Abstract
Background
There are several treatment options for managing acute asthma exacerbations (sustained worsening of symptoms that do not subside with regular treatment and require a change in management). Guidelines advocate the use of inhaled short acting beta2‐agonists (SABAs) in children experiencing an asthma exacerbation. Anticholinergic agents, such as ipratropium bromide and atropine sulfate, have a slower onset of action and weaker bronchodilating effect, but may specifically relieve cholinergic bronchomotor tone and decrease mucosal edema and secretions. Therefore, the combination of inhaled anticholinergics with SABAs may yield enhanced and prolonged bronchodilation.
Objectives
To determine whether the addition of inhaled anticholinergics to SABAs provides clinical improvement and affects the incidence of adverse effects in children with acute asthma exacerbations.
Search methods
We searched MEDLINE (1966 to April 2000), EMBASE (1980 to April 2000), CINAHL (1982 to April 2000) and reference lists of studies of previous versions of this review. We also contacted drug manufacturers and trialists. For the 2012 review update, we undertook an 'all years' search of the Cochrane Airways Group's register on the 18 April 2012.
Selection criteria
Randomized parallel trials comparing the combination of inhaled anticholinergics and SABAs with SABAs alone in children (aged 18 months to 18 years) with an acute asthma exacerbation.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We used the GRADE rating system to assess the quality of evidence for our primary outcome (hospital admission).
Main results
Twenty trials met the review eligibility criteria, generated 24 study comparisons and comprised 2697 randomised children aged one to 18 years, presenting predominantly with moderate or severe exacerbations. Most studies involved both preschool‐aged children and school‐aged children; three studies also included a small proportion of infants less than 18 months of age. Nine trials (45%) were at a low risk of bias. Most trials used a fixed‐dose protocol of three doses of 250 mcg or two doses of 500 mcg of nebulized ipratropium bromide in combination with a SABA over 30 to 90 minutes while three trials used a single dose and two used a flexible‐dose protocol according to the need for SABA.
The addition of an anticholinergic to a SABA significantly reduced the risk of hospital admission (risk ratio (RR) 0.73; 95% confidence interval (CI) 0.63 to 0.85; 15 studies, 2497 children, high‐quality evidence). In the group receiving only SABAs, 23 out of 100 children with acute asthma were admitted to hospital compared with 17 (95% CI 15 to 20) out of 100 children treated with SABAs plus anticholinergics. This represents an overall number needed to treat for an additional beneficial outcome (NNTB) of 16 (95% CI 12 to 29).
Trends towards a greater effect with increased treatment intensity and with increased asthma severity were observed, but did not reach statistical significance. There was no effect modification due to concomitant use of oral corticosteroids and the effect of age could not be explored. However, exclusion of the one trial that included infants (< 18 months) and contributed data to the main outcome, did not affect the results. Statistically significant group differences favoring anticholinergic use were observed for lung function, clinical score at 120 minutes, oxygen saturation at 60 minutes, and the need for repeat use of bronchodilators prior to discharge from the emergency department. No significant group difference was seen in relapse rates.
Fewer children treated with anticholinergics plus SABA reported nausea and tremor compared with SABA alone; no significant group difference was observed for vomiting.
Authors' conclusions
Children with an asthma exacerbation experience a lower risk of admission to hospital if they are treated with the combination of inhaled SABAs plus anticholinergic versus SABA alone. They also experience a greater improvement in lung function and less risk of nausea and tremor. Within this group, the findings suggested, but did not prove, the possibility of an effect modification, where intensity of anticholinergic treatment and asthma severity, could be associated with greater benefit.
Further research is required to identify the characteristics of children that may benefit from anticholinergic use (e.g. age and asthma severity including mild exacerbation and impending respiratory failure) and the treatment modalities (dose, intensity, and duration) associated with most benefit from anticholinergic use better.
Keywords: Adolescent; Child; Child, Preschool; Humans; Infant; Administration, Inhalation; Adrenergic beta‐2 Receptor Agonists; Adrenergic beta‐2 Receptor Agonists/administration & dosage; Adrenergic beta‐2 Receptor Agonists/therapeutic use; Asthma; Asthma/drug therapy; Atropine; Atropine/administration & dosage; Atropine/therapeutic use; Cholinergic Antagonists; Cholinergic Antagonists/administration & dosage; Cholinergic Antagonists/therapeutic use; Disease Progression; Drug Therapy, Combination; Drug Therapy, Combination/methods; Hospitalization; Hospitalization/statistics & numerical data; Ipratropium; Ipratropium/administration & dosage; Ipratropium/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Combined inhaled anticholinergics and beta2‐agonists for initial treatment of acute asthma in children
Background In an asthma attack, the airways (small tubes in the lungs) narrow because of inflammation (swelling), muscle spasms and mucus secretions. Other symptoms include wheezing, coughing and chest tightness. This makes breathing difficult. Reliever inhalers typically contain short‐acting beta2‐agonists (SABAs) that relax the muscles in the airways, opening the airways so that breathing is easier. Anticholinergic drugs work by opening the airways and decreasing mucus secretions. Review question We looked at randomised controlled trials to find out whether giving inhaled anticholinergics plus SABAs (instead of SABAs on their own) in the emergency department provides benefits or harms in children having an asthma attack. Key results We found that children with a moderate or severe asthma attack who were given both drugs in the emergency department were less likely to be admitted to the hospital than those who only had SABAs. In the group receiving only SABAs, on average 23 out of 100 children with acute asthma were admitted to hospital compared with an average of 17 (95% CI 15 to 20) out of 100 children treated with SABAs plus anticholinergics. Taking both drugs was also better at improving lung function. Taking both drugs did not seem to reduce the possibility of another asthma attack. Fewer children treated with anticholinergics reported nausea and tremor, but no significant group difference was observed for vomiting. Quality of the evidence and further research Most of the studies were in preschool‐ and school‐aged children; three studies also included a small proportion of infants under 18 months of age, although there was no evidence that inclusion of these infants with wheezy episodes affected the results. Nine trials (45%) were at a low risk of bias and we regarded the evidence for hospitalisation as high quality. Physicians can administer the dose of anticholinergic and SABA in several different ways; as a single dose, or as a certain number of doses or more flexibly. Most of the trials gave the children two or three doses and we think that more research is needed to improve characterization of children that benefit from, and the most effective number and frequency of doses of, anticholinergic treatment.
Summary of findings
Summary of findings for the main comparison. Anticholinergic and short‐acting beta2‐agonists versus beta2‐agonists alone (all protocols) for initial treatment of acute asthma in children.
| Anticholinergic and shorth‐acting beta2‐agonists (SABA) versus SABA alone (all protocols) for initial treatment of acute asthma in children | ||||||
| Patient or population: children with initial treatment of acute asthma Settings: emergency department Intervention: anticholinergic and SABA versus SABA alone (all protocols) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Anticholinergic and SABA versus SABA alone (all protocols) | |||||
| Primary outcome: hospital admissions hospital admissions | 23 per 100 | 17 per 100 (15 to 20) | RR 0.73 (0.63 to 0.85) | 2497 (19 studies) | ⊕⊕⊕⊕ high | 3 studies had no admissions so did not contribute to the RR. |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SABA: short‐acting beta‐agonists. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Asthma is caused by inflammation in the airways and bronchoconstriction, which makes it difficult to breathe and leads to wheezing and breathlessness. The underlying inflammation causes the lining of the airways to secrete mucus, which also obstructs the free flow of air through the lungs. The bronchoconstriction is alleviated by using bronchodilators such as short acting beta2‐agonists (SABAs), while the underlying inflammation can be treated with regular inhaled corticosteroids. However, symptoms may flare up in response to an asthma trigger (e.g. virus, dust, pollen) leading to an asthma exacerbation. It is not known why the inflammation, secretions and bronchoconstriction occur. Asthma exacerbations can be life threatening and may require more medications than are used on a day‐to‐day basis.
Description of the intervention
The initial management of acute paediatric asthma exacerbations in children focuses on the rapid relief of bronchospasm using inhaled or nebulized bronchodilators (BTS 2011; GINA 2011). Children who do have moderate or severe asthma and those who do not respond sufficiently to bronchodilators to relieve symptoms require the addition of oral or intravenous glucocorticoids (BTS 2011; GINA 2011; Lougheed 2012). SABAs are clearly the most effective bronchodilators due to their rapid onset of action and the magnitude of achieved bronchodilation (Sears 1992; Svedmyr 1985; Teoh 2012). Anticholinergic agents, such as ipratropium bromide and atropine sulfate, have a slower onset of action and weaker bronchodilating effect, but may specifically relieve cholinergic bronchomotor tone and decrease mucosal edema and secretions (Chapman 1996; Gross 1988; Silverman 1990). Thus, the combination of inhaled anticholinergics with SABAs may yield enhanced and prolonged bronchodilation.
National and international guidelines recommend the addition of anticholinergics to inhaled SABAs in people with an acute severe asthma exacerbation and suggest consideration of this therapy in people with moderate exacerbations (BTS 2011; GINA 2011).
Why it is important to do this review
The first version of this Cochrane review published in 1997 found a significant reduction in hospital admissions in school‐aged children with severe exacerbations receiving intensive anticholinergic treatment. The review was updated in 2000 with the addition of three new trials further strengthening the initial conclusions (Plotnick 2000). With the identification of seven new studies, the 2012 update aims to examine the conclusions of the earlier version of this review and explore if characteristics of participants or treatment are associated with increased benefit.
Objectives
To determine whether the addition of inhaled anticholinergics to inhaled SABAs provides clinical improvement and affects the incidence of adverse effects in children with acute asthma exacerbations.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) conducted in an emergency department setting, comparing the combination of an inhaled anticholinergic drug and SABA versus a SABA alone in the treatment of an acute asthma exacerbation.
Types of participants
Children aged 18 months to 18 years presenting to an emergency department with an acute exacerbation of asthma. Where studies included children younger than 18 months, we excluded them if the younger children contributed more than 5% of the total study population (post‐hoc decision).
Types of interventions
Treatment group: single or repeated doses of nebulized or inhaled short‐acting anticholinergics plus SABAs.
Control group: single or repeated doses of nebulized or inhaled placebo plus SABAs.
Types of outcome measures
Primary outcomes
Hospital admission.
Secondary outcomes
Change from baseline in % predicted forced expiratory volume in one second (FEV1) (60 and 120 minutes after the last combined anticholinergic and SABA inhalation).
Percent change from baseline in FEV1 (60 and 120 minutes after the last combined inhalation).
Change from baseline in respiratory resistance (60 and 120 minutes after the last combined inhalation).
Change from baseline in clinical score (60 and 120 minutes after the last combined inhalation).
Oxygen saturation (60 and 120 minutes after the last combined inhalation).
Need for repeated bronchodilator treatments after the intervention/placebo protocol, prior to disposition.
Need for systemic corticosteroids.
Adverse effects such as nausea, vomiting and tremor.
Relapse rate.
Search methods for identification of studies
Electronic searches
For the previous version of this review, MEDLINE (1966 to April 2000), EMBASE (1980 to April 2000) and CINAHL (1982 to April 2000) were searched using the following MeSH, full text and keyword terms: [asthma, wheez* or respiratory sounds] and [random*, trial*, placebo*, comparative study, controlled study, double‐blind, single‐blind] and [child* or infan* or adolescen* or pediatr* or paediatr*] and [emergenc* or acute*] and [ipratropium* or anticholinerg* or atropin*].
For the 2012 review update, we undertook an 'all years' search of the Cochrane Airways Group Register of Trials with the following terms: anticholinergic* or anti‐cholinergic* or atropine* or ipratropium or tiotropium or oxitropium or Aclidinium) AND ((beta* and agonist) or bronchodilat* or salbutamol or fenoterol or albuterol or terbutaline or metaproterenol) and (child* or paediat* or pediat* or adolesc* or infan* or toddler* or bab* or young* or preschool* or "pre school*" or pre‐school* or newborn* or "new born*" or new‐born* or neo‐nat* or neonat*.
Searching other resources
We reviewed bibliographies of all trials and review articles identified through searches to identify potentially relevant citations. We contacted the manufacturer of ipratropium bromide, Boehringer Ingelheim, for the original review and the update, to identify other published or unpublished trials. We contacted trialists working in the field of paediatric asthma to identify potentially relevant trials.
Data collection and analysis
Selection of studies
In the updated search, one review author (BG) examined each new citation (title and abstract) identified through one of the above strategies and classified as clearly included, possibly included or clearly not an RCT. We retrieved and assessed full‐text articles of all citations identified as definite or possible RCTs, irrespective of language of publication. BG and Toby Lasserson (former Managing Editor of the Cochrane Airways Group) assessed the full text independently to determine if the study met the inclusion criteria. We resolved any disagreement by consensus. Emma Welsh (Managing Editor of the Cochrane Airways Group) and Elizabeth Stovold (Trials Search Co‐ordinator of the Cochrane Airways Group) assisted in screening repeat literature searches prior to publication.
Data extraction and management
Two review authors independently extracted data from eligible studies and entered data into the Characteristics of included studies table.
Assessment of risk of bias in included studies
One review author (BG) and one member of the Cochrane Airways Group (TL or EJW) assessed the risk of bias. Agreement was reached independently in each case. We assessed the risk of bias as high, low or unclear in accordance with recommendation in the Cochrane Handbook for Systematic Reviews of Interventions for the following domains (Higgins 2011):
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding (performance bias and detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other bias.
Measures of treatment effect
We analyzed treatment effects for dichotomous outcomes as pooled risk ratios (RR). For continuous outcomes, we used the mean difference (MD) or the standardized mean difference (SMD) to estimate the pooled effect size. For example, the MD was reported for pulmonary function tests using the same unit of measure. We used the SMD, reported in 'standard deviation units', when the change in the same pulmonary function test was reported in different units (change in % predicted FEV1 and % change in FEV1). We presented pooled effect sizes along with the 95% confidence intervals (CI).
Dealing with missing data
We attempted to contact the study investigators or study sponsors to verify study methodology and extracted information and to provide additional data if necessary.
Assessment of heterogeneity
We used the I2 statistic for assessing the level of statistical heterogeneity between the results of the studies. We used the following levels of I2 as a guide:
0% to 40% may not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% considerable heterogeneity.
Assessment of reporting biases
We visually inspected funnel plot symmetry where we had more than 10 trials contributing data to a meta‐analysis in an effort to detect possible biases.
Data synthesis
We entered data into Review Manager 5.1 software (RevMan 2011). Treatment effects for dichotomous outcomes were analyzed and reported as pooled RRs using the fixed‐effect model (Greenland 1985), or, in case of substantial, unexplained heterogeneity, the random‐effects model (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We postulated a priori that three factors may potentially influence the magnitude or direction, or both, of the therapeutic response, namely:
the intensity of anticholinergic treatment;
co‐intervention with glucocorticoids; and
the severity of exacerbation (based on documented/reported severity by authors and by tertile of admission rate).
Therefore, RCTs were grouped according to the intensity of anticholinergic protocol (single‐dose regimen, multiple fixed‐dose regimen, multiple flexible‐dose regimen) and stratified on the presence/absence of systemic glucocorticoids. Whenever reported, the baseline % predicted FEV1 and hospital admission rate in the control groups were recorded as indicators of severity and examined for their potential interaction with therapeutic effect.
In order to evaluate the effect of baseline severity on the magnitude of response to the intervention, we stratified trials according to severity documented or reported by authors as per the original review. In addition, we ranked each study in increasing order of the control group admission rate as a proxy for severity. We then split these arbitrarily into tertiles, which we referred to as low, medium or high risk. If the admission rate for a particular study was not available, we did not rank that study.
For the 2012 update, we considered adding a subgroup analysis by age of child; however, the overlap in age ranges included in each study precluded this analysis. We considered also doing a subgroup analysis based on cumulative dose of ipratropium delivered. However, this analysis was potentially confounded by the severity of the asthma exacerbation of the children included in the trials as well as differences between the drug delivery methods (nebulizers versus metered‐dose inhalers (MDI) and spacers) and was thus omitted.
