Abstract
Background
The mitogen-activated protein kinase (MPK) cascade pathway represents a highly conserved signal transduction mechanism in plants, playing a crucial role in growth, development, and stress response. Nevertheless, systematic analysis on the MPK cascade genes in rubber trees remains unexplored.
Results
We conducted a comprehensive identification of the MPK cascade gene family of Hevea brasiliensis, identifying a total of 20 HbMPKs, 13 HbMPKKs, and 167 HbMAPKKKs genes. Through phylogenetic analysis and compared to Arabidopsis MPK cascade genes, HbMPKs and HbMPKKs were categorized into categorized four subgroups with no significant expansion or contraction, while the notably expanded HbMAPKKKs were divided into three subgroups: Raf, ZIK, and MEKK. Conserved motifs, gene structure, and motif analysis further bolster the validity of phylogenetic classification. Furthermore, expression profiling analysis based on public transcriptomic data revealed that these genes were differentially expressed in various tissues and differentially regulated in response to different stresses. Among them, the genes highly expressed in latex or the upregulated genes after tapped including HbMPK8, HbMPK12, HbMPK19, HbMPKK6, HbMPKK9, HbMPKKK15, HbMPKKK21 might be related to latex development and natural rubber (NR) yield. Through yeast two-hybrid assays, we successfully pinpointed 34 pairs of HbMPKKK-HbMPKK-HbMPK interaction modules. Integrating the interaction network and gene expression patterns, 12 potential HbMPK cascade signaling modules including HbMPKKK6/41/79-HbMPKK1-HbMPK9/12/15 and HbMPKKK6/14/21/41/79-HbMPKK9-HbMPK9/15 might involve in NR production and stress responses.
Conclusions
Our study comprehensively unveils the multidimensional characteristics of the MPK cascade gene family in rubber trees and successfully identifies its core signaling cascade module, laying a crucial foundation for future in-depth exploration of the biological functions of the MPK cascade signaling module in rubber trees.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-06615-6.
Keywords: MPK cascade, Hevea brasiliensis, Kinases, Gene family
Background
The mitogen-activated protein kinase (MPK) cascades are highly conserved and functionally significant signaling modules in eukaryotes [1, 2]. A complete MPK signaling cascade consists of three main components: MPKs, MPK kinases (MPKKs), and MPK kinase kinases (MPKKKs) [3]. MPKKKs are typically activated by G proteins or upstream MPKKK kinases, which subsequently activate downstream MPKKs through phosphorylation of two serine or threonine residues within the S/TxxxxxS/T motif [4]. MPKs are characterized by the TXY motif, and the upstream MPKKs activate MPKs by phosphorylating the tyrosine and threonine residues within this motif. Ultimately, activated MPKs phosphorylate specific substrates, triggering cellular responses to external signals [5]. The MPK cascades play crucial roles in plant growth and development [2, 6–8], as well as in responses to various biotic and abiotic stresses [2, 8–10]. In Arabidopsis thaliana, the YODA (YDA)-MKK4/MKK5-MPK3/MPK6 cascade module functions downstream of the ER/ERL receptor and upstream of SPCH/SCRM to negatively regulates the stomatal formation in the leaf epidermis [2], and suppresses the expression of the peptide ligand gene STOMAGEN to coordinate stomatal development in the mesophyll cells [11]. The MAPKKK19/20-MKK3-MPK1/2/7/14 cascade module functions as a mediator of the temperature and after-ripening signals to modulate Arabidopsis seed germination [12]. In rice, the OsMKKK10–OsMKK4–OsMPK6 cascade positively control the seed size, while disruption of OsMKKK10, OsMKK4, or OsMPK6 all lead to small and light grains, short panicles, and dwarf plants [2]. In soybean, MPK3/MPK6 phosphorylate the receptor-like cytoplasmic kinase CDL1, enhancing its stability and thereby increasing resistance to nematodes [13]. In wheat, the TaMKK2-TaMAPK6 cascade functions as a positive regulator to phosphorylate the core immune component TaSGT1, thereby conferring stripe rust resistance [14]. In tomato, SlMPK1/SlMPK2 phosphorylates the transcription factor SlBBX17, resulting in enhanced cold tolerance [15]. In cotton, GhMAPKK5 positively regulates the resistance against drought and salt stresses [16].
The rubber tree (Hevea brasiliensis Müll. Arg.) is the primary source of natural rubber (NR), supplying over 98% of the global demand [17, 18]. NR, known for its superior elasticity, impact resistance, and wear resistance, is widely used in industrial and medical applications, making it an irreplaceable raw material in many products [19]. The laticifers, located within the bark, are responsible for the synthesis and storage of NR [20]. The development of laticifers and the biosynthesis of NR have long been important topics in both botanical and economic research [21, 22]. Studies have shown that mechanical wounds can effectively promote the development of laticifer cells, with jasmonic acid (JA) and coronatine (COR), a JA mimic, positively regulating laticifers development and NR biosynthesis via MYC transcriptional factors [23–25], while exogenous applications of ethephon (ETH) increase latex production and flow [26]. However, frequent tapping can lead to tapping panel dryness (TPD), causing the laticifer cells lose part or even all latex production capacity, severely affecting NR yield [27]. Rubber trees are a tropical crop, yet China's rubber-producing regions are situated on the northern edge of the tropics, in non-traditional planting areas, and frequently encounter adverse environmental factors like low temperatures and drought [28]. The MPK cascade signaling pathway plays a crucial regulatory role in plant stress response, growth, and development, but its specific function in rubber trees remains incompletely elucidated.
To date, genome-wide analyses of the MPK cascade have been conducted in several plant species, including A. thaliana [1], Oryza sativa [29, 30] and Populus trichocarpa [31]. Generally, these three classes of kinases could divide into several defined subgroups based on sequence similarities and domain organizations. The fewer MPKs and MPKKs in plants were both classified into four subgroups, namely Group A to Group D, respectively [29–31]. Differently, plant MPKKKs form the largest class among the cascade, with 80 in Arabidopsis [1], 75 in rice [29, 30], and 104 in poplar [31]. The MPKKKs can fall into three subgroups including MEKK, Raf (rapidly accelerated fibrosarcoma), and ZIK (ZR1-interacting kinase) [4, 32]. Previously, Jin et al. have identified the MPK gene family in H. brasiliensis [33], but the MPKK and MPKKK gene families have not yet been identified or subjected to a comprehensive genome-wide analysis. The MPK signaling pathway transmits signals through a cascade of phosphorylation reactions (MPKKK—MPKK—MPK), serving as a key transmission chain for plants to receive extracellular signals and trigger a series of stress responses. Furthermore, increasing evidence suggests that the functions of MPKK and MPKKK in plants are equally crucial. Therefore, a more comprehensive and systematic analysis of the entire MPK cascade in rubber tree remains indispensable for further research.
In this study, we identified 20 HbMPKs, 13 HbMPKKs, and 167 HbMPKKKs based on a genome-wide search against H. brasiliensis genome. We further analyzed the chromosomal locations, conserved motifs, gene structures, gene duplications, and promoter cis-acting elements of these genes, as well as their expression profiles across various tissues of rubber trees, latex from tapped and untapped trees, healthy trees and TPD-affected trees, under hormone and cold treatments. Additionally, we cloned HbMPK cascade members exhibiting distinct expression patterns and detected the interaction profiles among HbMPKKK-HbMPKK-HbMPK proteins using yeast two-hybrid (Y2H) assays. By comparing these interaction networks and expression patterns, we discovered that several HbMPK signaling modules might play complex roles in the growth and development of rubber trees, as well as their response to abiotic stress. This study lays the foundation for a deeper understanding of the HbMPK cascade and its gene functional characterization.
Methods
Plant materials
The seven-year-old rubber tree cultivar “CATAS7-33–97” (H. brasiliensis cv. “CATAS7-33–97”) was created by Chinese Academy of Tropical Agricultural Sciences (CATAS) and grown at the Rubber Research Institute of the Chinese Academy of Tropical Agricultural Sciences (RRI-CATAS) experimental station in Danzhou City, Hainan Province, China. In this study, we collected rubber tree latex samples, which were harvested every four days, and immediately mixed these samples with pyrolysis buffer SL on ice (RNA prep Pure Plant Kit, TIANGEN BIOTECH CO., LTD., Beijing, China). The samples were then stored at -80 °C for RNA extraction.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from rubber tree latex, inner bark or leaf tissues using the RNA prep Pure Plant Kit (TIANGEN BIOTECH CO., LTD., Beijing, China) according to the manufacturer's protocol. The quality and quantity of RNA were assessed by spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Then the purified RNA was reversely transcribed in a PCR amplifier, and the first strand cDNA was synthesized using Thermo Scientific Revert Aid First Strand cDNA Synthesis (Thermo Fisher Scientific, MA, USA).
