Abstract
The contribution of forced oscillation technique (FOT), also called oscillometry, in diagnosis and follow-up of progressive pulmonary fibrosis (PPF) is not yet established. The aims of this monocentric retrospective study were to compare the FOT profile between patients suffering from PPF and stable non-idiopathic pulmonary fibrosis (IPF) interstitial lung diseases (ILDs), to look for a correlation between oscillometry and conventional function tests currently used for PPF follow-up and functional definition (forced vital capacity (FVC) and diffusing lung capacity (DLCO)) and correlation with ILD severity according to FVC. Compared to non-IPF stable ILDs (n = 96), PPF patients (n = 45) showed lower median resistance at 5Hz (Xrs5) values (during inspiratory phase: 0.31 versus -0.39 cmH2O/(L/sec), p = 0.019595). Xrs5 also showed moderate correlation with FVC and DLCO. Finally, among all ILDs (n = 160), Xrs5 showed correlation with disease severity according to FVC. These results suggest that, in conjunction with conventional pulmonary function tests, FOT could be an interesting tool to predict progressive course of fibrosing non-IPF ILDs. Its exact contribution to PPF diagnosis and follow-up needs to be determined by a prospective approach.
Keywords: Oscillometry, Forced oscillation technique, Pulmonary function tests, Interstitial lung diseases, Progressive pulmonary fibrosis, Idiopathic pulmonary fibrosis
Subject terms: Respiratory tract diseases, Respiratory signs and symptoms
Introduction
This study focuses on the potential role of forced oscillation technique (FOT), also called oscillometry, in progressive pulmonary fibrosis (PPF). PPF represents a subtype of patients suffering from intersitial lung diseases (ILD) of various etiologies but characterized by a common feature, similar to idiopathic pulmonary fibrosis (IPF), the progression of pulmonary fibrosis, which needs to be identified at an earliest stage in order to propose antifibrotic therapy. PPF was defined by the 2022 ATS/ERS/JRS/ALAT Clinical Practice Guidelines in non-IPF patients independently of the underlying physiological process presenting two of the three following criteria occurring within the last year with no alternative explanation: worsening respiratory symptoms, physiological progression and radiological progression. Physiological evidence of disease progression is defined as the presence of either of the following findings: absolute decline in forced vital capacity (FVC) ≥ 5% predicted or absolute decline in diffusing lung capacity of carbon monoxide (DLCO) (corrected for hemoglobin) of ≥10% predicted1.
At present, the definition and prognosis of PPF essentially relies on conventional pulmonary function tests (PFTs). Measuring FVC and DLCO can be burdensome for some patients as it requires their cooperation and sufficient breath, thereby reducing reproducibility. FOT represents an innovative alternative because it is a non-invasive, quick, reproductible and convenient method to perform both for operator and patient, avoiding any special breathing maneuver (measured during regular breathing), initially developed for obstructive lung diseases2–4. Pressures waves are applied at the mouth through a mouthpiece (with a nose clip in place), sur-imposing sinusoidal oscillations to spontaneous tidal breathing at different frequencies (in a range of 5 to 40Hz)4. Airflows and pressures are measured by a transducer, assessing mechanical properties of the respiratory system, called respiratory system impedance (Zrs). Zrs is not used in clinical practice but represented by its components, respiratory system resistance (Rrs) and reactance (Xrs), which it is linked to by the equation Zrs2 = Rrs2 + Xrs2 2–4.
Rrs measures airway resistance from the mouth to the small caliber bronchi. Rrs at 5Hz (Rrs5) captures the total airway resistance while Rrs at 20Hz (Rrs20) reflects the resistance of the large airways. Rrs values are also influenced, to a lesser extent, by the resistance of the pulmonary tissues, the chest wall and the airway heterogeneity2,4,5.
Xrs represents the energy stored and dissipated by the airways, lung tissue and thorax to waves passing through and stretching it. It is composed of inertance, the forces of the moving air column in the conducting airways, and capacitance, the elastic properties of the peripheral parts of the respiratory system. Capacitance dominates at lower frequencies, representing the energy absorbed by the distal airways, parenchyma and chest wall. By convention, capacitance is described by negative values. Thus, if the capacitive energy is impaired by fibrosis or hyperinflation, Xrs will be more negative at low oscillation frequencies2,5.
Values can be reported at a single frequency or over the frequency range of 5-40Hz and its indices are expressed as the mean values of whole-breath data and of each phase as inspiration and expiration.
