Abstract
The efficacy of combined vitamin K2 and D3 therapy on bone fusion outcomes following endoscopic lumbar surgery in osteoporotic patients remains unclear. This prospective study investigated the effects of combined vitamin K2 and D3 supplementation on fusion outcomes in osteoporotic patients undergoing endoscopic lumbar interbody fusion. Seventy-one patients were divided into two groups: the experimental group (n = 36) received vitamin K2 (45 mg/day), vitamin D3 (250 IU/day), and calcium (1.2 g/day), while the control group (n = 35) received only vitamin D3 (250 IU/day) and calcium (1.2 g/day) for 6 months postoperatively. The primary outcome was fusion rate assessed by CT and dynamic radiography. At 6 months postoperatively, the VK2 + VD3 group showed significantly higher complete fusion rates compared to the control group (91.67% vs. 74.29%, P = 0.044). Serum P1NP levels were significantly higher in the VK2 + VD3 group at 3 months postoperatively (P = 0.001). Both groups showed comparable improvements in clinical outcomes (JOA-BPEQ and ODI scores). While BMD changes were not statistically significant between groups, the VK2 + VD3 group showed a trend toward BMD improvement. These findings suggest that combined vitamin K2 and D3 supplementation may enhance early fusion outcomes in osteoporotic patients undergoing endoscopic lumbar interbody fusion, potentially offering a simple and effective adjunct therapy for improving surgical outcomes.
Keywords: Vitamin K2, Vitamin D3, Osteoporosis, Endoscopic lumbar interbody fusion, Spine surgery
Subject terms: Orthopaedics, Fracture repair, Quality of life
Introduction
As life expectancy increases and birth rates decline, global population aging becomes more severe, leading to an increasing prevalence of primary osteoporosis1. According to statistics, in China, the prevalence of osteoporosis among individuals aged 65 years and older is as high as 32.0%, and the proportion of low bone mass among those aged 50 years and older is 46.4%2. Consequently, the proportion of elderly patients with low bone mass or osteoporosis among those with lumbar degenerative diseases (e.g., lumbar disc herniation, spinal stenosis, and spondylolisthesis) is also increasing. Currently, spinal fusion surgery is an effective method for treating severe lumbar degenerative diseases, as it can relieve spinal cord or nerve root compression, alleviate clinical symptoms, and restore spinal stability through intervertebral fusion3–5. In recent years, endoscopic lumbar interbody fusion (ELIF) has gradually replaced traditional open fusion surgery due to its advantages of minimal invasiveness, faster recovery, and fewer complications6,7. However, in patients with osteoporosis, the significant reduction in bone quality and density poses numerous challenges to endoscopic intervertebral fusion, including low fusion rates, bone graft collapse, loosening of internal fixation, and pseudoarthrosis formation8. These issues not only affect the long-term surgical outcomes but also increase the need for reoperation. Therefore, improving the bone fusion rate in osteoporotic patients following endoscopic lumbar interbody fusion has become an important topic in clinical research.
Vitamins K2 and D3 play crucial roles in bone metabolism. Vitamin K2, particularly its subtype MK-7, acts as a cofactor for γ-glutamyl carboxylase. This enzyme is involved in the γ-carboxylation of bone matrix proteins, such as osteocalcin. This process enhances the calcium-binding capacity of these proteins, promoting calcium deposition in bones and thereby increasing bone density9. In the body, vitamin D3 is converted to its active form, 1,25-dihydroxyvitamin D3, which promotes the absorption of calcium and phosphorus. This maintains blood calcium levels and supports bone mineralization10. Studies have found that combining vitamins K2 and D3 in the treatment of osteoporosis may have additive or synergistic effects11,12. Moreover, this combination is cost-effective and could serve as a simple and effective adjunct therapy to improve surgical outcomes and patient prognosis.
Currently, systematic clinical research on the impact of combined vitamin K2 and D3 treatment on spinal fusion outcomes following endoscopic lumbar surgery is lacking. Therefore, this study aims to evaluate the effects of combined vitamin K2 and D3 treatment on fusion rate, bone metabolism, bone density, and clinical symptoms in osteoporotic patients with lumbar degenerative disease undergoing endoscopic interbody fusion. This study seeks to provide a new adjunct therapeutic strategy for spinal endoscopic surgery.
Materials and methods
Ethical statement
This prospective, non-randomized controlled clinical trial was conducted. The protocol was approved by the Medical Ethics Committee of Shandong Wendeng Orthopedic Hospital (Approval Number: LL20210902) and registered with the Chinese Clinical Trial Registry on 16/01/2025 (Registration Number: ChiCTR2500096051). All patients provided written informed consent. We confirm that all methods were conducted in accordance with relevant guidelines and regulations.
