. Jeffrey Cummings reports a relationship with Joy Chambers-Grundy Endowment that includes: funding grants. Jeffrey Cummings has patent Neuropsychiatric Inventory with royalties paid to Jeffrey Cummings. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
JC is supported by NIGMS grant P20GM109025; NIA R35AG71476; NIA R25AG083721-01; Alzheimer’s Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment; Joy Chambers-Grundy Endowment.
Footnotes
This article is part of a special issue on Alzheimer's Disease published in Neurotherapeutics.
References
- 1.Cummings J. Anti-amyloid monoclonal antibodies are transformative treatments that redefine Alzheimer's disease therapeutics. Drugs. 2023;83(7):569–576. doi: 10.1007/s40265-023-01858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings J., Zhou Y., Lee G., Zhong K., Fonseca J., Cheng F. Alzheimer's disease drug development pipeline: 2024. Alzheimers Dement (N Y) 2024;10(2) doi: 10.1002/trc2.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe D.J. The advent of Alzheimer treatments will change the trajectory of human aging. Nat Aging. 2024;4(4):453–463. doi: 10.1038/s43587-024-00611-5. [DOI] [PubMed] [Google Scholar]
- 4.Hampel H., Hardy J., Blennow K., Chen C., Perry G., Kim S.H., et al. The amyloid-beta pathway in Alzheimer's disease. Mol Psychiatr. 2021;26(10):5481–5503. doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hippius H., Neundorfer G. The discovery of Alzheimer's disease. Dialogues Clin Neurosci. 2003;5(1):101–108. doi: 10.31887/DCNS.2003.5.1/hhippius. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ono K., Watanabe-Nakayama T. Aggregation and structure of amyloid beta-protein. Neurochem Int. 2021;151 doi: 10.1016/j.neuint.2021.105208. [DOI] [PubMed] [Google Scholar]
- 8.Boxer A.L., Sperling R. Accelerating Alzheimer's therapeutic development: the past and future of clinical trials. Cell. 2023;186(22):4757–4772. doi: 10.1016/j.cell.2023.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pontecorvo M.J., Lu M., Burnham S.C., Schade A.E., Dage J.L., Shcherbinin S., et al. Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(12):1250–1259. doi: 10.1001/jamaneurol.2022.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDade E., Cummings J.L., Dhadda S., Swanson C.J., Reyderman L., Kanekiyo M., et al. Lecanemab in patients with early Alzheimer's disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi: 10.1186/s13195-022-01124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teunissen C.E., Verberk I.M.W., Thijssen E.H., Vermunt L., Hansson O., Zetterberg H., et al. Blood-based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66–77. doi: 10.1016/S1474-4422(21)00361-6. [DOI] [PubMed] [Google Scholar]
- 12.Solopova E., Romero-Fernandez W., Harmsen H., Ventura-Antunes L., Wang E., Shostak A., et al. Fatal iatrogenic cerebral beta-amyloid-related arteritis in a woman treated with lecanemab for Alzheimer's disease. Nat Commun. 2023;14(1):8220. doi: 10.1038/s41467-023-43933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampel H., Vassar R., De Strooper B., Hardy J., Willem M., Singh N., et al. The beta-secretase BACE1 in Alzheimer's disease. Biol Psychiatry. 2021;89(8):745–756. doi: 10.1016/j.biopsych.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe M.S. Unraveling the complexity of gamma-secretase. Semin Cell Dev Biol. 2020;105:3–11. doi: 10.1016/j.semcdb.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hur J.Y. gamma-Secretase in Alzheimer's disease. Exp Mol Med. 2022;54(4):433–446. doi: 10.1038/s12276-022-00754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings J., Osse A.M.L., Cammann D., Powell J., Chen J. Anti-amyloid monoclonal antibodies for the treatment of Alzheimer's disease. BioDrugs. 2024;38(1):5–22. doi: 10.1007/s40259-023-00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu H., Liu B., Li L., Lemere C.A. Microglia do not take up soluble amyloid-beta peptides, but partially degrade them by secreting insulin-degrading enzyme. Neuroscience. 2020;443:30–43. doi: 10.1016/j.neuroscience.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mary A., Mancuso R., Heneka M.T. Immune activation in Alzheimer disease. Annu Rev Immunol. 2024;42(1):585–613. doi: 10.1146/annurev-immunol-101921-035222. [DOI] [PubMed] [Google Scholar]