Identification and chromosomal distribution of HbMPK cascade genes in H. brasiliensis
Download the whole-genome annotation and sequence data of H. brasiliensis from the NCBI database (https://www.ncbi.nlm.nih.gov/) [34]. To identify putative MPKs cascade genes in H. brasiliensis, the MPKs cascade proteins in A. thaliana from The Arabidopsis Information Resource (TAIR) database(https://www.arabidopsis.org/) [35] were used as queries to search against the H. brasiliensis protein sequences by Basic Local Alignment Search Tool for Proteins (BLASTP) program with an e-value of 1e-5 and identity of 30% as the threshold. Subsequently, the candidates were submitted to Simple Modular Architecture Research Tool (SMART) (https://smart.embl.de/) [36] with a cut-off value of 0.001, NCBI Conserved Domain Database tool (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) [37] (e-value = 0.001) and InterPro databases (https://www.ebi.ac.uk/interpro/) [38] (e-value = 0.001) to verify the kinase domain. Based on the H. brasiliensis gff3 file, the chromosome positions of HbMPK cascade genes were plotted using MapChart (https://www.wur.nl/en/show/Mapchart.htm) [39].
Diverse physical and chemical parameters of HbMPKs cascade genes, including the number of amino acids, molecular weight, theoretical isoelectric points, instability index, aliphatic index, and grand average of hydropathicity, were evaluated by Expert Protein Analysis System (ExPASy) (https://www.expasy.org/) [40].
Phylogenetic analysis of HbMPK cascade genes
The protein sequences of MPK cascade genes from H. brasiliensis and A. thaliana were aligned using MUSCLE [41]. The MPK and MPKK gene families were analyzed using neighbor-joining (NJ) trees constructed in TreeBest [42] with 1000 bootstrap replications, whereas the MPKKK gene family was analyzed using a maximum likelihood (ML) tree constructed in IQ-TREE [43]. The HbMPK cascade gene families of H. brasiliensis were classified based on the phylogenetic tree. The resulting phylogenetic trees were visualized and edited using the Interactive Tree Of Life (iTOL) platform (https://itol.embl.de/) [44].
Conserved domain, motifs and gene structure analysis
Conserved domains were identified using the NCBI Batch CD-Search tool (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi) [45]. Conserved motifs of MPK cascade proteins were analyzed by Online MEME software (https://meme-suite.org/meme/) with the parameter: number of motifs to select = 12 [46]. The structural information, including exon, intron, CDS, and UTR were obtained from the Gff3 annotation file of H. brasiliensis genome. Finally, the conserved domains, motifs and gene structures were visualized using TBtools [47].
Collinearity analysis and gene duplication
Gene duplication events between HbMPK cascade genes were analysed using the Multiple Collinearity Scan toolkit (MCScanX) (http://chibba.pgml.uga.edu/mcscan2/) with default parameters [48]. Collinearity relationship of MPK cascade genes within H. brasiliensis and between H. brasiliensis and A. thaliana were also analyzed using the MCScanX. Circos was used to plot collinearity curves of MPK cascade genes [49].
Cis-acting element analysis in promoter regions
The region 2000 bp upstream of the initiation codon (ATG) was regarded as the promoter sequence for each HbMPK cascade genes. The promoter sequences were extracted using TBtools and then submitted to PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [50] to detect the cis-acting regulatory elements. Subsequently, the results were visualized and analyzed using TBtools [47].
Gene expression analysis of HbMPK cascade genes using public RNA-Seq data
Previously released RNA-seq datasets of H. brasiliensis were used to analyze the expression profiles of HbMPK cascade genes. The expression patterns of these genes across different tissues, including virgin latex (SAMC376659), tapped latex (SAMC376661), cambium region (SAMC376662), inner bark (SAMC376658), leaves (SAMC376664), female flowers (SAMC376660), and male flowers (SAMC376663), were obtained from the China National Center for Bioinformation (CNCB) (https://ngdc.cncb.ac.cn/). Additionally, transcriptome data from TPD-affected trees (SAMN03078534) and healthy trees (SAMN03078533) were retrieved from the Chinese Academy of Tropical Agricultural Sciences (CATAS) (http://hevea.catas.cn/home/index) [51]. Furthermore, RNA-seq data from methyl jasmonate (MeJA) treatment (PRJNA353743), ETH treatment (PRJNA310171), and low-temperature stress (PRJNA432826) were also accessed from CATAS (http://hevea.catas.cn/home/index). All expression data were normalized as Transcripts Per Million (TPM) values [52]. The differentially expressed genes (DEGs) were carried out using DESeq2 [53] and were defined under the thresholds of log2FC (fold change) > 1 (FDR < 0.05). The expression profiles of HbMPK cascade genes were visualized as heatmaps using TBtools [47].
Plasmid construction and Y2H assay
For the Y2H assay, the full-length cDNA sequences of HbMPK cascade genes were amplified and fused into pGADT7 (AD) or pGBKT7 (BD) vectors to generate BD-HbMPKs, AD-HbMPKKs, and BD-HbMPKKKs constructs, respectively. The primers of HbMPK cascade genes were listed in Table S1. The constructed BD-fused vectors with empty pGADT7 vector were co-transformed into Y2HGold (Sangon Biotech, Shanghai, China) yeast cells to determine self-activation. The transformed cells containing both AD-HbMPKKs and BD-HbMPKs or BD-HbMPKKKs constructs were grown on synthetic-defined (SD) media lacking Leu and Trp (SD/-LT) and SD/-Leu/-Trp/-His/-Ade (SD/-AHLT) plates at 30 °C for 3–7 days to determine the interaction. The co-transformed yeast colonies that were able to grow on the selective SD/-AHLT media indicate the interaction between the two tested proteins occurred. If the self-activation existed, Aureobasidin A (AbA) with the concentration of 200 ng/ml was added into SD/-AHLT media to inhibit it. The co-transformation of pGBKT7-P53, or pGBKT7-Lam with the pGADT7-T was used as the positive and negative controls, respectively.
Results
Identification of HbMPK cascade genes in H. brasiliensis
Using BLASTP screening combined with domain verification, a total of 20 HbMPKs, 13 HbMPKKs, and 167 HbMPKKKs were identified in the H. brasiliensis genome. Based on their chromosomal positions, these HbMPK cascade genes were named accordingly (Tables S2-S4). Chromosome localization analysis revealed that the 20 HbMPK genes were unevenly distributed across 11 chromosomes, while the 13 HbMPKK genes were mapped to 10 chromosomes, with each chromosome containing one to two members. The 167 HbMPKKK genes were distributed across 18 chromosomes, with the highest concentration on chromosome NC_079500.1 (Fig. 1).
Fig. 1.
Distribution of HbMPK cascade genes in H. brasiliensis genome. A The HbMPK genes were unevenly distributed on 11 chromosomes. B The HbMPKK genes were distributed on 10 chromosomes. C For HbMPKKK genes, 155 HbMPKKKs were unevenly distributed across 18 chromosomes, while 12 HbMPKKKs were located on 4 scaffolds. The values (Mb) on the left side of the chromosome indicated the gene positions on the chromosome
Analysis of physical and chemical properties revealed that the protein lengths (number of amino acids) of HbMPKs ranges from 365 to 615, with molecular weights between 42.15 and 70.01 kDa, and theoretical isoelectric points (pI) ranging from 4.64 to 9.62. The protein length of HbMPKKs varies from 138 to 518, with molecular weights between 15.46 and 57.68 kDa, and pI values ranging from 4.53 to 9.55. For HbMPKKKs, the protein length spans from 150 to 1432, with molecular weights between 16.82 and 155.01 kDa, and pI values ranging from 4.27 to 9.88. Further properties of the HbMPK cascade genes, including instability indices, aliphatic indices, and grand average of hydropathicity, are also presented in Tables S2-S4.