Sensitivity analysis
We performed sensitivity analyses to examine the effect on results of excluding unpublished trials, those with poor methodological quality and studies that included a small proportion of children under 18 months of age (less than 5% of total study participants).
Results
Description of studies
Results of the search
For the 2012 update, the search retrieved 223 references for screening (18 April 2012). We excluded studies that had already been assessed for inclusion in the first version of the review and obtained 19 citations for full‐text scrutiny. These citations referred to 19 studies (see Appendix 1 for details of previous search results) and seven new trials met the eligibility criteria for inclusion.
Included studies
Trial protocol/dose regimen
Overall, there were 20 included trials and three trials generated additional comparisons. Two studies provided stratified data subgrouped by severity (Qureshi 1998; Zorc 1999), and one study evaluated two different dosing strategies against control (Schuh 1995). Overall, there were 24 comparisons. We subgrouped trials according to the intensity of the anticholinergic protocol (see Table 2). Six trials on 570 children tested a single dose of ipratropium bromide added to multiple (two to six) doses of SABAs given every 20 to 30 minutes. We termed these the 'single‐dose protocol'.
1. Study treatments.
| Protocol | Study | Age range (years) | Delivery device | Dose of ipratropium bromide | Number of doses | Total dose delivered |
| Single dose | Beck 1985 | 6‐17.5 | Nebulized | 250 mcg | 1 | 250 mcg |
| Chakraborti 2006 | 5‐15 | MDI and spacer | 80 mcg | 1 | 80 mcg | |
| Cook 1985 | 12‐18 | Nebulized | 1‐2 mL of 0.025% solution | 1 | 1‐2 mL of 0.025% solution | |
| Ducharme 1998 | 3‐17 | Nebulized | 250 mcg | 1 | 250 mcg | |
| Phanichyakam 1990 | 4‐14 | MDI and spacer | 40 mcg | 1 | 40 mcg | |
| Schuh 1995 (single) (single dose) | 5‐17 | Nebulized | 250 mcg | 1 | 250 mcg | |
| Multiple fixed‐dose protocol | Benito Fernandez 2000 | 5 months to 16 years | Nebulized | 250 mcg | 2 | 500 mcg |
| BI [pers comm] | 2‐10 | Nebulized | 500 mcg | 3 | 1500 mcg | |
| Iramain 2011 | 2‐18 | Nebulized | 500 mcg children over 20 kg 250 mcg children under 20 kg |
6 | 3000 mcg or 1500 mcg | |
| Peterson 1996 | 5‐12 | Nebulized | 250 mcg | 2 | 500 mcg | |
| Qureshi 1997 | 6‐18 | Nebulized | 500 mcg | 2 | 1000 mcg | |
| Qureshi 1998 (moderate) | 2‐18 | Nebulized | 500 mcg | 2 | 1000 mcg | |
| Qureshi 1998 (severe) | 2‐18 | Nebulized | 500 mcg | 2 | 1000 mcg | |
| Reisman 1988 | 5‐15 | Nebulized | 250 mcg | 3 | 750 mcg | |
| Schuh 1995 (multiple dose) | 5‐17 | Nebulized | 250 mcg | 3 | 750 mcg | |
| Sharma 2004 | 6‐14 | Nebulized | 250 mcg | 3 | 750 mcg | |
| Sienra Monge 2000 | 8‐15 | MDI and spacer | 120 mcg | 2 | 240 mcg | |
| Watanasomsiri 2006 | 3‐15 | Nebulized | 250 mcg | 3 | 750 mcg | |
| Watson 1988 | 6‐17 | Nebulized | 250 mcg | 2 | 500 mcg | |
| Zorc 1999 (mild) | 1‐17 | Nebulized | 500 mcg | 2 | 1000 mcg | |
| Zorc 1999 (moderate) | 1‐17 | Nebulized | 500 mcg | 2 | 1000 mcg | |
| Zorc 1999 (severe) | 1‐17 | Nebulized | 500 mcg | 2 | 1000 mcg | |
| Multiple flexible‐dose protocol | Calvo 1998 | 5‐14 | MDI and spacer | 20 mcg | Max 7 | 140 mcg (max) |
| Guill 1987 | 13 months to 13 years | Nebulized | 0.05‐0.1 mg/kg | Max 3 | 0.15‐0.3 mg/kg (max) |
Max: maximum; MDI: metered‐dose inhaler.
In 16 intervention arms with 2191 children, doses of ipratropium bromide were administered at multiple fixed time points after the trial began. We termed these trials 'multiple fixed‐dose protocol'. The doses of ipratropium bromide varied from 120 mcg to 500 mcg and the number of doses ranged between two and six over 30 to 90 minutes.
Two studies including 84 children added a dose of anticholinergic to every SABA, leaving the number of inhalations determined by the child's need. These were termed 'multiple flexible‐dose protocol'. Calvo 1998 used a fixed dose of ipratropium bromide (20 mcg) given up to seven times in two hours. Treatment was stopped after the child's clinical score dropped below a predetermined threshold. Guill 1987 used a fixed dose of atropine sulfate (0.05 to 0.1 mg/kg) up to three times at 20‐minute intervals. Treatment was continued until symptoms were controlled or the subject was admitted to hospital.
With one exception that used atropine (Guill 1987), ipratropium bromide was used as the anticholinergic agent.
Participants
Three studies with children younger than 18 months were included (Benito Fernandez 2000; Guill 1987; Phanichyakam 1990). Only Benito Fernandez 2000 reported data for the primary outcome and we tested whether inclusion of this study would unduly affect the analysis (although unlikely, as the mean age of children in the study was 5.7 years).
Outcomes
The most frequently reported outcomes were hospital admission rate and spirometric measurements. Nineteen intervention arms (from 16 studies) reported our primary outcome of hospital admission rate. Of note, 84% of the weight in this outcome was contributed by trials focusing on children with documented or reported moderate or severe airway obstruction and 87% of the weight was contributed by trials in the higher and middle tertile of admission rate. Not all trials considered each outcome. The reporting of adverse and side effects was variable. Adverse effects such as hypertension or tachycardia were reported so infrequently that they could not be considered in this review. Whenever reported, we extracted side effects such as nausea, vomiting and tremor, which may interfere with children's compliance. All reported outcomes are listed in the Characteristics of included studies table.
Defining severity using admission rate
No single baseline spirometric characteristic was consistently reported across all studies. Hence insufficient lung function data were provided to enable us to determine the asthma severity of children at the start of trials. We ascertained asthma severity in two ways.
First, as per the original protocol, we stratified severity using lung function or clinical score at baseline and used both the eligibility/exclusion criteria and the severity reported by authors to confirm our assessment (see Table 3).
2. Severity based on review author assessment of trial report.
| Study | MEAN FEV1/PEFR (inclusion criteria) | MEAN score | Trial report description of severity | Review authors severity classification | Age |
| Beck 1995 | FEV 30‐31% (eligibility: FEV1 < 50% pred) | Not reported | Severe | Severe | 6 years‐upper range not reported |
| Benito‐Fernandez 2000 | Mean PEF: 45% predicted | 4.5 on a 5‐point score | Moderate to severe | Severe | 5 months to 16 years |
| BI (pers comm) | Unclear if measured | severity score of 13 (unknown scale) | Severe | Severe | 2‐10 years |
| Calvo (1998) | PEF = 69‐71% (excluded if PEF > 80% pred.) | 12‐point TAL score: 5.6‐6 (moderate) | Moderate | Moderate | 5‐14 years |
| Chakraborti (2006) | FEV1 69‐71 ± 37% (S+IB) and 57 ± 21% (S alone) | 10‐point clinical asthma score 1.83 (S+IB) vs. 2.07 (S alone) | Mild and moderate | Mild and moderate | 5‐15 years |
| Ducharme (1998) | Children requiring continuous nebulizations of salbutamol were excluded | Mild and moderate | Mild and moderate | 3‐7 years | |
| Iramain (2011) | FEV1 60‐64%/PEF 62‐60% | 15‐point pulmonary score: 12.3 | Moderate and severe | Moderate and severe | 2‐18 years |
| Pederson (1996) | Mean not reported (eligibility FEV1 < 70% pred.) | Not reported | Not reported | Moderate and severe | 5‐12 years |
| Qureshi (1997) | Mean FEV1 and PEF at baseline 37% and 34% (eligibility FEV1 < 50% pred.) | Not reported | Severe | Severe | 6‐18 years |
| Qureshi (1998) (sev) | Subgroup data provided by author: FEV1 34% (eligibility FEV1 < 50% pred.) |
12‐15 on 15‐point asthma score) | Severe | Severe | 2‐18 years |
| Qureshi (1998) (mod) | Subgroup data provided by author: FEV1 55% (eligibility: 50% < FEV1 < 70% pred.) |
8‐11 on a 15‐point Clinical score | Moderate | Moderate | 2‐18 years |
| Reisman (1998) | FEV1 33‐40% (eligibility; FEV1 < 55% pred.) | ‐ | Severe | Severe | 5‐15 years |
| Schuh (1995) | mean FEV1‐34% (eligibility; FEV1 < 50% pred.) | wheezing score 2.2‐2.5 on max of 3 | Severe | Severe | 5‐17 years |
| Sharma (2004) | PEF 35% pred. (excluded PEF < 30% pred. and signs of impending respiratory disease | Wheezing score 2.5 to 2.6 (max = 3) | Moderate | Severe | 6‐14 years |
| Sienra monge (2000) | FEV1: 1 L | not reported | Moderate to severe | Unrated | 8‐15 years |
| Watson (1988) | Mean FEV1 not reported (eligibility FEV1: 30‐70% pred.) | 5.7‐6 on a scale of 0 to 12 | Moderate and severe | Moderate and severe | 6‐17 years |
| Watanasomsiri (2006) | Mean % pred. PEFR at baseline 27‐30% in the subset of people cooperating with the forced expiratory technique)(PEFR was not an inclusion criteria) no exclusion or inclusion based on severity | 6.42 on a scale of 0 to 12 | Proportion of moderate asthma: 73% and of severe asthma: 27% | Moderate and severe | 3‐15 years |
| Zorc (1999) | Subgroups data obtained from authors | Not reported | ‐ | 3 strata (mild, moderate and severe) | 1‐17 years |
FEV1: forced expiratory volume in one second; IB: ipratropium bromide; max: maximum; PEF: peak expiratory flow; PEFR: peak expiratory flow rate; pred.: predicted; S: salbutamol; y.o.: years old.
Eight studies were judged as 'severe' (Benito Fernandez 2000; BI [pers comm]; Qureshi 1997; Qureshi 1998 (severe); Reisman 1988; Schuh 1995 (multiple); Sharma 2004; Zorc 1999 (severe)), four studies as 'moderate and severe' (Iramain 2011; Peterson 1996; Watanasomsiri 2006; Watson 1988), three studies as moderate (Calvo 1998; Qureshi 1998 (moderate); Zorc 1999 (moderate)), two studie as 'mild and moderate ' (Chakraborti 2006; Ducharme 1998), and one study as 'mild' (Zorc 1999 (mild)) asthma.
Sienra Monge 2000 did not provide enough information to confirm the authors' judgment and was left unrated (it did not provide data for the primary outcome).
Next, we used the control group event rate as a proxy for severity. Studies were ranked by the order of the admission rate of the control group, and divided into three groups and labelled as high, medium and low admission rates (see Table 4).
3. Studies grouped by control group event rate as a proxy for severity.
| Protocol | Study |
Admission rate (control group) |
Baseline population spirometry
characteristics (where available) |
Admission rate ‐ ranking |
| Multiple fixed‐dose protocol | Benito Fernandez 2000 | 0.53 | PEFR (%) mean 44.8 | High |
| Qureshi 1998 (severe) | 0.52 | PEFR < 50% predicted | High | |
| Schuh 1995 (multiple) | 0.46 | FEV1 < 50% predicted | High | |
| Qureshi 1997 | 0.45 | PEFR < 50% predicted | High | |
| Iramain 2011 | 0.43 | PEF or FEV1 < 60% predicted | High | |
| Zorc 1999 (severe) | 0.41 | Not reported | Medium | |
| Peterson 1996 | 0.30 | FEV1 < 70% predicted | Medium | |
| Zorc 1999 (moderate) | 0.26 | Not reported | Medium | |
| Reisman 1988 | 0.23 | FEV1 < 55% predicted | Medium | |
| Sharma 2004 | 0.16 | % predicted PEFR 34.4% (mean) | Medium | |
| Qureshi 1998 (moderate) | 0.09 | PEFR < 70% predicted | Low | |
| Watanasomsiri 2006 | 0.09 | % PEFR 29.2 | Low | |
| BI [pers comm] | 0.09 | Not reported | Low | |
| Zorc 1999 (mild) | 0.07 | Not reported | Low | |
| Watson 1988 | 0.00 | Not reported | Low | |
| Single‐dose protocol | Schuh 1995 (single) | 0.46 | FEV1 < 50% predicted | High |
| Ducharme 1998 | 0.17 | Respiratory resistance | Medium | |
| Chakraborti 2006 | 0.00 | FEV1 < 80% | Low |
FEV1: forced expiratory volume in one second; PEF: peak expiratory flow; PEFR: peak expiratory flow rate.
Ranking of severity based on admission rate in the control group corresponded somewhat but not perfectly to ranking based on mean baseline % predicted FEV1 in the studies that reported it or authors' reports. Ranking on admission rate permitted the inclusion of studies that did not use spirometry or did not report mean baseline % predicted FEV1. The ranking admission rate varied from 0 to 0.53.
Studies from the multiple dose‐fixed and single‐dose protocols provided hospital admission data and were, therefore, ranked in this manner.
We define six intervention arms from 'multiple dose‐fixed protocol' trials as a high admission rate (Benito Fernandez 2000; Iramain 2011; Qureshi 1997; Qureshi 1998 (severe); Schuh 1995 (multiple); Schuh 1995 (single)), six as medium admission rate (Ducharme 1998; Peterson 1996; Reisman 1988; Sharma 2004; Zorc 1999 (moderate); Zorc 1999 (severe)), and six as low admission rate (BI [pers comm]; Chakraborti 2006; Qureshi 1998 (moderate); Watanasomsiri 2006; Watson 1988; Zorc 1999 (mild)). We were unable to rank six studies because admission rates were not provided (Beck 1985; Calvo 1998; Cook 1985; Guill 1987; Phanichyakam 1990; Sienra Monge 2000).
Excluded studies
We excluded 30 studies; common reasons for exclusion of studies were: studies on people with chronic or stable asthma (N = 13), hospitalised people (N = 6), inappropriate protocols (e.g. different SABAs in each arm or SABAs at different doses) (N = 4), adults (N = 3), infants (N = 2), non‐randomized control trials (N = 2) and conference abstracts (N = 2). Full details are given in the Characteristics of excluded studies table.
Risk of bias in included studies
The judgment for each risk of bias domain for each study is included in the Characteristics of included studies table and an overview is given in Figure 1. Methodology of nine of the 19 trials was confirmed by the authors (Calvo 1998; Cook 1985; Ducharme 1998; Guill 1987; Peterson 1996; Qureshi 1997; Qureshi 1998 (severe); Schuh 1995; Zorc 1999 (moderate)).
1.

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
Allocation
We considered 10 studies to be at low risk of selection bias: randomisation was performed using computer‐generated random numbers in six studies (Chakraborti 2006; Ducharme 1998; Guill 1987; Iramain 2011; Peterson 1996; Zorc 1999 (mild)), tables of random numbers in three trials (Qureshi 1997; Qureshi 1998; Schuh 1995 (multiple); Schuh 1995 (single)), and one trial used block randomisation (Benito Fernandez 2000). Nine studies did not describe the method of randomisation and were classified as unclear risk of bias, one study used consecutive assignment and was judged to be at high risk of bias (Calvo 1998).
We considered 14 trials to be at low risk of bias for allocation concealment; 12 studies used number‐coded solutions supplied by the pharmacy (Beck 1985; Benito Fernandez 2000; Calvo 1998; Ducharme 1998; Iramain 2011; Peterson 1996; Qureshi 1997; Qureshi 1998 (moderate); Reisman 1988; Schuh 1995 (single); Watanasomsiri 2006; Zorc 1999 (mild)), one study used opaque consecutive numbered envelopes containing assignment (Guill 1987), and one study "used a person not involved in the study" (Chakraborti 2006). Allocation concealment was at unclear risk of bias in the remaining six studies.
