Abstract
While mostly de novo truncating variants in SCAF4 were recently identified in 18 individuals with variable neurodevelopmental phenotypes, knowledge on the molecular and clinical spectrum is still limited. We assembled data on 50 novel individuals with SCAF4 variants ascertained via GeneMatcher and personal communication. With detailed evaluation of clinical data, in silico predictions and structural modeling, we further characterized the molecular and clinical spectrum of the autosomal dominant SCAF4-associated neurodevelopmental disorder. The molecular spectrum comprises 25 truncating, eight splice-site and five missense variants. While all other truncating variants were classified as pathogenic/likely pathogenic, significance of one C-terminal truncating variant, one splice-site variant and the missense variants remained unclear. Three missense variants in the CTD-interacting domain of SCAF4 were predicted to destabilize the domain. Twenty-three variants occurred de novo, and variants were inherited in 13 cases. Frequent clinical findings were mild developmental delay with speech impairment, seizures, and skeletal abnormalities such as clubfoot, scoliosis or hip dysplasia. Cognitive abilities ranged from normal IQ to severe intellectual disability (ID), with borderline to mild ID in the majority of individuals. Our study confirms the role of SCAF4 variants in neurodevelopmental disorders and further delineates the associated clinical phenotype.
Subject terms: Genetics research, Autism spectrum disorders
Introduction
Both initiation and proper termination of transcription are crucial for cell function and survival [1, 2]. While transcription initiation via RNA polymerase II has already been explored in depth, the regulation of elongation and mRNA termination are less well understood. One factor involved in termination of RNA polymerase II mediated transcription is the SR-related CTD-associated factor 4 (SCAF4) (MIM: 616023). SCAF4 in its longest isoform consists of 20 exons and encodes a splicing factor containing the well conserved N-terminal CTD-interacting domain (CID), as well as an arginine/serine rich domain, and an RNA binding motif [3]. With its CTD-interacting domain, SCAF4 binds to the C-terminal domain of the largest subunit of RNA polymerase II, encoded by POLR2A, another gene implicated in a neurodevelopmental disorder [4–6]. Together with SCAF8, SCAF4 is responsible for preventing early mRNA termination by suppressing the use of alternative Poly-A sites during transcription and also secures termination at the right site [7]. Heterozygous alterations of SCAF4 have been shown to lead to deregulation of more than 9000 genes, including some involved in gene regulation and RNA processing [8].
Recently, variants in SCAF4 have been reported to cause a neurodevelopmental disorder (NDD) (Fliedner-Zweier syndrome, MIM#620511) [8–10]. In 2020, eight truncating, two splice-site and one missense variant were reported in 11 individuals with variable neurodevelopmental phenotypes, including mild intellectual disability, seizures, and various organ or skeletal anomalies [8]. Since then, only seven additional individuals carrying a SCAF4 variant and with intellectual disability, epilepsy, and cardiac defects have been reported [9–11].
We now have assembled a cohort of 50 further affected individuals with variants in SCAF4 (Fig. 1A) and thus considerably broaden the molecular and clinical spectrum of the SCAF4-associated NDD.
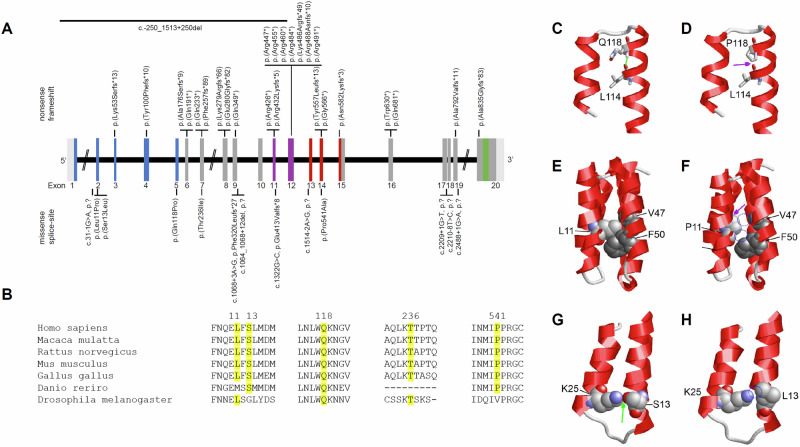
Fig. 1. SCAF4 variants in individuals with Fliedner-Zweier syndrome.
