Abstract
The number of colorectal cancer (CRC) patients is steadily growing worldwide, particularly in developing nations. Nonetheless, recent advances in early detection studies and therapy alternatives have reduced CRC mortality in affluent countries, despite rising incidence. Gut microbiota and their metabolites may contribute to tumor growth and reduced therapeutic efficacy. This preliminary study sought to uncover metabolic fingerprints in colorectal cancer patients. It also emphasizes the correlation between the gut microbiome, microbial metabolism, and altered metabolites in CRC. In this study, stool samples from 20 CRC patients and matched healthy controls were enrolled. Untargeted metabolomics approach based on an ultra-high-performance liquid chromatography high-resolution mass spectrometry (UHPLC-MS/MS) were applied. Statistical approaches, pathway enrichment analysis, and network analysis were employed to unleash CRC perturbed metabolic pathways and putative biomarkers. The study identified a distinct manually curated metabolite profile that is substantially linked to CRC. The steroidogenesis, aspartate, tryptophan (Trp), and urea cycle were the most significant pathways that concurrently contributed to CRC.Prominently, among other pathways, Trp metabolism was identified as a critical pathway, indicating a possible connection between the development of CRC and gut microbiota. In a nutshell the notable resulted metabolites reveal auspicious biomarkers for the initial diagnosis as well as surveilling of CRC progression. This preliminary study highlights the potential involvement that gut bacteria may contribute in CRC patients. Further investigation into the composition of the gut microbiome associated with this metabolic profile may lead to the identification of novel biomarkers for early detection and possible targets for treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00726-025-03448-3.
Keywords: Colorectal cancer, Gut microbiota, Microbial tryptophan metabolism, Metabolomics, LC–MS
Introduction
Colorectal cancer (CRC) is one of the most prevalent types of cancer. In 2018, it was estimated that CRC is the third most frequent cancer, representing 10.2% of all new cancer cases and the second-leading cause of cancer-related deaths, accounting for 9.2% of all cancer deaths worldwide (Bray et al. 2018; Gold et al. 2022; Tenesa and Dunlop 2009). In Egypt, it is the seventh most common cancer, estimated to have affected around 5000 people in 2015 (3.24% of all cancers) (Elhadidy and Haydara 2022). Although the overall rate is relatively low (around 6 per 100,000 men and 4.9 per 100,000 women), a concerning trend exists in younger people developing CRC in Egypt, likely due to adopting an occidental lifestyle (Ibrahim et al. 2014; Mounir et al. 2022). An observation that warrants further research to be conducted.
Various procedures and tests are currently used for prognosis, and staging of CRC, ranging from invasive procedures (e.g.; colonoscopy and tissue biopsy) to non-invasive stool tests (e.g.; fecal occult blood (FOBT)) (Glynne-Jones et al. 2017). Nevertheless, FOBT's ability to diagnose advanced adenomas of CRC in the proximal and distal colon is limited, with a sensitivity of around 24% and 32%, respectively (Lu et al. 2019). Therefore, clinicians are forced to utilize more invasive procedures to identify pre-malignant and malignant lesions in most cases. Due to the invasiveness of CRC diagnosis, the choice of screening method often depends on patient preference, availability, and healthcare system capabilities. Highlighting the lack of standardization approaches and difficulty in early disease detection (Ogunwobi et al. 2020).
The emergence of high-throughput “omics” (genomics, transcriptomics, proteomics, and metabolomics) led to the development of comprehensive alternatives for CRC diagnosis. Unlike other omics, metabolomics studies the biochemical processes involved in metabolites (Gold et al. 2022). The latter provides a comprehensive characterization of the interactions between genes and the environment. In essence, the study of metabolomics, provides a snapshot of the body's biochemical processes (Lin et al. 2016). This approach provides more insights into the perturbed pathways during the pathogenesis of CRC. Diverse types of bio-specimens could be implemented in metabolomics profiling, such as blood, urine, and stool. This opens a door for the possible finding a non-invasive novel biomarker for the early detection of CRC (Coker et al. 2022; Shtossel et al. 2024).
Profiling the small molecules in the stool of CRC patients includes sugars, lipids, amino acids, vitamins, and dNTPs of DNA. Nowadays, nuclear magnetic resonance (NMR), gas chromatography (GC), and high-resolution liquid chromatography (HPLC) are used as powerful technologies in metabolomics studies (Song et al. 2018). Numerous studies unraveled the involvement of gut microbes and their secondary metabolites in CRC (e.g.; short-chain fatty acids (SCFAs), indole, vitamin K…etc.) (Ivanisevic and Want 2019; Lin et al. 2016). Many of these metabolites were incriminated in CRC pathogenesis and elicit serious adverse effects through various metabolic pathways (Ivanisevic and Want 2019; Lin et al. 2016; Zheng et al. 2011). Taken together, metabolomics fingerprinting of stool puzzles out the microbiome-host interactions throughout identifying the CRC-associated perturbed metabolic pathways (Duizer and De Zoete 2023; Gold et al. 2022). As stool is attached to the lining of the colon (colorectal epithelium), it contains a high concentration of many endogenous metabolites derived from microbes-host co-metabolism (Schwabe and Jobin 2013). Moreover, stool carries a huge number of colon epithelial cells (approximately 1.5 million per gram of stool) from the colorectal tumor and colonic mucosa (Iyengar et al. 1991). This makes it a valuable noninvasive source for unraveling tissue-specific metabolic biomarkers.
Many studies have disclosed metabolic fingerprints that differentiate between CRC patients and healthy controls (Chan et al. 2009; Qiu et al. 2014). These findings may help in staging CRC pathogenesis, response to treatment regimens, and follow-up of patients. Metabolic pathway analysis of CRC revealed perturbation in several pathways, specifically, aminoacyl-tRNA biosynthesis, the biosynthesis of branched-chain amino acids (valine, leucine, and isoleucine), tryptophan (Trp), and butanoate metabolism (Gold et al. 2022). Notably, the Trp metabolic disorders might mediate the onset and progression of the CRC (Yu et al. 2024). Leading to the accumulation of metabolites that exacerbate the worseness of metastasis and immune bypassing (Santhanam et al. 2016; Trézéguet et al. 2021; Yu et al. 2024).
Of note, studies support the potentiality of microbial-derived metabolites such as; SCFAs, 2-keto butyric acids, indoles, and amino acids as key biomarkers for the differentiation between CRC patients and healthy controls (Bezabeh et al. 2009; Monleón et al. 2009). In particular, microbial-derived oncometabolites such as lactate, l-2-hydroxyglutarate, succinate, fumarate, and d-2-hydroxyglutarate exacerbate cancer progression (Ternes et al. 2020, 2022; Zhang et al. 2021). Debatable reports revealed their dual anti-carcinogenic and pro-tumorigenic properties, which might depend on a variety of factors. Additionally, different studies report varying levels of sensitivity and specificity for these metabolites, leading to discordant findings regarding their diagnostic utility (Ternes et al. 2022; Zhang et al. 2021). Although microbial-derived metabolites show promising evidence as biomarkers for discriminating CRC patients from healthy controls, an additional investigation is needed to tackle the challenges associated with variability, complexity, and standardization of these cadidates (Zhang et al. 2021).
This research embarked on identifying the unique metabolic patterns in the stool of Egyptian patients with colorectal cancer (CRC). The goal was to provide preliminary non-invasive biomarkers for CRC prognosis by examining the relationship between the gut microbiome, its metabolic activities, and the resulting changes in fecal metabolites.
Material and methods
Participants
In this study, 20 participants were divided into two equal groups: CRC patients (n = 10, males) and healthy controls (n = 10 males). Each group was matched for age and gender. The mean age of participants in the two groups was 53.9 ± 4.8 and 53.5 ± 3.7 years, respectively. Fecal samples were collected from patients undergoing colonoscopy and histological evaluation at Kobri Elkoba Hospital, Cairo, Egypt. CRC patients were staged according to the Union for International Cancer Control (UICC)–Tumor-Node-Metastasis (TNM) Staging System (Chen et al. 2021). The study excluded patients who had radiation or chemotherapy, antibiotics, NSAIDs, statins, or probiotics within two months of starting the study, or had confirmed diseases other than colorectal cancer. Regarding the healthy control cohort, they revealed normal blood laboratory tests, endoscopic assessment, and/or diagnostic imaging with no past history of disease or genetic abnormalities. The clinical and pathological characteristics of the participants are provided in Table S1. This study was approved by the Armed Force College of Medicine (AFCM) ethical board (No. 91, 2021). All experiments were performed in accordance with the ethical standards of the declaration of Helsinki. An informed consent was obtained from all subjects in this study.
Sample collection
Fresh stool samples were collected before surgery or bowel preparation. Samples were collected promptly, and frozen at − 80 °C until extracted.
Reagents and chemicals
Acetonitrile and ammonium formate 99% were purchased from Sigma-Aldrich (Sigma-Aldrich Co., St. Louis, MO, Germany), while methanol, formic acid, and sodium hydroxide were acquired from Thermo-Fisher (Thermo-Fisher Scientific, Loughborough, UK). Additionally, the water was obtained from Thermo-Fisher (Thermo-Fisher Scientific, Waltham, MA, USA). All of the solvents are HPLC grade.
