Abstract
Acute myeloid leukemia (AML) with KMT2A rearrangement (KMT2A-r) is associated with poor prognosis, but the benefit of allogeneic hematopoietic stem cell transplantation (allo-HSCT) for KMT2A-r AML is unclear. We reviewed adult AML patients treated within the TROPHY group and identified 292 cases of KMT2A-r AML, 254 (87.0%) of whom achieved first complete remission (CR1) and 192 (75.6%) of CR1 patients underwent allo-HSCT. We show that allo-HSCT is an independent favorable prognostic factor in CR1 patients for both overall survival (OS) (hazard ratio [HR] = 0.56, 95% confidence interval [CI]: 0.45–0.69, P < 0.001) and cumulative incidence of relapse (CIR) (HR = 0.01, 95% CI: 0.005–0.04, P < 0.001). Among allo-HSCT recipients, survival outcomes were comparable between patients with KMT2A::MLLT3 and those with other 11q23/KMT2A rearrangements (3-year OS: 74.3% vs. 77.5%, P = 0.97; 3-year event-free survival [EFS]: 55.2% vs. 62.2%, P = 0.34; 3-year CIR: 24.4% vs. 20.8%, P = 0.32). Both multiparameter flow cytometry-based measurable residual disease (MFC-MRD) and KMT2A-r MRD determined by quantitative PCR prior to allo-HSCT were associated with worse transplant outcomes. Multivariable analysis identified detectable KMT2A-r MRD at allo-HSCT as a significant risk factor for reduced EFS (HR = 2.46, 95% CI: 1.32–4.60, P = 0.005). These findings confirm the survival benefit of allo-HSCT in adult patients with KMT2A-r AML and underscore the prognostic value of KMT2A-r MRD prior to transplantation.
Subject terms: Acute myeloid leukaemia, Bone marrow transplantation
Highlights
Patients with KMT2A-rearranged (KMT2A-r) acute myeloid leukemia (AML) benefit from allogeneic hematopoietic stem cell transplantation (allo-HSCT) in first complete remission (CR1).
While KRAS mutations are novel adverse prognostic factors in KMT2A-r AML, allo-HSCT mitigates their adverse effects on survival.
PCR-based quantification of KMT2A-r measurable residual disease (MRD) prior to allo-HSCT is a superior surrogate for transplant prognosis than multiparameter flow cytometry-based MRD assessment.
Introduction
KMT2A (also known as mixed-lineage leukemia, MLL) rearrangements (KMT2A-r) are among the most frequent chromosomal abnormalities in acute myeloid leukemia (AML), accounting for approximately 3–7% of de novo AML cases in adults [1–10]. More than 130 gene partners have been identified with KMT2A-r in leukemias, and the prognosis of patients with KMT2A-r AML varies depending on the specific KMT2A fusion partner [11]. Consequently, KMT2A-r fusion patterns are currently utilized for risk stratification in AML [12]. Patients with KMT2A::MLLT3 tend to have better outcomes compared to those with other 11q23/KMT2A rearrangements, as demonstrated in several studies of both adult [4, 6, 8] and pediatric AML [13] patients treated with intensive chemotherapy. Meanwhile, data from a large cohort of pediatric AML showed that KMT2A::AF1p was associated with favorable clinical outcomes, while KMT2A::AFDN and KMT2A::AF10 correlated with poorer outcomes, independent of other prognostic factors [14]. As such, the 2022 European LeukemiaNet (ELN) risk stratification system classifies AML patients with KMT2A::MLLT3 into the intermediate-risk group, whereas those with all other balanced 11q23/KMT2A rearrangements are categorized as adverse risk [12].
Most AML patients with KMT2A-r (excluding those with KMT2A::MLLT3) are referred for allogeneic hematopoietic stem cell transplantation (allo-HSCT) after achieving first complete remission (CR1) due to its adverse-risk profile. The role of allo-HSCT in AML with KMT2A-r, however, remains controversial. A retrospective study by the international Berlin-Frankfurt-Munster Study group demonstrated that allo-HSCT in CR1 reduced relapse risk in pediatric AML patients with KMT2A-r [15]. Among 193 pediatric patients transplanted in CR1, the relapse risk was reduced (HR = 0.5, 95% CI: 0.4–0.8, P = 0.00096) in the high-risk group but not in the non-high-risk group. Furthermore, and consistent with previous findings [16, 17], allo-HSCT in CR1 did not improve overall survival (OS). Overall, the limited data on the impact of allo-HSCT in adult AML patients with KMT2A-r means that its benefits are unclear [18].
Measurable residual disease (MRD) is strongly associated with a high risk of relapse and poor outcomes [19, 20]. Multiparameter flow cytometry-based MRD (MFC-MRD) detection after induction therapy serves as a robust predictor of relapse and supports risk-directed post-remission strategies, including allo-HSCT in CR1 [21, 22]. MRD in most AML cases with KMT2A-r can also be evaluated by quantitative PCR, using specific KMT2A fusion genes with a sensitivity of 1×10−5 (KMT2A-r MRD) [12]; however, the consistency between the prognostic value of MFC-MRD and KMT2A-r MRD remains unclear. Nevertheless, Shi et al. reported that achieving MRD negativity in CR1 (defined as MFC-MRD < 1% and KMT2A-r MRD < 0.0001%) before allo-HSCT predicted higher OS, improved relapse-free survival (RFS), and lower cumulative incidence of relapse (CIR) [23]. Mo et al. separately analyzed 177 acute leukemia patients with KMT2A-r and found that KMT2A-r MRD positivity before allo-HSCT correlated with post-HSCT KMT2A positivity [24]. Specifically, the 4-year CIR following allo-HSCT was significantly higher in the pre-HSCT KMT2A-r expression ≥0.1% group (53.7%) compared with the KMT2A-r negative group (15.1%).
In this study, we conducted a retrospective, multicenter analysis of a large cohort of adult AML patients with KMT2A-r to evaluate the role of allo-HSCT, characterize the concurrent genetic mutational landscape, and compare the prognostic significance of MFC-MRD and/or KMT2A-r MRD at the time of allo-HSCT.
Material and methods
Patients
This multicenter retrospective study was collaboratively designed by Wuhan Tongji Hospital, Shanghai Ruijin Hospital, Peking University Institute of Hematology, and Wuhan Union Hospital, China. Consecutive patients diagnosed with AML between January 2017 and October 2023 were screened for eligibility. Patients were included if they were aged 14 years or older and presented with de novo AML and KMT2A-r. Patients were excluded if they were under 14 years of age, did not meet the ELN 2022 classification criteria for AML with KMT2A-r, had therapy-related AML or a history of myelodysplastic syndrome, and/or had incomplete medical records.
A total of 294 patients met the above criteria, and 292 were enrolled in the whole cohort for analysis, with the exception of two patients whose fusion partner could not be identified. An analysis in a subgroup of 254 patients achieved CR1 was performed, including a further evaluation of the 192 patients who underwent allo-HSCT. The final follow-up was conducted on June 30, 2024.
Ethics approval and consent to participate
The protocol was IRB-approved at all participating institutions, including the Institutional Review Board of Tongji hosptial, Tongji Medical College, Huazhong University of Science and Technology (No.TJ-IRB202504003) where the data was collated and analyzed and adhered to the principles of the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent for the use of clinical data was obtained from all patients as part of an ongoing quality improvement program.