Patient selection and study measures
Between October 2021 and October 2023, patients were recruited from the Department of Minimally Invasive Spine Surgery at Shandong Wendeng Orthopedic Hospital. After strict screening based on inclusion and exclusion criteria, 77 patients were enrolled. Patients were non-randomly allocated to two groups based on patient preference, economic factors (as vitamin K2 was not covered by insurance), and clinical judgment considering factors such as T-score severity, patient-reported pain and disability (JOA-BPEQ and ODI scores), and the degree of nerve compression observed on imaging. During the follow-up period, six participants dropped out. Five patients refused outpatient examinations and could not be followed up, and one patient in the control group withdrew due to an acute cerebral hemorrhage three months after surgery. Ultimately, the experimental group (VK2 + VD3) included 36 patients who received vitamin K2 (45 mg/day), chosen based on prior studies demonstrating efficacy in osteoporosis without adverse effects, along with vitamin D3 (250 IU/day) and calcium (1.2 g/day). The control group included 35 patients who received vitamin D3 (250 IU/day) and calcium (1.2 g/day) as basic treatment. All medications were administered starting 1 week after surgery and continued for six months. Patients were advised to maintain usual diet and sunlight exposure, though these were not strictly controlled. All enrolled patients underwent single-channel endoscopic lumbar interbody fusion with percutaneous pedicle screw fixation, performed by the same experienced deputy chief physician. A single PEEK cage and autologous laminectomy bone were used for intervertebral fusion.No other bone graft substitute were applied.
Inclusion criteria: (1) Patients aged between 50 and 80 years; (2) Patients diagnosed with lumbar spinal stenosis, degenerative spondylolisthesis, or isthmic spondylolisthesis based on clinical symptoms and imaging findings from MRI, CT, and X-ray examinations; (3) Patients confirmed to have osteoporosis (T-score ≤ − 2.5) via dual-energy X-ray absorptiometry (DXA), based on relative guidelines; (4) Patients in whom conservative treatment was ineffective and who require single-channel endoscopic lumbar interbody fusion with percutaneous pedicle screw fixation.
Exclusion criteria: (1) Patients with a history of previous lumbar surgery or requiring multi-level lumbar fusion; (2) Patients who have been using medications that affect bone metabolism long-term (e.g., bisphosphonates, PTH analogs); (3) Patients who are allergic or intolerant to vitamin K2 or D3; (4) Patients currently undergoing warfarin treatment were excluded due to the potential interaction between warfarin and vitamin K2, which could affect the anticoagulant effect of warfarin and increase the risk of bleeding or thromboembolic events; (5) Patients with severe cardiovascular or pulmonary diseases that preclude surgical tolerance.exhibited secondary osteoporosis due to metabolic disorders, chronic corticosteroid use, or other underlying conditions.
Evaluation of intervertebral fusion
At 3 and 6 months postoperatively, osseous union was assessed by three independent, blinded physicians using dynamic radiography and three-dimensional computed tomography (CT) scans. In case of disagreement, a consensus was reached through discussion. Fusion grading was performed according to the system of Bridwell et al.13. Bone formation was categorized into three grades: Grade I, bridging bone bonding with both adjacent vertebral bodies (1 point); Grade II, bridging bone bonding with either the superior or inferior vertebral body (2 points); and Grade III, incomplete bony bridging (3 points). Osseous fusion was evaluated using two CT slices: the central slices of the cage in both coronal and sagittal views. Complete intervertebral osseous fusion was defined as segmental angular motion less than 5° on dynamic X-ray images and the presence of complete superior and inferior fusions. Based on our previous study, both coronal and sagittal CT slices in the central cage region were evaluated as Grade I, each receiving a score of 1 (for a total score of 2). Cases with incomplete intervertebral osseous union were given a total score ranging from 3 to 6.
Evaluation of clinical outcomes
Clinical and neurological symptoms were examined using the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOA-BPEQ)14 and the Oswestry Disability Index (ODI)15 before surgery and at 3 days, 3 months, and 6 months postoperatively. The JOA-BPEQ is composed of several parameters—subjective symptoms, clinical signs, daily activity limitations, and bladder function—scored from 0 (worst) to 29 (best) points.
Assessment of osteoporosis
To evaluate bone metabolism, laboratory tests measured the serum concentrations of procollagen type I N-terminal propeptide (P1NP) and C-terminal telopeptide (β-CROSS) in non-fasting states at baseline (preoperatively) and at 3 months postoperatively. P1NP and β-CROSS serve as biomarkers for bone formation and bone resorption, respectively16. Femoral neck bone mineral density (BMD) was measured using dual-energy X-ray absorptiometry (DXA) before surgery and at 6 months postoperatively.
