Abstract
The synthesis, structure and thermal properties of a one-dimensional coordination polymer based on calcium bis(dimethylphosphate) (CaDMP) are reported. The rod-like particles of CaDMP crystallized from water in a monoclinic space group P21/c, and contained Ca[O2P(OCH3)2]2 polymeric chains consisting of the octahedrally coordinated Ca2+ ions bridged by the tridentate dimethylphosphate ligands. The variable temperature powder X-ray diffraction measurements showed that this structure undergoes anisotropic thermal expansion upon heating, with no polymorphic transitions occurring up to 190 °C. Thermolysis of CaDMP began around 260 °C leading to the formation of calcium condensed phosphates and volatile oxophosphorus species. A detailed differential scanning calorimetry (DSC) analysis, combined with a fitting of the experimental data to the Avrami or Liu–Mo kinetic models, revealed that CaDMP accelerates isothermal and non-isothermal crystallization of isotactic polypropylene (iPP). DSC and wide-angle X-ray scattering measurements confirmed the presence of α-iPP and β-iPP crystal domains in the systems loaded with CaDMP particles. The crystallographic analysis indicated that β-iPP polymorph formed via epitaxial crystallization on the surface of CaDMP crystals. Mechanical tests proved that the CaDMP-containing composites exhibited better ductility and impact strength than neat iPP.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-99757-4.
Keywords: Hybrid polymer, Calcium diorganophosphate, Isotactic polypropylene, Crystallization, Nucleating agent, Polymer composite
Subject terms: Coordination chemistry, Materials chemistry, Coordination polymers, Mechanical properties, Polymer characterization, Solid-state chemistry
Introduction
Nowadays, nucleating agents (NAs) are very important components of many polymeric materials. They increase the crystallization rate of polymers and promote the formation of large amounts of small crystallites, which have a beneficial effect on the optical and mechanical properties of the polymer compositions. Moreover, a properly selected NA can also influence the structure of the resulting crystalline phase formed by the polymer matrix1. An example of such polymer is isotactic polypropylene (iPP), that can form several polymorphic crystalline phases (i.e., the α, β, γ, ε polymorphs) composed of identical 31 helices exhibiting (TG)3 or (TG٭)3 conformation and periodicity of ca. 6.50 Å (3 iPP monomeric units within a single turn), but differing in a spatial packing of iPP macromolecules (i.e., packing polymorphism)2.
Under typical processing conditions used in industrial applications of iPP, a monoclinic polymorph of iPP (α-iPP) (cell parameters a = 6.65 Å, b = 20.96 Å, c = 6.50 Å, β = 99.3°; melting temperature of ca. 170 °C) is usually produced2. The α-iPP NAs include inorganic fillers (e.g., CaCO3, talc, wollastonite, or metal-doped silicates)3–5 or metal salts of cyclic carboxylic acids (e.g., benzoates6 or derivatives of hexahydrophthalic acid7. Other NAs that are able to significantly increase the crystallization temperature during melt crystallization of α-iPP and reduce its haziness at low concentrations are based on several groups of organic or metal-organic compounds such as acetalized cyclic sorbitol derivatives8, substituted aromatic heterocyclic organophosphates4,8–10, metal dicarboxylates11,12 and phosphonates13,14, metal complexes with glycerol15, substituted mono- and dihydrazides16, as well as some homologues of N,N′-dicyclohexylcarboxamide17 or trisamides18.
A metastable β form of iPP (β-iPP) is characterized by polymer helices packed within a trigonal unit cell described by the lattice parameters a = b = 11.03 Å and c = 6.50 Å, and has a melting point about 20 °C lower than α-iPP2. Although the appearance of β-crystals in iPP can be stimulated by crystallization under appropriately selected shear–stress conditions or temperature gradients, in order to obtain high contents of this phase, it is necessary to use special β-iPP nucleating agents (β-NAs)1. The most frequently applied are commercially available additives known under the trade names such as Hostaperm® (γ-quinacridone)19, NJSTAR® NU-100 and TMB-5 (N,N’-dicyclohexyl-substituted aryl bisamides)20, as well as rare earth metals-based WBG-II (a bimetallic complex of calcium and lanthanum having a general formula of CaxLa1−x(L1)m(L2)n, where L1 and L2 are dicarboxylic acids and amide-type ligands, respectively) and NAB-83 (a tetrahydrophthalic anhydride complex of selected divalent metals with an undisclosed composition)21. Various other additives having a significant β-iPP nucleating efficiency can be divided into several groups of chemical compounds: bisamides (e.g., secondary amides derived from aliphatic dicarboxylic acids17,22 and N-substituted derivatives of phthalamide bearing cyclohexyl or phenyl groups22–25, trisamides26, as well as metal salts of monocarboxylic acids (e.g., p-n-alkylbenzoate-alumoxanes27, acyclic aliphatic dicarboxylic acids (e.g., salts of divalent metals with malonic, glutaric, adipic, pimelic, suberic or azelaic acids)12,28–31, cyclic dicarboxylic acids32,33, or phthalic acid34 and its hydrogenated derivatives7,35,36. In addition, several inorganic compounds (e.g., surface-functionalized or neat metal oxides, hydroxides37–41 and salts42,43, neat or organically-modified silicate-type minerals (e.g., montmorillonite44, wollastonite45, and halloysite nanotubes46, metal particles (e.g., silver nanoparticles47 have also been reported as being capable of inducing the β-iPP crystallization under specific processing conditions. Interestingly, carbonaceous (nano)structures (i.e., pimelate-modified graphene nanoplatelets or graphene oxide particles48,49 and several organic polymers (e.g., liquid crystalline polymers50, hyperbranched polyesters51, biomimetic polydopamine52, or polystyrene53 exhibit similar nucleating properties towards β-iPP. A more detailed account of known β-iPP NAs can be found in a recent review by Wu et al.54.
β-iPP provides some advantageous thermal and mechanical properties of final materials, e.g. a higher heat distortion temperature, impact strength and toughness1,55, or better ductility and drawability56, in comparison to the products containing only the α-iPP domains. β-iPP also exhibits better resistance to both thermal and UV-induced photooxidative degradation55. However, its presence in iPP also has some drawbacks, the most important of which occurs under tensile stress conditions (i.e., a reduced values of Young’s modulus and yield stress) and can effectively limit industrial applications of the materials incorporating β-iPP phase56–58. An attractive option to solve these problems is to combine a stiffness of α-iPP with a toughness of β-iPP in one material, either thru utilization of a mixture of α-NA and β-NA (the so-called α/β compound NA)59,60, or a single NA exhibiting a dual nucleating ability (i.e., NA promoting the formation of both iPP polymorphs)57,61,62. It seems that the latter method is easier to apply in the industry since in most cases one can tailor the properties of the resulting material by proper changes of such processing conditions as the NA concentration in the iPP matrix and time-temperature protocols57,61, whereas in the case of the α/β compound NA one has also to control the α-NA/β-NA mass ratio and homogeneity of their mixture60. In fact, many of the up-to-date commercialized β-NAs exhibit dual nucleating activity21.
Several calcium salts of phosphoric acid diesters (CaDOPs) have been already described in the literature, for example those bearing aliphatic groups63,64, phenyl moieties65,66 or substituted aromatic rings65,67–70. However, a full crystallographic analysis has been carried out only for a few of them, namely those with [(tBuO)2PO2−]64, (PhO)2PO2− or (p-NO2PhO)2PO2− ligands65. It should be noted that in the case of homometallic systems (i.e., CaDOPs containing only calcium as the metallic centers of coordination), a common structural feature is the formation of 1D polymeric chains made of the octahedrally coordinated Ca2+ cations bridged by bidentate diorganophosphate groups and saturating their coordination sphere with oxygen ligands from water molecules64,65. The potential applications of CaDOPs range from additives impeding biomineralization65, through components of ion-selective electrodes67,68 and catalysts in asymmetric organic synthesis69, to precursors of calcium phosphate-based ceramic materials64. In addition to that, their usefulness in polymer technology have also been verified, for example as catalysts in ring-opening polymerization of cyclic carbonates and lactones66, or the already mentioned α-iPP NAs70. Recently, we have found that calcium bis(dimethylphosphate) (CaDMP) is an effective additive for iPP that produces a significant amount of the β-iPP phase under non-isothermal conditions71, and acts as a flame retardant72.
In this paper, we report on the synthesis, crystallographic structure and thermal transitions of CaDMP, as well as present the results indicating its effect on the crystallization of iPP. To the best of our knowledge, CaDMP is the first structurally characterized CaDOP hybrid polymer that does not contain any auxiliary ligands, and can be successfully applied as the α/β nucleating agent for iPP. Studies on its structure provided data helpful in determining the role of epitaxy in the β-iPP nucleation process and helped establish the structural features at the root of the β-iPP polymorph formation.
Experimental section
Materials and reagents
Unless stated otherwise, all chemicals and reagents were purchased from commercial sources and used without further purification: trimethyl phosphate (TMP) (≥ 98%, Merck Schuchardt OHG), calcium carbonate (99+%, Sigma-Aldrich), diethyl ether (pure p.a., Chempur). iPP Moplen HF501N (a homopolymer without any additives, Melt Flow Index 10 g (10 min)−1, density 900 kg m) was provided by LyondellBasell and used as a polymer matrix to prepare composites.
Preparative procedures
Synthesis of calcium dimethylphosphate
CaDMP was synthesized according to the following procedure: CaCO3 (30.00 g, 297 mmol), TMP (87.12 g, 609 mmol), and redistilled water (550 mL) were placed in a 1 L round-bottom flask equipped with a magnetic stirring bar and reflux condenser. The mixture was vigorously stirred and heated under reflux for 24 h. After the reaction completion and cooling to room temperature, the post-reaction mixture was filtered off and the clear and colourless filtrate was concentrated on a rotary evaporator at 60 °C, resulting in the formation of a large amount of white crystalline solid. After dispersing in Et2O (150 mL), the crude insoluble product was isolated via filtration on a Büchner funnel and washed with Et2O (3 × 50 mL). After a two-step drying procedure (24 h at 60 °C in a vacuum oven, followed by 6 h at 120 °C in an air dryer) 72.64 g of anhydrous CaDMP was obtained (reaction yield of 84%).
Preparation of neat iPP and its composites with CaDMP
Polypropylene materials filled with CaDMP were prepared by a two-step melt blending method. In the first step, the iPP masterbatches containing 20 wt% of CaDMP were prepared using a HAAKE PolyLab Rheometer (Thermo Fisher Scientific Inc., San Jose, CA, USA) working at 190 °C (rotor speed 100 rpm, duration 10 min.). In the second step, the obtained masterbatches were blended with a neat iPP powder at the predetermined mass ratios using a IM-15 laboratory conical twin screw extruder (ZAMAK, Skawina, Poland) (temperatures at 4 working zones: 170, 175, 180 and 185 °C, motor speed 100–200 rpm) coupled with a IMM-15 laboratory injection moulding machine (ZAMAK, Skawina, Poland) (barrel temperature 180 °C, injection pressure 7 MPa, form temperature 80 °C). The obtained iPP-based composites contained 0.2, 0.5, 1.0, 2.0 or 5.0 wt% of CaDMP. Henceforth, they will be denoted as iPP/xCaDMP, where x stands for the number describing content of CaDMP (in wt%) within the respective composite).
The sample of neat iPP was prepared from iPP powder by applying the same processing conditions as in the case of the composites.
Characterization methods
Elemental analysis
The hydrogen and carbon contents were determined using a Vario EL III elemental analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany). The measurements were carried out at 1,150 °C under helium/oxygen atmosphere. The obtained results are presented in Table S1 in the Supplementary Information (SI).
Flame atomic absorption spectroscopy (FAAS)
The calcium content was determined by means of FAAS using Aanalyst 800 atomic absorption spectrometer (Perkin-Elmer, Inc., Shelton, Connecticut, USA). The atomization of the sample was carried out in the acetylene–nitrous oxide flame. Prior to the FAAS analysis the sample was subjected to a microwave mineralisation in nitric acid using a UltraWAVE apparatus (Milestone Srl, Sorisole, BG, Italy). The obtained results are presented in Table S1 in SI.