Phylogenetic relationships of proteins encoded by HbMPK cascade genes
To analyze the evolutionary relationships among the HbMPK, HbMPKK, and HbMPKKK genes in H. brasiliensis and A. thaliana, two NJ phylogenetic trees and one ML phylogenetic were constructed using protein sequences from the MPK cascade gene families of both species. The results showed that the HbMPKs could be divided into four groups: A (HbMPK1, 3, 11), B (HbMPK4, 6, 7, 8, 10, 18), C (HbMPK2, 12), and D (HbMPK5, 9, 13, 14, 15, 16, 17, 19, 20) (Fig. 2A, Table 1). Similarly, the HbMPKKs were classified into four groups: A (HbMPKK2, 3, 6, 10, 11), B (HbMPKK1), C (HbMPKK7), and D (HbMPKK4, 5, 8, 9, 12, 13) (Fig. 2B, Table 1). The HbMPKKK gene family has the largest number of members and was divided into three subtypes: MEKK, RAF, and ZIK (Fig. 2C, Table 1).
Fig. 2.
Phylogenetic relationships of MPK cascade genes from H. brasiliensis and A. thaliana. A Phylogenetic tree of MPK genes in H. brasiliensis and A. thaliana. B Phylogenetic tree of MPKK genes in H. brasiliensis and A. thaliana. C Phylogenetic tree of MPKKK genes in H. brasiliensis and A. thaliana. Different groups are highlighted in distinct colors. The MPK cascade genes of H. brasiliensis in blue circles, and A. thaliana in red circles
Table 1.
Classification and comparison of the MPK cascade genes of H. brasiliensis and A. thaliana
| MPK cascade genes | Subfamily | H. brasiliensis | A. thaliana |
|---|---|---|---|
| MPKs | Group A | 3 | 3 |
| Group B | 6 | 5 | |
| Group C | 2 | 4 | |
| Group D | 9 | 8 | |
| Total | 20 | 20 | |
| MPKKs | Group A | 5 | 3 |
| Group B | 1 | 1 | |
| Group C | 1 | 2 | |
| Group D | 6 | 4 | |
| Total | 13 | 10 | |
| MPKKKs | MEKK | 66 | 21 |
| Raf | 81 | 48 | |
| ZIK | 20 | 11 | |
| Total | 167 | 80 |
In addition, we identified the conserved domains of the HbMPK, HbMPKK, and HbMPKKK gene families (Fig. S1). Among the four groups of the HbMPK family, group D contains eight HbMPK genes, all possess the catalytic domain of the serine/threonine kinase mitogen-activated protein kinases (STKc_TDY_MAPK). Groups A, B, and C have the catalytic structure of STKc_TEY_MAPK except for HbMPK4. In the Raf subfamily of the HbMPKKK family, the majority of members (72 of 85) contain the STKc_MAP3K-like domain. Additionally, some Raf subfamily members possess an ANKYR domain at the N-terminus and others feature LRR or PP2Cc domains at the C-terminus. In the ZIK subfamily, all proteins except HbMPKKK37 and HbMPKKK59 contain the STKc_WNK domain.
Conserved motifs and gene structures analysis
Using the MEME tool, 12 conserved motifs were identified in the MPK cascade proteins of H. brasiliensis (Fig. S1). In the motif analysis of HbMPK, all HbMPK proteins contained motifs 1, 2, 3, 5, 6, and 9, with motifs 1, 2, and 3 featuring functional characteristic domains. Motif 1 contained the core catalytic site, Serine/threonine-protein kinase, active site (V/I/A) xHRDLKPxNxLx(L/A/I), in which the conserved aspartic acid was essential for enzymatic catalytic activity [54]. Both motifs 2 and 3 have a protein kinase domain that can catalyze the phosphorylation of amino acids. In the motif analysis of HbMPKK, all HbMPKK proteins contain motifs 1, 7, and 9. Motifs 1, 2, 3, 4, and 5 all include functional domains associated with protein kinase activity, with motif 4 also containing a conserved site related to ATP binding ((L/I) GxGxGGxVxKVxHKxxxxxxALK). In HbMPKKK, the motif distribution varied among different subtypes, however, similar motif compositions were observed within the same subtype. Motif 2 contains the Serine/threonine-protein kinase active site domain, and was present in all HbMPKKK subtypes except HbMPKKK4, HbMPKKK33, HbMPKKK59, and HbMPKKK133.
To better understand the structure of MPK cascade gene transcripts in H. brasiliensis, we summarized the organizations of exon, intron, coding sequences (CDS), and untranslated region (UTR) (Fig. S1 and Tables S2-S4). The HbMPKs possessed 2 to 10 introns and 3 to 11 exons. Apart from HbMPK4, which lacked 3′-UTR, all the other 19 HbMPKs possessed both 5′-UTR and 3′-UTR regions. There was significant variation in the number of introns among different groups of HbMPKKs. Most members of group A contained 7 or 8 introns, group B had 10, while groups C and D did not contain any introns. In HbMPKKKs, the number of exons for Raf ranges from 2 to 25, and introns range from 1 to 24, MEKK has exons ranging from 1 to 18, with 19 members lacking introns; whereas ZIK shows a relatively even distribution of exons and introns, ranging from 3 to 8 and 2 to 7, respectively.
Collinearity relationship analysis of HbMPK cascade genes
The results of the gene duplication event and collinearity analysis clarified the amplification patterns of the HbMPK cascade genes within species and its conservation across different species. Initially, the origins of duplicated HbMPK cascade genes were categorized into four types of events: tandem, proximal, WGD/segmental, and dispersed (Table S5). Among the 20 HbMPK genes, 17 were duplicated from WGD/segmental, 3 from dispersed, and 1 from proximal. Among the 13 HbMPKK genes, 6 originated from WGD/segmental, 5 from dispersed, and 2 from proximal. For the HbMPKKK genes, the majority (60.48%, 101/167) were duplicated through WGD/segmental, 10.78% (18/167) originated from proximal, while dispersed and tandem events each accounted for 14.37% (24/167). These results suggested that WGD/segmental duplication appeared to be the primary driving force behind the expansion of the H. brasiliensis HbMPK gene family.
Moreover, a collinearity map was generated within the HbMPK cascade genes to identify gene pairs (Fig. 3). The results showed the presence of 12 paralogous gene pairs in HbMPKs, 3 paralogous gene pairs in HbMPKKs, and 80 paralogous gene pairs in HbMPKKKs. In the collinearity analysis with A. thaliana, there are 2 orthologs gene pairs between HbMPKs and AtMPKs, 4 orthologs gene pairs between HbMPKKs and AtMPKKs, and 9 orthologs gene pairs between HbMPKKKs and AtMPKKKs (Fig. S2).
Fig. 3.
