Abstract
Growing evidence highlights the critical role of chromatin remodeling in tumor development and progression. This study explores the relationship between chromatin remodeling-related genes (CRRGs) and breast cancer (BRCA). Public databases were used to retrieve the TCGA-BRCA and GSE20685 datasets. CRRGs were sourced from prior studies. Prognosis-associated CRRGs were identified using univariate Cox regression analysis. TCGA-BRCA BRCA samples were grouped into CRRG-related subtypes through consensus clustering analysis. Differential expression analysis was conducted in TCGA-BRCA (BRAC vs. control) and among subtypes to identify differentially expressed genes (DEGs). Candidate genes were obtained through the intersection of these DEGs. Prognostic genes were selected using univariate Cox and least absolute shrinkage and selection operator (LASSO) regression analyses. Independent prognostic factors were identified, and a nomogram model was developed. Functional enrichment, immune infiltration, clinical relevance, and drug sensitivity analyses were subsequently performed. TCGA-BRCA BRCA samples were classified into two CRRG-related subtypes (clusters 1 and 2) based on prognosis-associated CRRGs. A total of 141 candidate genes were identified by intersecting 250 DEGs (cluster 1 vs. cluster 2) with 3,145 DEGs (BRCA vs. control). Five prognostic genes—LHX5, ZP2, GABRQ, APOA2, and CLCNKB—were selected, and a prognostic risk model was developed. In clinical samples, APOA2 (P = 0.0290) and GABRQ (P = 0.0132) expression were significantly up-regulated, CLCNKB (P < 0.0001) and ZP2 (P = 0.0445) expression were significantly down-regulated, while LHX5 (P = 0.1508) expression did not present a significant difference. A nomogram was created, and calibration and Receiver Operating Characteristic (ROC) curves demonstrated its superior predictive ability for BRCA. Gene Set Variation Analysis (GSVA) revealed 16 pathways, such as “mTORC1 signaling” and “E2F targets,” were enriched in the high-risk group, while 9 pathways, including “estrogen response early” and “myogenesis,” were enriched in the low-risk group. Additionally, significant differences in immune cell types, including CD8+ T cells and follicular helper T cells, were observed between the two risk groups. The risk score was also significantly associated with six drugs, including AZD1332 1463 and SB505124 1194. This study presents a prognostic model based on five genes (LHX5, ZP2, GABRQ, APOA2, and CLCNKB) for BRCA, offering a novel perspective on the link between CRRGs and BRCA.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-025-01661-8.
Keywords: Breast cancer, Chromatin remodeling, Prognostic model, Immune microenvironment, Nomogram
Introduction
Breast cancer (BRCA) is a heterogeneous malignancy characterized by the uncontrolled proliferation of breast epithelial cells, driven by various carcinogenic factors. It is among the most prevalent cancers in women globally, with a rising annual incidence, posing a significant threat to patient health and causing substantial psychological distress [1–3]. Based on molecular and histological features, BRCA can be classified into three subtypes: hormone receptor-positive (estrogen receptor [ER+] or progesterone receptor [PR+]), human epidermal receptor 2 (HER2+)-positive, and triple-negative breast cancer (TNBC). Although the precise etiology of BRCA remains unclear, studies suggest a multifactorial origin involving genetic, environmental, and age-related factors [4–7]. Current treatment options for BRCA include surgical resection, chemoradiotherapy, endocrine therapy, and targeted molecular therapies. Prognosis is influenced by patient age, axillary lymph node involvement, tumor size, histological characteristics (particularly grade and lymphovascular invasion), hormone receptor status, and HER2 expression. Although these factors provide valuable insights for prognostic assessment, they fail to fully capture the individual clinical outcomes, as patients with similar profiles may experience varying prognoses. Recent advancements in molecular techniques, particularly gene expression profiling, have enhanced prognostic prediction and therapeutic response evaluation. However, a definitive set of prognostic indicators for BRCA remains elusive [8, 9]. Thus, the identification of novel prognostic genes is critical for improving patient prognosis and outcomes.
Chromatin remodeling refers to the dynamic reorganization of chromatin structure between condensed and transcriptionally accessible states. This process involves both covalent modifications to chromatin components (such as histones and DNA) and non-covalent modifications by ATP-dependent chromatin remodeling complexes (e.g., SWI/SNF, INO80, ISWI) [10, 11]. These modifications regulate the accessibility of transcription factors to genomic DNA, thereby modulating gene expression, a vital process for maintaining cellular homeostasis and regulating essential physiological functions. Abnormalities in chromatin remodeling have been implicated in cancer development, therapeutic resistance, and genomic reprogramming during tumorigenesis [12, 13]. For instance, the chromatin remodeling factor ARID2 inhibits hepatocellular carcinoma metastasis by recruiting DNMT1 to the Snail promoter, promoting DNA methylation and suppressing Snail transcription [14]. Additionally, Nexturastat A modulates gene expression and transcriptional patterns in TNBC cells, activating immune response-related genes by altering HDAC6 binding and chromatin structure [15]. However, the prognostic significance of chromatin remodeling-related genes (CRRGs) in BRCA has yet to be explored.
Leveraging publicly available BRCA-related data, this study employs bioinformatics techniques to identify CRRGs associated with prognosis in patients with BRCA and develop a prognostic model. Additionally, it explores the role of these genes in the immune microenvironment and signaling pathway regulation, offering insights into their potential molecular mechanisms and therapeutic implications in BRCA. The flow of this study was shown in Fig. S1.
Materials and methods
Source of data
Clinical and transcriptomic data for BRCA were retrieved from the TCGA-BRCA cohort of the TCGA database (https://portal.gdc.cancer.gov/), comprising 1,097 BRCA and 114 control samples. The GSE20685 dataset, sourced from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/), includes transcriptomic data from 327 BRCA samples. A total of 49 CRRGs were identified from previous studies (Table S1) [16].
Identification of CRRGs-related subtypes
Univariate Cox regression analysis was conducted on these 49 CRRGs using the ‘survival’ package (v 3.2–13) [17], with a significance threshold of P < 0.05 to identify prognosis-related CRRGs. A forest plot of the univariate Cox analysis was generated using the ‘forestplot’ package (v 2.0.1).
