Highlights
-
•
Cerebrotendinous Xanthomatosis (CTX) is a rare genetic disease of bile synthesis.
-
•
Up to 30% of CTX patients have epilepsy.
-
•
We show Fixation-Off Sensitivity (FOS) and eye-closure induced seizures in CTX.
Keywords: Cerebrotendinous Xanthomatosis (CTX), Fixation-Off Sensitivity (FOS), Epilepsy, Electroencephalography (EEG), CYP27A1 Gene
Abstract
Cerebrotendinous Xanthomatosis (CTX) is a rare autosomal recessive condition resulting in accumulation of cholesterol and cholestanol due to disrupted bile synthesis. Affected tissues include brain, tendons, skin, bone, lungs, and eyes. We report a clinical case presenting with epilepsy, which has been described, however with a particular EEG appearance that appears novel with Fixation-Off Sensitivity (FOS). The patient’s EEG showed significant buildup of abnormal slowing and frontally predominant generalized epileptiform discharges when her eyes were closed, and in contrast essentially normal tracings while eyes were open, eventually showing electrographic evolution and generating a bilateral tonic-clonic seizure. Genetic testing confirmed the diagnosis of CTX, and CTX-specific treatment with chonodeoxycholic acid was initiated in addition to anti-seizure medication.
1. Introduction
Cerebrotendinous Xanthomatosis (CTX) is a rare autosomal recessive condition that was first described in 1937 by van Bogaert [1] in France and later confirmed to be caused by a mutation in the CYP27A1 gene [2]. This mutation results in deficiency of the sterol 27-hydroxylase enzyme, a component of bile acid synthesis. This leads to accumulation of cholesterol and cholestanol within the body and resultant dysfunction in several tissues.
Herein, we discuss a patient who presented with epilepsy and a particular EEG finding which has not yet been described in CTX: upon closing her eyes, there was immediate and significant buildup of discharges and polymorphic delta slowing, time-locked with cognitive dysfunction and eventually evolving electrographically to generate a bifrontal seizure with spread to bilateral tonic-clonic activity.
2. Epidemiology and natural history
Cerebrotendinous Xanthomatosis is rare, and as such data is limited; a recent literature review identified 194 published cases with which one may define epidemiology and natural history. Prevalence is estimated at 3 to 8 per 100,000 individuals, highest among Jews of Moroccan descent. Onset is typically in late childhood though diagnosis lags into the 4th decade. The most frequently reported symptoms (>50%) include ataxia, pyramidal symptoms, peripheral neuropathy, chronic diarrhea, cognitive dysfunction and/or developmental delay, skin/tendinous xanthomas, and cataracts. Less frequently (<30%), seizures, scoliosis, pes cavus, extrapyramidal syndrome, myoclonus, pseudobulbar syndrome, or osteoporosis [[3], [4], [5]].
Regarding seizures, there is limited data published to define prevalence or seizure types. As recently as 1991, epilepsy was not considered a feature of CTX at all [6]. In literature review for the writing of this report, seizure type and prevalence varied. The most often quoted percentage of epilepsy in CTX was ∼ 30 % [4,7]. Some authors claimed CTX rarely causes refractory seizures, while others reported medication resistance at first presentation [8,9].
If left untreated, death occurs in middle age after a pseudobulbar phase. [3,4]. Treatment with chenodeoxycholic acid (CDCA) has been shown to improve dementia, EEG abnormalities, pyramidal/cerebellar symptoms, and imaging abnormalities [10]. This treatment is a direct replacement of the naturally occurring human bile acid, as the enzyme deficiency prevents these patients from making their own. A consensus paper in 2021 recommended early treatment as prognosis may be improved [11].
3. Typical MRI and EEG findings
A radiological review in 2000 reported imaging findings from 24 patients with CTX. Most commonly, nonspecific periventricular white matter abnormalities were seen. The most characteristic reported finding was abnormal signal intensity in the basal ganglia and infratentorial region, similar in appearance to other metabolic diseases, though also with involvement of gray matter (basal ganglia, dentate nucleus). All were hyperintense on T2-weighted images with the exception of the dentate nucleus lesions. The authors noted the lack of specificity of these findings [12].
There are few detailed descriptions of EEG findings; a study in 1984 found that 10 of 13 patients had marked derangements with irregular slow theta and delta waves and frequent bursts of high-voltage activity. With CDCA treatment, EEG showed normalization or less theta wave activity in 8 of those 13 patients. No mention was made as to whether the patients had seizures or epilepsy [10].
