Abstract
With the extensive utilization of antifungal drugs, the drug resistance of Candida albicans is progressively intensifying, and the effect of empirical treatment for C. albicans infection is not evident. There is an urgent need for novel strategies and methods for the treatment of C. albicans infection. Our study utilized the previously constructed C. albicans Ire1 double gene deletion strain to explore the influence of the Ire1 on endoplasmic reticulum (ER) stress and pathogenicity of C. albicans through drug stress phenotype testing, biofilm and hyphomycete formation testing, and mouse systemic infection testing. The results indicate that Ire1 is involved in maintaining the integrity of the C. albicans cell wall and influencing the hyphal formation ability of C. albicans. Concurrently, the deletion of the Ire1 increased the sensitivity of C. albicans to the ER stress agents tunicamycin and dithiothreitol and diminished the biofilm formation ability of C. albicans in vitro, resulting in significant inhibition of the growth of C. albicans. In mouse models, the deletion of Ire1 completely nullified the virulence and pathogenicity of C. albicans in the tail vein infection. In conclusion, Ire1 might be a key target for the potential development of new therapeutic drugs and vaccines.
Keywords: Candida albicans, Ire1, endoplasmic reticulum, virulence
1. Introduction
Candida is an opportunistic pathogen that is prevalently present in the peripheral environment and constitutes the microflora of normal human skin and gut, with a carriage rate reaching up to 60% in healthy individuals [1,2,3,4].
Candidiasis typically occurs as a consequence of excessive proliferation or abnormal colonization of candidiasis due to local or general impairments in the host’s defense mechanisms [3]. In recent years, with the advancement of science and technology, medical conditions and the medical environment have continued to improve; however, this has been accompanied by an increase in the incidence and mortality of invasive candidiasis, with a crude mortality rate of 46–75% and an attributable mortality rate of 10–49% [1,5,6,7,8]; thus, it has drawn extensive concern from clinicians.
With the extensive utilization of antifungal drugs, the drug resistance of Candida albicans is also escalating [9,10,11,12,13,14]. The efficacy of empirical treatment for C. albicans infection is not conspicuous, and novel strategies for the treatment of C. albicans infection are urgently required in the clinical setting. Hence, to explore the virulence factors and/or pathogenic factors of C. albicans to identify new key targets and provide a theoretical foundation for the discovery and development of new therapeutic drugs and vaccines.
2. Materials and methods
2.1. Strains and reagents
The standard strains SN152 and Ire1 gene knockout strains (Ire1Δ/Δ) of C. albicans were maintained in the laboratory of Bethune International Peace Hospital. Yeast extract and peptone were purchased from OXOID company; YPD culture medium (self-prepared), RPMI-1640, and d-anhydrous glucose were obtained from Solarbio; agar powder was purchased from Beijing Aoboxing; fluconazole (FLU), itraconazole (ITZ), calcium fluoride white (CFW), and Congo red (CGR) were purchased from MCE.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations and institutional policies and in accordance with the tenets of the Helsinki Declaration and has been approved by the Ethics Committee of the 980Th Hospital of PLA Joint Logistical Support Force (Bethune International Peace Hospital) (2023-KY-93).
2.2. Methods
2.2.1. Activation of C. albicans
Standard strains SN152 and Ire1Δ/Δ and clinical strains of C. albicans were resuscitated and inoculated onto Candida Comagia chromogenic medium and then cultured at 35°C and 5% CO2 for 48 h. A single colony with green, smooth, and good independent growth on the Candida Comagia chromogenic medium was selected using an aseptic inoculation ring and inoculated into 5 mL of YPD medium. The colony was fully mixed in a vortex mixer and incubated overnight in a shaker at 35°C at 160 rpm so that it was in the logarithmic growth phase.
2.2.2. Experiment on liquid growth of C. albicans
(1) C. albicans strains SN152 and Ire1Δ/Δ were added to 5 mL YPD, respectively, activated in a constant temperature shaking table at 35°C overnight, and then, the initial value of OD600 was adjusted to 0.1 with fresh YPD, which was evenly divided into two 50 mL sterile centrifuge tubes.