Consensus clustering, an established unsupervised classification method, was applied to the 1,097 BRCA samples from TCGA-BRCA based on the prognosis-related CRRGs, using the ‘ConsensusClusterPlus’ package (v 1.60.0) [18], to identify CRRG-related subtypes. To assess the distribution of samples across these subtypes, principal component analysis (PCA) was performed. Kaplan–Meier (K-M) survival curves for each subtype were plotted based on overall survival (OS) to determine survival differences among the subtypes. The expression levels of prognosis-related CRRGs were also examined across different clinical characteristics and subtypes.
Selection of differentially expressed genes (DEGs)
Differential expression analysis was conducted for the TCGA-BRCA cohort (BRCA vs. control) and across subtypes using the ‘edgeR’ package (v 3.36.0) [19] to identify differentially expressed genes (DEGs), with a significance threshold of FDR < 0.05 and |logFC|≥ 0.5. Volcano and heat maps of the DEGs were visualized using the ‘ggplot2’ (v 3.3.5) [20] and ‘pheatmap’ (v 1.0.12) [21] packages, respectively. Overlap analysis between DEGs from BRCA versus control and between subtypes was performed to identify intersected genes, which were considered candidate genes. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses for these candidate genes were conducted using the ‘clusterProfiler’ package (v 4.0.2) [22], with statistical significance defined as P.adjust < 0.05 and count ≥ 1.
Development of the risk model
The 1,081 BRCA samples with OS data from TCGA-BRCA were randomly split into a training set (757 samples) and a test set (324 samples) in a 7:3 ratio. Univariate Cox and least absolute shrinkage and selection operator (LASSO) regression analyses were performed on the training set to identify prognostic genes, with LASSO conducted using the ‘glmnet’ package (v 4.1-3) [23]. A risk model was subsequently constructed, with the formula for calculating the risk score as follows:
In this formula, Coef and X represent the coefficients and gene expression values, respectively. Based on the median risk score, each BRCA sample was assigned a risk score using this formula, categorizing the samples into high- and low-risk groups. Risk curves for the two groups and heat maps of prognostic gene expression were generated using ‘ggplot2.’ To assess survival differences between the risk groups, K-M survival analysis was conducted using the ‘survminer’ package (v 0.4.9). To validate the predictive power of the risk model, receiver operating characteristic (ROC) curves for 1-, 3-, and 5-year survival were plotted using the Kaplan–Meier ROC curve (v 1.0.3) [24]. Further, the risk model was validated in both the TCGA-BRCA and GSE20685 datasets.
Development of a nomogram
Risk scores and 10 clinical characteristics (age, radiation therapy, tumor stage, margin status, race, pathologic T, N, and M stages, menopause status, and surgical approach) were incorporated into univariate and multivariate Cox regression analyses, with a threshold of P < 0.05. Independent prognostic factors were identified, and a nomogram model was constructed. Calibration and ROC curves for the nomogram were created to assess its predictive accuracy.
Enrichment analysis
Gene set variation analysis (GSVA) was performed by first downloading the hallmark gene set via the ‘msigdbr’ package (v 7.4.1). GSVA was then applied to the BRCA samples using the hallmark pathways. Differential analysis between GSVA scores of the two risk groups was conducted with the ‘limma’ package, using the low-risk group as the reference, and thresholds set at P < 0.05 and |t|> 2, to identify significantly different hallmark pathways. In the high-risk group, pathways were activated with t > 0, whereas in the low-risk group, pathways were activated with t < 0. A heat map was created to show clustering of risk scores and significantly different pathways. Gene set enrichment analysis (GSEA) was conducted for both risk groups using the ‘clusterProfiler’ package (v 4.0.2) [22] and ‘org.Hs.eg.db’ (v 3.13.0).
In addition, single-gene GSEA of prognostic genes in the TCGA-BRCA cohort was performed using the “clusterProfiler” R package (v4.15.0.3). First, expression associations between genes were assessed using Pearson correlation coefficients, and a list of correlation coefficients was obtained. Subsequently, GSEA analysis was performed for each prognostic gene based on the KEGG database. Finally, GSEA analysis was performed for each prognostic gene. Ten Hallmark pathways (five up-regulated and five down-regulated) that were significantly enriched were screened according to a preset threshold (P < 0.05 and |NES|> 1).
Clinical characteristics analysis
The association between the risk score and BRCA clinical characteristics was explored by comparing risk score levels across clinical subgroups using the Wilcoxon test. The clinical characteristics analyzed included age (> 60 and ≤ 60 years), pathological M (M0, M1, and MX), radiation therapy (yes/no), pathological T (T1, T2, T3, T4, and NX), stage (stage I, II, III, IV, and V), and pathological N (N0, N1, N2, N3, and NX). The relationships between subgroups, survival status, and risk scores in the training set were visualized through Sankey diagrams and heat maps, generated using the ‘ggalluvial’ package (v 0.12.3).
Genomic variation analysis and immune micro-environment analysis
Genomic mutation differences between the two risk groups were explored by analyzing mutation types in BRCA samples using the ‘maftools’ package (v 2.8.05), based on somatic mutation data from the training set. Tumor mutation burden (TMB) scores were calculated for each BRCA sample based on the number of somatic mutations. Differences in TMB scores between the two risk groups were assessed using the Wilcoxon test. Spearman correlation analysis was performed to examine the relationship between TMB scores and risk scores.
The relative abundance of immune-infiltrating cells was assessed using the CIBERSORT algorithm [25]. The rank sum test was employed to determine differences in immune cell infiltration between the two risk groups (P < 0.05). Immune cell scores between the groups were compared using ‘ggplot2.’ Spearman correlation analysis was conducted to investigate the relationship between risk scores and significantly different immune cells. Furthermore, immune checkpoint expression levels were compared between the two risk groups using the Wilcoxon test. The proportions of stromal and immune cells in the samples from both risk groups were estimated using the ESTIMATE method, based on gene expression data from the training set.
The tumor immune dysfunction and exclusion (TIDE) and drug sensitivity analyses
In the TCGA-BRCA cohort, TIDE scores, T-cell dysfunction scores, and T-cell exclusion scores were calculated using the TIDE database (http://tide.dfci.harvard.edu), and differences between the two risk groups were analyzed separately.