A Exon structure of SCAF4 (NM_020706.2, NP_065757.1) with newly identified variants and a larger intragenic deletion. Domains color coded according to ensemble, white for non-coding exonic regions, gray for coding exons and color for annotated domains (blue: CTD-interacting domain (CID), purple: Ser/Arg rich domain (SR), red: RNA binding motif (RRM), green: Pro/Gln rich domain (PQ)) [34]. B Conservation of missense variants from Homo sapiens to Drosophila melanogaster. All variants are fully conserved in tetrapods. Variant positions are highlighted in yellow. Alignment was performed with Clustalw2 [35, 36] with the following reference sequences: Homo sapiens: NP_065757.1, Macaca mulatta: XP_014988521.2, Rattus norvegicus: NP_001032424.2, Mus musculus: NP_001404016.1, Gallus gallus: NP_001012840.1, Danio rerio: NP_001373157.1, Drosophila melanogaster: NP_001097394.1. Effect of the p.(Gln118Pro), p.(Leu11Pro) and p.(Ser13Leu) exchanges on the structure of the CTD-interacting domain. C In wt-SCAF4, the amide hydrogen of Q118 forms a hydrogen bond with L114 (green line). D In the Q118P variant, this hydrogen bond cannot be formed because proline lacks an amide proton. Instead, steric clashes occur between the cyclic sidechain of P118 and L114 (magenta arrow), which are expected to decrease helix stability. E L11 of wildtype SCAF4 forms hydrophobic sidechain interactions with V47 and F50 (shown in gray). F In the L11P variant, these hydrophobic interactions cannot be formed by the cyclic sidechain of the proline residue, which is predicted to destabilize the structure of the CTD-interacting domain. Lacking hydrophobic interactions in the variant are highlighted by blue dotted circles. In addition, the cyclic proline sidechain induces steric clashes within the helix (magenta arrow), which further decrease protein stability. G S13 of wildtype SCAF4 forms a sidechain hydrogen bond with K25. Both residues are shown in space-filled presentation and colored according to the atom types; the hydrogen bond between the sidechain oxygen (red) of S13 and the sidechain nitrogen (blue) of K25 is indicated by a green arrow. H In the p.(Ser13Leu) variant, this hydrogen bond cannot be formed by the nonpolar sidechain of L13, which is predicted to destabilize the structure of the CTD-interacting domain. The site of the lacking hydrogen bond is highlighted by a blue dotted circle.
Materials and methods
Molecular and clinical data
Affected individuals were ascertained through international collaboration using GeneMatcher [12] and personal communication. Clinical data were collected using a standardized excel template (Supplemental Table S1). Testing by (trio) exome or genome sequencing was either performed in a diagnostic setting, not requiring a specific ethical approval, or in a research setting with ethical approval from institutional review boards of the respective centers (Commission d'éthique de la recherché canton Vaud, Lahore College for Women University, Kantonale Ethikkomission Zürich, Kantonale Ethikkommission Bern, Technical University of Munich, Harvard (IRB-P00032816), Supplemental Table S1). Segregation analysis for familial variants was performed by Sanger sequencing or by quantitative RT-PCR (Supplemental Table S1). Variants were classified according to ACMG criteria [13] plus current ClinGen sequence variant interpretation recommendations (PS2/PM6 version 1.1; splicing recommendations [14]). Information on additional variants and the phenotypes of the affected individuals is detailed in Supplemental Table S2. Consent for publication of genetic and clinical data, as well as photographs was obtained from the individuals, their parents, or legal guardians, respectively. The study complied with the principles set out in the Declaration of Helsinki.
