Abstract
This study evaluates the role of differential artery-vein (AV) analysis in optical coherence tomography angiography (OCTA) for treatment outcome prediction of diabetic macular edema (DME). Deep learning AV segmentation in OCTA enabled the robust extraction of quantitative AV features, including perfusion intensity density (PID), blood vessel density (BVD), vessel skeleton density (VSD), vessel area flux (VAF), blood vessel caliber (BVC), blood vessel tortuosity (BVT), and vessel perimeter index (VPI). Support vector machine (SVM) classifiers were employed to predict changes in best-corrected visual acuity (BCVA) and central retinal thickness (CRT). Comparative analysis revealed that differential AV analysis significantly enhanced prediction performance, with BCVA accuracy improved from 70.45% to 86.36% and CRT accuracy enhanced from 68.18% to 79.55% compared to traditional OCTA analysis. These findings underscore the potential of AV analysis as a transformative tool for advancing personalized therapeutic strategies and improving clinical decision-making in managing DME.
1. Introduction
Diabetic macular edema (DME) represents the leading cause of vision loss in individuals with diabetic retinopathy (DR) [1]. The pathogenesis of DME involves complex interactions between ischemic, inflammatory, and vascular mechanisms, predominantly driven by alterations in the retinal vasculature [2]. Previously, laser photocoagulation was the standard treatment for DME, offering vision stabilization but limited improvement in visual acuity [3]. Anti-vascular endothelial growth factor (VEGF) therapy has emerged as a cornerstone treatment, aiming to reduce VEGF-mediated vascular permeability and thereby alleviate macular swelling [4]. Treatment efficacy is typically assessed based on two primary criteria: improvements in best-corrected visual acuity (BCVA), reflecting functional recovery, and reductions in central retinal thickness (CRT), indicating anatomical resolution of macular edema. However, variability in treatment outcomes highlights the need for a deeper understanding of the underlying vascular pathophysiology [5].
Optical coherence tomography angiography (OCTA) has become a transformative tool in retinal imaging, offering non-invasive, high-resolution visualization of retinal microvasculature. This modality provides quantitative metrics that facilitate the assessment of vascular abnormalities and perfusion deficits in DME. OCTA-derived biomarkers, such as the foveal avascular zone (FAZ) area, FAZ volume, capillary non-perfusion area (NPA), vessel skeleton density (VSD), blood vessel density (BVD), vessel tortuosity (BVT), and vessel caliber (BVC), have been shown to correlate with disease severity and visual acuity in DR and DME patients [6–12]. Additionally, recent studies have identified several OCTA biomarkers to predict visual outcomes in DME patients undergoing treatment, including the deep-to-superficial layers flow ratio [13], BVD [8,14–16], FAZ area [15], and the presence of microaneurysms in the nasal macula [17]. These imaging biomarkers offer valuable insights into predicting treatment efficacy and developing personalized therapeutic strategies.
Recent advancements in machine learning-driven artery-vein (AV) analysis of OCTA images have greatly enhanced the understanding of DR and its associated complications, including DME [18–23]. By incorporating AV analysis, OCTA enables precise differentiation between arterial and venous regions, uncovering their respective alterations correlated with pathophysiological conditions. A recent study demonstrated that integrating AV features into machine learning classifiers significantly improved the classification of DR stages [24]. These findings highlight the potential of differential AV analysis to enhance the accuracy of disease classification and the prediction of treatment outcomes.
This study investigates the potential of differential AV analysis in OCTA for objective prediction of anti-VEGF treatment outcomes in DME. By leveraging deep learning AV segmentation, this research evaluates predictive accuracy through the extraction of AV biomarkers, including BVD, BVC, BVT, perfusion intensity density (PID), vessel skeleton density (VSD), vessel area flux (VAF), and vessel perimeter index (VPI). This work highlights the potential of differential AV analysis in OCTA to advance personalized therapeutic strategies and refine clinical decision-making in managing retinal vascular diseases.
2. Methods
2.1. Data acquisition
The study received ethical approval from the Institutional Review Board (IRB) at National Taiwan University Hospital (NTUH) and the University of Illinois Chicago (UIC). It adhered to the principles outlined in the Declaration of Helsinki. This study included 44 eyes of DME from 36 patients treated with either intravitreal ranibizumab or aflibercept at NTUH, Taipei, Taiwan. The clinical and demographic characteristics of the study participants, including age, sex, DR stage, baseline BCVA, and CRT, are summarized in Table 1. Only patients aged 18 or older, with a clinical diagnosis of DME confirmed by optical coherence tomography (OCT), and who had not received anti-VEGF treatment within the past three months, met the inclusion criteria. A total of 61.4% (27/44) of the included eyes were treatment-naïve. Among the remaining 17 eyes, 13 had received prior anti-VEGF treatment for DME, and 4 had been treated for vitreous hemorrhage. Additionally, one eye had received a subtenon injection of triamcinolone acetonide more than three months before enrollment. Of the 13 eyes previously treated for DME, 6 had received three or more anti-VEGF injections, and 4 of these were classified as refractory DME cases, defined as CRT decrease < 20% or CRT > 300 µm after treatment. Notably, no patient had received anti-VEGF or steroid treatment within three months prior to enrollment.