Oscillometry is a promising tool in the diagnosis, prognosis, and management of ILDs but its clinical application is still less established than in obstructive diseases2–4. Current literature data demonstrate that patients with ILD have increased Rrs, increased frequency dependence of Rrs and more negative Xrs values at low frequencies, consistent with increased elastance2,6–8. FOT is currently more of a clinical research tool in ILDs and remains difficult to implement in clinical practice. The literature is sparse, focusing mainly on limited cohorts of patients and IPF. Apart from a recent study by Liang et al. 9, there are few data comparing ILD with other pulmonary diseases and no standardized cut-offs, making it difficult to accurately interpret results9. To the best of our knowledge, no study has looked specifically at the role of FOT in PPF population, despite the fact that conventional PFTs, which are sometimes difficult to perform and less convenient than FOT, play a major role in the early detection of progressive phenotypes among ILD patients and prediction of their evolution.
The aim of this study was to determine whether FOT is a reliable tool to predict the progressive course of fibrosing ILDs by demonstrating that certain values differ between patients with PPFs versus stable non-IPF ILDs. In addition, it was intended to look for a correlation between FOT and PFT values currently used in the follow-up of ILDs and PPF functional definition (FVC and DLCO). Finally, correlation was investigated between ILD severity according to FVC (by dividing patients into 3 severity groups) and FOT values.
Methods
Population
This is a single-center retrospective study. Data were collected retrospectively among patients with fibrosing ILD diagnoses (according to a multidisciplinary discussion among experienced clinical experts in the field of ILDs), evaluated in a tertiary ILD center in the pneumology clinic of CHU Liège between 11th January 2021 and 13th January 2023. The inclusion criteria were a fibrosing ILD diagnosis of any type (established by a multidisciplinary discussion including at least pulmonologists and radiologists with specific expertise in this field) and the availability of at least one conventional PFT and FOT performed during the same visit. There was no exclusion criterion.
IPF and PPF were defined based on the 2022 ATS/ERS/JRS/ALAT guidelines on Idiopathic Pulmonary Fibrosis and Progressive Pulmonary Fibrosis1.
Patients with fibrosing ILDs not fulfilling either IPF or PPF definitions were classified as “stable non-IPF patients”.
The protocol was approved by the ethics committee of University Hospital of Liège (Comité d'Éthique Hospitalo-Facultaire Universitaire de Liège (707), study refrence: 2022/20). The need to obtain informed consent was waived by the ethics committee of University Hospital of Liège. All methods were performed in accordance with the relevant guidelines and regulations.
FOT analysis
FOT was performed using a Resmon PRO FULL V3 FOT system, software version 21.5.0, following the European Respiratory Society technical standards10. Values were assessed at 5Hz, as measurements averaged over several tidal breaths (whole-breath analysis) and as measurements separately averaged during inspiration and expiration. The percentage of predicted value given by the software was calculated using the prediction equation developed by Oostveen et al. 11. This equation takes into account the age, sex, height and weight of healthy Caucasian subjects aged between 18 and 80 years.
Statistics
For comparison between PPF and stable patients non-IPF ILDs, the unpaired t-test was used for PFT data given the normal distribution of these data, and the Mann–Whitney test for FOT data selected due to the non-normal distribution of these data.
Spearman rank correlation method was used in order to correlate forced expired volume in one second (FEV-1), FVC and DLCO values with FOT values, as it is suitable for non-linear variables. Spearman correlation coefficient is referred to as “r”.
All patients were categorized into 3 groups according to their FVC value (≥ 80%, ≥ 60 and < 80% and < 60% of predicted values). FOT values were compared between those groups, using Kruskall Wallis test and Dunn’s post hoc test (reflecting the variability in group data distributions).
FOT results are expressed as median (interquartile range) and PFTs an mean ± standard derivation.
P-value < 0.05 was considered to be statically significative.
Statistical analyses were performed using TIBCO Statistica, v. 13.5.0, TIBCO Software Inc, Palo Alto, CA, USA and graphs using GraphPad Prism software version 9.0.0 for Windows, GraphPad Software, San Diego, California, USA.
Results
Demographic, treatments and functional characteristics
Among 160 patients with fibrosing ILDs, 21 (13%) had IPF and 139 (87%) had non-IPF ILDs which included 94 (59%) stable ILDs and 45 (28%) PPFs (Fig. 1).
Fig. 1.