The 50% inhibitory concentration (IC50) values for 198 chemotherapy or targeted therapy drugs from the GDSC database (https://www.cancerrxgene.org/) were calculated for BRCA samples using the ‘oncoPredict’ package (v 0.2) to compare IC50 values between the two risk groups. The Spearman correlation between the top three IC50 values and risk scores was also evaluated.
Expression profiling of prognostic genes
To assess the potential of prognostic genes as pan-cancer biomarkers, the TIMER database (http://timer.cistrome.org/) was used to analyze the expression of these genes across various cancer types. Additionally, a protein–protein interaction (PPI) network was constructed for prognostic genes and CRRGs using the STRING database (https://string-db.org/).
Expression validation of prognostic genes
In the TCGA-BRCA cohort, the expression levels of prognostic genes were analyzed in both BRCA and control samples. Moreover, 10 pairs of BRCA tissue and adjacent normal tissue samples were collected from patients with BRCA, and expression analysis of the prognostic genes was performed using quantitative real-time polymerase chain reaction (qRT-PCR). Informed consent was obtained from all participants, and the study was approved by the Ethics Committee of Shanxi Provincial Cancer Hospital (Approval Number: KY2024117).
RNA extraction from tissue samples was performed using TRIzol, following the manufacturer's protocol. Reverse transcription was then carried out to synthesize cDNA from the extracted RNA. Quantitative PCR amplification was performed over 40 cycles in a CFX96 real-time quantitative PCR system with the following conditions: 95 °C for 1 min, followed by 20 s at 95 °C, 20 s at 55 °C, and 30 s at 72 °C. The relative expression levels of each prognostic gene were calculated using the 2−∆∆CT method, with GAPDH as the internal control. To ensure the reliability of the results, the qRT-PCR experiments were performed in triplicate, with three internal controls for each sample. The final expression value for each sample was derived from the average of three replicate wells. The qRT-PCR primers used are listed in Table 1.
Table 1.
The qRT-PCR primers
| Gene | Forward primer | Reverse primer |
|---|---|---|
| LHX5 | GTGCAAAGACGACTACCTGAG | CGGTCCGTACAGGATGACAC |
| ZP2 | ACACTGTTTTACGGAGCAGC | CCCAGCTGAAACGGAAGGAT |
| GABRQ | AAGAGTCATTGCCCGCTACC | ATTGGAGAGGAAGGTGGAGGT |
| CLCNKB | AAATCCCACCTTGGGCAGAG | TGGACACCAGCTCTGGGAAC |
| APOA2 | GGAGCTTTGGTTCGGAGACA | TAACCAGTTCCGTTCCAGCC |
| GAPDH | CGAAGGTGGAGTCAACGGATTT | ATGGGTGGAATCATATTGGAAC |
Results
Identification of two CRRGs-related subtypes through consistent clustering analysis
Following univariate Cox proportional hazards regression analysis of the 49 CRRGs, four prognosis-related CRRGs (MTA1, INO80B, HDAC2, and SMARCD3) were identified (Fig. 1A). Consistent clustering analysis results indicated that the cumulative distribution function (CDF) curve showed the most significant decline when K = 2, leading to the classification of TCGA-BRCA samples into two CRRG-related subtypes (clusters 1 and 2, Fig. 1B). PCA revealed a distinct separation of the two subtypes (Fig. 1C). K-M survival analysis demonstrated significant survival differences between the two subtypes, with cluster 1 exhibiting a better prognosis (Fig. 1D). Moreover, the expression of the four prognosis-related CRRGs significantly differed between the two subtypes (Fig. 1E).
Fig. 1.
Identification of breast cancer (BRCA) subtypes based on chromatin remodeling-related genes. A Forest plot from univariate Cox regression analysis of signature genes. B (Left) Heatmap showing sample clustering for k = 2. (Middle) Cumulative distribution function. (Right) Delta area curve. C PCA results for the two subtypes. D Kaplan–Meier (K–M) survival curve comparing the two subtypes. E Heatmap showing the expression of four prognosis-related CRRGs between the two subtypes
Identification of candidate genes were identified
For the 512 samples in cluster 1 and 569 samples in cluster 2 from the TCGA dataset, the R package edgeR (v 3.36.0) was used to compare gene expression differences between the two subtypes. A total of 250 DEGs (cluster 1 vs. cluster 2) were identified, including 27 upregulated and 223 downregulated DEGs (Fig. 2A, B). Additionally, 3,145 DEGs (BRCA vs. control) were identified in TCGA-BRCA, comprising 1392 upregulated and 1753 downregulated DEGs (Fig. 2C, D). These results were further visualized using volcano plots and heat maps. A total of 141 candidate genes were obtained by intersecting the 250 DEGs (cluster 1 vs. cluster 2) and 3145 DEGs (BRCA vs. control), as shown in the Venn diagram (Fig. 2E). GO enrichment analysis revealed that the candidate genes were significantly enriched in 115 GO terms and one KEGG pathway. Prominent GO terms included “regulation of endocrine processes,” “endocrine hormone secretion,” “receptor ligand activity,” “hormone activity,” and “signaling receptor activator activity” (Fig. 2F). KEGG analysis identified “neuroactive ligand-receptor interaction” as the enriched pathway (Fig. 2F).
Fig. 2.
