Abstract
Study Design
Retrospective cohort study.
Objectives
This study aimed to identify factors influencing postoperative outcomes of cervical spondylotic myelopathy (CSM) in individuals with cerebral palsy (CP).
Methods
Data from admitted individuals were retrospectively reviewed. Individuals whose modified Barthel index score, assessed at least 6 months after surgery, declined by 1 or more grades compared to their preoperative score were classified into the poor outcome (PO) group. Multivariate logistic regression analysis was performed to assess risk factors for poor postoperative outcomes.
Results
Of the 73 participants, 15 were in the PO group and 58 in the non-PO group. Duration (OR 1.99, 95% CI 1.25-3.65, P = .01), signal change grade 2 (OR 10.44, 95% CI 1.32-118.01, P = .034), and spinal cord compression ratio, M2 (OR 0.85, 95% CI, 0.73-0.96, P = .02) on preoperative MRI were identified as significant factors associated with the risk of poor postoperative outcomes. Based on the receiver operating characteristic curve analysis, the cutoff values for duration and cord compression metric were determined as 2 years (AUC = 0.689, 95% CI 0.532-0.845) and 76.2% (AUC = 0.841, 95% CI 0.696-0.987), respectively.
Conclusions
This study identified key predictors of poor postoperative outcomes in individuals with CP undergoing surgery for CSM. Symptom duration exceeding 2 years, signal change grade 2, and spinal cord compression ratio below 76.2% on preoperative MRI were found to be predictors of poor outcome. These results underscore the importance of early intervention and detailed preoperative radiological assessment to improve surgical outcomes in this population.
Keywords: cerebral palsy, cervical spondylotic myelopathy, spinal cord compression, magnetic resonance imaging, prognostic factor, prognosis
Introduction
Cerebral palsy (CP) is a non-progressive motor disability resulting from disturbances in the developing fetal or infant brain. 1 However, despite having non-progressive characteristics, individuals with CP can experience clinical deterioration and unexpected complications owing to various secondary conditions. 2 Cervical stenosis can lead to compression of the cervical spinal cord, resulting in loss of function and neurological deficits. 3 Compared with individuals without CP, those with CP are more likely to develop early onset cervical degenerative disc disease and have a higher frequency of listhetic instability in the midcervical spine.4-6
Cervical spondylotic myelopathy (CSM), characterized by spinal cord compression due to spondylotic degenerative changes, is one of the most common forms of degenerative cervical myelopathy.7,8 In individuals with cerebral palsy (CP), the cervical spine is particularly susceptible to early-onset cervical spondylotic myelopathy (CSM) due to long-standing abnormal biomechanics.4,9 Excessive spondylotic changes, combined with involuntary movements, poor head control, and abnormal posture, contribute to cervical malalignment, canal stenosis, neural compression, and dynamic instability. 10 As a result, individuals with CP often develop cervical abnormalities such as segmental instability, kyphotic deformities, and premature disc degeneration. 11 Conservative treatments for CSM in individuals with CP are often ineffective; therefore, surgical intervention is usually considered.12-14 The primary goal of surgery is to adequately decompress the spinal canal and nerve roots, provide stability, and correct the alignment of the cervical spine.15-17 However, owing to the nature of CP, which includes the complexity of the deformity and involuntary neck movements, long-term surgical outcomes are not always favorable.16,18
In the non-CP population, several predictors of postoperative outcomes for CSM have been identified. Preoperative neurological status is a strong indicator of prognosis.19,20 Old age is known as a poor prognostic factor. 21 Furthermore, several factors such as a long duration of symptoms and great compression of the spinal cord on magnetic resonance imaging (MRI) are known negative predictors.22,23
However, research on the postoperative predictors of CSM in the CP population is lacking. Owing to their clinical characteristics, such as the complexity of the deformity and involuntary neck movements, we anticipate that they may exhibit different patterns compared with general predictors. Therefore, in this study, we aimed to identify the predictors of long-term prognosis after surgery for CSM in individuals with CP.
Methods
Participants
The medical records of individuals with CP who received CSM surgery between January 2008 and August 2023 were retrospectively reviewed. This study was approved by our institutional review board (4-2024-0643). The inclusion criteria were as follows: individuals with CP who visited our center with a diagnosis of CSM and those who underwent surgery for CSM. The exclusion criteria for all the individuals were as follows: a prior history of cervical spine surgery; lack of preoperative cervical plain radiographs or MRI images, or images with significantly poor image quality preventing necessary measurements; insufficient preoperative and postoperative 6-month activity of daily living assessment results; missing medical records and test results; and postoperative functional impairment due to causes other than myelopathy (Figure 1).
Figure 1.
Flowchart of participant selection. Of 154 participants, 81 were excluded, leaving 73 participants for analysis.
Data Assessment
Clinical characteristics including sex, type of CP, hypertension, diabetes mellitus, type of cervical surgery, age, duration of symptoms (specifically motor weakness), height, weight, and body mass index (BMI) were collected. Functional status was determined using the modified Barthel index (MBI), which indicates independence in activities of daily living.