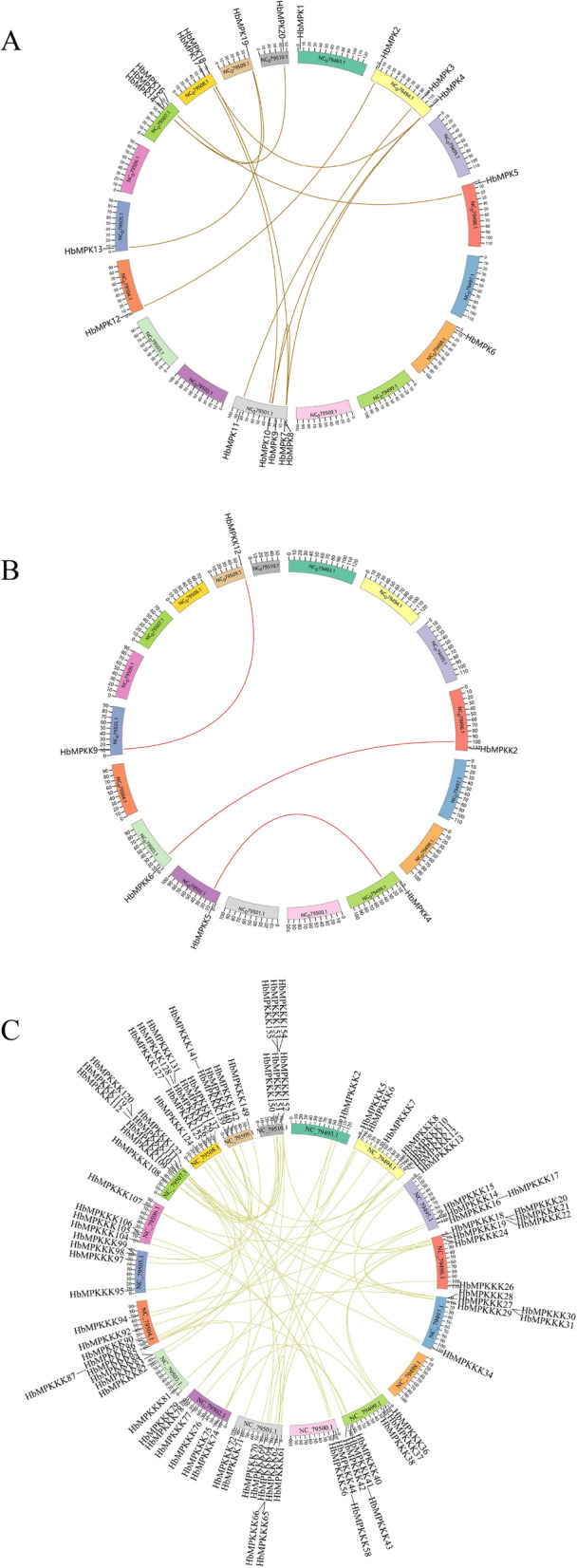
Intraspecific collinearity analysis of HbMPK cascade genes. A Collinearity analysis of HbMPKs. B Collinearity analysis of HbMPKKs. C Collinearity analysis of HbMPKKKs. Colored blocks represent different chromosomes, and gene names were labeled around the circular genome. Curved lines indicate collinear gene pairs within the genome
Identification of cis-acting elements in HbMPK cascade genes
The cis-acting elements in the promoter regions of all HbMPK cascade genes were identified using PlantCARE (Fig. S3). Besides the core elements, these predicted cis-acting elements could be categorized into four types: development, stress, hormone, and light responsive (Tables S6-S7). A total of 28 light-responsive elements were predicted, with the three most frequent being Box-4 (95%, 190 out of 200), G-Box (73%), and GT1-motif (63%). Among the hormone-responsive elements, the most common was the abscisic acid (ABA) response element (ABRE, 68%), followed by MeJA response elements (including CGTCA-motif and TGACG-motif, both at 63%), salicylic acid response elements (TCA-motif, 47%), and auxin response elements (TGA-motif, 34%). Additionally, several gibberellin response elements were identified, such as P-box (33%), TATC-box (20%), and GARE-motif (19%). Several stress-responsive elements (such as ARE, MBS, and LTR) related to anaerobic, drought, and low-temperature responses were identified in 168, 83, and 70 HbMPK cascade genes, respectively. In addition, some development-responsive elements related to meristem expression (CAT box, 38%), zein metabolism regulation (O2 site, 26%), endosperm expression (15%), and circadian control (13%) were identified. These results suggest that these abundant cis-acting elements might regulate the expression of HbMPK cascade genes in H. brasiliensis during development and in response to light, stress, and hormones.
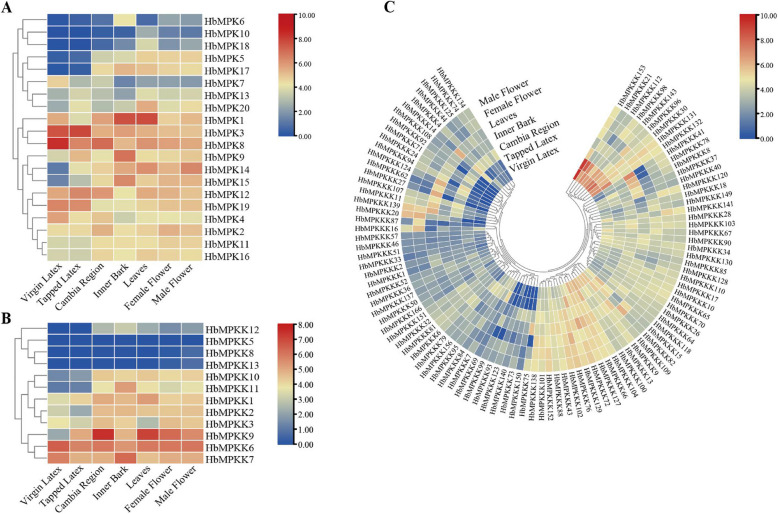
Expression profiling of HbMPK cascade genes across various tissues
To better understand the gene expression of the HbMPK cascade genes in different tissues, we obtained relevant transcriptome datasets from CNCB and displayed through heat maps (Fig. 4 and Table S8). It was noted that the cascade genes were differentially and preferentially expressed in various tissues. Among them, HbMPK3, HbMPK8, HbMPK12, HbMPK19, HbMPKK6, HbMPKK7, HbMPKKK153, and HbMPKKK21 were significantly expressed in both virgin latex and tapped latex, which might be related to NR production and should be taken more attention in future work. HbMPK8, HbMPKK9, HbMPKK6, and HbMPKKK14 showed high expression in the cambium, while HbMPK1, HbMPK3, HbMPK9, HbMPK15, HbMPKK7, and HbMPKKK41 exhibited high expression in the bark. Additionally, HbMPK1, HbMPKK9, HbMPKK6, and HbMPKKK27 were highly expressed in the leaves. Based on this analysis, we found that the genes HbMPK1, HbMPK3, HbMPK8, HbMPKK6, HbMPKK7, and HbMPKK9 showed high expression across multiple tissues, suggesting that they may play important roles in critical signaling nodes.
Fig. 4.
Expression profiling of HbMPK cascade genes across different tissues. A Tissue-specific expression levels of HbMPKs. B Tissue-specific expression levels of HbMPKKs. C Tissue-specific expression levels of HbMPKKKs. The color scale represents expression levels, ranging from blue (low expression) to red (high expression)
Expression analysis of HbMPK cascade genes in tapped and virgin latex, healthy and TPD-affected trees
In the expression analysis of latex from regularly tapped trees and virgin trees, a total of 10 HbMPK, 6 HbMPKK, and 80 HbMPKKK genes exhibited differential expression (Fig. 5A and Table S9). Among these, 4 HbMPK genes were down-regulated and 6 were up-regulated. 4 HbMPKK genes were down-regulated and 2 were up-regulated, while 34 HbMPKKK genes were down-regulated and 46 were up-regulated. It was noteworthy that HbMPK14, HbMPK15, HbMPKK9, HbMPKKK124, HbMPKKK140, and HbMPKKK14 were significantly upregulated after tapped, implying their potential involvement in stress responses and metabolic regulation triggered by mechanical damage, particularly in signaling pathways related to rubber synthesis.
Fig. 5.
Expression analysis of HbMPK cascade genes in tapped and virgin latex, healthy and TPD-affected trees. A Expression patterns in tapped latex and virgin latex. B Expression patterns in healthy trees and TPD-affected trees. The color scale represents expression levels, ranging from blue (low expression) to red (high expression). Differentially expressed genes are marked with red (up-regulated) and blue (down-regulated) annotations
Additionally, we conducted a comparative analysis of gene expression in healthy trees and TPD-affected trees (Fig. 5B and Table S10). The results showed that HbMPK20, HbMPKKK20, HbMPKKK25, HbMPKKK42, HbMPKKK59, and HbMPKKK87 were down-regulated in TPD-affected trees, while others, like HbMPK8, HbMPK9, HbMPK12, HbMPKK9, HbMPKKK71, and HbMPKKK14, were up-regulated. The down-regulated genes may indicate their functional role in healthy conditions, whereas their function could be suppressed in pathological states. Conversely, the up-regulated genes may play protective or regulatory roles in response to adverse signals.
Expression analysis of HbMPK cascade genes under MeJA, ETH and cold treatment
MeJA and ETH are two important hormones widely present in plants, playing a crucial role in stress response and rubber yield regulation in rubber trees [55, 56]. We analyzed the expression changes of the HbMPK cascade genes under treatments with these two hormones (Fig. S4A-B and Tables S11-S12). Under different concentrations of MeJA treatment, the expression of several genes gradually decreased with increasing concentration (HbMPK6, HbMPK18, and HbMPKKK24) or increased (HbMPKKK78, HbMPKKK124, and HbMPKKK126). This indicates that the regulatory effect of MeJA on the expression of MPK cascade genes in rubber trees is complex, with certain genes exhibiting concentration-specific responses to MeJA. Most members of the HbMPK and HbMPKKK gene families exhibited significant differential expression under ETH treatment, particularly among members of the HbMPKKK family. With extended treatment time, several HbMPKKK genes (HbMPKKK48, HbMPKKK51, HbMPKKK52, HbMPKKK53, and HbMPKKK54) showed significant up-regulation. This suggests that these genes may play an important role in the physiological responses and adaptation processes of rubber trees under ETH stimulation.