Sample Pre-processing for metabolomics analysis
Stool samples were subjected to metabolite extraction. Twenty fecal samples (10 healthy controls and 10 CRC) were thawed on ice, all samples were aliquoted to 100 mg. For extraction, in brief with minor changes, samples were treated with 400 µL of extraction solvent (acetonitrile: methanol, 3:1) (Jain et al. 2019) for 10 min. The mixture was vortexed and swirled at 20 °C, ultrasonicated for 15 min (Elma Elmasonic Ultrasonic cleaning unit P, Elma, Germany). Then, samples were centrifuged at 12,000 rpm at 4 °C (Tomy MX-207 High Speed Refrigerated Micro Centrifuge, Tomy Kogyo Co., Ltd, Japan) for 10 min. Three hundred µL of supernatant was transferred to a sterile Eppendorf tube, and the pellet was re-extracted with 400 µL of the above mentioned extraction solvent. The samples were then ultrasonicated for another 15 min at 20 °C before being centrifuged for 10 min at 12,000 rpm at 4 °C. The supernatant was then pooled with the initial extraction in a sterile Eppendorf tube source (Ahmed et al. 2022; Sameh et al. 2023).
The samples were then dried by a SpeedVac (Concentrator plus Eppendorf, Germany) at 30 °C and reconstituted in a solvent consisting of water, methanol, and acetonitrile in a 2:1:1 ratio respectively. The extracts were analyzed using Liquid Chromatography with tandem mass spectrometry (LC–MS/MS). A detailed sample preparation roadmap is illustrated in supplementary information (Fig. S1).
Analysis of UHPLC-MS/MS using Information Dependent Acquisition (IDA)
Information Dependent Acquisition (IDA) was used for LC–MS/MS analysis of the collected specimens in both positive and negative ionization modes (Decaestecker et al. 2004). ExionLC™ AC UHPLC system (AB SCIEX, Concord, Canada) with Acquity XSelect HSS T3 analytical column 2.1 × 150 mm, 2.5 µm (Waters Co, Milford, US) combined with Triple TOF 5600+ mass spectrometer (AB SCIEX, Concord, Canada) was used for the analysis.
Regarding the chromatographic separation, 5µL of each sample was injected for 35 min employing gradient elution at a steady flow rate of 0.3 mL/min. The mobile phase's solutions were as follows: solution (A); 5 mM ammonium formate in 1% methanol (pH 3.0) for positive mode elution, solution (B); 5 mM ammonium formate in 1% methanol (pH 8.0) for negative mode elution, and solution(C); acetonitrile 100%. Gradient elution was planned with the following sequence: 0% C for 1.0 min, 0–90% C in 20 min, 90% for 4.0 min, 90–0% C in 1.0 min, and lastly, 3.0 min of re-equilibration with 0% C. Positive-ion (ESI+) as well as negative-ion (ESI−) modes of mass spectrometric analysis were carried out using a DuoSpray™ ion source (Ahmed et al. 2022; Sameh et al. 2023).
IDA acquisition with dynamic background subtraction was used to detect metabolites within the samples by setting TOF -MS scan from 50 to 1000 Da accumulated in 30 ms followed by MS/MS on the 15 most intense precursor ions from 50 to 1000 Da using a fixed 50 Da transition window. The accumulation time for each MS/MS acquisition was 50ms, and the collision energies were 35 V and − 35 V for positive and negative mode, respectively. The overall cycle time was 0.6502 s. Analyst TF (v 1.7.1) was used to acquire MS and MS/MS spectra (Sameh et al. 2023).
Quality control of samples
Equal quantities of 15 µL of each sample were pooled to generate quality control QCs (QC- pool); equal volumes of 30 µL of samples from the healthy group (QC- healthy); and equal volumes of 30 µL of samples from the CRC group (QC-CRC). The QC samples were used to evaluate technical consistency and relative standard deviation (RSD %) (Hao et al. 2018). An automated calibration delivery system performed mass calibration every 2 h using either positive or negative APCI calibration solution (AB SCIEX). Blank specimens were employed to clean up any possible carry over and to assess the quality of all runs.
Building database for metabolites identification
To handle the big data of the Human Metabolome Database (HMDB) for untargeted metabolomics experiments, an in-house machine learning prototype algorithm was developed to retrieve and validate spectral data using R scripts (unpublished data). As a result, a high-resolution customized HMDB for fecal metabolites was established. Several crucial modifications were performed including: 1) inclusion of only experimental data from LC–MS experiments to ensure methodological consistency. 2) Selection of experimental adduct types; and 3) restriction to endogenous metabolites found in feces to directly reflect our focus on metabolites derived from gut microbiota and the host. This selective approach aimed to refine the dataset to metabolites most pertinent to CRC pathophysiology, thereby enhancing the accuracy of the fecal metabolomics profiling. The curated metabolite information enabled a deeper investigation into metabolic alterations linked to the fecal metabolites. This allowed us to identify central pathways and metabolites associated with disease development and interactions with the gut microbiome.
Metabolomics Data Analysis
The identification of metabolites was conducted using the MS-DIAL 4.9.2 platform (Tsugawa et al. 2015). As a search space, the in-house built database was employed. To provide greater confidence to the identified metabolites at both parents and fragment ions levels (Tsugawa et al. 2015), manual validation was applied using PeakView 2.2 with MasterView 1.1 program (AB SCIEX) packages (Ahmed et al. 2022). As search criteria, precursor ion XIC signal to noise ratio (S/N) > 10, sample: blank > 5, and a precursor mass tolerance of 10 ppm were applied. Resulted output was subjected to statistical analysis and biological screening (Sameh et al. 2023).
Data pre-processing and statistical analysis
All pre-processing, statistical analysis, and visualization were conducted using R (version 4.2.2) (Chan 2018). Metabolite's abundance data file was merged in one file (Gnp table) using MS-DIAL software and subjected to pre-processing steps (Tsugawa et al. 2015). First, probabilistic quotient normalization (PQN) was conducted for all samples before applying any filtration criteria (Gotsmy et al. 2022). The filtration criteria were applied by removing metabolite features having missing values ≥ 60% per group. Duplicated metabolite features were filtered by keeping the high intensity features. To address the missing values, the data was estimated by randomly choosing a value within ± 10% of the group's median (Overmyer et al. 2021; Wei et al. 2018). Auto-scaling was applied before the statistical analysis (Goodacre et al. 2007). The shared metabolites were subjected to normality testing using Shapiro–Wilk test with a p-value ≤ 0.05 (Mishra et al. 2019). To determine the differentially significant metabolites (DEMs), Wilcoxon Mann–Whitney test was used. Additional cut-offs were added to determine the DEMs including the Log2 fold change (FC) ≥ 1 and adjusted p value (FDR) ≤ 0.05 calculated by false discovery rate (FDR) test (Neuhäuser 2011). Furthermore, multivariate statistical analysis was conducted using principal component analysis (PCA), partial least square discriminant analysis (PLS-DA), and hierarchical clustering analysis (HCA).
Metabolite set enrichment analysis (MSEA) and weighted gene coexpression network analysis (WGCNA)
MSEA was used to determine the significantly perturbed pathways using Metaboanalyst 6.0 (Pang et al. 2024). To identify patterns of co-expressed metabolites, weighted gene co-expression network analysis was applied using WGCNA package v1.72 based on the metabolomics data (Langfelder and Horvath 2008). WGCNA is an open access R software package (Langfelder and Horvath 2008). A sample tree plot was constructed to detect outlier samples. First, a signed metabolite correlation matrix with a soft threshold beta parameter was set at β = 3 to build the topological overlap matrix (TOM). A minimum module size of 20 metabolites was chosen and a hierarchical clustering was employed using dynamic tree cut (DTC) algorithm and the modules were constructed with a cut height of 0.75. Second, to identify modules associated with clinical conditions, the module eigengenes/eigenmetabolites (the first principal component representing each module) were correlated with the clinical data using biweight midcorrelation (Darzi et al. 2021). Additionally, a Wilcoxon test was performed on these modules (eigengenes/eigenmetabolites) to identify the modules that significantly distinguish between CRC and healthy control groups, with FDR ≤ 0.05 as the significance cut-off (Feng et al. 2022). Metscape 3.1, a plugin for Cytoscape, was utilized to present a metabolite-metabolite interaction network (Karnovsky et al. 2012). The networks were constructed by importing metabolite concentration data into the Metscape interface.
Results
Shotgun metabolomics profiling
The fecal metabolomics of CRC patients and healthy controls were screened to determine the DEMs that differentiate between the two cohorts. LC–MS/MS was used to profile the fecal metabolomics. The relative standard deviation (RSD%) of the QCs were all less than 20% (Table S2), supporting the reliability and reproducibility of our analytical method. For the identification of the metabolites, the primary search of fecal metabolites against the high-resolution Human Metabolome Database (HMDB) revealed a total of 4,082 features in the negative mode and 1,160 features in the positive mode (Table S3). Following application of the pre-processing criteria (ppm error cut-off, removing duplicates, filtration, and manual curation), 216 metabolites had passed the analysis criteria. Substantially, 170 metabolites were relatively quantified (shared) in the two cohorts. Twenty metabolites were uniquely determined in the CRC cohort (did not pass the threshold in the counterpart group), while twenty-six metabolites were uniquely determined in healthy controls (as mentioned in Table S4).