(2) Adding 2 μg/mL tunicamycin to one tube and not adding to the other tube, cultured for 20 h.
(3) Determining the strain of OD600 value and understand the strain growth situation.
2.2.3. Liquid mycelium induction of C. albicans
The overnight-activated C. albicans SN152 and Ire1Δ/Δ strains were collected by centrifuge at 5,000 rpm. The bacteria were washed with sterile phosphate buffered saline (PBS) for 2–3 times and then re-suspended in the mycelium induction medium with RPMI 1640, with the initial OD600 adjusted to approximately 0.05. After incubation at 160 rpm in a constant temperature shaking table at 35°C for 2 h, the bacteria were collected, and the mycelial growth status of each strain was observed by wet tablets and Gram staining microscopy.
2.2.4. The ability of C. albicans to form biofilm in vitro was detected by crystal violet staining
The activated C. albicans SN152 and Ire1Δ/Δ strains were collected by centrifugation at 5,000 rpm, washed with sterile PBS for 2–3 times, and then re-suspended in RPMI 1640 medium. The initial OD600 was adjusted to approximately 0.1, and 100 μL/well was added to the 96-well plate. After 24 h culture, the culture medium was aspirated and washed with 200 μL/well PBS for 3 times. After fixation with 100 μL/well methanol for 15 min, the methanol was aspirated and allowed to dry. After staining with 100 μL/well 1% crystal violet for 5 min, the remaining crystal violet was aspirated and washed with 200 μL/well PBS several times until rinsed. The plate was dried upside down in a 37°C incubator. 100 μL/well of 33% glacial acetic acid was dissolved at 37°C for 30 min and mixed well. The OD595 value was detected and recorded.
2.2.5. Determination of C. albicans stress phenotype
The growth of C. albicans SN152 and Ire1Δ/Δ under diverse pressure conditions was observed through spot testing.
(1) C. albicans SN152 and Ire1Δ/Δ were, respectively, added to 5 mL YPD and were oscillated overnight in a constant temperature shaking table at 35°C for activation. Subsequently, the OD600 value of the bacterial solution was adjusted to approximately 0.1 with fresh YPD and was cultured at 160 rpm in a shaking table at 35°C for 4–6 h to reach the logarithmic stage.
(2) The bacteria reaching the logarithmic stage were centrifuged and collected at 5,000 rpm. Then, the centrifuged bacteria were washed with sterile normal saline for 2–3 times, suspended in the normal saline, and the OD600 was adjusted to 0.2.
(3) Dilute OD600 to 0.2 bacterial solution by double ratio (10 times gradient) and dilute four gradients.
(4) Take 2.5 μL of the bacterial solution per gradient, place it on the labeled solid pressure medium plate in the order of points from high to low concentration, and seal the plate with a sealing film.
(5) The plate was placed upside down in a constant temperature and humidity incubator at 35°C for approximately 2–3 days. Subsequently, the growth of each strain was observed and photographed for record keeping.
2.2.6. Determination of systemic infection ability in mice
(1) Seven-week-old female BALB/C mice were randomly divided into the normal saline group, the SN152 group, and the Ire1Δ/Δ group (nine mice per group) for the subsequent establishment of the experimental model via tail vein injection.
(2) The overnight-activated C. albicans SN152 and Ire1Δ/Δ strains were collected by centrifugation at 5,000 rpm, washed with sterile normal saline for 2–3 times, and then suspended in sterile normal saline and adjusted to an OD600 of 0.5 (approximately 5 × 107 CFU/mL). The suspension of C. albicans was injected into the tail vein of each group of mice through tail vein injection, 100 μL each (5 × 106 CFU/mL).
(3) The growth and death of mice in each group were periodically recorded starting from the second day after the completion of the tail vein injection. The death data of mice in each group were statistically analyzed, and the survival curve was drawn using GraphPad Prism 9 software.
