Summary
We present the clinical results of a phase 2 trial combining neoadjuvant docetaxel, cisplatin, 5 Flourouracil, and the PD-L1 inhibitor avelumab in locally advanced gastro-esophageal adenocarcinoma (GEA). Fifty-one patients receive neoadjuvant therapy with 50 proceeding to surgery. Grade 3–4 adverse events occur in 40%; complete/major pathological response is found in 7/50 (14%) and 9/50 (18%), with 2-year disease-free survival of 67.5%. There is no correlation between tumor regression and PD-L1 or mismatch repair (MMR) status. Multiplex immunohistochemistry and longitudinal single-cell transcriptomic profiling reveal alterations in certain innate immune cell populations, particularly noting an M2-tumor-associated macrophage (M2-TAM) proliferation in non-responding tumors. These findings describe the effective nature of this treatment regimen for GEA and reveal associated features of the inflammatory milieux associated with response to chemo-immunotherapy. The specific character of the inflammatory environment in non-responders may, in the future, help personalize treatment. This study was registered at ClinicalTrials.gov (NCT03288350).
Keywords: gastroesophageal adenocarcinoma, surgery, chemotherapy, immunotherapy, single cell transcriptomics, spatial proteomics, tumor associated macrophage, immune microenvironment, pathologic response
Graphical abstract

Highlights
-
•
Neoadjuvant avelumab and DCF are safe and efficacious in this group of patients
-
•
pCR occurs in 14% of patients, and 2-year DFS is 67.5%
-
•
Pre-treatment M2-TAM proliferation is associated with poor treatment response
-
•
MIF and CD86 expression among M2-TAMs may explain this trend
In this non-randomized trial of neoadjuvant docetaxel, cisplatin, fluorouracil, and avelumab for locally advanced esophageal adenocarcinoma, Alcindor et al. find adverse events, complete pathological response, and two-year disease-free survival among 40%, 14%, and 67.5% of patients, respectively. M2-tumor-associated macrophage proliferation occurs among non-responders suggesting a potential mechanism of treatment resistance.
Introduction
Gastro-esophageal cancer ranks seventh in incidence and sixth in mortality among cancers worldwide with gastro-esophageal adenocarcinoma (GEA) being the most common subtype in the Western hemisphere.1 For patients with locally advanced GEA, randomized data have shown that neoadjuvant treatment offers better survival outcomes compared with surgery alone.2,3 Although neoadjuvant chemoradiotherapy is a suitable option, as evidenced by the FLOT4 trialneoadjuvant docetaxel-based chemotherapy has rapidly become a predominant standard of care.4 However, despite the improved efficacy of neoadjuvant docetaxel, approximately 40% of primary lesions will fail to respond to systemic treatment.5
Following the documented survival benefit associated with the addition of immune checkpoint inhibition to chemotherapy in recurrent or metastatic GEA,6,7,8 support for this therapeutic modality in the neoadjuvant setting is emerging. Both single-arm and randomized trials have demonstrated that the addition of neoadjuvant anti-PD-L1 therapy was associated with impressive rates of major pathological response (MPR) and pathological complete response (pCR) of 33%–70% and 15%–45%, respectively.9,10 However, more conservative pCR rates of between 3.7% and 11.5% associated with other anti-PD-L1 or with anti-PD-1 agents suggest that the ideal regimen has yet to be defined.11,12,13 This is also reflected in survival outcomes as neoadjuvant pembrolizumab failed to improve event-free survival when combined with cisplatin-based chemotherapy in the KEYNOTE-585 trial.14 In comparison, adjuvant nivolumab improved disease-free survival (DFS) following neoadjuvant chemoradiotherapy and esophagectomy in the CheckMate 577 trial.15
Avelumab (healthcare business of Merck KGaA, Darmstadt, Germany) is an intravenously administered, fully human anti-PD-L1 immunoglobulin G1 monoclonal antibody. By blocking the intercellular interaction between PD-L1 on tumor cells and PD-1 on T cells and other immune cells, immunosuppression is curtailed and effector T cells lysis of tumor cells encouraged.16 Although the survival benefit of avelumab has been demonstrated in patients with advanced urological and dermatological malignancies,17,18,19 its role in GEA is underexplored, particularly in the neoadjuvant setting.
Here we report the results of a single-arm phase 2 trial investigating safety, efficacy, and immunological correlations of neoadjuvant docetaxel, cisplatin, and 5FU combined with avelumab (aDCF) in patients with resectable, locally advanced GEA. The primary objective of this study was to describe the association of preoperative aDCF with pCR as this outcome has been linked to excellent survival in other trials of chemo-immunotherapy. The secondary objectives were to describe the two-year DFS and incidence of grade 3/4 adverse events (AEs) associated with this specific regimen. A post hoc analysis of the tissue inflammatory microenvironment with a specific focus on M2 tumor-associated macrophages (M2-TAMs) is also described.
Results
Patient and tumor characteristics
A flow diagram of patient inclusion into the study is displayed in Figure 1. Between February 2018 and April 2022, 51 patients were enrolled. One patient withdrew consent to partake in the study following the administration of neoadjuvant aDCF, leaving an effective population of 50 who underwent surgery. The demographic, clinical, and pathological data of the 51 patients enrolled in this study are described in Table 1. With a median age of 64 years (range 18–79), 45 (88%) were males. There were 35 (69%) with an ECOG (Eastern Cooperative Oncology Group) performance status of 0, and the median Charlson comorbidity index was 4 (2–7). Most of the tumors were in the esophagus or gastro-esophageal junction (Siewert I/II = 41/51, 80%) with 5/51 (10%) in each of the cardia and subcardia/stomach. Eighty-eight percent of patients have cT3 lesions, and 61% were clinically lymph node positive (cN+) on pre-treatment staging investigations. Signet ring cells were found in 10/51 (20%) of patients, and 26/50 (51%) had poorly differentiated or undifferentiated lesions.
Figure 1.
A CONSORT diagram showing patient inclusion in the study
Fifty-one patients were enrolled of which 50 underwent surgery. There were 37 patients who continued to receive adjuvant therapy.
Table 1.
Demographic, clinical, and tumor characteristics of the 51 patients enrolled in the study
| Variable | N/median (%/range) N = 51 |
|---|---|
| Age (years) | 64 (18–79) |
| Gender | |
| male | 45 (88%) |
| female | 6 (12%) |
| ECOG status | |
| 0 | 35 (69%) |
| 1 | 16 (31%) |
| Dysphagia | |
| 0 | 14 (27%) |
| 1 | 21 (41%) |
| 2 | 14 (27%) |
| 3 | 2 (4%) |
| Primary tumor site | |
| esophagus/gastroesophageal junction (I, II) | 41 (80%) |
| gastroesophageal junction III/cardia | 5 (10%) |
| gastric/subcardia/stomach | 5 (10%) |
| Clinical tumor stage | |
| T1b | 1 (2%) |
| T2 | 2 (4%) |
| T3 | 45 (88%) |
| T4 | 3 (6%) |
| Clinical nodal stage | |
| N0 | 20 (39%) |
| ≥N1 | 31 (61%) |
| Histological grade | |
| well differentiated | 3 (6%) |
| moderately differentiated | 22 (43%) |
| poorly differentiated | 26 (51%) |
| Signet ring features | 10/51 (20%) |
| Barrett’s esophagus | 26 (51%) |
| Charlson comorbidity index | 4 (2–7) |
| Median follow-up (months) | 26.5 (5–74) |
Dysphagia scores: 0 = no dysphagia: able to eat normal diet, 1 = moderate passage: able to eat some solid foods, 2 = poor passage: able to eat semi-solid foods, 3 = very poor passage: able to swallow liquids only.
A total of 49/51 (96%) patients received all 4 preoperative cycles of aDCF with two patients not completing neoadjuvant treatment due to serious AEs. One patient suffered from pneumonia (grade 4), and another from immune-related hepatic failure and sepsis (grade 3).
Safety and feasibility
The data for the 51 patients included in the safety-evaluable analysis are presented in Table S1. Using the National Cancer Institute-Common Toxicity Criteria for Adverse Events version 3.0 (CTCAE),20 grade 3/4 treatment-related AEs occurred in 20/50 (40%) of patients. The most common grade 3–4 AE was neutropenia (20/51, 20%). Common grade 1–2 side effects included fatigue (75%), alopecia (33%), and constipation (29%). In four cases, dose reductions of the neoadjuvant therapy were required. This included 5FU for grade 3 diarrhea (1/51) and grade 3 oral mucositis (1/51), docetaxel for grade 2 fatigue (1/51), and cisplatin for grade 3 hypomagnesemia (1/51). The administration of neoadjuvant aDCF was delayed in 17 patients due to grade 3–4 neutropenia (10/51, 20%), grade 2 diarrhea (1/51), grade 1–2 arthralgia (5/51), and grade 3 myalgia (1/51). There was no mortality secondary to treatment with aDCF.
Surgical outcomes
After preoperative immunochemotherapy, no radiological disease progression was observed on the preoperative restaging investigations, and all 50 patients proceeded to surgery. The type of operative procedure, incidence of postoperative complications, and data regarding the adjuvant arm of systemic therapy are described in Table S2. For the 50 patients who underwent surgery, the median time between finishing treatment and surgery was 53 days (22–158 days). The most common surgical procedure performed was an Ivor Lewis esophagectomy (40/50, 80%) followed by total gastrectomy (4/50, 8%) and left thoraco-abdominal esophago-gastrectomy, McKeown esophagectomy, and subtotal gastrectomy (2/50, 4% each). There were 14/50 patients with Clavien-Dindo postoperative complications ≥3 within 30 days of surgery with the most common complications being anastomotic leak/conduit necrosis (9/50, 18%) and aspiration pneumonia (7/50, 14%) with 7/50 (14%) patients requiring reoperation. The 30-day mortality rate was 0%, although one patient died 47 days after surgery from septic complications resulting in a 90-day mortality rate of 2%. An R0 resection was achieved in 48/50 (96%) patients. The median lymph node yield was 36 (13–78) while the median number of positive lymph nodes was 1 (0–21).
There were 30/50 patients (60%) who initiated adjuvant therapy and received at least 2 cycles, and 24/50 (48%) completed all 4 cycles of adjuvant aDCF. All 50 patients in the efficacy population had surgery with curative intent.
Efficacy and association of putative biomarkers of response
Pathological response results are detailed in Table S3. Using the College of American Pathologists protocol (version 4.1) and the Modified Ryan Scheme for tumor regression score,21 seven patients (14%) showed pCR (tumor regression grade, TRG 0) and two (4%) had near-complete pathological response with microscopic residual disease (TRG 1). Therefore, a total of 9/50 (18%) had an MPR (TRG 0/1). Moderate response (TRG 2) occurred in 16/50 (32%) and poor response (TRG 3) occurred in 25/50 (50%).
A mismatch repair protein deficiency (MMRd) was found in 8/47 (17%) of samples tested, and two of these patients (25%) had pCR. There were 26/50 (52%) of patients with ypT3 disease and 48%, 26%, 12%, and 14% with ypN stage 0, 1, 2, and 3, respectively. With regards to PD-L1 status, 27/45 (60%) patients had a combined positive score (CPS) ≥ 5, and 17/45 (38%) had CPS ≥10. HER2 positivity was found in 3/46 (7%) patients. As shown in Table 2, none of these biomarkers correlated with the grade of pathological response. Using a conservative cutoff of 70% for Tumor Proportion Score (TPS) expression, based on data comparing immunohistochemistry (IHC) assays and data among patients with lung cancer,22 no association with pathological response was found.
Table 2.