Adverse events
Safety was investigated by the reporting of adverse events. Any reports of serious adverse events required patients to be withdrawn from the study.
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 20.0, IBM SPSS Inc., Chicago, USA). A sample size of 70 patients (35 per group) was estimated to detect a 20% difference in fusion rates with 80% power and α = 0.05, based on previous studies. Quantitative data were presented as mean ± standard deviation and compared using the t-test for inter-group differences and one-way ANOVA for time-point comparisons within groups. Categorical data were compared using the Chi-square test. A p-value less than 0.05 was considered statistically significant.
Results
Seventy-seven participants were screened, with 71 completing the six-month follow-up (36 in the VK2 + VD3 group and 35 in the control group). There were no significant differences between the two groups in terms of age, sex, BMI, disease type, or surgical segment (Table 1). No postoperative complications related to lumbar interbody fusion were reported in either group, including pseudoarthrosis, cage migration, or pedicle screw fracture or loosening.
Table 1.
Baseline characteristics of the patients.
| Variables | VK2 + VD3 Group (n = 36) | Control Group (n = 35) | P value |
|---|---|---|---|
| Age (years) | 54.7 ± 8.2(50–72) | 55.2 ± 9.4(50–74) | 0.812 |
| Male/female | 11/25 | 12/23 | 0.737 |
| BMI(kg/m2) | 25.37 ± 4.19(17.0–33.8) | 26.04 ± 4.52(17.1–35.1) | 0.519 |
| Disease | 0.146 | ||
| Lumbar spinal stenosis without spondylolisthesis | 20 | 25 | |
| Degenerative spondylolisthesis | 7 | 4 | |
| Isthmic spondylolisthesis | 9 | 6 | |
| surgical segment | 0.752 | ||
| L3-4 | 2 | 1 | |
| L4-5 | 21 | 23 | |
| L5/S1 | 13 | 11 | |
| Femoral BMD(T-score) | |||
| Femoral neck | − 2.58 ± 0.83 | − 2.56 ± 0.88 | 0.919 |
| Total proxima | − 2.18 ± 1.01 | − 2.09 ± 0.96 | 0.731 |
Analysis of fusion rate
Intervertebral fusion rates differed significantly between the VK2 + VD3 group and the control group (Table 2). At 3 months postoperatively, complete fusion was achieved in 31 patients (86.11%) in the VK2 + VD3 group, compared to 23 patients (65.71%) in the control group. At 6 months postoperatively, complete fusion was achieved in 33 patients (91.67%) in the VK2 + VD3 group, while 26 patients (74.29%) in the control group achieved complete fusion. At both 3 and 6 months postoperatively, the VK2 + VD3 group had significantly higher complete fusion rates compared to the control group (P = 0.012 and P = 0.044, respectively). Representative cases from both groups are shown in Fig. 1A–H.
Table 2.
Intervertebral Fusion Rates at 3 and 6 Months Postoperatively.
| Classification (3/6 Months Postop) | VK2 + VD3 Group (n = 36) | Control Group (n = 35) | P value (3 months) | P value (6 months) |
|---|---|---|---|---|
| Complete fusion, N (%) | 31(86.11%)/33(91.67%) | 23(65.71%)/26(74.29%) | 0.012 | 0.044 |
| Incomplete fusion, N (%) | 5(13.89%)/3(8.33%) | 12(34.29%)/9(25.71%) |
Fig. 1.
Representative Cases Based on 3D CT Assessment. (A–D) A 68-year-old female patient from the VK2 + VD3 group with L4 spondylolisthesis, presenting with right lower limb pain and low back pain. (A and B) 3 months postoperatively; (C and D) 6 months postoperatively. (E–H) A 70-year-old female patient from the control group with L4-L5 spinal stenosis, presenting with bilateral lower limb stiffness and low back pain. (E and F) 3 months postoperatively; (G and H) 6 months postoperatively.
Analysis of clinical outcomes
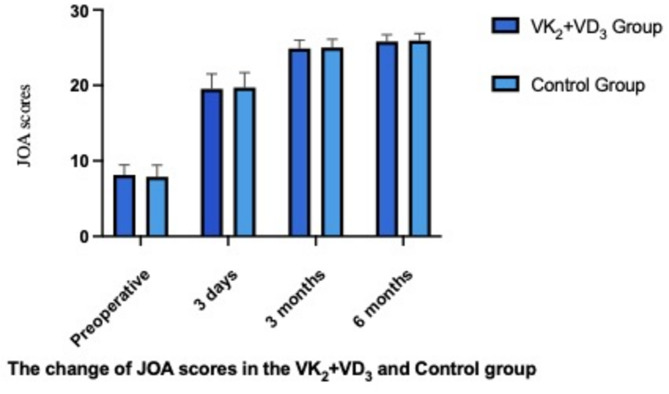
Compared to the preoperative status, JOA-BPEQ and ODI scores showed significant improvement at each postoperative time point in both groups (P < 0.05). No significant differences were observed between the two groups at any time point postoperatively (P > 0.05). See Table 3, Figs. 2 and 3 for further details.