Single-crystal X-ray crystallography
For the purpose of the X-ray crystallographic analysis the clear, aqueous filtrate obtained from the post-reaction mixture was subjected to a slow, partial evaporation at room temperature in a stream of air. A single crystal suitable for the X-ray diffraction studies was selected from the thus crystallized solid under a polarizing microscope. Subsequently, it was mounted in inert oil and transferred to the cold gas stream of the κ-CCD Gemini A Ultra diffractometer (Oxford Diffraction Company, Wroclaw, Poland). Cell refinement, as well as data collection, reduction and analysis were performed with the CrysAlisPRO software. The crystal data were processed in Olex273, solved with the ShelXT structure solution program using Intrinsic Phasing74 and refined with the SHELXL−2018/3 program refinement package using least squares minimization75. The structure was refined using the hklf 5 routine on-merohedral twins resulting in a BASF value of 0.5044(1). The twin law is a twofold rotation axis around the [001] crystallographic direction in direct space. Hydrogen atoms were added to the structure model at geometrically idealized coordinates and refined as riding atoms. The experimental parameters and crystal data for CaDMP are summarized in Table S2, S3 and S4 in SI.
Powder X-ray diffraction (PXRD) analysis
PXRD pattern of CaDMP thermolyzate was recorded at room temperature on a D8 Advance automated diffractometer (Bruker, Karlsruhe, Germany) equipped with a Lynx-Eye position-sensitive detector using Cu-Kα radiation (λ = 1.5406 Å). The data were collected in the Bragg–Brentano (θ/2θ) horizontal geometry (flat reflection mode) between the 2θ angles of 8° and 60° in steps of 0.03°, with 2 s per step (total time 384 s).
Wide-angle X-ray scattering (WAXS) analysis
WAXS measurements were performed on a URD-6 diffractometer (Seifert-FPM GmbH, Freiberg, Germany) equipped with copper target X-ray tube (λ = 1.5418 Å) operated at 40 kV and 30 mA. Cu-Kα radiation was monochromized with a graphite filter. WAXS curves were recorded in a symmetrical reflection step scanning mode, over the 2θ range of 3–60°, with the step size of 0.1° and the registration time of 20 s per step, using a scintillation counter. The samples of iPP or its composites with CaDMP subjected to the WAXS measurements had the shape of the 100 μm-thick foils produced by pressing the previously injection-moulded items for 2 min between two copper plates (at 190 °C and 5 MPa) using a hand press QC677A (Cometech Testing Machines Co., LTD, Taichung, Taiwan), and subsequent cooling to room temperature for 5 min under the mass of 2 kg.
Variable temperature PXRD (VT-PXRD) analysis
VT-PXRD measurements above room temperature were carried out on a D8 Discover instrument (Bruker, Germany) equipped with a VANTEC-1 position-sensitive detector and a DCS-350 heating stage (Anton Paar) with a temperature stability of 1 K, using Cu-Kα radiation and a step size of 0.0122°. The data were collected between the 2θ angles of 3° and 60°.
Fourier-transform infrared (FTIR) spectroscopy
FTIR analysis was conducted on a Nicolet iS5 spectrometer equipped with an iD7 diamond attenuated total reflectance accessory (Thermo Electron Scientific Instruments LLC, Madison, WI, USA)). The sample or background spectra consisted of 16 scans (1.6 s per scan).
Nuclear magnetic resonance (NMR) spectroscopy
1H and 31P NMR spectra were recorded in CD3OD on a Mercury 400 MHz spectrometer (Varian Inc., Palo Alto, CA, USA)) operating at 400.11 and 161.96 MHz, respectively. Chemical shifts are reported relative to the residual solvent signal (1H NMR) or 85% H3PO4(aq) (31P NMR). The measurements were carried out at room temperature.
Solid-state magic angle spinning (MAS) NMR spectroscopy
Standard solid-state 31P cross-polarized MAS NMR measurements were conducted on an Avance III 400 Wide Bore spectrometer (Bruker, Germany) at 22 °C with a spinning rate of 8 kHz and resonance frequency of 161.98 MHz. Data processing was performed using Bruker TopSpin software.
Differential scanning calorimetry (DSC)
DSC measurements of CaDMP were carried out on a DSC Q200 apparatus (TA Instruments, New Castle, DE, USA). The following temperature program was applied: heating run from −150 °C to 220 °C followed by a cooling run to −150 °C and another heating run from −150 °C to 220 °C. During all heating/cooling runs the temperature was changed at a steady rate of 10 °C min–1.
DSC measurements of the injection-moulded samples of neat iPP or its composites with CaDMP were carried out on a Mettler-Toledo DSC1 differential scanning calorimeter (Mettler Toledo GmbH, Greifensee, Switzerland) under a nitrogen atmosphere. The sample weights were between: 2.89 and 5.19 mg (for non-isothermal measurements), 3.66 and 4.28 mg (for isothermal measurements), or 2.20 and 5.35 mg (for isothermal stepwise crystallization measurements). One of the following time–temperature programs was applied, depending on the type of the investigated crystallization process:
for a non-isothermal crystallization: heating run from room temperature to 190 °C followed by a cooling run to 0 °C and another heating run from 0 °C to 190 °C. During all heating runs, the temperature was changed at a steady rate of 10 °C min–1, whereas the cooling run was carried out at a cooling rate prechosen from the 3–10 °C min–1 range;
for an isothermal crystallization: heating run from room temperature to 200 °C at 10 °C min, isothermal run at 200 °C for 15 min, followed by a cooling run to 132 °C with a rate of 20 °C min–1 and isothermal crystallization at that temperature for 939–5400 s (depending on the time required for a complete evolution of the crystallization exotherm). After crystallization completion, the sample was heated to 200 °C at the 5 °C min–1 rate. Please note that during a cooling run no first- or second-order thermal transitions were observed even for the composite system with the highest CaDMP content;
for a stepwise isothermal crystallization: heating run from room temperature to 200 °C at 10 °C min–1, isothermal run at 200 °C for 15 min, followed by a cooling run to 132 °C with a rate of 20 °C min–1 and isothermal crystallization at that temperature for a predefined time. Subsequently, the temperature was increased to 140 °C at a rate of 20 °C min–1 and then to 200 °C at a rate of 5 °C min–1.
Thermogravimetry (TG) in air
TG analysis of CaDMP was performed in a stream of synthetic air using a Mettler-Toledo TGA/DSC1 thermogravimetric analyser equipped with a SDTA sensor (Mettler Toledo GMBH, Greifensee, Switzerland). The sample (12.018 mg) was heated from 30 °C to 900 °C, at a heating rate of 10 °C min–1.
Simultaneous thermal analysis (STA)
Volatile thermal decomposition products and thermal effects of the processes occurring during heating were studied by TG coupled with differential thermal analysis (DTA) and quadrupole mass spectrometry (QMS) using a STA 449 C Jupiter apparatus (Netzsch, Selb, Germany) coupled with a QMS 403 C Aeolos quadrupole mass spectrometer (Netzsch, Selb, Germany). The sample was placed in Al2O3 crucible and heated in a stream of argon (flow rate of 90 mL min–1) from 30 °C to 1,000 °C at a heating rate of 5 °C min–1.
Scanning electron microscopy (SEM)
SEM images of the samples were taken using a Helios 5 PFIB scanning electron microscope (Thermo Fisher Scientific, Hillsboro, OR, USA) equipped with a high-resolution Elstar field emission electron column, a Phoenix ion column with inductively coupled Xe+ plasma, and a set of Elstar detectors for secondary electrons (standard ETD detector placed in the chamber) and intra-column TLD (for high-resolution immersion imaging), ABS/CBS scatter electrons detector, STEM3 + detector, and ICE detector for ion imaging. During the measurements, the working distance of ca. 4.0 mm and the accelerating voltage of the electron beam of 5 kV were used.
Before the SEM measurement, all samples were sputtered with a 10 nm-thick Au layer using a CCU-010 high-vacuum sputtering machine (Safematic GmbH, Zizers, Switzerland).
Mechanical tests of the injection-moulded specimens
Tensile tests based on the PN-EN ISO 527 standard were carried out on an Instron 33R universal machine (Instron, Norwood, MA, USA), with a crosshead speed of 100 mm min–1. The results presented in the manuscript are the average of the measurements of 3 samples.
Charpy impact tests based on the PN-EN ISO 179 standard were conducted on a Zwick HIT 50P apparatus (Zwick Roell, Ulm, Germany) equipped with the 0.5 J pendulum. The results presented in the manuscript are the average of the measurements of 3 samples.
Results and discussion
CaDMP synthesis and thermal stability
CaDMP was synthesized according to a modified method that previously had been utilized successfully by our research group in the case of its zinc-containing analogue76. Compared to the methods already reported in the literature, our hydrothermal pathway offered significant advantages since it proceeded with a very high yield (typically exceeding 80%), utilized commercially available, non-hazardous reagents, and did not require any special reaction conditions or apparatus (e.g., inert atmosphere, anhydrous organic solvents, pressure vessels). It was based on a simple acid-base type reaction taking place in aqueous medium between CaCO3 and dimethyl phosphate generated in situ via hydrolysis of one phosphoester group from TMP molecule. The intermediate product, namely CaDMP hydrate, easily underwent dehydration to anhydrous CaDMP even at room temperature, but drying in a stream of hot air helped to complete that process.
The presence of only one type of ligands (e.g., (CH3O)2PO2− groups) within CaDMP structure was confirmed by the results of elemental analysis (carbon, hydrogen and calcium contents, see Table S1 in SI), which showed a very good agreement (e.g., up to 4.4% of a relative difference) with the values theoretically calculated for the polymer with a general formula of Ca[O2P(OCH3)2]2. FTIR spectrum of CaDMP (Fig. S1 in SI) also lacked any absorption bands related to a free or coordinated water, such as those ascribed to either the O–H stretching (a spectrum region above 3,000 cm–1) or HOH bending (at ca. 1,640 cm–1) modes. It corresponded very well to the infrared absorption spectrum of barium dimethylphosphate77, showing the presence of the aliphatic C–H stretching and bending bands (2,840–3,000 cm–1 and 1,440−1,465 cm–1 regions, respectively), as well as four absorption signals located at 1,230, 1,088, 806 and 743 cm–1 that could be ascribed to the asymmetric and symmetric stretching vibrations within the phosphate moiety: the first two of them arise from the P–O bonds involved in calcium coordination, whereas those below 1,000 cm−1 were related to the phosphoester linkages (their spectral position is less sensitive to the change of the metal cation in the analysed compound)76–78. The two most prominent bands observed on the FTIR spectrum of CaDMP (at 1,037 and 1,060 cm) resulted from the stretching modes of the C–O bonds77,78.
Further evidence of the purity of CaDMP was given by the observation of single resonance peaks on its 31P NMR spectra recorded either in the solid state (δP = 0.01 ppm, Fig. S2 in SI) or in solution (δP = 4.09 ppm, Fig. S3 in SI). These findings suggested that at the resolution of both 31P NMR methods all phosphorus nuclei of CaDMP are in the same chemical environment and no traces of an unreacted TMP, or products of further hydrolysis of phosphoester bonds could be detected. Interestingly, 1H NMR spectrum of CaDMP (Fig. S4 in SI) contained two peaks located at 3.58 and 3.55 ppm, with integrals of an equal value. We have previously reported the same phenomenon (e.g., “double” methyl protons’ NMR signal and one population of magnetically distinct phosphorus nuclei) in the case of zinc bis(dimethylphosphate) NMR spectra76, so it can be assumed that in a strongly polar solvent CaDMP exhibited a similar behaviour as the former: it underwent depolymerization to monomeric species or free ions. From that point of view, the occurrence of two 1H NMR methyl signals could result from phosphorus-proton spin-spin coupling, especially that such type of coupling via 3 bonds and characterized by the coupling constants around 10 Hz has been already reported for various dimethylphosphates, including barium salt79.