Distribution of patients based on tumor regression grade and pathological and radiological features
| Pathological complete and major pathological response N = 9 |
Non-major pathological response N = 41 |
p valuea |
|||
|---|---|---|---|---|---|
| TRG 0 N = 7 |
TRG 1 N = 2 |
TRG 2 N = 16 |
TRG 3 N = 25 |
||
| Histological grade: well differentiated moderately differentiated poorly differentiated |
1 (14.3%) 1 (14.3%) 5 (71.4%) |
0 (0.0%) 1 (50.0%) 1 (50.0%) |
0 (0.0%) 9 (56.3%) 7 (43.7%) |
2 (8.0%) 10 (40.0%) 13 (52.0%) |
0.376 |
| PDL1 (CPS) <1 1≥ to <5 ≥5 to <10 ≥10 unknown |
0 (0.0%) 2 (28.6%) 2 (28.6%) 2 (28.6%) 1 (14.3%) |
0 (0.0%) 2 (100.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
0 (0.0%) 1 (6.3%) 4 (25.0%) 9 (56.3%) 2 (12.5%) |
4 (16.0%) 9 (36.0%) 4 (16.0%) 6 (24.0%) 2 (8.0%) |
0.439 0.943 0.751 0.341 |
| PDL1 (TPS) low (<70) high (≥70) unknown |
6 (85.7%) 0 (0.0%) 1 (14.3%) |
2 (100.0%) 0 (0.0%) 0 (0.0%) |
13 (81.2%) 1 (6.3%) 2 (12.5%) |
23 (92.0%) 0 (0.0%) 2 (8.0%) |
>0.999 |
| MMR status preserved deficient unknown |
4 (57.1%) 2 (28.6%) 1 (14.3%) |
2 (100.0%) 0 (0.0%) 0 (0.0%) |
12 (75.0%) 3 (18.8%) 1 (6.3%) |
23 (92.0%) 3 (12.0%) 1 (4.0%) |
0.601 |
| HER2 status positive negative unknown |
0 (0.0%) 5 (71.4%) 2 (28.6%) |
1 (50.0%) 1 (50.0%) 0 (0.0%) |
0 (0.0%) 15 (93.8%) 1 (6.3%) |
2 (8.0%) 22 (88.0%) 1 (4.0%) |
0.398 |
| Change in SUVmax >35% <35% |
5 (71.4%) 2 (28.6%) |
1 (50.0%) 1 (50.0%) |
13 (81.2%) 3 (18.8%) |
14 (56.0%) 11 (44.0%) |
>0.999 |
Comparing MPR vs. non-MPR status.
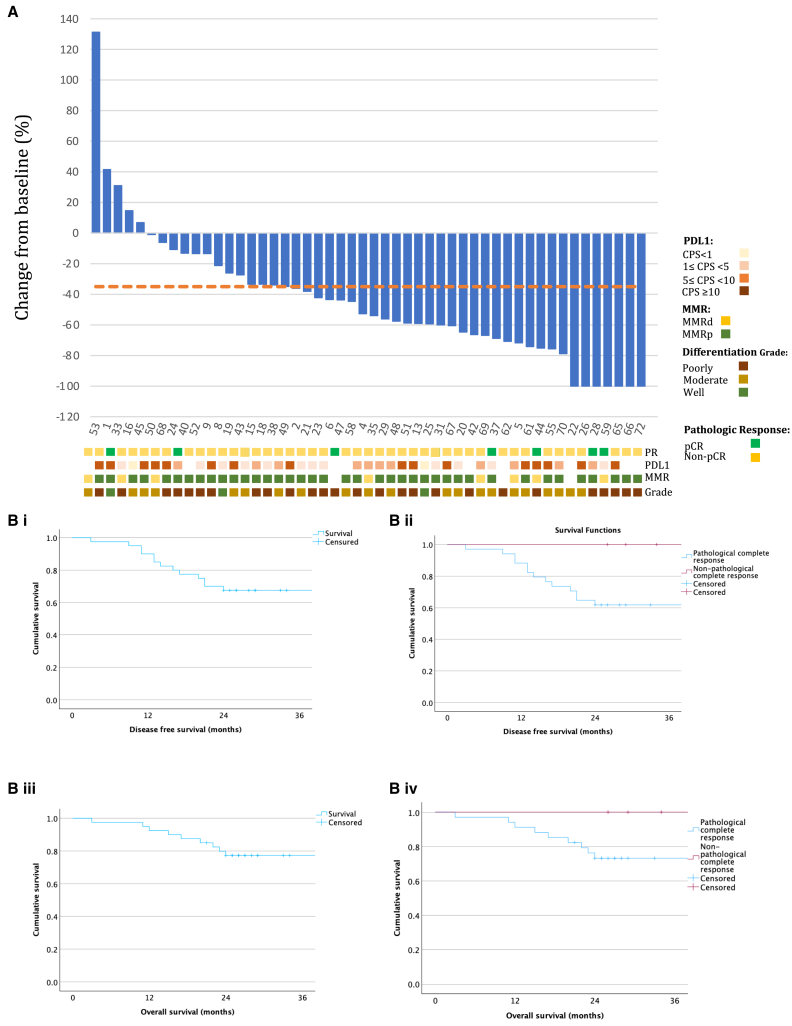
Figure 2 displays a waterfall plot of objective FDG ((18F)flourodeoxyglucose)-PET (Positon Emisssion Tomography) response as defined by the percentage change in the maximum standardized update value (SUVmax) between the pre- and post-treatment scan. The majority of patients had a reduction in SUVmax after neoadjuvant aDCF. In keeping with previous literature of a clinically relevant PET-CT response among patients treated with neoadjuvant chemotherapy,23 a decrease of at least 35% in the maximum SUVmax was noted in 33/50 patients (66%). However, this reduction in maximum SUVmax was not associated with pathological response (Table 3).
Figure 2.
Stratified radiological and survival outcomes of the cohort
(A) A waterfall plot showing the change in the maximum standardized uptake values (SUVmax) correlated with pathological response, PD-L1 status, MMR status, and grade of differentiation for the entire cohort (n = 50).
(B) (i) Kaplan-Meier plot of DFS for the cohort as a whole (median DFS not met) and (ii) stratified by pCR status until (median DFS not met, log rank [Mantel-Cox] = 0.091) 36 months after histological confirmation. Similar plots were created for OS for the cohort as a whole (iii) (median OS not met) and stratified by pCR status (median OS not met, log rank [Mantel-Cox] = 0.175).
Survival outcomes
Between the date of histological diagnosis and the closure of data collection on the 16th of May 2023, the median follow-up for the cohort was 26.5 months (5–74). The median OS for the cohort was not met. The two-year DFS and OS for the entire cohort were 68% (27/40 patients) and 78% (31/40), respectively. The median time to disease recurrence was 13 months (range 7–44). There was a trend toward improved two-year DFS and OS when stratifying the cohort by pCR status (6/6, 100% vs. 21/34, 62%, p = 0.091 and 6/6, 100% vs. 25/34, 74%, p = 0.175, respectively) (Figures 2Bi–2Biv). Conversely, MPR status was associated with a significant improvement in 2-year DFS (8/8, 100% vs. 19/32, 59%, p = 0.029) (Figure S2i). A trend toward improved two-year OS when stratifying according to MPR status was noted (8/8, 100.0% vs. 23/32, 72%, p = 0.100) (Figure S2ii). OS was also not affected by mismatch repair (MMR) status (Figure S2iii).
Multiplex immunohistochemistry and spatial analysis: Link between pathological response and altered immune cell density
To investigate how aDCF influences the dynamic behavior and spatial distribution of immune cells, we gathered tissue specimens at various time points during treatment. Pre-treatment biopsies referred to treatment-naive samples while mid-treatment biopsies were retrieved endoscopically after the completion of 2 of the 4 rounds of neoadjuvant aDCF. Conversely, the post-treatment samples were taken from the surgical specimen at the time of resection. For the purposes of this post hoc analysis, and due to the relatively small number of patients with pCR, samples with MPR (TRG0/1) were compared with moderate responders (TRG2) and poor responders (TRG3). Once stratified by pathological response, CD8+ T cell, M2-TAM, and cytotoxic T lymphocyte (CTL) cell density (cells/mm2) were determined in pre-treatment and post-treatment samples. There were 42 pre-treatment and 36 post-treatment specimens available for multiplex immunohistochemistry (mIHC) spatial analysis. As shown in Figure 3A, a representative selection of composite OPAL-6-stained microphotographs of paired patient-matched pre-treatment and post-treatment samples was used to assess changes in the tumor immune microenvironment in relation to treatment response.
Figure 3.
Multiplex imaging density and proximity analyses stratified by response to neoadjuvant therapy
(A) Seven-colored composite microphotographs (20X, scale: 50 μm) of representative paired treated and untreated sections of MPR, TRG2, and TRG3 responders (left to right column).
(B) (i) The results of a spatially resolved analysis of mIHC-defined immune markers using Halo software. Density of M2-TAM (CD68+CD163+), potential regulatory T cells (TREG Foxp3+), CD8+T cells, and CTLs (CD8+granzymeB+) was measured stratified by TRG (TRG0/1 n = 7, TRG 2 n = 11, and TRG3 n = 18). A significant reduction in the percentage change in M2-TAM density was associated with MPR (two-way ANOVA, Tukey’s correction for multiple comparison, p = 0.038). Though non-significant, increment in mean changes in CTLs and CD8+T cells densities was correlated with favorable outcome. All boxplots are shown with the horizontal line denoting the median, vertical line denoting the 95% confidence interval, and “+” denoting the mean. (B) (ii) Spatial analysis of the measured immune cells indicated a predictive role of intra-tumoral M2-TAM content in pre-treatment gastro-esophageal cancer tissues stratified by TRG (TRG0/1 n = 7, TRG 2 n = 13, and TRG3 n = 21). The mean density of pre-existing, intra-tumoral (infiltrated) M2-TAM among patients with MPR was significantly higher compared to that of poor responders (TRG3) (unpaired t test with Welch’s correction, p = 0.024). (B) (iii) Calculation of the average distance of M2-TAM from the tumor core stratified by TRG (TRG0/1 n = 7, TRG 2 n = 13, and TRG3 n = 21). M2-TAMs were found to be significantly further from tumor cells present in TRG0/1 patients’ specimen compared to that of TRG3 patients (Welch’s unpaired two tailed t test, p = 0.005.
(C) Forty-two formalin-fixed paraffin-embedded (FFPE) specimens from pre-treatment biopsies were available. Patients were arranged (left to right) in a heatmap based on their pathological responses. Clinical and post-treatment TNM stages, tumor location, objective response to treatment, and survival were also stratified. High or low subgroups of M2-TAM, TREG, and CD8+T cells were determined using cohort medians. Analysis revealed that 22 out of 42 patients harbor high pre-existing M2-TAM (>median = 390.8 cells/mm2). In total, 63% of these patients had ≥500 M2-TAM/mm2, and 93% of such patients were pathological non-responders (TRG2 and TRG3). Similar analysis for potential TREG and CD8+T cells was conducted and also displayed in the heatmap (last 3 rows).
The mean percentage changes in these cell densities (cells/mm2) are presented in Figure 3Bi and Table S4i. When compared to baseline M2-TAM density on the treatment-naive sample, aDCF treatment was associated with reduced M2-TAM density in all MPR patients’ resection tissues. Presented as mean with the 95% confidence interval (CI), a significant difference was noted in the mean percentage reduction of M2-TAM density between pre- and post-treatment MPR samples when compared to the same of TRG3 samples (in MPR 70% reduction, 95% CI −87 to −54 vs. in TRG3 102% increase, 95% CI −106 to 318, two-way ANOVA, Tukey’s correction for multiple comparison, p = 0.038).
A general increase in the total density of CD8+ T cells during neoadjuvant treatment was observed with improved pathological response (Figure 3Bi). Similarly, a trend toward a reduction in the mean percentage increase of total CD8+ T cell density when comparing MPR with TRG 3 was also noted (304% increase, 95% CI 68 to 675 vs. 105% increase, 95% CI 0.4 to 210).
Prognostic spatial distribution of pro- and anti-tumor immune cells in treatment-naive specimens
Differences in the spatial distribution of selected immune cells relative to the tumor core were analyzed using Halo. The tumor core was defined by an expert pathologist (P.-O.F.) on the hematoxylin and eosin slide of the biopsy specimen and confirmed by staining positively for CK7. Once stratified by pathological response, the densities of intra-tumoral M2-TAM (CD68+CD163+), CTLs (CD8+GNZB+), and TREG (Foxp3+) were calculated in the pre-treatment samples (Figure 3Bii; Table S4ii). All samples from patients that would subsequently have an MPR following treatment had an M2-TAM density of <500 counts/mm2 within tumor cores on their corresponding treatment-naive biopsy. The intra-tumoral M2-TAM density of MPR treatment-naive samples was significantly less than that of TRG3 samples (212.8 counts/mm2, 95% CI 116.2–452.9 vs. 397.0 counts/mm2, 95% CI 180.3–780.0, Welch’s unpaired two tailed t test, p = 0.024). Only 1/14 (7.1%) patient with an M2-TAM density in the top quartile (>500 per μm2) had an MPR whose lesion was also MMRd (Figure 2). As noted in Figure 3C, there seemed to be no difference between the incidence of M2-TAM density >500 cells/mm2 when comparing patients with, and without, clinical lymph node metastases (8/19 cN+ versus 11/22 cN−).