Table 3.
Clinical outcomes (JOA-BPEQ and ODI Scores) before and after surgery.
| Time | VK2 + VD3 group (n = 36) | Control group (n = 35) | P value |
|---|---|---|---|
| JOA-BPEQ | |||
| Preoperative | 8.13 ± 1.32 | 7.89 ± 1.54 | 0.507 |
| 3 days postoperative | 19.54 ± 1.96* | 19.72 ± 1.98* | 0.732 |
| 3 months postoperative | 24.89 ± 1.11* | 25.01 ± 1.09* | 0.611 |
| 6 months postoperative | 25.82 ± 0.88* | 25.94 ± 0.92* | 0.610 |
| ODI | |||
| Preoperative | 45.60 ± 11.15 | 46.01 ± 11.53 | 0.894 |
| 3 days postoperative | 14.71 ± 4.38* | 15.02 ± 4.67* | 0.755 |
| 3 months postoperative | 10.93 ± 3.82* | 11.20 ± 3.93* | 0.726 |
| 6 months postoperative | 4.88 ± 2.91* | 4.91 ± 2.98* | 0.964 |
*P < 0.05, comparison of indicators at each postoperative time point versus preoperative scores.
Fig. 2.
Histograms of JOA-BPEQ Scores (P > 0.05, comparison between the two groups).
Fig. 3.
Histograms of ODI Scores (P > 0.05, comparison between the two groups).
Analysis of bone density and bone metabolism markers
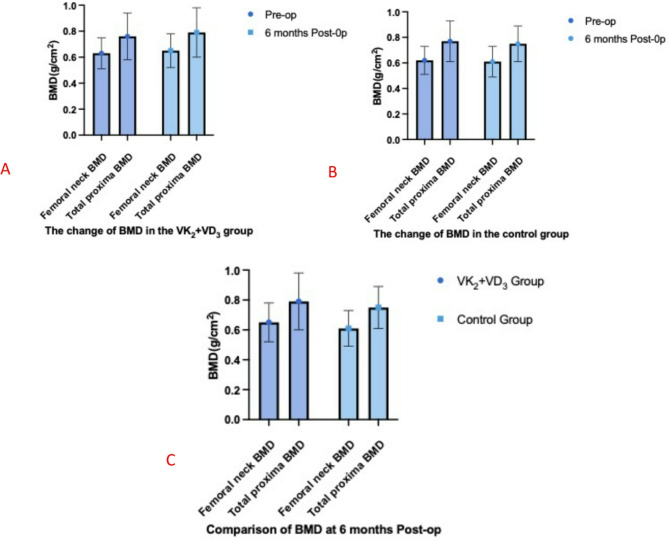
The changes in BMD are shown in Table 4 and Fig. 4A–C. Preoperatively, there were no significant differences in BMD at the femoral neck and total hip between the two groups (P = 0.684 and 0.894, respectively). At 6 months postoperatively, femoral neck BMD increased by 3.17% in the VK2 + VD3 group (P = 0.278), while it decreased by 1.61% in the control group (P = 0.604). Total hip BMD increased by 3.95% in the VK2 + VD3 group (P = 0.174), while it decreased by 2.62% in the control group (P = 0.479). There were no significant differences in femoral neck and total hip BMD between the preoperative and 6-month postoperative values within each group, nor were there significant differences between the two groups at 6 months postoperatively (P = 0.170 and 0.130, respectively).
Table 4.
Comparison of BMD and bone metabolism markers between the two groups.
| Time | VK2 + VD3 Group (n = 36) | Control Group (n = 35) | P value |
|---|---|---|---|
| Femoral neck BMD (g/cm2) | |||
| Preoperative | 0.63 ± 0.12 | 0.62 ± 0.11 | 0.684 |
| 6 months postoperative | 0.65 ± 0.13 | 0.61 ± 0.12 | 0.170 |
| Total proxima BMD (g/cm2) | |||
| Preoperative | 0.76 ± 0.18 | 0.77 ± 0.16 | 0.894 |
| 6 months postoperative | 0.79 ± 0.19 | 0.75 ± 0.14 | 0.130 |
| Serum P1NP(ug/L) | |||
| Preoperative | 48.12 ± 19.72 | 47.73 ± 18.26 | 0.931 |
| 3 months postoperative | 94.32 ± 38.26 | 67.91 ± 36.56 | 0.001 |
| Serum β-CROSS (mU/dL) | |||
| Preoperative | 0.64 ± 0.18 | 0.63 ± 0.27 | 0.831 |
| 3 months postoperative | 0.62 ± 0.21 | 0.59 ± 0.24 | 0.500 |
Fig. 4.