- 19.Berriat F., Lobsiger C.S., Boillee S. The contribution of the peripheral immune system to neurodegeneration. Nat Neurosci. 2023;26(6):942–954. doi: 10.1038/s41593-023-01323-6. [DOI] [PubMed] [Google Scholar]
- 20.van Dyck C.H. Anti-amyloid-β monoclonal antibodies for Alzheimer's disease: pitfalls and promise. Biol Psychiatry. 2018;83(4):311–319. doi: 10.1016/j.biopsych.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cynis H., Frost J.L., Crehan H., Lemere C.A. Immunotherapy targeting pyroglutamate-3 Abeta: prospects and challenges. Mol Neurodegener. 2016;11(1):48. doi: 10.1186/s13024-016-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowe S.L., Duggan Evans C., Shcherbinin S., Cheng Y.J., Willis B.A., Gueorguieva I., et al. Donanemab (LY3002813) phase 1b study in Alzheimer's disease: rapid and sustained reduction of brain amyloid measured by florbetapir F18 imaging. J Prev Alzheimers Dis. 2021;8(4):414–424. doi: 10.14283/jpad.2021.56. [DOI] [PubMed] [Google Scholar]
- 23.Soderberg L., Johannesson M., Gkanatsiou E., Nygren P., Fritz N., Zachrisson O., et al. Amyloid-beta antibody binding to cerebral amyloid angiopathy fibrils and risk for amyloid-related imaging abnormalities. Sci Rep. 2024;14(1) doi: 10.1038/s41598-024-61691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg S.M., Bax F., van Veluw S.J. Amyloid-related imaging abnormalities: manifestations, metrics and mechanisms. Nat Rev Neurol. 2025 doi: 10.1038/s41582-024-01053-8. [DOI] [PubMed] [Google Scholar]
- 25.Grimm H.P., Schumacher V., Schafer M., Imhof-Jung S., Freskgard P.O., Brady K., et al. Delivery of the Brainshuttle amyloid-beta antibody fusion trontinemab to non-human primate brain and projected efficacious dose regimens in humans. mAbs. 2023;15(1) doi: 10.1080/19420862.2023.2261509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn B., Stein P., Temple R., Cavazzoni P. An appropriate use of accelerated approval - aducanumab for Alzheimer's disease. N Engl J Med. 2021;385(9):856–857. doi: 10.1056/NEJMc2111960. [DOI] [PubMed] [Google Scholar]
- 27.Dunn B., Stein P., Cavazzoni P. Approval of aducanumab for Alzheimer disease-the FDA's perspective. JAMA Intern Med. 2021;181(10):1276–1278. doi: 10.1001/jamainternmed.2021.4607. [DOI] [PubMed] [Google Scholar]
- 28.Sevigny J., Chiao P., Bussiere T., Weinreb P.H., Williams L., Maier M., et al. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 29.Budd Haeberlein S., Aisen P.S., Barkhof F., Chalkias S., Chen T., Cohen S., et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197–210. doi: 10.14283/jpad.2022.30. [DOI] [PubMed] [Google Scholar]
- 30.Swanson C.J., Zhang Y., Dhadda S., Wang J., Kaplow J., Lai R.Y.K., et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Abeta protofibril antibody. Alzheimers Res Ther. 2021;13(1):80–94. doi: 10.1186/s13195-021-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dyck C.H., Swanson C.J., Aisen P., Bateman R.J., Chen C., Gee M., et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhury S., Chowdhury N.S. Novel anti-amyloid-beta (Abeta) monoclonal antibody lecanemab for Alzheimer's disease: a systematic review. Int J Immunopathol Pharmacol. 2023;37 doi: 10.1177/03946320231209839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen S., van Dyck C.H., Gee M., Doherty T., Kanekiyo M., Dhadda S., et al. Lecanemab Clarity AD: quality-of-life results from a randomized, double-blind phase 3 trial in early Alzheimer's disease. J Prev Alzheimers Dis. 2023;10(4):771–777. doi: 10.14283/jpad.2023.123. [DOI] [PubMed] [Google Scholar]