The MBI scores were collected for all individuals both before surgery and again more than 6 months after surgery. The MBI scores were categorized into 5 groups as follows: 0-24 for total dependency, 25-49 for severe dependency, 50-74 for moderate dependency, 75-90 for mild dependency, and 91-100 for minimal dependency (Figure 2).24,25 Individuals were classified into the poor outcome (PO) group if their level of independence in daily living, measured by the MBI, declined when comparing their preoperative score with the score collected more than 6 months after surgery; otherwise, they were classified into the non-PO group.
Figure 2.
Cut-off values of the Modified Barthel Index based on levels of dependency in activities of daily living. The Modified Barthel Index can be classified into 5 levels of dependency based on the scores.
Preoperative Radiologic Evaluation
Preoperative radiological parameters were obtained from plain radiography and MRI. Plain radiography was used to measure the C0-2 Cobb angle (neutral position), C2-7 Cobb angle (flexion and extension), T1 slope (neutral position), and C2-7 sagittal vertical axis (neutral position). The T1 slope was defined as the angle between the horizontal plane and the line parallel to the superior T1 endplate. The C2-7 sagittal vertical axis was defined as the distance between the plumb line dropped from the center of C2 and the posterosuperior aspect of C7. Additionally, C2-7 range of motion was calculated by subtracting the Cobb angle during flexion from that during extension. 26
MRI was used to measure the grade and extent of signal intensity in the spinal cord on T2-weighted images in the sagittal plane. T2-weighted signal intensities (T2SI) in the spinal cord at the narrowest level were classified into grades 0-2 on a sagittal view (grade 0, none; grade 1, light or obscure; grade 2, intense or bright). 27 Additionally, the extent of T2SI in the spinal cord in the sagittal view was classified as none, focal, or diffuse (spanning more than 1 vertebral level). Three metrics were calculated from the MRI images using both sagittal and axial cuts (Figure 3). M1 was obtained from the sagittal view and calculated as the canal diameter at the level with the most severe canal stenosis divided by the average canal diameter above and below it. 28 M2, which is also derived from the sagittal view, represents the spinal cord diameter at the level with the most severe spinal cord compression divided by the average of the spinal cord diameters above and below. 28 M3 was calculated by dividing the height at the narrowest point of the spinal cord by the greatest distance along the spinal cord. 29
Figure 3.
Three metrics were measured in magnetic resonance images. (A) M1. M1 was calculated as the canal diameter at the level with the most severe canal stenosis divided by the average of the canal diameters above and below it. (B) M2. M2 was calculated as the spinal cord diameter at the level with the most severe spinal cord compression divided by the average of the spinal cord diameters above and below it. (C) M3. M3 was calculated by dividing the height at the narrowest point of the spinal cord by the greatest distance along the spinal cord.
Statistical Analysis
Statistical analyses were performed using the R Statistical Software version 4.4.0 (R Foundation for Statistical Computing, Vienna, Austria). Simple descriptive statistics were used to characterize the participants and distribution of the variables. For continuous variables, data are presented as means ± standard deviations and were analyzed using an independent t test. We verified the normality of the variables using the Shapiro–Wilk test. If the normality assumption was satisfied, we conducted an independent t test and reported the results as means ± standard deviations. If the normality assumption was not satisfied, we conducted a non-parametric Mann–Whitney U test and reported the results as medians (interquartile ranges). The chi-square and Fisher’s exact tests were used for categorical variables. A multivariate logistic regression analysis was performed to predict poor postoperative outcomes. Model performance was evaluated by constructing receiver operating characteristic (ROC) curves and computing the area under the curve (AUC). Significance was set at P < .05.
Results
Normality Tests for Continuous Variables
The Shapiro–Wilk test was conducted to verify the normality of continuous variables. The variables that satisfied the normality test were age (P = .056), weight (P = .194), T1S (P = .066), and C2-7 sagittal vertical axis (P = .688). Meanwhile, height (P = .019), body mass index (P = .001), duration (P < .001), C0-2 Cobb angle (neutral position) (P < .001), C2-7 Cobb angle (flexion) (P = .041), C2-7 Cobb angle (extension) (P = .006), C2-7 ROM (P < .001), M1 (P = .007), M2 (P < .001), M3 (P = .012), MBI (pre-op) (P < .001), and MBI (post-op 6-months) (P < .001) did not meet the normality assumption.