For a comprehensive investigation of the biochemical classes of the identified metabolites, the MSEA for the chemical structures was conducted (as shown in Fig. S2). This analysis highlights the distribution of various chemical classes within the studied cohort. Markedly, the Fatty Acyls (in blue), Carboxylic acids and derivatives (in dark grey), and Organooxygen compounds (in light yellow) constitute a significant portion of the profile with FDR = 1.89E-03, 1.81E-08, and 2.82E-06, respectively. Other chemical classes were presented in smaller proportions include Azoles, Benzene and substituted derivatives, Diazines, Glycerophospholipids, Indoles and derivatives, Organonitrogen compounds, Phenylpropanoic acids, Prenol lipids, Purine nucleosides, Pyrimidine nucleosides, Pyrimidine nucleotides, and Steroids and steroid derivatives.
Metabolic differences between CRC patients and the healthy controls cohort
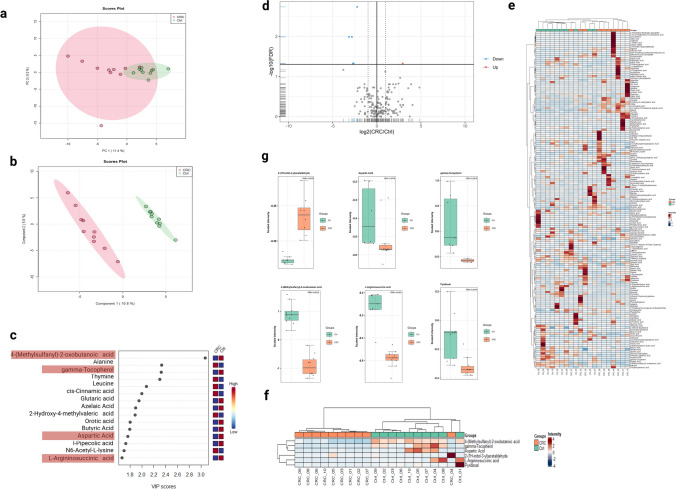
To unravel the potential variations between the CRC patients and the healthy controls in this initial cohort, unsupervised multivariate PCA was employed. The latter did not show any discernible pattern of separation between the two groups (Fig. 1a). In light of this, a PLS-DA model was employed. PLS-DA successfully discriminated against the CRC versus healthy control group (Fig. 1b). Accuracy and permutation testing revealed that clustering was of statistical significance (Fig. S3). PLS-DA variable importance in projection (VIP) considers how much each PLS-DA loading contributes to explaining the target variable (Y) across different dimensions, and combines this information into a single importance score. The VIP plot identified the top 15 discriminatory variables, including 4-(Methylsulfanyl)-2-oxobutanoic acid, alanine, gamma-tocopherol, thymine, and leucine (Fig. 1c).
Fig. 1.
Bioinformatics analysis for the fecal metabolomics profile of the CRC cohort compared to healthy controls. a, b Principal component analysis (PCA) and partial least square discriminant analysis (PLS-DA) for the metabolomics profile, respectively. Metabolite abundances were auto-scaled before the analysis. c Variable importance in projection (VIP) score plot for the CRC and healthy control cohorts displays the top 15 most important metabolite features identified by PLS-DA. Colored boxes on right indicate relative concentration of corresponding metabolite for the CRC and healthy controls. The red highlighted metabolite features are significantly down-regulated DEMs (FDR ≤ 0.05, log2 (FC) ≥ 1.5). d Volcano plot showing the log2 (fold change) between the CRC and healthy controls (y-axis), and – log10 (FDR) calculated by Wilcoxn rank-sum test to show the significant DEMs. The blue color represents significant down-regulated metabolites, while the red color represents significant up-regulated metabolites (FDR ≤ 0.05, log2 (FC) ≥ 1.5). e Hierarchical cluster analysis (HCA) heatmap plot for the profiled metabolites between the CRC and healthy control groups. f Hierarchical cluster analysis (HCA) heatmap plot highlights the significant DEMs (FDR ≤ 0.05, log2 (FC) ≥ 1.5). g Box plot representation for the significant DEMs (FDR ≤ 0.05, log2 (FC) ≥ 1.5)
We further demonstrate the high metabolomic diversity between CRC and healthy control groups (Fig. 1d) using volcano plot considering P value and fold change. The analysis revealed 6 significant DEMs (FDR ≤ 0.05, log2 (FC) ≥ 1.5) between both experimental groups. 2-(1H-indol-3-yl) acetaldehyde was the only up-regulated metabolite with a 2.9-fold change, while 5 were down-regulated. These metabolites were 4-(Methylsulfanyl)-2-oxobutanoic acid, L-aspartic Acid, pyridoxal, gamma-Tocopherol, and L-Argininosuccinic acid (Table 1).
Table 1.
Significant differential expressed metabolites (DEMs) in CRC cohort as compared to healthy controls
| Metabolite ID | Metabolite name | q-value (FDR) | Log 2 (Fold change) | Regulation |
|---|---|---|---|---|
| HMDB0000052 | L-Argininosuccinic acid | 0.010294 | – 3.1665 | down |
| HMDB0000191 | Aspartic Acid | 0.04757 | – 2.6223 | down |
| HMDB0001190 | 2-(1H-indol-3-yl)acetaldehyde | 0.04757 | 2.9238 | up |
| HMDB0001492 | gamma-Tocopherol | 0.010294 | – 2.8342 | down |
| HMDB0001545 | Pyridoxal | 0.04757 | – 2.72 | down |
| HMDB0001553 | 4-(Methylsulfanyl)-2-oxobutanoic acid | 0.0018403 | – 2.237 | down |
The heatmaps and hierarchical clustering of metabolites reveal significant differences in abundance between the two cohorts for all 170 metabolites (Fig. 1e). Additionally, the significant metabolites (FDR ≤ 0.05, log2 (FC) ≥ 1.5) are highlighted in Fig. 1f. While the individual expression of the significant metabolites was depicted, using box plots (Fig. 1g).
Integration of biological dysfunctions with metabolic pathways in CRC pathogenesis
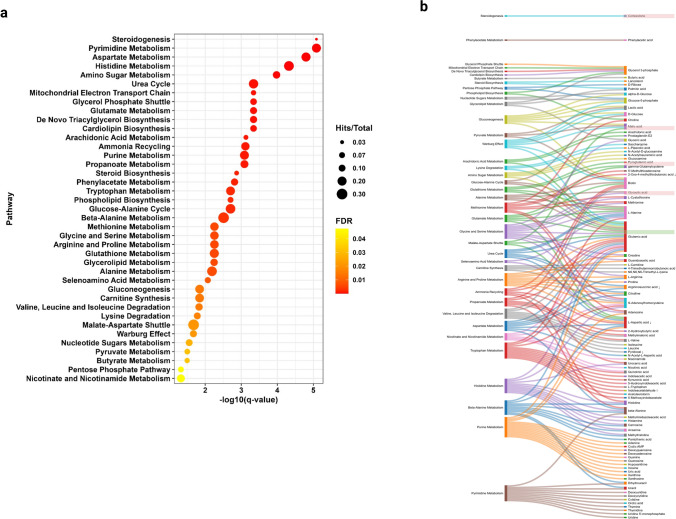
Metabolite Set Enrichment Analysis (MSEA) revealed several metabolic pathways altered in the stool of CRC patients. The significance of altered pathways was determined by the number of metabolites ‘’hits’’, enrichment impact, and FDR ≤ 0.05. Thirty-nine metabolic pathways were altered (FDR ≤ 0.05). The urea cycle, pyrimidine metabolism, aspartate, histidine, and amino sugar metabolism were among the top 18 significant pathways (FDR ≤ 0.01) (Fig. 2a). The analysis of differential metabolites revealed their impact in pathways related to the Malate-Aspartate Shuttle, the urea cycle, glutamate metabolism, tryptophan metabolism, amino acid degradation, and purine metabolism as illustrated by the Sankey diagram (Fig. 2b). These findings revealed that major alterations in metabolic pathway patterns occurred as the tumor progressed.
Fig. 2.