- 34.Sims J.R., Zimmer J.A., Evans C.D., Lu M., Ardayfio P., Sparks J., et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–527. doi: 10.1001/jama.2023.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barakos J., Purcell D., Suhy J., Chalkias S., Burkett P., Marsica Grassi C., et al. Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with anti-amyloid beta therapy. J Prev Alzheimers Dis. 2022;9(2):211–220. doi: 10.14283/jpad.2022.21. [DOI] [PubMed] [Google Scholar]
- 36.Salloway S., Chalkias S., Barkhof F., Burkett P., Barakos J., Purcell D., et al. Amyloid-Related Imaging Abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79(1):13–21. doi: 10.1001/jamaneurol.2021.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummings J., Rabinovici G.D., Atri A., Aisen P., Apostolova L.G., Hendrix S., et al. Aducanumab: appropriate use recommendations update. J Prev Alzheimers Dis. 2022;9(2):221–230. doi: 10.14283/jpad.2022.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings J., Apostolova L., Rabinovici G.D., Atri A., Aisen P., Greenberg S., et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10(3):362–377. doi: 10.14283/jpad.2023.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bateman R.J., Smith J., Donohue M.C., Delmar P., Abbas R., Salloway S., et al. Two phase 3 trials of gantenerumab in early Alzheimer's disease. N Engl J Med. 2023;389(20):1862–1876. doi: 10.1056/NEJMoa2304430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sur C., Kost J., Scott D., Adamczuk K., Fox N.C., Cummings J.L., et al. BACE inhibition causes rapid, regional, and non-progressive volume reduction in Alzheimer's disease brain. Brain. 2020;143(12):3816–3826. doi: 10.1093/brain/awaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleisher A.S., Truran D., Mai J.T., Langbaum J.B., Aisen P.S., Cummings J.L., et al. Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology. 2011;77(13):1263–1271. doi: 10.1212/WNL.0b013e318230a16c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alves F., Kalinowski P., Ayton S. Accelerated brain volume loss caused by anti-beta-amyloid drugs: a systematic review and meta-analysis. Neurology. 2023;100(20):e2114–e2124. doi: 10.1212/WNL.0000000000207156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfefferbaum A., Sullivan E.V., Mathalon D.H., Shear P.K., Rosenbloom M.J., Lim K.O. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19(5):1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 44.Dieleman N., Koek H.L., Hendrikse J. Short-term mechanisms influencing volumetric brain dynamics. Neuroimage Clin. 2017;16:507–513. doi: 10.1016/j.nicl.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belder C.R.S., Boche D., Nicoll J.A.R., Jaunmuktane Z., Zetterberg H., Schott J.M., et al. Brain volume change following anti-amyloid beta immunotherapy for Alzheimer's disease: amyloid-removal-related pseudo-atrophy. Lancet Neurol. 2024;23(10):1025–1034. doi: 10.1016/S1474-4422(24)00335-1. [DOI] [PubMed] [Google Scholar]
- 46.Mintun M.A., Lo A.C., Duggan Evans C., Wessels A.M., Ardayfio P.A., Andersen S.W., et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691–1704. doi: 10.1056/NEJMoa2100708. [DOI] [PubMed] [Google Scholar]
- 47.Collij L.E., Bollack A., La Joie R., Shekari M., Bullich S., Roe-Vellve N., et al. Centiloid recommendations for clinical context-of-use from the AMYPAD consortium. Alzheimers Dement. 2024;20(12):9037–9048. doi: 10.1002/alz.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shcherbinin S., Evans C.D., Lu M., Andersen S.W., Pontecorvo M.J., Willis B.A., et al. Association of amyloid reduction after donanemab treatment with tau pathology and clinical outcomes: the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(10):1015–1024. doi: 10.1001/jamaneurol.2022.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jagust W.J., Landau S.M. Alzheimer's Disease Neuroimaging I. Temporal dynamics of beta-amyloid accumulation in aging and Alzheimer disease. Neurology. 