Table 1. Clinical and Demographic Characteristics of Study Participants a .
| Baseline | Post-Treatment | |
|---|---|---|
| Number of subjects (n) | 36 | 36 |
| Male | 16 | 16 |
| Female | 20 | 20 |
| Number of eyes (n) | 44 | 44 |
| Right | 19 | 19 |
| Left | 25 | 25 |
| Age (years) | 60.47 ± 10.14 | 60.80 ± 10.14 |
| Age range | 33 - 79 | 33 - 79 |
| DR stage of eyes | ||
| Moderate | 14 | 14 |
| Severe | 11 | 11 |
| PDR | 19 | 19 |
| BCVA (LogMAR) | 0.5690 ± 0.3478 | 0.3578 ± 0.2857 |
| CRT (µm) | 413.81 ± 119.80 | 315.39 ± 82.33 |
DR = diabetic retinopathy; PDR = proliferative DR; BCVA = best corrected visual acuity; CRT = central retinal thickness.
Diagnostic criteria for DME required DR with focal or diffuse leakage in the macular area, as documented by fluorescein angiography, along with macular edema characterized by retinal thickening, intraretinal cysts, hyperreflective foci, or subretinal fluid, as shown by OCT. In this study, retinal thickening was defined as a central subfield thickness of 300 µm or more, and all enrolled eyes met this criterion. Intraretinal cysts, hyperreflective foci, and subretinal fluid were identified within the central 1.5 mm area in the vertical or horizontal B-scan OCT images. At enrollment, 95.45% (42/44) of eyes exhibited intraretinal cysts, 63.6% (28/44) had hyperreflective foci, and 31.8% (14/44) presented with subretinal fluid.
Exclusion criteria included other retinal diseases, a history of ocular surgery within the past six months, or significant media opacities affecting image quality. All patients received three consecutive monthly intravitreal injections of either ranibizumab or aflibercept as part of the loading treatment. No patients received a combination of both drugs during the treatment period. Baseline and post-treatment BCVA measurements and OCTA imaging were performed one month after the third injection. Treatment response was evaluated based on two separate criteria: BCVA improvement and CRT improvement. BCVA improvement was defined as a decrease of more than 0.1 LogMAR, while CRT improvement was classified as a decrease of more than 10%. These classifications helped to assess both functional and anatomical responses to the treatment. Deidentified OCTA images and clinical datasets, with all personally identifiable information removed, were acquired for retrospective analysis with IRB approval. For this retrospective study, the requirement for patient informed consent was waived, and the study adhered to patient privacy and confidentiality guidelines established by the IRB.
OCTA images were acquired using the AngioVue SD-OCT device (Optovue, Fremont, CA, USA) with a 3 mm × 3 mm macular scan protocol. The OCT device had a 70,000 Hz A-scan rate, ∼5 µm axial resolution, and ∼10 µm lateral resolution for 3 mm × 3 mm scans. En face images of the superficial vascular plexus (SVP), segmented from the inner limiting membrane (ILM) to 10 µm below the inner plexiform layer (IPL), were used in this study.
2.2. Feature extraction
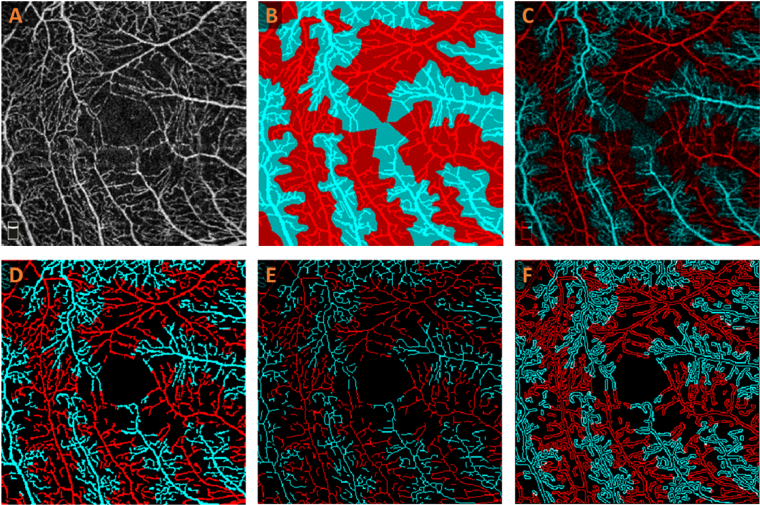
Our recently demonstrated MF-AV-Net [22] was employed for AV segmentation in OCTA (Fig. 1(A)) to generate AV maps, represented by the dark blue and red vessels in Fig. 1(B). To generate AVA maps, a k-nearest neighbor (kNN) classifier is applied to classify arterial and venous regions [23], which are illustrated by the light blue and red regions, respectively (Fig. 1(B)). Multiplying the OCTA image by the AVA map results in an OCTA-AV map (Fig. 1(C)), which contains the intensity information of an OCTA image while distinguishing arterial and venous areas in separate red and blue channels. Using this method, vessels of various orders and scales visible in the OCTA images can be classified as either arteries or veins. The OCTA layer indicator area, located in the bottom left corner of the OCTA image, is excluded from the OCTA-AV map shown in Fig. 1(C).