Taking advantage of the spatial resolution data, proximity analysis (see method details) was performed to quantify numbers and average distance of M2-TAM, TREG, and CTLs within a 50 μm proximity of tumor cells in treatment-naive specimens (see Figure S3). M2-TAMs were significantly closer to tumor cells in patients with TRG3 compared to MPR samples (Figure 3Biii, 15.2 μm, 95% CI 12.87–18.9 vs. 24.2 μm, 95% CI 19.78–35.05, Welch’s unpaired two tailed t test, p = 0.005). This suggests a mitigation of the response to neoadjuvant aDCF if M2-TAMs are accumulated in greater numbers and closer proximity to tumor cells. Overall, the data implied a potential for increased intercellular communication between immune-suppressive M2-TAM and cancer cells in tumors that responded poorly to neoadjuvant aDCF.
To further assess the relationship between M2-TAM, TREG, and CTL subpopulations, a nearest-neighbor analysis was also performed on treatment-naive specimens (Table S3iii). There was an inverse relationship between the proximity of FoxP3+ TREG and M2-TAM such that poorer response was associated with closer cell-cell proximity (Spearman correlation r = 0.968, p = <0.001–0.007). Conversely, for CTL, a constant proximity to M2-TAM was maintained irrespective of subsequent disease response.
A diagram proposing a prognostic scheme for aDCF treatment based on the intra-tumoral density and proximity to tumor cells of M2-TAM, CTL, and TREG is shown in Figure S4.
Single-cell transcriptomics of biopsy samples
We obtained fresh tissue samples from 11/50 patients for single-cell RNA transcriptomic sequencing (scRNA-seq) on the 10× Genomics Chromium platform. This yielded a total of 20 samples, 10 pre-treatment samples, 5 mid-treatment samples, and 5 post-treatment samples. Stratified by pathological response, there were 2 TRG1 samples, 4 TRG2 samples, and 14 TRG3 samples. All samples were confirmed histologically to have been taken from an area that contained esophageal adenocarcinoma (EAC) by pathological assessment. After quality control (see methods), 44,118 cells with usable transcriptomes were identified. To align the scRNA-Seq analysis with the IHC results, we focused on identifying CTL, M2-TAM, and TREG cells, based on the same markers as used for the IHC. Thus, after batch correction and dimension reduction of cell transcriptomes (see methods) and mapping each cell to a 2D plane (YMAP), we labeled the cells for these three cell types (Figure 4A), which encompassed 5,237 cells, with 2,702 CTLs (CD8+GZMB+), 2,105 M2-TAMs (CD68+CD163+/CD206+), and 425 TREGs (CD4+FOXP3+). The top 10 differentially expressed genes in each of these cell types were identified with a view to further describe the transcriptomic identity and function of these clusters (Figure 4B). For instance, C1QA, which has been associated with cancer progression and T cell exhaustion, was expressed by the M2-TAM among many samples.
Figure 4.
Single-cell analysis highlighting differential gene expression and cellular distribution among specific clusters of interest
(A) A series of UMAPs showing the spatial reduction of cells included from the 20 samples in the scRNA-seq analysis including (i) stratified by cell types, (ii) time in the treatment cycle, (iii) sample number, (iv) response to neoadjuvant therapy, and (v) cell type allocation: CTL (CD8+GZMB+), TREG (CD4+FOXP3+), and M2-TAM (CD68+CD163+/CD206+) cells from all collected samples (n = 20).
(B) A heatmap showing the top 10 genes (weighted by lowest adjusted p value) per cluster of M2-TAM, TREG, and CTL cell clusters among all samples (n = 20).
(C) A bar plot showing the percentage distribution of M2-TAM, TREG, and CTL cells stratified by sample, response to treatment, and stage in the treatment cycle (treatment pre n = 10, treatment mid n = 2, and treatment post n = 5) with the corresponding number of cells per sample.
(D) A transposition of the same data showing the combined cell distribution across all samples stratified by cell type and response to therapy.
Location of these three cell types on the UMAP space relative to all the cell types is presented in Figure S5i–S5iii. When these maps were further stratified by response to neoadjuvant therapy (Figure S6), it becomes evident that the CTL cells of samples of the TRG3 response group had transcriptomes distinct from those of the TRG1 sample (Figure S7). The absolute cell counts stratified by sample and pathological response are shown in Table S5. Motivated by our findings in IHC of differential cell type abundance across response groups, we first examined the changes in cell type proportions over the course of treatment. The changing cell proportions are displayed in Figure 4C, including the absolute cell count of each sample. The percentage of M2-TAM within TRG1 pre-treatment samples appears to be lower with a higher proportion of CTL in comparison to treatment-naive TRG2/3 samples. This is in keeping with the finding of M2-TAM enrichment in non-responders described earlier on immunohistochemistry. We note that the bulk of the cells in the analysis originated from post-treatment samples in keeping with the higher tissue yield from the resection specimens (Figure 4D).
Characterizing transcriptomes of M2-TAM of poor responders
Considering the association with the changing density and proximity of M2-TAM seen on the spatial analysis earlier and pathological outcome, the changing transcriptomes in the M2-TAM throughout the treatment cycle of TRG3 samples was then explored. A comparative analysis with MPR samples was not possible since no MPR post-treatment samples were available for analysis because of lack of a residual tumor mass.
When considering TRG3 patients only, the bulk of the cells in the analysis originated from post-treatment samples most likely because these samples were harvested from the surgical specimen rather than endoscopic biopsy (Figure 5A). Therefore, to correct for vastly different cell numbers, gene expression analysis was performed as a pseudobulk (summing of all the cells followed by normalization for cell number) for comparing treatment-naive and post-treatment TRG3 samples. Differential expression analysis with differential expression analysis (DESeq) is shown in the volcano plot in Figure 5B. After filtering (see method details), 49 differentially expressed genes remained that were statistically significant with an adjusted p value of <0.05. The heatmap displaying the differential expression of these genes in the M2-TAM cells in TRG3 samples throughout the treatment cycle is displayed in Figure 5C. There were 3 patients for whom samples at each time point were available. The top 10 differentially expressed genes, based on adjusted p value, from the analysis of the cohort as a whole were identified, and the log-normalized count of these genes plotted for each of these three patients stratified by the time the biopsy was taken (Figure S8). These data suggest that the transcriptomic profile found among patients with TRG3 was present throughout the treatment cycle including within treatment-naive specimens.
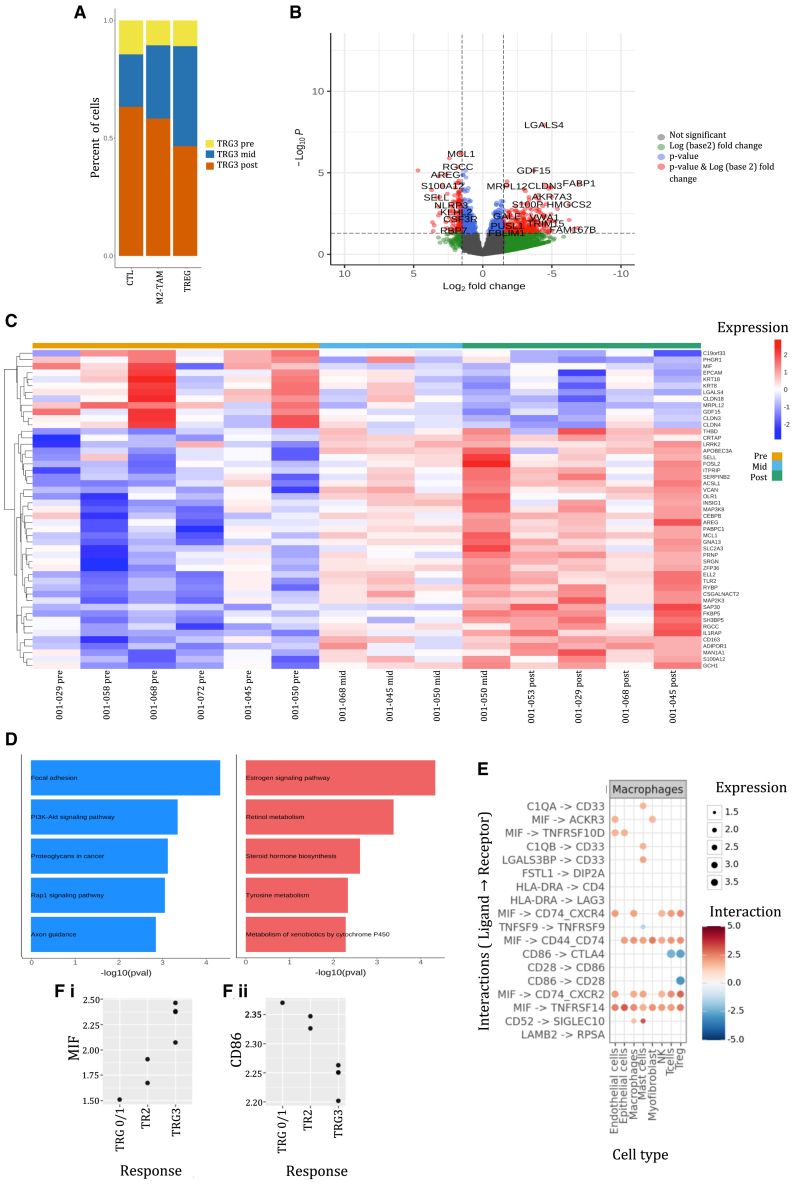
Figure 5.
Exploring single-cell characteristics of M2-TAMs among poor responders to neoadjuvant therapy
(A) For patients with a TRG3, the number of cells allocated to the M2-TAM, TREG, and CTL clusters was summated and the percentage distribution calculated at each time point in the treatment cycle (treatment pre, n = 6, treatment mid, n = 3, and treatment post n = 5).
(B) A volcano plot of an unmatched comparison of differentially expressed genes among M2-TAM (CD68+CD163+/CD206+) cells of patients with TRG3 lesions between the treatment pre (n = 6) and treatment post (n = 5) samples using a LogFold change cutoff of +/−1.5 and p value of <0.05.
(C) A heatmap of differentially expressed genes of M2-TAM (CD68+CD163+/CD206+) among patients with TRG3 lesions, comparing treatment pre (n = 6) and treatment mid (n = 3) with treatment post (n = 5) with a LogFold change cutoff of +/−1.5 and p value of <0.05.
(D) Functional enrichment pathways of differentially expressed genes of treatment-naive versus post-treatment TRG3 samples with upregulated (blue) and downregulated (red) genes.
(E) A plot demonstrating ligand-receptor differential gene expression between M2-TAM and other TIME cell lineages among TRG3 samples (n = 14).
(F) A dot plot comparing (i) MIF and (ii) CD68 expression among M2-TAMs stratified by TRG status (MPR n = 1, TRG 2 n = 2, and TRG 3 n = 3).
Treatment-naive TRG3 M2-TAMs were enriched with proinflammatory and pro-migratory genes that have been associated with poor survival in cancer (C19orf33, migration inhibitory factor [MIF], MRPL12, GDF15, CLDN3, and CLDN4). In comparison, post-treatment samples were enriched in genes for macrophage infiltration (CRTAP and SELL), activation of inflammasome complexes and secretion of cytokines (IL1RAP, LRRK2, and FOSL2), malignant cell infiltration and proliferation (ADIPOR1, SAP30, RGCC, and FKBP5), and antioxidant properties that reduce oxidative stress and prevent cellular death (GCH1). The enrichment profile of treatment-naive vs. post-treatment TRG3 samples was further interrogated using DEnrichPlot. This revealed upregulation of adhesion proteins and signaling pathways including P13K-Akt, Rap1, and Ras. Conversely, estrogen and retinol metabolism pathways were downregulated (Figure 5C).