The results of TG analysis (Fig. S5 and Table S5 in SI) showed that in air CaDMP was thermally stable up to 260–294 °C, depending on the selected criterion of the beginning of its thermolysis: the lowest value characterized the temperature of a bending point on the TG curve (Tb = 257.7 °C), whereas both the extrapolated onset temperature (Tonset = 275.7 °C) and the temperature at which CaDMP sample lost the first 2% of its initial mass (T98% = 279.4 °C) were higher. Oxidative degradation proceeded via several overlapping steps (a maximum rate of decomposition was observed around T = 299 °C) during which the sample lost almost half of its mass and transformed into a mixture of inorganic calcium condensed phosphates, among which the crystalline phases of calcium metaphosphate and calcium pyrophosphate could be identified by means of PXRD studies (see Fig. S6 in SI) – phase identification was performed using the PDF-2 database80. Interestingly, the quadrupole mass spectrometry (QMS) spectra of the gaseous products evolved during CaDMP pyrolysis (Fig. S7 in SI) showed a group of signals characteristic of the oxophosphorus species. A similar situation has been reported in the case of aluminum tris(dimethylphosphate) (AlDMP) pyrolysis81. In fact, the QMS spectra of CaDMP and AlDMP closely match each other suggesting that both these compounds undergo pyrolytic degradation according to the same set of chemical transformations, e.g., a homolytic P–OCH3 bond scission and subsequent rearrangements of the newly liberated methyl and methoxyl radicals, resulting in the formation of identical volatiles that included dimethyl ether, methanol, as well as TMP and its products of thermal degradation81. It is worth noting, that these findings have also been confirmed by the FTIR detection of TMP in the liquid phase obtained after a condensation of volatiles formed during CaDMP pyrolysis in nitrogen72.
Crystal structure of CaDMP and unit cell parameters at different temperatures
A single-crystal X-ray diffraction studies revealed that CaDMP crystallizes from water solution as a 1D coordination polymer in a monoclinic space group P21/c – the crystal data and experimental conditions are summarized in Table S2, S3 and S4 (SI).
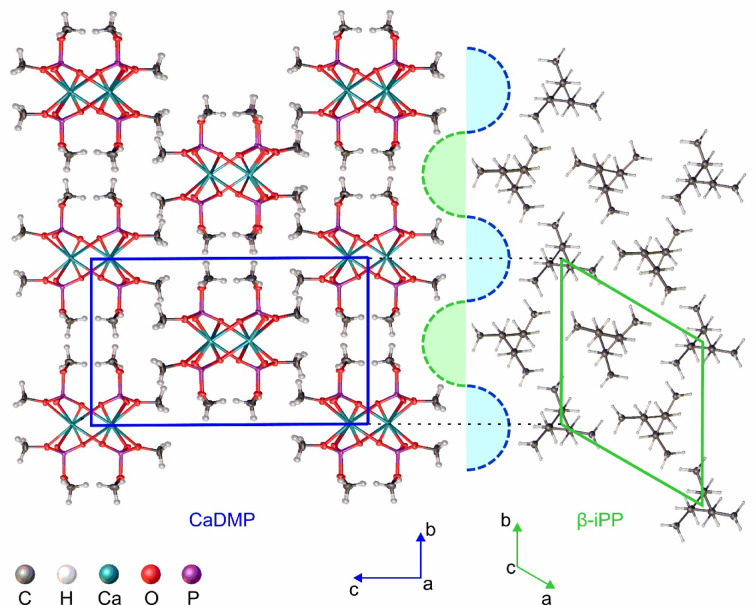
The asymmetric unit of CaDMP, comprising one calcium cation and two dimethylphosphate (DMP) ligands, is depicted in Fig. S8 (SI). In the crystal structure of CaDMP calcium cations are surrounded by six DMP moieties occupying eight coordination sites of the metal center (Fig. S8 in SI). Each Ca2+ ion is linked with the adjacent one via a bridging binding mode of the tridentate DMP ligand thus forming a 1D polymer, as shown in Fig. 1. One methoxy group of each DMP anion remains uncoordinated and acts as a terminal group isolating the neighbouring polymeric chains (Fig. S9 in SI). SEM images showed that 1D polymeric chains of CaDMP organized into a few micrometre long, rod-like particles having a hexagonal cross-section (Fig. S10 in SI).
Fig. 1.
Structure of the 1D polymeric chain of CaDMP in a view along the crystallographic z-axis. For the clarity of presentation hydrogen and carbon atoms are omitted.
In accordance with the convention recommended by IUPAC82, the methyl substituents around both crystallographically distinct phosphorus centers within CaDMP structure adopt a thermodynamically stable gauche–gauche conformation (G+G+)78 since their dihedral angles are within the 65–83° and 62–70° regions. The analysis by means of the bond–valence vector (BVV) model83 (see SI for a detailed description of its protocol) indicated almost no structural strains in the geometry of calcium and phosphorus coordination polyhedra, since the values of the length of the resultant BVV calculated for them were on a very low level (ca. 0.04 and 0.10 valence units for phosphorus and calcium, respectively, Table S6 in SI). These structural features were enough to create crystallographically distinct PO4 tetrahedra, that were, however, indistinguishable from one another in the 31P MAS NMR spectrum of CaDMP (see Fig. S2 in SI).
It should be noted, that the structural analysis of CaDMP was carried out at −153 °C, which was significantly lower than the temperature usually applied during β-iPP crystallization (ca. 120–140 °C). A knowledge that all materials are prone to temperature-driven structural transformations (e.g., thermal expansion/contraction and/or polymorphic transitions), combined with the fact that a successful epitaxial growth of the β-iPP phase on the surface of NAs particles requires a good matching between their crystal lattice parameters23,36,47, prompted us to investigate the impact of temperature on the CaDMP structure. DSC curves recorded for CaDMP between −150 and 220 °C (Fig. S11 in SI) did not contain any signals attributable to thermal effects of the first- or second-order phase transitions suggesting that, within this temperature region, CaDMP remained solid and existed in a single polymorphic form. The same conclusions could be derived from the PXRD diffractograms recorded at different temperatures (i.e., the results of a variable temperature PXRD, VT-PXRD, measurements) – Fig. S12 and Table S7 in SI contain data on the CaDMP crystal lattice parameters obtained by means of a careful indexing of the observed X-ray reflections. As can be seen, the unit cell of CaDMP underwent a typical expansion on heating from room temperature to 190 °C (or contraction when being cooled from that temperature), albeit this process was characterized by a large anisotropy. The susceptibility of the CaDMP lattice parameters to the temperature changes increased in the order of a- < c- < b-parameter, as shown by the values of the linear thermal expansion coefficients (αi) along the chosen crystallographic i direction calculated according to the previously described method84: for the VT-PXRD heating mode αa, αb and αc equalled to 5.84(92) ×10–6, 11.41(20) ×10–5 and 2.46(9) ×10–5K–1, respectively, whereas the volumetric thermal expansion coefficient βV had the value of 14.51(29) ×10–5 K–1 (see Table S8 in SI). It should be noted that the values of CaDMP lattice parameters and unit cell volume calculated at −153 °C using Eqs. (S10)–(S13) (SI), were very close to those directly determined for CaDMP monocrystal (in each case the difference was less than 1% of the experimental value), which not only proved the correctness of these equations but was also a clear evidence that within the investigated temperature region (e.g., between −153 and 190 °C) CaDMP did not reorganize its crystal structure.
iPP-nucleating properties of CaDMP
Isothermal crystallization
The impact of CaDMP on the isothermal crystallization of iPP was investigated at 132 °C for the samples of neat iPP and its composites loaded with 0.2–5.0 wt% of the filler. Figure 2; Table 1 present the DSC data on the evolution of crystallization exotherms during an isothermal cycle, as well as a melting behaviour of the thus crystallized iPP phases. Abbreviations: ΔHc, total – total enthalpy of crystallization (an overall area of the crystallization exotherm) calculated with respect to the mass of polymer in the sample; Tm1 and Tm2 – melting temperatures recorded during heating of the previously crystallized samples; 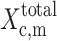 – overall absolute degree of crystallinity of the sample calculated based on the melting endotherms (see a detailed description in SI);
– overall absolute degree of crystallinity of the sample calculated based on the melting endotherms (see a detailed description in SI); 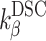 – fraction of the β-iPP crystals within the iPP crystal phase determined by means of DSC.
– fraction of the β-iPP crystals within the iPP crystal phase determined by means of DSC.
Fig. 2.
DSC curves of neat iPP and its composites with different loadings of CaDMP recorded during: (a) an isotherm at 132 °C, and (b) a subsequent heating to 200 °C (with a heating rate of 10 °C min–1).
Table 1.
Selected DSC parameters estimated during isothermal crystallization carried out at 132 °C for neat iPP and its composites with cadmp.
| CaDMP content/wt% | ΔHc, total/J g–1 |
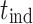 /s /s |
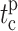 /s /s |
 /s /s |
Tm1/°C | Tm2/°C |
 /% /% |
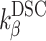
|
|---|---|---|---|---|---|---|---|---|
| isothermal step |
heating step after isothermal crystallization |
|||||||
| 0.0 | 95.96 | 481 | 1,742 | 1,599.7 | – | 166.07 | 61.1 | – |
| 0.2 | 80.19 | 155 | 427 | 331.2 | 152.97 | 165.76 | 57.1 | 0.56 |
| 0.5 | 81.97 | 114 | 315 | 237.6 | 153.01 | 165.79 | 57.9 | 0.52 |
| 1.0 | 83.81 | 78 | 243 | 198.7 | 153.65 | 165.75 | 56.8 | 0.52 |
| 2.0 | 79.30 | 46 | 128 | 89.8 | 151.63 | 164.32 | 53.1 | 0.57 |
| 5.0 | 82.88 | 34 | 102 | 72.2 | 151.94 | 164.82 | 56.2 | 0.47 |
As can be seen in Fig. 2, the isothermal crystallization of neat iPP at 132 °C was a time-consuming process and required more than 1 h for its completion. Incorporating CaDMP particles into iPP substantially increased the rate at which the latter crystalized, since with increasing CaDMP content the crystallization exotherm became narrower and moved to the lower values region on the time axis. This observation was also supported by the changes in the values of all time parameters usually determined for the isothermal crystallization of polymers, namely the induction time (a period of time from the beginning of the isotherm to the first deviation of the DSC signal from the baseline, tind), time of the exotherm maximum (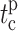 ), and the crystallization exotherm’s full width at half maximum (
), and the crystallization exotherm’s full width at half maximum (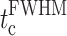 ). From the data presented in Table 1 it is evident that with increasing content of CaDMP the values of all these parameters were lowered compared to neat iPP (by 68–96% depending on both the type of a parameter and the filler’s loading in the composite). The best effects were observed for the system with the highest content (i.e., 5 wt%) of CaDMP, in which both the nucleation stage of the crystallization process was accelerated (e.g., its tind was lowered to 34 s from 481 s characterizing the unmodified iPP matrix), and the overall rate of the iPP crystal phase growth increased (e.g., ca. 22 times reduction in its
). From the data presented in Table 1 it is evident that with increasing content of CaDMP the values of all these parameters were lowered compared to neat iPP (by 68–96% depending on both the type of a parameter and the filler’s loading in the composite). The best effects were observed for the system with the highest content (i.e., 5 wt%) of CaDMP, in which both the nucleation stage of the crystallization process was accelerated (e.g., its tind was lowered to 34 s from 481 s characterizing the unmodified iPP matrix), and the overall rate of the iPP crystal phase growth increased (e.g., ca. 22 times reduction in its  in comparison to neat iPP).
in comparison to neat iPP).
In order to carry out a more detailed analysis of the iPP isothermal crystallization kinetics we applied a widely-recognized mathematical modeling method based on the Avrami–Evans theory that relates the relative volumetric degree of crystallinity of the sample (Xt) to the time elapsed since the beginning of the crystallization exotherm formation85. By taking into account the overall time of the DSC isothermal cycle (t) and tind, the basic and simplest form of the Avrami–Evans equation can be expressed by Eq. (1)85:
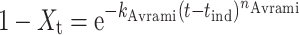 |
1 |
or its linearized (double logarithmic) version (Eq. (2)):
 |
2 |
where nAvrami is the Avrami index related to the dimensionality of the growing crystals and nucleation mode, whereas kAvrami denotes the Avrami overall crystallization rate constant referring to both nucleation and crystal growth stages. It should be noted that kAvrami is given in units of 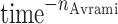 and for comparison purposes should be normalized to the nAvrami–independent parameter KAv (expressed in units of time–1) by elevating its value to the power nAvrami−1 (i.e.,
and for comparison purposes should be normalized to the nAvrami–independent parameter KAv (expressed in units of time–1) by elevating its value to the power nAvrami−1 (i.e., 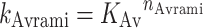 ). Moreover, for the known values of nAvrami, kAvrami and Xt= 0.5 a solution of Eq. (1) gives Eq. (3) defining the theoretical half crystallization time (
). Moreover, for the known values of nAvrami, kAvrami and Xt= 0.5 a solution of Eq. (1) gives Eq. (3) defining the theoretical half crystallization time (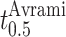 )85.