Pathway enrichment analysis for the metabolomics profile using Metaboanalyst 6.0. a Quantitative enrichment analysis (MSEA) for the whole profile, the pathway name (x-axis) and the –log10 FDR (y-axis). All the represented pathways had passed FDR cut-off (FDR > 0.05). The color of the dot corresponds to the value of FDR, while the size of the dot corresponds to the ratio of hit metabolites per total metabolites in the pathway. b Sankey diagram linking the pathway (on the left side) to the hits/metabolites (on the right side). The red highlighted metabolite features are significantly down-regulated DEMs, while the green highlighted metabolite features are significantly up-regulated DEMs (FDR > 0.05)
Weighted metabolite co-expression networks (WMCNA) and their modules
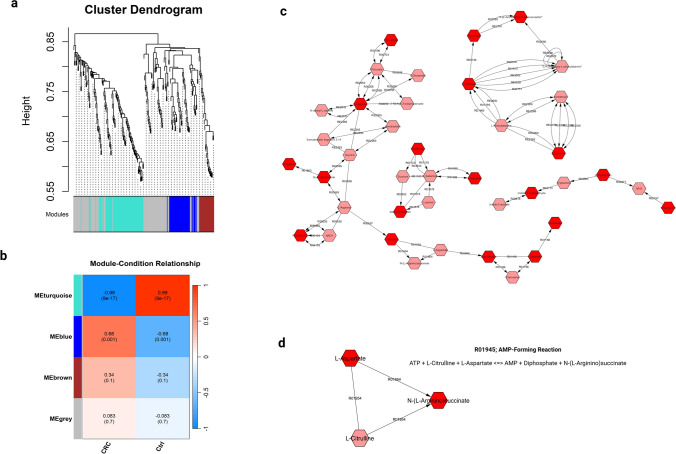
Weighted gene co-expression network analysis (WGCNA) or analogously weighted metabolite co-expression networks (WMCNA) uses correlations to analyze and depict connections between metabolites. A total of 216 metabolites were considered candidate metabolites, which were used for constructing the WMCNA of both cohorts (CRC and healthy controls). First, the WGCNA R package was used to draw a sample tree plot to check whether the profiled metabolites include outlying samples or not. As shown in the samples tree (Fig.S4a), no outlier samples were spotted. To reach scale-free topology, the soft threshold beta parameter was set at β = 3, the choice of this power was based on the elbow and mean connectivity (Fig.S4b and S4c). Second, a signed network with β = 3, minimum module size = 20, and merging threshold = 0.25 were used to construct a topological overlap matrix (TOM) (Fig.S4d). TOM is not only considering direct connections between metabolites, but also how these metabolites connect to other metabolites in the network (indirect connections). Thirdly, using TOM a dissimilarity matrix is derived (1 − TOM) to cluster metabolites, metabolites were classified into distinct clusters called “metabolites modules/eigenmetabolite” (Fig.S4d). Module assignment is determined through hierarchical clustering approach to identify groups of metabolites with similar abundance patterns (eigen metabolites) (as shown in Fig. 3a). The densely correlated metabolite modules were determined based on the average linkage hierarchical clustering on the whole profile using the matrix.
Fig. 3.
Network analysis for the fecal metabolomics profile of CRC and healthy controls. a Cluster dendrogram of metabolite features determined from the WGCNA R package. Metabolites were clustered based on the average linkage calculation from the dissimilarity of topology overlap matrix (TOM). A total of 216 metabolites were assigned to four modules (turquoise, blue, brown, and grey). The assigned modules color are represented at the bottom of the graph. b Module-condition correlation between the assigned modules, CRC, and control groups. Each row corresponds to a module eigenmetabolite and each column to a condition/group. Each cell contains the corresponding correlation and p-value. The cells are color-coded by the correlation coefficient value according to the color legend. c Metscape network for the MEblue module eigenmetabolites and the uniquely identified metabolites in CRC group. d Metscape network for the significant DEMs, showing only the direct interaction between L-Argininosuccinic acid and Aspartic Acid. The dark red metabolites corresponding to metabolites in the input of the analysis, while light red metabolites corresponding to intermediate metabolites (not in the input of the analysis)
The analysis revealed four metabolite modules; MEturquoise, MEblue, MEbrown, and MEgrey (the grey module was utilized for housing metabolites that were not co-expressed with other modules) as shown in Fig. 3a. The MEblue and MEturquoise were the largest modules containing 43 and 50 metabolites, respectively, while the MEbrown contained 25 metabolites only (with a reference to Table S5). The association of these modules with phenotypes were determined, and the relationship between clinical traits (CRC or healthy controls) and modules were shown in Fig. 3b. Only two modules revealed significant associations with clinical traits, MEturquoise and MEblue. The MEturquoise was significantly co-expressed (p value = 9E−17) and negatively correlated to the CRC (r = − 0.99). While, the MEblue was significantly co-expressed (p value = 0.001) and positively correlated to the CRC (r = 0.68).
Metabolite-metabolite interaction network analysis based on WMCNA
Based on WMCNA and module assignment, we constructed a metabolite-metabolite interaction network of pathways using data from MetScape 3.1 (Fig. 3c). As the MEblue 4 module has a significant correlation with CRC phenotype, we prioritize it to construct metabolite-metabolite interaction network. Eigen Metabolites of the MEblue module (positively correlated to CRC, n = 43) and unique metabolites in CRC group (did not pass the threshold in healthy control group, n = 22) were mapped in accordance with their implicated metabolic pathways in order to visualize metabolic networks perturbed in CRC pathogenesis. The network has been narrowed down significantly to remove intermediate metabolites that were not in the input. The network showed direct and indirect interactions between metabolites. Interestingly, Tyramine, 4-(2-aminoethyl)-1,2-benzenediol, and L-Tyrosine showed direct interactions with each other and indirect interaction with L-Leucine. Similarly, L-Isoleucine, L-Tryptophan, and indole-3-acetaldehyde (significantly upregulated) showed indirect interactions with each other. Besides, Raffinose, Galactitol, and alpha-D-Glucose showed indirect interactions with each other. Notably, (S)-Lactate, L-Alanine, Creatine, Guanidinoacetate, Nicotinamide, L-Citrulline, beta-Alanine, L-Histidine, and Urocanate showed direct and indirect interactions with each other.
Furthermore, to determine the interactions between DEMs (L-Argininosuccinic acid, Aspartic Acid, gamma-tocopherol, Pyridoxal, 4-(Methylsulfanyl)-2-oxobutanoic acid), and (2-(1H-indol-3-yl) acetaldehyde)) a network was built to include the six DEMs. Similar to the previous analysis, the network was refined to remove intermediate metabolites that were not in the input. Only, the L-Argininosuccinic acid and Aspartic Acid showed direct interaction through the AMP-forming reaction, highlighting the perturbation in this reaction in CRC pathogenesis (Fig. 3d).
Discussion:
In this study, we investigated metabolic fingerprints of CRC patients’ cohort using untargeted metabolomics screening of stool samples. Twenty subjects were separated into two equal groups: CRC patients and healthy controls. Each group was age- and gender-matched. Participants in both groups had mean ages of 53.9 ± 4.8 and 53.5 ± 3.7 years, respectively. We reported six metabolites that were significantly different between CRC and healthy controls (FDR ≤ 0.05, log2 (FC) ≥ 1.5) between both experimental groups. As a result of the aforementioned, the level of 2-(1H-indol-3-yl) acetaldehyde was up regulated, conversely, 4-(Methylsulfanyl)-2-oxobutanoic acid, L-aspartic Acid, pyridoxal, gamma-tocopherol, and L-argininosuccinic acid were dramatically downregulated.
To further develop our comprehension of the metabolites identified, we conducted MSEA pathway analysis to connect the significant metabolites with their respective pathways. To the best of our knowledge, this work provides a pioneering investigation into the regulation of 2-(1H-indol-3-yl) acetaldehyde, a metabolite originating from the Trp metabolic pathway associated with gut microbiota was present at 2.9 times higher levels in individuals with CRC. One of the key micro environmental variables linked to the occurrence of intestinal malignancies is intestinal microbiota. The function of intestinal microbiota in carcinomatosis, particularly in CRC, has currently been established by a number of studies (Belcheva et al. 2015; Zhao et al. 2017). These results emphasize the importance of gut microbiota metabolites such as indoles, short-chain fatty acids, and fatty acids, which have a considerable impact on host immunity and cancer progression, as well as a role in regulating both (Sridharan et al. 2014; Sun et al. 2018). Trp metabolism provides a number of compounds that are crucial for host physiology. It is disrupted in many disorders, particularly neurological, metabolic, infectious, intestinal ailments, and cancer cells, making it a potential pharmaceutical target (Stone 2020). When gut microorganisms including Fusobacteria, Enterobacteriaceae, and Clostridia deplete Trp metabolism in CRC patients, Trp catabolism shifts away from serotonin production and toward pro-tumorigenic kynurenine metabolites (Wang et al. 2012). Thus, understanding the link between microbial Trp metabolism, gut microbiota, and CRC will help identify new targets for anticancer drugs and improve tumor detection and therapy.
Concerning pyrimidine, aspartate, histidine, glutamate, and purine, amino acids have received a lot of interest as one of the many chemicals that could play a key role in cancer etiology (Wei et al. 2020). Our findings indicate that L-aspartic acid is significantly downregulated in CRC patients. This result aligns with previous studies demonstrating a substantial decrease in L-aspartic acid levels in the stool of CRC patients (Liu et al. 2023a, b). L- aspartic acid is a urea cycle metabolite that also contributes in gluconeogenic processes. It contains reducing equivalents in the malate-aspartate shuttle, which uses the easy interconversion of aspartate and oxaloacetate (Holeček 2023). A recent study suggests that aspartic acid and glutamic acid may exert their anti-tumor effects by interacting with N-methyl-D-aspartate receptors (NMDARs), ultimately inhibiting tumor cell proliferation (Yamaguchi et al. 2016).