2021;96(9):e1347–e1357. doi: 10.1212/WNL.0000000000011524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farrell M.E., Jiang S., Schultz A.P., Properzi M.J., Price J.C., Becker J.A., et al. Defining the lowest threshold for amyloid-PET to predict future cognitive decline and amyloid accumulation. Neurology. 2021;96(4):e619–e631. doi: 10.1212/WNL.0000000000011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dore V., Krishnadas N., Bourgeat P., Huang K., Li S., Burnham S., et al. Relationship between amyloid and tau levels and its impact on tau spreading. Eur J Nucl Med Mol Imag. 2021;48(7):2225–2232. doi: 10.1007/s00259-021-05191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knopman D.S., Knapp M.J., Gracon S.I., Davis C.S. The Clinician Interview-Based Impression (CIBI): a clinician's global change rating scale in Alzheimer's disease. Neurology. 1994;44(12):2315–2321. doi: 10.1212/wnl.44.12.2315. [DOI] [PubMed] [Google Scholar]
- 53.Desai A.K., Grossberg G.T., Sheth D.N. Activities of daily living in patients with dementia: clinical relevance, methods of assessment and effects of treatment. CNS Drugs. 2004;18(13):853–875. doi: 10.2165/00023210-200418130-00003. [DOI] [PubMed] [Google Scholar]
- 54.Food and Drug Administration . Guidance for industry. U.S. Department of health and human services; Food and drug administration; center for drug evaluation and research (CDER) Center for Biologics Evaluation and Research (CBER); Rockville, Maryland: 2024.; 2024. Early Alzheimer's disease: developing drugs for treatment. [Google Scholar]
- 55.Andrews J.S., Desai U., Kirson N.Y., Zichlin M.L., Ball D.E., Matthews B.R. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer's disease clinical trials. Alzheimers Dement (N Y). 2019;5:354–363. doi: 10.1016/j.trci.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cummings J. Meaningful benefit and minimal clinically important difference (MCID) in Alzheimer's disease: open peer commentary. Alzheimers Dement (N Y) 2023;9(3) doi: 10.1002/trc2.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dickson S.P., Wessels A.M., Dowsett S.A., Mallinckrodt C., Sparks J.D., Chatterjee S., et al. 'Time Saved' as a demonstration of clinical meaningfulness and illustrated using the donanemab TRAILBLAZER-ALZ Study findings. J Prev Alzheimers Dis. 2023;10(3):595–599. doi: 10.14283/jpad.2023.50. [DOI] [PubMed] [Google Scholar]
- 58.Hartz S.M., Schindler S.E., Streitz M.L., Moulder K.L., Mozersky J., Wang G., et al. Assessing the clinical meaningfulness of slowing CDR-SB progression with disease-modifying therapies for Alzheimers disease. Alzheimers Dement (N Y) 2025;11(1) doi: 10.1002/trc2.70033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petersen R.C., Aisen P.S., Andrews J.S., Atri A., Matthews B.R., Rentz D.M., et al. Expectations and clinical meaningfulness of randomized controlled trials. Alzheimers Dement. 2023;19(6):2730–2736. doi: 10.1002/alz.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tahami Monfared A.A., Tafazzoli A., Ye W., Chavan A., Zhang Q. Long-term health outcomes of lecanemab in patients with early Alzheimer's disease using simulation modeling. Neurol Ther. 2022;11(2):863–880. doi: 10.1007/s40120-022-00350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pittock R.R., Aakre J.A., Castillo A.M., Ramanan V.K., Kremers W.K., Jack C.R., Jr., et al. Eligibility for anti-amyloid treatment in a population-based study of cognitive aging. Neurology. 2023;101(19):e1837–e1849. doi: 10.1212/WNL.0000000000207770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canu E., Rugarli G., Coraglia F., Basaia S., Cecchetti G., Calloni S.F., et al. Real-word application of the AT(N) classification and disease-modifying treatment eligibility in a hospital-based cohort. J Neurol. 2024;271(5):2716–2729. doi: 10.1007/s00415-024-12221-7. [DOI] [PubMed] [Google Scholar]