)85.
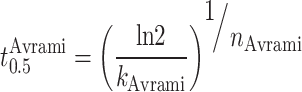 |
3 |
In the case of neat iPP and its composites with CaDMP we calculated the Xt values at various periods of isothermal crystallization process by integrating their DSC exotherms and subsequently applying Eq. (4):
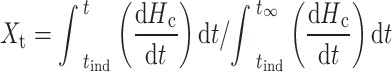 |
4 |
where dHc refers to the measured enthalpy of crystallization during an infinitesimal period of time dt and t∞ is the time required for the completion of the crystallization process. By plotting ln[−ln(1 − Xt)] versus ln(t − tind) we obtained the values of both nAvrami and ln(kAvrami) as the slopes and intercepts of the resulting straight lines, respectively. Figure 3 shows the variation of Xt with the time of isothermal crystallization and the Avrami plots applied in the linear fitting procedure, whereas Table 2 contains the Avrami kinetic parameters calculated for the investigated polymeric systems (R2 is the coefficient of determination providing information about the goodness of fit of the Avrami linear regression model,  and
and  denote crystallization half time calculated based on the Avrami–Evans theory in accordance to Eq. 3 and estimated from the experimental datapoints presented in Fig. 3, respectively, rounded to the nearest integer). It should be noted that, in accordance with the guidelines proposed in the literature86, the linear fitting was carried out only for the data corresponding to the Xt values between 0.03 and 0.20, since it minimized errors related to either a low value of the heat flow signal at the initial period of the iPP isothermal crystallization, or a nonlinearity of the Avrami plots occurring during the secondary crystallization stage.
denote crystallization half time calculated based on the Avrami–Evans theory in accordance to Eq. 3 and estimated from the experimental datapoints presented in Fig. 3, respectively, rounded to the nearest integer). It should be noted that, in accordance with the guidelines proposed in the literature86, the linear fitting was carried out only for the data corresponding to the Xt values between 0.03 and 0.20, since it minimized errors related to either a low value of the heat flow signal at the initial period of the iPP isothermal crystallization, or a nonlinearity of the Avrami plots occurring during the secondary crystallization stage.
Fig. 3.
Kinetics of the isothermal crystallization of neat iPP and its composites with various CaDMP loadings: (a) variation of Xt with isothermal crystallization time, (b) Avrami plots from which nAvrami and kAvrami were obtained, (c) changes in the overall crystallization rate (reciprocal crystallization half time). (1) neat iPP; (2) iPP/0.2CaDMP; (3) iPP/0.5CaDMP; (4) iPP/1CaDMP; (5) iPP/2CaDMP; (6) iPP/5CaDMP.
Table 2.
Kinetic parameters calculated based on the Avrami-Evans theory applied to isothermal crystallization (T = 132 °C) of neat iPP and its composites with cadmp.
| CaDMP loading/wt% | n Avrami | kAvrami/
|
R 2 | KAv /min–1 |

|

|
|---|---|---|---|---|---|---|
| 0.0 | 2.144(1) | 8.23(2) ×10–4 | 0.99991 | 0.0364(1) | 1,389 | 1,342 |
| 0.2 | 2.285(2) | 0.01831(3) | 0.99993 | 0.1736(4) | 294 | 287 |
| 0.5 | 2.351(3) | 0.03336(5) | 0.99991 | 0.2355(6) | 218 | 213 |
| 1.0 | 2.385(3) | 0.05111(5) | 0.99993 | 0.2874(5) | 179 | 176 |
| 2.0 | 2.575(5) | 0.2518(6) | 0.99989 | 0.5854(11) | 89 | 88 |
| 5.0 | 2.636(5) | 0.4107(12) | 0.99992 | 0.7135(13) | 73 | 72 |
Analysis of the Xt versus (t − tind) curves (Fig. 3) clearly indicated that with increasing concentration of CaDMP within the iPP composite the system required less time to reach any given value of Xt, thus suggesting that the overall rate of crystallization was also increased. This phenomenon could be quantified by tracking the changes in the values of two important kinetic parameters: the already mentioned kAvrami (or its nAvrami-independent alternative, KAv), as well as crystallization half time (a time that elapsed from the onset of crystallization exotherm until the relative crystallinity of the system reached half of its total level) whose inverse represents the overall rate of isothermal crystallization of iPP85. The data presented in Table 2; Fig. 3 show that even the smallest amount of CaDMP (i.e., 0.2 wt%) caused a 22-times increase in kAvrami (which equals to an almost fivefold increase in KAv) and reduced the value of 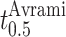 by ca. 79% compared to neat iPP. This general trend continued for the other composite systems, so that the one containing 5 wt% CaDMP exhibited the kAvrami, KAv and
by ca. 79% compared to neat iPP. This general trend continued for the other composite systems, so that the one containing 5 wt% CaDMP exhibited the kAvrami, KAv and 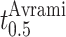 values of 0.4107(12)
values of 0.4107(12) 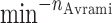 , 0.7135(13) min–1 and 73 s, respectively. For that composite the overall rate of isothermal crystallization equalled ca. 0.82 min–1, whereas at the same temperature conditions the neat iPP sample crystallized with a rate of ca. 0.04 min–1 (Fig. 3). It should be noted, that the differences between the values of half crystallization times calculated from the Avrami equations (
, 0.7135(13) min–1 and 73 s, respectively. For that composite the overall rate of isothermal crystallization equalled ca. 0.82 min–1, whereas at the same temperature conditions the neat iPP sample crystallized with a rate of ca. 0.04 min–1 (Fig. 3). It should be noted, that the differences between the values of half crystallization times calculated from the Avrami equations (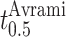 ) and those estimated from the experimental data (
) and those estimated from the experimental data ( ) were very small (ca. 1.1–3.5% of the
) were very small (ca. 1.1–3.5% of the  values), which proved a high goodness of fit of the adopted mathematical model85.
values), which proved a high goodness of fit of the adopted mathematical model85.
Interestingly, the incorporation of CaDMP into iPP was accompanied by a small (ca. 13–17%) decrease in the total enthalpy of isothermal crystallization (see Table 1). In our opinion, this phenomenon could be explained in terms of a lower absolute crystallinity of the composite samples, and/or the changes in the polymorphic type of iPP crystal phases that were formed therein. In fact, in the case of the iPP/CaDMP samples the DSC heating curves recorded directly after their isothermal crystallization (see Fig. 2) showed the occurrence of two partially overlapping endothermal transitions located around 152 and 165 °C, contrary to the neat iPP sample that underwent only one melting process at ca. 166 °C (Table 1). Based on the literature data2, the process proceeding at higher temperature can be ascribed to the melting of α-iPP, whereas that occurring at lower temperature – to the melting of β-iPP. Thus, one could conclude that the neat iPP sample contained only the former polymorph, whereas the iPP/CaDMP composites were a mixture of the α- and β-iPP crystal phases. Based on the DSC procedure proposed in the literature46, we approximated the contribution of β-iPP to the overall absolute degree of iPP crystallinity ( ) of the investigated materials (β-iPP fraction,
) of the investigated materials (β-iPP fraction,  ) defined by Eq. 5:
) defined by Eq. 5:
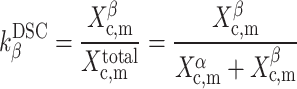 |
5 |
where 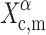 and
and 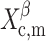 are the relative crystallinities of the α- and β-iPP phase, respectively (for a more detailed description of this procedure see Fig. S13 in SI). The obtained values of
are the relative crystallinities of the α- and β-iPP phase, respectively (for a more detailed description of this procedure see Fig. S13 in SI). The obtained values of 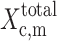 and
and 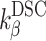 (Table 1) suggest that the incorporation of CaDMP into iPP had a negligible effect on the overall ability of iPP to formation of the crystalline domains, since the
(Table 1) suggest that the incorporation of CaDMP into iPP had a negligible effect on the overall ability of iPP to formation of the crystalline domains, since the 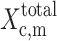 values of the iPP/CaDMP composites were on a comparable level with that of the unmodified iPP. However, the presence of CaDMP particles caused that approximately half of the obtained polymer crystals exhibited the β-iPP structure.
values of the iPP/CaDMP composites were on a comparable level with that of the unmodified iPP. However, the presence of CaDMP particles caused that approximately half of the obtained polymer crystals exhibited the β-iPP structure.
It is worth noting, that the kinetics of isothermal crystallization of the α- and β-iPP phases cannot be evaluated separately during a conventional calorimetric experiment due to a lack of separation of their respective exotherms. In order to overcome this problem, we applied the isothermal stepwise crystallization (ISC) procedure85. The samples had been isothermally crystallized for different periods of time to attain different degrees of crystallinity, and then their polymorphic compositions were investigated from the melting endotherms recorded during a subsequent heating steps. The results of these experiments for the iPP/0.2CaDMP and iPP/5CaDMP composites are shown in Fig. 4. Based on these data, one can conclude that regardless of the CaDMP content both polymorphs of iPP were formed simultaneously and could be detected from the beginning of the isothermal crystallization process. However, in the case of the system with the lowest NA loading the content of each iPP polymorph increased in a comparable manner, with β-iPP dominating the crystal domains only at the highest crystallinity conversion. On the other hand, in iPP/5CaDMP α-iPP prevailed over β-iPP throughout the entire process, albeit the difference between their contents diminished with the progress of isothermal crystallization. The reason for such behavior is still unknown and needs further experimental investigation.
Fig. 4.
Isothermal stepwise crystallization. Variation of the α-iPP and β-iPP contents (expressed as a degree of crystallinity of a given crystal phase i, 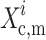 ) with a progress of the overall crystallization in the iPP-based composites with: (a) 0.2 wt% and (b) 5 wt% of CaDMP.
) with a progress of the overall crystallization in the iPP-based composites with: (a) 0.2 wt% and (b) 5 wt% of CaDMP.
Crystallization under non-isothermal conditions
The process of crystallization under non-isothermal conditions was investigated for neat iPP and its composites with CaDMP by means of DSC during cooling from the melt (at a cooling rate of 10 °C min–1). The type of the resulting crystal domains was analysed the same way as in the case of isothermal crystallization, i.e., thru their melting endotherms occurring during a subsequent heating cycle. Figure 5 presents the DSC curves recorded during both these cycles, while some of the characteristic parameters of crystallization are summarized in Table 3: Tini – initial temperature of crystallization;  and
and  – maximum and full width at half maximum of the exothermic peak, respectively;
– maximum and full width at half maximum of the exothermic peak, respectively;  – crystallization half time estimated from the experimental datapoints (its value rounded to the nearest integer);
– crystallization half time estimated from the experimental datapoints (its value rounded to the nearest integer); 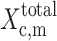 – absolute degree of crystallinity of the sample calculated based on the melting endotherms;
– absolute degree of crystallinity of the sample calculated based on the melting endotherms; 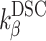 – fraction of the β-iPP crystals within the iPP crystal phase.
– fraction of the β-iPP crystals within the iPP crystal phase.
Fig. 5.
Normalized DSC traces of neat iPP and its composites with different loadings of CaDMP recorded during: (a) a cooling cycle, and (b) a subsequent heating to 190 °C. Temperature change of 10 °C min. For the clarity of presentation only the temperature regions corresponding to the iPP crystallization or melting are shown.
Table 3.