4. Case report and EEG findings
A 21-year old African-American woman was admitted to our Epilepsy Monitoring Unit (EMU). She had a history of mild intellectual disability, undergoing an Individual Education Program (IEP) during school, and was given extended time to complete scholastic activities. She had a single febrile seizure at age 2 with a high fever. She was diagnosed with “absence” seizures at age 2 and “grand mal” seizures as a teenager. Several EEGs throughout adolescence were suggestive of generalized epilepsy, showing generalized spike-and-wave discharges. An MRI brain was normal.
During her EMU stay, antiseizure medications (ASMs) were weaned. Home medications at that time included levetiracetam, lamotrigine, cenobamate, and clonazepam. Her initial EEG on days 1–3 showed persistent interictal bilateral posterior sharply contoured 2–3 Hz delta slowing with a left-sided predominance and also occasional generalized spike-and-wave discharges with a bifrontal and slightly left-sided predominance, at times occurring in bursts of 2–3 Hz. During photic stimulation, there was no photoparoxysmal response.
By day 4, all medications had been weaned, though several of her home medications with long half lives would still have had low level serum concentrations. At this point, a new EEG phenomena emerged: prominent polymorphic delta slowing with abundant generalized discharges, noted only when eyes were closed.
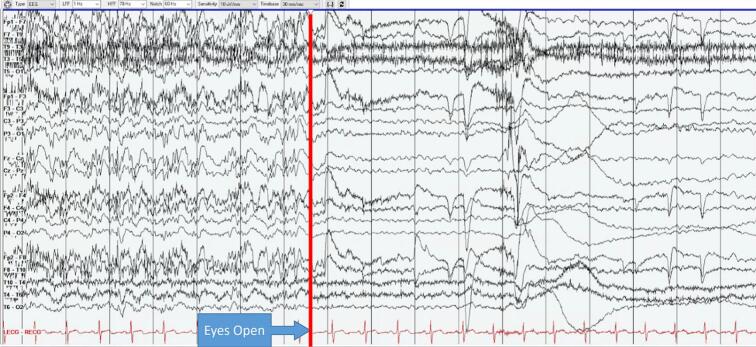
Careful review of video in correlation with EEG showed that when the patient's eyes were open, EEG showed a similar background to prior with beta, alpha, and theta frequencies and occasional epileptiform findings. However, every single time she closed her eyes for longer than 1 eyeblink (roughly 0.5 s), there was immediate emergence of bifrontal sharp semirhythmic alpha, abundant frontally predominant generalized discharges, and polymorphic diffuse delta slowing which would last as long as eyes were closed, up to 10 s or more. This phenomenon was persistent and invariably reproducible on Day 4 from 10:30 to 12:47 at which time she had a seizure (Fig. 1, Fig. 2).
Fig. 1.
Eye closure reveals emergence of slowing and epileptiform discharges (paroxysms). Sensitivity: 10 µV, LFF: 1 Hz, HFF: 70 Hz. Bipolar longitudinal montage.
Fig. 2.
Eye opening reveals abrupt resolution of slowing and epileptiform discharges. Sensitivity: 10 µV, LFF: 1 Hz, HFF: 70 Hz. Bipolar longitudinal montage.
At 12:37 staff came to perform bedside testing given the EEG abnormalities. The patient was unable to answer questions or follow commands whenever her eyes were closed. If her eyes opened, she could briskly follow all commands and answer questions without error (Fig. 3).
Fig. 3.
During testing, the patient closed her eyes and was unable to answer questions only while eyes were closed. Sensitivity: 10 µV, LFF: 1 Hz, HFF: 70 Hz. Bipolar longitudinal montage.
At 12:47 after bedside testing concluded, she rolled back over in bed to go to sleep, closing her eyes. This time, EEG showed generalized polyspike/wave complexes which became rhythmic at 1.5 Hz and finally organized into 6–8 Hz bilateral frontal lobe ictal pattern. She had an ictal cry and went quickly into a bilateral tonic-clonic seizure. The tonic-clonic phase ended after ∼ 30 s with offset to profound diffuse suppression (Fig. 4.1, Fig. 4.2).
Fig. 4.1.
The patient repositioned in bed (myogenic artifact) and closed her eyes. Discharges organized and became rhythmic, progressing to frontally predominant generalized spike and slow wave discharges accompanied clinically by a bilateral tonic clonic seizure. Sensitivity: 10 µV, LFF: 1 Hz, HFF: 70 Hz. Bipolar longitudinal montage.
Fig. 4.2.
Contiguous epoch following Fig. 4.1 exhibiting seizure evolution with spread to bilateral tonic/clonic activity. Sensitivity: 10 µV, LFF: 1 Hz, HFF: 70 Hz. Bipolar longitudinal montage.