Blinding
Sixteen studies claimed double‐blinding while two were described as triple‐blind (Ducharme 1998; Peterson 1996), and two were unblinded (Phanichyakam 1990; Sharma 2004). Twelve studies used an identical placebo in the control group, one study described a similarly looking intervention and placebo solutions (Cook 1985), and three studies did not provide details of the blinding method (BI [pers comm]; Sienra Monge 2000; Watson 1988). It was assumed that the person making the decision to admit to hospital was also blinded to the medication received but only one study described them as blinded (Schuh 1995).
Incomplete outcome data
Thirteen studies reported the presence/absence of participant withdrawal or dropout and the reasons for the attrition if applicable (BI [pers comm]; Calvo 1998; Cook 1985; Ducharme 1998; Guill 1987; Iramain 2011; Peterson 1996; Qureshi 1997; Qureshi 1998 (moderate); Schuh 1995; Watanasomsiri 2006; Watson 1988; Zorc 1999 (severe)). One study referred to 40 children in the abstract but presented data for only 30 children with no clear explanation (Sienra Monge 2000).
Selective reporting
One study recorded data for hospital admission but did not publish the data and commented that the "hospital admission data was not significant" (Beck 1985).
Effects of interventions
See: Table 1
First we present the primary outcome with all protocols, then we present the prespecified subgroup analyses (trial protocol, severity, co‐intervention of corticosteroid) followed by the secondary outcomes. We did not subgroup the secondary outcomes.
Primary outcome: admission to hospital
When anticholinergics were delivered in addition to SABAs, there was a significant decrease in the risk of hospital admission versus SABAs and placebo (RR 0.73; 95% CI 0.63 to 0.85; Figure 2). The primary analysis was inclusive of all study protocols (single dose, multiple‐fixed and multiple‐flexible); however, only studies using single and multiple‐fixed dose protocols reported data for this outcome. Fifteen studies (nineteen comparisons) reported data for this outcome, but the absence of admission in three studies mean that the pooled RR was based on data from 16 comparisons (2326 children). The result was statistically significant (P value < 0.0001) and there was no evidence of statistical heterogeneity between the study results (I2 = 0%). In the children receiving SABAs only, 23 people out of 100 were admitted to hospital compared with 17 (95% CI 15 to 20) out of 100 for children receiving SABAs plus anticholinergics (Figure 3). This represents an overall number needed to treat for an additional beneficial effect (NNTB) of 16 (95% CI 12 to 29).
2.

Forest plot of comparison: 1 Anticholinergic and short‐acting beta2‐agonists versus short‐acting beta2‐agonists alone (all protocols), outcome: 1.1 Primary outcome: hospital admissions.
3.

In the children on short‐acting beta2‐agonists only, 23 people out of 100 were admitted to hospital, compared with 17 (95% CI 15 to 20) out of 100 for children on short‐acting beta2‐agonists plus anticholinergics.
A funnel plot of the results for this outcome did not appear to indicate obvious signs of publication bias (Figure 4).
4.

Funnel plot of analysis 1.1.
Primary outcome: admission to hospital: subgroup analysis by study protocol
Subgrouping the studies by study protocol for the same outcome showed no statistically significant difference between subgroups (test for subgroup differences: Chi2 = 0.55, degrees of freedom (df) = 1, P value = 0.46, I2 = 0%), although the power was low due to marked group imbalances in weight (Analysis 1.2). Fifteen intervention arms including 1998 children were subgrouped into the multiple fixed‐dose protocol group. Of interest, anticholinergics delivered as a multiple fixed‐dose regimen in 11 studies on 1998 children led to a decrease in the risk of hospital admission by 28% compared with SABA alone (RR 0.72; 95% CI 0.61 to 0.84). This result was statistically significant (P value < 0.0001) with no evidence of heterogeneity (I2 = 0%). However, pooling data from only three studies (including 419 children) that used a single‐dose regimen showed no significant reduction in risk (RR 0.84; 95% CI 0.56 to 1.26). Studies grouped into the multiple flexible‐dose protocol did not provide data for this outcome. Due to subgroup imbalance in number and severity, we cannot firmly conclude on whether the intensity of therapy makes a difference in the magnitude of effect of treatment.
1.2. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 2 Primary outcome: hospital admissions subgrouped by trial protocol.
Primary outcome: admission to hospital: subgroup analysis by author's judgment of asthma severity and control group admission rate (multiple dose ‐ fixed protocol)
Efforts had been made in previous versions of this review to stratify studies by asthma severity based on baseline lung function or clinical score and validated by both the eligibility and exclusion criteria and the authors' assessment of severity (Table 3). The studies were classified as severe, moderate and severe, moderate, mild and moderate, and mild. There was no significant difference between the RRs in these severity subgroups (test for subgroup differences: Chi2 = 2.58, df = 4, P value = 0.63, I2 = 0%; Analysis 1.3). Admittedly, the number of subgroups may have decreased the power to detect statistically significant subgroup differences.
1.3. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 3 Primary outcome: hospital admissions subgrouped by review authors' judgment of trial report.
In the eight studies including 1188 children, judged as experiencing a severe asthma exacerbation, a 27% decrease in the risk of admission (RR 0.73; 95% CI 0.61 to 0.87) was observed with anticholinergic treatment.
In the four studies including 371 children, rated as having a moderate or severe asthma exacerbation, a 40% reduction in risk of hospitalisation (RR 0.60; 95% CI 0.41 to 0.89) was documented in favor of anticholinergic treatment. In the three studies with 463 children experiencing a moderate asthma exacerbation, no significant reduction in risk of hospitalisation (RR 0.77; 95% 0.49 to 1.22) was observed although the effect size was of similar magnitude to that in the preceding groups.
Two studies including 358 children experiencing mild and moderate asthma exacerbations showed a non‐significant reduction in hospital admission (RR 0.87; 95% CI 0.52 to 1.47).
In 117 children judged as experiencing a mild asthma exacerbation, there was no apparent beneficial effect on hospital admission with a wide confidence interval (RR 1.43; 95% CI 0.42 to 4.79).
In this review update, we subgrouped hospital admissions using our proxy measure for severity derived from the control group event rate for hospitalisation (see Table 4). The studies were grouped into tertiles corresponding to high, medium and low risk of admission (high risk, admission rate ≥ 43%; medium risk, admission rate 10% to 42%; and low risk < 10%). There was no significant difference between the RRs in the severity subgroups (test for subgroup differences: Chi2 = 1.68, df = 2, P value = 0.43, I2 = 0%).
Six studies on 669 children were grouped in the upper tertile with the highest control group admission rates: there was a 32% decrease in the risk of admission with the use of anticholinergics (RR 0.68; 95% CI 0.56 to 0.82; Analysis 1.4). In the six studies on 780 children in the medium‐risk group, the use of anticholinergics reduced the risk of admission by 25% (RR 0.75; 95% CI 0.57 to 0.99). In the subgroup with the lowest control group event rates that included six studies on 968 children, the use of anticholinergics did not significantly reduce the risk of hospital admission (RR 0.91; 95% CI 0.59 to 1.42). Comparing the control group weighted risk against the treatment group's risk, the NNTB in the high‐risk group was 7 (95% CI 5 to 12) versus 17 (95% CI 10 to 415) in the medium‐risk group and 139 (not statistically significant) in the low‐risk group (see Table 5).
1.4. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 4 Primary outcome: hospital admissions subgrouped by control group event rate (tertiles).
4. Number needed to treat for hospital admissions grouped by severity tertile.
| Risk tertile | Weighted control group risk | Risk ratio (95% CI) | Corresponding treatment group risk (95% CI) | NNTB (95% CI) |
| Low | 8.0% | 0.91 (0.59 to 1.42) | 7% (4 to 11) | 139 (not significant) |
| Medium | 24.1% | 0.75 (0.57 to 0.99) | 18% (14 to 24) | 17 (10 to 415) |
| High | 48.7% | 0.68 (0.56 to 0.82) | 33% (27 to 40) | 7 (5 to 12) |
Comparing the control group weighted risk against the treatment group's risk it can be seen that the NNTB in the high risk group was 7 versus 13 in the medium‐risk group and 131 in the low‐risk group.
CI: confidence interval; NNTB: number need to treat for an additional beneficial effect.
Irrespective of the methods to classify severity, the findings would suggest that, within this group of trials of children with predominantly moderate and severe asthma, those with the most severe airway obstruction and a baseline risk of admission of more than 40% showed the largest numerical benefit from the addition of anticholinergics, with an intermediate effect in those with moderate airway obstruction or a baseline risk of admission between 10% and 40% and no significant effect in the few trials pertaining only to children with mild asthma.
Primary outcome: admission to hospital: subgroup analysis by co‐intervention with corticosteroids
Studies were grouped by co‐intervention with corticosteroids (Analysis 1.5). There was no significant difference between the subgroups (test for subgroup differences: Chi2 = 0.66, df = 3, P value = 0.88, I2 = 0%). In the eight studies that administered corticosteroids to all children, the addition of an anticholinergic showed a reduction in the risk of hospital admission (RR 0.71; 95% CI 0.59 to 0.86). In the six studies that did not give corticosteroids to all children, the addition of an anticholinergic showed a significant reduction in risk (RR 0.67; 95% CI 0.47 to 0.94). In the three studies that administered corticosteroids at the physician's discretion, the point estimate was similar, but the confidence interval crossed unity (RR 0.77; 95% CI 0.54 to 1.10). This subgroup analysis would suggest that the effect of an anticholinergic was present, independently from the administration of systemic corticosteroids.
1.5. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 5 Primary outcome: hospital admissions subgrouped by co‐intervention of corticosteroid.
Secondary outcomes: lung function
Five studies on 402 children reported data for change in % predicted FEV1 across two study protocols (single‐ and multiple‐dose protocols) (Analysis 1.6). Children treated with an anticholinergic showed a significant improvement from baseline in % predicted FEV1 at 60 minutes compared to those treated with SABA alone (MD 10.08; 95% CI 6.24 to 13.92). Two studies on 117 children reported data for the same outcome at 120 minutes (Analysis 1.7), which also showed a statistically significant improvement in % predicted FEV1 in the treatment group versus the control group (MD 6.87; 95% CI 1.17 to 12.56). Fewer studies provided data for change in absolute FEV1 or in peak expiratory flow rate (PEFR) at 60 minutes (Analysis 1.8) or 120 minutes (Analysis 1.9); although in both cases appeared to favor the use of salbutamol plus an anticholinergic over salbutamol alone, it was only statistically significant at 60 minutes (SMD 0.57; 95% CI 0.25 to 0.88).
1.6. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 6 Change from baseline in % predicted FEV1, 60 minutes after the last of IB.
1.7. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 7 Change from baseline in % predicted FEV1, 120 minutes after last IB.
1.8. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 8 % Change in FEV1 or PEFR at 60 minutes after last IB (± 15 minutes).
1.9. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 9 % Change in FEV1 or PEFR at 120 minutes after last IB (± 30 minutes).
Secondary outcome: respiratory resistance
In a post hoc analysis of one trial examining 294 children with mild to moderate exacerbations, stratified on concurrent use of systemic corticosteroids, no statistically significant group difference in respiratory resistance observed at 60 minutes (Analysis 1.10) and at 120 minutes (Analysis 1.11) suggesting no significant influence of concurrent corticosteroids on the magnitude of effect associated with a single dose of ipratropium bromide (Ducharme 1998).
1.10. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 10 % Change in respiratory resistance at 60 minutes after IB (± 15 minutes).
1.11. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 11 % Change in respiratory resistance at 120 minutes after IB (± 30 minutes).
Secondary outcome: change in clinical score
No studies provided data on clinical score at 60 minutes; however, three studies on 934 children provided data for clinical score at 120 minutes. Of these three studies, only two included means with standard deviations. Combining these two results showed that subjects treated with an anticholinergic had a greater improvement in clinical score at 120 minutes (SMD ‐0.23; 95% CI ‐0.42 to ‐0.04; Analysis 1.12).
1.12. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 12 Change in clinical score at 120 minutes (± 30 minutes).
Secondary outcome: oxygen (O2) saturation at 60 minutes post therapy
In two trials reporting data for oxygen saturation there was a significant group difference at 60 minutes (RR 0.73; 95% CI 0.55 to 0.97; 415 children; Analysis 1.14), but not at 120 minutes (RR 1.10; 95% CI 0.76 to 1.59; 185 children; Analysis 1.15).
1.14. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 14 O2 saturation < 95% at 60 minutes (± 15 minutes).
1.15. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 15 O2 saturation < 95% at 120 minutes (± 30 minutes).
Secondary outcome: need for repeat bronchodilator treatment required after standard protocol prior to disposition
Nine studies on 1074 children reported data for the number of children who required repeat treatments of bronchodilator after the study protocol finished and before discharge. Subjects treated with the anticholinergic protocol were 13% less likely to require a repeat bronchodilator treatment before discharge (RR 0.87; 95% CI 0.79 to 0.97; Analysis 1.13).
1.13. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 13 Need for repeat bronchodilator treatment after standard protocol prior to disposition.
Secondary outcome: adverse events (tremor, vomiting and nausea)
Adverse events reported included tremor, vomiting and nausea. Analysis of nine studies on 524 children showed that children treated with the addition of an anticholinergic were significantly less likely to experience tremor than those treated with inhaled SABAs alone (RR 0.69; 95% CI 0.51 to 0.93; Analysis 1.17). Similarly, analysis of seven studies including 757 children showed that children treated with an anticholinergic were significantly less likely to suffer from nausea (RR 0.60; 95% CI 0.38 to 0.95; Analysis 1.19). Analysis of eight studies on 1230 children observed no statistically significant group difference on vomiting (RR 0.88; 95% CI 0.49 to 1.56; Analysis 1.18).
1.17. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 17 Tremor.
1.19. Analysis.
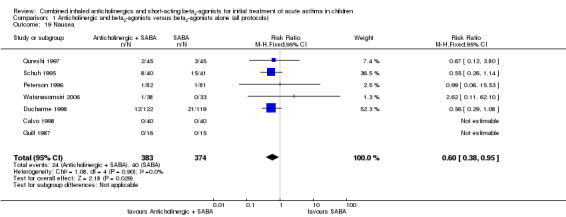
Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 19 Nausea.
1.18. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 18 Vomiting.
Secondary outcome: relapse
Ten studies on 1389 children reported the relapse rate of children initially discharged from the emergency department during the follow‐up period. No statistically significant difference between groups was observed (RR 1.07; 95% CI 0.68 to 1.68; Analysis 1.20).
1.20. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 20 Relapse.
Sensitivity analysis
Three sensitivity analyses were performed to examine the impact of unpublished data, the inclusion of studies with very young children and inclusion of trials with lower reported methodological quality.
Excluding the two unpublished studies (BI [pers comm]; Peterson 1996) from the primary outcome (all treatment protocols) did not alter the direction or significance level of the risk of admission (RR 0.72; 95% CI 0.61 to 0.84).
Of the three included studies that included children under 18 months of age (Benito Fernandez 2000; Guill 1987; Phanichyakam 1990), only Benito Fernandez 2000 contributed data to the primary outcome. Removing this study from the primary outcome (all treatment protocols) made very little difference to the overall result and no impact on the statistical significance (RR 0.74; 95% CI 0.63 to 0.86).
Excluding the five studies (Beck 1985; Calvo 1998; Chakraborti 2006; Phanichyakam 1990; Sharma 2004) with lower reported methodological quality and thus at higher risk of bias also did not alter the direction or significance level of the result (RR 0.74; 95% CI 0.64 to 0.86).
Discussion
Summary of main results
Primary outcome: hospital admissions
In children with predominantly moderate and severe asthma exacerbation, the combination of an anticholinergic and SABA significantly reduced the risk of hospital admission, the primary outcome of this review. This result was derived from 15 studies (19 comparisons) and 2497 children. Does this result alone justify the use of an anticholinergic in the treatment of all children with acute asthma? To investigate this further we performed several subgroup analyses and considered the validity of these results and their significance for practice.