Cytogenetic classification and genotyping
De novo AML cases were evaluated by the karyotype (G-, Q-, or R-banding), fluorescence in situ hybridization and reverse transcription PCR results to confirm KMT2A-r [25]. TaqMan-based real-time quantitative PCR (RT-qPCR) was used to test KMT2A::MLLT3 (MLL-AF9), KMT2A::AFDN (MLL-AF6), KMT2A::ELL (MLL-ELL), KMT2A::MLLT10 (MLL-AF10), KMT2A::MLLT1 (MLL-ENL), KMT2A::AFF1 (MLL-AF4), KMT2A::MLLT11 (MLL-AF1q), KMT2A::MLLT6 (MLL-AF17), KMT2A::SEPT6, KMT2A::SEPT9, KMT2A::ARHGEF12, and ABL1 transcript levels. RT-qPCR primers, probe sequences, and exon positions are provided in Supplementary Table S1. KMT2A fusion transcripts were amplified as previously described [26–31]. Sample processing, nucleic acid extraction, and next-generation sequencing (NGS) were performed as previously described [32]. NGS data generated using local custom diagnostic panels were available for 271 patients, representing 92.8% of the entire cohort. The mutational status of 54 protein-coding genes was assessed through targeted amplicon sequencing conducted on an Illumina MiSeq platform (see Supplementary Methods).
Treatment procedures
All patients typically underwent cytarabine/anthracycline-based induction chemotherapy, as per the Chinese Guidelines for the Diagnosis and Treatment of Adult Acute Myeloid Leukemia [33]. After CR, patients with or without allo-HSCT were typically administered an intermediate or high dose of cytarabine, according to MRD status and donor availability. In allo-HSCT, the primary preconditioning regimens included cytarabine, busulfan, cyclophosphamide, semustine, and anti-thymocyte globulin [34, 35]. The protocols for graft-versus-host disease (GVHD) and infection prophylaxis have been previously described [19, 36, 37], with further details provided in the Supplementary Methods. Maintenance therapy with HMA based regimen was considered for patients who were MRD-positive before transplant, had AML with high to very high DRI, and opted for maintenance treatment. Specific maintenance treatment regimens were provided in Supplementary Table S3.
MRD monitoring protocols
MRD, including both MFC-MRD and KMT2A-r MRD, was monitored prior to allo-HSCT, at 1, 2, 3, 4, 5, 6, 9, and 12 months post-allo-HSCT, and then at 6-month intervals thereafter [19, 37]. MFC-MRD was considered present when a cluster of >25 cells with leukemia-associated immunophenotypes (LAIP) and SSC characteristics identified in all plots of interest and carrying at least two LAIP markers identified at diagnosis was observed. When abnormal cells were identified, they were quantified as a percentage of the total CD45+ white cell events. MFC-MRD positivity was defined by leukemia-associated aberrant immunophenotypes and/or deviations from normal phenotypes at a threshold of 0.1% [38]. KMT2A-r MRD positivity was determined as ≥0.001% KMT2A-r mutant copies per ABL1 copies measured in bone marrow (BM) [39].
Data collection
Investigators at each participating hospital accessed institutional electronic medical records and clinical databases to collect data on patient demographics, diagnosis, concurrent gene mutation status, pre-allo-HSCT chemotherapy, transplant regimens, MRD status before and after allo-HSCT, and clinical outcomes. Two experienced physicians independently reviewed all collected data to ensure accuracy and consistency.
Definitions
CR was characterized by <5% BM blasts, no evidence of extramedullary disease, and absence of peripheral blood blasts. Relapse was defined as the presence of ≥5% BM blasts, reappearance of blasts in peripheral blood, development of extramedullary disease, or recurrence of pre-HSCT chromosomal abnormalities after achieving initial morphologic CR. Non-relapse mortality (NRM) referred to deaths that occurred without disease progression or relapse. Pre-transplant MRD positivity was defined as the presence of either MFC-MRD or KMT2A-r MRD before allo-HSCT. Event-free survival (EFS) events were defined as post-transplant MRD positivity, relapse, or death from any cause, whichever occurred first. RFS was defined as the duration of survival with continuous CR. CIR represented the time from CR to the first relapse, with death in CR treated as a competing event. OS events were defined as death from any cause.
Statistical analyses
Data were censored at the time of death or the last available follow-up. The primary outcome was the relapse rate, while secondary outcomes included EFS and OS. Patient characteristics are described as medians and ranges for continuous variables and as frequencies or percentages for categorical variables. Comparisons between groups were conducted using the Kruskal-Wallis test for continuous variables and using the χ² or Fisher’s exact tests for categorical data. The Kaplan-Meier estimator was used to calculate survival probabilities, and the cumulative incidence function was applied to calculate relapse incidence through competing risk analysis. When statistically significant, further pairwise comparisons between each group were conducted and Bonferoni- or BH-adjusted P values were calculated. In allo-HSCT cohort, the median time from diagnosis to transplantation was 5.43 months, with all patients undergoing transplantation within 1 year of diagnosis. To minimize immortal-time bias and ensure adequate sample sizes between groups, we set the landmark time at 6 months after diagnosis for both the transplantation and chemotherapy groups. Additionally, to assess the impact of MRD on OS in the early and late post-transplantation period, we defined an additional landmark time at 1 year after diagnosis. Univariable and multivariable Cox regression analyses were performed to identify potential prognostic factors impacting OS and EFS, while Fine-Gray analyses were used to assess factors influencing CIR. Allo-HSCT was included as a time-dependent covariate in the analyses. Variables with P > 0.1 were sequentially excluded from the model, and a P < 0.05 was considered statistically significant. Statistical analyses were conducted using R software (v4.2.0; https://www.r-project.org) and SPSS 26 (SPSS Inc., IBM, Armonk, NY, USA).
Results
Patient characteristics
The flow chart of the study is presented in Fig. 1. A total of 292 adult AML patients with KMT2A-r initially enrolled. Table 1 presents the clinical characteristics of these 292 patients and stratified by different KMT2A-r subtypes. The most common genetic rearrangement was KMT2A::MLLT3 (n = 96, 32.9%), followed by KMT2A::AFDN (n = 69, 23.6%), KMT2A::ELL (n = 61, 20.9%), KMT2A::MLLT10 (n = 34, 11.6%), and KMT2A::MLLT1 (n = 13, 4.5%). Other types of KMT2A-r, each observed in fewer than 10 patients, were categorized into the ‘other KMT2A-r’ group (n = 19, 6.5%). The frequency distribution of the KMT2A-r subtypes is depicted in Supplementary Figure S1.
Fig. 1. Flow diagram of patient enrollment.
Allo-HSCT, allogeneic hematopoietic stem cell transplantation. CR1, first complete remission.
Table 1.