Identification of candidate genes. A Volcano plot of DEGs between cluster 1 and cluster 2. B Heatmap of DEGs between cluster 1 and cluster 2. C Volcano plot of DEGs between BRCA and control samples. D Heatmap of DEGs between BRCA and control samples. E Venn diagram showing the overlap of DEGs (cluster 1 vs. cluster 2) and DEGs (BRCA vs. control), revealing the candidate genes. F Enrichment results of candidate genes, categorized into four parts: GO-biological process (BP), GO-molecular function (MF), GO-cellular component (CC), and KEGG pathways
Construction and validation of the risk model
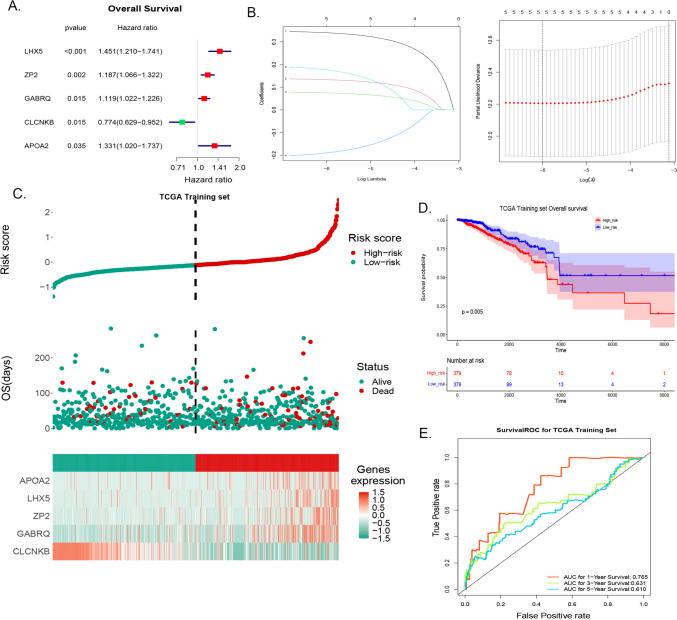
A total of five prognostic genes (LHX5, ZP2, GABRQ, and APOA2 as risk factors and CLCNKB as a protective factor) were identified through univariate Cox and LASSO analyses in the TCGA-BRCA cohort based on candidate genes (Fig. 3A, B), and a risk model was established. The risk score for each sample was calculated using the coefficients and prognostic gene expression levels in BRCA samples. Based on the median risk score, the BRCA samples were divided into two risk groups: high-risk and low-risk groups. The prognosis of the risk model was assessed in the TCGA-BRCA training set, and the results revealed that the expression levels of APOA2, LHX5, ZP2, and GABRQ were higher in the high-risk group, whereas CLCNKB expression was higher in the low-risk group (Fig. 3C). Additionally, K-M survival analysis showed that the high-risk group had a poorer prognosis (Fig. 3D). The AUC values for 1 year, 3 years, and 5 years were 0.765, 0.631, and 0.610, respectively, indicating that the risk model performed well (Fig. 3E).
Fig. 3.
Development of the risk model. A Forest plot of univariate Cox regression analysis for five candidate genes. B LASSO regression analysis for screening candidate genes. Left: Horizontal axis represents log (Lambda), and vertical axis shows gene coefficients; Right: Horizontal axis represents log (Lambda), and vertical axis displays cross-validation error. C Distributions of risk score, survival time, and gene expression in the training set. D Kaplan–Meier (K–M) curve for high- and low-risk groups in the training set. E Receiver operating characteristic (ROC) curve for the training set
To validate the model, analysis was conducted on the test set of TCGA-BRCA and GSE20685 datasets, yielding consistent results with the training set. APOA2, LHX5, ZP2, and GABRQ were expressed at higher levels in the high-risk group, while CLCNKB expression was elevated in the low-risk group (Figs. 4A, 5A). K-M survival curves confirmed that patients in the low-risk group had significantly longer survival times (Figs. 4B, 5B). The AUC values for the risk model were above 0.6 at 1, 3, and 5 years, confirming the model’s good generalization ability (Figs. 4C, 5C).
Fig. 4.
Evaluating the risk model in the test set. A Distributions of risk score, survival time, and gene expression. B K-M curve for high- and low-risk groups. C ROC curve
Fig. 5.
Evaluating the risk model using the GSE20685 dataset. A Distributions of risk score, survival time, and gene expression. B K-M curve for high- and low-risk groups. C ROC curve
Development of an effective nomogram model
Univariate Cox independent prognostic analysis identified risk score, age, and tumor stage as independent prognostic factors (Fig. 6A, B). A nomogram model was subsequently developed (Fig. 6C). The calibration curve demonstrated that the slopes for 1, 3, and 5 years were nearly equal to 1, indicating superior prediction accuracy of the model (Fig. 6D). The ROC curve revealed an AUC of 0.744 for the nomogram model, further suggesting its robust predictive capability (Fig. 6E).
Fig. 6.
Construction and validation of the nomogram. A Univariate Cox regression analysis for independent prognostic factors. B Multivariate Cox regression analysis for independent prognostic factors. C Prognostic nomogram for patients with BRCA. D Calibration curve for the nomogram. E ROC curve for the nomogram
Functional exploration in BRCA
GSVA identified 25 hallmark pathways with significant differences between the two risk groups (Fig. 7A). In the high-risk group, 16 hallmark pathways (e.g., “mTORC1 signaling,” “E2F targets,” “G2/M checkpoint,” and “unfolded protein response”) were significantly activated, while in the low-risk group, 9 hallmark pathways (e.g., “estrogen response early,” “myogenesis,” and “bile acid metabolism”) were prominently activated. The heat map displayed these significant differences in the activation of hallmark pathways between the two risk groups (Fig. 7B). GSEA revealed 11 KEGG pathways, including “natural killer cell-mediated cytotoxicity,” “steroid hormone biosynthesis,” “cell cycle,” and “primary immunodeficiency” (Fig. 7C).
Fig. 7.
Functional exploration in BRCA. A GSVA of hallmark pathways. B Heatmap displaying hallmark pathway activation in high- and low-risk groups. C GSEA results for high- and low-risk groups
Based on single-gene GSEA analysis, we found that APOA2 and LNX5 were co-enriched in Oxidative phosphorylation, suggesting a potential role in energy metabolism, while CLCNKB was enriched in the TNF signaling pathway, implying that it may be involved in inflammatory regulation. In addition, both GABRQ and CLCNKB were significantly associated with DNA replication, while APOA2 was significantly enriched in the protein processing in endoplasmic reticulum pathway. both GABRQ and ZP2 were significantly associated with Antigen processing and presentation, while GABRQ was also enriched in Nucleocytoplasmic transport. These findings provide important clues to elucidate the molecular mechanisms of prognostic genes in disease progression (Fig. S2A–E and Table S2–S6).
Correlation analysis of risk score with BRCA clinical characteristics and genomic variant analysis
The correlation between clinical characteristics and risk scores revealed significant differences in pathological T and N stages, with notable differences between T1 and T2, as well as between N0 and N1 (Fig. 8A). Sankey diagrams and heat maps were generated to illustrate the relationships among different subtypes, risk scores, and survival statuses (Fig. 8B). Additionally, genomic variant analysis indicated that the frequency of TP53 mutations was higher in the high-risk group (Fig. 8C). The risk score showed a positive correlation with TMB, with TMB scores being significantly higher in the high-risk group (Fig. 8D).