Pathological response-associated variation in intercellular communication
Finally, on the limited set of samples available, we assessed cell-cell communication between cell types using the expression of ligands and their cognate receptors in distinct cell type patterns in scRNA-seq data. Specifically we asked whether cell-cell interaction pairs were altered between the response groups. Using the CellPhone algorithms,24 we identified 53 differentially expressed ligand-receptor pairs with a majority involving M2-TAM (Figure 5E). Many of these altered communication channels were driven by MIF and were enriched in M2-TAM of TRG3 samples (Figure 5Fi), suggesting increased signaling between macrophages and all other cell types. Of note, a transcriptional signature suggestive of activation of an intercellular signaling axis based on MIF (on macrophages) and CD74 (on TREG) was found. This has been implicated in promoting tumor-infiltrating TREG and modulating response to checkpoint inhibitors and was enhanced in the TRG3 samples.25 Conversely, we detected a decreased interaction of the M2-TAM and T cells via CD86 (on macrophage) and the coactivator CD28 (on T cells) in the TRG3 samples (Figure 5Fii) based on the reduction of both respective transcripts in these cell types. This reduction of the T cell co-stimulatory receptor molecule CD28 engagement on T cells, including TREG, by its ligand CD86 on macrophages suggested a global lowering of T cell activation. While this seems to contradict the observed accumulation of TREG, it may be opposed by the decreased communication of CD86 with the negative regulator CTLA-4 in these non-responding patients, thereby unleashing the feedback control of TREG population.26
In summary, scRNA-seq findings replicated the enrichment in the poor responders (TRG3 samples) of TREG and M2-TAM, as observed in IHC. In addition, cell-cell communication network analysis supports the concept of a mutual interaction between M2-TAM and TREG that promotes tumor-infiltrating TREG and may contribute to immunosuppression and dampen the response to immune checkpoint inhibitors.27,28
Discussion
In this study, we have shown the feasibility of combined immune checkpoint inhibitor and chemotherapy (aDCF) in the treatment of GEA and favorable oncological efficacy. The study reached its statistical primary endpoint in terms of achieving pCR, thus corroborating the promising efficacy of aDCF as an experimental perioperative regimen for the treatment of locally advanced GEA. These results suggest an added benefit of combining the anti-PD-L1 monoclonal antibody, avelumab, with DCF since the 14% rate of pCR described here is higher than the 7% described with DCF alone as reported previously in our institutional cohort.29 Multiplex imaging analysis also suggests an association of the accumulation of M2-TAM and regulatory T cells with poor response to the combined therapy. Cell-cell communication network analysis afforded by scRNA-seq, which identifies putative pairs of interacting cell types based on expression of transcripts of ligands and their cognate receptor in the respective cells, also suggests that M2-TAMs, generally thought to be immune suppressive, represent a cell-cell communication hub, signaling to many stromal cells. In turn, this may account for a tumor immune microenvironment (TIME) that promotes immune evasion.
It is well established that tumor cell expression of PD-L1 interacts with PD-1 on T lymphocytes inhibiting their cytotoxic ability. This molecular interaction enables tumors to circumvent host immune surveillance. However, another critical immune checkpoint involves CTLA-4 that competes with CD28 for binding to CD80 and CD86 on antigen-presenting cells including macrophages. This interaction delivers co-stimulatory signals also essential for activating T lymphocytes. During immunosuppressive states, the greater affinity of CTLA-4 for CD80/CD86 is thought to downregulate the stimulatory effect of CD28.30 This explains why CTLA-4 inhibition, which removes the CD80/86 binding competition allowing proinflammatory CD28 upregulation, has been identified as a promising alternative treatment paradigm in gastrointestinal malignancies.31
Among the TRG3 samples presented here, we found reduction in CD86/CTLA-4 and CD86/CD28 interactions across various T cell populations, including TREG, and macrophages. These disruptions impair T cell proliferation and cytotoxic capabilities and builds on previous evidence describing the immunosuppressive environment found in the TIME of gastric cancer.32 Importantly, this “immune-cold” environment has been associated with poor response to systemic therapy and impaired survival outcomes among patients with locally advanced gastric adenocarcinoma.33 Although further research is needed on this topic, we suggest that this characterization of the TIME in part explains poor tumoral response to aDCF.
Additionally, the M2-TAMs found in poor responders were also found to have accentuated activation of the macrophage MIF-CD74 pathway. The release of the chemokine MIF by M2-TAM, which has been found during cancer progression, is thought to promote intra-tumoral accumulation of TREGs, which specifically overexpress CD74.34 In addition to promoting the polarization of macrophages into the M2 phenotype, MIF/CD74 interaction enhances the production of immunosuppressive molecules by M2-TAM and reduces their capacity to activate CTLs encouraging anti-tumor immunity. Importantly, M2-TAMs have been found to contribute to T cell dysfunction via the secretion of specific cytokines and metabolites affecting the therapeutic effect of anti-PD-L1 treatment.35,36 High M2-TAM concentration has also been associated with increased PD-L1 expression therefore providing another means of M2-TAM-derived resistance to anti-PD-L1 therapy.37,38 Our data also reveal that M2-TAMs interact with other elements of the TIME via interaction with TNFRSF14, a potent proinflammatory transmembrane protein expressed on multiple cells in the TIME. Downregulation of MIF interaction with members of the tumor necrosis factor superfamily has not been observed in the setting of neoadjuvant therapy resistance in gastrointestinal carcinoma.39
With regards to the safety of aDCF, the incidence of grade 3–4 treatment-related AEs of 40% described here was higher than the 20% described in both trial and real-world descriptions of other taxane-based regimens.4,40 While the incidence of neutropenia and diarrhea was similar to that described for flourouracil, leucovorin, oxaliplatin, docetaxel (FLOT), it is considerably less than the 78% of grade 3 or worse AEs found when combining perioperative FLOT with pembrolizumab in the KEYNOTE-585 trial.14 Interestingly, in this trial, the incidence of treatment-related complications did not vary between the arms implying that the AE profile is driven by the systemic chemotherapy and less so by the addition of immunotherapy. It is also interesting to note that, in comparison to the data presented here, only 33% of patients had grade 3/4 AEs when camrelizumab, apatinib, nab-paclitaxel, and S-1 were combined.10 Additionally, in this study too, the addition of immunotherapy did not seem to affect the incidence of treatment-related AEs. Therefore, we suggest that the side effect profile described here is in keeping with other studies based on taxane-based systemic therapy among this specific cohort of patients. Furthermore, whether adjuvant immunotherapy alone could offset AEs while still delivering potential pathological and survival benefits needs to be understood.
Despite these knowledge gaps, that 96% of patients recruited to the study completed all preoperative cycles and ultimately underwent surgery further highlights the feasibility of this regimen. Finally, the incidence of surgical complications among patients treated with aDCF was also equivalent to previously published data describing our institutional experience.41
Prospective data from both single-arm and randomized trials reveal a variation in the rate of pCR and MPR achieved. Among several single-arm phase 2 trials investigating the role of neoadjuvant chemotherapy and anti-PD-L1 therapy in the treatment of locally advanced GEA, pCR and MPR occur at rates between 17%–45% and 55%–70% respectively.9,42,43,44,45 Emerging data from several randomized trials confirm this trend. The recently published NEOSUMMIT-01, in which toripalimab was added to neoadjuvant SOX, described a pCR and MPR of 22.2% and 44.4%, respectively.46 The DANTE trial found that the addition of atezolizumab to FLOT was associated with a pCR of 24% vs. 15% (p = 0.032).47 Similarly, based on FLOT and durvalumab, the phase 3 MATTERHORN trial also described an increase in pCR (19% vs. 7%, p = <0.001) and MPR (27% vs. 14%, p = <0.001).48 Reflecting the greater efficacy of FLOT over alternative chemotherapeutic agents when pembrolizumab was added to cisplatin-based chemotherapy, pCR was more conservative (12.9% versus 8%, p = <0.001).14 Furthermore, when camrelizumab and apatinib were used in combination with nab-paclitaxel, a non-significant trend toward higher pCR was found (16.3% vs. 6.0%, p = 0.094).10
While an equivalent oncological efficacy has been described when comparing FLOT and DCF,41 the 14% pCR and 18% MPR found in the present cohort are lower than those of the docetaxel and anti-PD-L1 regimens described earlier. Despite these variations, it seems that survival outcomes among all patients who achieve pCR are similar irrespective of the regimen chosen. Moreover, when comparing the DFS and OS of the entire cohort to the aforementioned trials, the results are similar.
The intra-trial variation in the efficacy of different anti-PD-L1 therapies in terms of pathological response reflects, in part, the differing experience of the predictive value of pre-treatment PD-L1 expression in terms of response to treatment. For instance, a CPS >1 was associated with improved overall survival in patients with metastatic GEA treated with nivolumab or sintilimab in the CheckMate 6496 and ORIENT 1649 trials. In an exploratory analysis, using a similar cutoff data from the MATTEHORN study seems to show a positive relationship with pathological response. Data from the DANTE trial suggest that pCR was higher if the CPS score was ≥10. Similarly, MPR was doubled with a CPS > 5% among those treated with camrelizumab and apatinib10 and with toripalimab and chemotherapy.46
Conversely, CPS was not predictive of pathological or survival outcomes among recipients of neoadjuvant pembrolizumab and FLOT14 or atezolizumab when combined with neoadjuvant chemoradiotherapy.50 Specifically among patients with MMRd lesions, CPS > 1 was also not associated with pathological response following treatment with neoadjuvant tremelimumab and durvalumab.51 Despite the documented poor sensitivity of TPS in the setting of gastric cancer,52 even when a threshold of 1% is used to define positivity, the lack of correlation between CPS and TPS described in the current study further underscores the need to identify alternative biomarkers predictive of pathological response to anti-PD-L1 therapy with a view to personalizing treatment.53 Such a discovery is crucial as the achievement of pCR following the administration of combination therapy seems to be associated with improved survival outcomes.
By questioning the role of PD-L1 as a marker predictive of pathological response, efforts have focused on finding biological markers that predict treatment outcomes with a view personalizing treatment. Interest has turned toward prognostic features of the TIME, including M1/M2-TAM, which are closely associated with tumor progression and response to systemic therapy.54 Specifically, in the setting of gastric cancer, although a higher level of CD68+ macrophage infiltration has been found among responders to neoadjuvant treatment, response to PD-L1 blockage plus chemotherapy is characterized more specifically by an elevated ratio of M1 (CD68+CD11b+F4/80+CD206−) to M2 (CD68+CD11b+F4/80+CD206+) macrophages.55 The finding that there is a negative correlation between pro-tumoral M2-TAM density and response to therapy is well documented.56
The increased density of CD68+CD206+ M2-TAM in close proximity to tumoral cells has also been previously described, which in turn is associated with improved survivability of cancer cells.57,58 M2 polarization during co-culture with gastric cancer cells led to CXCL5 expression engendering chemoresistance via the activation of PI3K/AKT/mTOR pathways.59 Similar pathway upregulation was found in the pathway enrichment analysis described earlier. Unsurprisingly, the proximity of CD68+CD163+CD206+ T2-TAM to the tumor core has therefore been negatively associated with patient survival,60 particularly if there is PD-L1 enrichment of CD163+ M2-TAM.61 This is likely because M2-TAMs are thought to increase resistance to anti-PD-L1 therapy by binding to PD-1 and CTLA-4 on the surface of cytotoxic T cells, blocking the lymphocytes’ adaptive immune response and reducing the anti-cancer effect of immunotherapy.62 Chen et al. identified that increased CD68+STING+ density was negatively associated with objective response to anti-PD-L1 therapy among patients with gastric cancer.63 However, unlike in the results described in the present study, these findings were not correlated with pathological response.
We failed to find a relationship between MMRd and pathological response to treatment, a finding also described elsewhere.14,46 Understanding why there is a lack of association between MMRd status and favorable pathological response described in this cohort is challenging due to the limited data available comparing the outcomes of avelumab therapy in patients with MMRd versus MMRp lesions. In a limited study of patients with advanced endometrial cancer also treated with avelumab, the presence of MMRd was not associated with the incidence of objective response to treatment.64 Additional data from this study found that JAK1 and B2M mutations characterize MMRd lesions that failed to respond to avelumab.
This contrasts recent randomized data describing an enhanced pCR rate of 58.6% among MMRd patients treated with neoadjuvant nivolumab and ipilimumab as part of the NEONIPIGA study.65 Improved pathological response among MMRd lesions has also been described within other randomized trial and single-arm studies including the INFINITY trial.14,51,66 In patients with colorectal cancer, MMRd status has been associated with subtle changes in the TIME including a higher density of multiple infiltrating lymphocyte lineages,67 production of more neoantigens,68 and modulators of systemic inflammation.69 The enhanced inflammatory response inherent encompassing these tumors may explain why, in part, subgroup analyses of large cohorts of patients with early-stage GEA have reported no benefit in patients with MMRd tumors receiving chemotherapy alone.70 Although higher than in other recently published cohorts, the 14% incidence of MMRd within the study cohort is within the range of 4%–24% incidence within GEA described in a recent review.71 However, absence of samples prevented a single-cell analysis of this specific subgroup.
Considering the complexity of using the TIME to predict response to anti-PD-L1 therapy, interest has also turned to clinical tools that are more readily available. Changes in tumor metabolism on PET/CT have been demonstrated to be a strong predictor of pathological response following neoadjuvant chemotherapy.72 In the setting of esophageal squamous cell carcinoma, a similar relationship has also been described following treatment with neoadjuvant chemo-immunotherapy.73 Conversely, among patients with adenocarcinoma treated with neoadjuvant chemo-immunotherapy, changes in the SUV and total lesion glycolysis during treatment do not correlate with pathological outcomes with a high incidence of false negatives reported.9 The results presented here further highlight the limitation of this imaging modality among this specific group of patients in terms of discerning disease response to neoadjuvant therapy.