Regarding the gamma-tocopherol, to our current understanding, this is the first study to investigate the modulation of gamma-tocopherol in the stool of CRC patients. Antioxidant vitamin E (tocopherols) may protect against colon cancer through several cellular and molecular mechanisms (Campbell et al. 2003; Guan et al. 2012; Ju et al. 2010). According to recent research, gamma tocopherol may be able to reduce the risk of colon cancer linked to colitis while extending aspirin's anti-inflammatory action and reducing its side effects (K. Y. Liu et al. 2023a, b). It has been demonstrated that 5-fluorouracil (5-FU) and gamma-tocopherol co-treatment can increase chemotherapy's effectiveness against HT-29 colon cancer cells (Bazzaz et al. 2019). Furthermore, research indicates that gamma-tocopherol as well as, its metabolites alter the gut microbiota's composition, promoting Lactococcus and Bacteroides beneficial bacteria and preventing the depletion of Roseburia, which is linked to inflammatory bowel disorders (Yang et al. 2021). Consequently, the correlation between gamma-tocopherol and gut microbiota in CRC highlights the need of investigating the combined impacts of gamma-tocopherol on cancer prevention and microbiome modification for possible therapeutic approaches.
Our study revealed that pyridoxal, an active form of vitamin B6, has been found to be downregulated in CRC patients. This finding is in accordance with other studies which showed that serum pyridoxal was inversely associated with CRC risk (Gylling et al. 2017; Xu et al. 2022). It has been demonstrated that higher levels of pyridoxal-5'-phosphate are associated with a decreased risk of CRC (Lee et al. 2009). Our MSEA results also indicate that pyridoxal affects the valine, leucine, and isoleucine degradation pathways. Pyridoxal-5'-phosphate is indispensable for the initial steps of branched-chain amino acid (BCAAs) degradation. BCAAs, including valine, isoleucine, and leucine (Wang et al. 2023), which are essential for various metabolic processes, affecting energy production and nutrition consumption (Mann et al. 2021). The breakdown process starts with transamination, where specific transaminases convert BCAAs into their corresponding branched-chain α-keto acids. This reaction requires vitamin B6 as a cofactor, emphasizing its crucial role in the metabolism of these amino acids (Vanweert et al. 2022). Pyridoxal-5'-phosphate may act as a prophylactic against cancer progression as it has been previously demonstrated that pyridoxal up-regulates the messenger RNA for insulin-like growth factor-binding protein 1 in both hepatocellular carcinoma and colon cancer cells (Zhang et al. 2013). Furthermore, the correlations between vitamin B6 status and overall survival, disease-free survival, and recurrence risk have been also studied (Zhao et al. 2019). Improved overall survival is linked to higher preoperative vitamin B6 status in patients with stage I–III colorectal cancer (Holowatyj et al. 2022). Not only does Vitamin B6 affect colorectal cancer risk but also affects the response to treatment. It has been demonstrated that pyridoxine increased the effectiveness of 5-fluorouracil and folinic acid-based chemotherapy regimens against various tumor forms, including colorectal cancer (Machover et al. 2024).
Concerning the downregulation of argininosuccinic acid in the CRC group, this study is the first to investigate the regulation of argininosuccinic acid in CRC stool samples. Argininosuccinic is a vital intermediary in the arginine metabolic pathway, it affects the availability of arginine for immunological and cancer cells. This is a critical factor in the development of CRC, as cancer cells need arginine for survival, proliferation, and differentiation. The urea cycle also relies on argininosuccinic acid to synthesize arginine. High levels of argininosuccinic may enhance the efficacy of tumor immunotherapy in patients with CRC by guaranteeing tumor-infiltrating lymphocytes with an appropriate supply of arginine. (Husson et al. 2003; Karimian et al. 2019; Sun and Zhao 2022). According to our MSEA findings, argininosuccinic acid is involved in the arginine, proline, and aspartate metabolism, as well as the urea cycle pathways. It has been proved that the reduced expression of argininosuccinate synthase 1 (ASS1) in tumors is associated with a poor prognosis. This emphasizes the importance of ASS1 in regulating aspartate levels, which in turn affects nucleotide synthesis and cell growth (Lim et al. 2024). Studies have explored the interconnectedness of proline metabolism with arginine and aspartate. Proline is synthesized from glutamate and its metabolism is linked to cellular stress responses and signaling pathways, potentially interacting with arginine metabolism. Aspartate transaminase plays a crucial role in these pathways, facilitating amino group transfer reactions essential for amino acid biosynthesis (Han et al. 2021). Argininosuccinate is a vital component of the urea cycle, which is an important metabolic mechanism that eliminates nitrogenous waste generated by the degradation of proteins and other nitrogenous substances (Smith 2023). It has been demonstrated that CRC patients exhibit dysregulation of the urea cycle (Gao et al. 2024). Nitrogen diversion toward argininosuccinate synthase, carbamoyl-phosphate synthetase2, aspartate transcarbamylase, and dihydrooratase activation is caused by dysregulation of the urea cycle, which also increases pyrimidine production (Harrison et al. 2023; Wang et al. 2021). This leads to observable alterations in nitrogen metabolites in tumors and associated biofluids (Lee et al. 2018).
Of note, our study is the first to tackle how the levels of 4-(Methylsulfanyl)-2-oxobutanoic acid were altered in stool samples from CRC patients. The MSEA results showed that 2-oxo-4-methylthiobutanoic acid plays a crucial role in the methionine metabolism pathway. This compound serves as the direct precursor of methional, which is known to strongly induce apoptosis (Yang et al. 2020). Methional is also involved in various metabolic processes, particularly the synthesis of methionine. This conversion is essential for maintaining adequate methionine levels in biological systems, as methionine is a vital amino acid involved in numerous physiological functions, including protein synthesis and methylation reactions. These results emphasize the use of metabolomic studies in understanding the pathophysiology of colorectal cancer (CRC) and in devising diagnostic approaches, particularly with regard to the function of 4-(Methylsulfanyl)-2-oxobutanoic acid.
MSEA analysis also identified the top statistically significant perturbations in steroidogenesis, arachidonic acid metabolism, the glycerol phosphate shuttle, and de novo triacylglycerol biosynthesis. Steroidogenesis is the de novo production of steroid hormones from cholesterol such as corticosteroids and sex steroids, as well as neurosteroids, by a series of consecutive enzyme-catalyzed processes in the adrenal and gonads. Additionally, circulating steroid hormone precursors are further metabolized in certain peripheral organs (Louw-du Toit et al. 2017). It has been suggested that CRC incidence and development are influenced by androgens, proposing possible treatment targets (Banibakhsh et al. 2023). The arachidonic acid pathway plays a crucial role in the development and progression of CRC (Jones et al. 2003). This pathway involves the metabolism of arachidonic acid into various bioactive lipids, which are significant in inflammatory responses, cell signaling, and the tumor microenvironment (Martinez et al. 2021).
The Glycerol 3-phosphate shuttle is made up of two distinct enzymes: mitochondrial FAD-linked glycerol 3-phosphate dehydrogenase 2 (GPD2) and cytosolic NAD + -linked glycerol 3-phosphate dehydrogenase 1 (GPD1). Together, these two enzymes serve as a crucial link between the metabolism of lipids and glucose by acting as a NADH shuttle for mitochondrial bioenergetics (Mracek et al. 2013). It was reported that GPD1 activity was shown to be significantly lower in malignant colon and rectum tissues than in normal tissues (Oh et al. 2024). On the other hand, GPD2 expression and activity were increased in tissues and cell lines from prostate and liver cancer compared to normal equivalents (Chowdhury et al. 2005, 2007).
Regarding the phosphatidic acid pathway, commonly referred to as the production of triglycerides, is primarily linked to the liver and adipose tissue (Joshi et al. 2014). The higher saturation of membrane lipids in the CRC cell line HCT116 has been linked to de novo lipogenesis. Because of the enhanced activity of fatty acid synthase, saturated fatty acids are numerous and integrated into membrane phospholipids, which reduces the cells' susceptibility to free radicals and therapeutic penetration (Rysman et al. 2010). Moreover, increased fatty acid synthase (FASN) activity is linked to the stimulation of cellular respiration and the β-oxidation of endogenous lipids. The mammalian target of the rapamycin kinase (mTOR kinase) signaling pathway, which triggers the synthesis of proteins involved in growth, division, or angiogenesis during cancer and metastasis, is what causes these events (Chang et al. 2016; Francipane and Lagasse 2014; Gulhati et al. 2011; Zaytseva et al. 2015). Thus, comprehensive research is essential to identify key metabolites and their associated pathways for developing preventive and therapeutic techniques against CRC.
Concerning the WGCNA, it tackles the issue of correcting multiple hypothesis testing by summarizing thousands of genes/metabolites into smaller, functionally related groups (eigengenes). This allows researchers to analyze associations between these eigengenes and phenotypes, rather than focusing on individual genes. Similarly, we applied the WGCNA to construct WMCNA to determine the eigen metabolites and their correlation to the clinical conditions (Dileo et al. 2011; Pei et al. 2017). The significance correlation between MEblue eigenmetabolites and CRC clinical condition, suggests the importance of these metabolites in CRC pathogenesis. The eigenmetabolites of the MEblue module and unique metabolites in the CRC group were chosen for visualization of metabolic networks perturbed in CRC pathogenesis. Notably, the network revealed indirect interactions between L-isoleucine, L-tryptophan, and the upregulated indole-3-acetaldehyde. This finding aligns with previous studies highlighting the role of Trp metabolism in digestive system tumors, via mediating the tumor growth, metastasis, and immunomodulation (Santhanam et al. 2016; Trézéguet et al. 2021; Yu et al. 2024).