Antiseizure medications were administered after this seizure and the remainder of her EEG showed a background of slow delta with overriding alpha and beta activity. The aforementioned phenomena was no longer appreciable, indicating exquisite sensitivity of her EEG abnormalities to ASMs.
She was later seen in the genetics clinic and whole exome sequencing was performed along with her mother, who submitted a sample for duo testing. The results were positive for 3 findings and no VUS were reported. The positive findings included the following: 1) a 787 kb multi-gene deletion in 16p13.11 at coordinates GRCh37/hg19: chr16:15737239–15820210, for which the patient was heterozygous and inherited from her unaffected mother and 2) two separate heterozygous pathogenic mutations in CYP27A1, which were found to be on opposite alleles (in trans), one of which was inherited from her unaffected mother and the other either from her father or de novo. Two separate mutations in CYP27A1 in trans would lead to disease, as it has a recessive inheritance pattern. One variant was NM_000784.3: c.844 + 1 G > T:p? and the maternally inherited variant was NM_000784.3: c.886C > T: p.(Gln296Ter) (CAA > TAA). Cerebrotendinous xanthomatosis was confirmed with laboratory testing showing elevated levels of cholesterol, 7α-Hydroxy-4-cholesten-3-one, and 7α,12α-Dihydroxycholest-4-en-3-one, with otherwise normal complete blood count and complete metabolic panel.
An MRI of the patient’s brain was not able to be obtained.
Treatment was initiated with chenodeoxycholic acid (CDCA) and genetic counselling provided to the entire family, who might also undergo CDCA treatment pending individual risk/benefit discussions.
5. Discussion
While epilepsy is not considered a rare manifestation of cerebrotendinous xanthomatosis, the particular EEG findings we observed have not yet been described in this disease. Our literature review has revealed few detailed descriptions of EEG findings in CTX and as such further study on this topic is valuable.
It should be noted that the patient's genetic testing also revealed a coexisting 16p13.11 microdeletion. This genetic mutation has been associated with epilepsy in published literature, and we confirmed that this patient's deletion included a gene (NDE1) that has been strongly correlated with epilepsy in a large cross-sectional genome study [13].
There are several reasons making CTX the most likely explanation for our patient's symptoms. She has no other features of reported 16p13.11 phenotypes such as intellectual disability, microcephaly, schizophrenia, or facial dysmorphisms. It is also well published that this microdeletion has widely variable penetrance [14]. Our patient's mother carries the same 16p13.11 mutation and passed it down, including a deletion of the same NDE1 gene, and is not affected with epilepsy or any of the aforementioned signs/symptoms.
Our patient’s testing showed a single 16p13.11 microdeletion but two separate heterozygous mutations in the CYP27A1 gene; one from her mother and one either from her father or de novo. This has led to clinically measurable changes in cholesterol metabolism, which we used to confirm the diagnosis of CTX. While we cannot entirely rule out pathophysiologic changes from 16p13.11 microdeletion, there is more evidence in this case supporting clinically significant disease from CTX.
The EEG phenomenon we describe has been identified before and termed Fixation-Off Sensitivity (FOS), though the pathophysiology remains unclear. It is differentiated from Eyelid Closure Paroxysm which lasts only 1–4 s from eye closure. FOS has been described most frequently in Self-Limited epilepsy with Autonomic Seizures (SeLAS, a.k.a Panayiotopoulos Syndrome) and Photosensitive Occipital Lobe Epilepsy (POLE, a.k.a idiopathic childhood occipital epilepsy of Gastaut), less often in symptomatic occipital lobe epilepsy, eyelid myoclonia with absences, or benign childhood seizure susceptibility syndrome. Some patients exhibiting FOS have suffered focal insults, while others exhibit findings more consistent with diffuse networks of hyperexcitability [15]. Patients with CTX commonly exhibit white matter abnormalities [14], and similar findings have also been seen in patients with a photoparoxysmal response, which may indicate a shared pathophysiology between the two phenomena. Definitively identifying mechanisms underlying photosensitivity in epilepsy is similarly difficult, however both populations of patients include many with either structural abnormality and/or diffuse networks of hyperexcitability [16].
One possible pathophysiologic explanation would be an abnormality of the alpha-rhythm generators as postulated by Panayiotopoulos [17], however this would not be expected based on currently reported pathology in CTX since alpha-rhythm generators would presumably be found in cortex.