Taken together, these findings allude to the complex modulatory relationship that M2-TAMs have with the pathological response of GEA to neoadjuvant therapies. As such, the need to identify more robust and reliable biomarkers to guide precision treatment decisions and prognosticate response to neoadjuvant systemic therapy is desperately required.
Overall, the activity and safety of perioperative aDCF are promising, and we have alluded to the fundamental role that M2-TAM and TREG play in the microenvironment and the association with pathological response to neoadjuvant chemo-immunotherapy. While the survival outcomes of this study require longer-term data to robustly describe, we postulate that M2-TAM and TREG density and spatial location of the M2 cells are potentially useful in helping guide precision systemic chemo-immunotherapy in patients with locally advanced GEA.
Limitations of the study
There are some limitations to this study. The small sample size and the single-center and non-randomized nature of this study may affect the power of the study, particularly with regards to the high-dimensional data, notably due to the low availability of samples for single-cell analysis for patients with pCR. As a result, we were unable to compare between pathological responses and perform subset analysis on patients with MMRd and HER2 positivity. This is particularly pertinent considering that a number of patients in this trial were MMRd (n = 8), which has been associated with a distinct immune profile and response to immunotherapy. As such, the treatment and translational results for this specific cohort of patients may vary to those who are not MMRd. Unfortunately, we did not have a sufficient number of MMRd patients to extensively differentiate the immune microenvironment between MMRd and MMRp tumors. By combining cohorts with other studies, this comparison could be explored further in future work. The single-cell transcriptome data are further limited by the presence of some epithelial cell markers expressed within the identified clusters implying potential contamination at the sample processing stage. The limited availability of single-cell samples also precluded a thorough analysis of variables beyond TRG that may have affected elements of M2-TAM function. Extending to the density and spatial analyses, the limited number of patients with non-cT3 lesions prevented comparison within the TIME across lesions with varying depth of invasion. Selection and treatment bias are inherent in the study due to the single-center nature of the study. Finally, the immaturity of the survival data limits further analysis of survival outcomes stratified by variables identified in the analyses in this manuscript.
Resource availability
Lead contact
Requests for further information and resources should be directed to and will be fulfilled by the lead contact, Dr Lorenzo Ferri (Lorenzo.Ferri@mcgill.ca).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The RNA single-cell data have been deposited at the SRA repository as PRJNA1052389 and are publicly available as of the date of publication.
-
•
Multiplex imaging data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This research was financially supported by EMD Serono, Mississauga, Ontario, Canada, a business of Merck KGaA, Darmstadt, Germany, and was previously conducted under an alliance between the healthcare business of Merck KGaA, Darmstadt, Germany, and Pfizer. The authors also acknowledge the support of the McGill University Health Centre Foundation and the Montreal General Hospital Foundation, Canada. Some of the samples used in this study are based upon work supported by the Department of Defense, U.S. Army Medical Research Acquisition Activity, under grant number W81XWH-21-0623 (CA200572).
The funding source did not have any role on data analysis or interpretation of results. The finding source did not assist with drafting of the manuscript.
The healthcare business of Merck KGaA, Darmstadt, Germany, and Pfizer reviewed the manuscript for medical accuracy only before journal submission. The authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors.
Author contributions
Conceptualization: T.A., J.T., P.-O.F., S.P., M.S., N.B., S.B., S.H., V.S., and L.F. Methodology: T.A., J.T., P.-O.F., S.P., T.O., M.S., N.B., D.Z., C.M., J.C.-L., M.H., V.M., S.C.-B., A.S., G.E., M.F., G.A., A.E., R.S., S.B., M.P., S.H., V.S., and L.F. Software: J.T., M.S., and S.B. Validation: J.T., P.-O.F., S.P., T.O., and M.S. Formal analysis: T.A., J.T., P.-O.F., S.P., T.O., M.S., M.D., N.B., D.Z., C.M., J.C.-L., M.H., V.M., S.C.-B., A.S., G.E., M.F., G.A., A.E., R.S., S.B., M.P., S.H., V.S., and L.F. Investigation: T.A., J.T., P.-O.F., S.P., M.S., M.D., N.B., G.E., S.B., and L.F. Resources: T.A., P.-O.F., S.P., M.D., N.B., and S.B. Data curation: S.P., M.D., and S.B. Writing – original draft: T.A., J.T., P.-O.F., S.P., T.O., M.S., N.B., D.Z., C.M., J.C.-L., M.H., V.M., S.C.-B., A.S., G.E., M.F., G.O., A.E., R.S., S.B., M.P., S.H., V.S., and L.F. Writing – review and editing: T.A., J.T., P.-O.F., S.P., M.D., N.B., S.B., and L.F. Visualization: J.T., S.P., M.S., and M.D. Supervision: T.A. and L.F. Projected administration: M.D. and N.B. Funding application: T.A. and L.F.
Declaration of interests
T.A. has received research funding from EMD Serono and consultancy fees for BMS, Roche, Merck, Novartis, and Tiaho. The rest of the authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| hMLH1 | Roche Diagnostics | Cat#: G168-15, RRID:AB_1933569 |
| hMSH2 | Roche Diagnostics | Cat#: G219-1129, RRID:AB_2936886 |
| hMSH6 | Roche Diagnostics | Cat#: SP93, RRID:AB_2936885 |
| hPMS2 | Roche Diagnostics | Cat#: A16-4, RRID:AB_3669003 |
| PD-L1 | Agilent Technologies Canada Inc | Dako 73-10, RRID:AB_2833074 |
| CK7 | Roche Diagnostics | Cat#: 790–4462, RRID:AB_2861319 |
| CD8 | Agilent Technologies Canada Inc | Cat#: M7103, RRID:AB_2075537 |
| granzyme B | Ventana | Cat#: 760–4283, RRID:AB_2335967 |
| CD68 | Ventana | Cat#: 790–2931, RRID:AB_2335972 |
| CD163 | Roche Diagnostics | Cat#: 760–4437, RRID:AB_2335969 |
| FoxP3 | CST | Cat#: 9837, RRID:AB_2732885 |
| Biological samples | ||
| Gastro-esophageal tissue specimens | Human | N/A |
| Critical commercial assays | ||
| Qubit ssDNA HS Assay kit | ThermoFisher | Lot No: Q10212 |
| Chromium Single Cell 3′ Reagent Kits v3.1 | 10x Genomics | Chromium Next GEM Single Cell 3′ Reagent Kits (v3.1 User Guide) |
| Chromium Single Cell DNA Reagent Kits | 10x Genomics | Chromium Single Cell 3′ Reagent Kits User Guide (v3.1 Chemistry Dual Index) |
| KAPA library quantification kit | Roche | Cat#: 07960140001, Kit No: KK4824 |
| LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells | ThermoFisher | Cat#: L-3224 |
| DRAQ5 | ThermoFisher | Cat#: 65-0880-92 |
| MGIEasy Universal Library Conversion Kit (v1.0) | MGI | Cat#: 1000004155 |
| OPAL (6-plex) | Akoya Biosciences | NEL811001KT |
| Deposited data | ||
| RNA single cell data from human samples | This paper | SRA repository (PRJNA1052389) |
| Software and algorithms | ||
| R Project for Statistical Computing | R foundation | https://www.r-project.org/; RRID:SCR_001905 |
| SPSS | IBM | https://www.ibm.com/spss |
| Seurat v5.0 | Satija lab | https://satijalab.org/seurat/; RRID:SCR_016341 |
| GraphPad Prism 8 | http://www.adobe.com/products/illustrator.html | https://www.graphpad.com; RRID: SCR_002798 |
| Cell Ranger (v.3) | 10X Genomics | https://www.10xgenomics.com/support/software/cell-ranger/latest; RRID: SCR_017344 |
| DemuxFastqs | Fulcrum Genomics | https://github.com/fulcrumgenomics/fqtk |
| fastq-multx (v1.4.2) | MIT | https://github.com/brwnj/fastq-multx |
| Whitelist (v3) | 10x Genomics | 3M-february-2018.txt.gz |
| HALO HighPlex-FL module | Indica Labs, USA | https://indicalab.com/halo/halo-modules/highplex-fl/ |
| Tissue Classifier Add-on for HALO® | Indica Labs, USA | https://indicalab.com/halo/halo-modules/tissue-classifier-add-on/ |
| Spatial Analysis module | Indica Labs, USA | https://indicalab.com/halo/halo-modules/spatial-analysis/ |
Experimental model and study participant details
Enrolled patients
The study is registered on www.clinicaltrials.gov (NCT03288350) and has received approval from Health Canada and the hospital Research Ethics Board. All patients gave informed and written consent prior to enrollment. The full study protocol is available from the authors on request.
Fifty-one patients (45 Male and 6 Female) were enrolled in the study. Patients were characterized by absence of prior malignancy and auto-immune disease, and adequate physiological reserve to tolerate both systemic therapy and surgical procedure. Demographics of enrolled patients is included in results above and Table 1.
Method details
Primary and secondary endpoints
This is a single-center, single arm, phase II study conducted at the McGill University Health Center (Montreal, QC, Canada). This university affiliated tertiary center is a referral center for patients from throughout Quebec with esophagogastric cancer. The primary endpoint of this study was to describe the association of preoperative aDCF with pCR as this outcome has been linked to excellent survival in other trials of chemo-immunotherapy. The secondary endpoints were to describe the two-year disease-free survival (DFS) and incidence of grade 3/4 adverse events (AE) associated with this regimen. We also described a post hoc analysis of the tissue inflammatory microenvironment with a specific focus on M2 tumor associated macrophages.
Considerations regarding sample size are discussed below.
Screening procedures for staging routinely included contrast infused computed tomography of the chest, abdomen and pelvis and whole-body 18-FDG positron emotion tomography. Endoscopic ultrasound was selectively used and diagnostic laparoscopy only performed for sub-cardia gastric and Siewert type III gastro-esophageal junction tumors.
Inclusion and exclusion criteria
Patients thought to be eligible for inclusion in the study were verified according to the following inclusion criteria: Patients must have been 18 years or old and had biopsy confirmed adenocarcinoma of the esophagus, stomach or gastroesophageal junction; clinically staged II to IIIB (AJCC 8th edition,74 including T1b, N + Mx or T2-4, Nany, Mx) that was deemed to be surgically resectable; a World Health Organization performance status score of 0–1; normal full blood count, liver and renal test values. Patients were excluded from the study if they had a daily intake of prednisone greater than 10 mg (or equivalent) or recent use of other immunosuppressive medications within 7 days of enrollment into the trial, serious autoimmune or infectious diseases that could be exacerbated by immunotherapy, previous solid organ transplantation, contraindication to any of the chemotherapeutic agents in use in the protocol or prior systemic therapy for GEA. Patients were also excluded if they had known severe hypersensitivity reactions monocolonal antibodies or other elements of the DCF protocol, clinically significant cardiovascular disease, stroke or serious underlying arrythmia and known drug or alcohol abuse disorder.
Treatment
The experimental perioperative regimen consisted of 4 cycles of aDCF given every 2 weeks, before and after surgery. On day 1, after premedication (dexamethasone 12 mg intravenously (IV), aprepitant 125 mg orally), docetaxel was given at the dose of 40 mg IV/m2 over 1 h; cisplatin was administered at the dose of 40 mg IV/m2; 5-fluorouracil was infused over 48 h at the dose of 1000 mg/m2/day. On day 4, avelumab was injected IV at the dose of 10 mg/kg after oral intake of 650 mg acetaminophen and IV administration of 50 mg of diphenhydramine. The protocol was then amended to allow administration of all cancer drugs in one day, starting with avelumab. The use of prophylactic filgrastim was at the discretion of the investigator (TA). In practice, this related to delaying of a treatment cycle due to neutropenia or an episode of febrile neutropenia.
Toxicity was graded according to National Cancer Institute-Common Toxicity Criteria for Adverse Events (CTCAE), version 3.20 Administration of cisplatin, docetaxel, and 5-FU was delayed in case of diarrhea or mucositis of CTCAE grade 2 or greater, or thrombocytopenia <75,000/μL. For neutropenia <1500/μL, chemotherapy could be withheld or administered with G-CSF according to the investigator’s judgment. Cisplatin dose was reduced by 50% for decreased creatinine clearance to 50–60 mL/min and was discontinued if < 50 mL/min. Docetaxel was reduced by 25% in case of CTCAE grade 4 neutropenia persisting longer than 7 days or accompanied by fever and thrombocytopenia of CTCAE grade 3 or greater. The dose of docetaxel was reduced by 25% in case of CTCAE grade 4 neutropenia persisting longer than 7 days or accompanied by fever and thrombocytopenia of CTCAE grade 3 or greater. Docetaxel was discontinued in the case of severe hypersensitivity, bilirubin level more than 2 ULN, or of peripheral neuropathy of CTCAE grade 3 or greater. The dose of 5-FU was reduced by 25% at any time for CTCAE grade 3 or greater diarrhea or mucositis, and CTCAE grade 2 or greater hand-foot syndrome. Any drug was discontinued in the event of a delay by greater than 2 weeks due to drug-specific toxicity.