Basic clinical characteristics and clinical outcomes of the whole cohort.
| Characteristic | Whole cohort n = 292 | KMT2A::MLLT3 n = 96 | KMT2A::AFDN n = 69 | KMT2A::ELL n = 61 | KMT2A::MLLT10 n = 34 | KMT2A::MLLT1 n = 13 | Other KMT2A-r n = 19 | P value |
|---|---|---|---|---|---|---|---|---|
| Age, years (range) | 40.0 (15.0–72.0) | 37.5 (15.0–68.0) | 41.0 (15.0–70.0) | 42.0 (15.0–69.0) | 38.5 (16.0–72.0) | 46.0 (17.0–65.0) | 41.0 (28.0–67.0) | 0.706 |
| Age group, n (%) | ||||||||
| Younger (<60 y) | 261 (89.4) | 86 (89.6) | 62 (89.9) | 55 (90.2) | 31 (91.2) | 12 (92.3) | 15 (78.9) | 0.805 |
| Older (≥60 y) | 31 (10.6) | 10 (10.4) | 7 (10.1) | 6 (9.8) | 3 (8.8) | 1 (7.7) | 4 (21.1) | |
| Sex, n (%) | ||||||||
| Male | 133 (45.5) | 42 (43.75) | 34 (49.3) | 26 (42.6) | 19 (55.9) | 6 (46.2) | 6 (31.6) | 0.596 |
| Female | 159 (54.5) | 54 (56.25) | 35 (50.7) | 35 (57.4) | 15 (44.1) | 7 (53.8) | 13 (68.4) | |
| WBC count, ×109/L (range) | 14.2 (0.3–398.0) | 4.7 (0.3–315.7) | 55.2 (0.8–398.0) | 17.6 (1.2–248.0) | 6.1 (0.8–142.5) | 3.5 (0.9–311.2) | 8.8 (0.7–156.0) | <0.001c |
| WBC group, n (%) | ||||||||
| Low (<100) | 245 (83.9) | 81 (84.4) | 48 (69.6) | 56 (91.8) | 33 (97.1) | 11 (84.6) | 16 (84.2) | 0.003d |
| High (≥100) | 47 (16.1) | 15 (15.6) | 21 (30.4) | 5 (8.2) | 1 (2.9) | 2 (15.4) | 3 (15.8) | |
| Gene mutationsa, n (%) | N = 271 | N = 87 | N = 64 | N = 58 | N = 32 | N = 12 | N = 18 | |
| KRAS mutations | 45 (16.6) | 14 (16.1) | 13 (20.3) | 12 (20.7) | 2 (6.3) | 1 (8.3) | 3 (16.7) | 0.490 |
| NRAS mutations | 41 (15.1) | 14 (16.1) | 5 (7.8) | 11 (19.0) | 5 (15.6) | 2 (16.7) | 4 (22.2) | 0.432 |
| FLT3-ITD mutations | 23 (8.5) | 4 (4.6) | 6 (9.4) | 11 (19.0) | 1 (3.1) | 1 (8.3) | 0 (0.0) | 0.040e |
| PTPN11 mutations | 18 (6.6) | 4 (4.6) | 3 (4.7) | 6 (10.3) | 2 (6.3) | 0 (0.0) | 3 (16.7) | 0.336 |
| Courses of induction chemotherapy before CR1, median (range) | 1 (1–4) | 1 (1–4) | 1 (1–4) | 1 (1–4) | 1 (1–4) | 1 (1–3) | 1 (1–2) | 0.077 |
| Courses of consolidation chemotherapy, median (range) | 1 (0–10) | 1 (0–6) | 1 (0–6) | 2 (0–5) | 1 (0–4) | 2 (0–4) | 1 (0–10) | 0.511 |
| CR1, n (%) | ||||||||
| NE | 18b (6.2) | 7 (7.3) | 2 (2.9) | 4 (6.6) | 2 (5.9) | 1 (7.7) | 2 (10.5) | |
| No | 20 (6.8) | 6 (6.3) | 7 (10.1) | 3 (4.9) | 3 (8.8) | 1 (7.7) | 0 (0.0) | 0.827 |
| Yes | 254 (87.0) | 83 (86.5) | 60 (87.0) | 54 (88.5) | 29 (85.3) | 11 (84.6) | 17 (89.5) | |
| Allo-HSCT in CR1 | 192 (65.8) | 59 (61.5) | 48 (69.6) | 45 (73.8) | 22 (64.7) | 5 (38.5) | 13 (68.4) | 0.121 |
| Outcome, % (95% CI) | ||||||||
| 3-year OS | 59.4 (53.4–66.0) | 57.2 (46.9–69.6) | 61.0 (49.1–75.7) | 65.3 (53.6–79.6) | 59.3 (44.4–79.2) | 32.8 (13.3–81.0) | 58.6 (38.9–88.4) | 0.410 |
| 3-year CIR | 32.3 (26.0–38.7) | 34.0 (23.4–44.9) | 37.1 (22.2–52.0) | 21.3 (11.2–33.4) | 40.3 (21.6–58.3) | 58.8 (10.4–88.3) | 13.9 (2.0–37.0) | 0.175 |
AML acute myeloid leukemia, WBC white blood cell, Allo-HSCT allogeneic hematopoietic stem cell transplantation, CR1 first complete remission, NE nonevaluable for response, CI confidence interval, OS overall survival, CIR cumulative incidence of relapse.
aAssessed in available data.
b18 patients were not evaluated, 17 of whom dead without evaluation and 1 of whom not evaluated for personal reason.
cThe adjusted P value by Bonferoni method for AFDN was less than 0.001 compared to MLLT3 and 0.029 compared to MLLT10.
dThe adjusted P value by Bonferoni method for AFDN was 0.024 compared to ELL and 0.021 compared to MLLT10.
eNo statistical differences between groups after further pairwise comparison.
The median age at diagnosis was 40 years (range: 15–72), with 89.4% of patients aged ≤ 60 years. No significant differences in age distribution were found among the different KMT2A-r subtypes (P = 0.706). The complete remission (CR) rate was 87.0%. The median white blood cell (WBC) count at diagnosis was 14.2 × 109/L (range: 0.3–398.0). Hyperleukocytosis was most frequently observed in patients with KMT2A::AFDN compared to those with KMT2A::ELL (adjusted P = 0.024) and KMT2A::MLLT10 (adjusted P = 0.021), respectively.
Concurrent mutational landscape
Next-generation sequencing (NGS) was performed on 271 subjects (92.8%, 271/292) using a panel of up to 54 genes (Supplementary Table S2), identifying mutations in 36 genes. The median number of mutations per patient was one (range: 0–6). The most frequent mutation was KRAS (17%), followed by NRAS (15%), FLT3-ITD (8%), and PTPN11 (7%). 31.7% of patients (86/271 evaluable patients) harbored mutations in the RAS pathway, including KRAS, NRAS, PTPN11, BRAF, NF1, and KIT. Notably, mutations in KRAS and NRAS were not mutually exclusive, as 13 patients exhibited both mutations simultaneously. The distribution of mutations across different KMT2A-r subtypes is shown in Supplementary Fig. S2.
Among the subtypes, 17.2% of patients with KMT2A::ELL, 41.4% with KMT2A::MLLT3, 46.9% with KMT2A::MLLT10, 50% with KMT2A::MLLT1, and 51.6% with KMT2A::AFDN did not harbor any sequenced genetic mutations. The distribution of RAS pathway mutations (e.g., KRAS, NRAS, and PTPN11) did not significantly differ among the various rearrangement types (Table 1; data for KRAS is shown in Supplementary Fig. S3).
Clinical outcomes
The median OS for the entire cohort was 66.8 months (range: 0.68–102 months). The 3-year OS and CIR rates were 59.4% (95% CI: 53.4–66.0%) and 32.3% (95% CI: 26.0–38.7%), respectively. No significant differences in clinical outcomes were observed among patients with different types of KMT2A-r (Supplementary Fig. S4A-B, Table 1).