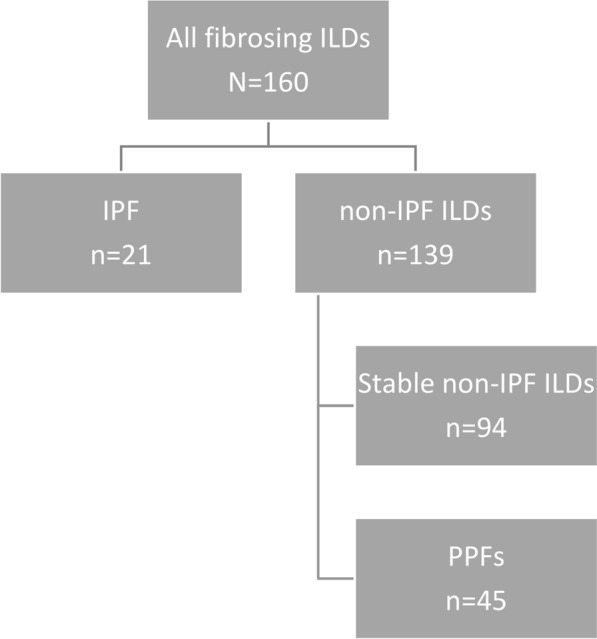
Flowchart . ILDs interstitial lung diseases, IPF idiopathic pulmonary fibrosis, PPFs progressive pulmonary fibrosis.
In comparison to stable patients, PPF patients included less non-smoker (46 patients (49%) versus 15 patients (33%), p-value (p) < 0.001) (Table 1).
Table 1.
Baseline characteristics (demography, treatments and PFTs) of all patients and comparison between PPFs and stables non-IPF ILDs.
| All patients N = 160 |
Stable non-IPF ILDs n = 94 |
PPFs n = 45 |
|
|---|---|---|---|
| Demography | |||
| Age (years) | 64 (53–72) | 62 (50–70) | 68 (59–75) |
| Biological sex (M/F) | 96/64 | 48/46 | 30/15 |
| BMI (kg/m2) | 27 ± 9.3 | 28 ± 8.5 | 26 ± 10.3 |
| Smoking (NS/ES/CS) | 61/63/22 | 46/26/15 | 15/37/7*** |
| Diagnosis | |||
| CTD-ILD (nb (%)) | 61 (38.1%) | 35 (37.2%) | 26 (57.8%) |
| fsarcoidosis (nb (%)) | 27 (16.9%) | 23 (24.5%) | 4 (8.9%) |
| IIP (nb (%)) | 19 (11.9%) | 13 (13.8%) | 6 (13.3%) |
| CPFE (nb (%)) | 3 (1.9%) | 1 (1.1%) | 2 (4.4%) |
| cHP (nb (%)) | 3 (1.9%) | 2 (2.1%) | 1 (2.2%) |
| Other ILDs | 26 (16.3%) | 20 (21.3%) | 6 (13.3%) |
| Treatments | |||
| IS (nb (%)) | 59 (36.9%) | 35 (37.2%) | 24 (53.3%) |
| Anti-fibrotic (nb (%)) | 10 (6.3%) | 0(0%) | 10 (22.2%) |
| OCS (nb (%)) | 58 (36.3%) | 32 (34%) | 26 (57.8%) |
| ICS (nb (%)) | 39 (24,3%) | 12 (12.8%) | 6 (13.3%) |
| LABA (nb (%)) | 34 (21.3%) | 18 (19.1%) | 10 (22.2%) |
| LAMA (nb (%)) | 5 (3%) | 5 (5.3%) | 1 (2.2%) |
| SABA/SAMA (nb (%)) | 18 (11,3%) | 7 (7.4%) | 5 (11.1%) |
| Pulmonary functional test | |||
| FEV-1 (%pred) | 83 ± 18 | 85 ± 16 | 76 ± 19** |
| FVC (%pred) | 82 ± 18 | 87 ± 16 | 74 ± 18*** |
| FEV-1/FVC (absolute ratio) | 79 ± 7 | 78 ± 8 | 80 ± 7* |
| MEF25-75 (%pred) | 97 ± 38 | 90 ± 33 | 95 ± 36 |
| TLC (%pred) | 86 ± 20 | 92 ± 20 | 77 ± 18** |
| DLCO (%pred) | 64 ± 22 | 70 ± 22 | 53 ± 19**** |
Continuous variables are Age is expressed as median (interquartile ranges). BMI and PFTs are expressed as mean ± standard deviation.