In vitro splice assay
To investigate a potential splice effect of the variant c.2210-8 T > C, an exon trapping mini-gene assay was performed. The sequence of exons 17 and 18 (primer pair 1, F: tgaatagaagttaactaccacctgt, R: attaattatctcccggtcaacattc) or exon 18 alone (primer pair 2, F: ctttagagcctgcgatggag, R: attaattatctcccggtcaacattc) of SCAF4 and the surrounding introns (primer pair 1: 5’ intron: 160 bp, 3’intron: 119 bp, primer pair 2: 5’ intron: 58 bp, 3’intron: 119 bp) were cloned into the empty pSPL3 vector (Thermo Fisher Scientific). The regions were amplified from genomic DNA, and site directed mutagenesis (Agilent) was used to introduce the specific variant into the plasmid. Constructs pSPL3-SCAF4_WT_1, pSPL3-SCAF4_Var_1, pSPL3-SCAF4_WT_2 and pSPL3-SCAF4_Var_2 were transiently transfected into HEK293 cells using the jetPrime transfection system (Polyplus Transfection). 48 h post transfection, RNA was isolated from the cells using the RNeasy Plus Mini Kit (Qiagen). cDNA was reversely transcribed using SuperScript II (Thermo Fisher Scientific). RT-PCR was performed using standard primers (F: TCTGAGTCACCTGGACAACC, R: ATCTCAGTGGTATTTGTGAGC) to amplify the product transcribed from the vector. Resulting products were analyzed using gel electrophoresis and by sequencing. Sequences were aligned and depicted with Benchling biology software (www.benchling.com).
Structural modeling
The effect of the p.(Leu11Pro), p.(Ser13Leu) and p.(Gln118Pro) missense variants was modeled using the crystal structure of the SCAF4 CTD-interacting domain (PDB:6XKB) [15]. Variants were modeled with SwissModel [16], and RasMol [17] was used for structure analysis and visualization.
Results
SCAF4 molecular spectrum
We assembled 50 individuals from 41 families with 39 different, novel variants in SCAF4 (Fig. 1A). Twenty-three of these variants occurred de novo, in 13 cases the variant was inherited, and in 14 individuals parents were not available for testing. In individual 15, the variant was detected as mosaic in 8% of the peripheral blood cells.
Identified variants included an intragenic deletion of exons 1–12, 25 truncating (nonsense or frameshift) variants, eight splice-site variants and five missense variants. Aberrant splicing was confirmed in mRNA for c.1068+3 A > G (individual 12) and for c.1322 G > C (individual 16) in the respective centers (Supplemental Table S1). In silico analysis of the remaining six splice-site variants using SpliceAI [18] and Pangolin [19] scores predicted a likely splice-site alteration for five variants in individuals 1, 13, 32, 39 and 42 (c.31-1 G > A, c.1064_1068+12del, c.1514-2 A > G, c.2209+1 G > T, and c.2488+1 G > A, respectively). The variant c.2210-8 T > C in individual 40 was predicted to not have an impact on splicing (Supplemental Table S4). In accordance, testing this variant in an in vitro splice assay did not indicate aberrant splicing (Supplemental Fig. S1). Truncating, including splice-site variants were distributed ubiquitously over the gene/protein, thus being compatible with a general loss-of-function (LoF) effect or haploinsufficiency (Fig. 1A). Of note, one of the protein truncating variants was located in the ultimate exon and was predicted to escape nonsense mediated mRNA decay.