Fig. 1.
Illustrating the feature extraction from an OCTA image. (A) OCTA image. (B) AVA map. (C) OCTA-AV map (D) Binarized OCTA-AV map (E) Skeletonized blood vessel map in OCTA-AV map. (F) Vessel perimeter map in OCTA-AV map.
For the OCTA-AV map (Fig. 1(C)), a Hessian-based multiscale Frangi filter [25] can be applied to enhance vascular flow information, followed by binarization to generate a vasculature map (Fig. 1(D)). This vasculature map can then be skeletonized (Fig. 1(E)), which removes pixels along the boundaries of vessels without breaking the continuity of the vessels [26]. A vessel perimeter map (Fig. 1(F)) can be created by detecting the vessel edges and removing pixels not adjacent to the vessel edges in the binarized image (Fig. 1(D)) [27].
Seven quantitative features were extracted from each OCTA image: PID, BVD, VSD, VAF, BVC, BVT, and VPI. These features are commonly used quantitative metrics and can be calculated for total, arterial, and venous vessels. This set can also be expanded to include additional quantitative features as needed. Potential correlations may exist among features such as BVD, VSD, PID, and VPI, as well as between VAF and BVC, based on their definitions. Our feature selection algorithm is designed to identify and select features that contribute most to classification performance. The detailed procedure for calculating PID is reported in our recent publication [23]. VAF is calculated following the procedure described by Abdolahi et al. [28] with the binarized OCTA-AV map in Fig. 1(D). Based on Fig. 1(D) to 1(F), we used the procedures described by Yao et al. [29] to calculate BVD, BVC, VSD, BVT, and VPI.
Without AVA segmentation, it is only possible to calculate features for the total vasculature, including both arteries and veins. However, with AVA segmentation in OCTA-AV maps, it becomes feasible to determine features specifically for arteries and veins. For example, the parameter PID can be calculated separately for the total area, arterial area, and venous area, and annotated as total PID (T-PID), arterial PID (A-PID), and venous PID (V-PID), respectively. Ratios between total, arterial, and venous features can also be defined, such as the arterial-venous ratio, arterial-total ratio, and venous-total ratio for each feature. For instance, the arterial-venous PID ratio is calculated by dividing A-PID by V-PID, the arterial-total PID ratio by dividing A-PID by T-PID, and the venous-total PID ratio by dividing V-PID by T-PID. Features for the total vasculature, such as T-PID, T-BVD, T-VAF, T-BVC, T-BVT, and T-VPI, represent quantitative measurements without differentiation between arterial and venous areas.
2.3. Classification models
In this study, we addressed two distinct predictive problems: assessing BCVA improvement and CRT improvement. Pearson correlation analysis (r = 0.331, p = 0.028) revealed a weak to moderate correlation between BCVA and CRT improvements, indicating that functional and anatomical responses to anti-VEGF therapy do not always align. Therefore, separate models are necessary for predicting anatomical and functional treatment outcomes. While both BCVA and CRT improvements are continuous variables, we opted for a classification approach to align with clinical practice, where distinguishing between “responders” and “non-responders” is often more actionable for patient management and treatment planning. This approach is commonly used in clinical trials as a secondary outcome to facilitate interpretation. For this study, BCVA improvement was defined as a decrease of more than 0.1 LogMAR, while CRT improvement was defined as a decrease of more than 10%. These thresholds were chosen based on established clinical significance and facilitate the translation of predictive outcomes into practical decision-making. By categorizing patients into binary outcome groups, our methodology aims to enhance the interpretability of the results and provide a more intuitive framework for assessing functional and anatomical treatment responses.