Patients with KMT2A::MLLT3 showed no significant differences in 3-year OS compared to those with 11q23/KMT2A rearrangements other than KMT2A::MLLT3 (57.2% vs. 60.5%, P = 0.81), nor in CIR (34.0% vs. 31.5%, P = 0.45) (Supplementary Fig. S4C-D). Patients harboring KMT2A::MLLT1, however, showed a trend towards worse clinical outcomes compared to their counterparts (3-year OS: 32.8% vs. 60.4%, P = 0.055; CIR: 58.8% vs. 31.4%, P = 0.087) (Supplementary Fig. S5A-B). When compared to KMT2A::ELL, KMT2A::MLLT1 exhibited significantly poorer outcomes, with a lower 3-year OS (32.8% vs. 65.3%, P = 0.024) and a higher CIR (58.8% vs. 21.3%, P = 0.047) (Supplementary Fig. S5C-D).
Impacts of genetic mutations on outcomes
We subsequently examined the impact of genetic mutations on clinical outcomes. Patients with KRAS mutations exhibited significantly poorer survival compared to those without KRAS mutations (3-year OS: 45.4% vs. 64.1%, P = 0.0073; Supplementary Fig. S6A). Additionally, patients harboring KRAS mutations showed a trend towards higher CIR (3-year CIR: 41.4% vs. 30.4%, P = 0.18; Supplementary Fig. S6B). By contrast, NRAS mutations did not seem to influence clinical outcomes (Supplementary Fig. S6C-D). Among the whole cohort, 13 patients had concurrent KRAS and NRAS mutations. The presence of an NRAS mutation, however, did not worsen the prognosis for patients with KRAS mutations (Supplementary Fig. S6E-F). Mutations in TP53 and PTPN11 were not associated with prognostic value in patients with KMT2A-r lesions (Supplementary Fig. S7C-F). Conversely, patients with FLT3-ITD mutations exhibited a trend towards poorer survival, with a lower 3-year OS (43.2% vs. 62.2%, P = 0.057; Supplementary Fig. S7A-B).
Outcomes of patients undergoing allo-HSCT in CR1
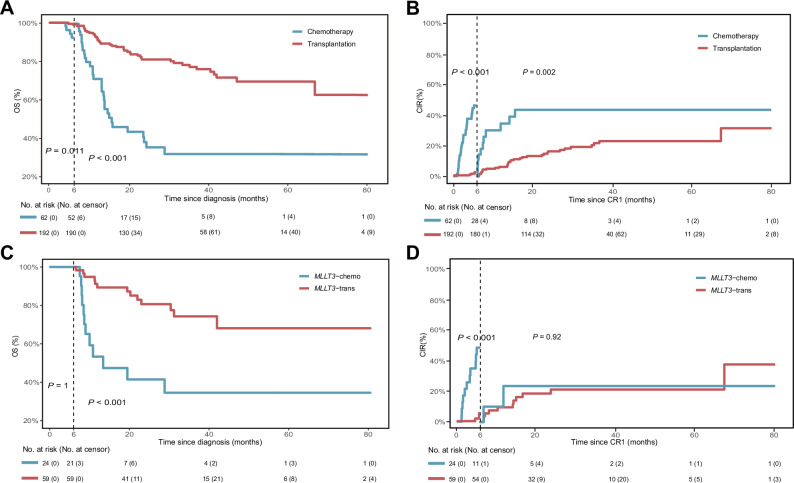
Among the general cohort of 292 patients, CR1 was achieved in 254 patients (87.0%), of these, 192 (75.6%) underwent allo-HSCT, while 62 (24.4%) received chemotherapy as post-remission therapy. No significant differences in CR rates were observed among patients with different types of KMT2A-r (P = 0.726, Table 1). Using the sixth month after diagnosis as the segmentation time point, 3-year OS and CIR rates were significantly better in patients who underwent allo-HSCT compared to those treated with chemotherapy alone. The OS rates were 77.3% (95% CI: 70.8–84.4%) vs. 31.7% (95% CI: 20.0–50.2%; P < 0.001), while the CIR rates were 20.4% (95% CI: 13.6–27.2%) vs. 43.5% (95% CI: 23.1–63.9%; P = 0.002) (Fig. 2A, B). The prognosis of patients who underwent allo-HSCT was significantly better than that of those without allo-HSCT across all KMT2A-r types (P < 0.05, Supplementary Figure S8; data for KMT2A::MLLT3 is shown in Fig. 2C, D).
Fig. 2. Impact of transplantation group vs. chemotherapy group on clinical outcomes in KMT2A-r AML after CR1.
The OS (A) and CIR (B) of patients who underwent allo-HSCT at CR1 compared to those who received chemotherapy only. The OS (C) and CIR (D) of patients with KMT2A::MLLT3 who underwent allo-HSCT at CR1 compared to those who received chemotherapy only. The transplantation group included patients who underwent allo-HSCT in CR1. The chemotherapy group included patients who received chemotherapy only in CR1. OS overall survival, CIR cumulative incidence of relapse, Allo-HSCT allogeneic hematopoietic stem cell transplantation, AML acute myeloid leukemia, CR1 first complete remission. Note: The 6th month after diagnosis was chosen as the landmark time. The curve does not show the censored points; the censored data are shown at the bottom along with the risk table.
Prognostic predictors for CR1 patients
A univariable Cox regression analysis was performed on patients who achieved CR1 (n = 254). The analysis identified several prognostic factors for OS, including KRAS mutations (HR = 1.71, 95% CI: 0.98–2.99, P = 0.06), the number of induction courses (>1 vs. 1: HR = 1.52, 95% CI: 0.96–2.39, P = 0.072), the number of consolidation courses (2-3 vs. 0-1: HR = 0.60, 95% CI: 0.37–0.98, P = 0.042), and allo-HSCT at CR1 (HR = 0.59, 95% CI: 0.50–0.70, P < 0.001). Prognostic factors for CIR included the number of induction courses (>1 vs. 1: HR = 2.03, 95% CI: 1.30–3.17, P = 0.002) and allo-HSCT at CR1 (HR = 0.02, 95% CI: 0.01–0.05, P < 0.001) (Supplementary Table S4).