Three of the five missense variants (p.(Leu11Pro), p.(Ser13Leu), p.(Gln118Pro)) localized to the CTD-interacting domain, while a fourth (p.(Pro541Ala)) resided in the RNA recognition motif (RRM). All five missense variants were predicted to be deleterious by at least six variant effect prediction scores (REVEL [20], M-CAP [21], CADD [22], GERP + + [23], SIFT [24], MutationTaster [25], and POLYPHEN [26]) (Supplemental Table S3), and affected amino acid residues were highly conserved (Fig. 1B). SCAF4 was predicted to be highly intolerant towards LoF variants (pLI = 1, observed/expected = 0.19, LOEUF = 0.29) [27], while this was not the case for missense variants (z = 1.48, o/e = 0.89). Structural modeling for the three missense variants in the CTD-interacting domain indicated that each of them significantly disrupt the domain structure (Fig. 1C–H). In two of the variants (p.(Leu11Pro), p.(Gln118Pro)) a proline emerged within an α-helix. The cyclic structure of the proline sidechain induced steric clashes within the helix, which is expected to decrease domain stability (Fig. 1C–F). For two of the variants, destabilization between adjacent helices is caused through the loss of hydrophobic (p.(Leu11Pro); Fig. 1E, F) or polar sidechain interactions (p.(Ser13Leu); Fig. 1G, H). Structural destabilization of the CTD-interacting domain has also been recently reported for a p.(Cys54Arg) variant [11]. Since the CTD-interacting domain interacts with the C-terminal heptapeptide repeat domain (CTD) of RNA polymerase II [7, 15], it is expected that disruption of the CTD-interacting domain structure will also strongly impair the interaction between SCAF4 and the polymerase.
Variants assembled in this study were not or only very infrequently (one to two allele counts) listed in the gnomAD database (v4.0) [28], detailed information can be found in supplemental Table S1. All but two (I40 (c.2210-8 T > C) and I43 (p.(Ala835Glyfs*83))) of the truncating and splice-site variants were classified as likely pathogenic or pathogenic according to current ACMG criteria [13] (Supplemental Table S1). Though there is some evidence for pathogenicity via in silico prediction, de novo occurrence and/or structural modeling also for the remaining variants, they were currently classified as variants of uncertain significance. Classification and applied criteria are detailed in Supplemental Table S1.
In 19 individuals, additional variants, either (likely) pathogenic or of uncertain significance in other disease genes were identified (Supplemental Table S2), of which at least some might contribute to the respective individual’s phenotype. For example, individual 6 harbored a maternally inherited variant in CLCN1 (MIM: 160800) (Myotonia congenita Thomsen), which might explain sleeping difficulties and muscular hypertonia [29]. A 9q33 deletion was found in individual 7, which is also associated with intellectual disability, epilepsy, ambiguous genitalia and nail patella syndrome [30]. Also, the inherited variant in TLK2 (MIM: 618050) in individual 50 might contribute to her intellectual disability and hypotonia [31].
Clinical spectrum
Developmental delay and/or intellectual disability (ID) were reported in all but three individuals in this study, who were tested because of epilepsy or muscular hypotonia and behavioral issues. Therefore, penetrance of SCAF4-associated phenotypes appears to be high but clinical presentation is variable. Cognitive impairment ranged from learning difficulties or mild ID in 36 individuals to severe ID in three individuals. In the familial cases with available data, six of eight transmitting parents were reported to have had or still have learning difficulties or mild behavioral abnormalities. Only two mothers were reported to be unremarkable regarding developmental delay or learning issues. Speech development appeared to be more severely affected than motor development, with impaired speech reported in 36 individuals and delayed walking reported only in ten individuals, respectively. Behavioral or psychiatric disturbances were frequently observed (40 individuals) and included aggression, attention deficit hyperactivity disorder, autism spectrum disorder and tics. Sleeping difficulties were reported in 16 individuals and feeding difficulties in 10. Seizures with an age of onset between 9 months and 13 years occurred in 20 individuals. Seizure types were both focal and generalized, and EEG anomalies included polymorphic wave patterns and multifocal spike waves. MRIs were performed in 27 cases and revealed non-specific abnormalities in eight of them such as periventricular leukomalacia and prominent ventricles, as well as Chiari malformation. Variable eye or skeletal anomalies were reported in 16 and 23 individuals each. Other abnormalities were infrequent and included muscular hypo- or hypertonia(14x) postnatal micro-(9x) or macrocephaly, (1x) urogenital, (11x) renal (4x) and cardiac anomalies (3x). Facial dysmorphism were reported in most of the individuals but were rather non-specific (Fig. 2). Individual 15 with the mosaic truncating variant presented with developmental delay in early childhood, some behavioral issues in infancy and a multicystic left kidney, a rather mild but similar presentation compared to individuals with germline variants.