We also analyzed the expression of MPK cascade genes under low-temperature stress (Fig. S4C and Table S13). There were 73 differentially expressed HbMPK cascade genes under cold treatment, over half of which (48 out of 73) were significantly up-regulated, indicating that they may play important roles in cold stress response. This further suggests that MPK cascade genes have potential significance in the environmental adaptation of rubber trees.
Interactions between HbMPK cascade family members
To identify the MPK cascade signaling transduction modules in rubber trees, we selected genes with significant expression patterns for interaction analysis. For example, HbMPK8 was highly expressed in latex from regularly tapped trees, HbMPKK9 showed high expression in the cambium, and HbMPKKK41 exhibited notable expression in the bark. In total, we chose 9 HbMPKs, 5 HbMPKKs, and 15 HbMPKKKs, which were highly-expressed in latex and other tissues, or differentially expressed under cold under MeJA, ETH and cold treatment (Table S14). The HbMPKs and HbMPKKKs were cloned into the BD vector, whereas the HbMPKKs were cloned into the AD vector. Initially, we performed self-activation assays, revealing that 9 HbMPKs and 5 HbMPKKs lacked self-activation activity. Conversely, HbMPKKK4 and HbMPKKK106 demonstrated pronounced self-activation activity, while HbMPKKK6 and HbMPKKK41 exhibited weaker self-activation.
Next, we paired HbMPKs with HbMPKKs, and HbMPKKs with HbMPKKKs, and then transformed them into the Y2HGold yeast cells and recorded their growth on SD/-AHLT medium (Fig. 6). In the interaction detection of HbMPKK-HbMPK pairing, we discovered that HbMPKK1 interacts with four proteins: HbMPK2, HbMPK9, HbMPK12, and HbMPK15. Following this, HbMPKK9 was found to interact with three MPKs, among which its interaction with HbMPK15 was relatively weak. HbMPKK2, HbMPKK3, and HbMPKK6 all interacted with HbMPK8. In the interaction detection of HbMPKKK-HbMPKK pairing, we found that HbMPKK9 interacted with four proteins: HbMPKKK14, HbMPKKK21, HbMPKKK30, and HbMPKKK79. Additionally, there is an interaction between HbMPKK1 and HbMPKKK79, while HbMPKK2, HbMPKK3, and HbMPKK6 all interacted with HbMPKKK101.
Fig. 6.
Y2H interaction analysis of HbMPK cascade proteins. Interactions between selected HbMPKKs and HbMPKs, and HbMPKKs and HbMPKKKs. Yeast cells carrying specific plasmid combinations were plated on SD/-Leu/-Trp (SD/-LT) and SD/ -Ade/-His/-Leu/-Trp (SD/-AHLT) medium. Positive control (+ , pGBKT7-P53 + pGADT7-T) and negative control (-, pGBKT7-Lam + pGADT7-T)
To inhibit self-activation in the yeast two-hybridization test system, we added Aureobasidin A (AbA) to the selective culture medium. With complete inhibition of self-activation activity, it was observed that both HbMPKKK6 and HbMPKKK41 could interact with HbMPKK1 and HbMPKK9 (Fig. S5).
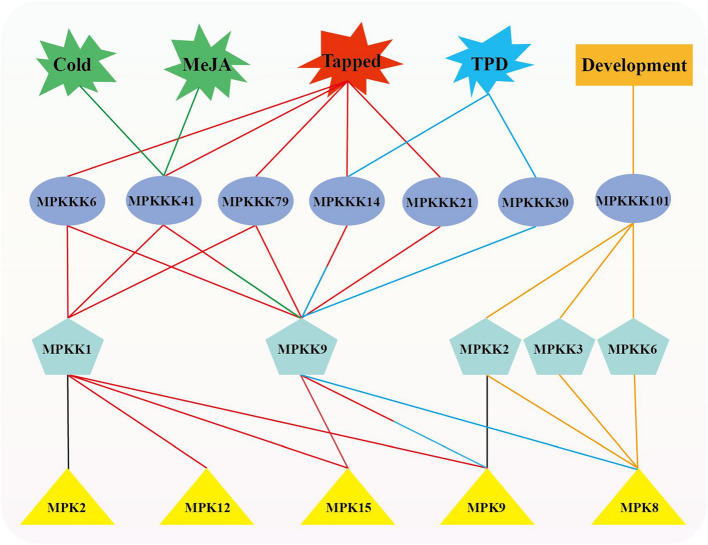
Comparison of the expression patterns between HbMPK cascade genes
To further elucidate the biological functions of the validated HbMPKKK-HbMPKK-HbMPK interactions, we integrated the interaction network and gene expression patterns to reveal a series of potential HbMPK cascade signaling modules (Fig. 7). These modules exhibit specific expression characteristics, such as high expression in specific tissues and induction under various conditions, including tapping, TPD, MeJA, and cold stress. For instance, MPKK6-MPK8 module genes were highly expressed in latex. HbMPKKK4/101-HbMPKK2-HbMPK8, HbMPKKK101-HbMPKK3-HbMPK8, HbMPKKK4/14/30-HbMPKK9-HbMPK8, and HbMPKK1-HbMPK2 were highly expressed in the cambium. HbMPKKK14/79-HbMPKK9 was highly expressed in leaves, and HbMPKKK6/41/79-HbMPKK1-HbMPK9/15 is highly expressed in bark. After undergoing tapping treatment, various modules exhibited significant expression changes. Specifically, HbMPKK2-HbMPK8 demonstrated downregulation, whereas HbMPKKK6/41/79-HbMPKK1-HbMPK9/12/15 and HbMPKKK6/14/21/41/79-HbMPKK9-HbMPK9/15 were significantly induced. Additionally, HbMPKKK14/30-HbMPKK9-HbMPK8/9 showed significant upregulation in TPD-affected trees, while HbMPKKK41-HbMPKK9 exhibited strongly induced expression under MeJA and cold treatment conditions. These discoveries offer crucial insights for further investigating the functions of HbMPK cascade signaling modules in various biological processes.
Fig. 7.
Interaction network of HbMPK cascade genes. The lines between HbMPK cascade genes represent their interactions. Different colors indicate induction under specific treatment conditions and potential biological responses: green lines represent induction by MeJA and cold treatment, red lines represent induction by tapped treatment, blue lines represent induction in TPD-affected trees, and orange lines represent potential involvement in the growth and development of rubber tree laticifers. Lines with two colors indicate involvement in multiple responses
Discussion
MPK cascades are crucial signaling modules in plants, primarily situated downstream of sensors or receptors, facilitating the cell response to external stimuli [7]. Among various species, MPKKK constitutes the largest component of the MPK cascade, followed by MPK, with the least being MPKK. For instance, 80 AtMPKKK, 10 AtMPKK, and 20 AtMPKs were identified in A. thaliana [1], 104 PtMPKKK, 11 PtMPKK, and 21 PtMPK in P. trichocarpa [31], and 65 JcMPKKK, 5 JcMPKK, and 12 JcMPK in Jatropha curcas [57]. There were 20 MPKs identified in rubber tree previously [32], however, other two components of the HbMPK cascade genes, HbMPKKKs and HbMPKKs, were not reported yet. Here we searched the rubber tree genome based on the identified Arabidopsis MPK cascade gene sequences, successfully identifying 167 HbMPKKK, 13 HbMPKK, and 20 HbMPK genes. Similarly to Arabidopsis, HbMPKKKs are categorized into three subtypes: MEKK, Raf, and ZIK, while both HbMPKK and HbMPK are divided into four groups, Group A-D (Table 1). Notably, the HbMPKKK gene family is significantly expanded compared to Arabidopsis AtMPKKKs, while there are no differences in MPKs and MPKKs. Furthermore, gene duplication events play a pivotal role in the evolution of plant genomes, often resulting in the emergence of new genes and genetic regulatory pathways [58, 59]. Our research reveals that WGD or segmental duplication events are the primary driving forces for the expansion of the HbMPKKK genes.