To ensure accurate classifications, we evaluated the performance of various classifiers, including decision trees, random forest, Gaussian naive Bayes, logistic regression, and SVMs. Among these, the SVM classifier with radial basis function (RBF) kernels demonstrated the best performance, achieving a mean accuracy that was 4% to 6% higher for different classifications than the second-best model, which was the random forest classifier. Model selection was based on classification accuracy and area under the curve (AUC). SVMs are commonly preferred for their effectiveness in handling high-dimensional spaces, where the number of features can exceed the number of samples, making them ideal for small to medium-sized datasets while minimizing the risk of overfitting. Given the characteristics of our research, which include high-dimensional data, binary classifications, and limited dataset size, the SVM classifiers stand out as the optimal choice. The SVM classifiers were configured with hyperparameters C = 2 for BCVA improvement prediction and C = 3 for CRT improvement prediction, with gamma set to ‘scale’ in both cases, to evaluate the impact of AV analysis on the prediction performance of DME treatment. Classification underwent a 5-fold cross-validation process. The performance of the SVM classifiers was assessed using four metrics: sensitivity, specificity, accuracy, and AUC of the receiver operating characteristic (ROC) curve. To identify the optimal subset of features that maximize classification accuracy, we applied sequential forward selection (SFS) [30], based on the greedy search algorithm. SFS begins with an empty set of features and iteratively adds the feature that most improves the accuracy of the classification model. This process continues until no further improvement is achieved, enhancing model simplicity and interpretability by reducing dimensionality.
3. Results
The PID, BVD, VSD, VAF, BVC, BVT, and VPI for total, arterial, and venous vessels, as well as their ratios across the entire image, were calculated using the OCTA-AV maps. To achieve the most robust classification performance, we employed the SFS algorithm and SVM classifiers, which identified the optimal subset of features that maximized classification accuracy. Before AV analysis, features derived from total vessels, such as T-PID, T-BVD, T-VSD, T-VAF, T-BVC, T-BVT, and T-VPI, were used as input to the classifier. After differential AV analysis, the classifier incorporated all features, including total, arterial, venous features, and their ratios, as inputs.
Table 2 presents the mean and standard deviation of performance metrics for BCVA and CRT improvement classifications before and after AV analysis. For BCVA improvement classification, differential AV analysis substantially enhanced performance metrics. Sensitivity increased from 79.31% to 89.66%, specifically improved from 53.33% to 80%, and accuracy rose from 70.45% to 86.36%. The AUC also showed a marked improvement, increasing from 72.45% to 84.2%. Similarly, for CRT improvement classification, AV analysis resulted in notable gains. Sensitivity improved from 65.52% to 82.76%, while specificity remained consistent at 73.33%. Accuracy increased from 68.18% to 79.55%, and the AUC showed a slight improvement from 81.99% to 82.21%. These results demonstrate the effectiveness of incorporating AV analysis in improving the classification performance for both BCVA and CRT improvement, highlighting its potential to enhance predictive accuracy and reliability in DME treatment evaluation.
Table 2. Performance comparison of classifications before and after AV analysis.
| Features from total vessels (Before AV analysis) | Features from total, arterial, and venous vessels and their ratios (After AV analysis) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Binary Classification | Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC (%) | Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC (%) |
| BCVA improvement | 79.31 | 53.33 | 70.45 | 72.45 | 89.66 | 80.00 | 86.36 | 84.2 |
| ±6.21 | ±8.06 | ±4.45 | ±5.18 | ±2.74 | ±4.26 | ±3.03 | ±3.78 | |
| CRT improvement | 65.52 | 73.33 | 68.18 | 81.99 | 82.76 | 73.33 | 79.55 | 82.21 |
| ±7.31 | ±5.61 | ±4.93 | ±4.27 | ±4.24 | ±4.74 | ±3.81 | ±4.16 | |
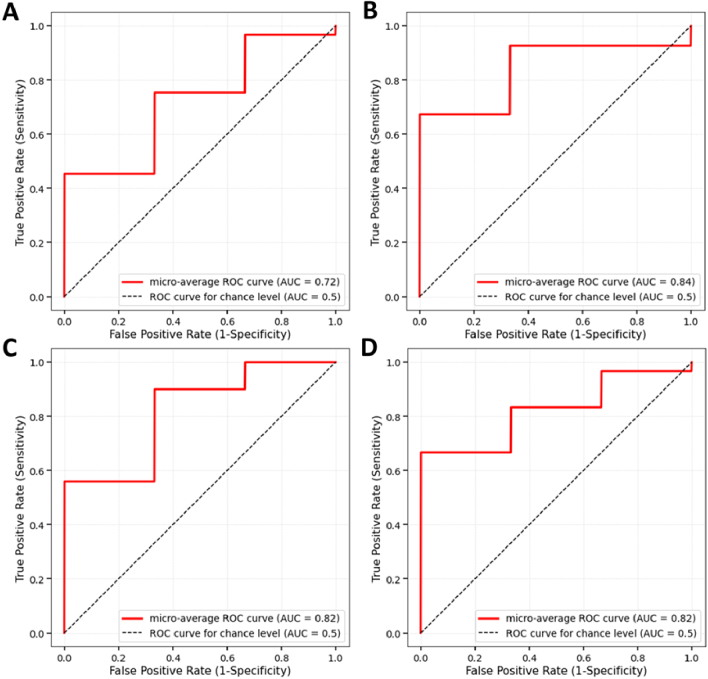
Table 3 presents the identified optimal feature subsets, ranked by their importance for BCVA and CRT improvement classifications. Figure 2 illustrates the ROC curves and the associated AUC values for BCVA and CRT improvement classifiers before and after AV analysis. These visualizations further emphasize the enhanced performance of the classifiers, particularly for BCVA improvement, following the incorporation of AV analysis.