Following completion of the neoadjuvant therapy, or if treatment was prematurely discontinued, patients were restaged with both a dedicated contrast infused CT of the chest, abdomen and pelvis and an FDG PET-CT scan performed 4–5 weeks after the end of the last neoadjuvant treatment cycle. Patients without distant metastases on restaging diagnostic imaging underwent resection within the following 2 weeks. Surgical approach was dictated by tumor location and patient performance status. Extended celiac lymphadenectomy (D2 dissection) was performed in all patients and en-bloc mediastinal dissection for esophageal cancers performed where applicable. Minimally invasive approaches were adopted when deemed appropriate by the managing surgeon. Post-operative complications were graded by the Thoracic Surgery Quality Improvement Classification,75 a modification of the Clavien-Dindo classification system.76 Postoperative treatment was commenced 6–12 weeks after surgical resection and followed a similar regimen as the neoadjuvant treatment. Patients were thereafter followed clinically and radiologically with a contrast infused CT of the chest, abdomen and pelvis every 3 months for the first two years, and every 6 months till completion of 5 years of follow-up. Upper endoscopy was performed 6 months for the first 3 years and then yearly for the following two years.
Pathological assessment and response grading
Processing of the specimen and assessment was performed with the College of American Pathologist (CAP) Protocols (version 4.1). As per CAP recommendations for esophageal carcinomas, the pre-operative therapy was graded according to the Modified Ryan Scheme for Tumor Regression Score.21
A score of 0 was given if no viable cancer cells were detected (pathological complete response, pCR), 1 if single cells or rare small groups of cancer cells were seen (near pCR), 2 in the presence of residual cancer with evident tumor regression but more than single cells (moderate response) and 3 if there was extensive residual cancer (poor response). Scores 0 and 1 together defined major pathologic response (MPR). Furthermore, ‘responders’ were defined as patients in whom TRG 0/1/2 was found whilst ‘non-responders’ referred to patients with TRG 3.
Immunohistochemistry was performed on formalin fixed paraffin embedded (FFPE) sections with the Roche Ventana Benchmark Ultra (Roche Diagnostics, Canada). Mismatch repair (MMR) immunohistochemistry was performed with pre-diluted dispensers for hMLH1(G168-15, Roche Diagnostics), hMSH2 (G219-1129, Roche Diagnostics), hMSH6(SP93, Roche Diagnostics) and hPMS2(A16-4, Roche Diagnostics), on the pre-treatment biopsies. Mismatch repair deficiency was defined as deficiency in one, or more, of the MMR proteins as per the CAP template for reporting results of biomarker testing of specimens from patients with carcinoma of the colon and rectum (version 1.2). Tumor PD-L1 expression was determined using Dako 73-10 clone (Agilent Technologies Canada Inc) using the Dako Autostainer Link 48 apparatus (Agilent Technologies). On-slide positive controls included tonsil and placenta. The Combined Positive Score (CPS) and Tumor Proportion Score (TPS) were both assessed.
Tissue collection and sample processing
Tissue was retrieved via endoscopic biopsy at several stages throughout the patient’s treatment pathway including treatment naive, mid-treatment (after two cycles of aDCF) and post-treatment post-resection samples. Using high-definition white light endoscopy to ensure the efficacy of sample collection, multiple biopsies were taken from the suspected lesion with an additional sample taken from the same location. The former was placed into a cold medium of RPMI (Invitrogen), Primocin (Invivogen) and gentamycin (Invitrogen) whilst the latter was formalin fixed and paraffin embedded (FFPE) for formal pathological analysis to confirm the presence of adenocarcinoma within the sample.
If carcinoma was pathologically confirmed, the sample was processed for single cell transcriptomic and OPAL 6 spatial analysis. A diagram describing the experiment workflow is displayed in Figure S1.
OPAL 6 and spatial analysis
FFPE sections were matched and analyzed using multiplex immunohistochemistry with an OPAL (6-plex) kit (NEL811001KT, Akoya Biosciences). The labeling for various primary antibodies included CK7 (Roche, #790–4462), CD8 (DAKO, #M7103), granzyme B (Roche, #760–4283), CD68 (Roche #790–2931), CD163 (Roche #760–4437) and FoxP3 (CST, #9837). A secondary antibody and OPAL dyes were then used and finally counterstained with spectral DAPI. A reference slide with single labels was also prepared. A LSM710 microscope (Zeiss) was used to capture slide images with 5–6 regions of interest per sample for each patient. The HALO software package (Indica Labs, USA) was used for imaging analysis. High cell density was defined as all values per μm2 greater than the median density for the treatment naive and post-treatment samples separately.
Using the HALO HighPlex-FL module pathologists blindly reviewed tissue and classified tissue as tumor or stoma excluding normal and fibrotic areas. Cell segmentation and individual cell marking were then performed and the Spatial Analysis Module of HALO was then used to determine the spatial relationship of tumor cells to either cytotoxic T cells, M2 macrophages or potential regulatory T cells. The proximity algorithm computed the number of cells within a given distance of the tumor cell (</>50μm).
Single cell transcriptomics
To achieve single cell disassociation, specimens were dissected minced and digested using 5 mL of Advanced DMEM/F12 containing 10 mg Collagenase Type 3 (Worthington) and 500 U Hyaluronidase (Sigma) in a Ctube (Miltenyi) using the gentleMACS Octo Dissociator (Miltenyi) and resuspended in PBS and 1MM of DDT. The suspension was passed through a 100um cell strainer (Fisher) and centrifuged (500xg at 5 min, 4°C). The cells were resuspended in 0.25% Trypsin-EDTA (Invitrogen) and subsequently incubated for 5 min at 37°C. The trypsin was then inactivated by adding 10% fetal bovine serum. After being re-centrifuged (500xg, 5 min, 4°C) the cell pellet was resuspended in 2.5U Dispase/10μg DNAse buffer and incubated for 5 min at 37°C which was then inactivated by adding PBS. The homogenate was then passed through a 40 μM strainer (Fisher) prior to being centrifuged again (500xg, 5 min, 4°C). Using ACK Lysing Buffer (Gibco) for 5 min at room temperate, red cells were lysed. After adding PBS, and being centrifuged again (500xg, 5 min, 4°C), the cell pellet was washed twice with 2% fetal bovine serum in PBS. The substrate was then processed via the 10x Genomics platform.
Single-cell RNA sequencing
The LightCycler 480 Real-Time PCR instrument (Roche) and the KAPA library quantification kit (Roche) were used to quantify cellular libraries with triplicate measurements. These quantification values were used for MGI library conversion and for Illumina sequencing normalization.
After 10XGenomics library construction, the MGIEasy Universal Library Conversion kit was used to ensure all outputs were compatible with MGI sequencers. This conversion circularizes the library by rolling circle amplification that creates a long DNA strand which individually fold into a tight nanoball where each library fragment results in a single DNA nanoball (clone).
The DNA nanoballs were quantified with Qubit ssDNA HS assay kit (ThermoFisher), normalized and loaded into the sequencing flowcells using an auto-loading methods (MGI-DL-200R autoloader). The flowcells have a functional surface that captures and immobilizes the DNA nanoballs in a grid like pattern. Two libraries were typically loaded per lane for the single cell RNA sequencing using the DNBSEQ-G400RS PE100 MGI kit with App-A primers on a DNBSEQ-G400 MGI sequencer. Single cell RNA libraries were sequenced with 28 cycles for Read 1, 150 cycles for Read 2 and 8 cycles for the i7 index. Color balancing was achieved with color-balanced single index adapters (10x Genomics) were used for libraries sequenced on MGI. These MGI runs were demultiplexed by fastq-multx (https://github.com/brwnj/fastq-multx) or fgbio/DemuxFastqs (http://fulcrumgenomics.github.io/fgbio/tools/latest/DemuxFastqs.html). In both instances we used a mismatch of 1. After polyA-trimming via cutadapt (v3.2)86, reads were pseudo-aligned to the GRCh38 reference transcriptome (ENSEMBL release 96) with kallisto (v0.46.2)87 using the default kmer size of 31. The pseudo-aligned reads were processed into a cell-by-gene count matrix using bustools (0.40.0) 88. Cell barcodes were altered using the whitelist (v3) provided by 10xGenomics. All further processing was done in Seurat (version 4.4).
Single cell quality control and processing
Seurat v.4.477 was used for all single cell analyses. Standard pre-processing steps were performed starting with quality control on the single cell transcriptomes. Cells with <1000 UMIs or <500 genes detected were removed. Cells with >20% mitochondrial RNA content were also excluded. Raw counts were then processed as in Zheng et al.78 using per-cell normalisation, followed by highly-variable gene selection (n = 4000 genes), log1p transform and z-scoring per gene. PCA was computed based on the z-scored values. A nearest neighbor graph (k = 15) was built on the first 50 PCs. UMAP embeddings and Leiden-clustering were calculated based on this nearest neighbor graph. Integrative analysis was performed using Canonical correlation analysis and cell doublets were then removed via DoubletFinder.79 This yielded 44118 high quality transcriptomes. The cell types were assigned to clusters based on expression >1% of CTL CD8+GZMB+ (CTL), CD4+FOXP3+ (TREG) and CD68+CD163+/CD206+ (M2-TAM). Samples were grouped according to their TRG status on final pathology (TRG1/2/3) stratified by retrieval point in the treatment pathway: Treatment naive, ‘pre’; mid-treatment, ‘mid’; and post-treatment, ‘post'. There were no samples from patients who achieved TRG0 available for analysis.
Differentially expressed genes between clusters were calculated via a Wald-test on raw counts. The ‘DittoSeq’ package was used to compute and visualise cell type proportions per sample, across treatment cycles and across response groups.80 DESeq2 was used to perform a pseudobulk differential expression analysis between ‘pre’, ‘mid’ and ‘post’ samples.81 Differentially expressed genes were identified using |LogFold change| >1.5 and an adjusted p value of <0.05. Finally, in order to explore gene enrichment, the DEenrichRPlot package82 was used with the ‘KEGG_2019_Human’ database to identify the top 5 overexpressed and underexpressed pathways among TRG3 ‘pre’ and ‘post’ samples.
Quantification and statistical analysis
To meet the primary objective and to detect an improvement of pCR from the 7% described in historical studies of DCF in patients with esophagogastric adenocarcinoma to a proposed incidence of 20%, a Simon 2-stage scheme was chosen to achieve a statistical power of 80%. Allowing for an α error of 5% the first stage of the study was considered successful if more than one out of the first 16 patients initially treated showed pCR. If this was met, accrual to a total of 50 patients was achieved with the study being considered positive if at more than 6 patients achieved pCR. Conversely, if no more than one patient has pCR among the first 16 enrolled, the study would be closed to further accrual.
Secondary endpoints were the two-year disease-free survival (DFS) and incidence of grade 3/4 avelumab related adverse events (AE) associated with this regimen. Exploratory endpoints were laboratory-based, hypothesis-generating experiments. This included using the intensity of PD-L1 expression on tumor cells (TPS score) or combined tumor and immune cells (CPS score), expression of mismatch repair (MMR) protein; and HER-2 status via immunohistochemistry, and genes revealed by single cell transcriptomic analysis to predict tumor response to the experimental regimen.
Categorical data is described as absolute numbers and percentages. Continuous demographic data is presented as median with range whilst data from the IHC analysis is presented as mean with 95% CI. Fisher’s exact test was used to compare categorical variables including HER2 and MMR status stratified by TRG category. This included the assessment of the percentage change in maximum SUV (SUVmax) which was generated by calculating the change in the post-treatment SUVmax and the pre-treatment SUVmax. Using >35% change as the cut-off for in this measurement, changes in the SUVmax could be compared between TRG categories.
For the comparison of continuous variables relating to demographic data, the Mann-Whitney-U test was used. To compare the changing density within the TIME between different TRG categories stratified by cell subtype, two-way ANOVA with Tukey’s correction was used. Finally, comparison of cellular density within the TIME also between different TRG categories, unpaired T-test with Welch’s correction was chosen. Samples were excluded from this analysis if they fell beyond the 95% CI. Finally, comparison of differentially expressed genes was computed using a Wald-test. Kaplan-Meier survival curves were generated with survival curves compared with Log Rank (Mantel-Cox). Median follow-up time was calculated using the reverse Kaplan Meier method. Starting from the date of pathological diagnosis, the data cutoff date to calculate disease free and overall survival was the 16th of May 2023.