Baseline Characteristics of Participants
The clinical characteristics of the study participants are presented in Table 1. Among the 73 participants, 15 were classified into the PO group, and the remaining 58 were classified into the non-PO group. No significant differences in sex (non-PO vs PO: male 53.4% vs 60.0%, P = .650) and type of CP (dyskinetic, 74.1% vs 66.7%; spastic, 19.0% vs 26.7%; mixed, 6.9% vs 6.7%, P = .784) were observed between the 2 groups. Moreover, the presence of hypertension and diabetes mellitus (3.4% vs 6.7%, P = .576), type of surgery performed (anterior and posterior fixation, 44.8% vs 46.7%; anterior fixation, 29.3% vs 26.7%; posterior fixation, 22.4% vs 26.7%; laminectomy without fixation, 3.4% vs 0, P = 1.000), and age (43.6 ± 7.9 years vs 44.4 ± 8.6 years, P = .729) were not significantly different between the 2 groups. Meanwhile, the duration of symptoms was longer in the PO group than in the non-PO group (0.7 [0.3, 1.4] years vs 2.0 [0.8, 2.8] years, P = .024). Height (1.6 (1.5, 1.7) m vs 1.6 (1.5, 1.7) m; P = .973), weight (55.2 ± 10.7 kg vs 53.5 ± 8.7 kg, P = .566), BMI (21.5 (18.7, 23.7) kg/m2 vs 20.4 (19.0, 22.9) kg/m2, P = .652), and preoperative MBI (66.0 (34.8, 89.5) vs 75.0 (61.5, 88.0), P = .235) were not significantly different between the 2 groups.
Table 1.
Clinical Characteristics of Participants.
| Non-PO (n = 58) | PO (n = 15) | P-Value | |
|---|---|---|---|
| Sex | .650 | ||
| Male | 31 (53.4%) | 9 (60.0%) | |
| Female | 27 (46.6%) | 6 (40.0%) | |
| Type of CP | .784 | ||
| Dyskinetic | 43 (74.1%) | 10 (66.7%) | |
| Spastic | 11 (19.0%) | 4 (26.7%) | |
| Mixed | 4 (6.9%) | 1 (6.7%) | |
| Hypertension | .504 | ||
| (−) | 56 (96.6%) | 14 (93.3%) | |
| (+) | 2 (3.4%) | 1 (6.7%) | |
| Diabetes mellitus | .504 | ||
| (−) | 56 (96.6%) | 14 (93.3%) | |
| (+) | 2 (3.4%) | 1 (6.7%) | |
| Surgery type | 1.000 | ||
| Anterior and posterior fixation | 26 (44.8%) | 7 (46.7%) | |
| Anterior fixation | 17 (29.3%) | 4 (26.7%) | |
| Posterior fixation | 13 (22.4%) | 4 (26.7%) | |
| Fixation (−) | 2 (3.4%) | 0 (0) | |
| Age (years) | 43.6 ± 7.9 | 44.4 ± 8.6 | .729 |
| Duration (years) | 0.7 (0.3, 1.4) | 2.0 (0.8, 2.8) | .024* |
| Height (m) | 1.6 (1.5, 1.7) | 1.6 (1.5, 1.7) | .973 |
| Weight (kg) | 55.2 ± 10.7 | 53.5 ± 8.7 | .566 |
| Body mass index (kg/m2) | 21.5 (18.7, 23.7) | 20.4 (19.0, 22.9) | .652 |
| Preoperative MBI | 66.0 (34.8, 89.5) | 75.0 (61.5, 88.0) | .235 |
Abbreviations: PO, poor outcome; CP, cerebral palsy; MBI, modified Barthel index.
Note: *indicates statistically significant P-values.
Comparison of Preoperative Radiologic Findings Between the PO and Non-PO Groups
Preoperative radiological findings are summarized in Table 2. None of the parameters measured on the plain radiographs showed significant differences. The C0-2 Cobb angle (neutral) was 15.9 (8.6, 26.6)° in the non-PO group and 22.7 (16.1, 29.9)° in the PO group (P = .243). The C2-7 Cobb angle (flexion) was 29.4 (17.0, 37.4)° and 34.2 (19.0, 42.9)° (P = .390), and the C2-7 Cobb angle (extension) was 23.5 (12.9, 40.3)° and 34.4 (22.3, 44.9)° (P = .152), respectively. The T1 slope was 23.4 ± 11.2° and 19.9 ± 10.4° (P = .276). The C2-7 sagittal vertical axis was 21.3 ± 19.7 mm and 20.4 ± 22.8 mm (P = .868). The C2-7 range of motion was −5.3 (−18.8, 18.4)° and 11.5 (−22.2, 22.2)° (P = .557).
Table 2.
Preoperative Radiologic Findings of Participants.
| Non-PO (n = 58) | PO (n = 15) | P-Value | |
|---|---|---|---|
| Plain radiograph findings | |||
| C0-2 cobb angle (neutral) (°) | 15.9 (8.6, 26.6) | 22.7 (16.1, 29.9) | .243 |
| C2-7 cobb angle (flexion) (°) | 29.4 (17.0, 37.4) | 34.2 (19.0, 42.9) | .390 |
| C2-7 cobb angle (extension) (°) | 23.5 (12.9, 40.3) | 34.4 (22.3, 44.9) | .152 |
| T1 slope (°) | 23.4 ± 11.2 | 19.9 ± 10.4 | .276 |
| C2-7 sagittal vertical axis (mm) | 21.3 ± 19.7 | 20.4 ± 22.8 | .868 |
| C2-7 range of motion (°) | −5.3 (−18.8, 18.4) | 11.5 (−22.2, 22.2) | .557 |
| MRI findings | |||
| T2SI grade | <.001* | ||
| Grade 0 | 15 (25.9%) | 3 (20.0%) | |
| Grade 1 | 41 (70.7%) | 5 (33.3%) | |
| Grade 2 | 2 (3.4%) | 7 (46.7%) | |
| T2SI type | .228 | ||
| None | 15 (25.9%) | 3 (20.0%) | |
| Focal | 26 (44.8%) | 4 (26.7%) | |
| Diffuse | 17 (29.3%) | 8 (53.3%) | |
| M1 | 75.0 (66.6, 82.6) | 64.5 (45.1, 67.8) | <.001* |
| M2 | 86.9 (80.4, 89.9) | 66.9 (56.8, 75.9) | <.001* |
| M3 | 72.4 (67.0, 77.9) | 62.4 (54.6, 66.2) | <.001* |
Abbreviations: PO, poor outcome; T2SI, T2-weighted signal intensities.