Different treatment protocols used in the trials
An anticholinergic was administered as a single dose, multiple doses according to a fixed protocol, or a flexible dosing regimen where the an anticholinergic was systematically added to every SABA treatment until sufficient clinical improvement for discharge. This fundamental difference in treatment protocols prompted the first subgroup analysis by study protocol. The majority of data came from trials using multiple doses of ipratropium added to inhaled SABA with a fixed protocol: these trials used doses between 250 mcg and 500 mcg usually via a nebulizer device, with all but one using two or three doses over 30 to 90 minutes. There was a 28% statistically significant reduction in the risk of hospital admission in favor of anticholinergics. The three trials using a single‐dose protocol showed a smaller effect size that was not statistically significant and there were no admission data in the two trials using a flexible protocol. In absence of significant group difference in this analysis and the absence of head‐to‐head comparison between treatment protocols, there is insufficient information to judge how these different treatment protocols compare with each other. Until further data are available, it seems reasonable to recommend repeated doses of ipratropium bromide in a fixed protocol for one to two hours.
Asthma exacerbation severity
Examining participant characteristics and study author's classification of severity, it was evident that the overwhelming majority of trials were conducted in children with moderate or severe asthma, or both (not unexpected for studies performed in emergency departments). When the trials were broken down into severity subgroups based on the rate of admission to hospital in the control group (given inhaled SABAs alone) or based on lung function and clinical score, there was no significant difference between the severity subgroups.
The strongest evidence for a 32% and 25% statistically significant reduction in the risk of admission to hospital with an additional inhaled anticholinergic was seen from the trials classified with a high and medium admission rate, respectively, and while no evidence of effect was noted in the subgroup with the lowest admission rates, the small sample in this latter subgroup reduced the precision of this observation. Seven children (95% CI 5 to 12) needed to be treated in the high admission rate subgroup to prevent one hospital admission versus 17 (95% CI 10 to 415) in the medium admission rate subgroup and 139 (not statistically significant) in the low admission rate subgroup (see Table 5). Similarly, although there was no overall statistically significant subgroup difference when severity was based on lung function and clinical score, there was evidence of effect in all severity subgroups but those classified in the mild and mild‐moderate severity subgroups. We underline that, irrespective of the severity classification used, as the CIs for these subgroups were overlapping, there was no significant difference in RR or risk difference between the severity subgroups in this group of predominantly moderate and severe children. Until further data are available, it seems prudent to recommend the addition of anticholinergics in children with moderate or severe exacerbations.
Use of systemic corticosteroids
As the use of corticosteroids for the treatment of acute asthma is widely established in practice, we explored the possibility of effect modification by co‐intervention with oral corticosteroids (BTS 2011; GINA 2011; Lougheed 2012; Rowe 2001). There was some variation in the use of corticosteroids between studies; corticosteroids were either not used, were systematically used or their use was left to the discretion of the attending physician. We found no statistically significant group difference between the subgroups that did or did not administer systemic corticosteroids and no apparent difference in the size of the effect of an anticholinergic. This suggests that the effect of anticholinergics is not influenced by co‐intervention with systemic corticosteroids, at least not within two to three hours of corticosteroid administration (mean time to decision to admit in studies contributing data to the primary outcome two to three hours). This may be due to the delayed onset of action of systemic corticosteroids three to four hours after administration (Rowe 2001).
Other subgroups
Further subgroup analyses were considered for the 2012 update, but not performed. Analysis by age of child was not possible because of the significant overlap in children's ages between studies (see Table 2) and reported data were not stratified by age group in individual studies. Total dose of treatment could not be reliably established as the majority of studies used nebulizer devices to deliver the drug. With such a wide age range of subjects it is unclear what proportion of the delivered dose reaches the child's airways (Rubin 2003), which would make any comparison difficult to interpret reliably.
Secondary outcomes
Wide ranges of physiological and spirometric parameters were reported among the studies. However, such was the range of parameters used by different studies that the amount of data that could be pooled were limited.
Statistically significant group differences favoring anticholinergic use were observed for lung function whether reported as 'change in % predicted FEV1' or as '% change in FEV1', clinical score at 120 minutes, oxygen saturation at 60 minutes and the need for repeat use of bronchodilators prior to discharge from the emergency department. No significant group difference was seen for the relapse rate.
Adverse events
The combination of anticholinergics and SABAs was associated with a statistically significant 30% and 39% reduced risk of nausea and tremor, respectively. No significant group difference in vomiting was observed. Clinically important adverse effects, such as tachycardia or hypertension, were reported too infrequently to permit aggregation. Thus, the addition of anticholinergics to SABAs decreased the incidence of commonly observed beta2‐adrenergic effects in children treated with SABAs only.
Overall completeness and applicability of evidence
The age range of subjects in the included studies ranged from four months to 18 years (with eight studies including preschool‐aged children). The diagnosis of asthma in young children represents a considerable challenge to clinicians and wheezy symptoms in children younger than 12 months may represent a different disease process (such as bronchiolitis) to asthma. The inclusion of studies with infants less than 12 months of age was carefully considered for its potential to confound the results. In the studies that included subjects less than 18 months of age (Benito Fernandez 2000; Guill 1987; Phanichyakam 1990), the population age range was examined and in each case less than 5% of the population were under 18 months. With such a degree of homogeneity within the studies, the impact, if any, on the overall review was therefore likely to have been very small. Sensitivity analysis removing the only study (Benito Fernandez 2000) that reported admission data of the three studies that included children less than one year of age, did not alter the direction or magnitude of effect for the primary outcome (RR 0.74; 95% CI 0.63 to 0.86).
With over half of the studies in this review being carried out in North America, the validity of study results to other countries should be considered. Large geographical variations in hospital admission rates have been predominantly attributed to differences in asthma diagnosis, use of daily prophylaxis, intensity of emergency therapy, admission criteria and bed availabilities (Homer 1996; Payne 1995). It is acknowledged in the UK and elsewhere, that admission rates in chronic disease may also be influenced by factors such as population deprivation and availability of primary care services (Saxena 2006). These factors may limit the external validity (generalizability) of study results, but as they would be applied similarly to both the treatment and control group within each study, this would not affect the internal validity of the observed effect. Interestingly, the absence of statistical heterogeneity of study results across trials provides reassurance to the robustness of study results across countries from which these studies originated.
Quality of the evidence
Like all systematic reviews, this meta‐analysis is limited by the quality of existing data (Khan 1996). This review examined studies for bias against fixed pre‐established criteria in keeping with current Cochrane review methodology. Potential biases were identified in five out of the 19 studies contributing data to our primary outcome. However, there were a far greater number of studies where the potential for bias was unclear. This could be a reflection of poor methodological reporting or poor methodology. Sensitivity analysis removing studies with potential bias did not change the direction and significance of the results for the primary outcome in any of the treatment protocols (Beck 1985; Calvo 1998; Chakraborti 2006; Phanichyakam 1990; Sharma 2004). Data from two unpublished studies (BI [pers comm]; Peterson 1996) were included in this review and sensitivity analysis showed that removing them from the analysis did not impact significantly on the overall result for the primary outcome.
Although only one study clearly described the physician making the decision to admit as blinded to the study medication, the double‐blinded nature of nearly all trials (18 out of 20 were double blinded) would imply that study physicians making such clinical decisions were also blinded to the study medication. This should be clearly reported in future trials.
Despite these possible limitations, we did not consider them to have affected the strength or direction of the results for our primary outcome and so we rated the quality of evidence for the effect on hospital admission as high. However, unanswered questions remain regarding the optimal dose, intensity of therapy and severity of asthma exacerbations in relation to the reduced risk of hospital admission.
Potential biases in the review process
We attempted to minimize selection bias in the review process by using a broad search strategy and having the extracted data checked by a member of the editorial base of the Cochrane Airways Group.
Agreements and disagreements with other studies or reviews
The present review summarizes the best evidence available to April 2012. The first update identified three new trials not included in the first version of this review published in 1997. This update has added seven new trials. The conclusion regarding the single‐dose protocol and the multiple dose‐fixed protocol with regard the primary outcome remains unchanged due to the absence of new trials. With four additional trials in the multiple dose‐fixed protocol, the findings of the previous review were confirmed and strengthened. Thus supporting the addition of multiple doses of anticholinergics to SABAs for reducing hospital admission and improving lung function in children not only with severe but also with moderate asthma exacerbations.
The findings of this review are similar to other reviews examining a paediatric population (Rodrigo 2005). In their review, Rodrigo et al included 16 studies examining the use of anticholinergics plus SABAs versus SABAs alone in childhood asthma. Of those studies, 15 were examined in this review. Timsit 2002 was excluded from this review as the protocol examined a different dose of SABA in each treatment arm. The review concluded that "the addition of multiple doses of inhaled ipratropium bromide to SABAs is indicated in the standard treatment in children, adolescents and adults with moderate to severe exacerbations of asthma in the emergency setting" (Rodrigo 2005). In line with our definition, Rodrigo 2005 defined severity by baseline spirometric testing (FEV1 or peak expiratory flow (PEF) 50% to 70% of predicted as moderate exacerbation and FEV1 or PEF less than 50% of predicted as a severe exacerbation or different clinical scores).
Authors' conclusions
Implications for practice.
The findings of this Cochrane review support the use of anticholinergics in addition to SABAs over SABAs alone in the management of acute asthma in children, with an anticipated 27% reduction in the risk of hospital admission. The absence of statistically significant subgroup difference in treatment response between severity subgroups or those receiving different treatment intensity (multiple fixed‐dose versus single‐dose) prevents firm conclusions regarding a possible effect modification associated with treatment intensity and asthma severity. Yet, given the large weight of the trials pertaining to children with moderate or severe asthma (with an under‐representation of those with mild asthma) and the absence of effect in the mildest subgroup, it seems prudent to recommend the systematic use of anticholinergics in children with moderate or severe asthma only. Similarly, as most of the evidence is derived from trials testing a multiple fixed‐dose protocol using three doses of 250 mcg or two doses of 500 mcg of ipratropium bromide administered by nebulizer over 60 to 90 minutes, in combination with a SABA, we would recommend this treatment strategy and duration. There are also insufficient data to comment on the efficacy of adding anticholinergic to every SABA treatment, that is, titrating the number of both therapies to the child's response, on the optimal duration of combination therapy, and no evidence to explore whether the anticholinergic dose should be adjusted to the child's age. The addition of anticholinergics to SABAs was associated with significantly less tremor and nausea than SABAs alone.
Implications for research.
Future trials should be designed to address five issues, the impact on the magnitude of effect of: (1) the severity of airway obstruction (mild asthma and impending respiratory failure); (2) child's age (preschooler‐aged versus school‐aged children); (3) intensity/flexibility of therapy (fixed versus flexible); (4) dose of anticholinergics (250 mcg versus 500 mcg) and (5) delivery devices (nebulization versus inhalation). This could be done by conducting head‐to head comparisons with different doses, treatment intensity (multiple fixed‐dose versus multiple flexible‐dose protocols) and different inhalation modalities (metered dose inhaler versus nebulization) or by stratifying on child's age and baseline severity or both. Indeed, future research must attempt to define the severity of asthma exacerbation reliably and reproducibly in the study population by standardized methods (e.g. spirometry, respiratory resistance or interrupter technique) or validated clinical scores, or both. Any such attempts should consider the difficulties of using spirometric parameters in a young population (Arets 2001), although it offers the most objective means to ascertain the severity of airway obstruction reliably (Silverman 2007). Alternate measures include use of clinical scores with validated cut‐offs for severity such as the Pediatric Respiratory Assessment Measure (Chalut 2000; Ducharme 2008).
Because systemic glucocorticoids are now the standard treatment of children with moderate and severe exacerbations, they should be systematically given with SABAs in future trials. Trials are needed to examine the effect of adding anticholinergics in a multiple flexible‐dose manner, that is, each time a SABA is deemed necessary.
Future trials should include more sensitive and reliable endpoints such as number of bronchodilator inhalations and oxygenation (during the emergency department treatment), duration of hospitalisation and duration of need for intensive (at less than four‐hour interval) bronchodilator inhalation (for hospitalised children) and attempt to measure time to full recovery in discharged participants, that is, the duration of symptoms, rescue SABA use, functional status and quality of life.
In addition, it seems urgent to assess the efficacy and duration of anticholinergics in children with impending respiratory failure at risk of admission or admitted to the intensive care unit.
What's new
| Date | Event | Description |
|---|---|---|
| 4 September 2013 | Amended | Typo in BG affiliation amended |
History
Protocol first published: Issue 1, 1996 Review first published: Issue 2, 1997
| Date | Event | Description |
|---|---|---|
| 18 April 2012 | New citation required and conclusions have changed | Seven new studies added (Benito Fernandez 2000; Chakraborti 2006; Iramain 2011; Sharma 2004; Sienra Monge 2000; Watanasomsiri 2006) making a total of 20 studies including 2697 children; methods for assessing risk of bias updated; summary of findings table added; conclusions changed. |
| 18 April 2012 | New search has been performed | Literature search re‐run |
| 22 July 2008 | Amended | Converted to new review format. |
| 25 April 2000 | New citation required and conclusions have changed | Three new trials were included in this update; two pertaining to the multiple dose ‐ fixed protocol (Qhreshi, 1998; Zorc, 1999) and one pertaining to the multiple dose ‐ flexible protocol (Calvo, 1998); one previously unpublished trial (Ducharme, 1995) was published and is hereafter referred to as Ducharme, 1998. The addition of these new trials confirmed previous findings about the efficacy of intensive anticholinergics and beta2‐agonist inhalations in acute severe pediatric asthma. It raised the possibility that intensive therapy may have some benefits for children with moderate exacerbations by reducing the number of inhalations required after the fixed protocol. Despite a new trial testing the multiple‐dose flexible protocol, the evidence is still insufficient to conclude about the benefits of the systematic addition of anticholinergics to every beta2‐agonist inhalation, irrespective of disease severity. No new trial was added to the single dose protocol, which remains unchanged. |
Acknowledgements
No source of funding was available for this review. We thank Laurie Plotnick for his contribution to the previous version of this review. We thank the Cochrane Airways Review Group, namely Stephen Milan, Anna Bara, Elizabeth Stovold, Toby Lasserson, Christopher Cates and Emma Welsh for the literature search and ongoing support and Dr Paul Jones for his constructive comments. We also thank Dr Terry Klassen and Dr David McGillivray for their invaluable suggestions and Dr Francisco Noya and L Nannini for translating our correspondence to and from Spanish‐speaking study authors. We are indebted to the trials' authors, namely Drs M.F. Guill, F.A. Qureshi, S. Schuh, K.P. Dawson, T. Klassen, J.J. Zorc and G.M. Calvo, who cooperated enthusiastically to our requests for information.
Appendices
Appendix 1. Archive of search results for review
Issue 3, 2000
Forty studies were reviewed in full text for possible inclusion; 37 studies were identified by the literature search and bibliographies and three studies were identified by contact with trialists. A total of 13 randomised controlled trials were selected for inclusion.