Fig. 2. Clinical photographs of affected individuals.
Note the broad nasal bridge in I2, I10, and I45.
In total, detailed clinical information on seven adults ( > 18 years) was available. Though data is not sufficient to draw detailed conclusions on natural history, mild developmental delay and mild cognitive presentation in infancy seems to correlate well with a mild manifestation in adulthood in terms of learning difficulties but capacities for a normal job and a family. No major, late-onset or progressive health issues were reported in any of the adult individuals.
The clinical phenotype associated with missense variants was not distinguishable from that associated with truncating variants. More detailed information on the clinical spectrum can be found in Table 1 and in Supplemental Table 1.
Table 1.
Clinical summary.
| Variant type | Truncating, this cohort, n = 45 | Truncating, previously published, n = 16 8–11 | Missense, this cohort, n = 5 | Missense previously published, n = 2 8,11 | Total |
|---|---|---|---|---|---|
| ID/DD | 1/1 | ||||
| mild | 32/44 | 11/15 | 4/5 | 47/64 (73%) | |
| moderate | 7/44 | 3/15 | 1/5 | 11/64 (17%) | |
| severe | 3/44 | 1/15 | 0/5 | 4/64 (6%) | |
| Seizures | 18/43 | 11/15 | 2/5 | 2/2 | 33/65 (51%) |
| Behavioral/psychiatric disturbances | 36/43 | 9/10 | 4/5 | 1/1 | 50/59 (85%) |
| Hypotonia | 6/40 | 4/8 | 3/5 | 13/53 (25%) | |
| Hypertonia | 4/40 | 1/8 | 1/5 | 6/53 (11%) | |
| Macrocephaly | 1/38 | 0/3 | 1/1 | 2/42 (5%) | |
| Microcephaly | 9/38 | 1/1 | 0/3 | 10/42 (24%) | |
| Eye abnormalities | 16/41 | 1/1 | 0/5 | 17/47 (36%) | |
| Facial dysmorphism | 28/42 | 2/2 | 1/5 | 31/49 (63%) | |
| Short stature | 9/42 | 1/5 | 10/47 (21%) | ||
| Sleeping difficulties | 15/40 | 1/1 | 1/5 | 17/46 (37%) | |
| Feeding difficulties | 9/37 | 1/1 | 1/5 | 11/43 (26%) | |
| Urogenital anomalies | 10/41 | 4/8 | 1/5 | 15/54 (28%) | |
| Renal anomalies | 4/34 | 4/6 | 0/5 | 8/45 (18%) | |
| Skeletal anomalies | 21/41 | 8/10 | 2/5 | 1/1 | 32/57 (56%) |
| Cardiac anomalies | 3/36 | 4/6 | 0/5 | 7/47 (15%) |
ID intellectual disability, DD developmental delay;
Discussion
By assembling 50 additional individuals, we further delineate the molecular and clinical spectrum of SCAF4-associated NDD (MIM#620511).