Note: *indicates statistically significant P-values.
Regarding MRI findings, significant differences in the T2SI grade (grade, 0 25.9% vs 20.0%; grade 1, 70.7% vs 33.3%; grade 2, 3.4% vs 46.7%, P < .001) were observed between the 2 groups. By contrast, the T2SI type did not show a significant difference (none, 25.9% vs 20.0%; focal: 44.8% vs 26.7%; diffuse, 29.3% vs 53.3%, P = .228). M1, M2, and M3 showed significant differences (M1, 75.0 (66.6, 82.6)% vs 64.5 (45.1, 67.7)%; M2, 86.9 (80.4, 89.9)% vs 66.9 (56.8, 75.9)%; M3, 72.4 (67.0, 77.9)% vs 62.4 (54.6, 66.2)%, all P < .001).
Univariate and Multivariate Analyses Regarding Poor Postoperative Outcomes
The risk of poor postoperative outcomes was positively correlated with disease duration (P = .016) and T2SI grade 2 (P = .005) (Table 3). It negatively correlated with M1 (P < .001), M2 (P < .001), and M3 (P = .001). Table 4 shows the results of the multivariate logistic regression analysis. After the adjustment, the risk of poor postoperative outcomes was significantly positively correlated with duration (OR, 1.990; 95% CI, 1.249-3.652; P = .009) and T2SI grade 2 (OR, 10.439; 95% CI, 1.316-118.006; P = .034). Meanwhile, the risk was significantly negatively correlated with M2 (OR, 0.854; 95% CI, 0.732-0.959; P = .017). The variables included in multivariate analysis did not exhibit multicollinearity.
Table 3.
Univariate Logistic Regression Analysis of Factors Associated With Poor Outcomes.
| Variable | Odds Ratio | 95% Confidence Interval | P-Value | |
|---|---|---|---|---|
| Sex | Male | (Reference) | ||
| Female | 0.765 | 0.230-2.401 | .650 | |
| Type of CP | Dyskinetic | (Reference) | ||
| Spastic | 1.564 | 0.374-5.730 | .512 | |
| Mixed | 1.075 | 0.052-8.313 | .951 | |
| Hypertension | (−) | (Reference) | ||
| (+) | 2.000 | 0.089-22.371 | .582 | |
| Diabetes mellitus | (−) | (Reference) | ||
| (+) | 2.000 | 0.089-22.371 | .582 | |
| Surgery type | Anterior and posterior fixation | (Reference) | ||
| Anterior fixation | 0.874 | 0.203-3.363 | .847 | |
| Posterior fixation | 1.143 | 0.260-4.528 | .851 | |
| Fixation (−) | N/A | N/A | ||
| Age | 1.013 | 0.944-1.095 | .724 | |
| Duration (years) | 1.504 | 1.094-2.161 | .016* | |
| Height (m) | 0.742 | 0.001-511.404 | .928 | |
| Weight (kg) | 0.983 | 0.925-1.039 | .561 | |
| Body mass index (kg/m2) | 0.956 | 0.798-1.120 | .602 | |
| Preoperative MBI | 1.017 | 0.996-1.042 | .146 | |
| C0-2 cobb angle (neutral) | 1.017 | 0.977-1.058 | .388 | |
| C2-7 cobb angle (flexion) | 1.017 | 0.972-1.067 | .464 | |
| C2-7 cobb angle (extension) | 1.019 | 0.987-1.053 | .235 | |
| T1 slope | 0.969 | 0.912-1.022 | .273 | |
| C2-7 sagittal vertical axis | 0.998 | 0.969-1.026 | .866 | |
| C2-7 range of motion | 1.006 | 0.982-1.032 | .614 | |
| T2SI grade | Grade 0 | (Reference) | ||
| Grade 1 | 0.610 | 0.132-3.261 | .531 | |
| Grade 2 | 17.500 | 2.770-169.882 | .005* | |
| T2SI type | None | (Reference) | ||
| Focal | 0.769 | 0.150-4.343 | .752 | |
| Diffuse | 2.353 | 0.563-12.264 | .263 | |
| M1 | 0.887 | 0.819-0.940 | <.001* | |
| M2 | 0.851 | 0.771-0.914 | <.001* | |
| M3 | 0.874 | 0.797-0.937 | .001* | |
Abbreviations: CP, cerebral palsy; MBI, modified Barthel index; T2SI, T2-weighted signal intensities.