Selected parameters estimated during non-isothermal crystallization of the iPP/CaDMP composites carried out at cooling/heating rates of 10 °C min–1.
| CaDMP content/wt% | ΔHc, total /J g |
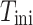 /°C /°C |
 /°C /°C |
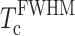 /°C /°C |
 /s /s |
Tm1/°C | Tm2/°C |
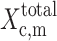 /% /% |

|
|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 94.70 | 127.47 | 113.74 | 8.24 | 79 | – | 163.57 | 58.4 | – |
| 0.2 | 91.79 | 129.09 | 118.78 | 5.22 | 64 | 148.60 | 163.55 | 58.6 | 0.37 |
| 0.5 | 93.33 | 130.59 | 120.28 | 4.96 | 66 | 149.06 | 163.38 | 59.4 | 0.43 |
| 1.0 | 90.20 | 128.48 | 121.91 | 5.16 | 45 | 150.76 | 164.69 | 60.5 | 0.54 |
| 2.0 | 91.77 | 129.59 | 124.27 | 4.10 | 36 | 149.71 | 163.16 | 58.8 | 0.54 |
| 5.0 | 98.45 | 130.92 | 124.91 | 4.49 | 41 | 150.28 | 163.47 | 59.7 | 0.43 |
In comparison with neat iPP, the crystallization exotherms of the systems filled with CaDMP became narrower and were shifted towards higher temperatures. Based on these findings, one can assume that under the investigated non-static thermal conditions a crystallization of the iPP matrix was facilitated upon incorporation of the CaDMP particles: a small (i.e. by ca. 1–4 °C) increase in the Tini values indicated slightly lower energy barriers during the nucleation stage, while the higher temperatures of the maximum heat release rate (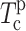 ) combined with a 36–50% decrease in the
) combined with a 36–50% decrease in the 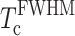 values could result from a larger number of crystallization nuclei being formed and/or the positively affected crystal growth rate of iPP. A combination of both these effects effectively accelerated the iPP crystal phase formation, since even at the lowest filler’s concentration (i.e. 0.2 wt% of CaDMP) the overall crystallization rate (approximated by the reciprocal crystallization half time) was increased by ca. 34% compared to the unmodified iPP. The extent of this change became even greater at higher CaDMP loadings, reaching a level of the 83–93% increase in the case of the samples containing 2–5 wt% of CaDMP. It is worth noting, that the presence of CaDMP during cooling of the molten iPP matrix promoted a formation of the α- and β-iPP crystals, as revealed by the bimodal melting profiles of the investigated composites (see Fig. 5). In fact, the recorded DSC melting curves, as well as crystallinity of the samples and the contents of each iPP polymorph (see
values could result from a larger number of crystallization nuclei being formed and/or the positively affected crystal growth rate of iPP. A combination of both these effects effectively accelerated the iPP crystal phase formation, since even at the lowest filler’s concentration (i.e. 0.2 wt% of CaDMP) the overall crystallization rate (approximated by the reciprocal crystallization half time) was increased by ca. 34% compared to the unmodified iPP. The extent of this change became even greater at higher CaDMP loadings, reaching a level of the 83–93% increase in the case of the samples containing 2–5 wt% of CaDMP. It is worth noting, that the presence of CaDMP during cooling of the molten iPP matrix promoted a formation of the α- and β-iPP crystals, as revealed by the bimodal melting profiles of the investigated composites (see Fig. 5). In fact, the recorded DSC melting curves, as well as crystallinity of the samples and the contents of each iPP polymorph (see 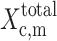 and
and 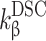 values presented in Table 3) are close to those estimated during a previously discussed process of isothermal crystallization.
values presented in Table 3) are close to those estimated during a previously discussed process of isothermal crystallization.
The results of preliminary studies discussed above, prompted us to a more detailed investigation of the impact of CaDMP on the kinetics of non-isothermal crystallization of iPP. For that purpose, neat iPP and its two composites (i.e. the systems filled with 0.2 wt% and 1.0 wt% of CaDMP) were selected and crystallized from melt at different cooling rates (φ, 3–10 °C min–1). The recorded crystallization exotherms were analyzed and integrated (see Table 4 and Fig. S14 in SI), providing the data for a calculation of the relative crystallinity as a function of temperature (XT) according to Eq. (6):
Table 4.
Selected parameters estimated during non-isothermal crystallization of iPP and its composites filled with 0.2 or 1.0 Wt% of CaDMP carried out at different cooling rates.
| CaDMP content/wt% | φ/°C min–1 | ΔHc, total/J g–1 |
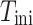 /°C /°C |
 /°C /°C |
 /°C /°C |
 /s /s |
CRP/°C–1 |
|---|---|---|---|---|---|---|---|
| 0.0 | 3 | 97.28 | 131.94 | 118.55 | 5.50 | 249 | 0.066(18) |
| 5 | 94.05 | 125.49 | 116.29 | 3.85 | 112 | ||
| 7 | 94.75 | 128.03 | 113.71 | 7.36 | 116 | ||
| 10 | 94.70 | 127.47 | 113.74 | 8.24 | 79 | ||
| 0.2 | 3 | 92.85 | 133.19 | 124.71 | 3.60 | 166 | 0.083(16) |
| 5 | 90.90 | 130.89 | 122.32 | 4.10 | 103 | ||
| 7 | 92.80 | 128.96 | 120.67 | 4.73 | 72 | ||
| 10 | 91.79 | 129.09 | 118.78 | 5.22 | 64 | ||
| 1.0 | 3 | 92.59 | 133.71 | 127.01 | 3.37 | 139 | 0.130(6) |
| 5 | 91.26 | 131.14 | 124.58 | 3.42 | 81 | ||
| 7 | 90.82 | 130.36 | 123.98 | 4.20 | 60 | ||
| 10 | 90.20 | 128.48 | 121.91 | 5.16 | 45 |
 |
6 |
where Tc or T∞ refer to the crystallization temperature at the time t or after the completion of the non-isothermal crystallization process, respectively. XT can be converted to a time-resolved Xt by applying Eq. (7). Fig. S14 (SI) presents the evolution of Xt with the non-isothermal crystallization time t for 3 materials mentioned above.
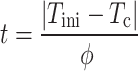 |
7 |
As can be seen in Table 4 for all tested materials, an increase in a cooling rate led to a shift of the crystallization peak to the lower temperature region, and its widening. At any given cooling rate, the estimated temperatures of the initiation and maximum rate of crystallization characterizing the systems modified with CaDMP were higher than those related to neat iPP, and this trend intensified with the increasing CaDMP content. All these findings proved that the crystallization of iPP accelerated in the presence of CaDMP, regardless of the rate of temperature change. In order to evaluate this more quantitatively, we analyzed the crystallization rate parameter (CRP), which can be considered as a factor representing the overall crystallization rate during a non-isothermal crystallization process. CRP was first introduced for polypropylene by Ma and coworkers, and defined as the slope of a straight line obtained after plotting the reciprocal of crystallization half time versus φ87. Our composite systems loaded with 0.2 or 1.0 wt% of CaDMP exhibited CRP values higher by ca. 45% or 85%, respectively, than that of the unmodified iPP, indicating a much faster crystallization (see Table 4, standard error of the CRP value is presented in parenthesis). This conclusion found further support in the results of a mathematical modeling of the non-isothermal crystallization kinetics according to the Liu and Mo methodology88.
At a given relative degree of crystallinity, the Liu and Mo model correlates the crystallization time t and the cooling rate φ, following a simplified Eq. (8):
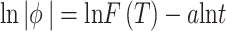 |
8 |
where a is the parameter containing information about the dimensionality of crystallization and nucleation mode88. At a given Xt, the 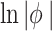 versus
versus 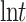 plot produces a straight line and the values of a and lnF(T) can be easily obtained as the slope and intercept of that line, respectively. F(T) is a temperature-dependent complex function that refers to the cooling rate value required by the crystallizing system to reach a certain value of Xt in a unit crystallization time. Because of that, its value can be treated as a good probe for an assessment of the overall crystallization rate: the lower it is, the faster the crystallization process proceeds. Fig. S15 (SI) presents the Liu and Mo plots acquired for the investigated iPP/CaDMP systems at Xt between 0.1 and 0.9, whereas Fig. 6 and Table S9 (SI) show the calculated values of a and F(T) kinetic parameters.
plot produces a straight line and the values of a and lnF(T) can be easily obtained as the slope and intercept of that line, respectively. F(T) is a temperature-dependent complex function that refers to the cooling rate value required by the crystallizing system to reach a certain value of Xt in a unit crystallization time. Because of that, its value can be treated as a good probe for an assessment of the overall crystallization rate: the lower it is, the faster the crystallization process proceeds. Fig. S15 (SI) presents the Liu and Mo plots acquired for the investigated iPP/CaDMP systems at Xt between 0.1 and 0.9, whereas Fig. 6 and Table S9 (SI) show the calculated values of a and F(T) kinetic parameters.
Fig. 6.
Non-isothermal crystallization of iPP and its composites with CaDMP: variation of the Liu and Mo kinetic parameter F(T) with Xt.
Data presented in Fig. 6, clearly proves that a distribution of the CaDMP particles within the iPP matrix reduced the value of F(T) and this trend intensified with increasing filler content: F(T)’s estimated for the material containing 1 wt% of CaDMP were decreased by ca. 32–42% compared to the unmodified polymer, depending on the chosen value of Xt. Such behavior indicated that the composite systems required lower undercooling (a driving force of crystallization) to reach the same value of Xt compared with neat iPP.
Wide-angle X-ray scattering (WAXS) measurements
In order to independently confirm the formation of the β-iPP crystalline phase upon the addition of CaDMP to iPP, the X-ray diffraction technique was applied. Fig. S16 (SI) shows experimental WAXS curves obtained for neat iPP and CaDMP, as well as their composites. Neat polymer exhibited only the reflections corresponding to the α-iPP polymorph – those with the highest intensity were located at the 2θ angles of 14.1°, 16.9°, 18.7°, 21.2°, or 21.8°, and resulted from X-ray diffractions occurring on the (110), (040), (130), (111) or (041) crystallographic planes, respectively2. On the other hand, all the WAXS profiles recorded for the composite materials exhibited an additional, distinct diffraction reflection with maximum around 2θ of 16.1°, which could be assigned solely to the β-iPP crystal phase, i.e., the diffraction incidents occurring on its (110) crystallographic plane2. Furthermore, the intensity of a diffraction reflection at 2θ ca. 21.2° was increased compared to the WAXS trace of neat iPP due to an overlapping of the strong (111) reflections characteristic for both the α and β polymorphs of iPP2. The presence of the X-ray signals characteristic for both crystalline forms of iPP confirmed the results of the previously discussed DSC measurements and proved that upon their incorporation into the polymer matrix CaDMP particles exhibited a nucleation ability toward the β-iPP polymorph.
It should be noted, that a careful deconvolution of the experimental WAXS profiles (a detailed description of this procedure can be found in SI, together with its graphical example presented in Fig. S17) allowed us to calculate both the overall crystallinity ( ) and the relative content of the β-iPP crystal phase (
) and the relative content of the β-iPP crystal phase (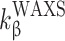 ). The latter parameter was determined for the two-phase (α-iPP + β-iPP) case, using an empirical equation proposed by Turner Jones and coworkers (Eq. (9))89:
). The latter parameter was determined for the two-phase (α-iPP + β-iPP) case, using an empirical equation proposed by Turner Jones and coworkers (Eq. (9))89:
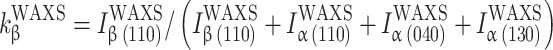 |
9 |
in which 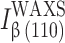 is the integral intensity of a diffraction reflection characteristic for the (110) plane of β-iPP, whereas
is the integral intensity of a diffraction reflection characteristic for the (110) plane of β-iPP, whereas 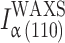 ,
,  and
and  are the integral intensities of the strongest reflections ascribed to the (110), (040) and (130) planes of the α-iPP crystal phase, respectively. Table 5 presents the results of all above-mentioned calculations.
are the integral intensities of the strongest reflections ascribed to the (110), (040) and (130) planes of the α-iPP crystal phase, respectively. Table 5 presents the results of all above-mentioned calculations.
Table 5.
Selected parameters evaluated using WAXS analysis of the crystal phases formed within the samples of neat iPP and its composites with cadmp.
| CaDMP content/wt% |
 ratio ratio |
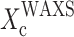 /% /% |
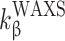
|
|---|---|---|---|
| 0.0 | 1.45 | 48.8 | nd |
| 0.2 | 0.85 | 52.6 | 0.17 |
| 0.5 | 0.78 | 53.5 | 0.14 |
| 1.0 | 0.57 | 53.8 | 0.36 |
| 2.0 | 0.47 | 52.6 | 0.38 |
| 5.0 | 0.48 | 57.1 | 0.27 |
A comparison of the data presented in Tables 3 and 5 led to a conclusion that the processing conditions applied during the preparation of the samples intended for WAXS did not substantially impede their overall crystallization degree since the  values were on a comparable level with those estimated from the DSC measurement carried out under non-isothermal conditions: upon an incorporation of the CaDMP particles only a small increase in the amount of the iPP crystalline phase was observed. On the other hand, a distribution of the α- and β-iPP crystallites was drastically changed, since the
values were on a comparable level with those estimated from the DSC measurement carried out under non-isothermal conditions: upon an incorporation of the CaDMP particles only a small increase in the amount of the iPP crystalline phase was observed. On the other hand, a distribution of the α- and β-iPP crystallites was drastically changed, since the 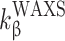 values were approximately 2–4 times lower than the
values were approximately 2–4 times lower than the 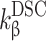 values determined by a calorimetric method. One can assume, that such a large dominance of the α-iPP polymorph was probably related to a different thermo-mechanical history of the samples subjected to the WAXS and DSC measurements (i.e., different temperature profiles and heat transportation within the samples during their preparation and measurement, as well as a slow β-iPP to α-iPP transformation during cooling and/or subsequent prolonged treatment of the samples at room temperature prior to the WAXS measurement).
values determined by a calorimetric method. One can assume, that such a large dominance of the α-iPP polymorph was probably related to a different thermo-mechanical history of the samples subjected to the WAXS and DSC measurements (i.e., different temperature profiles and heat transportation within the samples during their preparation and measurement, as well as a slow β-iPP to α-iPP transformation during cooling and/or subsequent prolonged treatment of the samples at room temperature prior to the WAXS measurement).