(4) On the second day following the tail vein injection, four mice in each group were killed by neck amputation. Subsequently, the right kidney was dissected, washed with sterile PBS, weighed, placed in a 1 mL sterile PBS EP tube, and homogenized with an ultrasonic breaker. The homogenate was diluted by multiple (10-fold) gradients, and 10 μL was applied onto a Candida chromogenic plate. After incubation at 35°C for 2–3 days, the number of C. albicans colonies on the plate was counted, and the load of C. albicans in mouse kidneys was calculated to indicate the colonization ability of C. albicans SN152 and Ire1Δ/Δ in mouse kidneys.
2.2.7. Pathological evaluation of mouse renal tissue injury
(1) The operation was identical to that in Section 2.2.6. After dissection, the left kidney was retrieved, promptly placed in neutral buffered formalin, labeled, and fixed at room temperature for 24 h.
-
(2) Preparation of slices
Slice and patch: The embedded wax blocks are sliced into 3–5 μm sections using a microslicer and then placed in a constant-temperature water bath. The sections are tightly pressed onto the pathological slides with tweezers, dried in a 45°C incubator, and sealed with neutral gum after staining.
(3) Hematoxylin–eosin (HE) staining
(4) Glycogen (PAS) staining
3. Results
3.1. Deletion of Ire1 affects the stress function of the endoplasmic reticulum (ER) of C. albicans
ER stress refers to the accumulation of unfolded or misfolded proteins in the ER caused by various reasons, which triggers the unfolded protein response [15,16,17]. Itamycin and dithiothreitol (DTT) can serve as an inducer of ER stress, resulting in a significant accumulation of misfolded proteins [18]. To explore the effect of Ire1 gene deletion on the ER function of C. albicans, we performed a DTT sensitive phenotype test of C. albicans SN152 (WT) and the Ire1 gene deletion strain Ire1Δ/Δ to 2 μg/mL itamycin and 20 μM by using the solid dilution point plate method. The phenotypic results are presented in Figure 1. According to the results, there was no difference in the growth of C. albicans in the SN152 (WT) group and the Ire1Δ/Δ group on the solid YPD plate.
Figure 1.
Growth phenotypes of SN152, Ire1Δ/Δ strains on (a) YPD, (b) 2 μg/mL tunicamycin, and (c) (20 μM DTT) drug plates.
However, after 2–3 days of culture with 2 μg/mL of eimycin and 20 μM of DTT, compared with the SN152 (WT) strain, the deletion of Ire1Δ/Δ strain was significantly more sensitive to 2 μg/mL of itamycin and 20 μM of DTT, and its growth was significantly inhibited.
Meanwhile, the growth of the SN152 (WT) and Ire1Δ/Δ strains was measured through a liquid growth test. The phenotypic results are presented in Figure 2. After adding 2 μg/mL of eimycin in the YPD liquid medium for 20 h, the OD600 of Ire1Δ/Δ decreased more significantly than that of SN152 (WT) (****P < 0.0001). Therefore, we contend that the Ire1 gene is involved in maintaining the normal function of the ER stress of C. albicans.
Figure 2.

Growth assay of SN152, Ire1Δ/Δ strains in YPD liquid medium containing tunicamycin (2 μg/mL).
3.2. The impact of Ire1 deletion on the transformation function of C. albicans yeast to mycelium
C. albicans is a typical dimorphic fungus that exhibits a variety of forms, such as yeast, pseudomycelium, and mycelium. It can switch between the yeast phase, the mycelium phase, and the pseudomycelium phase, and this morphological transformation is closely related to the virulence of C. albicans [3,19,20].
When the host immunity is low, the form of C. albicans undergoes a change, transforming from the yeast form to the mycelium form. The corresponding adhesion and invasion are enhanced, and it becomes easier to penetrate the host mucosal barrier, eventually causing invasive candidiasis. In severe cases, it may even threaten the life of the host.
SN152 (WT) and Ire1Δ/Δ strains were activated overnight and cultivated in RPMI 1640 mycelia induction medium at 37°C for 2 h. Subsequently, the mycelia development capacity of each strain was observed under the microscope through wet tablets and Gram staining. As depicted in Figure 3, the mycelium of the SN152 (WT) strain could develop normally after a 2-h culture. In contrast to the wild strain, yeast-type cells were the dominant morphology in the Ire1Δ/Δ strain, and only a few spores developed short mycelia presenting a pseudomycelia state. These results imply that the deletion of the Ire1 gene can severely affect the mycelial formation ability of C. albicans.