Note: *indicates statistically significant P-values.
Table 4.
Multivariate Logistic Regression Analysis of Factors Associated With Poor Outcomes.
| Variable | Adjusted Odds Ratio | 95% Confidence Interval | P-Value |
|---|---|---|---|
| Duration (years) | 1.990 | 1.249-3.652 | .009* |
| T2SI grade 2 | 10.439 | 1.316-118.006 | .034* |
| M1 | 0.981 | 0.844-1.168 | .816 |
| M2 | 0.854 | 0.732-0.959 | .017* |
| M3 | 0.959 | 0.819-1.109 | .582 |
Abbreviations: T2SI, T2-weighted signal intensities.
Note: *indicates statistically significant P-values.
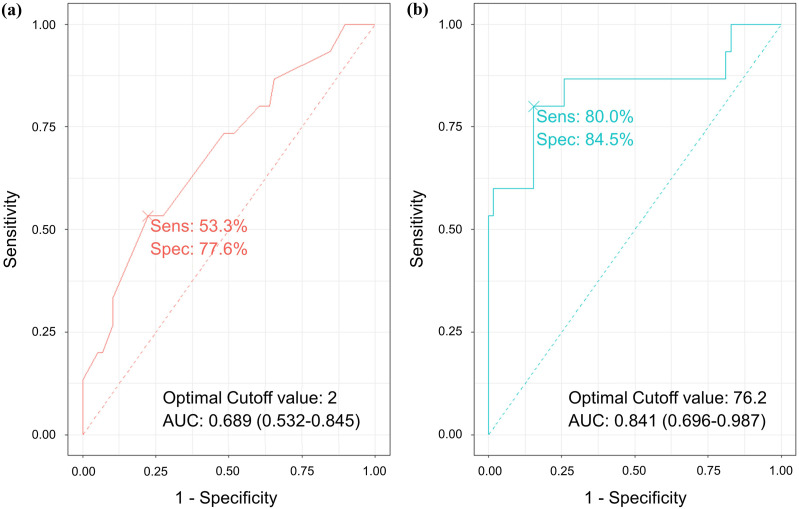
Optimal Cutoff Values for Duration and Cord Compression Ratio for Predicting Poor Postoperative Outcomes
Figure 4 shows the results of ROC analysis. The AUC acquired from the ROC curve for duration was 0.689 (95% CI, 0.532-0.845) for predicting the poor postoperative outcomes. The optimal value was determined to be 2.0 years (sensitivity, 53.3%; specificity, 77.6%). The AUC for M2 was 0.841 (95% CI, 0.696-0.987), and the optimal value was 76.2% (sensitivity, 80.0%; specificity 84.5%).
Figure 4.
Receiver operating characteristic curve analysis of (A) symptom duration and (B) spinal cord compression ratio to poor postoperative outcome. Sensitivity, specificity, optimal cutoff value, and area under the curve is presented.
Discussion
This study aimed to identify predictors of surgical outcomes in individuals with CP-associated CSM, a population with unique challenges due to underlying deformities and chronic abnormal movements. We found that symptom duration exceeding 2 years, T2-weighted signal intensity (T2SI) grade 2, and the spinal compression ratio (M2) were significant prognostic factors. These findings highlight the importance of early diagnosis and intervention, robust imaging evaluation, and multidisciplinary care to optimize outcomes in this complex patient group.
Early Surgical Intervention and Symptom Duration
A key finding of this study was the critical impact of symptom duration on postoperative outcomes. Patients with symptom durations exceeding 2 years demonstrated significantly worse outcome, as evidenced by declines in the MBI, a validated measure of functional independence. Prolonged compression of the spinal cord likely leads to irreversible neural damage, limiting the potential for functional recovery even after decompression.14,30
These results are consistent with broader research in CSM populations, emphasizing the importance of early diagnosis and intervention to mitigate progressive spinal cord injury.7,31 For individuals with CP, whose conditions are often compounded by involuntary movements and underlying deformities, the urgency for early intervention is particularly pronounced. Prolonged symptoms exacerbate ischemic and mechanical damage to neural elements, reducing the efficacy of subsequent surgical intervention.7,30
Clinical implications of these findings include the necessity for healthcare providers to maintain vigilance for signs of CSM in individuals with CP. Regular screening for changes in mobility, fine motor function, and continence—paired with timely imaging studies—can facilitate earlier diagnosis and surgical referral, potentially preventing further neurological deterioration. 7
Prognostic Role of T2-Wieghted Signal Intensity
T2SI on MRI emerged as a significant predictor of poor surgical outcomes. Patients with grade 2 T2SI, characterized by intense brightness on MRI, were more likely to experience reduced postoperative independence. These advanced T2SI changes reflect chronic spinal cord damage, such as gliosis, which are markers of irreversible neural injury.23,32
The predictive value of T2SI aligns with findings in non-CP populations, where higher grades of signal intensity on T2-weighted imaging correlate with diminished functional recovery post-surgery. 33 For clinicians, incorporating T2SI grading into preoperative evaluations offers a robust tool for identifying high-risk patients and tailoring surgical interventions accordingly.