Changes in Femoral Neck and Total Hip BMD Between the Two Groups. (A) Changes in BMD in the VK2 + VD3 group. (B) Changes in BMD in the Control Group. (C) Comparison of BMD at 6 Months Postoperatively Between the Two Groups.
The changes in bone metabolism markers are shown in Table 4 and Fig. 5A,B. Preoperatively, there were no significant differences in serum P1NP and β-CROSS levels between the two groups (P = 0.931 and 0.831, respectively). However, at 3 months postoperatively, serum P1NP levels in the VK2 + VD3 group significantly increased compared to the control group (P = 0.010). Serum β-CROSS levels decreased slightly in both groups, but the difference was not statistically significant (P = 0.500).
Fig. 5.
Changes in P1NP and β-CROSS Between the Two Groups. (A) Comparison of P1NP at 3 Months Postoperatively Between the Two Groups. (B) Comparison of β-CROSS at 3 Months Postoperatively Between the Two Groups.
Discussion
In this prospective clinical trial, we investigated the effects of combined vitamin K2 and D3 treatment on bone fusion rate, bone density, bone metabolism, and clinical outcomes in patients with osteoporosis undergoing endoscopic lumbar interbody fusion (ELIF). The results demonstrate that, compared with the control group, the VK2 + VD3 group exhibited significantly higher fusion rates and more favorable changes in bone metabolism markers. These findings provide new insights into adjunctive treatments for spinal endoscopic fusion in patients with osteoporosis.
The primary findings of this study are that the VK2 + VD3 combined treatment group exhibited significantly higher intervertebral fusion rates at 3 and 6 months postoperatively. Specifically, the fusion rates in the VK2 + VD3 group were 86.11% and 91.67% at 3 and 6 months, respectively, compared to 65.71% and 74.29% in the control group (P = 0.012 and 0.044, respectively). The fusion rates observed in our study, particularly in the VK2 + VD3 group, are higher than those reported in some previous studies17,18. This difference may be attributed to the inherent advantages of the endoscopic lumbar interbody fusion (ELIF) approach. Compared to traditional open surgery, ELIF is characterized by significantly less soft tissue trauma, reduced blood loss, faster patient recovery, and potentially more thorough endplate preparation. This meticulous endplate preparation, which is crucial for successful fusion, may create a more favorable environment for bone graft integration and new bone formation. In addition to the benefits conferred by the ELIF technique, the improved fusion rates can also be attributed to the synergistic effects of vitamins K2 and D3 on bone metabolism. Vitamin K2, as a cofactor for γ-glutamyl carboxylase, enhances the γ-carboxylation activity of bone matrix proteins such as osteocalcin, increasing their ability to bind calcium ions and promoting the deposition of calcium into the bone matrix with hydroxyapatite crystals19. Meanwhile, vitamin D3 upregulates the expression of calcium-binding proteins and osteogenic-related transcription factors, creating an optimal environment for bone mineralization20. Together, these vitamins act on multiple targets in the bone remodeling process, enhancing the formation and quality of new bone. Additionally, vitamins K2 and D3 may modulate the inflammatory microenvironment of bone tissue, reducing the production of inflammatory mediators, promoting tissue repair, and accelerating postoperative healing, thereby reducing the risk of complications such as pseudoarthrosis and loosening of internal fixation21.