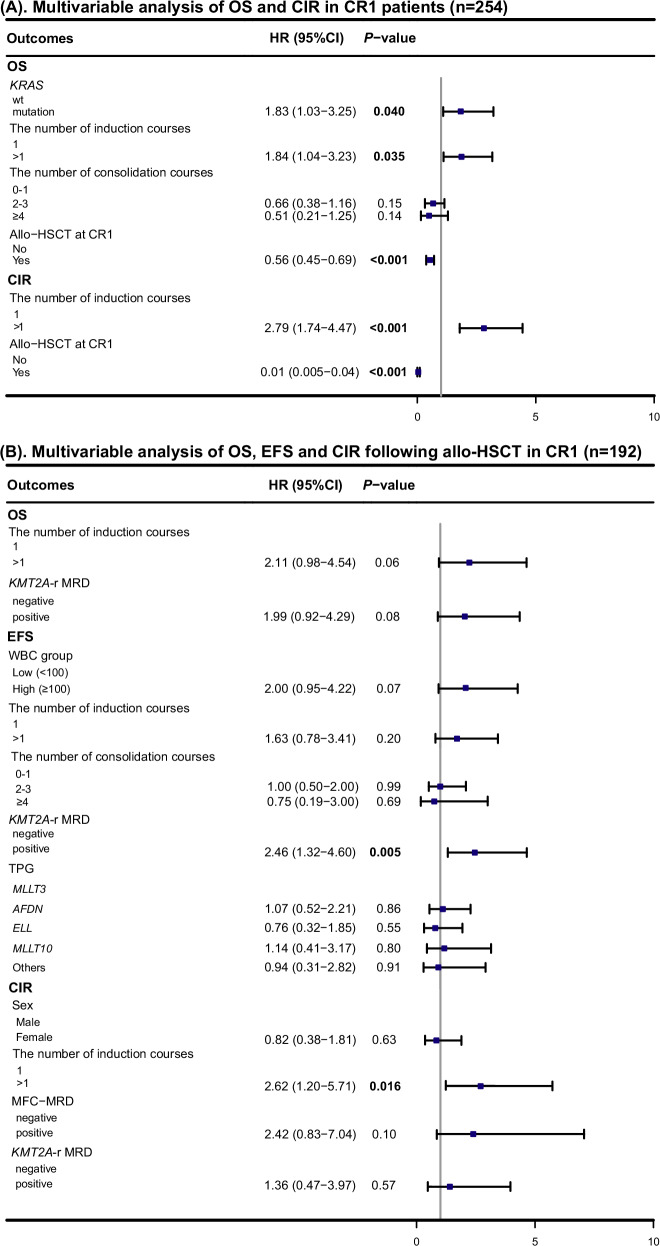
Multivariable Cox regression analysis incorporating these factors revealed that KRAS mutations (HR = 1.83, 95% CI: 1.03–3.25, P = 0.040) and the number of induction courses (>1 vs. 1: HR = 1.84, 95% CI: 1.04–3.23, P = 0.035) were adverse prognostic factors for OS. Conversely, allo-HSCT at CR1 (HR = 0.56, 95% CI: 0.45–0.69, P < 0.001) was a favorable prognostic factor for OS. For CIR, the number of induction courses (>1 vs. 1: HR = 2.79, 95% CI: 1.74–4.47, P < 0.001) was an adverse indicator, while allo-HSCT at CR1 remained a favorable factor (HR = 0.01, 95% CI: 0.005–0.04, P < 0.001) (Fig. 3A).
Fig. 3. Multivariable analysis of clinical outcomes.
Data are provided for CR1 patients (n = 254) (A) and patients who underwent allo-HSCT in CR1 (n = 192) (B). HR hazard ratio, CI confidence interval, OS overall survival, EFS event-free survival, CIR cumulative incidence of relapse, Allo-HSCT allogeneic hematopoietic stem cell transplantation, CR1 first complete remission, Wt wildtype, WBC white blood cell, TPG translocation partner gene, MRD measurable residual disease, MFC-MRD multiparameter flow cytometry-based MRD. Note: KMT2A-r MRD cases were evaluated with specific KMT2A fusion genes. Due to the small sample size of patients with MLLT1, this mutation was combined with the “Other” group for analysis. P-values were obtained by multivariable Cox regression for OS and EFS, or by the competing risk model for CIR unless otherwise specified. Allo-HSCT was included as a time-dependent covariate in the analysis.
MRD prior to allo-HSCT
We next evaluated the prognostic significance of MFC-MRD and KMT2A-r MRD prior to allo-HSCT. Among 168 evaluable patients, 35 (20.8%) exhibited detectable MFC-MRD, while 38 (28.8%) out of 132 evaluable patients tested positive for KMT2A-r MRD. Patients were stratified based on MFC-MRD and KMT2A-r MRD status at the time of allo-HSCT.
We found no significant differences in OS and EFS between patients with detectable MFC-MRD and those without (3-year OS: 67.4% vs. 77.3%, P = 0.30; 3-year EFS: 55.5% vs. 61.8%, P = 0.72, Fig. 4A, C). Further landmark analysis for OS found no statistically significant differences (Fig. 4B). Patients with detectable MFC-MRD, however, showed a significantly higher CIR, with a 3-year CIR of 42.1% compared to 15.3% for patients without detectable MFC-MRD (P = 0.0035, Fig. 4E).
Fig. 4. Impact of pre-transplant MRD on clinical outcomes.
The OS of patients with positive and negative MFC-MRD before transplantation (A), the landmark analysis assessing OS within 1 year and between 1 year and 3 years after diagnosis (B), as well as the EFS (C) and CIR (D). The OS (E), landmark analysis assessing OS (F), EFS (G), and CIR (H) of patients with positive and negative KMT2A-r MRD before transplantation. KMT2A-r MRD cases were evaluated with specific KMT2A fusion genes. OS overall survival, EFS event-free survival, CIR cumulative incidence of relapse, MRD measurable residual disease, MFC-MRD flow cytometry-based MRD. Note: The curve does not show the censored points; the censored data are shown at the bottom along with the risk table.
By contrast, detectable KMT2A-r MRD was associated with a substantial reduction in EFS among allo-HSCT recipients, with 3-year EFS rates of 39.7% for patients with detectable KMT2A-r MRD versus 69.9% for those without (P = 0.0011, Fig. 4G). KMT2A-r MRD positivity also correlated with an increased CIR (3-year CIR: 42.8% vs. 14.7%, P = 0.0084, Fig. 4H), although it did not result in significantly shorter OS (3-year OS: 75.6% vs. 82.4%, P = 0.085, Fig. 4E). Notably, detectable KMT2A-r MRD at the time of allo-HSCT primarily impacted OS in the late post-transplantation period (Fig. 4F).
For AML patients with KMT2A::MLLT3, detectable KMT2A-r MRD at allo-HSCT (25.6%, 11/43 evaluable patients) was associated with poorer survival outcomes, with a 3-year OS of 72.7% compared to 93.1% in patients without KMT2A-r MRD (P = 0.029, Supplementary Fig. S9A) and a 3-year EFS of 36.4% versus 66.7% (P = 0.047, Supplementary Fig. S9B); however, KMT2A-r MRD positivity in this group did not significantly increase CIR (3-year CIR: 30.7% vs. 16.2%, P = 0.44, Supplementary Fig. S9C).
We further stratified patients into four groups based on the combined status of MFC-MRD and KMT2A-r MRD: MFC-MRD−/KMT2A-r MRD−, MFC-MRD−/KMT2A-r MRD+, MFC-MRD+ /KMT2A-r MRD−, and MFC-MRD+/KMT2A-r MRD+. Comparative analysis among these groups revealed significant differences in EFS (3-year EFS: 70.1% vs. 32.2% vs. 66.7% vs. 57.8%, P = 0.00058, Supplementary Fig. S10B) and CIR (3-year CIR: 11.4% vs. 32.1% vs. 44.4% vs. 36.2%, P = 0.011, Supplementary Fig. S10C). No significant differences, however, were observed in OS (3-year OS: 84.5% vs. 70.7% vs. 62.2% vs. 88.2%, P = 0.082, Supplementary Fig. S10A).