Fig. 8.
Correlation analysis between risk score and clinical characteristics of BRCA. A Distribution differences of high- and low-risk groups across various clinical characteristics. B Sankey diagram (left) and heatmap (right) illustrating the relationship between subtypes, risk scores, and clinical characteristics. C Waterfall plot of genomic variant analysis in high- and low-risk groups. D (Left) Differences in TMB scores between high- and low-risk groups. (Right) Correlation between TMB score and risk score
Investigation of the immune microenvironment in BRCA
The immune microenvironment plays a pivotal role in tumor biology and has increasing relevance in predicting prognosis and treatment outcomes. An analysis of the abundance of 22 immune cell types in BRCA samples revealed significant differences between the two risk groups, as displayed in stacked plots (Fig. 9A). Nine immune cell types, including CD8+ T cells, activated memory CD4+ T cells, follicular helper T cells, and M2 macrophages, showed marked differences between the risk groups, highlighting the importance of the immune microenvironment in BRCA (Fig. 9B). The risk score was significantly correlated with eight differential immune cell types, showing a positive association with M1 macrophages and activated memory CD4+ T cells, and a negative association with resting mast cells and M2 macrophages (Fig. 9C). Immune checkpoints, which are immunosuppressive molecules expressed on immune cells to regulate immune activation and prevent autoimmunity, exhibited significant differences between the two risk groups. Key immune checkpoints, including CTLA4, BTLA, LAG3, and TIGIT, were notably different between the groups (Fig. 9D). Finally, ESTIMATE analysis, which evaluates the proportion of stromal and immune cells in tumor tissues, revealed that the immune score was significantly lower in the low-risk group. The risk score showed a significant association with the immune score, underscoring the relationship between risk scores and immune infiltration (Fig. 9E).
Fig. 9.
Immune microenvironment analysis. A Stacked graph depicting the proportion of immune cells in samples. B Comparison of immune cell proportions between high- and low-risk groups. C Correlation between risk score and immune cell types. D Expression of immune checkpoints in high- and low-risk groups. E ESTIMATE score differences between high- and low-risk groups
Immunotherapy and drug sensitivity analyses
The TIDE analysis revealed significant differences in T cell dysfunction and T cell exclusion scores between the two risk groups, with both scores lower in the low-risk group, suggesting that patients in the high-risk group may have a better response to immunotherapy (Fig. 10A). Chemotherapy remains a standard treatment for malignant tumors. To assess the drug sensitivity in both risk groups, a predictive model based on the GDSC database was used. Notable differences in the sensitivity to 146 chemotherapeutic agents were observed, with 143 drugs being more effective in the high-risk group (e.g., AZD1332 1463 and SB505124 1194), while three drugs showed higher sensitivity in the low-risk group (AZD7762 1022, Paclitaxel 1080, and Vinorelbine 2048), indicating that the high-risk group may derive greater survival benefit from chemotherapy (Fig. 10B). Furthermore, a significant correlation was found between the risk score and the IC50 values for the six drugs (the top three in terms of sensitivity for both high- and low-risk groups) (Fig. 10C, D).
Fig. 10.
Immunotherapy and drug sensitivity analysis. A Differences in TIDE scores between high- and low-risk groups. B Drug sensitivity analysis in high- and low-risk groups. C Top 3 drug sensitivities in high- and low-risk groups. The correlation between IC50 values of chemotherapy drugs and risk scores. D IC50 values of drugs with positive and negative correlations in high- and low-risk groups
Expression analysis of prognostic genes
Pan-cancer analysis revealed that five prognostic genes exhibited significant expression differences in head and neck squamous cell carcinoma (HNCS) and lung adenocarcinoma (LUAD), while four prognostic genes (APOA2, CLCNKB, GABRQ, and ZP2) were significantly different in kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIPR), and thyroid carcinoma (THCA). Three genes (GABRQ, LHX5, and ZP2) showed significant differences in uterine corpus endometrial carcinoma (UCEC) (Fig. 11A). Additionally, the PPI network analysis demonstrated interactions between LHX5, APOA2, and CRRGs (Fig. 11B). In TCGA-BRCA, APOA2 and GABRQ were significantly upregulated in BRCA samples compared to controls, while CLCNKB and ZP2 were downregulated (Fig. 11C). Furthermore, the qRT-PCR results suggested that APOA2 and GABRQ were upregulated genes and the expression of the CLCNKB and ZP2 gene expression were down-regulated, while LHX5 expression was not significantly different between the two groups (Fig. 11D).
Fig. 11.
Expression profile analysis of prognostic genes. A Expression of prognostic genes across multiple cancers. B PPI network of prognostic genes and chromatin remodeling-related genes. C Expression patterns of prognostic genes in the TCGA-BRCA cohort. D qRT-PCR validation of five prognostic genes in BRCA and adjacent normal tissues
Discussion
BRCA has the highest incidence among women globally. However, current markers and diagnostic tools fail to accurately predict prognosis and treatment responses in patients with BRCA. Abnormal chromatin remodeling plays a critical role in cancer progression, treatment resistance, and genome reprogramming in tumorigenesis. Yet, the prognostic significance of CRRGs in patients with BRCA has not been extensively studied. This research utilized transcriptomic and clinical data from patients with BRCA, along with CRRGs, sourced from public databases. Bioinformatics analyses were conducted to identify five chromatin remodeling-related prognostic genes in BRCA, followed by functional enrichment and immune analysis, offering insights for clinical diagnosis and treatment strategies.