Zhang et al.22 evaluated the effects of combined vitamin K2 and D3 treatment on early fusion rates in patients with reduced bone mass or osteoporosis undergoing transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF). The study included 78 patients, and the results showed that at 6 months postoperatively, the complete fusion rate in the VK2 + VD3 group was significantly higher than in the control group (91.18% vs. 71.43%, P = 0.036). Koshihara et al.23 confirmed in an animal model that vitamin K2 supplementation can enhance bone mineralization, and our study further provides clinical evidence of its synergistic effects in osteoporotic patients undergoing fusion surgery. In contrast, the control group, which received only vitamin D3 and calcium as basic treatment, showed some improvement in fusion rates but did not reach the level of the combined treatment group. This is consistent with the findings of Ravindra et al.24, who reported that the use of vitamin D3 alone has limited effects on bone metabolism, while the combination with vitamin K2 may have a stronger synergistic effect. Some studies, however, have reported less conclusive results regarding the combined effects of vitamin K2 and D3. For example, Yonemura et al.25 observed no significant additional benefit from combining VK2 with VD3 in preventing prednisolone-induced BMD loss. These discrepancies may be attributed to variations in study populations, dosages, treatment durations, outcome measures, or clinical settings. Therefore, while our findings, along with several others, suggest that combined vitamin K2 and D3 supplementation plays a positive role in enhancing early fusion outcomes after ELIF, this study contributes to the growing body of evidence supporting this approach; however, further research is necessary to resolve remaining uncertainties in the literature.
In terms of bone metabolism, the VK2 + VD3 group showed a significant increase in serum procollagen type I N-terminal propeptide (P1NP) levels at 3 months postoperatively (P = 0.01), indicating accelerated bone formation. Although the levels of C-terminal telopeptide of type I collagen β-isomer (β-CROSS) decreased in both groups, the difference between the groups was not statistically significant (P = 0.500). These results suggest that, during the early postoperative period, the combined treatment primarily exerts its effects by promoting bone formation rather than significantly inhibiting bone resorption. This likely reflects an initial anabolic effect of the combined vitamin K2 and D3 therapy, potentially shifting the bone remodeling balance towards net bone formation. However, it is important to note that bone remodeling is a complex process, and while our findings suggest a predominantly anabolic effect at 3 months, longer-term studies are needed to fully elucidate the sustained effects of the combined treatment on bone turnover balance, including both formation and resorption. This is consistent with the known mechanisms of vitamins K2 and D3 in promoting bone matrix synthesis and mineralization26. Previous studies have established P1NP and β-CROSS as reliable markers of bone turnover, and the observed changes in these markers further support the effectiveness of the combined vitamin K2 and D3 treatment in enhancing bone metabolism, particularly in patients with osteoporosis27.
Despite the lack of statistically significant differences in BMD between the two groups, the VK2 + VD3 group showed a numerical increase in femoral neck and total hip BMD at 6 months postoperatively. Specifically, femoral neck BMD increased by 3.17% in the VK2 + VD3 group compared to 1.02% in the control group. Total hip BMD increased by 3.95% in the VK2 + VD3 group compared to 1.56% in the control group. The discrepancy between the improved fusion rates and the lack of significant BMD changes can be attributed to several factors. First, spinal fusion represents a localized bone healing process occurring specifically at the intervertebral space, while BMD measurements via DXA reflect systemic, whole-body bone density. The combined vitamin K2 and D3 treatment may exert a more pronounced effect on the local microenvironment at the fusion site, promoting osteoblast activity and new bone formation within the cage and in the immediately surrounding bone. Second, as changes in BMD typically require a longer duration to become clinically evident28, the 6-month follow-up period of this study may be insufficient to detect significant differences. It is possible that with longer observation, differences in BMD might emerge. However, the observed numerical increases are consistent with the improvements in bone metabolism markers, suggesting that the combined treatment may have long-term benefits for bone density. This warrants further investigation in studies with extended follow-up periods.
In terms of clinical outcomes, both the VK2 + VD3 and control groups demonstrated significant improvements in JOA-BPEQ and ODI scores as early as 3 days postoperatively. Although substantial functional recovery is unlikely within this short timeframe after lumbar fusion surgery, these early improvements likely reflect the immediate effects of nerve decompression. The primary goal of the surgery is to alleviate pressure on the spinal cord and nerve roots, which can result in rapid pain reduction and improved neurological symptoms. Consequently, the observed changes at 3 days likely represent this immediate post-decompression effect, rather than significant structural changes associated with the fusion process. Despite these early improvements, no significant differences in JOA-BPEQ and ODI scores were observed between the groups at any time point. Although the VK2 + VD3 group exhibited advantages in bone metabolism and fusion rates, these benefits may not have fully translated into clinical score differences within the 6-month follow-up period. Bone fusion is a gradual process, and complete consolidation and remodeling may require longer than 6 months to fully impact functional outcomes. Previous studies have shown that improved fusion quality and bone metabolism can lead to better long-term functional recovery29, suggesting the need for extended follow-up to evaluate the potential clinical advantages of combined vitamin K2 and D3 treatment in this context. Furthermore, no serious complications, including pseudoarthrosis, cage migration, or pedicle screw fracture or loosening, were observed in either group during the postoperative period, suggesting the combined treatment strategy’s safety.