The most prominent feature is developmental delay and intellectual disability, mostly in the borderline to mild range, and language and speech delay. Mild manifestation in childhood seems to go in hand with a rather mild and favorable outcome in adulthood, many of the adult individuals presenting with learning difficulties to some degree but normal jobs and families. Also, behavioral issues such as attention deficit hyperactivity disorder, autistic features and aggressivity are frequently reported. While seizures have been previously reported in 60–100% of affected individuals [8–11], they occur only in 42% of individuals in this cohort, adding up to a total frequency of 51% in all reported individuals. An apparently higher prevalence in the initial reports might be due to a bias in the previously much smaller group. Seizures have been previously described as focal in affected individuals [10] and as reflex seizures in a zebrafish model [32]. With more cases known, the spectrum now seems to be more variable, including generalized epilepsy in this cohort and another report [11]. Other clinical aspects less commonly seen in this, larger cohort are hypotonia, cardiac, and renal anomalies, which were previously described in up to 67% of the individuals and now only appear in 7–20% (detailed information can be found in Table 1) [8–11]. Also for this observation a previous bias due to previously smaller case numbers is likely. Further frequent aspects of SCAF4-associated NDD are non-specific facial dysmorphism and skeletal abnormalities, while other morphological or organ abnormalities are noted only infrequently. This characterizes the SCAF4-associated phenotype as a rather mild and non-specific neurodevelopmental disorder with variable clinical expression and high, though not complete, penetrance. Due to a certain prevalence of cardiac, renal, urogenital and ophthalmological anomalies, a specific check for such abnormalities might be considered at time of diagnosis.
As the majority of identified disease-related variants in SCAF4 are truncating, this led to the prediction of haploinsufficiency as the most likely disease mechanism [8]. In accordance, most variants assembled in the current cohort are truncating as well. Nevertheless, in total seven missense variants were reported, five in this and two in previous studies [8, 11], most of them de novo and residing in functional domains, thus predicted to possibly also result in loss of protein function.
Interestingly, in our assembled cohort there was a relatively large number of inherited SCAF4 variants (13 cases). Most variant carrying parents or siblings in these familial cases also presented with similar neurodevelopmental phenotypes. This is in accordance with SCAF4-associated NDD being rather mild regarding the cognitive phenotype but might provide some challenges for variant filtering and interpretation in trio exome sequencing approaches.
The rather non-specific, mild phenotype might also complicate interpretation of additional variants in other disease related genes. These additional variants might be (re)classified as (likely) benign or deleterious in the future [33], the latter thus contributing to the variable phenotype, possibly within an oligogenic context.
To conclude, through assembling a larger cohort of individuals with variants in SCAF4, we were able to further delineate the phenotype of the SCAF4-associated NDD. Our findings support haploinsufficiency as the most likely pathomechanism.
Supplementary information
Acknowledgements
We thank all participating individuals and their families.
Author contributions
Conceptualization: CMS, CZ; Data Curation: CMS, HS, CZ; Formal Analysis: CMS, HS; Investigation: CMS, AR, CMB, IH, FA, UK, VMcN, LD, KS, AB, ARa, A-AS, BI, SM, MN, BC, WD, TB, TBH, RJF, AJM, TL, CRH-E, CWO, FM, ARe, NI, SN, EL, JAB, JH, TB, KMR, HZE, RP, FZ, JJK, KC, AS, M-AD, PMA, FR, SS, SR, EM, AK, SS, MJMN, RME, HA, PRH, LAD, HMHB, SMcK, AM, A-DI, RN-E, GB, OP, JK, SR, SSS, PG, SS, AQ, AR, YH, RS, BK, ZAM, ZT, SH-H, SKS, CGS, NJA, HF, AM-N, FTM-T, HS, CZ; Supervision: CZ; Visualization: CMS, HS; Writing-original draft: CMS, CZ; Writing-review and editing: CMS, AR, CMB, IH, FA, UK, VMcN, LD, KS, AB, ARa, A-AS, BI, SM, MN, BC, WD, TB, TBH, RJF, AJM, TL, CRH-E, CWO, FM, ARe, NI, SN, EL, JAB, JH, TB, KMR, HZE, RP, FZ, JJK, KC, AS, M-AD, PMA, FR, SS, SR, EM, AK, SS, MJMN, RME, HA, PRH, LAD, HMHB, SMcK, AM, A-DI, RN-E, GB, OP, JK, SR, SSS, PG, SS, AQ, AR, YH, RS, BK, ZAM, ZT, SH-H, SKS, CGS, NJA, HF, AM-N, FTM-T, HS, CZ.