It is worth noting, that in the case of the CaDMP-containing materials the intensity of the diffraction peaks related to the (110) and (040) crystallographic planes of α-iPP substantially changed compared to neat iPP: the former decreased its intensity, whereas the latter became a dominant one among the iPP-related reflections (see Fig. S16 (SI)). It suggests that during crystallization in these systems, an orientation of the α-iPP crystallites perpendicular to the (040) plane was favored. As reported in the literature90, a ratio of the intensities of these two diffraction peaks (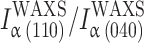 ) provides information on the extent of the orientation of the α-iPP crystals along its a– or b–unit cell directions. As can be seen from the data presented in Table 5, the substantially lower values of the
) provides information on the extent of the orientation of the α-iPP crystals along its a– or b–unit cell directions. As can be seen from the data presented in Table 5, the substantially lower values of the 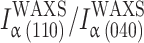 ratio estimated for all of the CaDMP-based composites indicated a preferred alignment of the α-iPP crystals in the b-axis direction.
ratio estimated for all of the CaDMP-based composites indicated a preferred alignment of the α-iPP crystals in the b-axis direction.
Mechanism of the β-iPP nucleation by CaDMP
The nucleating properties of different compounds towards the formation of β-iPP are usually explained based on a spatial organization and epitaxial growth of the iPP chains at the surface of the NA particles. This process requires the occurrence of an appropriate degree of geometrical matching between the NA lattice parameters and those of the β-iPP crystal phase, which can be quantified with the mismatch coefficient (fm) defined by Eq. 10:
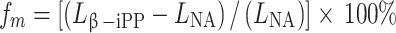 |
10 |
where Lβ−iPP and LNA refer to some specific distance (usually a unit cell parameter or its multiplication) in the crystal structure of β-iPP and NA, respectively23,24,36,47. Based on the empirical data, it is assumed that a value of fm higher than 15% indicates too much stress between both crystal phases effectively preventing epitaxy36,47,90. In the case of β-iPP and CaDMP, a favorable crystallographic matching (fm ≈ 0.11% at 30 °C) occurred only between the b-axis of the nucleator and a- or b-axis of the polymer – in both crystal structures these axes are perpendicular to the direction in which the respective polymeric chains (i.e., CaDMP or β-iPP) propagate. Taking this into account, we propose that during the first stage of an epitaxial crystallization iPP macromolecules got oriented parallel to the crystallographic a-direction of CaDMP and took position within the gaps formed by the methyl groups from CaDMP chains located at the vertices of the latter’s unit cell. Since the 31 screw axes of these iPP chains laid on the (020) crystallographic plane of CaDMP, the interchain distance specific for the β-iPP crystal phase (11.01 Å) was introduced to the system (Fig. 7). Due to the interactions with this first layer of iPP macromolecules the next layers of polymer chains underwent preorientation and were forced to “reproduce” spatial configuration of the β-iPP phase. It is worth noting, that one-dimensional matching of the iPP interchain distance with the gaps on the a–b crystallographic plane of CaDMP was crucial for the subsequent growth of the β-iPP phase, since it prevailed over the structural strains caused by an incompatibility between the periodicities of CaDMP and iPP chains (fm for the c- and a-lattice parameters of iPP and CaDMP, respectively, reaches value of ca. 18%). However, one cannot exclude the possibility that at the actual temperatures of the β-iPP crystallization this discrepancy may have got smaller due to the differences in β-iPP and CaDMP thermal expansion coefficients.
Fig. 7.
Proposed crystal lattice matching between CaDMP and β-iPP. The data for the β-iPP structure are taken from the work of Meille and coworkers91. Unit cell borders and crystallographic directions are indicated with solid lines and arrows, respectively.
Mechanical properties of the iPP/CaDMP composites
Considering that iPP is a construction material with a broad field of applications, the mechanical properties of neat iPP and its composites with 0.2–5.0 wt% of CaDMP were characterized in tensile and impact tests.
As can be seen in Table 6, the addition of CaDMP to iPP had no statistically significant effect on tensile strength of the obtained materials – only in the case of the system with the highest CaDMP content the observed ca. 5% reduction in tensile strength (in relation to neat iPP) exceeded the limits of measurement error. On the other hand, CaDMP significantly affected elongation at break of iPP, especially at lower ranges of the filler’s concentration: an introduction of only 0.2 wt% of CaDMP changed this parameter from 37% (neat iPP) to 280% (an increase by ca. 657%). The maximum of ductile properties was reached at the 0.5 wt% CaDMP loading, and further increasing the amount of CaDMP in the system resulted in a decrease in its elongation at break: the iPP/5CaDMP composite had it on a comparable level as neat iPP. Moreover, the presence of CaDMP also affected the behavior of the iPP matrix during impact, since all of the obtained iPP/CaDMP composites exhibited increased impact strength compared to the unmodified iPP.
Table 6.
Selected mechanical parameters of iPP and its composites with CaDMP estimated during tensile and impact tests.
| CaDMP content/wt% | Tensile strength/MPa | Elongation at break/% | Impact strength/kJ m |
|---|---|---|---|
| 0.0 | 37.7 ± 0.5 | 37 ± 8 | 5.57 ± 0.18 |
| 0.2 | 36.7 ± 0.6 | 280 ± 56 | 8.03 ± 0.35 |
| 0.5 | 36.4 ± 0.5 | 517 ± 51 | 7.58 ± 0.24 |
| 1.0 | 36.1 ± 2.5 | 190 ± 71 | 7.26 ± 0.61 |
| 2.0 | 36.9 ± 0.7 | 175 ± 7 | 7.83 ± 0.89 |
| 5.0 | 35.7 ± 0.9 | 49 ± 3 | 6.65 ± 0.13 |
Conclusion
The results of our studies on CaDMP indicate that its 1D particles consist of infinite polymeric chains built of octahedrally coordinated Ca2+ cations bridged by tridentate [(CH3O)2PO2]− ligands. In each dimethylphosphate ligand an oxygen atom of one of its methoxyl groups and oxide ligands from the P=O and dissociated PO-H moieties are involved in a coordination of Ca2+ centers. Because of that, CaDMP is free of any auxiliary ligands. This structure is very stable and upon heating up to the beginning of thermal decomposition, it does not undergo any structural transformations apart from a thermal expansion. Under oxidative conditions, CaDMP decomposes above ca. 260–295 °C forming a mixture of inorganic calcium condensed phosphates (i.e., calcium meta- and pyrophosphates).
The incorporation of even a small amount of CaDMP particles results in a substantial increase in both ductility and impact strength of the iPP polymer matrix (e.g., an increase in elongation at break and impact resistance by ca. 657% and 44%, respectively, for iPP/0.2CaDMP), with its tensile strength being practically unchanged. However, taking into account the values of kinetic parameters derived from non-isothermal crystallization experiments (which best reflect the real conditions occurring during processing of the iPP-based materials) and mechanical properties estimated for the injection moulded specimens, one can propose 2 wt% as the optimal level of CaDMP loading among the investigated iPP composites.
DSC and WAXS give evidence for the formation of a mixture of α-iPP and β-iPP crystal domains within the investigated composites. The values of 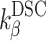 (0.4–0.6) show that CaDMP exhibits a non-selective, α/β nucleating properties towards iPP. A comparison of the CaDMP and β-iPP unit cell parameters shows an almost perfect one-dimensional matching of their crystal structures in directions perpendicular to the axes of propagation of their chains. However, one cannot exclude the possibility that at the actual temperature of epitaxial crystallization of β-iPP, some additional crystal lattice matching may occur due to the differences in thermal expansion coefficients of β-iPP and CaDMP.
(0.4–0.6) show that CaDMP exhibits a non-selective, α/β nucleating properties towards iPP. A comparison of the CaDMP and β-iPP unit cell parameters shows an almost perfect one-dimensional matching of their crystal structures in directions perpendicular to the axes of propagation of their chains. However, one cannot exclude the possibility that at the actual temperature of epitaxial crystallization of β-iPP, some additional crystal lattice matching may occur due to the differences in thermal expansion coefficients of β-iPP and CaDMP.
DSC data showed that under isothermal conditions one can observe a substantial increase in the Avrami overall crystallization rate constant of iPP, dependent on the CaDMP content in the system. Similarly, on cooling from the molten state, the evolution of crystallization heat in the iPP/CaDMP composites begins and reaches a maximum rate at temperatures a few Celsius degrees higher than in neat polymer; the resulting crystallization exotherms also become narrower than the reference one. One can conclude that the iPP non-isothermal crystallization kinetics are also positively affected by CaDMP particles. Mathematical modelling of this process following the Liu-Mo theory reveals that, with increasing CaDMP content, the composite systems requires lower supercooling to reach the same relative degree of crystallinity in a unit time, as showed by lower values of the Liu-Mo cooling function, F(T).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was partially funded by POB “Technologie Materiałowe” of Warsaw University of Technology within the Excellence Initiative: Research University (IDUB) programme under Grant Agreement 1820/3/Z0l/POB5/2021. Financial support from the National Science Centre, Poland (OPUS-11 research scheme, grant number 2016/21/B/ST5/00126) is also gratefully acknowledged.
Author contributions
Conceptualization: Maciej Dębowski, Mateusz Kullas, Krystyna Czaja; Methodology: Maciej Dębowski, Mateusz Kullas, Marcin Bączek, Maciej Dranka; Formal Analysis: Maciej Dębowski, Mateusz Kullas; Investigation: Maciej Dębowski, Mateusz Kullas, Beata Sacher-Majewska, Marcin Bączek, Maciej Dranka, Andrzej Ostrowski; Writing – original draft: Maciej Dębowski, Mateusz Kullas, Maciej Dranka, Zbigniew Florjańczyk; Writing – review & editing: Maciej Dębowski; Visualisation: Maciej Dębowski, Maciej Dranka; Funding acquisition: Maciej Dębowski; Resources: Maciej Dębowski, Mateusz Kullas, Krystyna Czaja; Supervision: Maciej Dębowski, Krystyna Czaja. All authors read and approved the final version of this manuscript.
Data availability
The authors declare that the data supporting the findings of this study are included in this published article (and its Supplementary Information files). Should any raw data files be needed, they are available from the corresponding author upon reasonable request.Crystallographic data for the structure reported in this paper have been deposited in the Cambridge Crystallographic Data Centre with CCDC number 2173285. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
Declarations
Competing interests
Maciej Dębowski, Mateusz Kullas, Krystyna Czaja and Beata Sacher-Majewska have patent application “Sposób nukleacji izotaktycznego polipropylenu” pending to Opole University / Warsaw University of Technology. All the remaining authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polymer crystallization with. and without nucleating agents. in Handbook of Nucleating Agents (ed. Wypych, G.) 33–51. 10.1016/B978-1-895198-93-5.50005-8 (ChemTec Publishing, 2016).
- 2.Auriemma, F., De Rosa, C., Malafronte, A., Scoti, M. & Di Girolamo, R. Solid state polymorphism of isotactic and syndiotactic polypropylene. in Polypropylene Handbook: Morphology, Blends and Composites (eds Karger-Kocsis, J. & Bárány, T.) 37–119 (Springer Nature Switzerland AG, Cham, 10.1007/978-3-030-12903-3_2. (2019).