Boxplots are shown with the horizontal line denoting the median, vertical line denoting the 95% confidence interval and ‘+’ denoting the mean. For scatter dot plots, each dot represents the respective intra-tumoral M2-TAM density and the corresponding median for each subgroup. Significant differences within these diagrams are highlighted with ‘∗’ or ‘∗∗’ respectively. All p values are two-sided with a value of <0.05 being considered statistically significant. All analyses were performed in R v.4.1.0 with ggplot2 which, along with SPSS version 29, was used to create all plots.
Additional resources
The study is registered on www.clinicaltrials.gov URL: https://clinicaltrials.gov/ct2/show/NCT03288350 (NCT03288350).
Published: April 15, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2025.102045.
Contributor Information
Thierry Alcindor, Email: thierry.alcindor@mcgill.ca.
Lorenzo Ferri, Email: lorenzo.ferri@mcgill.ca.
Supplemental information
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Boonstra J.J., Kok T.C., Wijnhoven B.P., van Heijl M., van Berge Henegouwen M.I., Ten Kate F.J., Siersema P.D., Dinjens W.N., van Lanschot J.J., Tilanus H.W., van der Gaast A. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer. 2011;11:181. doi: 10.1186/1471-2407-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hagen P., Hulshof M.C.C.M., van Lanschot J.J.B., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P.L., Richel D.J., Nieuwenhuijzen G.A.P., Hospers G.A.P., Bonenkamp J.J., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Al-Batran S.E., Homann N., Pauligk C., Goetze T.O., Meiler J., Kasper S., Kopp H.G., Mayer F., Haag G.M., Luley K., et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 5.Möhring C., Mańczak A., Timotheou A., Sadeghlar F., Zhou T., Mahn R., Monin M.B., Toma M., Feldmann G., Brossart P., Köksal M. Perioperative therapy with FLOT4 significantly increases survival in patients with gastroesophageal and gastric cancer in a large real-world cohort. Int. J. Cancer. 2023;153:609–622. doi: 10.1002/ijc.34511. [DOI] [PubMed] [Google Scholar]
- 6.Janjigian Y.Y., Shitara K., Moehler M., Garrido M., Salman P., Shen L., Wyrwicz L., Yamaguchi K., Skoczylas T., Campos Bragagnoli A., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rha S.Y., Oh D.Y., Yañez P., Bai Y., Ryu M.H., Lee J., Rivera F., Alves G.V., Garrido M., Shiu K.K., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181–1195. doi: 10.1016/S1470-2045(23)00515-6. [DOI] [PubMed] [Google Scholar]
- 8.Janjigian Y.Y., Kawazoe A., Bai Y., Xu J., Lonardi S., Metges J.P., Yanez P., Wyrwicz L.S., Shen L., Ostapenko Y., et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402:2197–2208. doi: 10.1016/S0140-6736(23)02033-0. [DOI] [PubMed] [Google Scholar]
- 9.Verschoor Y.L., van de Haar J., van den Berg J.G., van Sandick J.W., Kodach L.L., van Dieren J.M., Balduzzi S., Grootscholten C., IJsselsteijn M.E., Veenhof A.A.F.A., et al. Neoadjuvant atezolizumab plus chemotherapy in gastric and gastroesophageal junction adenocarcinoma: the phase 2 PANDA trial. Nat. Med. 2024;30:519–530. doi: 10.1038/s41591-023-02758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J.X., Tang Y.H., Zheng H.L., Ye K., Cai J.C., Cai L.S., Lin W., Xie J.W., Wang J.B., Lu J., et al. Neoadjuvant camrelizumab and apatinib combined with chemotherapy versus chemotherapy alone for locally advanced gastric cancer: a multicenter randomized phase 2 trial. Nat. Commun. 2024;15:41. doi: 10.1038/s41467-023-44309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J.L., Zhang B., Xu J.P., Qi L., Xin D., Wang L., Wang B.Z., Tian Y.T., Li Y., Huang J. Perioperative treatment and biomarker analysis of LP002, an anti-PD-L1 antibody, plus chemotherapy in resectable gastric and gastroesophageal junction cancer. Cancer Med. 2023;12:5639–5648. doi: 10.1002/cam4.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Z., Xie Y., Wang B., Zhu Y., Ke Y., Zhang W., Sun Y., Li Y., Wang C., Zhao D., Tian Y. Oxaliplatin and capecitabine (XELOX) plus toripalimab as perioperative treatment for locally advanced gastric or gastro-esophageal junction adenocarcinoma (Neo-capture): A single-arm, phase 2 study. J. Clin. Oncol. 2022;40:e16001. doi: 10.1200/JCO.2022.40.16_suppl.e16001. [DOI] [Google Scholar]
- 13.Liu Y.H.G., Li H., Zhao Y., Li Z., Zhuang J., Wang G., Li B., Wang J., Li Z., Xia Q. Camrelizumab combined with FLOFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma: Updated results of efficacy and safety. J. Clin. Oncol. 2021;39:4036. doi: 10.1200/JCO.2021.39.15_suppl.4036. [DOI] [Google Scholar]
- 14.Shitara K., Rha S.Y., Wyrwicz L.S., Oshima T., Karaseva N., Osipov M., Yasui H., Yabusaki H., Afanasyev S., Park Y.K., et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024;25:212–224. doi: 10.1016/S1470-2045(23)00541-7. [DOI] [PubMed] [Google Scholar]
- 15.Kelly R.J., Ajani J.A., Kuzdzal J., Zander T., Van Cutsem E., Piessen G., Mendez G., Feliciano J., Motoyama S., Lièvre A., et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021;384:1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 16.Collins J.M., Gulley J.L. Product review: avelumab, an anti-PD-L1 antibody. Hum. Vaccin. Immunother. 2019;15:891–908. doi: 10.1080/21645515.2018.1551671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powles T., Park S.H., Voog E., Caserta C., Valderrama B.P., Gurney H., Kalofonos H., Radulović S., Demey W., Ullén A., et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 18.Patel M.R., Ellerton J., Infante J.R., Agrawal M., Gordon M., Aljumaily R., Britten C.D., Dirix L., Lee K.W., Taylor M., et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia S., Nghiem P., Veeranki S.P., Vanegas A., Lachance K., Tachiki L., Chiu K., Boller E., Bharmal M. Real-world clinical outcomes with avelumab in patients with Merkel cell carcinoma treated in the USA: a multicenter chart review study. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2022-004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achrol A.S., Rennert R.C., Anders C., Soffietti R., Ahluwalia M.S., Nayak L., Peters S., Arvold N.D., Harsh G.R., Steeg P.S., Chang S.D. Brain metastases. Nat. Rev. Dis. Primers. 2019;5:5. doi: 10.1038/s41572-018-0055-y. [DOI] [PubMed] [Google Scholar]
- 21.Ryan R., Gibbons D., Hyland J.M.P., Treanor D., White A., Mulcahy H.E., O'Donoghue D.P., Moriarty M., Fennelly D., Sheahan K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141–146. doi: 10.1111/j.1365-2559.2005.02176.x. [DOI] [PubMed] [Google Scholar]
- 22.Grote H.J., Feng Z., Schlichting M., Helwig C., Ruisi M., Jin H., Scheuenpflug J., Gann C.N., Su Z., Reck M., et al. Programmed Death-Ligand 1 Immunohistochemistry Assay Comparison Studies in NSCLC: Characterization of the 73-10 Assay. J. Thorac. Oncol. 2020;15:1306–1316. doi: 10.1016/j.jtho.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Lordick F., Ott K., Krause B.J., Weber W.A., Becker K., Stein H.J., Lorenzen S., Schuster T., Wieder H., Herrmann K., et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 24.Efremova M., Vento-Tormo M., Teichmann S.A., Vento-Tormo R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 2020;15:1484–1506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 25.Fey R.M., Nichols R.A., Tran T.T., Vandenbark A.A., Kulkarni R.P. MIF and CD74 as Emerging Biomarkers for Immune Checkpoint Blockade Therapy. Cancers. 2024;16 doi: 10.3390/cancers16091773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marangoni F., Zhakyp A., Corsini M., Geels S.N., Carrizosa E., Thelen M., Mani V., Prüßmann J.N., Warner R.D., Ozga A.J., et al. Expansion of tumor-associated Treg cells upon disruption of a CTLA-4-dependent feedback loop. Cell. 2021;184:3998–4015.e19. doi: 10.1016/j.cell.2021.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Huang H., Lu J., Bi P., Wang F., Liu X., Zhang B., Luo Y., Li X. Tumor cells induced-M2 macrophage favors accumulation of Treg in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2017;10:8389–8401. [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W., Wei F.Q., Li W.J., Wei J.W., Zhong H., Wen Y.H., Lei W.B., Chen L., Li H., Lin H.Q., et al. A positive-feedback loop between tumour infiltrating activated Treg cells and type 2-skewed macrophages is essential for progression of laryngeal squamous cell carcinoma. Br. J. Cancer. 2017;117:1631–1643. doi: 10.1038/bjc.2017.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferri L.E., Ades S., Alcindor T., Chasen M., Marcus V., Hickeson M., Artho G., Thirlwell M.P. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann. Oncol. 2012;23:1512–1517. doi: 10.1093/annonc/mdr465. [DOI] [PubMed] [Google Scholar]
- 30.Borriello F., Sethna M.P., Boyd S.D., Schweitzer A.N., Tivol E.A., Jacoby D., Strom T.B., Simpson E.M., Freeman G.J., Sharpe A.H. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 31.Wang B., Qin L., Ren M., Sun H. Effects of Combination of Anti-CTLA-4 and Anti-PD-1 on Gastric Cancer Cells Proliferation, Apoptosis and Metastasis. Cell. Physiol. Biochem. 2018;49:260–270. doi: 10.1159/000492876. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda T., Wang Y.A. Gastric cancer immunosuppressive microenvironment heterogeneity: implications for therapy development. Trends Cancer. 2024;10:627–642. doi: 10.1016/j.trecan.2024.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira M.A., de Castria T.B., Ramos M.F.K.P., Dias A.R., Cardili L., de Moraes R.D.R., Zilberstein B., Nahas S.C., Ribeiro U., Jr., de Mello E.S. Cytotoxic T-lymphocyte-associated protein 4 in gastric cancer: Prognosis and association with PD-L1 expression. J. Surg. Oncol. 2021;124:1040–1050. doi: 10.1002/jso.26604. [DOI] [PubMed] [Google Scholar]
- 34.Noe J.T., Mitchell R.A. MIF-Dependent Control of Tumor Immunity. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.609948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong L., Chen C., Zhang Y., Guo P., Wang Z., Li J., Liu Y., Liu J., Chang R., Li Y., et al. The loss of RNA N(6)-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8(+) T cell dysfunction and tumor growth. Cancer Cell. 2021;39:945–957.e10. doi: 10.1016/j.ccell.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Prima V., Kaliberova L.N., Kaliberov S., Curiel D.T., Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA. 2017;114:1117–1122. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pu Y., Ji Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.874589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Liu L., Liu J., Dang P., Hu S., Yuan W., Sun Z., Liu Y., Wang C. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol. Cancer. 2023;22:58. doi: 10.1186/s12943-023-01725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y., Zhan S., Chen L., Sun M., Li M., Mou X., Zhang Z., Xu L., Xu Y. TNFSF14/LIGHT promotes cardiac fibrosis and atrial fibrillation vulnerability via PI3Kgamma/SGK1 pathway-dependent M2 macrophage polarisation. J. Transl. Med. 2023;21:544. doi: 10.1186/s12967-023-04381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giommoni E., Lavacchi D., Tirino G., Fornaro L., Iachetta F., Pozzo C., Satolli M.A., Spallanzani A., Puzzoni M., Stragliotto S., et al. Results of the observational prospective RealFLOT study. BMC Cancer. 2021;21:1086. doi: 10.1186/s12885-021-08768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tankel J., Ahmed N., Mueller C., Najmeh S., Spicer J., Mulder D., Cool-Lartigue J., Rousseau M., Frechette D., Sud S., et al. Docetaxel-Based Neoadjuvant Chemotherapy Followed by En Bloc Resection for Esophageal Adenocarcinoma: A 15-Year Retrospective Analysis from a Regional Upper Gastrointestinal Cancer Network. Ann. Surg Oncol. 2024;31:2461–2469. doi: 10.1245/s10434-023-14779-4. [DOI] [PubMed] [Google Scholar]