Funding
CZ was supported by a grant from the German Research Foundation/Deutsche Forschungsgemeinschaft (DFG, ZW184/6-1). ARa was supported by grants from the Swiss National Science Foundation (31003A_182632 and IZSTZ0_216615), ARu was supported by a grant from Instituto de Salud Carlos IIIFEDER, (PI19/01902). In addition, the collaboration in this study were facilitated by ERN ITHACA, one of the 24 European Reference Networks (ERNs) approved by the ERN Board of Member States, cofounded by European Commission. [EU Framework Partnership Agreement ID: 3HP-HP-FPA ERN-01-2016/739516]. Open access funding provided by University of Bern.
Data availability
Data supporting the findings of this study are provided in the manuscript or the supplementary material or is available from the corresponding author upon request.
Competing interests
HZE, RP, FZ and JK are employees of GeneDx, LLC. The remaining authors declare no conflicts of interest.
Ethics
The research protocol fulfilled the requirements of the local institutional ethics committee (Kantonale Ethikkommission Bern, 2021-01396). Consent for publication of genetic and clinical data, as well as photographs was obtained from the individuals, their parents, or legal guardians, respectively. The study complied with the principles set out in the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-024-01760-2.
References
- 1.Proudfoot NJ. Transcriptional termination in mammals: stopping the RNA polymerase II juggernaut. Science 2016;352:aad9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elkon R, Ugalde AP, Agami R. Alternative cleavage and polyadenylation: extent, regulation and function. Nat Rev Genet. 2013;14:496–506. [DOI] [PubMed] [Google Scholar]
- 3.Becker R, Loll B, Meinhart A. Snapshots of the RNA processing factor SCAF8 bound to different phosphorylated forms of the carboxyl-terminal domain of RNA polymerase II. J Biol Chem. 2008;283:22659–69. [DOI] [PubMed] [Google Scholar]
- 4.Haijes HA, Koster MJE, Rehmann H, Li D, Hakonarson H, Cappuccio G, et al. De novo heterozygous POLR2A variants cause a neurodevelopmental syndrome with profound infantile-onset hypotonia. Am J Hum Genet. 2019;105:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuryev A, Patturajan M, Litingtung Y, Joshi RV, Gentile C, Gebara M, et al. The C-terminal domain of the largest subunit of RNA polymerase II interacts with a novel set of serine/arginine-rich proteins. Proc Natl Acad Sci USA. 1996;93:6975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larochelle M, Hunyadkurti J, Bachand F. Polyadenylation site selection: linking transcription and RNA processing via a conserved carboxy-terminal domain (CTD)-interacting protein. Curr Genet. 2017;63:195–9. [DOI] [PubMed] [Google Scholar]
- 7.Gregersen LH, Mitter R, Ugalde AP, Nojima T, Proudfoot NJ, Agami R, et al. SCAF4 and SCAF8, mRNA Anti-Terminator Proteins. Cell 2019;177:1797–813.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fliedner A, Kirchner P, Wiesener A, van de Beek I, Waisfisz Q, van Haelst M, et al. Variants in SCAF4 Cause a neurodevelopmental disorder and are associated with impaired mRNA processing. Am J Hum Genet. 2020;107:544–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho LML, Pinto CF, de Oliveira Scliar M, Otto PA, Krepischi ACV, Rosenberg C. SCAF4-related syndromic intellectual disability. Am J Med Genet A 2023;191:570–4. [DOI] [PubMed] [Google Scholar]
- 10.Lin H, Chen YH. SCAF4 variants associated with focal epilepsy accompanied by multisystem disorders. Seizure. 2024;116:65–73. [DOI] [PubMed]
- 11.Hu Y, Zhang B, Chen L, He J, Yang L, Chen X. SCAF4 variants are associated with epilepsy with neurodevelopmental disorders. Seizure. 2024;116:113–8. [DOI] [PubMed]