Data and analyses
Comparison 1. Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: hospital admissions | 19 | 2497 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.63, 0.85] |
| 2 Primary outcome: hospital admissions subgrouped by trial protocol | 19 | 2497 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.63, 0.85] |
| 2.1 Single‐dose protocol | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.56, 1.26] |
| 2.2 Multiple fixed‐dose protocol | 15 | 1998 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.84] |
| 2.3 Multiple flexible‐dose protocol | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Primary outcome: hospital admissions subgrouped by review authors' judgment of trial report | 18 | 2497 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.63, 0.85] |
| 3.1 Severe | 8 | 1188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.87] |
| 3.2 Moderate‐severe | 4 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.89] |
| 3.3 Moderate | 3 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.49, 1.22] |
| 3.4 Mild‐moderate | 2 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.52, 1.47] |
| 3.5 Mild | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.42, 4.79] |
| 4 Primary outcome: hospital admissions subgrouped by control group event rate (tertiles) | 18 | 2417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.63, 0.85] |
| 4.1 High control group event rate (upper tertile) | 6 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.82] |
| 4.2 Medium control group event rate (middle tertile) | 6 | 780 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 0.99] |
| 4.3 Low control group event rate (lower tertile) | 6 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.59, 1.42] |
| 5 Primary outcome: hospital admissions subgrouped by co‐intervention of corticosteroid | 17 | 2407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.63, 0.84] |
| 5.1 Background corticosteroids | 8 | 1043 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.59, 0.86] |
| 5.2 No background corticosteroids | 6 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.47, 0.94] |
| 5.3 Variable (at physicians discretion) | 3 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.10] |
| 5.4 Not reported | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.48, 1.53] |
| 6 Change from baseline in % predicted FEV1, 60 minutes after the last of IB | 5 | 402 | Mean Difference (IV, Fixed, 95% CI) | 10.08 [6.24, 13.92] |
| 7 Change from baseline in % predicted FEV1, 120 minutes after last IB | 2 | 117 | Mean Difference (IV, Fixed, 95% CI) | 6.87 [1.17, 12.56] |
| 8 % Change in FEV1 or PEFR at 60 minutes after last IB (± 15 minutes) | 4 | 166 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.57 [0.25, 0.88] |
| 9 % Change in FEV1 or PEFR at 120 minutes after last IB (± 30 minutes) | 4 | 219 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.15, 0.39] |
| 10 % Change in respiratory resistance at 60 minutes after IB (± 15 minutes) | 1 | 294 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.07] |
| 10.1 Co‐intervention: corticosteroids during the previous 60 minutes | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 10.2 Co‐intervention: no corticosteroids | 1 | 224 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.02, 0.08] |
| 11 % Change in respiratory resistance at 120 minutes after IB (± 30 minutes) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 11.1 Co‐intervention: corticosteroids during the previous 120 minutes | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.12, 0.16] |
| 11.2 Co‐intervention: no corticosteroids | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 12 Change in clinical score at 120 minutes (± 30 minutes) | 3 | 934 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.42, ‐0.04] |
| 13 Need for repeat bronchodilator treatment after standard protocol prior to disposition | 9 | 1074 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.79, 0.97] |
| 14 O2 saturation < 95% at 60 minutes (± 15 minutes) | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.55, 0.97] |
| 15 O2 saturation < 95% at 120 minutes (± 30 minutes) | 2 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.76, 1.59] |
| 16 Need for corticosteroids in emergency department prior to disposition | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.08] |
| 17 Tremor | 9 | 524 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.51, 0.93] |
| 18 Vomiting | 8 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.49, 1.56] |
| 19 Nausea | 7 | 757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.95] |
| 20 Relapse | 10 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.68, 1.68] |
1.1. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 1 Primary outcome: hospital admissions.
1.16. Analysis.

Comparison 1 Anticholinergic and beta2‐agonists versus beta2‐agonists alone (all protocols), Outcome 16 Need for corticosteroids in emergency department prior to disposition.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beck 1985.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Hospital for sick children, Toronto, Canada DURATION OF STUDY: 7 months |
|
| Participants | N SCREENED: not available N RANDOMIZED: 28 N COMPLETED: 25 M = not available F = not available AGE: 6‐17.5 years BASELINE DETAILS: FEV1 < 50% predicted INCLUSION CRITERIA: acute attack, FEV1 < 50% predicted, able to perform spirometry consistently EXCLUSION CRITERIA: not available |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications during study)
FOLLOW‐UP PERIOD
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: % change in
FEV1 VITAL SIGNS: pulse, blood pressure, respiratory rate ADVERSE EFFECTS: tremor, vomiting ADMISSION: rate recorded but data not reported RELAPSE: within ? time CRITERIA FOR ADMISSION: not reported |
|
| Notes | Author (HL) contacted Confirmation by HL of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 children withdrawn due to protocol errors |
| Selective reporting (reporting bias) | High risk | Hospital admission data recorded but only reported as "not significantly different" |
| Other bias | Low risk | |
Benito Fernandez 2000.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Hospital de Cruces, Vizcaya Spain DURATION OF STUDY: 1 year |
|
| Participants | N SCREENED: not reported N RANDOMIZED: 102 N COMPLETED: 102 M = 67 F = 35 AGE: 5 months to 16 years (mean age 5.7 years) BASELINE DETAILS: both groups showed similar oxygen saturation and clinical scores at inclusion (oxygen saturations 93.5%, 4.45 and 4.35) (note: clinical score measured 0‐5) (described as moderate to severe group) INCLUSION CRITERIA: age 5 months to 16 years, severe acute asthma, previous recurrent wheezing episodes and previous asthma diagnosis EXCLUSION CRITERIA: acute or chronic pulmonary disease other than asthma, and children with their first wheezing episode |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTIONS
FOLLOW‐UP PERIOD
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: PEFR CLINICAL SCORE VITAL SIGNS: oxygen saturation NEED FOR ADDITIONAL SALBUTAMOL NEED TO SEEK MEDICAL ATTENTION IN 2 WEEKS HOSPITALIZATION CRITERIA FOR ADMISSION: not reported |
|
| Notes | Translated by Carlos Rodriguez Author not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | The department of pharmacy provided identical packages |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both solutions had an identical smell and physical appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in analysis (no withdrawals) |
| Selective reporting (reporting bias) | Unclear risk | Unable to ascertain |
| Other bias | Low risk | |
BI [pers comm].
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Philippines (multicenter) DURATION OF STUDY: 2002‐2003 |
|
| Participants | N SCREENED: not available N RANDOMIZED: 500 N COMPLETED: 500 M = not available F = not available AGE: 2‐10 years BASELINE DETAILS: not available INCLUSION CRITERIA: asthma exacerbation presenting to hospital EXCLUSION CRITERIA: children: with known or suspected hypersensitivity to study drugs, with medical condition that would contraindicate the use of beta2‐adrenergic or anticholinergic medications, with first wheezing episode only, with prior intubation for asthma for more than 24 hours, who used ipratropium in 6 hours prior to consultation, with concurrent stridor or possible presence of intrathoracic foreign body, with disease known to have chronic effect on respiratory function (e.g. CF or cardiac disease), requiring immediate resuscitation or airway intervention, with psychiatric disease or psychosocial problems, on other investigational drugs or have used any other investigational drugs within the past month |
|
| Interventions | PROTOCOL:
INTERVENTION GROUP
CONTROL GROUP
DEVICE
|
|
| Outcomes | ASTHMA SEVERITY SCORE NUMBER OF CHILDREN ASSESSED TO BE NEEDING HOSPITALIZATION AMOUNT OF ADDITIONAL RESCUE MEDICATION O2 SATURATION NUMBER OF DISCHARGED CHILDREN REVISITING THE EMERGENCY DEPARTMENT OR DOCTOR'S CLINIC WITHIN 24 HOURS CRITERIA FOR ADMISSION: not reported |
|
| Notes | Unpublished trial data supplied by Boehringer Ingelheim (Phil.) Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available; other information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double blind; other information not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals due to adverse events (1 in each group) |
| Selective reporting (reporting bias) | Unclear risk | Cannot ascertain this |
| Other bias | Unclear risk | Cannot ascertain this |
Calvo 1998.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Valdivia City, Chile DURATION OF STUDY: fall and winter |
|
| Participants | N SCREENED: not available N RANDOMIZED: 40 N COMPLETED: 40 M = 47 F = 33 AGE: 5‐14 years BASELINE DETAILS: PEF < 80% predicted and Tal Score > 5 INCLUSION CRITERIA: perform spirometry EXCLUSION CRITERIA: cardiac failure, lung disease, needed to be hospitalised, experienced a first acute bronchial obstruction, had hypersensitivity to the medications used, were treated 8 hours before the onset of this study |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: peak flow
CLINICAL
SCORE: Tal score
VITAL SIGNS: pulse,
respiratory rate
ADVERSE EFFECTS: tremor,
mydriatic reaction, dryness of the oral membrane,
pharyngeal irritation, nausea, vomiting
NUMBER OF CHILDREN NEEDING CORTICOSTEROIDS
ADMISSION CRITERIA FOR ADMISSION: not reported |
|
| Notes | Author (GMC) contacted Confirmation by GMC of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Consecutive assignment |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None identified |
Chakraborti 2006.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: paediatric chest clinic in Tertiary Hospital, North India DURATION OF STUDY: 1 year 6 months |
|
| Participants | N SCREENED: not available N RANDOMIZED: 60 N COMPLETED: 60 M = 36 F = 24 AGE: 5‐15 years BASELINE DETAILS: mild to moderate acute exacerbation of asthma (FEV1 < 80% predicted) INCLUSION CRITERIA: able to perform spirometry EXCLUSION CRITERIA: severe acute exacerbation, co‐existing cardiac or renal disease, intolerance to study medication, glaucoma, urinary retention, use of oral bronchodilator in last 12 hours |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL
TREATMENT PERIOD CO‐INTERVENTIONS
FOLLOW‐UP PERIOD
DEVICE
|
|
| Outcomes | HOSPITALIZATION PULMONARY FUNCTION TESTS: FEV1 , FEV1%, FVC, FVC%, FEF25‐75 , FEF%, FEV1/FVC, PEFR, PEFR% VITAL SIGNS: heart rate CLINICAL SCORE: wheeze score, accessory muscle score, clinical asthma score CRITERIA FOR ADMISSION: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocks of 6 |
| Allocation concealment (selection bias) | Low risk | "A person not involved in the study evaluation did the labelling" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "...similar looking MDIs..." "...10 resident doctors were asked to identify labelled MDIs as to which contained drug and placebo. Only 1 resident could identify the MDIs (placebo or ipratropium) correctly. 90% cases failed to identify" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data available for 60 children randomised |
| Selective reporting (reporting bias) | Low risk | All data relevant to outcomes in this review were reported in full |
| Other bias | High risk | Data only available for 30 minutes post‐treatment |
Cook 1985.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Christchurch Hospital, Christchurch, New Zealand DURATION OF STUDY: not available |
|
| Participants | N SCREENED: not available N RANDOMIZED: 48 N COMPLETED: 45 M = 26 F = 22 AGE: 18 months to 12 years BASELINE DETAILS: "moderately severe" INCLUSION CRITERIA: moderately severe asthma exacerbation EXCLUSION CRITERIA: children deemed to require intravenous therapy |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during study)
FOLLOW‐UP:
DEVICE
|
|
| Outcomes | CHANGE IN CLINICAL SCORE: overall score of wheeze, air entry and respiratory distress VITAL SIGNS: pulse, respiratory rate NEED FOR REPEAT TREATMENTS AFTER STANDARD PROTOCOL PRIOR TO DISPOSITION | |
| Notes | Co‐author (KPD) contacted Confirmation by KPD of methodology: obtained Confirmation of data extraction: pending For change in clinical score, no values for t = 0 minutes, therefore used t = 5 minutes as baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Three patients (1 in each group) required intravenous therapy and did not complete the trial. The results from these patients were excluded from the analysis" |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Ducharme 1998.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Montreal Children's Hospital, Canada DURATION OF STUDY: 30 months |
|
| Participants | N SCREENED: 858 N RANDOMIZED: 298 N COMPLETED: 298 M = 182 F = 116 AGE: 3‐17 years BASELINE DETAILS: "mild to moderate" asthma attack INCLUSION CRITERIA: exacerbation of mild or moderate severity requiring more than 1 nebulization of salbutamol. Ability to perform respiratory resistance consistently EXCLUSION CRITERIA: children with severe asthma, in whom short delay for documenting baseline respiratory resistance appeared unacceptable or in whom continuous nebulizations of salbutamol were prescribed |
|
| Interventions | PROTOCOL
INTERVENTION GROUP ‐ combination of:
CONTROL GROUP ‐ combination of:
CO‐INTERVENTION (other medications used during study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS:
% change in
RFo
CHANGE IN CLINICAL SCORE:
Wheezing score
O2
SATURATION
# REPEAT TREATMENTS REQUIRED AFTER
STANDARD PROTOCOL PRIOR TO DISPOSITION
VITAL
SIGNS: pulse
ADVERSE EFFECTS: tremor,
vomiting, nausea
NEED FOR CORTICOSTEROIDS
PRIOR TO DISCHARGE
ADMISSION
RELAPSE:
within 72 hours
RELAPSE
ADVERSE
EFFECTS: not described CRITERIA FOR ADMISSION: not reported |
|
| Notes | Author (FMD)
contacted Confirmation by FMD of methodology and data extraction obtained Factorial design ‐ tested 2 interventions simultaneously (frequent low doses of salbutamol + combination therapy with IB); no interaction observed between the 2 interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Coded solutions |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of unreported outcomes |
| Other bias | Low risk | No other bias identified |
Guill 1987.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Medical College of Georgia, USA DURATION OF STUDY: not available |
|
| Participants | N SCREENED: not available N RANDOMIZED: 44 N COMPLETED: 44 M = 26 F = 18 AGE: 13 months to 13 years BASELINE DETAILS: not available INCLUSION CRITERIA: bronchospasm for which bronchodilator treatment was considered necessary EXCLUSION CRITERIA: not available |
|
| Interventions | PROTOCOL
TEST GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: change in % predicted PEF CLINICAL SCORE: pulmonary index NUMBER OF REPEAT TREATMENTS REQUIRED PRIOR TO DISPOSITION ADVERSE EFFECTS: no details in study given but data confirmed ADMISSION: data not available RELAPSE: data not available | |
| Notes | Author (MFG) contacted Confirmation by MFG of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Opaque consecutive numbered envelopes containing assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebos used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial and were included in the analysis |
| Selective reporting (reporting bias) | Low risk | No evidence of unreported outcomes |
| Other bias | Low risk | No other bias identified |
Iramain 2011.
| Methods | STUDY DESIGN: parallel randomised double‐blind
trial LOCATION, NUMBER OF CENTERS: emergency department, location not reported DURATION OF STUDY: not available |
|
| Participants | N SCREENED: not available N RANDOMIZED: 106 N COMPLETED: 97 (49 salbutamol + ipratropium; 48 salbutamol + placebo) M = 49% F = 51% AGE: 2‐18 years BASELINE DETAILS: moderate to severe asthma INCLUSION CRITERIA: children presenting with moderate to severe asthma crises (episode of respiratory difficulty and wheezing associated with the use of intercostal muscles or subcostal retraction and fall in PEF and FEV1). EXCLUSION CRITERIA: mild asthma crisis, administration of corticosteroids in the previous 24 hours, presence of severe respiratory failure requiring admission to ICU or mechanical ventilation, history of respiratory failure requiring admission to ICU, cardiac or pulmonary malfunction, chronic lung disease (pulmonary bronchodysplasia, CF), stridor, foreign body aspiration, neurologic alterations or contraindications for the use of SABAs or anticholinergic medications |
|
| Interventions | PROTOCOL:
INTERVENTION GROUP
CONTROL GROUP
RUN‐IN PERIOD
CO‐INTERVENTIONS
FOLLOW‐UP
CRITERIA FOR ADMISSION
DEVICE
|
|
| Outcomes | FEV1 at 30, 60, 90, 120 and 240
minutes Hospital admission |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After obtaining informed consent from parents, patients were randomised using a computer generated sequence" |
| Allocation concealment (selection bias) | Low risk | Numbered bottles protect allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The hospital pharmacy department prepared two types of numbered plastic bottles.... The two solutions have the same smell, colour and fluid level in order to prevent differentiation. Neither the investigators nor the patients knew which solution the bottles contained" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts (2 from salbutamol + ipratropium due to tachycardia and 1 ICU admission; 1 from salbutamol due to tachycardia) |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found |
| Other bias | Unclear risk | None identified |
Peterson 1996.