In this study, bioinformatics analysis identified five genes—LHX5, ZP2, GABRQ, APOA2, and CLCNKB—as prognostic markers for BRCA. LHX5, a transcription factor, is highly expressed in various cancers. The protein it encodes interacts with nuclear receptors and DNA-binding proteins, playing a pivotal role in regulating processes such as cell proliferation, differentiation, and apoptosis. Recently, LHX5 has been implicated in the progression of bladder cancer across multiple stages [26, 27]. ZP2, primarily associated with the reproductive system, particularly the zona pellucida of eggs, is upregulated in numerous cancers. It regulates cell cycle progression, promotes cell proliferation, inhibits apoptosis, governs stem cell function, and activates the JNK signaling pathway [28]. A study highlighted the elevated expression of ZP2 in colon cancer cells, where it may enhance cell proliferation via the EXOSC5-ERK1/2-cyclinD1 signaling pathway [29]. Gamma-aminobutyric acid (GABA), the brain’s principal inhibitory neurotransmitter, activates the GABA-A receptor, composed of three subunit types, including GABRQ [30]. GABA receptors are expressed in various solid tumors and are involved in regulating cell proliferation and migration, positioning them as potential targets for cancer therapies [31]. GABRQ has been shown to be overexpressed in hepatocellular carcinoma, with GABA promoting tumor cell proliferation through GABRQ [32]. Moreover, GABRQ expression is closely associated with prognosis in clear cell renal cell carcinoma (ccRCC), where low levels may predict poor outcomes, providing new prognostic biomarkers for patients with ccRCC [33]. APOA2 encodes apolipoprotein A-II, the second most abundant protein in high-density lipoprotein (HDL) particles. Variations in APOA2 isoforms and abnormal expression levels are linked to several malignancies, particularly pancreatic cancer, where APOA2 isoform levels are elevated in the serum of patients with pancreatic cancer compared to healthy individuals [34, 35]. Recent studies have suggested that APOA2 may play a pivotal role in BRCA tumorigenesis and progression, especially in response to the chemotherapy drug paclitaxel [36, 37]. APOA2 likely influences tumor development, particularly through its involvement in lipid metabolism, oxidative stress promotion, and pro-inflammatory responses. Our study found that APOA2 expression was significantly higher in the high-risk group compared to the low-risk group, implying that APOA2 may contribute to BRCA growth by regulating lipid metabolism. CLCNKB, a member of the transmembrane protein and voltage-gated chloride channel families, exhibits relatively low expression in renal tumors, as evidenced by quantitative PCR analysis of specific renal tumor subtypes [38]. CLCNKB expression is also weak in esophageal cancer, where its low levels correlate with poor prognosis [39]. Although the direct interaction between CLCNKB and the tumor microenvironment (TME) remains unclear, its potential role in regulating ion channels and membrane potential suggests that it may indirectly affect electrolyte balance and cell signaling within the TME, ultimately influencing tumor cell behavior. In contrast to previous studies, our research developed a risk model based on five prognostic genes to assess prognosis, immune cell infiltration, and drug sensitivity, presenting a more robust and innovative approach that provides a foundation for further molecular mechanism research.
Our analysis, incorporating the five prognostic genes, revealed significant differences in immune cell infiltration, such as CD8+ T cells, and immune checkpoint expression, including CTLA4, between the high- and low-risk groups. The immune microenvironment is a critical component of tumor biology, and increasing evidence underscores its clinical and pathological relevance in predicting prognosis and therapeutic outcomes. Recently, immune checkpoint inhibitors (ICIs), particularly programmed cell death protein 1/programmed cell death 1 ligand 1 (PD-1/PD-L1) inhibitors, have demonstrated significant efficacy in treating over 20 solid tumors, including breast cancer. However, the response rate to PD-1/PD-L1 inhibitors remains moderate, typically ranging between 20 and 30% [40, 41], highlighting the limitations of monotherapy. Recent clinical studies of neoadjuvant ICIs in patients with triple-negative breast cancer(TNBC), which is a highly immune infiltrative tumor with poor prognosis, have improved pathological complete response rate and prolonged survival [42]. Although the use of combination of chemotherapy with ICIs has shown efficacy in TNBC, the discontinuation rate and serious adverse events of these treatments remains close monitoring [43]. In addition, antibody–drug conjugates (ADCs) have dramatically changed the breast cancer treatment landscape. The anti-TROP2 ADC Sacituzumab Govitecan has been approved for treatment of previously treated, metastatic TNBC patients. The novel ADC Datopotecan-deruxtecan (Dato-DXd) has recently shown encouraging results for TNBC [44, 45]. However, there is no reliable biomarker to predict efficacy and prognosis, leading to some patients receiving ineffective treatment. This current situation suggests the limitations of precision therapy in TNBC. Recently, a systematic review and meta-analysis assessed the prognostic significance of the neutrophil-to-eosinophil ratio (NER) across various cancer types, which may screen specific populations and guide more personalized treatment decisions [46]. Our study offers a model for identifying patients who are more likely to benefit from immunotherapy, contributing to personalized treatment strategies and potentially extending patient survival.
An important finding of this study is the interaction between LHX5, APOA2, and CRRGs within the PPI network. To explore these relationships further, the potential interactions between LHX5 and BPTF, as well as between APOA2 and SMARCD3, were investigated. LHX5 functions as a transcription factor, predominantly localized in the nucleus [47, 48]. BPTF, in turn, plays a critical role in BRCA development and activates the PI3K pathway, which is linked to key hallmarks of tumorigenesis, including cell cycle progression, chemoresistance, resistance to hypoxia, and metastasis [49, 50]. Future studies should focus on elucidating the relationship between LHX5 and the PI3K pathway mediated by BPTF. Additionally, SMARCD3 contributes to cell cycle progression by repressing p21 expression in hormone-positive (ER+) BRCA [51, 52]. Future research could explore the connection between APOA2-mediated lipid metabolism and SMARCD3-regulated cell cycle progression in ER+ BRCA.
However, this study has certain limitations. The dataset-based analysis may introduce biases in sample source and clinical background, as most samples come from specific regions or populations, potentially limiting the generalizability of the findings to other racial or regional contexts. Although clinical and tumor staging information is relatively complete, some missing data may affect the analysis' accuracy. Therefore, future studies should involve a more diverse sample pool to comprehensively capture the genetic characteristics and prognostic factors of BRCA. Meanwhile, we will establish standardized high- and low-risk group cut-off values and validation across larger, multicenter cohorts. Moreover, internal reference genes such as 18S rRNA were added to the qRT-PCR experiment to optimize the construction process of cDNA library, and the accuracy of data was improved by calculating the geometric average. At the same time, we will conduct chromatin immunoprecipitation experiments on the most prominent genes to further explore their regulatory mechanisms. In addition, we will also explore the effects of these prognostic genes on cell transformation and function through transfection experiments in primary breast cell lines. Finally, we will deeply analyze the expression patterns of these genes in different subpopulations, comprehensively consider more biological and clinical factors, and construct a prognostic model that integrates multi-level information, in order to improve the accuracy and reliability of prognosis prediction.