Table 3. Identified optimal feature subsets sorted based on their importance before and after AV analysis.
| Classification | Before or after AV analysis | Number of total features | Number of optimal features | Optimal feature subset |
|---|---|---|---|---|
| BCVA improvement | Before | 7 | 3 | T-BVT, T-VAF, T-PID |
| After | 42 | 4 | VT-PIDR, V-BVT, AV-BVTR, T-BVT | |
| CRT improvement | Before | 7 | 1 | T-BVC |
| After | 42 | 3 | T-BVC, V-VAF, T-VPI |
Fig. 2.
ROC curves for BCVA improvement classification: (A) before AV analysis and (B) after AV analysis; and CRT improvement classification: (C) before AV analysis and (D) after AV analysis.
4. Discussion
Differential AV analysis provides valuable information in understanding the vascular contributions to DME and predicting treatment outcomes with anti-VEGF therapy. Diabetic damage to the retina leads to a complex interplay of arterial ischemia, venous congestion, and capillary leakage, all of which contribute to DME and vision impairment. Histologically, diabetic macular ischemia is characterized by capillary dropout and the narrowing or obliteration of precapillary arterioles, which reduces blood flow downstream of the occlusion and impairs oxygen delivery regulated by retinal arteries, leading to ischemia and hypoxia [31]. In response to ischemia and hypoxia, the retina and retinal pigment epithelium (RPE) release high levels of VEGF [32]. Overexpression of VEGF resulting in hyperpermeability, vascular leakage, and DME development, as well as the growth of retinal, optic disk, and/or iris neovascularizations (NVs) [33].
Venous congestion plays a significant role in the development and persistence of DME [34]. Diabetes-induced microvascular dysfunction leads to venous dilation and tortuosity, increased venous pressure, and impaired venous outflow, exacerbating vascular leakage and fluid accumulation in the macula [34]. Increased venous pressure promotes capillary leakage and reduces perfusion efficiency, compounding the ischemic effects of arterial narrowing [35]. These venous abnormalities further exacerbate hypoxia by hindering the removal of deoxygenated blood and metabolic waste, perpetuating the cycle of VEGF overexpression and vascular permeability [2]. Moreover, systemic factors such as hypertension and increased blood viscosity in diabetic patients amplify venous congestion, worsening DME severity [36].
Anti-VEGF therapy aims to reduce VEGF levels, thereby decreasing vascular permeability and promoting fluid reabsorption [4]. Anti-VEGF therapy may have variable outcomes depending on the predominant vascular pathology [5]. These insights about the different roles of arterial and venous areas on DME and anti-VEGF treatment show the importance of differential AV analysis within OCTA images. As an in vivo imaging capability, OCTA enables non-invasive, high-resolution imaging of retinal vessels, providing an opportunity to differentiate arteries from veins and assess their respective roles in DME pathogenesis and treatment response.
In this study, we utilized the MF-AV-Net [22] architecture combined with a k-nearest neighbor (kNN) classifier for AVA segmentation within OCTA. The integration involved multiplying the OCTA image by the AVA map to generate OCTA-AV maps with preserved OCTA intensity information. OCTA-AV maps distinctly highlight arterial and venous areas in separate red and blue channels, enabling precise differentiation between arterial and venous regions. This segmentation was instrumental in deriving features specific to arteries, arterioles, and capillaries in arterial areas, as well as veins, venules, and capillaries in the venous areas. This approach enabled a detailed quantitative analysis of vascular features, capturing changes relevant to DME and providing insights into treatment outcomes. By leveraging the unique patterns in arterial and venous structures affected by DME, the model improved classification accuracy for treatment response. This methodology underscores the value of differential AV analysis in advancing the understanding and clinical management of DME and precise assessment of therapeutic efficacy.