Quantitative Evaluation Using the M2 Ratio
Quantitative metrics derived from preoperative MRI, such as the M2 ratio, offer important insights into the severity of spinal cord compression. The M2 ratio compares the diameter of the spinal cord at the most compressed level to adjacent levels, providing a quantifiable measure of compression. This study demonstrated that a lower values indicated a higher risk of poor outcome.
The identification of an optimal cutoff value of 76.2% further strengthens the clinical utility of the M2 ratio. Patients with M2 ratios above this threshold were significantly more likely to regain or maintain independence postoperatively. These findings are consistent with prior research emphasizing the prognostic importance of MRI-based compression metrics in CSM management.28,34
Although this study demonstrated the importance of MRI for postoperative outcomes, M1 and M3 did not serve as predictive factors despite significant differences between the non-PO and PO groups. Although canal stenosis can reflect the severity of CSM, it does not reflect the condition of the spinal cord, making it inadequate for predicting neurological outcomes or functional prognoses.
The use of objective metrics like the M2 ratio underscores the importance of detailed preoperative imaging in surgical planning. By stratifying patients based on the severity of compression, surgeons can make more informed decisions, optimizing surgical strategies.
Limited Impact of Demographic Factors
Interestingly, demographic and comorbid factors such as age, sex, body mass index, and conditions like hypertension and diabetes did not significantly influence surgical outcomes in this study. Similarly, the type of CP (spastic, dyskinetic, or mixed) and the surgical approach (anterior vs posterior fixation) were not associated with differences in recovery.
These findings contrast with trends observed in non-CP populations, where factors such as advanced age is often negative predictors of recovery.21,35 The unique clinical context of CP, characterized by intrinsic motor impairments, repeated and unusual movements such as dystonia, and spinal deformities, likely diminishes the influence of external demographic factors. Instead, disease-specific factors such as symptom duration and spinal cord compression metrics were more decisive in predicting outcomes.
Plain Radiography and Prognosis
Plain radiography is commonly used for preoperative evaluation of CSM. Plain radiographs are essential for assessing cervical lordosis or its loss and determining flexibility. They can also provide information on instability and spondylolisthesis more effectively than supine MRI, particularly for understanding repetitive trauma mechanisms in CSM. 36 However, despite their clinical importance, they are insufficient for predicting postoperative outcomes and prognoses.
In the previous study by Kim, 26 it was reported that the C2-7 Cobb angle (flexion), T1 slope, and C2-7 range of motion were significantly higher in the CP group during preoperative evaluation. However, since the study compared the CP group with the non-CP group, whereas our study focused on comparisons within the CP group, we were unable to determine whether these values had a significant impact on the postoperative results.
Potential Role of Neurophysiological Evaluation
Neurophysiological studies, including central motor conduction time (CMCT), somatosensory evoked potentials (SSEPs), and motor evoked potentials (MEPs) are valuable tools for evaluating the functional state of the spinal cord.37,38 In non-CP populations, abnormalities in these modalities not only aid in diagnosing myelopathy but also provide insight into disease severity and serve as predictors of clinical outcomes.37,38 However, their utility has not been well investigated in individuals with CP. Given the complex neurological profiles in this population, these modalities may offer significant value for more precise preoperative evaluation and postoperative prognostication. The absence of neurophysiological data in the present study represents a limitation that should be acknowledged.
Limitations and Future Directions
This study has several limitations. First, its retrospective design may limit the generalizability of the findings and lead to selection bias. Second, the relatively small sample size of individuals with CP limits the generalizability of our findings. Given the rarity of this population undergoing surgical intervention, multicenter collaborations are necessary to further validate the predictors identified in this study. Third, neurophysiological study data were not included, limiting our ability to assess their potential role in diagnosis and outcome prediction. Fourth, this study focused on outcomes beyond 6 months postoperatively but did not evaluate long-term prognoses. This follow-up period may not fully capture long-term functional trajectories or late surgical complications. Since late neurological deterioration can occur several years after surgery, data from more extended postoperative periods are needed. Fifth, osteopenia and osteoporosis, which are important considerations in CP populations, could not be adequately assessed due to a lack of data. Finally, the use of only the MBI score for functional evaluation limited the scope of outcome assessments.
Future research should involve a multi-center prospective design, recruiting larger cohorts of individuals with CP and concurrent CSM to evaluate long-term postoperative outcomes more comprehensively. Extended follow-up periods are essential to assess sustained functional recovery, potential delayed complications, and the durability of surgical benefits. Moreover, using a broader range of clinical scales, such as the modified Japanese Orthopedic Association scale and Nurick grade, will enhance the postoperative outcome evaluation. Additionally, the inclusion of patients undergoing conservative management will allow for comparisons of surgical vs non-surgical outcomes.