Figure 3.
Effect of Ire1Δ/Δ on the ability of C. albicans to form hyphae. (a) and (b) Wet film microscopy and (c) and (d) Gram staining microscopy.
3.3. Effect of Ire1 gene knockout on biofilm formation of C. albicans in vitro
Crystal Violet dye is an alkaline dye that can bind to the DNA in the nucleus, presenting a deep purple color. This enables the number of cells in the C. albicans biofilm to be expressed by the color depth, and the biofilm is quantified by measuring its absorbance at OD595. As shown in Figure 4, compared with SN152, the ability of the Ire1Δ/Δ strain to generate a biofilm was significantly reduced. These results suggest that the deletion of the Ire1 gene has a significant impact on the biofilm formation ability of C. albicans.
Figure 4.

Effect of deletion of the Ire1 gene on the ability of C. albicans to form biofilms.
3.4. Deletion of Ire1 results in an increased sensitivity to stress in the cell wall of C. albicans
The cell wall of C. albicans serves as the first barrier against host immunity and possesses multiple functions, such as secreting adhesion factors, promoting morphological transformation, and enabling biofilm formation. To detect the effect of the Ire1 gene on the formation or remodeling of the cell wall of C. albicans, we conducted solid dilution point plate experiments using cell wall stress agents, CFW, and CGR. CFW can bind to glycosides with β-links. The chitin and cellulose in the cell wall of C. albicans contain β-linked glycosides, and CFW binds to them and inhibits their synthesis. CGR can specifically bind to 1,3-β-d glucan in the cell wall of C. albicans, preventing the normal assembly of the cell wall. As shown in Figure 5, the Ire1 gene deletion strain was significantly more sensitive to the pressure plates containing CFW (50 μg/mL) and CGR (10 μg/mL) compared with the SN152 (WT) strain. This indicates that the Ire1 gene of C. albicans plays a role in the cell wall stress induced by CFW and CGR.
Figure 5.
Growth phenotypes of SN152, Ire1Δ/Δ strains on (a) (YPD), (b) (50 μg/mL CFW), and (c) (10 μg/mL CGR) plates.
3.5. The Ire1 influences resistance of C. albicans to antifungal drugs
Azoles constitute a group of antifungal compounds that exhibit a broad range of activities against the majority of Candida species. They are generally well tolerated, possess predictable drug–drug interactions and can be administered either intravenously or orally as systemic drugs. The azole antifungals inhibit seosterol 14α-demethylase, which is a crucial enzyme in the formation of ergosterol, a major component of fungal cell membranes [21]. We investigated the susceptibility of C. albicans strains, SN152 (WT) and Ire1Δ/Δ, to FLU and ITZ. As depicted in Figure 6, the Ire1 deletion strain was significantly more sensitive to FLU (2 μg/mL, 4 μg/mL) and ITZ (0.125 μg/mL, 0.25 μg/mL) than the SN152 (WT) strain.
Figure 6.
Growth phenotypes of SN152, Ire1Δ/Δ strains on (a) (YPD), (b) (2 μg/mL FLU), (c) (4 μg/mL FLU), (d) (0.125 μg/mL ITZ), and (e) (0.25 μg/mL ITZ) plates.
3.6. Effect of Ire1 gene deletion on the ability of C. albicans to infect systemically
The aforementioned experimental results showed that the deletion of the Ire1 gene significantly affected the mycelial formation and morphological transformation abilities of C. albicans. To further explore the effect of the Ire1 gene on C. albicans’s virulence in mice, the mouse systemic infection model was established by injecting the tail vein. For detailed information, refer to Section 2.2.12. The mice were infected with strains of C. albicans SN152 (WT) and Ire1Δ/Δ through tail vein injection at a dose of 5 × 106 per mouse.
3.6.1. Survival curve
As shown in Figure 7, mice injected with the SN152 (WT) strain began to die on the first day after injection and all died by the third day, with a 100% mortality rate. Mice injected with the Ire1Δ/Δ strain, like those in the normal saline group, grew well and none died during the 14-day observation. The results indicated that the deletion of the Ire1 gene caused C. albicans to completely lose its pathogenicity and virulence.