Using the OCTA-AV maps, we extracted seven quantitative features from each OCTA image at baseline: PID, BVD, VSD, VAF, BVC, BVT, and VPI. These features were calculated for total, arterial, and venous vessels, as well as their respective ratios across the entire image. This comprehensive feature set assesses the vascular characteristics in DME at baseline, offering valuable insights into the microvascular environment before treatment. Each feature captures distinct aspects of retinal vasculature: PID, BVD, and VSD quantify perfusion and density, while VAF, BVC, and BVT characterize structural vessel changes. VPI provides additional specificity by assessing perimeter irregularities. These baseline features serve as predictors in machine learning models to classify patients based on their potential functional responses, represented by BCVA, and anatomical responses, indicated by CRT, to anti-VEGF therapy. BCVA improvement was defined as a decrease of more than 0.1 LogMAR, while CRT improvement was characterized by a more than 10% decrease.
The diverse input features enable the classification model to capture a broader range of disease manifestations, enhancing its ability to predict treatment outcomes accurately. In medical imaging research, particularly with OCTA images, limited dataset sizes remain a common challenge since these images are not routinely obtained in clinical practice. While incorporating a more significant number of features provides a richer representation of the data, it also increases the dimensionality of the feature space. To manage the high dimensionality of this feature set and optimize model performance, we employed an SFS algorithm combined with SVM classifiers. SFS systematically identified the most relevant subset of features, enhancing classification accuracy and computational efficiency.
Incorporating AV analysis at baseline substantially enhanced the prediction of treatment outcomes in DME patients. Specifically, AV analysis improved the classification accuracy for BCVA and CRT improvements by 15.91% and 11.37%, respectively. Additional significant gains included an 11.75% increase in AUC, a 26.67% improvement in specificity, and a 10.35% rise in sensitivity for BCVA improvement classification, as well as a 17.24% boost in sensitivity for CRT improvement classification. Notably, while AUC values before and after AV analysis remained similar, the sensitivity and accuracy of CRT improvement classification were significantly higher after AV analysis. This suggests that while the overall discriminative capability of the model was maintained, AV analysis improved the identification of true positives. These compelling findings highlight the value of AV analysis in extracting features specific to arteries or veins, enabling effective prediction of treatment outcomes in DME patients.
The features listed in Table 3 were selected due to their strong association with vascular abnormalities in DME pathology and anti-VEGF treatment [37,38]. For example, BVT and BVC reflect structural abnormalities, while PID and VAF are indicative of vascular perfusion density. The inclusion of AV features and their ratios provides insights into differential vascular contributions, highlighting the significance of arterial ischemia and venous congestion in DME progression and treatment response. The SFS algorithm identified these features based on their significant contribution to enhancing classification accuracy in predicting treatment outcomes.
Despite its strengths, this study is not without limitations. The dataset size was relatively small, and some analyses included both eyes of individual patients, which could introduce potential inter-eye correlations. Future studies with larger, multicenter datasets are needed to validate these findings and explore the generalizability of differential AV features across diverse populations. Future studies can explore synthetic data generation techniques, such as adding Gaussian noise, to enhance model robustness and refine decision boundaries. Additionally, while this study focused on baseline features, longitudinal analyses could provide further insights into the temporal dynamics of AV changes and their relationship with treatment outcomes.
5. Conclusion
In conclusion, this study demonstrates that differential AV analysis in OCTA can significantly enhance the prediction of treatment outcomes in DME. Incorporating AV features improves machine learning performance in predicting functional BCVA responses and anatomical CRT responses following anti-VEGF therapy. These findings pave the way for more precise, personalized therapeutic strategies and offer valuable insights into the vascular mechanisms underlying DME and its treatment.
Funding
National Eye Institute10.13039/100000053 (P30 EY001792, R01 EY029673, R01 EY030101, R01 EY023522, R01 EY030842); Research to Prevent Blindness10.13039/100001818; Richard and Loan Hill Department of Biomedical Engineering, University of Illinois at Chicago10.13039/100019924.
Disclosures
No competing interest exists for any author.
Data availability
Data may be obtained from the authors upon reasonable request.