Previous studies have demonstrated that MPK cascade genes play crucial roles in various plant biological processes, including plant growth and development, as well as response to environmental stresses [2, 6–10]. In this study, we conducted a detailed analysis of the expression of MPK cascade genes in rubber trees across different tissues, under various conditions, and hormone treatments. The cascade genes were expressed differentially and preferentially in various tissues including latex, and differentially regulated in response to different stresses. Among them, the genes highly expressed in latex or the upregulated genes after tapped including HbMPK8, HbMPK12, HbMPK19, HbMPKK6, HbMPKK9, HbMPKKK15, HbMPKKK21 might be related to latex development and NR yield. Notably, HbMPK8 exhibits high expression in multiple tissues, particularly in the latex and cambium of untapped trees. The Arabidopsis homologues of HbMPK8, AtMPK4, AtMPK5, AtMPK11, AtMPK12, and AtMPK13, have been confirmed to be involved in plant immunity, environmental response, and growth and development [60, 61]. Additionally, research has indicated that HbMPK may be regulated by PSK signals, thereby participating in laticifer differentiation [43]. Therefore, we speculate that HbMPK8 may play a crucial role in laticifer differentiation. In the HbMPKK gene family, Group D comprises a total of 6 members, among which 5 members (HbMPKK4, HbMPKK5, HbMPKK8, HbMPKK12, HbMPKK 13) exhibit either no expression or extremely low expression levels in most tissues. Only HbMPKK9 demonstrates high expression across multiple tissues, including cambium, leaf, and male flower. These findings imply that HbMPKK9 may play a role in various tissues of rubber trees. Furthermore, we discovered that HbMPKK9 expression is upregulated by ETH induction. Research on AtMKK9 indicates that it regulates ETH synthesis-related genes by activating downstream MPK3 and MPK6 signaling pathways, thereby enhancing the role of ETH in plant stress responses [62, 63]. We speculate that HbMPKK9 may also be involved in the regulation of ethylene signaling in rubber trees and play a pivotal role in stress resistance reactions.
Plant MPK interacts with upstream and downstream protein components to form a signaling cascade, thereby regulating various physiological responses. In this study, we identified multiple rubber tree MPK cascade signaling modules through Y2H experiments. The results indicate that members of the same subfamily typically exhibit similar interaction patterns. For instance, HbMPKK2, HbMPKK3, and HbMPKK6, all belonging to Group A, can interact with HbMPK8 and HbMPKKK101, forming HbMPKKK101-HbMPKK2/3/6-HbMPK8 modules. This interaction pattern aligns with the Arabidopsis homologous gene interaction module AtMEKK1-AtMPKK1/2-AtMPK4 [64]. Furthermore, we discovered that HbMPKK1 and HbMPKK9 can interact with multiple HbMPKKK and HbMPK proteins, respectively, establishing HbMPKKK6/41/79-HbMPKK1-HbMPK2/9/12/15 modules and HbMPKKK6/14/21/30/41/79-HbMPKK9-HbMPK8/9/15 modules. Gene expression analysis related to these interaction modules revealed that all members of the HbMPKKK6/41/79-HbMPKK1-HbMPK9/12/15 module were significantly upregulated under tapping treatment. Similarly, the HbMPKKK6/14/21/41/79-HbMPKK9-HbMPK9/15 modules also demonstrated similar expression patterns induced by tapping. Previously, Jeam et al. reported that the MPK3K14-MPKK3-MPK1/2/7/14 cascade module in Arabidopsis is activated by damage-induced JA signaling [65]. The signal transduction module observed in this study aligns with the JA signaling pathway triggered by damage in Arabidopsis. This finding suggests that these two modules, HbMPKKK6/41/79-HbMPKK1-HbMPK9/12/15 and HbMPKKK6/14/21/41/79-HbMPKK9-HbMPK9/15 might be involved in NR production and stress responses. Since the potential interaction modules were confirmed by Y2H assays, which might be with false positive frequency. To further verify the interaction, more approaches including luciferase complementation imaging and Co-immunoprecipitation assays [66] will be introduced. And the underlying mechanism the HbMPK cascade regulating NR and stress adaptation in rubber tree is being under investigation and will be uncovered in the future.
Conclusions
This study comprehensively unveiled the multidimensional features of the MPK cascade gene family in rubber trees. Furthermore, potential MPK cascade interaction modules were identified through Y2H experiments and expression analysis, offering crucial insights into the functional elucidation of MPK cascade pathways in rubber trees. These findings offer pivotal candidate genes and signaling pathway modules for future functional validation studies and genetic enhancement of rubber trees.
Supplementary Information
Supplementary Material 1: Fig. S1. Conserved motifs, domains and gene structure analysis of the HbMPK cascade gene family. Fig. S2. Interspecies collinearity analysis of MPK cascade genes in H. brasiliensis and A. thaliana. Fig. S3. Cis-acting element analysis of HbMPK cascade genes. Fig. S4. Expression analysis of HbMPK cascade genes under MeJA, ETH and cold treatment. Fig. S5. Interaction between HbMPKKK6 and HbMPKKK41 with HbMPKKs in a yeast two-hybrid assay.
Supplementary Material 2: Table S1. Primer sequences used in this study. Table S2. Basic information of the HbMPK genes. Table S3. Basic information of the HbMPKK genes. Table S4. Basic information of the HbMPKKK genes. Table S5. Classification of duplication origin information for HbMPK cascade genes. Table S6. Cis-acting element identified in the promoter of HbMPK cascade genes. Table S7. Function and category of each cis-acting element identified in the promoter of HbMPK cascade genes. Table S8. The TPM values of HbMPK cascade genes expressed in different tissues. Table S9. The TPM values of HbMPK cascade genes expressed in tapped and virgin latex. Table S10. The TPM values of HbMPK cascade genes expressed in healthy trees and TPD-affected trees. Table S11. The TPM values of HbMPK cascade genes expressed under MeJA treatment. Table S12. The TPM values of HbMPK cascade genes expressed under ETH treatment. Table S13. The TPM values of HbMPK cascade genes expressed under cold treatment. Table S14. The representative HbMPK cascades genes with significant expression characteristics.
Acknowledgements
We thank Xiwei Liao, Changyuan Liao and Yan Yan for their warm support. We thank the National Rubber Tree Germplasm Repository and the Hainan Innovation Base for providing the accession of Hevea germplasm resources and the Natural Rubber New Planting Materials for the current research. We also acknowledge the support from the Outstanding Talent Team Program of Hainan Province for the present work.
Abbreviations
- AbA
Aureobasidin A
- ETH
Ethephon
- kDa
Kilodalton
- MeJA
Methyl jasmonate
- MPK
Mitogen-activated protein kinase
- MPKK
Mitogen-activated protein kinase kinase
- MPKKK
Mitogen-activated protein kinase kinase kinase
- NR
Natural rubber
- Raf
Rapidly accelerated fibrosarcoma
- TPD
Tapping panel dryness
- ZIK
ZR1-interacting kinase
- Y2H
Yeast two-hybrid
Authors’ contributions
XMD and HZL conceived and designed the paper. XJH, MMS and JQC analyzed the experiment data. XJH wrote the manuscript. XMD and HZL revised the manuscript. XJH, XYD and LQ collected the samples and performed the experiments. All authors approved the final manuscript.
Funding
This work was supported by Guangxi Minzu University Research Fund (2021KJQD18), The Xiangsi Lake Youth Scholar Innovation Team of Guangxi Minzu University (2023GXUNXSHQN03), National Natural Science Foundation of China (31800578), National Key Research and Development Program (2023YFA0914800), National Training Program of Innovation and Entrepreneurship for Undergraduates (202310608030), the Chinese Academy of Tropical Agricultural Sciences for Science and Technology Innovation Team of National Tropical Agricultural Science Center (CATASCXTD202413) and the Hainan Provincial Natural Science Foundation of China (325CXTD621).