* p-value < 0.05; **p-value < 0.01; ***p-value < 0.001; ****p-value < 0.0001 comparing stable non-IPF ILDs to PPF.
cHP chronic hypersensitivity pneumonitis, CPFE combined fibrosis and emphysema, CS current smoker, CTD-ILD connective tissue disease-associated interstitial lung disease, DLCO diffusion lung capacity for carbon monoxide, ES ex-smoker, FEV-1 forced expired volume in 1 s, fsarcoidosis fibrosing sarcoidosis, FVC forced vital capacity, IIP idiopathic interstitial pneumonia, ILDs interstitial lung diseases, ICS inhaled corticosteroids, IPF idiopathic pulmonary fibrosis, IS immunosuppressors, kg/m2 kilogram per square meter, LABA long-acting beta agonists, LAMA long-acting muscarinic antagonists, M/F male/female, MEF25-75 mean flow between 25 and 75% of FVC, NS non-smoker, OCS oral corticosteroids, PFT pulmonary function test, PPF progressive pulmonary fibrosis, SABA short-acting beta agonists, SAMA short-acting muscarinic antagonists, sGaw specific airway conductance, TLC total lung capacity, %pred % of predicted value.
Non-IPF ILDs included connective tissue disease-associated interstitial lung disease (CTD-ILD; n = 61), fibrosing sarcoidosis (fsarcoidosis; n = 27), idiopathic interstitial pneumonia (IIP; n = 19), combined fibrosis and emphysema (CPFE; n = 3), chronic hypersensitivity pneumonitis (cHP; n = 3) and other ILDs (n = 26). CTD-ILD was the most frequent condition both in PPF and stable patients.
Compared to stable patients, PPF had statistically significantly lower FEV-1 (76 ± 19 versus 85 ± 16, p = 0.005), FVC (74 ± 18 versus 87 ± 16, p < 0.001), total lung capacity (TLC) (77 ± 18 versus 92 ± 20, p = 0.006) and DLCO (53 ± 19 versus 70 ± 22, p < 0.001) values.
FOT value comparison between PPF and stable non-IPF ILDs
In comparison to stable patients, PPFs showed significantly lower median Xrs at 5Hz (Xrs5) during inspiratory phase (0.31 cmH2O/(L/sec) versus -0.39 cmH2O/(L/sec), p = 0.0120) (Table 2).
Table 2.
Comparison of FOT values between non-IPF ILDs and IPFs.
| All patients N = 160 |
Stable non-IPF ILDs n = 139 |
IPF n = 21 |
|
|---|---|---|---|
|
Rrs5 Inspiratory (cmH2O/(L/sec)) |
0.38 (-0.35–1.18) | 0.33 (-0.28–1.25) | 0.41 (-0.64–1.15) |
| Rrs5 Inspiratory (%pred) | 111 (91–139) | 109 (93–142) | 112 (84–137) |
|
Rrs5 Expiratory (cmH2O/(L/sec)) |
1.01 (0.08–1.75) | 0.98 (0.2–1.75) | 0.84 (-0.34–1.8) |
| Rrs5 Expiratory (%pred) | 132 (104–163) | 131 (105–161) | 126 (93–166) |
|
Rrs5 Whole breath (cmH2O/(L/sec)) |
0.71 (0.05–1.57) | 0.7 (0.17–1.58) | 0.49 (-0.4–1.57) |
| Rrs5 Whole breath (%pred) | 122 (100–154) | 122 (105–155) | 115 (89–154) |
| Xrs5 Inspiratory (cmH2O/(L/sec)) | -0.01 (-1.2–0.8) | 0.31 (-0.61–0.99) | -0.39 (-1.42–0.53)* |
| Xrs5 Inspiratory (%pred) | 100 (73–141) | 91 (67–121) | 115 (82–149)* |
| Xrs5 Expiratory (cmH2O/(L/sec)) | -0.1 (-1.96–1.01) | 0.24 (-2.17–1.14) | -0.23 (-1.69–0.97) |
| Xrs5 Expiratory (%pred) | 101 (66–179) | 90 (61–182) | 106 (67–179) |
| Xrs5 Whole breath(cmH2O/(L/sec)) | -0.08 (-1.89–0.8) | 0.13 (-1.4–1.07) | -0.52 (-2.12–0.78) |
| Xrs5 Whole breath (%pred) | 103 (71–170) | 96 (63–151) | 116 (74–170) |
Continuous variables are expressed as median (interquartile ranges).
*p-value < 0,05 comparing stable ILDs to PPFs.
FOT forced oscillation technique, ILD interstitial lung disease, PPF progressive pulmonary fibrosis, Rrs5 respiratory system resistance at 5Hz, Xrs5 respiratory system reactance at 5Hz, cmH2O/(L/sec) centimeter of water per liter per second
Correlation between FOT and pulmonary function tests
As shown in Fig. 2, a correlation was found between certain FOT values and FEV-1, FVC and DLCO values.