While clinical prediction models have been developed and validated for CSM in the general population, 20 similar models have not yet been established for patients with CP. Therefore, future studies should also aim to develop sophisticated tools that can guide early diagnosis and surgical decision-making in this population. Moreover, the integration of neurophysiologic testing may enhance diagnostic accuracy and improve the overall management of this complex patient population.
Conclusions
Predictors for poor postoperative outcomes of CSM in individuals with CP were identified in this study. The duration of symptoms, specifically motor weakness, T2SI grade 2, and spinal compression ratio (M2) on preoperative MRI were found to be associated with long-term surgical outcomes. We determined that the cut-off values for the duration of symptoms (specifically motor weakness) and spinal compression ratio (M2) were 2 years and 76.2%, respectively. These predictive factors underscore the importance of early intervention and detailed preoperative radiological assessment to improve surgical outcomes. However, due to the limited existing research in this field, further studies are necessary to validate these findings and refine clinical decision-making for this unique patient population.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was supported by the Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare) (21A0202L1) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI22C1588).
Ethical Statement
Ethical Approval
This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea (4-2024-0643). Owing to the retrospective nature of the study, the requirement for informed consent was waived by the review board.
ORCID iDs
Su Ji Lee https://orcid.org/0000-0002-6376-0125
Jihye Hwang https://orcid.org/0009-0009-7983-2507
Min Gyu Kang https://orcid.org/0009-0002-2997-6990
Minjae Cho https://orcid.org/0000-0002-2245-9643
Yoon Ha https://orcid.org/0000-0002-3775-2324
Sung-Rae Cho https://orcid.org/0000-0003-1429-2684
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.*
References
- 1.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8-14. [PubMed] [Google Scholar]
- 2.Murphy KP. Cerebral palsy lifetime care – four musculoskeletal conditions. Dev Med Child Neurol. 2009;51(s4):30-37. [DOI] [PubMed] [Google Scholar]
- 3.Ando N, Ueda S. Functional deterioration in adults with cerebral palsy. Clin Rehabil. 2000;14(3):300-306. [DOI] [PubMed] [Google Scholar]
- 4.Harada T, Ebara S, Anwar MM, et al. The cervical spine in athetoid cerebral palsy. A radiological study of 180 patients. J Bone Joint Surg Br. 1996;78(4):613-619. [PubMed] [Google Scholar]
- 5.Hung CW, Matsumoto H, Ball JR, et al. Symptomatic cervical spinal stenosis in spastic cerebral palsy. Dev Med Child Neurol. 2020;62(10):1147-1153. [DOI] [PubMed] [Google Scholar]
- 6.Katsma M, Liu H, Pan X, Ryan KJ, Roye DP, Chambers HG. Management and treatment of musculoskeletal problems in adults with cerebral palsy: experience gained from two lifespan clinics. J Pediatr Rehabil Med. 2024;17(1):19-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40(12):E675-E693. [DOI] [PubMed] [Google Scholar]
- 8.Badhiwala JH, Ahuja CS, Akbar MA, et al. Degenerative cervical myelopathy - update and future directions. Nat Rev Neurol. 2020;16(2):108-124. [DOI] [PubMed] [Google Scholar]
- 9.Lee YJ, Chung DS, Kim JT, Bong HJ, Han YM, Park YS. Surgical treatments for cervical spondylotic myelopathy associated with athetoid cerebral palsy. J Korean Neurosurg Soc. 2008;43(6):294-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HC, Oh SH, Oh JK, Ha Y. Surgical strategies and perioperative considerations for cervical deformity with cerebral palsy: a comprehensive review of the literature. Neurospine. 2022;19(4):868-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YJ, Chung DS, Kim JT, Bong HJ, Han YM, Park YS. Surgical treatments for cervical spondylotic myelopathy associated with athetoid cerebral palsy. J Korean Neurosurg Soc. 2008;43(6):294-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azuma S, Seichi A, Ohnishi I, Kawaguchi H, Kitagawa T, Nakamura K. Long-term results of operative treatment for cervical spondylotic myelopathy in patients with athetoid cerebral palsy: an over 10-year follow-up study. Spine. 2002;27(9):943-948. [DOI] [PubMed] [Google Scholar]
- 13.Jameson R, Rech C, Garreau de Loubresse C. Cervical myelopathy in athetoid and dystonic cerebral palsy: retrospective study and literature review. Eur Spine J. 2010;19(5):706-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galivanche AR, Gillinov SM, Mercier MR, et al. In-hospital complications after cervical fusion in cases with versus without cerebral palsy. N Am Spine Soc J. 2022;12:100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim C-W, Hyun S-J, Kim K-J. Surgical impact on global sagittal alignment and health-related quality of life following cervical kyphosis correction surgery: systematic review. Neurospine. 2020;17(3):497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KN, Ahn PG, Ryu MJ, et al. Long-term surgical outcomes of cervical myelopathy with athetoid cerebral palsy. Eur Spine J. 2014;23(7):1464-1471. [DOI] [PubMed] [Google Scholar]