- 63.Rosenberg A., Ohlund-Wistbacka U., Hall A., Bonnard A., Hagman G., Ryden M., et al. beta-amyloid, tau, neurodegeneration classification and eligibility for anti-amyloid treatment in a memory clinic population. Neurology. 2022;99(19):e2102–e2113. doi: 10.1212/WNL.0000000000201043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howe M.D., Britton K.J., Joyce H.E., Menard W., Emrani S., Kunicki Z.J., et al. Clinical application of plasma P-tau217 to assess eligibility for amyloid-lowering immunotherapy in memory clinic patients with early Alzheimer's disease. Alzheimers Res Ther. 2024;16(1):154. doi: 10.1186/s13195-024-01521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banzi R., Camaioni P., Tettamanti M., Bertele V., Lucca U. Older patients are still under-represented in clinical trials of Alzheimer's disease. Alzheimers Res Ther. 2016;8:32. doi: 10.1186/s13195-016-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leinonen A., Koponen M., Hartikainen S. Systematic review: representativeness of participants in RCTs of acetylcholinesterase inhibitors. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0124500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franzen S., Smith J.E., van den Berg E., Rivera Mindt M., van Bruchem-Visser R.L., Abner E.L., et al. Diversity in Alzheimer's disease drug trials: the importance of eligibility criteria. Alzheimers Dement. 2022;18(4):810–823. doi: 10.1002/alz.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grill J.D., Flournoy C., Dhadda S., Ernstrom K., Sperling R., Molina-Henry D., et al. Eligibility rates among racially and ethnically diverse US participants in phase 2 and phase 3 placebo-controlled, double-blind, randomized trials of lecanemab and elenbecestat in early Alzheimer disease. Ann Neurol. 2024;95(2):288–298. doi: 10.1002/ana.26819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ossenkoppele R., van der Kant R., Hansson O. Tau biomarkers in Alzheimer's disease: towards implementation in clinical practice and trials. Lancet Neurol. 2022;21(8):726–734. doi: 10.1016/S1474-4422(22)00168-5. [DOI] [PubMed] [Google Scholar]
- 70.Hansson O., Blennow K., Zetterberg H., Dage J. Blood biomarkers for Alzheimer's disease in clinical practice and trials. Nat Aging. 2023;3(5):506–519. doi: 10.1038/s43587-023-00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cummings J.L., Osse A.M.L., Kinney J.W., Cammann D., Chen J. Alzheimer's disease: combination therapies and clinical trials for combination therapy development. CNS Drugs. 2024;38(8):613–624. doi: 10.1007/s40263-024-01103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nordvall G., Lundkvist J., Sandin J. Gamma-secretase modulators: a promising route for the treatment of Alzheimer's disease. Front Mol Neurosci. 2023;16 doi: 10.3389/fnmol.2023.1279740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu Y.J., Watts R.J. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. 2013;10(3):459–472. doi: 10.1007/s13311-013-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rezai A.R., D'Haese P.F., Finomore V., Carpenter J., Ranjan M., Wilhelmsen K., et al. Ultrasound blood-brain barrier opening and aducanumab in Alzheimer's disease. N Engl J Med. 2024;390(1):55–62. doi: 10.1056/NEJMoa2308719. [DOI] [PubMed] [Google Scholar]
- 75.Reiman E.M., Cummings J.L., Langbaum J.B., Mattke S., Alexander R.C. A chance to prevent Alzheimer's disease sooner than you think. Lancet Neurol. 2024;23(2):144–145. doi: 10.1016/S1474-4422(23)00508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aisen P., Bateman R.J., Crowther D., Cummings J., Dwyer J., Iwatsubo T., et al. The case for regulatory approval of amyloid-lowering immunotherapies in Alzheimer's disease based on clearcut biomarker evidence. Alzheimers Dement. 2025;21(1) doi: 10.1002/alz.14342. [DOI] [PMC free article] [PubMed] [Google Scholar]