Fig. 2.
Spearman rank correlations between FEV-1, FVC and DLCO values and Rrs5 and Xrs5 values. FEV1 forced expired volume in 1 s, %pred % of predicted value, p p-value, r Spearman coefficient, R5 resistance at 5Hz, X5 reactance at 5Hz, in inspiratory, ex expiratory, ns non significant.
FEV-1 values were significantly correlated with Rrs5 (p < 0.0001, = 0.0016 and = 0.0063 respectively for inspiratory, expiratory and whole breath values; Spearman correlation coefficient (r) = -0.310, -0.250 and -0.271 respectively for inspiratory, expiratory and whole-breath values) and Xrs5 (p < 0.0001 for inspiratory, expiratory and whole breath values; r = -0.517, -0.483 and -0.533 respectively for inspiratory, expiratory and whole-breath values).
Similarly, FVC values correlated significantly with Rrs5 (p < 0.0004, 0.0064 and 0.0038 respectively for inspiratory, expiratory and whole breath values; r = -0.279, -0.217 and -0.230 respectively for inspiratory, expiratory and whole-breath values) and Xrs5 (p < 0.0001 for inspiratory, expiratory and whole breath values; r = -0.587, -0.507 and -0.564 respectively for inspiratory, expiratory and whole-breath values).
Concerning DLCO values, correlation was found concerning Xrs5 (inspiratory (p < 0.0001, r = -0.330), expiratory (p = 0.0050, r = -0.225) and whole-breath (p = 0.0013, r = -0.258)) but no correlation was found concerning Rrs5.
See supplementary material for correlation table (sTable 1).
FOT values according to disease severity
All patients (N = 160), were divided into 3 groups according to their FVC value, reflecting disease severity: < 60% (n = 19), between 60 and 80% (n = 52) and ≥ 80% of predicted values (n = 93) and Xrs5 values were lower according to severity (Table 3 and Fig. 3).
Table 3.
FOT values according to FVC values (≥ 80%, ≥ 60 and < 80% and < 60% of predicted values).
| FVC ≥ 80%pred n = 93 |
FVC ≥ 60% & < 80%pred n = 52 |
FVC < 60%pred n = 19 |
|
|---|---|---|---|
|
Rrs5 Inspiratory (cmH2O/(L/sec)) |
0.21 (-0.42–0.81) | 0.73 (0.02–1.42) | 1.25 (-0.26–1.73) |
| Rrs5 (%pred) | 106 (90–126) | 122 (101–149) | 142 (93–159) |
|
Rrs5 Expiratory (cmH2O/(L/sec)) |
0.79 (0.03–1.52) | 1.21 (0.25–2.14) | 1.58 (0.08–2.19) |
|
Rrs5 Expiratory (%pred) |
124 (101–150) | 141 (109–183) | 156 (102–183) |
|
Rrs5 Whole breath (cmH2O/(L/sec)) |
0.59 (-0.16–1.21) | 1.19 (0.14–1.93) | 1.57 (-0.15–1.96) |
|
Rrs5 Whole breath (%pred) |
118 (96–140) | 134 (103–168) | 152 (96–173) |
|
Xrs5 Inspiratory (cmH2O/(L/sec)) |
0.53 (-0.12–1.17) | -0.8 (-1.68–0.45)**** | -2.73 (-3.22–1.32)++++° |
|
Xrs5 Inspiratory (%pred) |
82 (66–105) | 122 (85–158)*** | 187 (164–212)++++°° |
|
Xrs5 Expiratory (cmH2O/(L/sec)) |
0.74 (-0.22–1.3) | -0.43 (-3.1–0.53)**** | -2.27 (-3.9–1.39)++++ |
|
Xrs5 Expiratory (%pred) |
76 (48–106) | 114 (81–224)**** | 181 (153–249)++++ |
|
Xrs5 Whole breath (cmH2O/(L/sec)) |
0.52 (-0.11–1.29) | -0.79 (-2.75–0.31) | -2.36 (-3.16–1.85) |
|
Xrs5 Whole breath (%pred) |
83 (57–104) | 128 (88–197)**** | 197 (170–222)++++° |
Continuous variables are expressed as median (interquartile ranges).
***p-value < 0.001; ****p-value < 0.0001 comparing FVC ≥ 80% to FVC between 60 and 80%.
++++p-value < 0.0001 comparing FVC ≥ 80% to FVC < 60%.
°p-value < 0,05; °°p-value < 0,01 comparing to FVC < 60% to FVC between 60 and 80%.