Conclusions
In summary, this study tentatively explored metabolites alteration with CRC and potential biomarkers. The identification of six metabolites as putative CRC biomarkers suggests that these metabolites may be useful for CRC screening and monitoring. The sample size is limited; our analysis is yet preliminary. Larger sample cohorts from multicenter analyses ought to be examined in the future, and standards validation will be required.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr. Amro Abou-Elmagd, Gastroenterology Department, Armed Force College of Medicine, Cairo, Egypt, for providing the samples for this study. Also, we thank the administration of the Science, Technology & Innovation Funding Authority (STDF) for the fundraising that allowed purchasing the server required to handle the extensive computational demands.
Author contributions
A. R. and T. K. A. performed the experiments and analysis, interpreted the results, and wrote the manuscript. M. A. assisted with data analysis. M. M. conducted the mass spectrometry and ensured quality control. A. B., A. M., and W. A. contributed to writing parts of the manuscript. S. M. supervised the study, provided lab equipment, and drafted the manuscript. S. E. conceptualized the idea, designed the experiment, secured funding, and drafted the manuscript. All authors reviewed the paper.
Funding
The work presented here is funded by the Armed Force College of Medicine, Cairo, Egypt, and it is partially supported by Science, Technology & Innovation Funding Authority (STDF) under grant (AI 42547).
Data availability
The metabolomics and metadata reported in this paper are available via [Metabolights,https://www.ebi.ac.uk/metabolights/editor/study/MTBLS11640] study identifier [MTBLS11640]. The data presented in this study are available upon request from the corresponding author.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical statements
An informed consent was obtained from all subjects in this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the the Armed Force College of Medicine (AFCM) ethical board (No. 91, 2021), and with the 1964 Helsinki declaration. Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Asmaa Ramzy and Taghreed Khaled Abdelmoneim are equally contributed to this study.
References
- Ahmed EA, El-Derany MO, Anwar AM, Saied EM, Magdeldin S (2022) Metabolomics and lipidomics screening reveal reprogrammed signaling pathways toward cancer development in non-alcoholic steatohepatitis. Int J Mol Sci. 10.3390/ijms24010210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banibakhsh A, Sidhu D, Khan S, Haime H, Foster PA (2023) Sex steroid metabolism and action in colon health and disease. J Steroid Biochem Mol Biol 233:106371. 10.1016/j.jsbmb.2023.106371 [DOI] [PubMed] [Google Scholar]
- Bazzaz R, Bijanpour H, Pirouzpanah SMB, Yaghmaei P, Rashtchizadeh N (2019) Adjuvant therapy with gamma-tocopherol-induce apoptosis in HT-29 colon cancer via cyclin-dependent cell cycle arrest mechanism. J Biochem Mol Toxicol 33(11):e22399. 10.1002/jbt.22399 [DOI] [PubMed] [Google Scholar]
- Belcheva A, Irrazabal T, Martin A (2015) Gut microbial metabolism and colon cancer: can manipulations of the microbiota be useful in the management of gastrointestinal health? BioEssays 37(4):403–412. 10.1002/bies.201400204 [DOI] [PubMed] [Google Scholar]
- Bezabeh T, Somorjai R, Dolenko B, Bryskina N, Levin B, Bernstein CN, Jeyarajah E, Steinhart AH, Rubin DT, Smith IC (2009) Detecting colorectal cancer by 1H magnetic resonance spectroscopy of fecal extracts. NMR Biomed 22(6):593–600. 10.1002/nbm.1372 [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Campbell S, Stone W, Whaley S, Krishnan K (2003) Development of gamma (gamma)-tocopherol as a colorectal cancer chemopreventive agent. Crit Rev Oncol Hematol 47(3):249–259. 10.1016/s1040-8428(03)00042-8 [DOI] [PubMed] [Google Scholar]
- Chan BKC (2018) Data analysis using R programming. In: Chan BKC (ed) Biostatistics for human genetic epidemiology. Springer International Publishing, pp 47–122. 10.1007/978-3-319-93791-5_2 [Google Scholar]
- Chan ECY, Koh PK, Mal M, Cheah PY, Eu KW, Backshall A, Cavill R, Nicholson JK, Keun HC (2009) Metabolic profiling of human colorectal cancer using high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and gas chromatography mass spectrometry (GC/MS). J Proteome Res 8(1):352–361. 10.1021/pr8006232 [DOI] [PubMed] [Google Scholar]
- Chang L, Wu P, Senthilkumar R, Tian X, Liu H, Shen X, Tao Z, Huang P (2016) Loss of fatty acid synthase suppresses the malignant phenotype of colorectal cancer cells by down-regulating energy metabolism and mTOR signaling pathway. J Cancer Res Clin Oncol 142(1):59–72. 10.1007/s00432-015-2000-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Collins G, Wang H, Toh JWT (2021) Pathological Features and Prognostication in Colorectal Cancer. Curr Oncol 28(6):5356–5383. 10.3390/curroncol28060447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SK, Gemin A, Singh G (2005) High activity of mitochondrial glycerophosphate dehydrogenase and glycerophosphate-dependent ROS production in prostate cancer cell lines. Biochem Biophys Res Commun 333(4):1139–1145. 10.1016/j.bbrc.2005.06.017 [DOI] [PubMed] [Google Scholar]
- Chowdhury SK, Raha S, Tarnopolsky MA, Singh G (2007) Increased expression of mitochondrial glycerophosphate dehydrogenase and antioxidant enzymes in prostate cancer cell lines/cancer. Free Radic Res 41(10):1116–1124. 10.1080/10715760701579314 [DOI] [PubMed] [Google Scholar]
- Coker OO, Liu C, Wu WKK, Wong SH, Jia W, Sung JJY, Yu J (2022) Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarkers. Microbiome 10(1):35. 10.1186/s40168-021-01208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzi M, Gorgin S, Majidzadeh-A K, Esmaeili R (2021) Gene co-expression network analysis reveals immune cell infiltration as a favorable prognostic marker in non-uterine leiomyosarcoma. Sci Rep 11(1):2339. 10.1038/s41598-021-81952-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaestecker TN, Vande Casteele SR, Wallemacq PE, Van Peteghem CH, Defore DL, Van Bocxlaer JF (2004) Information-dependent acquisition-mediated LC-MS/MS screening procedure with semiquantitative potential. Anal Chem 76(21):6365–6373. 10.1021/ac0492315 [DOI] [PubMed] [Google Scholar]
- Dileo MV, Strahan GD, Den Bakker M, Hoekenga OA (2011) Weighted correlation network analysis (WGCNA) applied to the tomato fruit metabolome. PLoS ONE 6(10):e26683. 10.1371/journal.pone.0026683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duizer C, De Zoete MR (2023) The role of microbiota-derived metabolites in colorectal cancer. Int J Mol Sci 24(9):8024. 10.3390/ijms24098024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhadidy A, Haydara T (2022) Increase young age incidence of colorectal carcinoma among cohort of Egyptian population. Al-Azhar Int Med J 3(1):71–75 [Google Scholar]
- Feng T, Lai C, Zhong D, Luo L, Zou H, Wang G, Yang Q, Yao Y, Huang X (2022) Weighted gene co-expression network analysis reveals prognostic and diagnostic significance of PAQR4 in patients with early and late hepatocellular carcinoma. J Gastroint Oncol 13(2):768–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francipane MG, Lagasse E (2014) mTOR pathway in colorectal cancer: an update. Oncotarget 5(1):49–66. 10.18632/oncotarget.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Mei Z, Liu Z, Zhu D, Yuan H, Zhao R, Xu K, Zhang T, Jiang Y, Suo C, Chen X (2024) Association between serum urea concentrations and the risk of colorectal cancer, particularly in individuals with type 2 diabetes: A cohort study. Int J Cancer 154(2):297–306. 10.1002/ijc.34719 [DOI] [PubMed] [Google Scholar]
- Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, Arnold D, Committee EG (2017) Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(4):22–40 [DOI] [PubMed] [Google Scholar]
- Gold A, Choueiry F, Jin N, Mo X, Zhu J (2022) The Application of metabolomics in recent colorectal cancer studies: a state-of-the-art review. Cancers 14(3):725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre R, Broadhurst D, Smilde AK, Kristal BS, Baker JD, Beger R, Bessant C, Connor S, Capuani G, Craig A, Ebbels T, Kell DB, Manetti C, Newton J, Paternostro G, Somorjai R, Sjöström M, Trygg J, Wulfert F (2007) Proposed minimum reporting standards for data analysis in metabolomics. Metabolomics 3(3):231–241. 10.1007/s11306-007-0081-3 [Google Scholar]
- Gotsmy M, Brunmair J, Büschl C, Gerner C, Zanghellini J (2022) Probabilistic quotient’s work and pharmacokinetics’ contribution: countering size effect in metabolic time series measurements. BMC Bioinform 23(1):379. 10.1186/s12859-022-04918-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan F, Li G, Liu AB, Lee MJ, Yang Z, Chen YK, Lin Y, Shih W, Yang CS (2012) δ-and γ-Tocopherols, but not α-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev Res 5(4):644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T, Evers BM (2011) mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res 71(9):3246–3256. 10.1158/0008-5472.CAN-10-4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylling B, Myte R, Schneede J, Hallmans G, Haggstrom J, Johansson I, Ulvik A, Ueland PM, Van Guelpen B, Palmqvist R (2017) Vitamin B-6 and colorectal cancer risk: a prospective population-based study using 3 distinct plasma markers of vitamin B-6 status. Am J Clin Nutr 105(4):897–904. 10.3945/ajcn.116.139337 [DOI] [PubMed] [Google Scholar]