Data availability
The materials are available from the corresponding author on reasonable request. The data generated or analyzed during this study are included in the text or supplementary material. The chromosome-scale genome of H. brasiliensis was obtained from the NCBI database under accession number PRJNA945562 (https://www.ncbi.nlm.nih.gov/), while the A. thaliana genome was retrieved from the TAIR database (http://www.arabidopsis.org/). RNA-seq data from various H. brasiliensis tissues, including virgin latex (SAMC376659), tapped latex (SAMC376661), cambium region (SAMC376662), inner bark (SAMC376658), leaves (SAMC376664), female flowers (SAMC376660), and male flowers (SAMC376663), were obtained from the CNCB (https://ngdc.cncb.ac.cn/). Additionally, transcriptome data from TPD-affected trees (SAMN03078534), healthy trees (SAMN03078533), MeJA treatment (PRJNA353743), ETH treatment (PRJNA310171), and low-temperature stress (PRJNA432826) were retrieved from the CATAS (http://hevea.catas.cn/home/index).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaojuan Huang and Manman Sun contributed equally to this work.
Contributor Information
Xiaomin Deng, Email: dxmbio822@163.com.
Hongze Liao, Email: hzliao@gxmzu.edu.cn.
References
- 1.Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J. 2008;413(2):217–26. [DOI] [PubMed] [Google Scholar]
- 2.Zhang MM, Zhang SQ. Mitogen-activated protein kinase cascades in plant signaling. J Integr Plant Biol. 2022;64(2):301–41. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol. 2010;61:621–49. [DOI] [PubMed] [Google Scholar]
- 4.Jonak C, Okresz L, Bogre L, Hirt H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol. 2002;5(5):415–24. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Su J, Zhang Y, Xu J, Zhang S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr Opin Plant Biol. 2018;45(Pt A):1–10. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Zhang S. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 2015;20(1):56–64. [DOI] [PubMed] [Google Scholar]
- 7.Komis G, Šamajová O, Ovečka M, Šamaj J. Cell and developmental biology of plant mitogen-activated protein kinases. Annu Rev Plant Biol. 2018;69:237–65. [DOI] [PubMed] [Google Scholar]
- 8.Sun T, Zhang Y. MAP kinase cascades in plant development and immune signaling. EMBO Rep. 2022;23(2):e53817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Movahedi A, Hwarari D, Dzinyela R, Ni S, Yang L. A close-up of regulatory networks and signaling pathways of MKK5 in biotic and abiotic stresses. Crit Rev Biotechnol. 2025;45(2):473–90. [DOI] [PubMed] [Google Scholar]
- 10.Wu G, Wang W. Recent advances in understanding the role of two mitogen-activated protein kinase cascades in plant immunity. J Exp Bot. 2024;75(8):2256–65. [DOI] [PubMed] [Google Scholar]
- 11.Wu M, Wang S, Ma P, Li B, Hu H, Wang Z, Qiu Q, Qiao Y, Niu D, Lukowitz W, Zhang S, Zhang M. Dual roles of the MPK3 and MPK6 mitogen-activated protein kinases in regulating Arabidopsis stomatal development. Plant Cell. 2024;36(10):4576–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otani M, Tojo R, Regnard S, Zheng L, Hoshi T, Ohmori S, Tachibana N, Sano T, Koshimizu S, Ichimura K, Colcombet J, Kawakami N. The MKK3 MAPK cascade integrates temperature and after-ripening signals to modulate seed germination. Proc Natl Acad Sci U S A. 2024;121(28):e2404887121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Zhu Q, Tan Y, Deng M, Zhang L, Cao Y, Guo X. Mitogen-activated protein kinases MPK3 and MPK6 phosphorylate receptor-like cytoplasmic kinase CDL1 to regulate soybean basal immunity. Plant Cell. 2024;36(4):963–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shu W, Yan T, Jing S, Gan P, Wang J, Hu Z, Zhao J, Fan X, Kang Z, Tang C, Wang X. Wheat MAPK cascade mediates SGT1 nuclear entry targeted by a stripe rust effector. J Integr Plant Biol. 2025. 10.1111/jipb.13888. [DOI] [PubMed]
- 15.Song J, Lin R, Tang M, Wang L, Fan P, Xia X, Yu J, Zhou Y. SlMPK1- and SlMPK2-mediated SlBBX17 phosphorylation positively regulates CBF-dependent cold tolerance in tomato. New Phytol. 2023;239(5):1887–902. [DOI] [PubMed] [Google Scholar]
- 16.Ding R, Li J, Wang J, Li Y, Ye W, Yan G, Yin Z. Molecular traits of MAPK kinases and the regulatory mechanism of GhMAPKK5 alleviating drought/salt stress in cotton. Plant Physiol. 2024;196(3):2030–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang C, Yang M, Fang Y, Luo Y, Gao S, Xiao X, An Z, Zhou B, Zhang B, Tan X, Yeang HY, Qin Y, Yang J, Lin Q, Mei H, Montoro P, Long X, Qi J, Hua Y, He Z, Sun M, Li W, Zeng X, Cheng H, Liu Y, Yang J, Tian W, Zhuang N, Zeng R, Li D, He P, Li Z, Zou Z, Li S, Li C, Wang J, Wei D, Lai CQ, Luo W, Yu J, Hu S, Huang H. The rubber tree genome reveals new insights into rubber production and species adaptation. Nat Plants. 2016;2(6):16073. [DOI] [PubMed] [Google Scholar]
- 18.Supriya R, Priyadarshan PM. Genomic technologies for Hevea breeding. Adv Genet. 2019;104:1–73. [DOI] [PubMed] [Google Scholar]
- 19.Cherian S, Ryu SB, Cornish K. Natural rubber biosynthesis in plants, the rubber transferase complex, and metabolic engineering progress and prospects. Plant Biotechnol J. 2019;17(11):2041–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian WM, Yang SG, Shi MJ, Zhang SX, Wu JL. Mechanical wounding-induced laticifer differentiation in rubber tree: an indicative role of dehydration, hydrogen peroxide, and jasmonates. J Plant Physiol. 2015;182:95–103. [DOI] [PubMed] [Google Scholar]
- 21.Tian W, Zhang X, Gao X, Chen Y. Relationship between the number of tapping-induced secondary laticifer lines and rubber yield among Hevea germplasm. Front Agric Sci Eng. 2016;3(4):363. [Google Scholar]
- 22.Tan Y, Cao J, Tang C, Liu K. Advances in genome sequencing and natural rubber biosynthesis in rubber-producing plants. Curr Issues Mol Biol. 2023;45(12):9342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng X, Guo D, Yang S, Shi M, Chao J, Li H, Peng S, Tian W. Jasmonate signalling in the regulation of rubber biosynthesis in laticifer cells of rubber tree. Hevea brasiliensis J Exp Bot. 2018;69(15):3559–71. [DOI] [PubMed] [Google Scholar]
- 24.Guo D. A myelocytomatosis transcription factor from Hevea brasiliensis positively regulates the expression of the small rubber particle protein gene. Ind Crop Prod. 2019;133:90–7. [Google Scholar]
- 25.Zhang SX, Wu SH, Chao JQ, Yang SG, Bao J, Tian WM. Genome-wide identification and expression analysis of MYC transcription factor family genes in rubber tree (Hevea brasiliensis Muell. Arg.). Forests. 2022;13(4):531. [Google Scholar]
- 26.Florez-Velasco N, Ramos VF, Magnitskiy S, Balaguera-López H. Ethylene and jasmonate as stimulants of latex yield in rubber trees (Hevea brasiliensis): molecular and physiological mechanisms. A systematic approximation review. Adv Agrochem. 2024;3(4):279–88. [Google Scholar]
- 27.Venkatachalam P, Thulaseedharan A, Raghothama K. Identification of expression profiles of tapping panel dryness (TPD) associated genes from the latex of rubber tree (Hevea brasiliensis Muell. Arg.). Planta. 2007;226(2):499–515. [DOI] [PubMed] [Google Scholar]
- 28.JX WANG. Physiological responses of two rubber tree clones with differential cold-tolerant potential to cold stress. J Rubber Res. 2017;20(2):117–29. [Google Scholar]
- 29.Rao KP, Richa T, Kumar K, Raghuram B, Sinha AK. In Silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res. 2010;17(3):139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamel LP, Nicole MC, Sritubtim S, Morency MJ, Ellis M, Ehlting J, Beaudoin N, Barbazuk B, Klessig D, Lee J, Martin G, Mundy J, Ohashi Y, Scheel D, Sheen J, Xing T, Zhang S, Seguin A, Ellis BE. Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006;11(4):192–8. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Liang X, Lin M, Lan Y, Xiang Y, Yan H. Comprehensive analysis of MAPK gene family in Populus trichocarpa and physiological characterization of PtMAPK3-1 in response to MeJA induction. Physiol Plant. 2023;175(1):e13869. [DOI] [PubMed] [Google Scholar]
- 32.Wrzaczek M, Hirt H. Plant MAP kinase pathways: how many and what for? Biol Cell. 2001;93(1–2):81–7. [DOI] [PubMed] [Google Scholar]