References
- 1.Sakata K., Funatsu H., Harino S., et al. , “Relationship of macular microcirculation and retinal thickness with visual acuity in diabetic macular edema,” Ophthalmology 114(11), 2061–2069 (2007). 10.1016/j.ophtha.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 2.Shin E. S., Sorenson C. M., Sheibani N., “Diabetes and retinal vascular dysfunction,” J. Ophthalmic Vision Res. 9, 362 (2014). 10.4103/2008-322X.143378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Régnier S., Malcolm W., Allen F., et al. , “Efficacy of anti-VEGF and laser photocoagulation in the treatment of visual impairment due to diabetic macular edema: a systematic review and network meta-analysis,” PLoS One 9(7), e102309 (2014). 10.1371/journal.pone.0102309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urias E. A., Urias G. A., Monickaraj F., et al. , “Novel therapeutic targets in diabetic macular edema: beyond VEGF,” Vision Res. 139, 221–227 (2017). 10.1016/j.visres.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 5.Cheema A. A., Cheema H. R., “Diabetic Macular Edema Management: A Review of Anti-Vascular Endothelial Growth Factor (VEGF) Therapies,” Cureus 16, e52676 (2024). 10.7759/cureus.52676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abtahi M., Le D., Ebrahimi B., et al. , “Differential capillary and large vessel analysis improves OCTA classification of diabetic retinopathy,” Invest. Ophthalmol. Visual Sci. 65(10), 20 (2024). 10.1167/iovs.65.10.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz M., Díez-Sotelo M., Gómez-Ulla F., et al. , “Automatic visual acuity estimation by means of computational vascularity biomarkers using oct angiographies,” Sensors 19(21), 4732 (2019). 10.3390/s19214732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh Y.-T., Alam M. N., Le D., et al. , “OCT angiography biomarkers for predicting visual outcomes after ranibizumab treatment for diabetic macular edema,” Ophthalmology Retina 3(10), 826–834 (2019). 10.1016/j.oret.2019.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y. K., Fung N. S. K., Chan J. C., et al. , “OCTA biomarkers in adults aged 50 and above: a prospective and cross-sectional community-based study,” BMC Ophthalmol. 23(1), 71 (2023). 10.1186/s12886-023-02815-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dadzie A. K., Le D., Abtahi M., et al. , “Normalized blood flow index in optical coherence tomography angiography provides a sensitive biomarker of early diabetic retinopathy,” Trans. Vis. Sci. Tech. 12(4), 3 (2023). 10.1167/tvst.12.4.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q., Zhang W., Zhu H., et al. , “Foveal avascular zone volume: a new index based on optical coherence tomography angiography images,” Retina 41(3), 595–601 (2021). 10.1097/IAE.0000000000002890 [DOI] [PubMed] [Google Scholar]
- 12.Uchitomi D., Murakami T., Dodo Y., et al. , “Disproportion of lamellar capillary non-perfusion in proliferative diabetic retinopathy on optical coherence tomography angiography,” Br. J. Ophthalmol. 104(6), 857–862 (2020). 10.1136/bjophthalmol-2019-314743 [DOI] [PubMed] [Google Scholar]
- 13.Park Y. G., Park Y.-H., “Quantitative Analysis of Retinal Microvascular Perfusion and Novel Biomarkers of the Treatment Response in Diabetic Macular Edema,” J. Diabetes Res. 2020, 1–8 (2020). 10.1155/2020/2132037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elnahry A. G., Noureldine A. M., Abdel-Kader A. A., et al. , “Optical coherence tomography angiography biomarkers predict anatomical response to bevacizumab in diabetic macular edema,” Diabetes, Metab. Syndr. Obes.: Targets Ther. 15, 395–405 (2022). 10.2147/DMSO.S351618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basiony A. I., Mohamed Gad Marey H., Abdel Fattah A. M. E., et al. , “Predictive value of optical coherence tomography angiography in management of diabetic macular edema,” BMC Ophthalmol. 24(1), 429 (2024). 10.1186/s12886-024-03540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hein M., Vukmirovic A., Constable I. J., et al. , “Angiographic biomarkers are significant predictors of treatment response to intravitreal aflibercept in diabetic macular edema,” Sci. Rep. 13(1), 8128 (2023). 10.1038/s41598-023-35286-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatano M., Higashijima F., Yoshimoto T., et al. , “Evaluation of microaneurysms as predictors of therapeutic response to anti-VEGF therapy in patients with DME,” PLoS One 17(11), e0277920 (2022). 10.1371/journal.pone.0277920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alam M., Le D., Son T., et al. , “AV-Net: deep learning for fully automated artery-vein classification in optical coherence tomography angiography,” Biomed. Opt. Express 11(9), 5249–5257 (2020). 10.1364/BOE.399514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao M., Guo Y., Hormel T. T., et al. , “A Deep Learning Network for Classifying Arteries and Veins in Montaged Widefield OCT Angiograms,” Ophthalmology Science 2(2), 100149 (2022). 10.1016/j.xops.2022.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X., Yang P., Wang H., et al. , “AV-casNet: Fully Automatic Arteriole-Venule Segmentation and Differentiation in OCT Angiography,” IEEE Trans. Med. Imaging 42(2), 481–492 (2023). 10.1109/TMI.2022.3214291 [DOI] [PubMed] [Google Scholar]