| Methods | RANDOMIZATION
METHOD: computer generated
random numbers
MEANS: number‐coded solutions
supplied by the pharmacy
BLINDING:
triple‐blinding, identical placebo
WITHDRAWAL/DROPOUT: described LOCATION: Canada |
|
| Participants | N = 163 (IB + salbutamol N = 82, salbutamol only N = 81) AGE: 5‐12 years BASELINE SEVERITY: < 70% predicted FEV1 OTHER: ability to perform spirometry consistently | |
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during study)
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: change in % predicted
FEV1, change in predicted PEF
O2 SATURATION
# REPEAT
TREATMENTS REQUIRED AFTER STANDARD PROTOCOL PRIOR TO
DISPOSITION
VITAL SIGNS: blood pressure,
pulse, respiratory rate
ADVERSE EFFECTS:
rash, dizziness, hyperkinesia, hypertonia, tremor,
conjunctivitis, taste perversion, vomiting,
abdominal pain, nausea, coughing, epistaxis,
pneumonia, pneumothorax, asthma exacerbation,
headache, chest pain, fever, pain, sinusitis,
stridor, bacterial infection
NEED FOR
CORTICOSTEROIDS PRIOR TO DISPOSITION
ADMISSION CRITERIA FOR ADMISSION: not reported RELAPSE: within 72 hours |
|
| Notes | Unpublished multicenter trial Co‐investigator (TK) contacted and granted permission to use unpublished data Confirmation by TK of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Cannot be ascertained |
| Selective reporting (reporting bias) | Unclear risk | Cannot be ascertained |
| Other bias | Unclear risk | Insufficient details provided (unpublished data) |
Phanichyakam 1990.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Ramathibodi Hospital, Bangkok, Thailand DURATION OF STUDY: not available |
|
| Participants | N SCREENED: not available N RANDOMIZED: 20 N COMPLETED: 20 M = 14 F = 6 AGE: 4 months to 15 years BASELINE DETAILS: not available INCLUSION CRITERIA: not available EXCLUSION CRITERIA: not available |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: % change in FEV1, % change in PEF VITAL SIGNS: blood pressure, heart rate, respiratory rate ADVERSE EFFECTS: 'ocular', 'secretion‐drying', 'facial‐flushing' | |
| Notes | Contact of 3 authors attempted but unsuccessful Confirmation of methodology and data extraction not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Each child received either 0.5 mg (2 puffs) of inhaled terbutaline or inhaled terbutaline 0.5 mg + inhaled IB 0.04mg (2 puffs) 15 minutes later" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | No unreported outcomes identified |
| Other bias | Low risk | No other bias identified |
Qureshi 1997.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Hospital of The King's Daughters, Virginia, USA DURATION OF STUDY: 11 months |
|
| Participants | N SCREENED: not available N RANDOMIZED: 90 N COMPLETED: 90 M = 55 F = 35 AGE: 6‐18 years BASELINE DETAILS: < 50% predicted PEFR INCLUSION CRITERIA: asthma exacerbation: difficulty breathing, wheezing and worsening of the child's usual symptoms or deterioration of pulmonary functions. The ability to perform reliable pulmonary function testing EXCLUSION CRITERIA: evidence of an intrathoracic foreign body, high clinical suspicion of pneumonia, concurrent stridor, any disease process known to chronically affect respiratory function |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during the study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS:
Change in predicted
FEV1,
Change in predicted
PEF
O2 SATURATION
VITAL
SIGNS: blood pressure, heart rate, respiratory
rate
ADVERSE EFFECTS: increasing
breathlessness, dry mouth, palpitations, blurred
vision, dilation of pupils, nausea, vomiting, mental
confusion, dizziness, headache, back and chest
pain
# REPEAT TREATMENTS REQUIRED AFTER
STANDARD PROTOCOL PRIOR TO DISPOSITION
ADMISSION CRITERIA FOR ADMISSION: oxygen saturations < 94% RELAPSE: within 48 hours |
|
| Notes | Author (FQ)
contacted Confirmation by FQ of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | No unreported outcomes identified |
| Other bias | Low risk | No other sources of bias identified |
Qureshi 1998.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Hospital of The King's Daughters, Virginia, USA DURATION OF STUDY: 8 months |
|
| Participants | N SCREENED: not available N RANDOMIZED: 480 N COMPLETED: 434 M = 248 F = 186 AGE: 2‐18 years (163 moderate group) BASELINE DETAILS: < 70% predicted PEFR INCLUSION CRITERIA: asthma exacerbation: increased difficulty breathing, wheezing and worsening of the child's usual symptoms or deterioration of PEFR EXCLUSION CRITERIA: treatment with ipratropium within 6 hours before the visit, a disease known to have a chronic effect on respiratory function, concurrent stridor, possible presence of an intrathoracic foreign body, a medical condition that would contraindicate the use of beta2 adrenergic or anticholinergic medications, or need for immediate resuscitation or airway intervention |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during the study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: % change in PEFR CHANGE IN CLINICAL SCORE O2 SATURATION # NEBULIZER TREATMENTS UNTIL DISPOSITION TIME TO DISPOSITION VITAL SIGNS: pulse, respiratory rate ADMISSION CRITERIA FOR ADMISSION: "objective changes in clinical and pulmonary function and oxygen saturations in air" (saturations > 94% in air were accepted) RELAPSE |
|
| Notes | Author (FQ) contacted Confirmation by FQ of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In 46 children, symptoms resolved before the second dose of the study medication. 18 received a single dose of albuterol and improved. Of the remaining 26 children who had a moderate exacerbation, the split between treatment groups was even (14 and 12). This does not affect the results for outcome: hospital admission, but it may introduce bias in clinical scores since data would be collected on those children more likely to experience a change in their scores over the course of the trial protocol |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Qureshi 1998 (moderate).
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Hospital of The King's Daughters, Virginia, USA DURATION OF STUDY: 8 months |
|
| Participants | N SCREENED: not available N RANDOMIZED: 480 N COMPLETED: 434 M = 248 F = 186 AGE: 2‐18 years (163 moderate group) BASELINE DETAILS: < 70% predicted PEFR INCLUSION CRITERIA: asthma exacerbation: increased difficulty breathing, wheezing and worsening of the child's usual symptoms or deterioration of PEFR EXCLUSION CRITERIA: treatment with ipratropium within 6 hours before the visit, a disease known to have a chronic effect on respiratory function, concurrent stridor, possible presence of an intrathoracic foreign body, a medical condition that would contraindicate the use of beta2 adrenergic or anticholinergic medications, or need for immediate resuscitation or airway intervention |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during the study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: % change in PEFR CHANGE IN CLINICAL SCORE O2 SATURATION # NEBULIZER TREATMENTS UNTIL DISPOSITION TIME TO DISPOSITION VITAL SIGNS: pulse, respiratory rate ADMISSION CRITERIA FOR ADMISSION: "objective changes in clinical and pulmonary function and oxygen saturations in air" (saturations > 94% in air were accepted) RELAPSE |
|
| Notes | Author (FQ) contacted Confirmation by FQ of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In 46 children symptoms resolved before the second dose of the study medication. 18 received a single dose of albuterol and improved. Of the remaining 26 children who had a moderate exacerbation, the split between treatment groups was even (14 and 12). This does not affect the results for outcome: hospital admission, but it may introduce bias in clinical scores since data would be collected on those children more likely to experience a change in their scores over the course of the trial protocol |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Qureshi 1998 (severe).
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Hospital of The King's Daughters, Virginia, USA DURATION OF STUDY: 8 months |
|
| Participants | N SCREENED: not available N RANDOMIZED: 480 N COMPLETED: 434 M = 248 F = 186 AGE: 2‐18 years (271 severe group) BASELINE DETAILS: < 50% predicted PEFR INCLUSION CRITERIA: asthma exacerbation: increased difficulty breathing, wheezing and worsening of the child's usual symptoms or deterioration of PEFR EXCLUSION CRITERIA: treatment with ipratropium within 6 hours before the visit, a disease known to have a chronic effect on respiratory function, concurrent stridor, possible presence of an intrathoracic foreign body, a medical condition that would contraindicate the use of beta2 adrenergic or anticholinergic medications, or need for immediate resuscitation or airway intervention |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during the study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: % change in PEFR CHANGE IN CLINICAL SCORE O2 SATURATION # NEBULIZER TREATMENTS UNTIL DISPOSITION TIME TO DISPOSITION VITAL SIGNS: pulse, respiratory rate ADMISSION CRITERIA FOR ADMISSION: "objective changes in clinical and pulmonary function and oxygen saturations in air" (saturations > 94% in air were accepted) RELAPSE |
|
| Notes | Author (FQ)
contacted Confirmation by FQ of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In 46 children symptoms resolved before the second dose of the study medication. 18 received a single dose of albuterol and improved. Of the remaining 26 children who had a moderate exacerbation, the split between treatment groups was even (14 and 12). This does not affect the results for outcome: hospital admission, but it may introduce bias in clinical scores since data would be collected on those children more likely to experience a change in their scores over the course of the trial protocol |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Reisman 1988.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Hospital for Sick Children, Toronto, Canada DURATION OF STUDY: 12 months |
|
| Participants | N SCREENED: not available N RANDOMIZED: 25 N COMPLETED: 24 M = not available F = not available AGE: 5‐15 years BASELINE DETAILS: < 55% predicted FEV1 INCLUSION CRITERIA: children who could perform spirometry and < 55% predicted FEV1 EXCLUSION CRITERIA: not available |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: change in % predicted
FEV1
CHANGE IN WHEEZING
SCORE
ADMISSION
CRITERIA FOR
ADMISSION: not reported RELAPSE: not reported ADVERSE EFFECTS: tremor, vomiting |
|
| Notes | Author (HL) contacted Confirmation of methodology and data extraction not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information available |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 subject not completing the study was too tired to co‐operate with the spirometry |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Schuh 1995.
| Methods | STUDY DESIGN (parallel, cross‐over): Parallel LOCATION, NUMBER OF CENTERS: Hospital for Sick Children, Toronto, Canada DURATION OF STUDY: 2 years 2 months |
|
| Participants | N SCREENED: 1586 N RANDOMIZED: 79 N COMPLETED: unclear M = not available F = not available AGE: 5‐17 years BASELINE DETAILS: FEV1 < 50% predicted INCLUSION CRITERIA: acute asthma attack, were able to perform the pulmonary function testing reliably, FEV1 < 50% predicted EXCLUSION CRITERIA: children with 1st wheezing episode, used IB in last 4 hours prior to study start, previous PICU admission, concurrent cardiopulmonary disease, near death and requiring immediate intervention, known hypersensitivity to study drugs. |
|
| Interventions | PROTOCOL
TEST GROUPS
CONTROL GROUP
RUN‐IN PERIOD: none CO‐INTERVENTION (other medications used during study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS ‐ change in % predicted FEV1 OXYGEN SATURATION CHANGE IN CLINICAL SCORE: accessory muscle, wheeze, dyspnoea and overall # REPEAT TREATMENTS REQUIRED AFTER STANDARD PROTOCOL PRIOR TO DISPOSITION VITAL SIGNS: heart rate, respiratory rate ADVERSE EFFECTS: nausea, tremor, conjunctivitis, coughing spasm with syncope ADMISSION: described 'children with respiratory distress' RELAPSE: within 72 hours | |
| Notes | Author (SS) contacted Confirmation of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 parent changed his mind and demanded withdrawal before the start of experimental therapy. "Seven patients had to stop the trial prematurely at either 60 or 80 minutes; three children (one each in groups 1, 2 and 3) stopped cooperating before the 120 minute measurement, and three children in group 2 and one child in group 3 were too ill to continue. Two patients had missing data at 80 minutes (because of vomiting) but both had recovered by 120 minutes" |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Schuh 1995 (multiple).
| Methods | See table Schuh 1995 | |
| Participants | N SCREENED: 1586 N RANDOMIZED: 81 N COMPLETED: unclear M = not available F = not available AGE: 5‐17 years BASELINE DETAILS: FEV1 < 50% predicted |
|
| Interventions | PROTOCOL
TEST GROUPS
CONTROL GROUP
RUN‐IN PERIOD
CO‐INTERVENTION (other medications used during study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS ‐ change in % predicted FEV1 OXYGEN SATURATION CHANGE IN CLINICAL SCORE: accessory muscle, wheeze, dyspnoea and overall # REPEAT TREATMENTS REQUIRED AFTER STANDARD PROTOCOL PRIOR TO DISPOSITION VITAL SIGNS: heart rate, respiratory rate ADVERSE EFFECTS: nausea, tremor, conjunctivitis, coughing spasm with syncope ADMISSION: described 'children with respiratory distress' RELAPSE: within 72 hours | |
| Notes | Author (SS) contacted Confirmation of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 parent changed his mind and demanded withdrawal before the start of experimental therapy. "Seven patients had to stop the trial prematurely at either 60 or 80 minutes; three children (one each in groups 1, 2 and 3) stopped cooperating before the 120 minute measurement, and three children in group 2 and one child in group 3 were too ill to continue. Two patients had missing data at 80 minutes (because of vomiting) but both had recovered by 120 minutes" |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Schuh 1995 (single).
| Methods | See table Schuh 1995 | |
| Participants | N SCREENED: 1586 N RANDOMIZED: 79 N COMPLETED: unclear M = not available F = not available AGE: 5‐17 years BASELINE DETAILS: FEV1 < 50% predicted INCLUSION CRITERIA: acute asthma attack, were able to perform the pulmonary function testing reliably, FEV1 < 50% predicted EXCLUSION CRITERIA: children with first wheezing episode, used IB in last 4 hours prior to study start, previous PICU admission, concurrent cardiopulmonary disease, near death and requiring immediate intervention, known hypersensitivity to study drugs |
|
| Interventions | PROTOCOL
TEST GROUPS
CONTROL GROUP
RUN‐IN PERIOD
CO‐INTERVENTION (other medications used during study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS ‐ Change in % predicted FEV1 OXYGEN SATURATION CHANGE IN CLINICAL SCORE: accessory muscle, wheeze, dyspnoea and overall # REPEAT TREATMENTS REQUIRED AFTER STANDARD PROTOCOL PRIOR TO DISPOSITION VITAL SIGNS: heart rate, respiratory rate ADVERSE EFFECTS: nausea, tremor, conjunctivitis, coughing spasm with syncope ADMISSION: described 'children with respiratory distress' RELAPSE: within 72 hours | |
| Notes | Author (SS) contacted Confirmation of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Allocation concealment (selection bias) | Low risk | Identical placebo |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 1 parent changed his mind and demanded withdrawal before the start of experimental therapy. "Seven patients had to stop the trial prematurely at either 60 or 80 minutes; three children (one each in groups 1, 2 and 3) stopped cooperating before the 120 minute measurement, and three children in group 2 and one child in group 3 were too ill to continue. Two patients had missing data at 80 minutes (because of vomiting) but both had recovered by 120 minutes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None identified |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | |
Sharma 2004.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: India, 1 center |
|
| Participants | N SCREENED: not available N RANDOMIZED: 50 N COMPLETED: 50 M = not available F = not available AGE: 6‐14 years BASELINE DETAILS: % PEFR 34% (mean baseline of study population) INCLUSION CRITERIA: acute exacerbation of bronchial asthma defined as increasing cough, inability to speak in sentences and drink, wheezing and chest recession EXCLUSION CRITERIA: children with life‐threatening or severe attack characterized by cyanosis, silent chest or poor air entry, marked dyspnoea so that a child was unable to speak even 3 or 4 words, PEFR < 30% for height, received bronchodilator 6 hours prior to admission, previous ITU admission |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
RUN‐IN PERIOD
CO‐INTERVENTIONS
FOLLOW‐UP
DEVICE
|
|
| Outcomes | IMPROVEMENT IN % PREDICTED PEFR RESPIRATORY RATE WHEEZE SCORE USE OF ACCESSORY MUSCLES SCORES HOSPITAL ADMISSION RATE: reported ADMISSION RATE: not reported ADVERSE EVENTS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The study was not blinded..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Sienra Monge 2000.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Children's Hospital of Mexico, Mexico DURATION OF STUDY: not available |
|
| Participants | N SCREENED: 40 N RANDOMIZED: 30 N COMPLETED: 30 M = 18 F = 12 AGE: 8‐15 years BASELINE DETAILS: not available INCLUSION CRITERIA: acute asthma presenting to the emergency department (mild to severe according to the Woodran Scale) EXCLUSION CRITERIA: not available |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION
FOLLOW‐UP
DEVICE
|
|
| Outcomes | CHANGE IN FEV1 HOSPITALIZATION RATE ADMISSION CRITERIA: not reported |
|
| Notes | Translated by Luis Nannini | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 40 children referred to in the abstract but results are given for a study population of 30 |
| Selective reporting (reporting bias) | Unclear risk | Unable to ascertain this |
| Other bias | Low risk | None identified |
Watanasomsiri 2006.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: Thammasat University Hospital, Thailand DURATION OF STUDY: 2 years 5 months |
|
| Participants | N SCREENED: not available N RANDOMIZED: 74 N COMPLETED: 71 M = not available F = not available AGE: 3‐15 years BASELINE DETAILS: % PEFR 29.2 (baseline characteristics of study group) INCLUSION CRITERIA: clinical diagnosis of asthma, moderate to severe asthma attack (children younger than 5 years were required to have 3 or more episodes of wheezing before the presenting illness) EXCLUSION CRITERIA: first‐time episode of wheeze; co‐existent cardiac, renal or other chronic pulmonary disease; bronchopulmonary dysplasia; intolerance to study drugs; glaucoma or urinary retention. Children who had used IP within 24 hours, used oral corticosteroids within 3 days and required immediate resuscitation or airway intervention were also excluded from the study |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTIONS
FOLLOW‐UP PERIOD
DEVICE
|
|
| Outcomes | CHANGE IN CLINICAL SCORE PULMONARY FUNCTION TESTS: % change in actual PEFR from baseline, changes in % predicted PEFR VITAL SIGNS: respiratory rate, heart rate, oxygen saturation NEED FOR ADDITIONAL SALBUTAMOL DOSES HOSPITALIZATION ADMISSION CRITERIA: not reported RELAPSE: within 3 days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Low risk | "Solutions supplied by pharmacy", "Solutions drawn up an independent nurse..." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "...parents and physicians were unaware of vial contents" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 children withdrawn for technical reasons (errors in protocol) 1 child withdrawn by parents |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Watson 1988.