- 3.Dai, X. et al. The synergistic effect of an organic phosphate salt nucleating agent and CaCO3 in isotactic polypropylene. Polym. Bull.81, 7157–7169. 10.1007/s00289-023-05051-6 (2024). [Google Scholar]
- 4.Wu, X. et al. Synergistic effect of organic phosphate salt nucleating agents and inorganic fillers on isotactic polypropylene. J. Therm. Anal. Calorim.148, 13375–13384. 10.1007/s10973-023-12634-y (2023). [Google Scholar]
- 5.Bednarek, W. H., Ciesielczyk, F., Odalanowska, M., Paukszta, D. & Piasecki, A. Effect of lanthanum-modified magnesium silicate on isotactic polypropylene crystallization in composite materials during shear flow. Polym. Eng. Sci.60, 1856–1865. 10.1002/pen.25422 (2020). [Google Scholar]
- 6.Zhang, Y. F., Hou, H. H. & Guo, L. H. Effects of Cyclic carboxylate nucleating agents on nucleus density and crystallization behavior of isotactic polypropylene. J. Therm. Anal. Calorim.131, 1483–1490. 10.1007/s10973-017-6669-6 (2018). [Google Scholar]
- 7.Zhang, Y. F., Li, D. & Chen, Q. J. Preparation and nucleation effects of nucleating agent hexahydrophthalic acid metal salts for isotactic polypropylene. Colloid Polym. Sci.295, 1973–1982. 10.1007/s00396-017-4176-8 (2017). [Google Scholar]
- 8.Abreu, A. A., Talabi, S. I. & de Lucas, A. Influence of nucleating agents on morphology and properties of injection-molded polypropylene. Polym. Adv. Technol.32, 2197–2206. 10.1002/pat.5252 (2021). [Google Scholar]
- 9.Long, L. et al. Joint effects of molecular structure and crystal morphology of organophosphate monovalent salts on nucleated isotactic poly(propylene). J. Polym. Res.23, 206. 10.1007/s10965-016-1102-6 (2016). [Google Scholar]
- 10.Long, L., He, W., Li, J., Qin, S. & Yu, J. Nucleation ability of nonmetallic organophosphate derivatives in isotactic polypropylene. J. Therm. Anal. Calorim.127, 2283–2291. 10.1007/s10973-016-5790-2 (2017). [Google Scholar]
- 11.Mani, M. R., Chellaswamy, R., Marathe, Y. N. & Pillai, V. K. Enhanced nucleation of polypropylene by metal–organic frameworks (MOFs) based on aluminium dicarboxylates: influence of structural features. RSC Adv.6, 1907–1912. 10.1039/C5RA22764J (2015). [Google Scholar]
- 12.Ren, X. Q., Zhang, Y. F., He, J. & Li, Y. Nucleation effect of adipic acid metal salts in isotactic polypropylene. J. Therm. Anal. Calorim.135, 3321–3328. 10.1007/s10973-018-7515-1 (2019). [Google Scholar]
- 13.Jiang, X., Zhao, S., Meng, X. & Xin, Z. Effect of the metal phenylphosphonates on the nonisothermal crystallization and performance of isotactic polypropylene. J. Polym. Sci. Part. B: Polym. Phys.57, 161–173. 10.1002/polb.24767 (2019). [Google Scholar]
- 14.Zhang, X. et al. Effect of the lanthanum and cerium phenylphosphonates on the crystallization and mechanical properties of isotactic polypropylene. J. Polym. Res.28, 124. 10.1007/s10965-021-02486-1 (2021). [Google Scholar]
- 15.Bhatia, A., Jayaratne, V. N., Simon, G. P., Edward, G. H. & Turney, T. W. Nucleation of isotactic polypropylene with metal monoglycerolates. Polymer59, 110–116. 10.1016/j.polymer.2014.12.038 (2015). [Google Scholar]
- 16.Zhang, Y. F. & Mao, J. J. Effect of chemical structure of Hydrazide compounds on nucleation effect in isotactic polypropylene. J. Polym. Res.26, 277. 10.1007/s10965-019-1970-7 (2019). [Google Scholar]
- 17.Horváth, F. et al. Effect of N,N′-dicyclohexyldicarboxamide homologues on the crystallization and properties of isotactic polypropylene. ACS Omega. 6, 9053–9065. 10.1021/acsomega.1c00064 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, Y. F., Zhou, P. Z., Guo, L. H. & Hou, H. H. The relationship between crystal structure and nucleation effect of 1,3,5-benzenetricarboxylic acid tris(phenylamide) in isotactic polypropylene. Colloid Polym. Sci.295, 619–626. 10.1007/s00396-017-4030-z (2017). [Google Scholar]
- 19.Barczewski, M., Matykiewicz, D. & Hoffmann, B. Effect of Quinacridone pigments on properties and morphology of injection molded isotactic polypropylene. Int. J. Polym. Sci.2017 (7043297). 10.1155/2017/7043297 (2017).
- 20.Varga, J. & Menyhárd, A. Effect of solubility and nucleating duality of N,N′-dicyclohexyl-2,6- Naphthalenedicarboxamide on the supermolecular structure of isotactic polypropylene. Macromolecules40, 2422–2431. 10.1021/ma062815j (2007). [Google Scholar]
- 21.Kang, J. et al. New Understanding in the influence of melt structure and β-nucleating agents on the polymorphic behavior of isotactic polypropylene. RSC Adv.4, 29514–29526. 10.1039/C4RA04231J (2014). [Google Scholar]
- 22.Xia, M. Q. & Zhang, Y. F. The relation between chemical structure of branched amide nucleating agents and nucleation effect in isotactic polypropylene. J. Therm. Anal. Calorim.145, 3053–3066. 10.1007/s10973-020-09860-z (2021). [Google Scholar]
- 23.Wang, Z. et al. Probing into the epitaxial crystallization of Β form isotactic polypropylene: from experimental observations to molecular mechanics computation. J. Polym. Sci. Part. B: Polym. Phys.55, 418–424. 10.1002/polb.24293 (2017). [Google Scholar]
- 24.Xiang, J., Li, Y., Zhong, J. R., Lu, C. H. & Zhang, Y. F. Influence of chemical structures of bisamide nucleating agents on the crystallization behavior and properties of isotactic polypropylene. J. Therm. Anal. Calorim.148, 2417–2428. 10.1007/s10973-022-11874-8 (2023). [Google Scholar]
- 25.Cheng, H., Shang, M., He, Y., Shentu, B. & Gao, Z. Synthesis and effect of N,N’-diphenylterephthalamide on crystallization of isotactic polypropylene. Polym. Sci. Ser. B. 62, 473–482. 10.1134/S1560090420050036 (2020). [Google Scholar]
- 26.Kersch, M., Schmidt, H. W. & Altstädt, V. Influence of different beta-nucleating agents on the morphology of isotactic polypropylene and their toughening effectiveness. Polymer98, 320–326. 10.1016/j.polymer.2016.06.051 (2016). [Google Scholar]
- 27.Mani, M. R., Chellaswamy, R., Marathe, Y. N. & Pillai, V. K. New Understanding on regulating the crystallization and morphology of the β-polymorph of isotactic polypropylene based on carboxylate-alumoxane nucleating agents. Macromolecules49, 2197–2205. 10.1021/acs.macromol.5b02466 (2016). [Google Scholar]
- 28.Ren, X. Q. & Zhang, Y. F. Effects of different metal salts of aliphatic Dicarboxylic acids on the formation of β-crystalline form in isotactic polypropylene. J. Therm. Anal. Calorim.137, 563–573. 10.1007/s10973-018-7958-4 (2019). [Google Scholar]
- 29.Dou, Q. & Xue, J. Effect of an in-situ nucleating agent on the polymorphs and mechanical properties of isotactic polypropylene. J. Macromol. Sci. Part. B: Phys.54, 947–961. 10.1080/00222348.2015.1019601 (2015). [Google Scholar]
- 30.Qin, W. et al. Zinc pimelate as an effective β-nucleating agent for isotactic polypropylene at elevated pressures and under rapid cooling rates. Polym. Cryst.3, e10132. 10.1002/pcr2.10132 (2020). [Google Scholar]
- 31.Zhang, X., Qin, W., Wu, T. & Zhao, S. A new insight into the formation of β-crystals of isotactic polypropylene induced by zinc Dicarboxylic acids. Polymer262, 125500. 10.1016/j.polymer.2022.125500 (2022). [Google Scholar]
- 32.Pan, C. et al. A novel β-nucleating agent for isotactic polypropylene. J. Therm. Anal. Calorim.134, 2029–2040. 10.1007/s10973-018-7768-8 (2018). [Google Scholar]
- 33.Zhao, S., Liu, K., Zhou, S., Shi, Y. & Xin, Z. A novel self-dispersed Β nucleating agent for isotactic polypropylene and its unique nucleation behavior and mechanism. Polymer132, 69–78. 10.1016/j.polymer.2017.10.024 (2017). [Google Scholar]
- 34.Cheng, S. et al. Crystalline characteristic effect of in situ interaction between ZnO and Pht on inducing Β nucleation of isotactic polypropylene. Polym. Cryst.2022 (7067888). 10.1155/2022/7067888 (2022).
- 35.Liu, J. & Pan, Y. Nucleation and crystallization behavior of isotactic polypropylene induced by zinc tetrahydrophthalate. Polym. Bull.79, 4563–4580. 10.1007/s00289-021-03717-7 (2022). [Google Scholar]
- 36.Sun, Y. et al. Structural relationships between zinc hexahydrophthalate and the Β phase of isotactic polypropylene. Ind. Eng. Chem. Res.59, 18529–18538. 10.1021/acs.iecr.0c03366 (2020). [Google Scholar]
- 37.Gao, H. et al. Modification of polypropylene with nanosized α-Al2O3 powders prepared by the mechanochemical method. Mod. Phys. Lett. B. 32, 1850296. 10.1142/S0217984918502962 (2018). [Google Scholar]
- 38.Liu, J. & Liang, H. Heterogeneous nucleation and self-nucleation of isotactic polypropylene with addition of nano-ZnO. J. Therm. Anal. Calorim.146, 2115–2126. 10.1007/s10973-020-10446-y (2021). [Google Scholar]
- 39.Zhang, Z., Wang, C., Junping, Z. & Mai, K. β-Nucleation of Pimelic acid supported on metal oxides in isotactic polypropylene. Polym. Int.61, 818–824. 10.1002/pi.4148 (2012). [Google Scholar]
- 40.Gallegos-Medrano, K. K. et al. Influence of chain length, particle size, and thermal treatment of Dicarboxylic acid-functionalized titanium dioxide filler in polypropylene. J. Mater. Res.36, 1718–1729. 10.1557/s43578-021-00168-8 (2021). [Google Scholar]
- 41.Li, M., Zhang, Z., Wu, L., Zhang, Y. & Mai, K. β-Crystallization and mechanical properties of aluminum hydroxide-filled polypropylene composites. Polym. Compos.40, E194–E201. 10.1002/pc.24595 (2019). [Google Scholar]
- 42.Shi, W. et al. Isothermal crystallization behaviors of isotactic polypropylene with ultrasonically-modified calcium sulfate whisker as β-nucleating agent. Polym. Eng. Sci.62, 2219–2229. 10.1002/pen.26002 (2022). [Google Scholar]
- 43.Zhang, Z., Wang, C., Meng, Y. & Mai, K. Synergistic effects of toughening of nano-CaCO3 and toughness of β-polypropylene. Compos. Part. A. 43, 189–197. 10.1016/j.compositesa.2011.10.008 (2012). [Google Scholar]
- 44.Cao, J., Wen, N. & Zheng, Y. The Preparation of calcium pimelate modified OMMT from natural Ca-montmorillonite and its application as β-nucleating agent for polypropylene. Polym. Test.65, 352–359. 10.1016/j.polymertesting.2017.11.031 (2018). [Google Scholar]
- 45.Ding, Q., Zhang, Z., Dai, X., Li, M. & Mai, K. Effect of hybrid wollastonite with different nucleation and morphology on the crystallization and mechanical properties of polypropylene. Polym. Compos.40, E638–E646. 10.1002/pc.24925 (2019). [Google Scholar]
- 46.Liu, M., Guo, B., Du, M., Chen, F. & Jia, D. Halloysite nanotubes as a novel β-nucleating agent for isotactic polypropylene. Polymer50, 3022–3030. 10.1016/j.polymer.2009.04.052 (2009). [Google Scholar]
- 47.Hybiak, D. & Garbarczyk, J. Silver nanoparticles in isotactic polypropylene (iPP). Part I. Silver nanoparticles as metallic nucleating agents for b-iPP polymorph. Polimery59, 585–591. 10.14314/polimery.2014.585 (2014). [Google Scholar]
- 48.Villarreal-Martínez, D., Gutiérrez-Páramo, H. E., Martínez-Hernández, A. L., Velasco-Santos, C. & Almendarez-Camarillo, A. Induction of the β-phase in isotactic polypropylene with Pimelic acid modified graphene nanoplatelets. FlatChem38, 100472. 10.1016/j.flatc.2023.100472 (2023). [Google Scholar]
- 49.Broda, J., Baczek, M., Fabia, J., Binias, D. & Fryczkowski, R. Nucleating agents based on graphene and graphene oxide for crystallization of the β-form of isotactic polypropylene. J. Mater. Sci.55, 1436–1450. 10.1007/s10853-019-04045-y (2020). [Google Scholar]
- 50.Liu, Y., Yao, X., Fan, C., Wang, B. & Hu, J. Effect of a smectic liquid crystal polymer as new β-nucleating agent on crystallization structure, melting, and rheological behavior of isotactic polypropylene. Polym. Bull.76, 2915–2929. 10.1007/s00289-018-2505-9 (2019). [Google Scholar]
- 51.Liu, J., Liang, H., Li, C. & Hu, F. Study of phenyl-terminated hyperbranched polyester as a special β-nucleating agent on the toughness of isotactic polypropylene. Polym. Eng. Sci.59, E133–E143. 10.1002/pen.25004 (2019). [Google Scholar]
- 52.Liu, S. et al. Polydopamine particles as a β-nucleating agent and antioxidant for isotactic polypropylene. Chem. Eng. J.363, 1–12. 10.1016/j.cej.2019.01.118 (2019). [Google Scholar]
- 53.Wang, C., Lee, J. L., Landingin, J. & Ko, C. H. β-Modification of isotactic polypropylene induced by electrospun isotactic polystyrene fibers. Polymer176, 236–243. 10.1016/j.polymer.2019.05.049 (2019). [Google Scholar]
- 54.Wu, B. et al. β-Nucleated polypropylene: preparation, nucleating efficiency, composite, and future prospects. Polymers15, 3107. 10.3390/polym15143107 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Application of nucleating. agents in specific polymers. in Handbook of Nucleating Agents (ed. Wypych, G.) 127–181. 10.1016/B978-1-895198-93-5.50012-5 (ChemTec Publishing, 2016).