Prognostic predictors for patients received allo-HSCT in CR1
Among patients who received allo-HSCT at CR1 (n = 192), no significant differences in 3-year OS (P = 0.96), EFS (P = 0.26), and CIR (P = 0.32) were observed based on the type of KMT2A-r lesions (Supplementary Fig. S11A-C). The clinical outcomes of allo-HSCT recipients with KMT2A::MLLT3 were comparable to those with other 11q23/KMT2A rearrangements (3-year OS: 74.3% vs. 77.5%, P = 0.97; 3-year EFS: 55.2% vs. 62.2%, P = 0.34; 3-year CIR: 24.4% vs. 20.8%, P = 0.32, Supplementary Fig. S11D-F). Notably, allo-HSCT mitigated the adverse effects of KRAS mutations. Patients with KRAS mutations who underwent allo-HSCT exhibited outcomes comparable to those with wild-type KRAS in terms of OS (3-year OS: 68.2% vs. 78.9%, P = 0.15, Supplementary Fig. S12A), EFS (3-year EFS: 52.5% vs. 61.0%, P = 0.29, Supplementary Fig. S12B) and CIR (3-year CIR: 27.2% vs. 20.0%, P = 0.40, Supplementary Fig. S12C). Univariate analyses of transplantation recipients’ prognosis are summarized in Supplementary Table S5. In multivariable analysis, no significant risk factors for OS were identified among allo-HSCT recipients (Fig. 3B). Additionally, detectable KMT2A-r MRD at allo-HSCT (HR = 2.46, 95% CI: 1.32–4.60, P = 0.005) was a significant risk factor for shortened EFS in this cohort. For CIR, the number of induction courses (>1 vs. 1: HR = 2.62, 95% CI: 1.20–5.71, P = 0.016) remained an adverse indicator.
Discussion
KMT2A-r are recurrent genetic abnormalities in AML that affect a minority of adult AML patients [12, 40]. Our cohort represents the largest study to date involving adult AML patients with KMT2A-r. Using data from this cohort, we aimed to determine whether KMT2A-r AML patients benefit from allo-HSCT in CR1 and evaluate the prognostic value of KMT2A-r MRD prior to allo-HSCT. Consistent with previous reports, the incidence of KMT2A-r AML was significantly higher in patients < 60 years-of-age, with a median age of 40 years in our cohort [4, 41–43]. KMT2A::MLLT3 (i.e., t(9;11)(p22;q23)) was the most prevalent rearrangement, followed by KMT2A::AFDN (i.e., t(6;11)(q27;q23)) and KMT2A::ELL (i.e., t(11;19)(q23;p13.1)). While some studies have combined patients with KMT2A::ELL and KMT2A::MLLT1 (i.e., t(11;19)(q23;p13.3)) into a single subgroup for analysis, our findings demonstrate that each rearrangement exhibits distinct clinical features and outcomes; therefore, these rearrangements should not be regarded as a single entity.
The prognostic impact of different fusion partners in KMT2A-r AML has been studied for several decades, yet the results remain inconclusive [4, 6, 8, 14]. In the latest ELN2022 classification system, the presence of KMT2A::MLLT3 was established as the key criterion for classifying all patients with this translocation into the intermediate-risk group. This classification does not account for patient age or potentially co-existing rare, concurrent adverse-risk mutations. The findings of our study cohort, therefore, do not support the general view that KMT2A::MLLT3 is associated with a relatively favorable prognosis. Notably, some studies suggest that the prognostic advantage of KMT2A::MLLT3 may be limited to specific patient subgroups, particularly younger patients (<60 years) with de novo AML [41]. Interestingly, Bill et al. reported an uneven distribution of KRAS mutations among the various KMT2A-r subtypes, with 47% of their patients with t(6;11) harboring KRAS mutations, compared to only 3% of those with t(9;11). We found a similar distribution of KRAS mutations across the different translocations in our cohort. Given the adverse prognostic impact of KRAS mutations (Supplementary Fig. S13), this could explain the more favorable outcomes observed in patients with t(9;11) in the Bill et al. study [41]. Consistent with our observations, two independent research groups reported a KRAS mutation prevalence of approximately 20% in patients with t(9;11). These studies also did not identify significant differences in OS among patients with distinct fusion partners [42, 44]. Thus, the heterogeneous results documented in the literature regarding the favorable outcomes of KMT2A::MLLT3 AML may be attributed to a bias in the distribution of KRAS mutations rather than the fusion partner itself. Further studies are now necessary to validate this hypothesis.
With respect to clinical outcomes, we found that adult patients with KMT2A-r AML exhibit shorter OS when carrying a KRAS mutation, regardless of the specific type of KMT2A fusion partner. KRAS mutations were recently described as an independent adverse prognostic factor in pediatric KMT2A-r AML by the Japanese Pediatric Leukemia/Lymphoma Study Group, a finding validated in a smaller cohort of adult KMT2A-r patients [45]. The HARMONY AML study, involving 205 adult KMT2A-r patients, also reported that among those under 60 years of age with de novo AML and KRAS mutations, OS was significantly poorer [44]. Some studies, however, have not identified KRAS mutations as an adverse prognostic factor for OS [46], which contrasts with our findings and those previously reported. This discrepancy may stem from the relatively small sample sizes in those studies and potential biases in the distribution of KRAS mutations. Notably, and in contrast to earlier reports, we did not observe TP53 mutations as an adverse prognostic factor in KMT2A-r AML, likely due to the relatively lower frequency of TP53 mutations in our study cohort.
The role of allo-HSCT in patients with KMT2A-r AML remains a subject of debate, largely due to the limited data on its benefits in adult KMT2A-r AML patients. The results of our study demonstrate that transplantation at CR1 is associated with significantly better prognosis compared to consolidation chemotherapy alone. Interestingly, a recent study by the International Berlin-Frankfurt-Münster Study Group on pediatric KMT2A-r AML found that allo-HSCT during CR1 did not improve OS; instead, FCM-MRD negativity at the end of induction 2 was highly protective [15]. Additionally, our findings and those of other independent research groups confirm that patients with detectable MFC-MRD at the point of allo-HSCT exhibit a significantly higher probability of recurrence compared to those with undetectable MFC-MRD [47]. These findings underscore the importance of achieving deeper remission prior to allo-HSCT. Given the lack of innovative, low-toxicity treatment options and the inherent toxicity of traditional therapies, however, an excessive emphasis on achieving pre-transplant MRD negativity may not necessarily result in improved outcomes. Menin inhibitors, a novel class of agents targeting the biology of KMT2A-r and NPM1 mutant leukemias, represent a promising addition to treatment regimens aimed at enhancing clinical outcomes [48, 49].
As a retrospective study, a key limitation of our work is its inability to definitively establish whether MRD eradication leads to improved post-HSCT outcomes. Nonetheless, data from our cohort showed that patients with KRAS mutations who underwent allo-HSCT achieved comparable OS and EFS to those without KRAS mutations. This finding suggests that allo-HSCT may mitigate the adverse effects of KRAS mutations.
In our study, patients with undetectable MFC-MRD at allo-HSCT exhibited a significantly higher probability of disease control compared to those with detectable MFC-MRD, with a threshold of 0.1%. Furthermore, the prognostic value of KMT2A-r MRD requires further clarification, as most cases of KMT2A-r AML can also be assessed using specific KMT2A fusion genes. These fusion gene assays offer superior sensitivity and reliability compared to MFC-MRD [50]. Indeed, our data demonstrated KMT2A-r MRD has greater sensitivity in predicting transplant outcomes than MFC-MRD.
Sun Loo et al. conducted a retrospective study of 64 patients with KMT2A-r AML and found that, using quantitative PCR, patients with KMT2A-r MRD ≥ 0.001% at allo-HSCT exhibited 2-year OS and CIR rates of 39% and 75%, respectively [39]. These patients had significantly shorter OS (P = 0.012) and higher CIR (P = 0.0004) compared to patients without KMT2A-r MRD. Similarly, we observed that patients with KMT2A-r MRD had higher CIR and significantly shorter EFS; however, we could not definitively establish the impact of pre-transplant KMT2A-r MRD positivity on OS.