- 12.Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36:928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker LC, Hoya M, Wiggins GAR, Lindy A, Vincent LM, Parsons MT, et al. Using the ACMG/AMP framework to capture evidence related to predicted and observed impact on splicing: Recommendations from the ClinGen SVI Splicing Subgroup. Am J Hum Genet. 2023;110:1046–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou M, Ehsan F, Gan L, Dong A, Li Y, Liu K, et al. Structural basis for the recognition of the S2, S5-phosphorylated RNA polymerase II CTD by the mRNA anti-terminator protein hSCAF4. FEBS Lett. 2022;596:249–59. [DOI] [PubMed] [Google Scholar]
- 16.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 1997;18:2714–23. [DOI] [PubMed] [Google Scholar]
- 17.Sayle RA, Milner-White EJ. RASMOL: biomolecular graphics for all. Trends Biochem Sci. 1995;20:374. [DOI] [PubMed] [Google Scholar]
- 18.Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, et al. Predicting splicing from primary sequence with deep learning. Cell 2019;176:535–48.e24. [DOI] [PubMed] [Google Scholar]
- 19.Zeng T, Li YI. Predicting RNA splicing from DNA sequence using Pangolin. Genome Biol. 2022;23:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagadeesh KA, Wenger AM, Berger MJ, Guturu H, Stenson PD, Cooper DN, et al. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat Genet. 2016;48:1581–6. [DOI] [PubMed] [Google Scholar]
- 22.Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47:D886–D94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a high fraction of the human genome to be under selective constraint using GERP + +. PLoS Comput Biol. 2010;6:e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–6. [DOI] [PubMed] [Google Scholar]
- 26.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alfoldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020;581:434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Francioli LC, Goodrich JK, Collins RL, Kanai M, Wang Q, et al. A genomic mutational constraint map using variation in 76,156 human genomes. Nature 2024;625:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun C, Tranebjaerg L, Torbergsen T, Holmgren G, Van Ghelue M. Spectrum of CLCN1 mutations in patients with myotonia congenita in Northern Scandinavia. Eur J Hum Genet. 2001;9:903–9. [DOI] [PubMed] [Google Scholar]
- 30.Ehret JK, Engels H, Cremer K, Becker J, Zimmermann JP, Wohlleber E, et al. Microdeletions in 9q33.3-q34.11 in five patients with intellectual disability, microcephaly, and seizures of incomplete penetrance: is STXBP1 not the only causative gene? Mol Cytogenet. 2015;8:72. [DOI] [PMC free article] [PubMed]
- 31.Reijnders MRF, Miller KA, Alvi M, Goos JAC, Lees MM, de Burca A, et al. De novo and inherited loss-of-function variants in TLK2: clinical and genotype-phenotype evaluation of a distinct neurodevelopmental disorder. Am J Hum Genet. 2018;102:1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okudan ZV, Ozkara C. Reflex epilepsy: triggers and management strategies. Neuropsychiatr Dis Treat. 2018;14:327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fowler DM, Rehm HL. Will variants of uncertain significance still exist in 2030? Am J Hum Genet. 2024;111:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin FJ, Amode MR, Aneja A, Austine-Orimoloye O, Azov AG, Barnes I, et al. Ensembl 2023. Nucleic Acids Res. 2023;51:D933–D41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38:W695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are provided in the manuscript or the supplementary material or is available from the corresponding author upon request.