- Han M, Zhang C, Suglo P, Sun S, Wang M, Su T (2021) l-Aspartate: an essential metabolite for plant growth and stress acclimation. Molecules 26(7):1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Wang J, Page D, Asthana S, Zetterberg H, Carlsson C, Okonkwo OC, Li L (2018) Comparative evaluation of MS-based metabolomics software and its application to preclinical Alzheimer’s disease. Sci Rep 8(1):9291. 10.1038/s41598-018-27031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Webb WL, Rammu H, Lane N (2023) Prebiotic synthesis of aspartate using life’s metabolism as a guide. Life (Basel). 10.3390/life13051177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holeček M (2023) Roles of malate and aspartate in gluconeogenesis in various physiological and pathological states. Metab Clin Exp. 10.1016/j.metabol.2023.155614 [DOI] [PubMed] [Google Scholar]
- Holowatyj AN, Ose J, Gigic B, Lin T, Ulvik A, Geijsen A, Brezina S, Kiblawi R, van Roekel EH, Baierl A, Bohm J, Bours MJL, Brenner H, Breukink SO, Chang-Claude J, de Wilt JHW, Grady WM, Grunberger T, Gumpenberger T, Ulrich CM (2022) Higher vitamin B6 status is associated with improved survival among patients with stage I-III colorectal cancer. Am J Clin Nutr 116(2):303–313. 10.1093/ajcn/nqac090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A (2003) Argininosuccinate synthetase from the urea cycle to the cirtulline-NO cycle. Eur J Biochem 270:1987–1999 [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H (2014) Cancer incidence in Egypt: results of the national population-based cancer registry program. J Cancer Epidemiol 2014:1–18. 10.1155/2014/437971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanisevic J, Want EJ (2019) From samples to insights into metabolism: uncovering biologically relevant information in LC-HRMS metabolomics data. Metabolites 9(12):308. 10.3390/metabo9120308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar V, Albaugh GP, Lohani A, Nair PP (1991) Human stools as a source of viable colonic epithelial cells. FASEB J 5(13):2856–2859. 10.1096/fasebj.5.13.1655550 [DOI] [PubMed] [Google Scholar]
- Jain A, Li XH, Chen WN (2019) An untargeted fecal and urine metabolomics analysis of the interplay between the gut microbiome, diet and human metabolism in Indian and Chinese adults. Sci Rep 9(1):9191. 10.1038/s41598-019-45640-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R, Adel-Alvarez L-A, Alvarez OR, Broaddus R, Das S (2003) Arachidonic acid and colorectal carcinogenesis. Mol Cell Biochem 253(1):141–149. 10.1023/A:1026060426569 [DOI] [PubMed] [Google Scholar]
- Joshi M, Eagan J, Desai NK, Newton SA, Towne MC, Marinakis NS, Esteves KM, De Ferranti S, Bennett MJ, McIntyre A, Beggs AH, Berry GT, Agrawal PB (2014) A compound heterozygous mutation in GPD1 causes hepatomegaly, steatohepatitis, and hypertriglyceridemia. Eur J Hum Genet 22(10):1229–1232. 10.1038/ejhg.2014.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, Yang CS (2010) Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 31(4):533–542. 10.1093/carcin/bgp205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimian J, Hadi A, Salehi-Sahlabadi A, Kafeshani M (2019) The effect of arginine intake on colorectal cancer: a systematic review of literatures. Clin Nutr Res 8(3):209–218. 10.7762/cnr.2019.8.3.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky A, Weymouth T, Hull T, Tarcea VG, Scardoni G, Laudanna C, Sartor MA, Stringer KA, Jagadish HV, Burant C, Athey B, Omenn GS (2012) Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics 28(3):373–380. 10.1093/bioinformatics/btr661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinform 9(1):559. 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Li H, Giovannucci E, Lee IM, Selhub J, Stampfer M, Ma J (2009) Prospective study of plasma vitamin B6 and risk of colorectal cancer in men. Cancer Epidemiol Biomarkers Prev 18(4):1197–1202. 10.1158/1055-9965.EPI-08-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Adler L, Karathia H, Carmel N, Rabinovich S, Auslander N, Keshet R, Stettner N, Silberman A, Agemy L, Helbling D, Eilam R, Sun Q, Brandis A, Malitsky S, Itkin M, Weiss H, Pinto S, Kalaora S, Erez A (2018) Urea cycle dysregulation generates clinically relevant genomic and biochemical signatures. Cell 174(6):1559–1570. 10.1016/j.cell.2018.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LQJ, Adler L, Hajaj E, Soria LR, Perry RB-T, Darzi N, Brody R, Furth N, Lichtenstein M, Bab-Dinitz E, Porat Z, Melman T, Brandis A, Malitsky S, Itkin M, Aylon Y, Ben-Dor S, Orr I, Pri-Or A, Erez A (2024) ASS1 metabolically contributes to the nuclear and cytosolic -mediated DNA damage response. Nat Metab 6(7):1294–1309. 10.1038/s42255-024-01060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Ma C, Liu C, Wang Z, Yang J, Liu X, Shen Z, Wu R (2016) NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget 7(20):29454–29464. 10.18632/oncotarget.8762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, Wang Q, Nakatsu CH, Jones-Hall Y, Jiang Q (2023a) Combining gamma-tocopherol and aspirin synergistically suppresses colitis-associated colon tumorigenesis and modulates the gut microbiota in mice, and inhibits the growth of human colon cancer cells. Eur J Pharmacol 946:175656. 10.1016/j.ejphar.2023.175656 [DOI] [PubMed] [Google Scholar]
- Liu Y, Lau HC, Yu J (2023b) Microbial metabolites in colorectal tumorigenesis and cancer therapy. Gut Microbes 15(1):2203968. 10.1080/19490976.2023.2203968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw-du-Toit R, Storbeck K-H, Cartwright M, Cabral A, Africander D (2017) Progestins used in endocrine therapy and the implications for the biosynthesis and metabolism of endogenous steroid hormones. Mol Cell Endocrinol 441:31–45. 10.1016/j.mce.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Lu M, Luo X, Li N, Chen H, Dai M (2019) Diagnostic accuracy of fecal occult blood tests for detecting proximal versus distal colorectal neoplasia: a systematic review and meta-analysis. Clin Epidemiol 11:943–954. 10.2147/clep.s213677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machover D, Almohamad W, Castagne V, Desterke C, Gomez L, Goldschmidt E (2024) Treatment of patients with carcinomas in advanced stages with 5-fluorouracil, folinic acid and pyridoxine in tandem. Sci Rep 14(1):12054. 10.1038/s41598-024-62860-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G, Mora S, Madu G, Adegoke OAJ (2021) Branched-chain amino acids: catabolism in skeletal muscle and implications for muscle and whole-body metabolism. Front Physiol 12:702826. 10.3389/fphys.2021.702826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JA, Skiba MB, Chow HS, Chew WM, Saboda K, Lance P, Ellis NA, Jacobs ET (2021) A protective role for arachidonic acid metabolites against advanced colorectal adenoma in a phase III trial of selenium. Nutrients. 10.3390/nu13113877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Pandey CM, Singh U, Gupta A, Sahu C, Keshri A (2019) Descriptive statistics and normality tests for statistical data. Ann Card Anaesth 22(1):67–72. 10.4103/aca.ACA_157_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monleón D, Morales JM, Barrasa A, López JA, Vázquez C, Celda B (2009) Metabolite profiling of fecal water extracts from human colorectal cancer. NMR Biomed 22(3):342–348. 10.1002/nbm.1345 [DOI] [PubMed] [Google Scholar]
- Mounir A, Hassan MA, Selim MA, Mahmoud IA (2022) Epidemiology of colorectal cancer, incidence, survival, and risk factors: cairo university center of oncology and nuclear medicine experience. Egypt J Hosp Med 89(2):7061–7070 [Google Scholar]
- Mracek T, Drahota Z, Houstek J (2013) The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys Acta 1827(3):401–410. 10.1016/j.bbabio.2012.11.014 [DOI] [PubMed] [Google Scholar]
- Neuhäuser M (2011) Wilcoxon–Mann–Whitney test. Springer, pp 1656–1658. 10.1007/978-3-642-04898-2_615 [Google Scholar]