By comparing outcomes before and after the 1-year landmark, we identified a potential influence of KMT2A-r MRD at allo-HSCT on OS. This influence might reflect the implementation of more rigorous pretreatment regimens, effective maintenance therapies, or pre-emptive interventions (e.g., donor lymphocyte infusion) for patients with KMT2A-r MRD at allo-HSCT. These factors likely contributed to the sustained survival observed in these patients during the early post-transplantation period. Further studies are now needed to elucidate the precise mechanisms underlying this phenomenon. Additionally, it should be noted that discrepancies between different MRD assays pose challenges in clinical decision-making and require standardization to optimize patient care.
In conclusion, our study highlights the distinctive mutational patterns in KMT2A-r adult AML, with the RAS pathway being the most commonly affected. Notably, KRAS mutations were associated with a significantly higher risk of poor prognosis. Furthermore, the prognostic relevance of KMT2A::MLLT3 may be closely linked to the distribution of KRAS mutations. Although KMT2A::MLLT3 is classified as intermediate risk by ELN, our results suggest that allo-HSCT should be an integral part of the treatment strategy upon achieving CR1 for all patients with KMT2A-r, regardless of fusion partner or translocation. KMT2A-r MRD at allo-HSCT proved to be more sensitive than MFC-MRD for predicting prognosis, providing critical insights for optimizing the timing of transplantation, guiding transplant strategies, and selecting post-transplant maintenance therapies. Future studies are now warranted to further elucidate the biology of KMT2A-r AML, enabling the identification of more effective therapeutic targets and ultimately improving patient outcomes.
Supplementary information
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFA1101500 to Y.C.,2022YFC2502600 to X.H., 2022YFC2502606 to X.M.), the National Natural Science Foundation (82170206, 82470213 to X.H.) and funding from the Project of Disciplines of Excellence (20234Z0002 to X.H.).
Author contributions
YC, HX, WS and XM were responsible for designing the study design and revising the manuscript strictly for important content. RZ, HH, YZ, YX and JH collected the clinical data. RZ, HH, YC, HX and WS analyzed the data and wrote the manuscript. XM, CJ, LW, HL, ZP, GW and YY interpreted the results and provided feedback. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ruoxuan Zhang, Huihong Huang, Yi Zhang, Yi Xia.
Contributor Information
Xiaodong Mo, Email: moxiaodong@bjmu.edu.cn.
Wei Shi, Email: shiwei076@hust.edu.cn.
Xiaoxia Hu, Email: hxx12276@rjh.com.cn.
Yang Cao, Email: caoyangemma@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-025-01293-x.
References
- 1.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325–36. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65. [DOI] [PubMed] [Google Scholar]
- 3.Grimwade D, Mrozek K. Diagnostic and prognostic value of cytogenetics in acute myeloid leukemia. Hematol Oncol Clin North Am. 2011;25:1135–61. [DOI] [PubMed] [Google Scholar]
- 4.Mrozek K, Heinonen K, Lawrence D, Carroll AJ, Koduru PR, Rao KW, et al. Adult patients with de novo acute myeloid leukemia and t(9; 11)(p22; q23) have a superior outcome to patients with other translocations involving band 11q23: a cancer and leukemia group B study. Blood. 1997;90:4532–8. [PubMed] [Google Scholar]
- 5.Cox MC, Panetta P, Lo-Coco F, Del Poeta G, Venditti A, Maurillo L, et al. Chromosomal aberration of the 11q23 locus in acute leukemia and frequency of MLL gene translocation: results in 378 adult patients. Am J Clin Pathol. 2004;122:298–306. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Kantarjian H, Pierce S, Faderl S, O’Brien S, Qiao W, et al. Prognostic significance of 11q23 aberrations in adult acute myeloid leukemia and the role of allogeneic stem cell transplantation. Leukemia. 2013;27:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krauter J, Wagner K, Schafer I, Marschalek R, Meyer C, Heil G, et al. Prognostic factors in adult patients up to 60 years old with acute myeloid leukemia and translocations of chromosome band 11q23: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2009;27:3000–6. [DOI] [PubMed] [Google Scholar]
- 8.Mrozek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–36. [DOI] [PubMed] [Google Scholar]
- 9.Schoch C, Schnittger S, Klaus M, Kern W, Hiddemann W, Haferlach T. AML with 11q23/MLL abnormalities as defined by the WHO classification: incidence, partner chromosomes, FAB subtype, age distribution, and prognostic impact in an unselected series of 1897 cytogenetically analyzed AML cases. Blood. 2003;102:2395–402. [DOI] [PubMed] [Google Scholar]
- 10.Harrison CJ, Hills RK, Moorman AV, Grimwade DJ, Hann I, Webb DK, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol. 2010;28:2674–81. [DOI] [PubMed] [Google Scholar]
- 11.Meyer C, Larghero P, Almeida Lopes B, Burmeister T, Gröger D, Sutton R, et al. The KMT2A recombinome of acute leukemias in 2023. Leukemia. 2023;37:988–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Climent JA, Espinosa R 3rd, Thirman MJ, Le Beau MM, Rowley JD. Abnormalities of chromosome band 11q23 and the MLL gene in pediatric myelomonocytic and monoblastic leukemias. Identification of the t(9;11) as an indicator of long survival. J Pediatr Hematol Oncol. 1995;17:277–83. [DOI] [PubMed] [Google Scholar]
- 14.Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114:2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Weelderen RE, Klein K, Harrison CJ, Jiang Y, Abrahamsson J, Arad-Cohen N, et al. Measurable Residual Disease and Fusion Partner Independently Predict Survival and Relapse Risk in Childhood KMT2A-Rearranged Acute Myeloid Leukemia: A Study by the International Berlin-Frankfurt-Munster Study Group. J Clin Oncol. 2023;41:2963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollard JA, Guest E, Alonzo TA, Gerbing RB, Loken MR, Brodersen LE, et al. Gemtuzumab Ozogamicin Improves Event-Free Survival and Reduces Relapse in Pediatric KMT2A-Rearranged AML: Results From the Phase III Children’s Oncology Group Trial AAML0531. J Clin Oncol. 2021;39:3149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pigazzi M, Masetti R, Bresolin S, Beghin A, Di Meglio A, Gelain S, et al. MLL partner genes drive distinct gene expression profiles and genomic alterations in pediatric acute myeloid leukemia: an AIEOP study. Leukemia. 2011;17:560–3. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Liu QF, Qin YZ, Liu DH, Xu LP, Jiang B, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of patients with mixed-lineage-leukemia-rearranged acute leukemia: Results from a prospective, multi-center study. Am J Hematol. 2014;89:130–6. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Huo W, Huang J, Yang Y, Wang Y, Chang Y, et al. MRD positivity was the poor prognostic factor for adverse-risk AML patients with allogeneic hematopoietic stem cell transplantation: a multicenter TROPHY study. Blood Cancer J. 2024;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert J, Lambert J, Thomas X, Marceau-Renaut A, Micol JB, Renneville A, et al. Early detection of WT1 measurable residual disease identifies high-risk patients, independent of transplantation in AML. Blood Adv. 2021;5:5258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu S, Lin T, Nie D, Zhang Y, Sun Z, Zhang Q, et al. Dynamic assessment of measurable residual disease in favorable-risk acute myeloid leukemia in first remission, treatment, and outcomes. Blood Cancer J. 2021;11:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venditti A, Piciocchi A, Candoni A, Arena V, Palmieri R, Filì C, et al. Risk-adapted MRD-directed therapy for young adults with acute myeloid leukemia: 6-year update of the GIMEMA AML1310 trial. Blood Adv. 2024;8:4410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang B, Zhao Y, Luo Y, Yu J, Chen Y, Ye B, et al. Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation in Adult Patients With Acute Myeloid Leukemia Harboring KMT2A Rearrangement and Its Prognostic Factors. Cell Transpl. 2024;33:9636897231225821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Minimal residual disease monitoring and preemptive immunotherapies for frequent 11q23 rearranged acute leukemia after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2021;100:1267–81. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Xing S, Zhang H, Mao X, Xiao M, Wang Y. FISH improves risk stratification in acute leukemia by identifying KMT2A abnormal copy number and rearrangements. Sci Rep. 2022;12:9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iijima-Yamashita Y, Matsuo H, Yamada M, Deguchi T, Kiyokawa N, Shimada A, et al. Multiplex fusion gene testing in pediatric acute myeloid leukemia. Pediatr Int. 2018;60:47–51. [DOI] [PubMed] [Google Scholar]
- 27.Panagopoulos I, Andersen K, Eilert-Olsen M, Zeller B, Munthe-Kaas MC, Buechner J, et al. Therapy-induced Deletion in 11q23 Leading to Fusion of KMT2A With ARHGEF12 and Development of B Lineage Acute Lymphoplastic Leukemia in a Child Treated for Acute Myeloid Leukemia Caused by t(9;11)(p21;q23)/KMT2A-MLLT3. Cancer Genomics Proteom. 2021;18:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerveira N, Lisboa S, Correia C, Bizarro S, Santos J, Torres L, et al. Genetic and clinical characterization of 45 acute leukemia patients with MLL gene rearrangements from a single institution. Mol Oncol. 2012;6:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doubek M, Palasek I, Pospisil Z, Borsky M, Klabusay M, Brychtova Y, et al. Detection and treatment of molecular relapse in acute myeloid leukemia with RUNX1 (AML1), CBFB, or MLL gene translocations: frequent quantitative monitoring of molecular markers in different compartments and correlation with WT1 gene expression. Exp Hematol. 2009;37:659–72. [DOI] [PubMed] [Google Scholar]
- 30.Andersson A, Höglund M, Johansson B, Lassen C, Billström R, Garwicz S, et al. Paired multiplex reverse-transcriptase polymerase chain reaction (PMRT-PCR) analysis as a rapid and accurate diagnostic tool for the detection of MLL fusion genes in hematologic malignancies. Leukemia. 2001;15:1293–300. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmeister LM, Orhan E, Walter C, Niktoreh N, Hanenberg H, von Neuhoff N. et al. Impact of KMT2A Rearrangement and CSPG4 Expression in Pediatric Acute Myeloid. Leukemia. Cancers. 2021;13:4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J-i, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–15. [DOI] [PubMed] [Google Scholar]
- 33.Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. [Chinese guidelines for diagnosis and treatment of adult acute myeloid leukemia (not APL) (2023)]. Zhonghua Xue Ye Xue Za Zhi. 2023;44:705–12. [DOI] [PMC free article] [PubMed]
- 34.Wang Y, Liu QF, Lin R, Yang T, Xu YJ, Mo XD, et al. Optimizing antithymocyte globulin dosing in haploidentical hematopoietic cell transplantation: long-term follow-up of a multicenter, randomized controlled trial. Sci Bull (Beijing). 2021;66:2498–505. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Zhao Y, Jiang C, Han D, Pan Z, Zhang Z, et al. Diagnostic efficiency of metagenomic next-generation sequencing for suspected infection in allogeneic hematopoietic stem cell transplantation recipients. Front Cell Infect Microbiol. 2023;13:1251509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Y, Zhang C, Zhao X, Huang J, Zhang Z, Jiang C, et al. Allogeneic hematopoietic stem cell transplantation for adult acute myeloid leukemia patients with nucleoporin 98 (NUP98) gene rearrangements: a real-world study in China. Bone Marrow Transpl. 2023;58:1413–5. [DOI] [PubMed] [Google Scholar]
- 38.Heuser M, Freeman SD, Ossenkoppele GJ, Buccisano F, Hourigan CS, Ngai LL. et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2021;138:2753–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loo S, Potter N, Ivey A, O'Nions J, Moon R, Jovanovic J, et al. Pretransplant MRD detection offusion transcripts is strongly prognostic in KMT2A-rearranged acute myeloid leukemia. Blood.2024;144:2554–7. [DOI] [PubMed] [Google Scholar]
- 40.Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bill M, Mrózek K, Kohlschmidt J, Eisfeld AK, Walker CJ, Nicolet D, et al. Mutational landscape and clinical outcome of patients with de novo acute myeloid leukemia and rearrangements involving 11q23/KMT2A. Proc Natl Acad Sci USA. 2020;117:26340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossmann V, Schnittger S, Poetzinger F, Kohlmann A, Stiel A, Eder C, et al. High incidence of RAS signalling pathway mutations in MLL-rearranged acute myeloid leukemia. Leukemia. 2013;27:1933–6. [DOI] [PubMed] [Google Scholar]
- 43.Pigneux A, Labopin M, Maertens J, Cordonnier C, Volin L, Socié G, et al. Outcome of allogeneic hematopoietic stem-cell transplantation for adult patients with AML and 11q23/MLL rearrangement (MLL-r AML). Leukemia. 2015;29:2375–81. [DOI] [PubMed] [Google Scholar]
- 44.Hernández-Sánchez A, González T, Sobas M, Sträng E, Castellani G, Abáigar M, et al. Rearrangements involving 11q23.3/KMT2A in adult AML: mutational landscape and prognostic implications - a HARMONY study. Leukemia. 2024;38:1929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuo H, Yoshida K, Nakatani K, Harata Y, Higashitani M, Ito Y, et al. Fusion partner-specific mutation profiles and KRAS mutations as adverse prognostic factors in MLL-rearranged AML. Blood Adv. 2020;4:4623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Issa GC, Zarka J, Sasaki K, Qiao W, Pak D, Ning J, et al. Predictors of outcomes in adults with acute myeloid leukemia and KMT2A rearrangements. Blood Cancer J. 2021;11:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jentzsch M, Grimm J, Bill M, Brauer D, Backhaus D, Schulz J, et al. Prognostic relevance of remission and measurable residual disease status in AML patients prior to reduced intensity or non-myeloablative allogeneic stem cell transplantation. Blood Cancer J. 2021;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klossowski S, Miao H, Kempinska K, Wu T, Purohit T, Kim E, et al. Menin inhibitor MI-3454 induces remission in MLL1-rearranged and NPM1-mutated models of leukemia. J Clin Investig. 2020;130:981–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Issa GC, Aldoss I, DiPersio J, Cuglievan B, Stone R, Arellano M, et al. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature. 2023;615:920–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mo X-D, Lv M, Huang X-J. Preventing relapse after haematopoietic stem cell transplantation for acute leukaemia: the role of post-transplantation minimal residual disease (MRD) monitoring and MRD-directed intervention. Br J Haematol. 2017;179:184–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.