- 56.Nitta, K., Takashima, T., Nitta, K. & Takashima, T. Tensile properties in β-modified isotactic polypropylene. In: Polypropylene - Polymerization and Characterization of Mechanical and Thermal Properties (eds. Wang, W. & Zeng, Y.) 10.5772/intechopen.83348 (IntechOpen, 2018).
- 57.Horváth, F., Gombár, T., Varga, J. & Menyhárd, A. Crystallization, melting, supermolecular structure and properties of isotactic polypropylene nucleated with dicyclohexyl-terephthalamide. J. Therm. Anal. Calorim.128, 925–935. 10.1007/s10973-016-6057-7 (2017). [Google Scholar]
- 58.Karger-Kocsis, J. & Varga, J. Effects of β-α transformation on the static and dynamic tensile behavior of isotactic polypropylene. J. Appl. Polym. Sci.62, 291–300. (1996). [Google Scholar]
- 59.He, J., Zhang, Y. F., Ren, X. Q. & Li, Y. Nucleation effect of Α/β compound nucleating agents based on 1,3,5-benzenetricarboxylic acid tris(cyclohexylamide) in isotactic polypropylene. Polym. Bull.76, 5559–5575. 10.1007/s00289-018-02671-1 (2019). [Google Scholar]
- 60.Dong, M. et al. An effective Α/β nucleating agent compound for the Preparation of polypropylene. Int. Polym. Process.33, 164–170. 10.3139/217.3361 (2018). [Google Scholar]
- 61.Horváth, F., Bodrogi, D., Hilt, B., Pregi, E. & Menyhárd, A. Organogelators with dual β- and α-nucleating ability in isotactic polypropylene. J. Therm. Anal. Calorim.147, 9451–9468. 10.1007/s10973-022-11203-z (2022). [Google Scholar]
- 62.Menyhárd, A. et al. Self-organization of micro reinforcements and the rules of crystal formation in polypropylene nucleated by non-selective nucleating agents with dual nucleating ability. Polym. Cryst.3, e10136. 10.1002/pcr2.10136 (2020). [Google Scholar]
- 63.Otto, F. P. & Flynn, E. W. Socony Mobil Oil Company, Inc.,. Mixed metal complex salts of phosphoric acid esters and mineral oil compositions containing the same. US Patent 2790765 (1957).
- 64.Verma, S. & Murugavel, R. Di-tert-butylphosphate derived thermolabile calcium organophosphates: precursors for Ca(H2PO4)2, Ca(HPO4), α-/β-Ca(PO3)2, and nanocrystalline Ca10(PO4)6(OH)2. Inorg. Chem.59, 13233–13244. 10.1021/acs.inorgchem.0c01591 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Moradian-Oldak, J., Frolow, F., Addadi, L. & Weiner, S. Interactions between acidic matrix macromolecules and calcium phosphate ester crystals: relevance to carbonate apatite formation in biomineralization. Proc. R Soc. Lond. Ser. B. 247, 47–55. 10.1098/rspb.1992.0008 (1992). [DOI] [PubMed] [Google Scholar]
- 66.Chen, S. et al. Metallic organophosphate catalyzed bulk ring-opening polymerization. Polym. Chem.9, 732–742. 10.1039/C7PY02086D (2018). [Google Scholar]
- 67.Craggs, A. et al. The Preparation of mono- and di-[4-(n-octyl)phenyl]- and -[4-(1,1,3,3-tetramethylbutyl)phenyl]phosphoric acids for use in PVC calcium ion-selective electrodes. J. Inorg. Nucl. Chem.40, 1483–1487. 10.1016/0022-1902(78)80455-2 (1978). [Google Scholar]
- 68.Delduca, P. G., Moody, G. J. & Thomas, J. D. R. The Preparation of mono-and di-[2-nitro-4-(1,1,3,3-tetramethylbutyl) phenyl]-phosphoric acids and their calcium salts for use in PVC calcium ion selective electrodes. J. Inorg. Nucl. Chem.42, 467–468. 10.1016/0022-1902(80)80029-7 (1980). [Google Scholar]
- 69.Chen, X. et al. Chiral metal-organic frameworks with tunable catalytic selectivity in asymmetric transfer hydrogenation reactions. Nano Res.14, 466–472. 10.1007/s12274-020-2905-7 (2021). [Google Scholar]
- 70.Zhang, Y. F. & Xin, Z. Effects of substituted aromatic heterocyclic phosphate salts on properties, crystallization, and melting behaviors of isotactic polypropylene. J. Appl. Polym. Sci.100, 4868–4874. 10.1002/app.23209 (2006). [Google Scholar]
- 71.Kullas, M., Czaja, K., Dębowski, M. & Sacher-Majewska B. Sposób Nukleacji Izotaktycznego Polipropylenu. Polish Patent Application P.440535 (Opole University & Warsaw University of Technology, 2022).
- 72.Kullas, M., Dębowski, M. & Czaja, K. Flame retardancy in isotactic polypropylene modified with crystalline coordination polymers based on calcium, zinc or aluminum dimethyl phosphates. Polym. Degrad. Stab.221, 110675. 10.1016/j.polymdegradstab.2024.110675 (2024). [Google Scholar]
- 73.Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst.42, 339–341. 10.1107/S0021889808042726 (2009). [Google Scholar]
- 74.Sheldrick, G. M. SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A: Found. Adv.71, 3–8. 10.1107/S2053273314026370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C: Struct. Chem.71, 3–8. 10.1107/S2053229614024218 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dębowski, M. et al. Dynamic supramolecular polymers based on zinc bis(diorganophospate)s: synthesis, structure and transformations in solid state and solutions. Polymers14, 3407. 10.3390/polym14163407 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taga, K., Miyagai, K., Hirabayashi, N., Yoshida, T. & Okabayashi, H. Vibrational spectra and normal coordinate analysis of barium dimethyl, diethyl and Ethyl Methyl phosphates. J. Mol. Struct.245, 1–11. 10.1016/0022-2860(91)87001-X (1991). [Google Scholar]
- 78.Florián, J., Baumruk, V., Štrajbl, M., Bednárová, L. & Štěpánek, J. IR and Raman spectra, conformational flexibility, and scaled quantum mechanical force fields of sodium dimethyl phosphate and dimethyl phosphate anion. J. Phys. Chem.100, 1559–1568. 10.1021/jp9520299 (1996). [Google Scholar]
- 79.Kainosho, M., Nakamura, A. & Tsuboi, M. Phosphorus-proton spin-spin coupling in the P–O–C–H group. A comparison of Cyclic and acyclic systems. Bull. Chem. Soc. Jpn. 42, 1713–1718. 10.1246/bcsj.42.1713 (1969). [Google Scholar]
- 80.Powder Diffraction File Database PDF-2. Cards 03-0348 (calcium metaphosphate), 17–0500 (calcium metaphosphate), 09-0346 (calcium pyrophosphate).International Centre for Diffraction Data. (1998).
- 81.Dębowski, M. et al. Thermally induced structural transformations of linear coordination polymers based on aluminum tris(diorganophosphates). Dalton Trans.47, 16480–16491. 10.1039/C8DT02672F (2018). [DOI] [PubMed] [Google Scholar]
- 82.Torsion angle. in IUPAC Compendium of Chemical Terminology. International Union of Pure and Applied Chemistry. 10.1351/goldbook.T06406 (2006).
- 83.Zachara, J. Novel approach to the concept of bond-valence vectors. Inorg. Chem.46, 9760–9767. 10.1021/ic7011809 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Dębowski, M. et al. 1D and 2D hybrid polymers based on zinc phenylphosphates: synthesis, characterization and applications in electroactive materials. RSC Adv.11, 7873–7885. 10.1039/D0RA09493E (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pérez-Camargo, R. A., Liu, G. M., Wang, D. J. & Müller, A. J. Experimental and data fitting guidelines for the determination of polymer crystallization kinetics. Chin. J. Polym. Sci.40, 658–691. 10.1007/s10118-022-2724-2 (2022). [Google Scholar]
- 86.Lorenzo, A. T., Arnal, M. L., Albuerne, J. & Müller, A. J. DSC isothermal polymer crystallization kinetics measurements and the use of the avrami equation to fit the data: guidelines to avoid common problems. Polym. Test.26, 222–231. 10.1016/j.polymertesting.2006.10.005 (2007). [Google Scholar]
- 87.Zhang, R., Zheng, H., Lou, X. & Ma, D. Crystallization characteristics of polypropylene and low ethylene content polypropylene copolymer with and without nucleating agents. J. Appl. Polym. Sci.51, 51–56. 10.1002/app.1994.070510106 (1994). [Google Scholar]
- 88.Liu, T., Mo, Z., Wang, S. & Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci.37, 568–575. 10.1002/pen.11700 (1997). [Google Scholar]
- 89.Jones, A. T., Aizlewood, J. M. & Beckett, D. R. Crystalline forms of isotactic polypropylene. Makromol Chem.75, 134–158. 10.1002/macp.1964.020750113 (1964). [Google Scholar]
- 90.Wang, J., Dou, Q., Chen, X. & Li, D. Crystal structure and morphologies of polypropylene homopolymer and propylene-ethylene random copolymer: effect of the substituted 1,3,5-benzenetrisamides. J. Polym. Sci. Part. B: Polym. Phys.46, 1067–1078. 10.1002/polb.21440 (2008). [Google Scholar]
- 91.Meille, S. V., Ferro, D. R., Brueckner, S., Lovinger, A. J. & Padden, F. J. Structure of β-isotactic polypropylene: A long-standing structural puzzle. Macromolecules27, 2615–2622. 10.1021/ma00087a034 (1994). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are included in this published article (and its Supplementary Information files). Should any raw data files be needed, they are available from the corresponding author upon reasonable request.Crystallographic data for the structure reported in this paper have been deposited in the Cambridge Crystallographic Data Centre with CCDC number 2173285. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.