| Methods | RANDOMIZATION: method and means not described BLINDING: double‐blind, not described WITHDRAWAL/DROPOUT: none JADAD's QUALITY SCORE: 3 |
|
| Participants | N: 31 AGE: 6‐17 years BASELINE SEVERITY: 30‐70% predicted FEV1 COUNTRY: Canada OTHER: ability to perform spirometry consistently | |
| Interventions | PROTOCOL
TEST GROUP
CONTROL GROUP
CO‐INTERVENTION
FOLLOW‐UP
DEVICE
|
|
| Outcomes | PULMONARY FUNCTION TESTS: change in % predicted FEV1, % change in FEV1 OXYGEN SATURATION: no details given CHANGE IN CLINICAL SCORE: pulmonary Index, no details given ADVERSE EFFECTS: tremor, "no adverse effects" but none other specified ADMISSION: described RELAPSE: not mentioned | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no other information available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Unable to ascertain this reliably |
| Other bias | Low risk | None identified |
Zorc 1999.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: John Hopkins Hospital, Baltimore, USA DURATION OF STUDY: 1 year |
|
| Participants | N SCREENED: 1215 N RANDOMIZED: 427 N COMPLETED: 427 M = 298 F = 129 AGE: 1‐17 years BASELINE DETAILS: initial severity score of 1‐3, excluded children if required initial therapy in addition to the critical pathway ‐ initial severity score was incomplete/missing for 14% of the total 427 children of the full study INCLUSION CRITERIA: children who were to be treated according to a standardized emergency department protocol for acute asthma EXCLUSION CRITERIA: mild illness; decision not to treat on critical pathway; severe presentation requiring additional therapy; pretreatment with corticosteroids or IB; a history of glaucoma, CF or sickle cell disease |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during the study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | # NEBULIZER TREATMENTS UNTIL DISPOSITION
TIME
TO DISPOSITION
ADMISSION: to ward, to ICU ADMISSION CRITERIA: not reported RELAPSE |
|
| Notes | Author (MP) contacted Confirmation by MP of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | Trial protocol not reviewed |
| Other bias | Low risk | None identified |
Zorc 1999 (mild).
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: John Hopkins Hospital, Baltimore, USA DURATION OF STUDY: 1 year |
|
| Participants | N SCREENED: 1215 N RANDOMIZED: 427 N COMPLETED: 427 M = 298 F = 129 AGE: 1‐17 years MILD GROUP N = 117 BASELINE DETAILS: initial severity score of 1‐3, excluded children if required initial therapy in addition to the critical pathway ‐ initial severity score was incomplete/missing for 14% of the total 427 children of the full study INCLUSION CRITERIA: children who were to be treated according to a standardized emergency department protocol for acute asthma EXCLUSION CRITERIA: mild illness; decision not to treat on critical pathway; severe presentation requiring additional therapy; pretreatment with corticosteroids or IB; a history of glaucoma, CF or sickle cell disease |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during the study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | # NEBULIZER TREATMENTS UNTIL DISPOSITION
TIME
TO DISPOSITION
ADMISSION: to ward, to ICU ADMISSION CRITERIA: not reported RELAPSE |
|
| Notes | Author (MP) ‐ contacted Confirmation by MP of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | Trial protocol not reviewed |
| Other bias | Low risk | None identified |
Zorc 1999 (moderate).
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTERS: John Hopkins Hospital, Baltimore, USA DURATION OF STUDY: 1 year |
|
| Participants | N SCREENED: 1215 N RANDOMIZED: 427 N COMPLETED: 427 M = 298 F = 129 AGE: 1‐17 years MODERATE GROUP: N = 194 BASELINE DETAILS: initial severity score of 4‐6, excluded if required initial therapy in addition to the critical pathway ‐ initial severity score was incomplete/missing for 14% of the total 427 children of the full study INCLUSION CRITERIA: children who were to be treated according to a standardized emergency department protocol for acute asthma EXCLUSION CRITERIA: mild illness; decision not to treat on critical pathway; severe presentation requiring additional therapy; pretreatment with corticosteroids or IB; a history of glaucoma, CF or sickle cell disease |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during the study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | # NEBULIZER TREATMENTS UNTIL DISPOSITION
TIME
TO DISPOSITION
ADMISSION: to ward, to ICU ADMISSION CRITERIA: not reported RELAPSE |
|
| Notes | Author (MP) contacted Confirmation by MP of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | Trial protocol not reviewed |
| Other bias | Low risk | None identified |
Zorc 1999 (severe).
| Methods | STUDY DESIGN: Parallel LOCATION, NUMBER OF CENTERS: John Hopkins Hospital, Baltimore, USA DURATION OF STUDY: 1 year |
|
| Participants | N SCREENED: 1215 N RANDOMIZED: 427 N COMPLETED: 427 M = 298 F = 129 AGE: 1‐17 years SEVERE GROUP: N = 51 BASELINE DETAILS: initial severity score of 7‐9, excluded children if respiratory failure or required initial therapy in addition to the critical pathway ‐ initial severity score was incomplete/missing for 14% of the total 427 children of the full study INCLUSION CRITERIA: children who were to be treated according to a standardized emergency department protocol for acute asthma EXCLUSION CRITERIA: mild illness; decision not to treat on critical pathway; severe presentation requiring additional therapy; pretreatment with corticosteroids or IB; a history of glaucoma, CF or sickle cell disease |
|
| Interventions | PROTOCOL
INTERVENTION GROUP
CONTROL GROUP
CO‐INTERVENTION (other medications used during the study)
FOLLOW‐UP
DEVICE
|
|
| Outcomes | # NEBULIZER TREATMENTS UNTIL DISPOSITION
TIME
TO DISPOSITION
ADMISSION: to ward, to ICU ADMISSION CRITERIA: not reported RELAPSE |
|
| Notes | Author (MP) contacted Confirmation by MP of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Number‐coded solutions supplied by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | Trial protocol not reviewed |
| Other bias | Low risk | None identified |
CF: cystic fibrosis; F: female; FEF: forced expiratory flow; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; IB: ipratropium bromide; ICU: intensive care unit; ITU: intensive therapy unit; M: male; MDI: metered‐dose inhaler; PEF: peak expiratory flow; PEFR: peak expiratory flow rate; PICU: paediatric intensive care unit; q: over (e.g. q15 means over 15 minutes); UDV: unit dose vial; y.o.: years old.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anthracopoulos 2005 | Study compared anticholinergic agents and salbutamol versus higher dose of salbutamol alone |
| Boner 1987 | Study on people with stable asthma |
| Bratteby 1986 | Study on people with chronic asthma |
| Browne 2002 | Hospitalized participants treated with IV salbutamol and placebo versus IV salbutamol and anticholinergics |
| Caubet 1989 | Study on people with chronic asthma |
| Craven 2001 | Acute inpatient setting |
| Davis 1984 | Study on people with stable asthma |
| Delacourt 1994 | The study was not a randomised controlled trial |
| DeStefano 1989 | Study on people with chronic asthma |
| Ekwo 1978 | The study was not a randomised controlled trial |
| Freeman 1989 | The study pertained to participants who were already admitted |
| Friberg 1989 | Study on people with chronic asthma |
| Goggin 2001 | Hospitalized children |
| Greenough 1986 | Study on people with chronic asthma |
| Groggins 1981 | Study on people with stable asthma |
| Hodges 1981 | The study pertained to infants |
| Kumaratne 2003 | Significant number of participants under 18 months (mean: 19‐27 months, SD 20‐23) |
| Lenney 1986 | Study on people with chronic asthma |
| Mann 1982 | Study on people with chronic asthma |
| O'Driscoll 1989 | Age range: 17‐81 years |
| Paredes 1994 | Conference abstract ‐ incomplete data given |
| Ralston 2005 | Different SABAs used in each treatment arm |
| Rayner 1987 | The study pertained to people who were already admitted |
| Stokes 1983 | The study did not combine anticholinergic inhalations and SABAs inhalations |
| Storr 1986 | The study pertained to people who were already admitted |
| Timsit 2002 | Different doses of SABA in treatment groups |
| Vichyanond 1990 | Study on people with chronic asthma |
| Ward 1981 | Age range: 15‐79 year olds |
| Ward 1985 | Age range: 14‐75 years |
| Wilson 1984 | Study on people with chronic asthma |
IV: intravenous; SABA: short‐acting beta‐agonists; SD: standard deviation.
Differences between protocol and review
We updated the following methods in the present update (2013)
Removed presentation of subgroup by co‐treatment with corticosteroids and applied this and additional subgroups to the primary outcome only.
Combined all comparisons originally presented by protocol (single dose, multiple fixed dose and multiple flexible doses) into a single comparison.
We updated the 'Risk of bias' assessment from Jadad to the 'Risk of bias' tables currently recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Contributions of authors
BG: drafted the current version of the update.
FMD: designed and supervised the conduct of the original review, reviewed, interpreted and approved the updated review.
Sources of support
Internal sources
NHS Research and Development, UK.
External sources
No sources of support supplied
Declarations of interest
Francine Ducharme has received travel support, research funds and fees for speaking from Novartis, Taketa (formally Nycomed) and Merck Frosst Inc and served on an advisory board for Novartis.
Edited (no change to conclusions)
References
References to studies included in this review
Beck 1985 {published data only}
- Beck R. Use of ipratropium bromide by inhalation in the treatment of acute asthma in children. Clinical experience [in French] [Utilisation du bromure d'ipratropium par voie inhalée pour le traitement de l'asthme aigu chez l'enfant]. Archives de Pediatrie 1995;2 Suppl(2):145‐8. [DOI] [PubMed] [Google Scholar]
- Beck R, Robertson C, Galdes‐Sebaldt M, Levison H. Combined salbutamol and ipratropium bromide by inhalation in the treatment of severe acute asthma. Journal of Pediatrics 1985;107:605‐8. [DOI] [PubMed] [Google Scholar]
Benito Fernandez 2000 {published data only}
- Benito Fernandez J, Mintegui Raso S, Sanchez Echaniz J, Vazquez Ronco MA, Pijoan Zubizarreta JI. Efficacy of early administration of nebulized ipratropium bromide in children with asthmatic crisis [in Spanish]. Anales Espanoles de Pediatria 2000;53(3):217‐22. [PubMed] [Google Scholar]
BI [pers comm] {published data only}
- Boehringer Ingelheim Pharmaceuticals. A comparison of Combivent UDV (ipratropium 500mcg and salbutamol 2.5mg) and salbutamol UDV alone (2.5mg). Personal communication from Boehringer Ingelheim 2009.
Calvo 1998 {published data only}
- Calvo GM, Calvo AM, Marin HF, Moya GJ. Is it useful to add an anticholinergic treatment to beta2‐adrenergic medication in acute asthma attack. Journal of Investigative Allergology Clinical Immunology 1998;8(1):30‐4. [PubMed] [Google Scholar]
Chakraborti 2006 {published data only}
- Chakraborti A, Lodha R, Pandey RM, Kabra SK. Randomized controlled trial of ipratropium bromide and salbutamol versus salbutamol alone in children with acute exacerbation of asthma. Indian Journal of Pediatrics. 2006;73(11):979‐83. [DOI] [PubMed] [Google Scholar]
Cook 1985 {published data only}
- Cook JJ, Fergusson DM, Dawson KP. Ipratropium and fenoterol in the treatment of acute asthma. Pharmatherapeutica 1985;4(6):383‐6. [PubMed] [Google Scholar]
Ducharme 1998 {unpublished data only}
- Ducharme FM, Davis GM. Randomized controlled trial of ipratropium bromide and frequent low doses of salbutamol in the treatment of mild and moderate acute asthma. Journal of Pediatrics 1998;133(4):479‐85. [DOI] [PubMed] [Google Scholar]
Guill 1987 {published data only}
- Guill MF, Maloney MJ, DuRant RH. Comparison of inhaled metaproterenol, inhaled atropine sulfate and their combination in treatment of children with acute asthma. Annals of Allergy 1987;59:367‐71. [PubMed] [Google Scholar]
Iramain 2011 {published data only}
- Iramain R, Lopez‐Herce J, Coronel J, Spitters C, Guggiari J, Bogado N. inhaled salbutamol plus ipratropium in moderate and severe asthma crises in children. Journal of Asthma 2011;48:298‐303. [DOI] [PubMed] [Google Scholar]
Peterson 1996 {unpublished data only}
- Peterson R, Wensley D, Mitchell I, Klassen T, Lamarre J, Rivard G, et al. Boehringer Ingelheim Trial No 2442430.3. Boehringer Ingelheim 1994.