- 17.Lee JJ, Oh SH, Jeong YH, et al. Surgical strategies for cervical deformities associated with neuromuscular disorders. Neurospine. 2020;17(3):513-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimokawa N, Sato H, Matsumoto H, Takami T. Complex revision surgery for cervical deformity or implant failure. Neurospine. 2020;17(3):543-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin JJ, Jin BH, Kim KS, Cho YE, Cho WH. Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy. Acta Neurochir. 2010;152(10):1687-1694. [DOI] [PubMed] [Google Scholar]
- 20.Tetreault LA, Côté P, Kopjar B, Arnold P, Fehlings MG, AOSpine North America and International Clinical Trial Research Network . A clinical prediction model to assess surgical outcome in patients with cervical spondylotic myelopathy: internal and external validations using the prospective multicenter AOSpine North American and international datasets of 743 patients. Spine J. 2015;15(3):388-397. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda Y, Shibata T, Oki S, Kawatani Y, Mashima N, Oishi H. Outcomes of surgical treatment for cervical myelopathy in patients more than 75 years of age. Spine. 1999;24(6):529-534. [DOI] [PubMed] [Google Scholar]
- 22.Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187. [DOI] [PubMed] [Google Scholar]
- 23.Wilson JRF, Badhiwala JH, Moghaddamjou A, Martin AR, Fehlings MG. Degenerative cervical myelopathy; A review of the latest advances and future directions in management. Neurospine. 2019;16(3):494-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campagnini S, Liuzzi P, Mannini A, et al. Cross-validation of predictive models for functional recovery after post-stroke rehabilitation. J Neuroeng Rehabil. 2022;19(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cawood J, Visagie DS, Mji DG. Impact of post-stroke impairments on activities and participation as experienced by stroke survivors in a Western cape setting. S Afr J Occup Ther. 2016;46(2):10-15. [Google Scholar]
- 26.Kim HC, Jeon H, Jeong YH, et al. Factors affecting postoperative complications and outcomes of cervical spondylotic myelopathy with cerebral palsy: a retrospective analysis. J Korean Neurosurg Soc. 2021;64(5):808-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yukawa Y, Kato F, Yoshihara H, Yanase M, Ito K. MR T2 image classification in cervical compression myelopathy: predictor of surgical outcomes. Spine. 2007;32(15):1675-1678. [DOI] [PubMed] [Google Scholar]
- 28.Sritharan K, Chamoli U, Kuan J, Diwan AD. Assessment of degenerative cervical stenosis on T2-weighted MR imaging: sensitivity to change and reliability of mid-sagittal and axial plane metrics. Spinal Cord. 2020;58(2):238-246. [DOI] [PubMed] [Google Scholar]
- 29.Abudouaini H, Liu H, Wang B, et al. Outcome and predictive factors in rapid progressive cervical spondylotic myelopathy: a retrospective case-control study. Clin Neurol Neurosurg. 2020;198:106226. [DOI] [PubMed] [Google Scholar]
- 30.Kato S, Fehlings M. Degenerative cervical myelopathy. Curr Rev Musculoskelet Med. 2016;9(3):263-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma S, Sial A, Sima S, Diwan A. Clinical signs and symptoms for degenerative cervical myelopathy: a scoping review of case-control studies to facilitate early diagnosis among healthcare professionals with stakeholder engagement. Spinal Cord. 2025;63(3):171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández de Rota JJ, Meschian S, Fernández de Rota A, Urbano V, Baron M. Cervical spondylotic myelopathy due to chronic compression: the role of signal intensity changes in magnetic resonance images. J Neurosurg Spine. 2007;6(1):17-22. [DOI] [PubMed] [Google Scholar]
- 33.Uchida K, Nakajima H, Sato R, et al. Multivariate analysis of the neurological outcome of surgery for cervical compressive myelopathy. J Orthop Sci. 2005;10(6):564-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naderi S, Özgen S, Pamir MN, Özek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49. [DOI] [PubMed] [Google Scholar]
- 35.Madhavan K, Chieng LO, Foong H, Wang MY. Surgical outcomes of elderly patients with cervical spondylotic myelopathy: a meta-analysis of studies reporting on 2868 patients. Neurosurg Focus. 2016;40(6):E13. [DOI] [PubMed] [Google Scholar]
- 36.Iyer A, Azad TD, Tharin S. Cervical spondylotic myelopathy. Clin Spine Surg. 2016;29(10):408-414. [DOI] [PubMed] [Google Scholar]
- 37.Yu D, Chang MC, Jeon I, Kim SW. Diagnostic and prognostic significance of preoperative evoked potential tests in degenerative cervical myelopathy. Spine J. 2024;24(1):87-93. [DOI] [PubMed] [Google Scholar]
- 38.Reddy RP, Singh-Varma A, Chang R, et al. Transcranial motor evoked potentials as a predictive modality for postoperative deficit in cervical spine decompression surgery – a systematic review and meta-analysis. Global Spine J. 2024;14(5):1609-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.*