Figure 7.
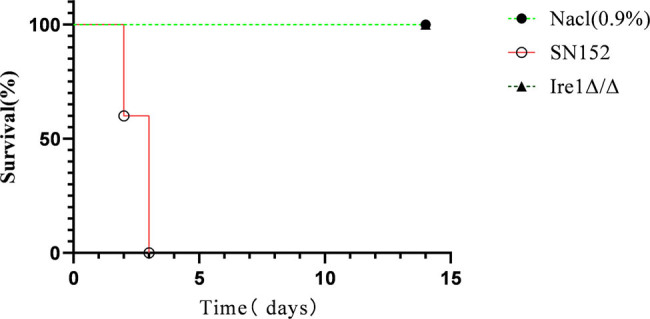
Survival curves of BALB/c mice after tail vein injection of C. albicans.
3.6.2. Analysis of bacterial load in kidney tissue
In the mouse model of bloodstream infection, the kidney is the main target organ attacked by C. albicans. To detect the effect of the Ire1 gene on the virulence of C. albicans infection, we counted and analyzed the bacterial amount in the kidneys of mice injected with C. albicans for 24 h. The specific operation steps are presented in Section 2.2.12. The experimental results are shown in Figure 8. After mice were infected with the C. albicans SN152 (WT) strain, the bacterial count in kidney tissue was 1.9 × 107/g, while after mice were infected with the Ire1Δ/Δ strain, the bacterial amount in kidney tissue was 0, completely losing the ability of colonization in mouse kidneys.
Figure 8.

Determination of bacterial load in mouse kidney at 24 h.
3.6.3. Pathological analysis of kidney tissue
HE and PAS staining were used to stain the kidney tissue of mice infected with C. albicans. The appearance of mouse kidney tissue is presented in Figure 9. The kidneys of C. albicans Ire1Δ/Δ and saline mice appeared reddish, while those of SN152 (WT)-infected mice appeared gray.
Figure 9.
Appearance of kidney tissue in (a) saline, (b) Ire1Δ/Δ, and (c) SN152 groups.
3.6.3.1. HE staining of pathological sections of kidney
The experimental results are presented in Figure 10. The Ire1Δ/Δ group and the normal saline group had normal kidney tissues, with no pathological changes or inflammatory cell infiltration observed. The pathological sections of mice infected with the SN152 (WT) strain indicated that the main pathological changes were inflammatory cell infiltration, and the renal interstitium, renal tubules, and glomeruli were invaded, dissolved, and destroyed to varying degrees.
Figure 10.
HE staining of kidney tissue (a) saline, (b) Ire1Δ/Δ, and (c) SN152 groups.
3.6.3.2. Renal tissue PAS staining
The experimental results are presented in Figure 11. The Ire1Δ/Δ group and the normal saline group had normal kidney tissues, and no C. albicans invasion or inflammatory cell infiltration was observed. A large number of mycelial C. albicans were found in the renal tubules and calyces of mice infected with the SN152 (WT) strain. Thus, we believe that the deletion of the Ire1 gene causes C. albicans to completely lose its ability to invade mouse kidney tissue. The Ire1 gene is an important factor in maintaining the virulence of C. albicans.
Figure 11.
PAS staining of kidney tissue (a) saline, (b) Ire1Δ/Δ, and (c) SN152 groups.
4. Discussion
Candida is an opportunistic pathogen that exists widely in the surrounding environment and is a microflora of normal human skin and gut, with a carriage rate of up to 60% in healthy people [1]. Candidiasis usually occurs due to overpropagation or abnormal colonization of Candida as a result of local or general defects in host defense mechanisms.
Candidiasis is a broad term that encompasses fungal infections of the skin, mucous membranes, and deep organs (intraperitoneal, thoracic, bone and joint, brain, heart, kidney, liver, spleen, etc.). It can occur at any age, while invasive candidiasis mainly refers to candidemia and deep organ infection.