The findings of this study have significant clinical implications for patients with osteoporosis, particularly in the context of endoscopic lumbar interbody fusion (ELIF). ELIF, with its advantages of minimal invasiveness, rapid recovery, and fewer complications, is increasingly becoming the primary treatment modality for lumbar degenerative diseases30. However, the low bone density and fragility in osteoporotic patients often result in lower fusion rates, limiting the widespread application of ELIF in this population31. The combined treatment of vitamins K2 and D3, by significantly improving bone fusion rates and bone metabolism, provides strong support for enhancing the surgical outcomes in these patients. This not only enhances the efficacy and safety of ELIF in osteoporotic patients but also promotes its broader application in this high-risk population. Moreover, vitamins K2 and D3 are cost-effective, safe, and well-tolerated by patients. Compared with other expensive anti-osteoporosis medications such as bisphosphonates or biologics, the combined treatment offers significant economic advantages32. This makes it a more accessible and practical option for improving the surgical outcomes and overall quality of life in patients with osteoporosis33.
Despite the positive clinical and metabolic outcomes, several limitations warrant consideration. The non-randomized, single-center design introduces a risk of selection bias. Patients were allocated based on baseline condition, preference, and economic factors, potentially leading to unmeasured differences between groups that could overestimate the treatment effect. The small sample size (n = 71) limits statistical power and generalizability, increasing the risk of Type II error. The 6-month follow-up, while adequate for early fusion assessment, is insufficient for long-term evaluation of BMD, fracture risk, or clinical outcomes, as bone remodeling is a slow process. Uncontrolled factors like diet and sun exposure could also confound the results.Furthermore, we did not systematically differentiate between primary and secondary osteoporosis, which could influence the response to vitamin K2 and D3 supplementation. Finally, the applicability to patients with other systemic diseases or more severe osteoporosis requires further investigation. Future research should address these limitations through larger, multi-center, randomized controlled trials with longer follow-up and rigorous control of confounding variables. Additionally, investigating the potential synergistic effects of combining vitamin K2 and D3 with other anti-osteoporotic medications, such as teriparatide, bisphosphonates, denosumab, or romosozumab, could be particularly beneficial, especially in patients with greater bone remodeling potential.
Conclusion
Combined vitamin K2 and D3 treatment significantly improved early bone fusion rates in osteoporotic patients undergoing endoscopic lumbar interbody fusion for lumbar degenerative diseases. This treatment also positively affected bone formation markers, although its long-term effects on bone mineral density remain to be determined. While appearing economical and potentially effective as an adjunctive therapy, further research, specifically randomized controlled trials, is crucial to confirm its long-term efficacy, safety, and optimal application in the perioperative management of osteoporotic patients.
Author contributions
Conceived and designed the study: S.F.Collected data: F.W.,Y.D.and X.H. Analyzed the data: F.W.,R.S. and Z.W. Wrote the paper: Y.W.All authors reviewed the manuscript.
Funding
This study was funded by the Joint TCM Science & Technology Projects of National Demonstration Zones for Comprehensive TCM Reform (GZY-KJS-SD-2023-031).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vos, T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet380, 2163–2196 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Health Commission of the People’s Republic of China. National Health and Health Committee’s text on the media communication meeting on October 19, 2018. http://www.nhc.gov.cn/wjw/xwdt/201810/d816a5c72f6b45e399a1e7214642cd47.shtml (Accessed 11 May 2021).