%pred % of predicted value, FOT forced oscillation technique, FVC forced vital capacityRrs5, respiratory system resistance at 5Hz, Xrs5 respiratory system reactance at 5Hz; cmH2O/(L/sec), centimeter of water per liter per second.
FVC forced vital capacity, %pred % of predicted value, R5 resistance at 5Hz, X5 reactance at 5Hz, in inspiratory, ex expiratory; ns non significant.
Fig. 3.
FOT values according to FVC. * p-value < 0,05; ** p-value < 0,01; ***p-value < 0,001; ****p-value < 0,0001. FVC forced vital capacity, %pred % of predicted value, R5 resistance at 5Hz, X5 reactance at 5Hz, in inspiratory, ex expiratory, ns non significant
Compared to patients with FVC < 60%, the ones with FVC between 60 and 80% had lower inspiratory Xrs5 expressed as predicted values (187% versus 122% of predicted values, p = 0.0073) and whole-breath Xrs5 (-2.36 versus -0.79 cmH2O/(L/sec), p = 0.0113 and 197% versus 128% of predicted values, p = 0.0359).
Likewise, compared to patients with FVC < 60%, the ones with FVC above 80% had lower X rs5 values (inspiratory (-2.73 versus -0.8 cmH2O/(L/sec), p < 0.0001, 187% versus 122% of predicted values, p < 0.0001) expiratory (-2.72 versus -0.43 cmH2O/(L/sec), p < 0.0001 and 181% versus 114% of predicted values, p < 0.0001) and whole-breath (-2.36 versus -0.79 cmH2O/(L/sec), p < 0.0001 and 197% versus 128% of predicted values, p < 0.0001).
Again, compared to patients with FVC between 60 and 80%, the ones with FVC ≥ 80% had lower Xrs5 values (inspiratory (-0.8 versus 0.53 cmH2O/(L/sec), p < 0.0001, 122% versus 82% of predicted values, p = 0.0002) expiratory (-0.43 versus 0.74 cmH2O/(L/sec), p < 0.0001 and 114% versus 76% of predicted values, p < 0.0001) and whole-breath (-0.79 versus 0.52 cmH2O/(L/sec), p < 0.0001 and 128% versus 83% of predicted values, p < 0.0001).
Demographic characteristics and diagnoses according to FVC values are described in sTable 2 (see Supplementary Material).
Of interest, we did not find any significant difference between FOT at baseline and PFT modifications over time (Figure 1 in Supplementary Material).
Discussion
These data show that in a monocentric retrospective cohort of 160 patients with fibrosing ILDs, PFT values correlate moderately with FOT values. Indeed, FEV-1, FVC and DLCO values (expressed as predicted values), commonly used for longitudinal monitoring of ILD patients, correlated moderately with inspiratory, expiratory and whole-breath Xrs5. This consistently implies that patients with lower DLCO and FVC values display more impaired lung compliance secondary to lung fibrosis which is known to alternate both lung volume and gas exchange. FEV-1 and FVC values (expressed as predicted values), in addition to being related with Xrs values, were also moderately related with inspiratory, expiratory and whole-breath Rrs5, indicating higher total airway resistance.
The link between FOT and PFT values has been demonstrated in numerous studies involving patients with obstructive lung diseases (such as chronic obstructive pulmonary disease and asthma). In particular, Rrs5 and Xrs5 values have been shown to be related to FEV-1 and FVC values2,5,12. There are fewer studies involving patients suffering of ILD but van Noord et al. already showed in 1989 that in these patients, TLC (expressed as absolute value) and vital capacity (VC) (expressed as predicted value) were correlated with mean Xrs values and the slopes of Xrs and Rrs as a function of frequency (8).
Consistent with our findings, some recent studies focusing on IPF, CPFE and ILD patients demonstrated correlation between Xrs5 and PFT values, in particular DLCO and FVC12–15. Mori et al. and Ishikawa et al., both focusing on IPF patients, also showed correlation between VC, FVC and FEV-114,16.
Furthermore, Fuji et al. demonstrated that inspiratory resonant frequency (Fres) (the frequency where capacitance and inertance make equal and opposite contribution to impedance) was significantly correlated with the composite physiological index, which is a score predicting the fibrosis severity on high-resolution computed tomography (HRCT) in patients suffering from IPF17.
Finally, two studies (focusing on systemic sclerosis and rheumatoid arthritis) found a positive association between reactance and Fres values and interstitial lung abnormalities on HRCT18,19.