- Ogunwobi OO, Mahmood F, Akingboye A (2020) Biomarkers in colorectal cancer: current research and future prospects. Int J Mol Sci 21(15):5311. 10.3390/ijms21155311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Mai XL, Kim J, de Guzman ACV, Lee JY, Park S (2024) Glycerol 3-phosphate dehydrogenases (1 and 2) in cancer and other diseases. Exp Mol Med 56(5):1066–1079. 10.1038/s12276-024-01222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer KA, Shishkova E, Miller IJ, Balnis J, Bernstein MN, Peters-Clarke TM, Meyer JG, Quan Q, Muehlbauer LK, Trujillo EA, He Y, Chopra A, Chieng HC, Tiwari A, Judson MA, Paulson B, Brademan DR, Zhu Y, Serrano LR, Jaitovich A (2021) Large-scale multi-omic analysis of COVID-19 severity. Cell Syst 12(1):23–40. 10.1016/j.cels.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z, Lu Y, Zhou G, Hui F, Xu L, Viau C, Aliya P, David LS, Xia LS (2024) MetaboAnalyst 60: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 10.1093/nar/gkae253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei G, Chen L, Zhang W (2017) WGCNA Application to Proteomic and Metabolomic Data Analysis. Elsevier, Cham, pp 135–158. 10.1016/bs.mie.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Cai G, Zhou B, Li D, Zhao A, Xie G, Li H, Cai S, Xie D, Huang C, Ge W, Zhou Z, Xu LX, Jia W, Zheng S, Yen Y, Jia W (2014) A distinct metabolic signature of human colorectal cancer with prognostic potential. Clin Cancer Res 20(8):2136–2146. 10.1158/1078-0432.ccr-13-1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniels VW, Machiels J, Vanderhoydonc F, Smans K, Waelkens E, Verhoeven G, Swinnen JV (2010) De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res 70(20):8117–8126. 10.1158/0008-5472.CAN-09-3871 [DOI] [PubMed] [Google Scholar]
- Sameh M, Khalaf HM, Anwar AM, Osama A, Ahmed EA, Mahgoub S, Ezzeldin S, Tanios A, Alfishawy M, Said AF, Mohamed MS, Sayed AA, Magdeldin S (2023) Integrated multiomics analysis to infer COVID-19 biological insights. Sci Rep 13(1):1802. 10.1038/s41598-023-28816-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam S, Alvarado DM, Ciorba MA (2016) Therapeutic targeting of inflammation and tryptophan metabolism in colon and gastrointestinal cancer. Transl Res 167(1):67–79. 10.1016/j.trsl.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Jobin C (2013) The microbiome and cancer. Nat Rev Cancer 13(11):800–812. 10.1038/nrc3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtossel O, Koren O, Shai I, Rinott E, Louzoun Y (2024) Gut microbiome-metabolome interactions predict host condition. Microbiome 12(1):24. 10.1186/s40168-023-01737-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M (2023) Chapter 9: metabolic, epigenetic functions and correlations with phenotype. In: Smith M (ed) The regulatory genome in adaptation, evolution, development, and disease. Academic Press, London, pp 217–242. 10.1016/B978-0-443-15352-5.00008-X [Google Scholar]
- Song EM, Byeon J-S, Lee SM, Yoo HJ, Kim SJ, Lee S-H, Chang K, Hwang SW, Yang D-H, Jeong J-Y (2018) Fecal fatty acid profiling as a potential new screening biomarker in patients with colorectal cancer. Dig Dis Sci 63(5):1229–1236. 10.1007/s10620-018-4982-y [DOI] [PubMed] [Google Scholar]
- Sridharan GV, Choi K, Klemashevich C, Wu C, Prabakaran D, Pan LB, Steinmeyer S, Mueller C, Yousofshahi M, Alaniz RC, Lee K, Jayaraman A (2014) Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat Commun 5:5492. 10.1038/ncomms6492 [DOI] [PubMed] [Google Scholar]
- Stone TW (2020) Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J Neurochem 152(6):627–649. 10.1111/jnc.14907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Zhao X (2022) Argininosuccinate synthase 1, arginine deprivation therapy and cancer management. Front Pharmacol 13:935553. 10.3389/fphar.2022.935553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zhang H-L, Zhang A-H, Zhou X-H, Wang X-Q, Han Y, Yan G-L, Liu L, Wang X-J (2018) Network pharmacology combined with functional metabolomics discover bile acid metabolism as a promising target for mirabilite against colorectal cancer. RSC Adv 8(53):30061–30070. 10.1039/C8RA04886J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenesa A, Dunlop MG (2009) New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet 10(6):353–358. 10.1038/nrg2574 [DOI] [PubMed] [Google Scholar]
- Ternes D, Karta J, Tsenkova M, Wilmes P, Haan S, Letellier E (2020) Microbiome in colorectal cancer: how to get from meta-omics to mechanism? Trends Microbiol 28(5):401–423. 10.1016/j.tim.2020.01.001 [DOI] [PubMed] [Google Scholar]
- Ternes D, Tsenkova M, Pozdeev VI, Meyers M, Koncina E, Atatri S, Schmitz M, Karta J, Schmoetten M, Heinken A, Rodriguez F, Delbrouck C, Gaigneaux A, Ginolhac A, Nguyen TTD, Grandmougin L, Frachet-Bour A, Martin-Gallausiaux C, Pacheco M, Letellier E (2022) The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat Metab 4(4):458–475. 10.1038/s42255-022-00558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trézéguet V, Fatrouni H, Merched AJ (2021) Immuno-metabolic modulation of liver oncogenesis by the tryptophan metabolism. Cells 10(12):3469. 10.3390/cells10123469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, Kanazawa M, VanderGheynst J, Fiehn O, Arita M (2015) MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods 12(6):523–526. 10.1038/nmeth.3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanweert F, Schrauwen P, Phielix E (2022) Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr Diabetes 12(1):35. 10.1038/s41387-022-00213-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L (2012) Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J 6(2):320–329. 10.1038/ismej.2011.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zheng X, Liu B, Xia Y, Xin Z, Deng B, He L, Deng J, Ren W (2021) Aspartate metabolism facilitates il-1β production in inflammatory macrophages. Front Immunol 12:753092. 10.3389/fimmu.2021.753092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao X, Ma Y, Yang Y, Ge S (2023) The effects of vitamin B6 on the nutritional support of BCAAs-enriched amino acids formula in rats with partial gastrectomy. Clin Nutr 42(6):954–961. 10.1016/j.clnu.2023.04.018 [DOI] [PubMed] [Google Scholar]
- Wei R, Wang J, Su M, Jia E, Chen S, Chen T, Ni Y (2018) Missing value imputation approach for mass spectrometry-based metabolomics data. Sci Rep. 10.1038/s41598-017-19120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Liu X, Cheng C, Yu W, Yi P (2020) Metabolism of amino acids in cancer. Front Cell Dev Biol 8:603837. 10.3389/fcell.2020.603837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Fang YJ, Che MM, Abulimiti A, Huang CY, Zhang CX (2022) Association of serum pyridoxal-5’-phosphate, pyridoxal, and par with colorectal cancer risk: a large-scale case-control study. Nutrients. 10.3390/nu14122389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Yamamoto K, Sato Y, Inoue S, Morinaga T, Hirano E (2016) Combination of aspartic acid and glutamic acid inhibits tumor cell proliferation. Biomed Res 37(2):153–159. 10.2220/biomedres.37.153 [DOI] [PubMed] [Google Scholar]
- Yang X-Y, Li X-Z, Zhang S-N (2020) Urinary metabolomic signatures in reticular oral lichen planus. Heliyon 6(5):e04041. 10.1016/j.heliyon.2020.e04041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhao Y, Im S, Nakatsu C, Jones-Hall Y, Jiang Q (2021) Vitamin E delta-tocotrienol and metabolite 13’-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice. J Nutr Biochem 89:108567. 10.1016/j.jnutbio.2020.108567 [DOI] [PubMed] [Google Scholar]
- Yu L, Lu J, Du W (2024) Tryptophan metabolism in digestive system tumors: unraveling the pathways and implications. Cell Commun Signal. 10.1186/s12964-024-01552-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaytseva YY, Harris JW, Mitov MI, Kim JT, Butterfield DA, Lee EY, Weiss HL, Gao T, Evers BM (2015) Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 6(22):18891–18904. 10.18632/oncotarget.3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Suidasari S, Hasegawa T, Yanaka N, Kato N (2013) High concentrations of pyridoxal stimulate the expression of IGFBP1 in HepG2 cells through upregulation of the ERK/c-Jun pathway. Mol Med Rep 8(4):973–978. 10.3892/mmr.2013.1629 [DOI] [PubMed] [Google Scholar]
- Zhang W, An Y, Qin X, Wu X, Wang X, Hou H, Song X, Liu T, Wang B, Huang X, Cao H (2021b) Gut microbiota-derived metabolites*** in colorectal cancer: the bad and the challenges. Front Oncol 11:739648. 10.3389/fonc.2021.739648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ni Y, Su M, Li H, Dong F, Chen W, Wei R, Zhang L, Guiraud SP, Martin FP, Rajani C, Xie G, Jia W (2017) High throughput and quantitative measurement of microbial metabolome by gas chromatography/mass spectrometry using automated alkyl chloroformate derivatization. Anal Chem 89(10):5565–5577. 10.1021/acs.analchem.7b00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L-G, Shu X-O, Li H-L, Gao J, Han L-H, Wang J, Fang J, Gao Y-T, Zheng W, Xiang Y-B (2019) Prospective cohort studies of dietary vitamin B6 intake and risk of cause-specific mortality. Clin Nutr 38(3):1180–1187. 10.1016/j.clnu.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Xie G, Zhao A, Zhao L, Yao C, Chiu NHL, Zhou Z, Bao Y, Jia W, Nicholson JK, Jia W (2011) The footprints of gut microbial-mammalian co-metabolism. J Proteome Res 10(12):5512–5522. 10.1021/pr2007945 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The metabolomics and metadata reported in this paper are available via [Metabolights,https://www.ebi.ac.uk/metabolights/editor/study/MTBLS11640] study identifier [MTBLS11640]. The data presented in this study are available upon request from the corresponding author.