Conclusions
In conclusion, this research identified five prognostic features related to chromatin remodeling in BRCA and conducted a series of analyses, including functional enrichment, offering a new perspective on prognostic features based on chromatin remodeling. Further in vitro and in vivo studies are necessary to validate our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the editors for their valuable feedback on this manuscript.
Author contributions
Jing Feng and Yinghao Liu analyzed and organized the data; Zhiqiang Chen wrote and revised this manuscript; Danni Zhao generated the figure and tables. Xiaodong Gu, and Yu Wang designed, revised, and supervised the study. All authors reviewed and approved the final manuscript.
Funding
This study was supported by the Science and Education Cultivation Fund of the National Cancer and Regional Medical Center of Shanxi Provincial Cancer Hospital (QH2023003).
Data availability
The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was conducted in accordance with the principles outlined in the 1975 Declaration of Helsinki. The research protocol was reviewed and approved by the Ethics Committee of Shanxi Provincial Cancer Hospital. All participants provided informed consent prior to their involvement in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Katsura C, Ogunmwonyi I, Kankam HK, et al. Breast cancer: presentation, investigation and management. Br J Hosp Med. 2022;83(2):1–7. 10.12968/hmed.2021.0459. [DOI] [PubMed] [Google Scholar]
- 2.Khadijeh B, Jafar K, Zeinab Z, et al. Breast cancer: biology, biomarkers, and treatments. Int Immunopharmacol. 2020;84:106535. 10.1016/j.intimp.2020.106535. [DOI] [PubMed] [Google Scholar]
- 3.Agnieszka K, Kamińska M, Katarzyna S, et al. Primary and secondary prevention of breast cancer. Ann Agric Environ Med. 2006;24:549–53. 10.26444/aaem/75943. [DOI] [PubMed] [Google Scholar]
- 4.Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer. 2019;11:151–64. 10.2147/BCTT.S176070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsburg O, Yip C-H, Brooks A, et al. Breast cancer early detection: A phased approach to implementation. Cancer. 2020;126(Suppl 10):2379–93. 10.1002/cncr.32887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br J Radiol. 2022;95(1130):20211033. 10.1259/bjr.20211033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang T, Li W, Huang T, et al. Genetic testing enhances the precision diagnosis and treatment of breast cancer. Int J Mol Sci. 2023;24(23):16607. 10.3390/ijms242316607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braden AM, Stankowski RV, Engel JM, et al. Breast cancer biomarkers: risk assessment, diagnosis, prognosis, prediction of treatment efficacy and toxicity, and recurrence. Curr Pharm Des. 2014;20(30):4879–98. 10.2174/1381612819666131125145517. [DOI] [PubMed] [Google Scholar]
- 9.Noor F, Noor A, Ishaq AR, et al. Recent advances in diagnostic and therapeutic approaches for breast cancer: a comprehensive review. Curr Pharm Des. 2021;27(20):2344–65. 10.2174/1381612827666210303141416. [DOI] [PubMed] [Google Scholar]
- 10.Kaur J, Daoud A, Eblen ST. Targeting chromatin remodeling for cancer therapy. Curr Mol Pharmacol. 2019;12(3):215–29. 10.2174/1874467212666190215112915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richard CC, Sandoval GJ, Soares LMM, et al. Mammalian SWI/SNF chromatin remodeling complexes: emerging mechanisms and therapeutic strategies. Trends Genet. 2020;36(12):936–50. 10.1016/j.tig.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Wang J, Lian Y, et al. Linking long non-coding RNAs and SWI/SNF complexes to chromatin remodeling in cancer. Mol Cancer. 2017;16(1):42. 10.1186/s12943-017-0612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F-L, Li D-Q. Targeting chromatin-remodeling factors in cancer cells: promising molecules in cancer therapy. Int J Mol Sci. 2022;23(21):12815. 10.3390/ijms232112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Cao H-J, Ma N, et al. Chromatin remodeling factor ARID2 suppresses hepatocellular carcinoma metastasis via DNMT1-Snail axis. Proc Natl Acad Sci U S A. 2020;117(9):4770–80. 10.1073/pnas.1914937117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bing Lu, Qiu Ru, Wei J, Wang Li, Zhang Q, et al. Phase separation of phospho-HDAC6 drives aberrant chromatin architecture in triple-negative breast cancer. Nat Cancer. 2024;5(11):1622–40. 10.1038/s43018-024-00816-y. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Yang H, Xiang Z, et al. Study on bone-like microstructure design of carbon nanofibers/polyurethane composites with excellent impact resistance. Nanomaterials. 2022;12(21):3830. 10.3390/nano12213830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsay IS, Ma S, Fisher M, et al. Model selection and prediction of outcomes in recent onset schizophrenia patients who undergo cognitive training. Schizophr Res Cogn. 2017;11:1–5. 10.1016/j.scog.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkerson MD, Hayes DN. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26(12):1572–3. 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustavsson EK, Zhang D, Reynolds RH, et al. ggtranscript: an R package for the visualization and interpretation of transcript isoforms using ggplot2. Bioinformatics. 2022;38(15):3844–6. 10.1093/bioinformatics/btac409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuguang Gu, Daniel H. Make interactive complex heatmaps in R. Bioinformatics. 2022;38(5):1460–2. 10.1093/bioinformatics/btab806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu G, Wang L-G, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7. 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Lu F, Yin Y. Applying logistic LASSO regression for the diagnosis of atypical Crohn’s disease. Sci Rep. 2022;12(1):11340. 10.1038/s41598-022-15609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–44. 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 25.Gregor S, Francesca F, Markus L. Immunedeconv: an R package for unified access to computational methods for estimating immune cell fractions from bulk RNA-sequencing data. Methods Mol Biol. 2020;2120:223–32. 10.1007/978-1-0716-0327-7_16. [DOI] [PubMed] [Google Scholar]