- 3.Deyo, R. A., Gray, D. T., Kreuter, W. & Collins, R. N, United States trends in lumbar fusion surgery for degenerative conditions. Spine30, 1441–1445 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Katz, J. N. Lumbar spinal fusion: Surgical rates, costs, and complications. Spine20(Suppl.), 84S (1995). [PubMed] [Google Scholar]
- 5.Soldozy, S. et al. Diagnostic, surgical, and technical considerations for lumbar interbody fusion in patients with osteopenia and osteoporosis: A systematic review. Brain Sci.11, 241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng, B. et al. Efficacy and safety of unilateral biportal endoscopy versus other spine surgery: A systematic review and meta-analysis. Front. Surg.9, 911914 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim, J. E. et al. Comparison of minimal invasive versus biportal endoscopic transforaminal lumbar interbody fusion for single-level lumbar disease. Clin. Spine Surg.34, E64–E71 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebata, S. et al. Role of weekly teriparatide administration in osseous union enhancement within six months after posterior or transforaminal lumbar interbody fusion for osteoporosis-associated lumbar degenerative disorders: A multicenter, prospective randomized study. JBJS99, 365–372 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Li, W. et al. Vitamin K2 stimulates MC3T3-E1 osteoblast differentiation and mineralization through autophagy induction. Mol. Med. Rep.19, 3676–3684 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazemian, E. et al. Effect of supplemental vitamin D3 on bone mineral density: A systematic review and meta-analysis. Nutr. Rev.81, 511–530 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Skalny, A. V., Aschner, M., Tsatsakis, A. & Rink, L. Role of vitamins beyond vitamin D3 in bone health and osteoporosis. Int. J. Mol. Med.53, 9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuang, X. et al. The combination effect of vitamin K and vitamin D on human bone quality: A meta-analysis of randomized controlled trials. Food Funct.11, 3280–3297 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Bridwell, K. H. et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects?. Spine20, 1410–1418 (1995). [PubMed] [Google Scholar]
- 14.Ohtori, S. et al. Evaluation of low back pain using the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire for lumbar spinal disease in a multicenter study: Differences in scores based on age, sex, and type of disease. J. Orthop. Sci.15, 86–91 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Fairbank, J. C., Couper, J., Davies, J. B. & O’Brien, J. P. The Oswestry low back pain disability questionnaire. Physiotherapy66, 271–273 (1980). [PubMed] [Google Scholar]
- 16.Nakamura, T. et al. Randomized teriparatide [human parathyroid hormone (PTH) 1–34] once-weekly efficacy research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J. Clin. Endocrinol. Metab.97, 3097–3106 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Huang, X. et al. Comparison of surgical invasiveness, hidden blood loss, and clinical outcome between unilateral biportal endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative disease: A retrospective cohort study. BMC Musculoskelet. Disord.24, 274 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.León, J. F. R. et al. Standalone lordotic endoscopic wedge lumbar interbody fusion (LEW-LIF™) with a threaded cylindrical peek cage: Report of two cases. J. Spine Surg.6(Suppl 1), S275–S282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myneni, V. D. & Mezey, E. Regulation of bone remodeling by vitamin K2. Oral Dis.23, 1021–1028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uenishi, K. et al. Stimulation of intestinal calcium absorption by orally administrated vitamin D3 compounds: A prospective open-label randomized trial in osteoporosis. Osteoporos. Int.29, 723–732 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalooeifard, R. et al. The effect of vitamin D deficiency on outcomes of patients undergoing elective spinal fusion surgery: A systematic review and meta-analysis. Int. J. Spine Surg.16, 53–60 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, W. et al. Concurrent treatment with vitamin K2 and D3 on spine fusion in patients with osteoporosis-associated lumbar degenerative disorders. Spine47, 352–360 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Koshihara, Y. et al. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J. Endocrinol.176, 339–348 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Ravindra, V. M. et al. Vitamin D levels and 1-year fusion outcomes in elective spine surgery: A prospective observational study. Spine40, 1536–1541 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Yonemura, K., Fukasawa, H., Fujigaki, Y. & Hishida, A. Protective effect of vitamins K2 and D3 on prednisolone-induced loss of bone mineral density in the lumbar spine. Am. J. Kidney Dis.43, 53–60 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Liu, J. K. & Chen, L. M. Mechanisms of vitamins K2 and D3 in bone mineralization. Nutr. Biochem.65, 107532 (2019). [Google Scholar]
- 27.Chen, X. Y. & Zhang, Y. Z. P1NP and β-CROSS as markers for bone turnover in osteoporosis. Clin. Chem. Lab. Med.59, 150–158 (2021). [Google Scholar]
- 28.Ushirozako, H. et al. Weekly teriparatide administration and preoperative anterior slippage of the cranial vertebra next to fusion segment < 2 mm promote osseous union after posterior lumbar interbody fusion. Spine44, E288–E297 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Lee, S. H. et al. Enhanced spinal fusion quality leads to improved long-term outcomes in osteoporotic patients. J. Orthop. Surg.28, 2309499020931543 (2020). [Google Scholar]
- 30.Smith, R., Johnson, M. & Brown, J. Endoscopic lumbar interbody fusion: A review of its clinical outcomes and advantages. J. Neurosurg. Spine29, 289–298 (2018). [Google Scholar]
- 31.Johnson, M., Smith, R. & Brown, J. Low bone density and fusion rates in osteoporotic patients undergoing lumbar interbody fusion. Spine44, 821–828 (2019). [Google Scholar]
- 32.Brown, J., Smith, R. & Johnson, L. Economic evaluation of vitamin K2 and D3 supplementation in osteoporosis management. J. Bone Miner. Res.34, 891–902 (2019). [Google Scholar]
- 33.Chen, Y., Wang, X. & Li, H. Cost-effectiveness of vitamin K2 and D3 in osteoporosis: A systematic review. Osteoporos. Int.32, 1871–1882 (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.