- 33.Jin X, Zhu L, Yao Q, Meng X, Ding G, Wang D, Xie Q, Tong Z, Tao C, Yu L, Li H, Wang X. Expression profiling of mitogen-activated protein kinase genes reveals their evolutionary and functional diversity in different rubber tree (Hevea brasiliensis) cultivars. Genes (Basel). 2017;8(10):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng H, Song X, Hu Y, Wu T, Yang Q, An Z, Feng S, Deng Z, Wu W, Zeng X, Tu M, Wang X, Huang H. Chromosome-level wild Hevea brasiliensis genome provides new tools for genomic-assisted breeding and valuable loci to elevate rubber yield. Plant Biotechnol J. 2023;21(5):1058–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M, Karthikeyan AS, Lee CH, Nelson WD, Ploetz L, Singh S, Wensel A, Huala E. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 2012;40(Database issue):D1202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letunic I, Doerks T, Bork P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40(Database issue):D302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 2020;48(D1):D265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blum M, Chang HY, Chuguransky S, Grego T, Kandasaamy S, Mitchell A, Nuka G, Paysan-Lafosse T, Qureshi M, Raj S, Richardson L, Salazar GA, Williams L, Bork P, Bridge A, Gough J, Haft DH, Letunic I, Marchler-Bauer A, Mi H, Natale DA, Necci M, Orengo CA, Pandurangan AP, Rivoire C, Sigrist CJA, Sillitoe I, Thanki N, Thomas PD, Tosatto SCE, Wu CH, Bateman A, Finn RD. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021;49(D1):D344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voorrips RE. MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93(1):77–8. [DOI] [PubMed] [Google Scholar]
- 40.Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012;40(Web Server issue):W597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Coghlan A, Ruan J, Coin LJ, Heriche JK, Osmotherly L, Li R, Liu T, Zhang Z, Bolund L, Wong GK, Zheng W, Dehal P, Wang J, Durbin R. TreeFam: a curated database of phylogenetic trees of animal gene families. Nucleic Acids Res. 2006;34(Database issue):D572-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchler-Bauer A, Bryant SH. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 2004;32(Web Server issue):W327-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(1):W202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, Kissinger JC, Paterson AH. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lescot M, Déhais P, Thijs G. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chao J, Wu S, Shi M, Xu X, Gao Q, Du H, Gao B, Guo D, Yang S, Zhang S, Li Y, Fan X, Hai C, Kou L, Zhang J, Wang Z, Li Y, Xue W, Xu J, Deng X, Huang X, Gao X, Zhang X, Hu Y, Zeng X, Li W, Zhang L, Peng S, Wu J, Hao B, Wang X, Yu H, Li J, Liang C, Tian WM. Genomic insight into domestication of rubber tree. Nat Commun. 2023;14(1):4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Cole PA. Catalytic mechanisms and regulation of protein kinases. Methods Enzymol. 2014;548:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J-P, Hu J, Liu Y-H, Yang C-P, Zhuang Y-F, Guo X-L, Li Y-J, Zhang L. Transcriptome analysis of Hevea brasiliensis in response to exogenous methyl jasmonate provides novel insights into regulation of jasmonate-elicited rubber biosynthesis. Physiol Mol Biol Plants. 2018;24(3):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wan J, Fan R, Yang W, Wei F, Gao H, Wei H, Qiu J. New insights into ethylene-induced latex flow in a dose-dependent manner in rubber tree. Ind Crops Prod. 2024;222:120012. [Google Scholar]
- 57.Wang H, Gong M, Guo J, Xin H, Gao Y, Liu C, Dai D, Tang L. Genome-wide identification of Jatropha curcas MAPK, MAPKK, and MAPKKK gene families and their expression profile under cold stress. Sci Rep. 2018;8(1):16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawton-Rauh A. Evolutionary dynamics of duplicated genes in plants. Mol Phylogenet Evol. 2003;29(3):396–409. [DOI] [PubMed] [Google Scholar]
- 59.Magadum S, Banerjee U, Murugan P, Gangapur D, Ravikesavan R. Gene duplication as a major force in evolution. J Genet. 2013;92(1):155–61. [DOI] [PubMed] [Google Scholar]
- 60.Thulasi Devendrakumar K, Li X, Zhang Y. MAP kinase signalling: interplays between plant PAMP- and effector-triggered immunity. Cell Mol Life Sci. 2018;75(16):2981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Zelicourt A, Colcombet J, Hirt H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016;21(8):677–85. [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Li Y, Wang Y, Liu H, Lei L, Yang H, Liu G, Ren D. Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J Biol Chem. 2008;283(40):26996–7006. [DOI] [PubMed] [Google Scholar]
- 63.He C, Liew LC, Yin L, Lewsey MG, Whelan J, Berkowitz O. The retrograde signaling regulator ANAC017 recruits the MKK9-MPK3/6, ethylene, and auxin signaling pathways to balance mitochondrial dysfunction with growth. Plant Cell. 2022;34(9):3460–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell. 2004;15(1):141–52. [DOI] [PubMed] [Google Scholar]
- 65.Sozen C, Schenk ST, Boudsocq M, Chardin C, Almeida-Trapp M, Krapp A, Hirt H, Mithofer A, Colcombet J. Wounding and insect feeding trigger two independent mapk pathways with distinct regulation and kinetics. Plant Cell. 2020;32(6):1988–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao HZ, Zhu MM, Cui HH, Du XY, Tang Y, Chen LQ, Ye D, Zhang XQ. MARIS plays important roles in Arabidopsis pollen tube and root hair growth. J Integr Plant Biol. 2016;58(11):927–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Fig. S1. Conserved motifs, domains and gene structure analysis of the HbMPK cascade gene family. Fig. S2. Interspecies collinearity analysis of MPK cascade genes in H. brasiliensis and A. thaliana. Fig. S3. Cis-acting element analysis of HbMPK cascade genes. Fig. S4. Expression analysis of HbMPK cascade genes under MeJA, ETH and cold treatment. Fig. S5. Interaction between HbMPKKK6 and HbMPKKK41 with HbMPKKs in a yeast two-hybrid assay.
Supplementary Material 2: Table S1. Primer sequences used in this study. Table S2. Basic information of the HbMPK genes. Table S3. Basic information of the HbMPKK genes. Table S4. Basic information of the HbMPKKK genes. Table S5. Classification of duplication origin information for HbMPK cascade genes. Table S6. Cis-acting element identified in the promoter of HbMPK cascade genes. Table S7. Function and category of each cis-acting element identified in the promoter of HbMPK cascade genes. Table S8. The TPM values of HbMPK cascade genes expressed in different tissues. Table S9. The TPM values of HbMPK cascade genes expressed in tapped and virgin latex. Table S10. The TPM values of HbMPK cascade genes expressed in healthy trees and TPD-affected trees. Table S11. The TPM values of HbMPK cascade genes expressed under MeJA treatment. Table S12. The TPM values of HbMPK cascade genes expressed under ETH treatment. Table S13. The TPM values of HbMPK cascade genes expressed under cold treatment. Table S14. The representative HbMPK cascades genes with significant expression characteristics.
Data Availability Statement
The materials are available from the corresponding author on reasonable request. The data generated or analyzed during this study are included in the text or supplementary material. The chromosome-scale genome of H. brasiliensis was obtained from the NCBI database under accession number PRJNA945562 (https://www.ncbi.nlm.nih.gov/), while the A. thaliana genome was retrieved from the TAIR database (http://www.arabidopsis.org/). RNA-seq data from various H. brasiliensis tissues, including virgin latex (SAMC376659), tapped latex (SAMC376661), cambium region (SAMC376662), inner bark (SAMC376658), leaves (SAMC376664), female flowers (SAMC376660), and male flowers (SAMC376663), were obtained from the CNCB (https://ngdc.cncb.ac.cn/). Additionally, transcriptome data from TPD-affected trees (SAMN03078534), healthy trees (SAMN03078533), MeJA treatment (PRJNA353743), ETH treatment (PRJNA310171), and low-temperature stress (PRJNA432826) were retrieved from the CATAS (http://hevea.catas.cn/home/index).