- 26.Akhir MKAM, Choy SC, Abdullah MA, et al. The role of ISL1 and LHX5 LIM homeobox genes in bladder tumourigenesis. Malays J Med Sci. 2020;27(1):37–45. 10.21315/mjms2020.27.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamalakaran S, Varadan V, Giercksky Russnes HE, et al. DNA methylation patterns in luminal breast cancers differ from non-luminal subtypes and can identify relapse risk independent of other clinical variables. Mol Oncol. 2011;5(1):77–92. 10.1016/j.molonc.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa J, Pereira R, Oliveira J, et al. Structural and molecular analysis of the cancer prostate cell line PC3: oocyte zona pellucida glycoproteins. Tissue Cell. 2018;55:91–106. 10.1016/j.tice.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Kraus D, Glassmann A, Golletz C, et al. Zona pellucida protein 2 (ZP2) is expressed in colon cancer and promotes cell proliferation. Cancers. 2021;13(8):1759. 10.3390/cancers13081759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García-Martín E, Martínez C, Alonso-Navarro H, et al. Gamma-aminobutyric acid GABRA4, GABRE, and GABRQ receptor polymorphisms and risk for essential tremor. Pharmacogenet Genom. 2011;21(7):436–9. 10.1097/FPC.0b013e328345bec0. [DOI] [PubMed] [Google Scholar]
- 31.Huang D, Alexander PB, Li QJ, Wang XF. GABAergic signaling beyond synapses: an emerging target for cancer therapy. Trends Cell Biol. 2023;33(5):403–12. 10.1016/j.tcb.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y-H, Liu Y, Li Y-D, et al. GABA stimulates human hepatocellular carcinoma growth through overexpressed GABAA receptor theta subunit. World J Gastroenterol. 2012;18(21):2704–11. 10.3748/wjg.v18.i21.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee D, Ha M, Hong CM, et al. GABRQ expression is a potential prognostic marker for patients with clear cell renal cell carcinoma. Oncol Lett. 2019;18(6):5731–8. 10.3892/ol.2019.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honda K, Katzke VA, Hüsing A, et al. CA19–9 and apolipoprotein-A2 isoforms as detection markers for pancreatic cancer: a prospective evaluation. Int J Cancer. 2019;144(8):1877–87. 10.1002/ijc.31900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honda K, Srivastava S. Potential usefulness of apolipoprotein A2 isoforms for screening and risk stratification of pancreatic cancer. Biomark Med. 2016;10(11):1197–207. 10.2217/bmm-2016-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhowmick C, Rahaman M, Bhattacharya S, et al. Identification of hub genes to determine drug-disease correlation in breast carcinomas. Med Oncol. 2023;41(1):36. 10.1007/s12032-023-02246-9. [DOI] [PubMed] [Google Scholar]
- 37.Flote VG, Vettukattil R, Bathen TF, et al. Lipoprotein subfractions by nuclear magnetic resonance are associated with tumor characteristics in breast cancer. Lipids Health Dis. 2016;15:56. 10.1186/s12944-016-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murakami T, Sano F, Huang Y, et al. Identification and characterization of Birt-Hogg-Dubé associated renal carcinoma. J Pathol. 2007;211(5):524–31. 10.1002/path.2139. [DOI] [PubMed] [Google Scholar]
- 39.Dai J, Reyimu A, Sun A, et al. Establishment of prognostic risk model and drug sensitivity based on prognostic related genes of esophageal cancer. Sci Rep. 2022;12(1):8008. 10.1038/s41598-022-11760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Liu L, Liu J, et al. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. 2023;22(1):58. 10.1186/s12943-023-01725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pang K, Shi ZD, Wei LY, et al. Research progress of therapeutic effects and drug resistance of immunotherapy based on PD-1/PD-L1 blockade. Drug Resist Updates Rev Comment Antimicrob Anticancer Chemother. 2023;66:100907. 10.1016/j.drup.2022.100907. [DOI] [PubMed] [Google Scholar]
- 42.Rizzo A, Cusmai A, Acquafredda S, et al. KEYNOTE-522, IMpassion031 and GeparNUEVO: changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol. 2022;18(18):2301–9. 10.2217/fon-2021-1647. [DOI] [PubMed] [Google Scholar]
- 43.Rizzo A, Schipilliti FM, Di Costanzo F, et al. Discontinuation rate and serious adverse events of chemoimmunotherapy as neoadjuvant treatment for triple-negative breast cancer: a systematic review and meta-analysis. ESMO Open. 2023;8(6):102198. 10.1016/j.esmoop.2023.102198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caputo R, Buono G, Piezzo M, et al. Sacituzumab Govitecan for the treatment of advanced triple negative breast cancer patients: a multi-center real-world analysis. Front Oncol. 2024;14:1362641. 10.3389/fonc.2024.1362641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schipilliti FM, Drittone D, Mazzuca F, et al. Datopotamab deruxtecan: a novel antibody drug conjugate for triple-negative breast cancer. Heliyon. 2024;10(7):e28385. 10.1016/j.heliyon.2024.e28385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahin TK, Ayasun R, Rizzo A, et al. Prognostic value of neutrophil-to-eosinophil ratio (NER) in cancer: a systematic review and meta-analysis. Cancers. 2024;16(21):3689. 10.3390/cancers16213689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Q, Zhao Y. The diverse biofunctions of LIM domain proteins: determined by subcellular localization and protein-protein interaction. Biol Cell. 2007;99(9):489–502. 10.1042/BC20060126. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, He C, Hu X. LIM homeobox transcription factors, a novel subfamily which plays an important role in cancer (review). Oncol Rep. 2014;31(5):1975–85. 10.3892/or.2014.3112. [DOI] [PubMed] [Google Scholar]
- 49.Bezrookove V, Khan IA, Nosrati M, et al. BPTF promotes the progression of distinct subtypes of breast cancer and is a therapeutic target. Front Oncol. 2022;12:1011173. 10.3389/fonc.2022.1011173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao S, Zhang W, Ma J, et al. PHF6 recruits BPTF to promote HIF-dependent pathway and progression in YAP-high breast cancer. J Transl Med. 2023;21(1):220. 10.1186/s12967-023-04031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flajollet S, Lefebvre B, Cudejko C, et al. The core component of the mammalian SWI/SNF complex SMARCD3/BAF60c is a coactivator for the nuclear retinoic acid receptor. Mol Cell Endocrinol. 2007;270(1–2):23–32. 10.1016/j.mce.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Tropée R, de la PeñaAvalos B, Gough M, et al. The SWI/SNF subunit SMARCD3 regulates cell cycle progression and predicts survival outcome in ER+ breast cancer. Breast Cancer Res Treat. 2021;185(3):601–14. 10.1007/s10549-020-05997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.