In recent years, with the advancement of science and technology, medical conditions and the medical environment keep improving. However, the incidence and mortality of invasive candidiasis have increased, with a crude mortality of 46–75% and an attributable mortality of 10–49% [1,5,6,7,8], which has drawn wide attention from clinicians. At least 15 distinct Candida spp. can cause human diseases, but the majority of invasive infections are caused by five pathogens: C. albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei [1,2,6,7]. The most common fungus causing invasive candidiasis infections is C. albicans. When the immune mechanism of the host is impaired or the intestinal mucosal flora is imbalanced, C. albicans can change from colonization to opportunistic bacteria and cause infection.
At present, there are three clear mechanisms causing infection: (1) long-term use of broad-spectrum antibiotics leads to flora imbalance, inhibits normal bacteria, reduces the release of mucosal anti-Candida protective factors, and causes Candida overcloning and reproduction [2,22]. (2) Damage to gastrointestinal and cutaneous mucosal barriers. Gastrointestinal surgeries, perforations, and central vein catheterizations can cause mucosal barrier damage, allowing C. albicans colonized in these sites to enter the bloodstream through the damaged mucosa. (3) The use of immunosuppressants, such as chemotherapy-induced neutropenia and glucocorticoid therapy, can reduce the host’s immune and epidemic prevention abilities, resulting in C. albicans entering tissues or organs from the blood. Common parts are the liver, spleen, kidney, brain, and heart, which may be related to the rich blood flow in these parts, increasing the chance of invasion.
At present, the drugs commonly used in clinical practice mainly include acanthocinins, azole, and polyenes. However, with the widespread use of antifungal drugs, the drug resistance of C. albicans is also increasing, and the effect of traditional treatment methods is not significant, which can no longer fully meet the needs of clinical treatment of C. albicans infection. Therefore, the virulence factors or pathogenic factors of C. albicans were studied to find new key targets and provide a theoretical basis for finding and developing new therapeutic drugs and vaccines.
The protein structure of Ire1 is the most conserved in eukaryotes, including fungal members. It acts as an ER stress sensor and plays pleiotropic roles in ER stress response, antifungal tolerance, cell wall regulation, and virulence-related traits [15,16,23]. Therefore, studying the molecular mechanism of the Ire1 pathway influencing C. albicans hyphae formation and pathogenicity by regulating ER stress will help clarify the relationship between hyphae formation and pathogenic factors at the cellular and molecular levels, and assist in finding therapeutic targets for C. albicans infection.
The Ire1 gene is highly correlated with the pathogenic virulence of C. albicans. In the ER stress induced by tunicamycin and DTT, the Ire1 deletion strain IRE1Δ/Δ exhibited greater sensitivity to 2 μg/mL tunicamycin and 20 μM DTT than the SN152 (WT) strain, and its growth was significantly inhibited. In the RPMI 1640 hyphae induction training, the hypha growth and biofilm formation ability of Ire1Δ/Δ had obvious flaws, which affected the yeast-mycelial morphological transformation and adhesion ability of C. albicans. Ire1Δ/Δ exhibited increased sensitivity to azole antifungals such as FLU and ITZ, as well as cell wall stressors CFW and CGR, illustrating the precise role of Ire1 in regulating sensitivity to these antifungals and cell wall stressors.
The Ire1Δ/Δ strain was non-virulent in the tail vein-infected mouse model and completely lost its pathogenic capacity.
All experimental mice survived until the conclusion of the experiment without manifesting clinical symptoms, and no C. albicans was cultivated from the kidney tissue of the infected mice. No invasion of C. albicans and inflammatory cell infiltration were observed in the pathological sections of the renal tissue.
5. Conclusion
In conclusion, we constructed Sho1Δ/Δ and investigated the influence of the Ire1 gene on ER stress and the pathogenicity of C. albicans through drug stress phenotype testing, biofilm formation assays, hyphal growth assays, and systemic infection tests in mice. The deletion of the Ire1 gene decreased the ability of C. albicans to form hyphae and biofilms, impaired cell wall integrity, increased sensitivity to ER stress agents, and lost toxicity in a mouse tail vein injection model. These results demonstrate that the Ire1 gene maintains the virulence and pathogenicity of C. albicans and can serve as a key target for the potential development of new therapeutic drugs and vaccines.