Phanichyakam 1990 {published data only}
- Phanichyakam P, Kraisarin C, Sasisakulporn C. Comparison of inhaled terbutaline and inhaled terbutaline plus ipratropium bromide in acute asthmatic children. Asian Pacific Journal of Allergy and Immunology 1990;8:45‐8. [PubMed] [Google Scholar]
Qureshi 1997 {published data only}
- Qureshi FA, Zaritsky A, Lakkis H. Efficacy of nebulized ipratropium in severe asthmatic children. Annals of Emergency Medicine 1997;29:205‐11. [DOI] [PubMed] [Google Scholar]
Qureshi 1998 {published data only}
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New England Journal of Medicine 1998;339(15):1030‐5. [DOI] [PubMed] [Google Scholar]
Qureshi 1998 (moderate) {published data only}
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New England Journal of Medicine 1998;339(15):1030‐5. [DOI] [PubMed] [Google Scholar]
Qureshi 1998 (severe) {published data only}
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New England Journal of Medicine 1998;339(15):1030‐35. [DOI] [PubMed] [Google Scholar]
Reisman 1988 {published data only}
- Reisman J, Galdes‐Sebaldt M, Kazim F, Canny G, Levison H. Frequent administration by inhalation of salbutamol and ipratropium bromide in the initial management of severe acute asthma in children. Journal of Allergy & Clinical Immunology 1988;81:16‐20. [DOI] [PubMed] [Google Scholar]
Schuh 1995 {published data only}
- Schuh S, Johnson DW, Callahan S, Canny G, Levison H. Efficacy of frequent nebulized ipratropium added to frequent high‐dose albuterol therapy in severe childhood asthma. Journal of Pediatrics 1995;126:639‐45. [DOI] [PubMed] [Google Scholar]
Schuh 1995 (multiple) {published data only}
- Schuh S, Johnson DW, Callahan S, Canny G, Levison H. Efficacy of frequent nebulized ipratropium added to frequent high‐dose albuterol therapy in severe childhood asthma. Journal of Pediatrics 1995;126:639‐45. [DOI] [PubMed] [Google Scholar]
Schuh 1995 (single) {published data only}
- Schuh S, Johnson DW, Callahan S, Canny G, Levison H. Efficacy of frequent nebulized ipratropium added to frequent high‐dose albuterol therapy in severe childhood asthma. Journal of Pediatrics 1995;126:639‐45. [DOI] [PubMed] [Google Scholar]
Sharma 2004 {published data only}
- Sharma A, Madaan A. Nebulized salbutamol vs salbutamol and ipratropium combination in asthma. Indian Journal of Pediatrics 2004;71(2):121‐4. [DOI] [PubMed] [Google Scholar]
Sienra Monge 2000 {published data only}
- Sienra Monge JJ, Bermejo Guevara MA, Rio Navarro BE, Rosas Vargas MA, Reyes Ruiz NI. Degree and duration of bronchodilatation with an agonist beta 2 administered alone versus an agonist beta 2 administered with ipratropium bromide in children with acute asthma. Revista Alergia Mexico 2000;47(1):26‐9. [PubMed] [Google Scholar]
Watanasomsiri 2006 {published data only}
- Watanasomsiri A, Phipatanakul W. Comparison of nebulized ipratropium bromide with salbutamol versus salbutamol alone in acute asthma exacerbation in children [Abstract]. Chest 2004;126(4 Suppl):761S‐b. [DOI] [PubMed] [Google Scholar]
- Watanasomsiri A, Phipatanakul W. Comparison of nebulized ipratropium bromide with salbutamol vs salbutamol alone in acute asthma exacerbation in children. Annals of Allergy, Asthma, & Immunology 2006;96(5):701‐6. [DOI] [PubMed] [Google Scholar]
Watson 1988 {published data only}
- Watson WTA, Becker AB, Simmons FER. Comparison of ipratropium solution, fenoterol solution and their combination administered by nebulizer and face mask to children with acute asthma. Journal of Allergy & Clinical Immunology 1988;82:1012‐8. [DOI] [PubMed] [Google Scholar]
Zorc 1999 {published data only}
- Zorc JJ, Pusic MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4):748‐52. [DOI] [PubMed] [Google Scholar]
Zorc 1999 (mild) {published data only}
- Zorc JJ, Pusic MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4):748‐52. [DOI] [PubMed] [Google Scholar]
Zorc 1999 (moderate) {published data only}
- Zorc JJ, Pusic MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4):748‐52. [DOI] [PubMed] [Google Scholar]
Zorc 1999 (severe) {published data only}
- Zorc JJ, Pusic, MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4):748‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anthracopoulos 2005 {published data only}
- Anthracopoulos M, Karatza A, Davlouros PA, Beratis NG. Comparison of salbutamol (SAL) and combined salbutamol and ipratropium bromide (SAL+IB) nebulization therapy in acute asthma exacerbations (AAE) in paediatric patients. European Respiratory Journal 2000;16 Suppl 31:304s. [Google Scholar]
- Anthracopoulos MB, Karatza AA, Davlouros PA, Chiladakis JA, Manolis AS, Beratis NG. Effects of two nebulization regimens on heart rate variability during acute asthma exacerbations in children. Journal of Asthma 2005;42(4):273‐9. [DOI] [PubMed] [Google Scholar]
Boner 1987 {published data only}
- Boner AL, Stefano G, Niero E, Vallone G, Gaburro D. Salbutamol and ipratropium bromide solution in the treatment of bronchospasm in asthmatic children. Annals of Allergy 1987;58:54‐8. [PubMed] [Google Scholar]
Bratteby 1986 {published data only}
- Bratteby L‐E, Foucard T, Lonnerholm G. Combined treatment with ipratropium bromide and beta‐2‐adrenoceptor agonists in childhood asthma. American Review of Respiratory Disease 1986;68:239‐47. [PubMed] [Google Scholar]
Browne 2002 {published data only}
- Browne GJ, Trieu L, Asperen P. Randomized, double‐blind, placebo‐controlled trial of intravenous salbutamol and nebulized ipratropium bromide in early management of severe acute asthma in children presenting to an emergency department. Critical Care Medicine 2002;30(2):448‐53. [DOI] [PubMed] [Google Scholar]
Caubet 1989 {published data only}
- Caubet Y. Comparison of the effectiveness and the tolerance of the dosing aerosols of fenoterol/ipratropium against salbutamol in the asthmatic child [in French] [Comparaison de l'efficacite et de la tolerance des aerosols doseurs de l'association fenoterol/ipratropium contre le salbutamol chez le grand enfant asthmatique]. La Revue de Pediatrie 1989;25(2):74‐6. [Google Scholar]
Craven 2001 {published data only}
- Craven D, Kercsmar CM, Myers TR, O'Riordan MA, Golonka G, Moore S. Ipratropium bromide plus nebulized albuterol for the treatment of hospitalized children with acute asthma. Journal of Pediatrics 2001;138(1):51‐8. [DOI] [PubMed] [Google Scholar]
Davis 1984 {published data only}
- Davis A, Vickerson F, Worsley G, Mindorff C, Kazim F, Levison H. Determination of dose‐response relationship for nebulized ipratropium in asthmatic children. Journal of Pediatrics 1984;Dec:1002‐5. [DOI] [PubMed] [Google Scholar]
Delacourt 1994 {published data only}
- Delacourt C, Blic J, Lebourgeois M, Scheinmann P. Value of ipratropium bromide in asthma crisis in children [in French] [Interet du bromure d'ipratropium dans la crise d'asthme de l'enfant]. Archives de Pediatrie 1994;1:87‐92. [PubMed] [Google Scholar]
DeStefano 1989 {published data only}
- DeStefano G, Bonetti S, Bonizzato C, Valletta EA, Piacentini GL, Boner AL. Additive effect of albuterol and ipratropium bromide in the treatment of bronchospasm in children. Annals of Allergy 1989;65(4):260‐2. [PubMed] [Google Scholar]
Ekwo 1978 {published data only}
- Ekwo E, Weinberger M. Evaluation of a program for the pharmacologic management of children with asthma. Journal of Allergy & Clinical Immunology 1978;61(4):240‐7. [DOI] [PubMed] [Google Scholar]
Freeman 1989 {published data only}
- Freeman J, Landau LI. The effects of ipratropium bromide and fenoterol nebulizer solutions in children with asthma. Clinical Pediatrics 1989;28(12):556‐60. [DOI] [PubMed] [Google Scholar]
Friberg 1989 {published data only}
- Friberg S, Graff‐Lonnevig V. Ipratropium bromide (Atrovent) in childhood asthma: a cumulative dose‐response study. Annals of Allergy 1989;62:131‐4. [PubMed] [Google Scholar]
Goggin 2001 {published data only}
- Goggin N, Macarthur C, Parkin PC. Randomized trial of the addition of ipratropium bromide to albuterol and corticosteroid therapy in children hospitalized because of an acute asthma exacerbation. Archives of Pediatrics & Adolescent Medicine 2001;155(12):1329‐34. [DOI] [PubMed] [Google Scholar]
Greenough 1986 {published data only}
- Greenough A, Loftus Bg, Pool J, Price JF. Response to bronchodilators assessed by lung mechanics. Archives of Disease in Childhood 1986;61:1020‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A, Yuksel B, Everett L, Price JF. Inhaled ipratropium bromide and terbutaline in asthmatic children. Respiratory Medicine 1993;87:111‐4. [DOI] [PubMed] [Google Scholar]
Groggins 1981 {published data only}
- Groggins RC, Milner AD, Stokes GM. Bronchodilator effects of clemastine, ipratropium bromide, and salbutamol in preschool children with asthma. Archives of Disease in Childhood 1981;56:342‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodges 1981 {published data only}
- Hodges IGC, Groggins RC, Milner AD, Stokes GM. Bronchodilator effect of inhaled ipratropium bromide in wheezy toddlers. Archives of Disease in Childhood 1981;56:729‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumaratne 2003 {published data only}
- Kumaratne M, Gunawardane G. Addition of ipratropium to nebulized albuterol in children with acute asthma presenting to a pediatric office. Clinical Pediatrics 2003;42(2):127‐32. [DOI] [PubMed] [Google Scholar]
Lenney 1986 {published data only}
- Lenney W, Evans NAP. Nebulized salbutamol and ipratropium bromide in asthmatic children. British Journal of Diseases of the Chest 1986;80:59‐65. [DOI] [PubMed] [Google Scholar]
Mann 1982 {published data only}
- Mann NP, Hiller EJ. Ipratropium bromide in children with asthma. Thorax 1982;37:72‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Driscoll 1989 {published data only}
- O'Driscoll BR, Taylor RJ, Horsley MG, Chambers DK, Bernstein A. Nebulised salbutamol with and without ipratropium bromide in acute airflow obstruction. Lancet 1989;1(8652):1418‐20. [DOI] [PubMed] [Google Scholar]
Paredes 1994 {published data only}
- Paredes N, Sol M, Rio N, Sienra M. Comparative efficacy of salbutamol versus salbutamol with ipratropium bromide in acute asthma in children [Abstract]. XV International Congress of Allergology and Clinical Immunology: Annual Meeting of the European Academy of Allergology and Clinical Immunology. 1994:377.
Ralston 2005 {published data only}
- Ralston ME, Euwema MS, Knecht KR, Ziolkowski TJ, Coakley TA, Cline SM. Comparison of levalbuterol and racemic albuterol combined with ipratropium bromide in acute pediatric asthma: a randomized controlled trial. Journal of Emergency Medicine 2005;29(1):29‐35. [DOI] [PubMed] [Google Scholar]
Rayner 1987 {published data only}
- Rayner RJ, Cartlidge PHT, Upton CJ. Salbutamol and ipratropium in acute asthma. Archives of Disease in Childhood 1987;62:840‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stokes 1983 {published data only}
- Stokes GM, Milner AD, Hodges IGC, Henry RL. Nebulised ipratropium bromide in wheezy infants and young children. European Journal of Respiratory Diseases 1983;64:494‐8. [PubMed] [Google Scholar]
Storr 1986 {published data only}
- Storr J, Lenney W. Nebulised ipratropium and salbutamol in asthma. Archives of Disease in Childhood 1986;61:602‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Timsit 2002 {published data only}
- Timsit S, Sannier N, Bocquet N, Cojocaru B, Wille C, Boursiquot C, et al. Benefit of ipratropium bromide for the treatment of childhood asthma in the emergency department. Archives de Pediatrie 2002;9(2):117‐24. [DOI] [PubMed] [Google Scholar]
Vichyanond 1990 {published data only}
- Vichyanond P, Sladek WA, Sur S, Hill MR, Szefler SJ, Nelson HS. Efficacy of atropine methylnitrate alone and in combination with albuterol in children with asthma. Chest 1990;98:637‐42. [DOI] [PubMed] [Google Scholar]
Ward 1981 {published data only}
- Ward MJ, Fentem PH, Smith WH, Davies D. Ipratropium bromide in acute asthma. British Medical Journal Clinical Research Ed. 1981;282(6264):598‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 1985 {published data only}
- Ward MJ, Macfarlane JT, Davies D. A place for ipratropium bromide in the treatment of severe acute asthma. British Journal of Diseases of the Chest 1985;79(4):374‐8. [DOI] [PubMed] [Google Scholar]
Wilson 1984 {published data only}
- Wilson N, Dixon C, Silverman M. Bronchial responsiveness to hyperventilation in children with asthma: inhibition by ipratropium bromide. Thorax 1984;39:588‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Arets 2001
- Arets H, Brackel H, Ent C. Forced expiratory manoeuvres in children: do they meet ATS and ERS criteria for spirometry?. European Respiratory Journal 2001;18:655‐60. [DOI] [PubMed] [Google Scholar]
BTS 2011
- British Thoracic Society and Scottish intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline, 2011. www.brit‐thoracic.org.uk/guidelines (accessed 8 August 2013).
Chalut 2000
- Chalut DS, Ducharme FM, Davis GM. The Preschool Respiratory Assessment Measure (PRAM): a responsive index of acute asthma severity. Journal of Pediatrics 2000;137(6):762‐8. [DOI] [PubMed] [Google Scholar]
Chapman 1996
- Chapman K. An international perspective on anticholinergic therapy. American Journal of Medicine 1996;100:2‐4S. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Ducharme 2008
- Ducharme FM, Chalut D, Plotnick L, Savadie C, Kudrika D, Zhang Xun, et al. The Pediatric Respiratory Assessment Measure: a valid clinical score for assessing acute asthma severity from toddlers to teenagers. Journal of Pediatrics 2008;152(4):476‐80. [DOI] [PubMed] [Google Scholar]
GINA 2011
- Global INitiative for Asthma. Global strategy for asthma management and prevention, 2011. www.ginasthma.org (accessed 8 August 2013).
Greenland 1985
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41:55‐68. [PubMed] [Google Scholar]
Gross 1988
- Gross NJ. Ipratropium bromide. New England Journal of Medicine 1988;8:486‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Homer 1996
- Homer CJ, Szilagyi P, Rodewald L, Bloom SR, Greenspan P, Yazdgerdi S, et al. Does quality of care affect rates of hospitalization for childhood asthma?. Pediatrics 1996;98:18‐23. [PubMed] [Google Scholar]
Khan 1996
- Khan KS, Daya S, Jahad AR. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156:661‐6. [PubMed] [Google Scholar]
Lougheed 2012
- Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Canadian Respiratory Journal 2012; Vol. 19, issue 2:127‐64. [1916‐7245: (Electronic)] [DOI] [PMC free article] [PubMed]
Payne 1995
- Payne SM, Donahue C, Rappo P, McNamara JJ, Bass J, First L, et al. Variations in pediatric pneumonia and bronchitis/asthma admission rates. Is appropriateness a factor?. Archives of Pediatric and Adolescent Medicine 1995;149:162‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2011.
Rodrigo 2005
- Rodrigo G, Castro‐Rodriguez J. Anticholinergics in the treatment of children and adults with acute asthma: a systematic review with meta analysis. Thorax 2005;60:740‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2001
- Rowe BH, Spooner C, Ducharme F, Bretziaff J, Bota G. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002178] [DOI] [PubMed] [Google Scholar]
Rubin 2003
Saxena 2006
- Saxena S, George J, Barber J, Fitzpatrick J, Majeed A. Association of population and practice factors with potentially avoidable admissions rates for chronic diseases in London: cross sectional analysis. Journal of the Royal Society of Medicine 2006;99(2):81‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sears 1992
- Sears MR. Clinical application of beta‐agonists. Practical Allergy and Immunology 1992;7:98‐100. [Google Scholar]
Silverman 1990
- Silverman M. The role of anticholinergic antimuscarinic bronchodilator therapy in children. Lung 1990;168:304‐9. [DOI] [PubMed] [Google Scholar]
Silverman 2007
- Silverman RA, Flaster E, Enright PL, Simonson SG. FEV1 performance among patients with acute asthma: results from a multicenter clinical trial. Chest 2007;131(1):164‐71. [DOI] [PubMed] [Google Scholar]
Svedmyr 1985
- Svedmyr N. A beta2‐adrenergic agonist for use in asthma pharmacology, pharmacokinetics, clinical efficacy and adverse effects. Pharmacotherapy 1985;5:109‐26. [DOI] [PubMed] [Google Scholar]
Teoh 2012
- Teoh L, Cates C, Hurwitz M, Acworth JP, Asperen P, Chang AB. Anticholinergic therapy for acute asthma in children. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD003797.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Plotnick 2000
- Plotnick L, Ducharme F. Combined inhaled anticholinergics and beta2‐agonists for initial treatment of acute asthma in children. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD000060] [DOI] [PubMed] [Google Scholar]