When patients in this study were divided into three severity groups based on FVC (< 60%, between 60 and 80% and ≥ 80%, expressed as predicted values), the Xrs5 values were significantly different between the groups. This means that Xrs5 is considered to be an accurate predictor of the functional severity of a patient with fibrosing ILD based on FVC. Logically, the more severe the patient is considered to be, the more his pulmonary compliance is impaired. Similarly, van Noord et al., divided their cohort of 54 ILD patients according to their TLC value (predicted value above or below 80%) and the more severe group showed decreased Xrs values at low frequencies, unlike group displaying TLC values above 80%6. A recent study conducted by Matesanz-Lopez et al. classified their ILD patients as severe if DLCO and/or FVC value was less than 70% of predicted values and severe patients had more impaired Xrs5 (inspiratory, expiratory and whole-breath) values than others15.
In the present study, PPF patients, compared to stable non-IPF patients, showed lower Xrs5 inspiratory values, meaning that this tool could be interesting in conjunction with standard PFTs (which appear to be superior, comparing Tables 1 and 2) in order to discriminate patients with progressive pattern.
Due to the restricted number of patients with longitudinal data, we did not find any correlation between FOT values and PFT values over follow-up. Several other studies demonstrated correlation between GAP score (prognostic of survival among IPF patients) and oscillometry data14,20,21. Mori et al. showed that, in IPF patients, Xrs5 values was significantly associated with ≥ 10% FVC decline over 12 ± 3 months (odds ratio 0.137, 95% CI 0.021–0.875, p = 0.036)14. Finally, Ishikawa et al. demonstrated that Xrs had significant impact on survival among IPF patients16.
The main limitation of this study is its retrospective, monocentric design, including a restricted number of patients, and therefore reducing its generalizability. A future multicentric, longitudinal and prospective approach is required in order to determine more precisely the predictive, diagnostic and prognostic role of oscillometry in PPF compared with conventional FOT. It should include a large cohort of patients (with a real-life PPF population representative in terms of demography and underlying ILD etiology). This approach would appear to be of great interest given the expected benefit of FOT in clinical practice. Severely ill patients suffering from pulmonary fibrosis may be, in certain cases, unable to perform traditional PFT, either because of an inability to coordinate, understand the instructions or have sufficient breath. The reproducibility of FOT values is also of great concern given the need to define a functional fibrosis progression with precision and without confounding factors.
Another limitation of this study is due to the fact that one single frequency of FOT value (5Hz) is covered, and therefore does not include values such as the resonant frequency and the area of the Xrs curve. Plus, despite the growing literature on the subject, FOT measurements still lack standard reference values.
Conclusion
In this monocentric retrospective study including a restricted number of patients, the ones with progressive pulmonary fibrosis showed lower median inspiratory reactance at 5Hz, and thus pulmonary compliance, compared to patients with stable non-IPF fibrosing ILDs. FVC and DLCO values correlated moderately with Xrs5 values and Xrs5 was more impaired in patients with lower FVC. This implies that, in conjunction with PFTs, FOT which do not rely on patients’ collaboration, moderately correlate with severity and progressive phenotype. The exact contribution of this tool to the diagnosis and follow-up of PPF patients needs to be further determined in a prospective study involving a sufficient number of patients.
Supplementary Information
Abbreviations
- cHP
Chronic hypersensitivity pneumonitis
- CPFE
Combined fibrosis and emphysema
- CTD-ILD
Connective tissue disease-associated interstitial lung disease
- DLCO
Diffusing lung capacity of carbon monoxide
- FEV-1
Forced expired volume in one second
- Fres
Resonant frequency
- Fsarcoidosis
Fibrosing sarcoidosis
- FOT
Forced oscillation technique
- FVC
Forced vital capacity
- HRCT
High resolution computed tomography
- IIP
Idiopathic interstitial pneumonia
- ILD
Interstitial lung disease
- IPF
Idiopathic pulmonary fibrosis
- PFT
Pulmonary function test
- PPF
Progressive pulmonary fibrosis
- P
P-value
- R
Spearman correlation coefficient
- Rrs
Resistance
- Rrs5
Resistance at 5Hz
- Rrs20
Resistance at 20Hz
- Xrs
Reactance
- Xrs5
Reactance at 5Hz
- TLC
Total lung capacity
- VC
Vital capacity
- Zrs
Impedance
Author contributions
A.D and J.G. wrote the main manuscript text. M.H. prepared figures and performed statistical analyses. L.G, H.G. and X.C. participated to data collection. All authors read and approved the final manuscript.
