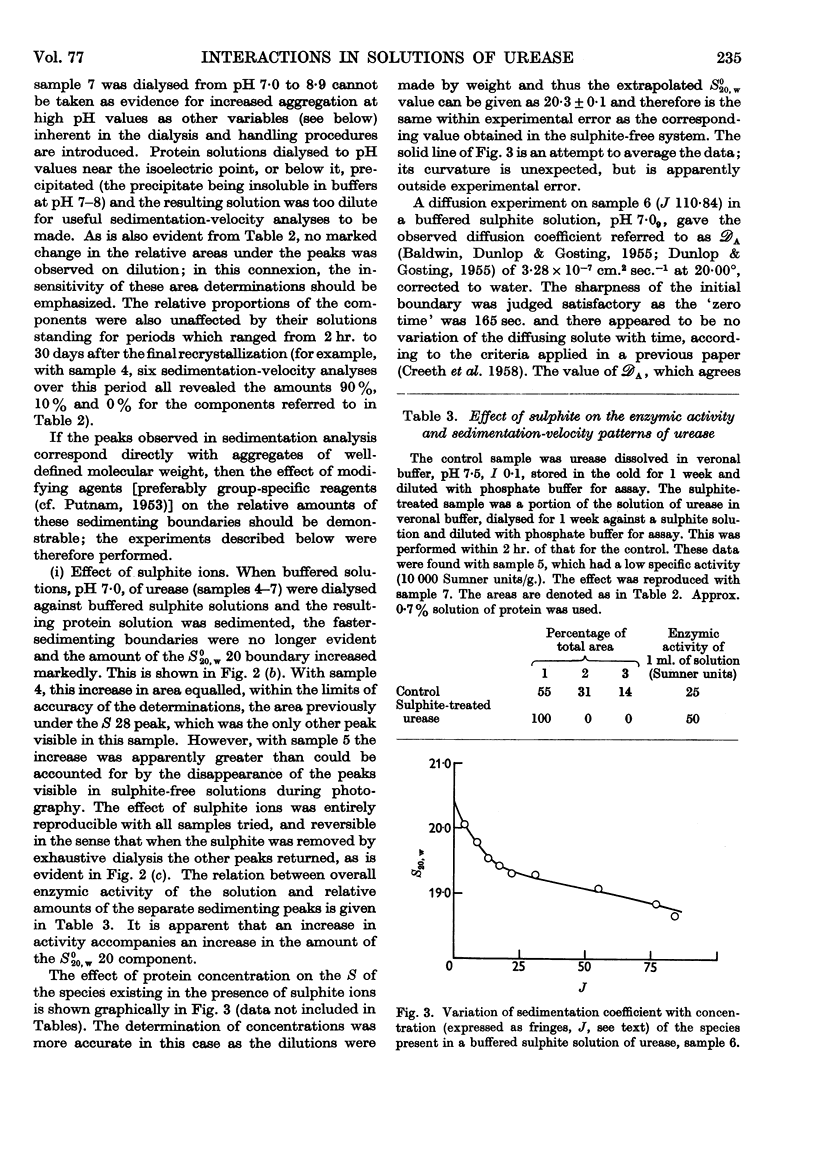
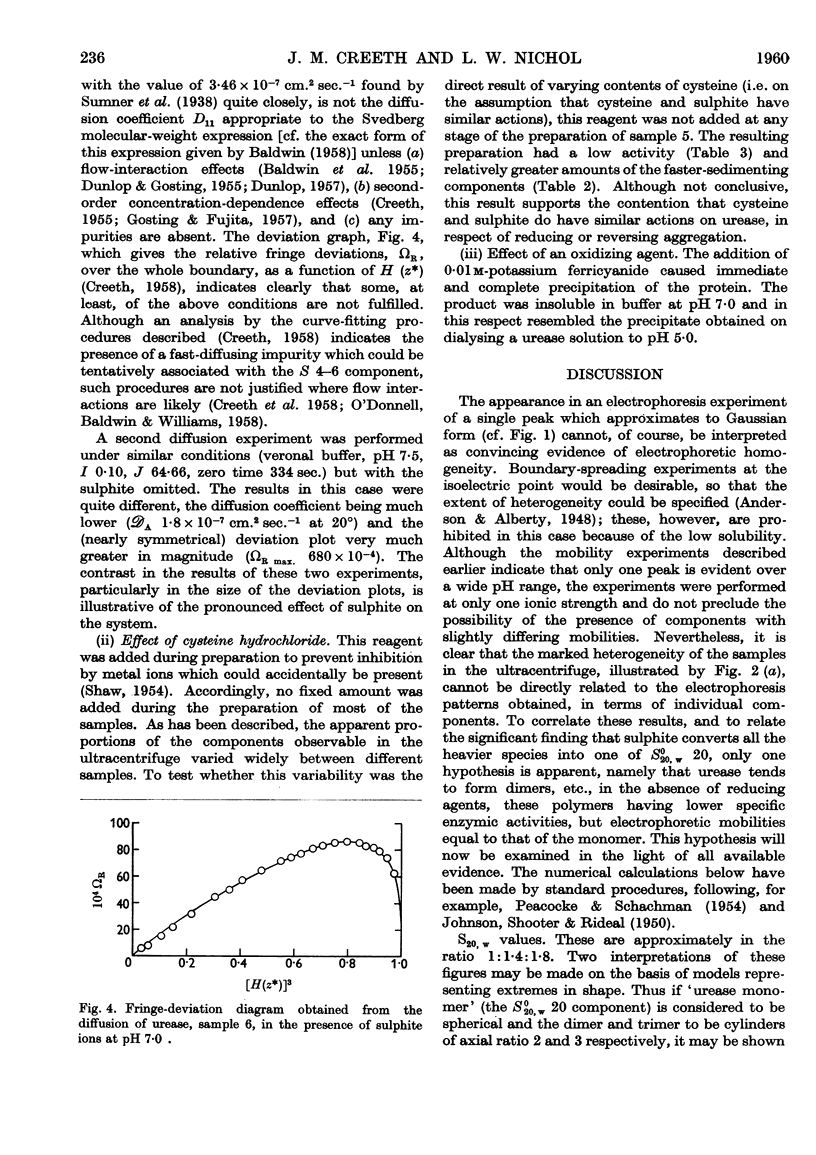
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRIGGS D. R., WOLF W. J. Studies on the cold-insoluble fraction of the water-extractable soybean proteins. I. Polymerization of the 11 S component through reactions of sulfhydryl groups to form disulfide bonds. Arch Biochem Biophys. 1957 Nov;72(1):127–144. doi: 10.1016/0003-9861(57)90180-7. [DOI] [PubMed] [Google Scholar]
- CECIL R., McPHEE J. R. A kinetic study of the reactions on some disulphides with sodium sulphite. Biochem J. 1955 Jul;60(3):496–506. doi: 10.1042/bj0600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CECIL R., McPHEE J. R. The estimation of thiols and disulphides by potentiometric titration with silver nitrate. Biochem J. 1955 Feb;59(2):234–240. doi: 10.1042/bj0590234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVERT H. E. The reversibility of the mercuric chloride inhibition of urease at varied initial urea concentrations. Arch Biochem Biophys. 1952 Nov;41(1):29–41. doi: 10.1016/0003-9861(52)90501-8. [DOI] [PubMed] [Google Scholar]
- HUGGINS C., TAPLEY D. F., JENSEN E. V. Sulphydryl-disulphide relationships in the induction of gels in proteins by urea. Nature. 1951 Apr 14;167(4250):592–593. doi: 10.1038/167592a0. [DOI] [PubMed] [Google Scholar]
- Henry R. J., Smith E. C. Use of Sulfuric Acid-Dichromate Mixture in Cleaning Glassware. Science. 1946 Nov 1;104(2705):426–427. doi: 10.1126/science.104.2705.426. [DOI] [PubMed] [Google Scholar]