- 42.Li N., Li Z., Fu Q., Zhang B., Zhang J., Wan X.B., Lu C.M., Wang J.B., Deng W.Y., Ma Y.J., et al. Efficacy and safety of neoadjuvant sintilimab in combination with FLOT chemotherapy in patients with HER2-negative locally advanced gastric or gastroesophageal junction adenocarcinoma: an investigator-initiated, single-arm, open-label, phase II study. Int. J. Surg. 2024;110:2071–2084. doi: 10.1097/JS9.0000000000001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin Y., Lin Y., Yang M., Lv J., Liu J., Wu K., Liu K., Li A., Shuai X., Cai K., et al. Neoadjuvant tislelizumab and tegafur/gimeracil/octeracil (S-1) plus oxaliplatin in patients with locally advanced gastric or gastroesophageal junction cancer: Early results of a phase 2, single-arm trial. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.959295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo H., Ding P., Sun C., Yang P., Tian Y., Liu Y., Lowe S., Bentley R., Li Y., Zhang Z., et al. Efficacy and safety of sintilimab plus XELOX as a neoadjuvant regimen in patients with locally advanced gastric cancer: A single-arm, open-label, phase II trial. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.927781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang H., Yu X., Li N., Kong M., Ma Z., Zhou D., Wang W., Wang H., Wang H., He K., et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2021-003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan S.Q., Nie R.C., Jin Y., Liang C.C., Li Y.F., Jian R., Sun X.W., Chen Y.B., Guan W.L., Wang Z.X., et al. Perioperative toripalimab and chemotherapy in locally advanced gastric or gastro-esophageal junction cancer: a randomized phase 2 trial. Nat. Med. 2024;30:552–559. doi: 10.1038/s41591-023-02721-w. [DOI] [PubMed] [Google Scholar]
- 47.Lorenzen S., Götze T.O., Thuss-Patience P., Biebl M., Homann N., Schenk M., Lindig U., Heuer V., Kretzschmar A., Goekkurt E., et al. Perioperative Atezolizumab Plus Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel for Resectable Esophagogastric Cancer: Interim Results From the Randomized, Multicenter, Phase II/III DANTE/IKF-s633 Trial. J. Clin. Oncol. 2024;42:410–420. doi: 10.1200/JCO.23.00975. [DOI] [PubMed] [Google Scholar]
- 48.Janjigian Y.Y., Van Cutsem E., Muro K., Wainberg Z., Al-Batran S.E., Hyung W.J., Molena D., Marcovitz M., Ruscica D., Robbins S.H., et al. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol. 2022;18:2465–2473. doi: 10.2217/fon-2022-0093. [DOI] [PubMed] [Google Scholar]
- 49.Xu J., Jiang H., Pan Y., Gu K., Cang S., Han L., Shu Y., Li J., Zhao J., Pan H., et al. Sintilimab Plus Chemotherapy for Unresectable Gastric or Gastroesophageal Junction Cancer: The ORIENT-16 Randomized Clinical Trial. JAMA. 2023;330:2064–2074. doi: 10.1001/jama.2023.19918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Ende T., de Clercq N.C., van Berge Henegouwen M.I., Gisbertz S.S., Geijsen E.D., Verhoeven R.H.A., Meijer S.L., Schokker S., Dings M.P.G., Bergman J.J.G.H.M., et al. Neoadjuvant Chemoradiotherapy Combined with Atezolizumab for Resectable Esophageal Adenocarcinoma: A Single-arm Phase II Feasibility Trial (PERFECT) Clin. Cancer Res. 2021;27:3351–3359. doi: 10.1158/1078-0432.CCR-20-4443. [DOI] [PubMed] [Google Scholar]
- 51.Raimondi A., Palermo F., Prisciandaro M., Aglietta M., Antonuzzo L., Aprile G., Berardi R., Cardellino G.G., De Manzoni G., De Vita F., et al. TremelImumab and Durvalumab Combination for the Non-OperatIve Management (NOM) of Microsatellite InstabiliTY (MSI)-High Resectable Gastric or Gastroesophageal Junction Cancer: The Multicentre, Single-Arm, Multi-Cohort, Phase II INFINITY Study. Cancers. 2021;13 doi: 10.3390/cancers13112839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moehler M., Dvorkin M., Boku N., Özgüroğlu M., Ryu M.H., Muntean A.S., Lonardi S., Nechaeva M., Bragagnoli A.C., Coşkun H.S., et al. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J. Clin. Oncol. 2021;39:966–977. doi: 10.1200/JCO.20.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashita K., Iwatsuki M., Harada K., Eto K., Hiyoshi Y., Ishimoto T., Nagai Y., Iwagami S., Miyamoto Y., Yoshida N., et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer. 2020;23:95–104. doi: 10.1007/s10120-019-00999-9. [DOI] [PubMed] [Google Scholar]
- 54.Basak U., Sarkar T., Mukherjee S., Chakraborty S., Dutta A., Dutta S., Nayak D., Kaushik S., Das T., Sa G. Tumor-associated macrophages: an effective player of the tumor microenvironment. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1295257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang X., Li M., Wu X., Guo T., Zhang L., Tang L., Jia F., Hu Y., Zhang Y., Xing X., et al. Neoadjuvant PD-1 blockade plus chemotherapy induces a high pathological complete response rate and anti-tumor immune subsets in clinical stage III gastric cancer. OncoImmunology. 2022;11 doi: 10.1080/2162402X.2022.2135819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svensson M.C., Svensson M., Nodin B., Borg D., Hedner C., Hjalmarsson C., Leandersson K., Jirström K. High Infiltration of CD68+/CD163- Macrophages Is an Adverse Prognostic Factor after Neoadjuvant Chemotherapy in Esophageal and Gastric Adenocarcinoma. J. Innate Immun. 2022;14:615–628. doi: 10.1159/000524434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng X., Weigert A., Reu S., Guenther S., Mansouri S., Bassaly B., Gattenlöhner S., Grimminger F., Pullamsetti S., Seeger W., et al. Spatial Density and Distribution of Tumor-Associated Macrophages Predict Survival in Non-Small Cell Lung Carcinoma. Cancer Res. 2020;80:4414–4425. doi: 10.1158/0008-5472.CAN-20-0069. [DOI] [PubMed] [Google Scholar]
- 58.Huang Y.K., Wang M., Sun Y., Di Costanzo N., Mitchell C., Achuthan A., Hamilton J.A., Busuttil R.A., Boussioutas A. Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry. Nat. Commun. 2019;10:3928. doi: 10.1038/s41467-019-11788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su P., Jiang L., Zhang Y., Yu T., Kang W., Liu Y., Yu J. Crosstalk between tumor-associated macrophages and tumor cells promotes chemoresistance via CXCL5/PI3K/AKT/mTOR pathway in gastric cancer. Cancer Cell Int. 2022;22:290. doi: 10.1186/s12935-022-02717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vayrynen S.A., Zhang J., Yuan C., Väyrynen J.P., Dias Costa A., Williams H., Morales-Oyarvide V., Lau M.C., Rubinson D.A., Dunne R.F., Kozak M.M. Composition, Spatial Characteristics, and Prognostic Significance of Myeloid Cell Infiltration in Pancreatic Cancer. Clin. Cancer Res. 2021;27:1069–1081. doi: 10.1158/1078-0432.CCR-20-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griguolo G., Tosi A., Dieci M.V., Fineberg S., Rossi V., Ventura A., Bottosso M., Bauchet L., Miglietta F., Jacob J., et al. A comprehensive profiling of the immune microenvironment of breast cancer brain metastases. Neuro Oncol. 2022;24:2146–2158. doi: 10.1093/neuonc/noac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y., Jia K., Sun Y., Zhang C., Li Y., Zhang L., Chen Z., Zhang J., Hu Y., Yuan J., et al. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment. Nat. Commun. 2022;13:4851. doi: 10.1038/s41467-022-32570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konstantinopoulos P.A., Luo W., Liu J.F., Gulhan D.C., Krasner C., Ishizuka J.J., Gockley A.A., Buss M., Growdon W.B., Crowe H., et al. Phase II Study of Avelumab in Patients With Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. 2019;37:2786–2794. doi: 10.1200/JCO.19.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.André T., Tougeron D., Piessen G., de La Fouchardière C., Louvet C., Adenis A., Jary M., Tournigand C., Aparicio T., Desrame J., Lièvre A. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2023;41:255–265. doi: 10.1200/JCO.22.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salah-Eddin Al-Batran S.L., Thuss-Patience P.C., Homann N., Schenk M., Lindig U., Vera H., Albrecht K., Goekkurt E., Haag G.M., Riera-Knorrenschild J., et al. A randomized, open-label, phase II/III efficacy and safety study of atezolizumab in combination with FLOT versus FLOT alone in patients with gastric cancer and adenocarcinoma of the oesophagogastric junction and high immune responsiveness: The IKF-S633/DANTE trial, a trial of AIO in collaboration with SAKK. J. Clin. Oncol. 2023;41:TPS4177. doi: 10.1200/JCO.2023.41.16_suppl.TPS4177. [DOI] [Google Scholar]
- 67.Park J.H., Powell A.G., Roxburgh C.S.D., Horgan P.G., McMillan D.C., Edwards J. Mismatch repair status in patients with primary operable colorectal cancer: associations with the local and systemic tumour environment. Br. J. Cancer. 2016;114:562–570. doi: 10.1038/bjc.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fridman W.H., Zitvogel L., Sautès-Fridman C., Kroemer G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 69.Giannakis M., Mu X.J., Shukla S.A., Qian Z.R., Cohen O., Nishihara R., Bahl S., Cao Y., Amin-Mansour A., Yamauchi M., et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;17:1206. doi: 10.1016/j.celrep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smyth E.C., Wotherspoon A., Peckitt C., Gonzalez D., Hulkki-Wilson S., Eltahir Z., Fassan M., Rugge M., Valeri N., Okines A., et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017;3:1197–1203. doi: 10.1001/jamaoncol.2016.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Velzen M.J.M., Derks S., van Grieken N.C.T., Haj Mohammad N., van Laarhoven H.W.M. MSI as a predictive factor for treatment outcome of gastroesophageal adenocarcinoma. Cancer Treat Rev. 2020;86 doi: 10.1016/j.ctrv.2020.102024. [DOI] [PubMed] [Google Scholar]
- 72.Yanagawa M., Tatsumi M., Miyata H., Morii E., Tomiyama N., Watabe T., Isohashi K., Kato H., Shimosegawa E., Yamasaki M., et al. Evaluation of response to neoadjuvant chemotherapy for esophageal cancer: PET response criteria in solid tumors versus response evaluation criteria in solid tumors. J. Nucl. Med. 2012;53:872–880. doi: 10.2967/jnumed.111.098699. [DOI] [PubMed] [Google Scholar]
- 73.Wang X., Yang W., Zhou Q., Luo H., Chen W., Yeung S.C.J., Zhang S., Gan Y., Zeng B., Liu Z., et al. The role of (18)F-FDG PET/CT in predicting the pathological response to neoadjuvant PD-1 blockade in combination with chemotherapy for resectable esophageal squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:4241–4251. doi: 10.1007/s00259-022-05872-z. [DOI] [PubMed] [Google Scholar]
- 74.Rice T.W., Patil D.T., Blackstone E.H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann. Cardiothorac. Surg. 2017;6:119–130. doi: 10.21037/acs.2017.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seely A.J.E., Ivanovic J., Threader J., Al-Hussaini A., Al-Shehab D., Ramsay T., Gilbert S., Maziak D.E., Shamji F.M., Sundaresan R.S. Systematic classification of morbidity and mortality after thoracic surgery. Ann. Thorac. Surg. 2010;90:936–942. doi: 10.1016/j.athoracsur.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 76.Clavien P.A., Sanabria J.R., Strasberg S.M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–526. [PubMed] [Google Scholar]
- 77.Satija R., Farrell J.A., Gennert D., Schier A.F., Regev A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng G.X.Y., Terry J.M., Belgrader P., Ryvkin P., Bent Z.W., Wilson R., Ziraldo S.B., Wheeler T.D., McDermott G.P., Zhu J., et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017;8 doi: 10.1038/ncomms14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGinnis C.S., Murrow L.M., Gartner Z.J. DoubletFinder: Doublet Detection in Single-Cell RNA Sequencing Data Using Artificial Nearest Neighbors. Cell Syst. 2019;8:329–337.e4. doi: 10.1016/j.cels.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bunis D.G., Andrews J., Fragiadakis G.K., Burt T.D., Sirota M. dittoSeq: universal user-friendly single-cell and bulk RNA sequencing visualization toolkit. Bioinformatics. 2021;36:5535–5536. doi: 10.1093/bioinformatics/btaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen E.Y., Tan C.M., Kou Y., Duan Q., Wang Z., Meirelles G.V., Clark N.R., Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The RNA single-cell data have been deposited at the SRA repository as PRJNA1052389 and are publicly available as of the date of publication.
-
•
Multiplex imaging data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.