- 21.Le D., Abtahi M., Adejumo T., et al. , “Deep learning for artery–vein classification in optical coherence tomography angiography,” Exp. Biol. Med. 248(9), 747–761 (2023). 10.1177/15353702231181182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abtahi M., Le D., Lim J. I., et al. , “MF-AV-Net: an open-source deep learning network with multimodal fusion options for artery-vein segmentation in OCT angiography,” Biomed. Opt. Express 13(9), 4870–4888 (2022). 10.1364/BOE.468483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abtahi M., Le D., Ebrahimi B., et al. , “An open-source deep learning network AVA-Net for arterial-venous area segmentation in optical coherence tomography angiography,” Commun. Med. 3(1), 54 (2023). 10.1038/s43856-023-00287-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abtahi M., Le D., Ebrahimi B., et al. , “Differential artery-vein analysis improves the OCTA classification of diabetic retinopathy,” Biomed. Opt. Express 15(6), 3889–3899 (2024). 10.1364/BOE.521657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frangi A. F., Niessen W. J., Vincken K. L., et al. , “Multiscale vessel enhancement filtering,” in Medical Image Computing and Computer-Assisted Intervention—MICCAI’98: First International Conference Cambridge, MA, USA, October 11–13, 1998 Proceedings 1(Springer, 1998), pp. 130–137. [Google Scholar]
- 26.Alam M., Toslak D., Lim J. I., et al. , “OCT feature analysis guided artery-vein differentiation in OCTA,” Biomed. Opt. Express 10(4), 2055–2066 (2019). 10.1364/BOE.10.002055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alam M., Thapa D., Lim J. I., et al. , “Quantitative characteristics of sickle cell retinopathy in optical coherence tomography angiography,” Biomed. Opt. Express 8(3), 1741–1753 (2017). 10.1364/BOE.8.001741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdolahi F., Zhou X., Ashimatey B. S., et al. , “Optical coherence tomography angiography–derived flux as a measure of physiological changes in retinal capillary blood flow,” Trans. Vis. Sci. Tech. 10(9), 5 (2021). 10.1167/tvst.10.9.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao X., Alam M. N., Le D., et al. , “Quantitative optical coherence tomography angiography: a review,” Exp. Biol. Med. 245(4), 301–312 (2020). 10.1177/1535370219899893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain A., Zongker D., “Feature selection: Evaluation, application, and small sample performance,” IEEE Trans. Pattern Anal. Machine Intell. 19(2), 153–158 (1997). 10.1109/34.574797 [DOI] [Google Scholar]
- 31.Tombolini B., Borrelli E., Sacconi R., et al. , “Diabetic macular ischemia,” Acta Diabetol 59(6), 751–759 (2022). 10.1007/s00592-021-01844-1 [DOI] [PubMed] [Google Scholar]
- 32.Beltramo E., Porta M., “Pericyte loss in diabetic retinopathy: mechanisms and consequences,” Curr. Med. Chem. 20(26), 3218–3225 (2013). 10.2174/09298673113209990022 [DOI] [PubMed] [Google Scholar]
- 33.Campochiaro P. A., Wykoff C. C., Shapiro H., et al. , “Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema,” Ophthalmology 121(9), 1783–1789 (2014). 10.1016/j.ophtha.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 34.Dimitrova G., Lutty G. A., “The role of retinal venous congestion in diabetic retinopathy,” SN Compr. Clin. Med. 3(4), 964–970 (2021). 10.1007/s42399-021-00809-3 [DOI] [Google Scholar]
- 35.Stewart M. W., “The expanding role of vascular endothelial growth factor inhibitors in ophthalmology,” in Mayo Clinic Proceedings (Elsevier, 2012), pp. 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLeod D. S., Lefer D. J., Merges C., et al. , “Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid,” The American Journal of Pathology 147(3), 642 (1995). [PMC free article] [PubMed] [Google Scholar]
- 37.Santamaría J., Caminal J. M., Cobos E., et al. , “Correlation between topographic vessel density and retinal thickness changes in patients with diabetic macular edema treated with anti-VEGF therapy: is it a suitable OCTA biomarker?” J. Pers. Med. 13(12), 1718 (2023). 10.3390/jpm13121718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santamaría J., Cobos E., Biarnes M., et al. , “Changes in vessel density patterns assessed with OCTA in patients with diabetic macular edema treated with anti-VEGF therapy,” Acta Diabetologica 61(11), 1385–1392 (2024). 10.1007/s00592-024-02290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be obtained from the authors upon reasonable request.