Footnotes
Funding information: This study was supported by the 980Th Hospital of PLA Joint Logistical Support Force science and technology innovation incubation plan project (FYJHQN202303) and Hebei Province medical science research project (20241197).
Author contributions: Conception and design were contributed by HZ, FW, and KJ; manuscript was drafted by HZ and LQ; provision of study materials or patients was contributed by BH, KJ, and FW; all experiments were completed by HZ, ML, HW, and MJ; data analysis and interpretation were contributed by HZ, LQ, HW, MJ, and MC; and FW and KJ revised the manuscript and were the supporters of the study. All authors contributed to the article and approved the submitted version.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Fukun Wang, Email: wangfk8@sina.com.
Keran Jia, Email: keranjia@sina.com.
References
- [1].McCarty TP, Pappas PG. Invasive candidiasis. Infect Dis Clin North Am. 2016;30(1):103–24. [DOI] [PubMed]
- [2].Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445–56. [DOI] [PubMed]
- [3].Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. [DOI] [PubMed]
- [4].Lass-Florl C, Kanj SS, Govender NP, Thompson 3rd GR, Ostrosky-Zeichner L, Govrins MA. Invasive candidiasis. Nat Rev Dis Primers. 2024;10(1):20. [DOI] [PubMed]
- [5].Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–208. [DOI] [PMC free article] [PubMed]
- [6].Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37(5):634–43. [DOI] [PubMed]
- [7].Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–17. [DOI] [PubMed]
- [8].Cleveland AA, Harrison LH, Farley MM, Hollick R, Stein B, Chiller TM, et al. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. PLoS One. 2015;10(3):e0120452. [DOI] [PMC free article] [PubMed]
- [9].Castanheira M, Messer SA, Rhomberg PR, Pfaller MA. Antifungal susceptibility patterns of a global collection of fungal isolates: results of the SENTRY Antifungal Surveillance Program (2013). Diagn Microbiol Infect Dis. 2016;85(2):200–4. [DOI] [PubMed]
- [10].Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J Clin Microbiol. 2011;49(1):396–9. [DOI] [PMC free article] [PubMed]
- [11].Vallabhaneni S, Cleveland AA, Farley MM, Harrison LH, Schaffner W, Beldavs ZG, et al. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: Data from a large multisite population-based candidemia surveillance program, 2008–2014. Open Forum Infect Dis. 2015;2(4):ofv163. [DOI] [PMC free article] [PubMed]
- [12].Arendrup MC, Perlin DS. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis. 2014;27(6):484–92. [DOI] [PMC free article] [PubMed]
- [13].Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56(12):1724–32. [DOI] [PMC free article] [PubMed]
- [14].Prasad R, Nair R, Banerjee A. Emerging mechanisms of drug resistance in Candida albicans. Prog Mol Subcell Biol. 2019;58:135–53. [DOI] [PubMed]
- [15].Kimata Y, Kohno K. Endoplasmic reticulum stress-sensing mechanisms in yeast and mammalian cells. Curr Opin Cell Biol. 2011;23(2):135–42. [DOI] [PubMed]
- [16].Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. [DOI] [PubMed]
- [17].Sircaik S, Roman E, Bapat P, Lee KK, Andes DR, Gow NAR, et al. The protein kinase Ire1 impacts pathogenicity of Candida albicans by regulating homeostatic adaptation to endoplasmic reticulum stress. Cell Microbiol. 2021;23(5):e13307. [DOI] [PMC free article] [PubMed]
- [18].Appenzeller-Herzog C, Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim Biophys Acta. 2008;1783(4):535–48. [DOI] [PubMed]
- [19].Jacobsen ID, Wilson D, Wachtler B, Brunke S, Naglik JR, Hube B. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther. 2012;10(1):85–93. [DOI] [PubMed]
- [20].Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12(7):317–24. [DOI] [PubMed]
- [21].Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366(9495):1435–42. [DOI] [PubMed]
- [22].Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, et al. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med. 2015;21(7):808–14. [DOI] [PMC free article] [PubMed]
- [23].Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6(1):28–32. [DOI] [PMC free article] [PubMed]