- JOHNSON P., SHOOTER E. M., RIDEAL E. K. The globulins of the ground nut. (Arachis hypogaea) II. Electrophoretic examination of the arachin system. Biochim Biophys Acta. 1950 Jun;5(3/4):376–396. doi: 10.1016/0006-3002(50)90184-3. [DOI] [PubMed] [Google Scholar]
- KUFF E. L., HOGEBOOM G. H., STRIEBICH M. J. The sedimentation behavior of alcohol dehydrogenase and urease in crude solutions. J Biol Chem. 1955 Jan;212(1):439–448. [PubMed] [Google Scholar]
- O'DONNELL I. J., BALDWIN R. L., WILLIAMS J. W. Correlation of the N=a reaction of thyroglobulin with the type of breakdown produced by papain. Biochim Biophys Acta. 1958 May;28(2):294–308. doi: 10.1016/0006-3002(58)90476-1. [DOI] [PubMed] [Google Scholar]
- OGSTON A. G., TILLEY J. M. Studies on the heterogeneity of crystallized beta-lactoglobulin. Biochem J. 1955 Apr;59(4):644–653. doi: 10.1042/bj0590644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEACOCKE A. R., SCHACHMAN H. K. Studies on the sedimentation behaviour of thymus deoxypentose nucleic acid with reference to its homogeneity, size and shape. Biochim Biophys Acta. 1954 Oct;15(2):198–210. doi: 10.1016/0006-3002(54)90060-8. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B. The inactivation of urease by deuterons and heat. Arch Biochem Biophys. 1952 Apr;36(2):328–335. doi: 10.1016/0003-9861(52)90418-9. [DOI] [PubMed] [Google Scholar]
- WILLS E. D. Enzyme inhibition by suramin and the measurement of the isoelectric points of some enzymes. Biochem J. 1952 Jan;50(3):421–425. doi: 10.1042/bj0500421. [DOI] [PMC free article] [PubMed] [Google Scholar]