Data availability
Datasets can be made accessible if needed by contacting the corresponding author. The content of the manuscript was presented as a poster at European Respiratory Society International Congress in September 2023, in Milan. The title of the poster was “Forced oscillation technique in progressive pulmonary fibrosis”.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The protocol was approved by the ethics committee of University Hospital of Liège (Comité d'Éthique Hospitalo-Facultaire Universitaire de Liège (707), study refrence: 2022/20). The need to obtain informed consent was waived by the ethics committee of University Hospital of Liège. All methods were performed in accordance with the relevant guidelines and regulations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-99857-1.
References
- 1.Raghu, G. et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med.205(9), e18–e47 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaminsky, D. A. et al. Clinical significance and applications of oscillometry. Eur. Respir. Rev.31, 210208 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirai, T. & Kurosawa, H. Clinical application of the forced oscillation technique. Intern Med.55(6), 559–566 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Desiraju, K. & Agrawal, A. Impulse oscillometry: the state-of-the-art for lung function testing. Lung India33, 410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bednarek, M., Grabicki, M., Piorunek, T. & Batura-Gabryel, H. Current place of impulse oscillometry in the assessment of pulmonary diseases. Respir. Med.170, 105952 (2020). [DOI] [PubMed] [Google Scholar]
- 6.van Noord, J. A. et al. Total respiratory resistance and reactance in patients with diffuse interstitial lung disease. Eur. Respir. J.2(9), 846–852 (1989). [PubMed] [Google Scholar]
- 7.Sokai, R. et al. Respiratory mechanics measured by forced oscillation technique in rheumatoid arthritis-related pulmonary abnormalities: frequency-dependence, heterogeneity and effects of smoking. Springerplus5, 335 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu, J. K. Y. et al. Correlation of respiratory oscillometry with CT image analysis in a prospective cohort of idiopathic pulmonary fibrosis. BMJ Open Respir. Res.9(1), e001163 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang, X. et al. Clinical application of oscillometry in respiratory diseases: an impulse oscillometry registry. ERJ Open Res.8(4), 00080–02022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King, G. G. et al. Technical standards for respiratory oscillometry. Eur. Respir. J.55(2), 1900753 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Oostveen, E. et al. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur. Respir. J.42(6), 1513–1523 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Mousa, H. & Kamal, E. Impulse oscillation system versus spirometry in assessment of obstructive airway diseases. Egypt J. Chest Dis. Tuberc.67(2), 106–112 (2018). [Google Scholar]
- 13.Yamamoto, Y. et al. Respiratory impedance is associated with ventilation and diffusing capacity in patients with idiopathic pulmonary fibrosis combined with emphysema. Int. J. Chron. Obstruct. Pulmon. Dis.17, 1495–1506 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori, Y. et al. Respiratory reactance in forced oscillation technique reflects disease stage and predicts lung physiology deterioration in idiopathic pulmonary fibrosis. Respir. Physiol. Neurobiol.275, 103386 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Matesanz-López, C., Raboso-Moreno, B., Saldaña-Pérez, L. E., Rodríguez-Nieto, M. J. & Río-Ramírez, M. T. Is lung function measured by oscillometry useful in interstitial lung diseases?. Open Respir. Arch.6(1), 100278 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa, T. et al. The impact of respiratory reactance in oscillometry on survival in patients with idiopathic pulmonary fibrosis. BMC Pulm. Med.24(1), 10 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii, M. et al. Inspiratory resonant frequency of forced oscillation technique as a predictor of the composite physiologic index in interstitial lung disease. Respir. Physiol. Neurobiol.207, 22–27 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Aronsson, D., Hesselstrand, R., Bozovic, G., Wuttge, D. M. & Tufvesson, E. Airway resistance and reactance are affected in systemic sclerosis. Eur. Clin. Respir. J.2, 28667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng, W. C. et al. Application of impulse oscillometry to detect interstitial lung disease and airway disease in adults with rheumatoid arthritis. BMC Pulm. Med.23(1), 331 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiyama, A. et al. Characteristics of inspiratory and expiratory reactance in interstitial lung disease. Respir. Med.107(6), 875–882 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Duman, D., Taştı, Ö. F. & Merve, T. F. Assessment of small airway dysfunction by impulse oscillometry (IOS) in COPD and IPF patients. Eur. Rev. Med. Pharmacol. Sci.27(7), 3033–3044 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets can be made accessible if needed by contacting the corresponding author. The content of the manuscript was presented as a poster at European Respiratory Society International Congress in September 2023, in Milan. The title of the poster was “Forced oscillation technique in progressive pulmonary fibrosis”.