Somewhat similar findings may be seen in epilepsy with eyelid myoclonia (EEM or Jeavons Syndrome), though with some subtle differences to our patient. In EEM, the background change upon eye closure is often well formed 3–6 Hz spike-and-wave (according to a 2020 review of the published EEG characteristics of EEM) [18], while our patient instead exhibited a background of more disorganized polymorphic delta slowing with intermixed nonrhythmic discharges and frontal sharp alpha. Our patient also notably had no eyelid myoclonus and does not exhibit other clinical similarities to EEM, making any theorized pathophysiological associations less convincing.
Regardless of underlying pathophysiology, the overall EEG findings we observed do indicate an extensive diffuse hyperexcitable network as seen in many other generalized epilepsy syndromes, as opposed to findings suggestive of a localization-related epilepsy.
6. Conclusion
Cerebrotendinous xanthomatosis (CTX) is a rare genetic disorder of cholesterol catabolism. Among other symptoms, patients may have epilepsy, and this patient’s EEG had a particular finding of disorganized and irritable background upon eyelid closure, without eyelid myoclonus, best termed Fixation-Off Sensitivity (FOS). This finding has not yet been described in CTX. Pathophysiology of this finding is unclear. Overall, these findings indicate an extensive diffuse hyperexcitable network as seen in other patients with generalized epilepsy syndromes.
CRediT authorship contribution statement
Zachary Mills: Writing – original draft, Visualization, Conceptualization. James Thomas Houston: Writing – review & editing, Visualization, Conceptualization. Ashley Thomas: Writing – review & editing, Visualization. Kelsey Shoenmeyer: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.van Bogaert L, Scherer HJ, Epstein E. Une forme cerebrale de la cholesterinose generalisee. Paris: Masson et Cie. 1937.
- 2.Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem. 1991 Apr 25;266(12):7779-83. PMID: 2019602; PMCID: PMC4449724. [PMC free article] [PubMed]
- 3.Lionnet C., Carra C., Ayrignac X., et al. Cerebrotendinous xanthomatosis: A multicentric retrospective study of 15 adults, clinical and paraclinical typical and atypical aspects. Rev Neurol. 2014;170(6–7) doi: 10.1016/j.neurol.2014.01.675. PMID: 24746394. [DOI] [PubMed] [Google Scholar]
- 4.Wong J.C., Walsh K., Hayden D., Eichler F.S. Natural history of neurological abnormalities in cerebrotendinous xanthomatosis. J Inherit Metab Dis. 2018 Jul;41(4):647–656. doi: 10.1007/s10545-018-0152-9. Epub 2018 Feb 26 PMID: 29484516. [DOI] [PubMed] [Google Scholar]
- 5.Federico A, Gallus GN. Cerebrotendinous Xanthomatosis. 2003 Jul 16 [updated 2022 Mar 17]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024. PMID: 20301583. [PubMed]
- 6.Arlazoroff A., Roitberg B., Werber E., Shidlo R., Berginer V.M. Epileptic seizure as a presenting symptom of cerebrotendinous xanthomatosis. Epilepsia. 1991 doi: 10.1111/j.1528-1157.1991.tb04705.x. PMID: 1915172. [DOI] [PubMed] [Google Scholar]
- 7.Salen G., Steiner R.D. Epidemiology, diagnosis, and treatment of cerebrotendinous xanthomatosis (CTX) J Inherit Metab Dis. 2017;40(6):771–781. doi: 10.1007/s10545-017-0093-8. Epub 2017 Oct 4 PMID: 28980151. [DOI] [PubMed] [Google Scholar]
- 8.Pedroso J.L., Pinto W.B., Souza P.V., Santos L.T., Abud I.C., Avelino M.A., et al. Early-onset epilepsy as the main neurological manifestation of cerebrotendinous xanthomatosis. Epilepsy Behav. 2012;24(3):380–381. doi: 10.1016/j.yebeh.2012.04.121. Epub 2012 May 30 PMID: 22658436. [DOI] [PubMed] [Google Scholar]
- 9.Kauffman M.A., Gonzalez-Morón D., Consalvo D., Kochen S. Cerebrotendinous xanthomatosis revealed in drug-resistant epilepsy diagnostic workup. Am J Med Sci. 2012;343(4):332–333. doi: 10.1097/MAJ.0b013e31823cf6d8. PMID: 22197981. [DOI] [PubMed] [Google Scholar]
- 10.Berginer V.M., Salen G., Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med. 1984;311(26):1649–1652. doi: 10.1056/NEJM198412273112601. PMID: 6504105. [DOI] [PubMed] [Google Scholar]
- 11.Stelten B.M.L., Dotti M.T., Verrips A., Elibol B., Falik-Zaccai T.C., Hanman K., et al. Expert opinion on diagnosing, treating and managing patients with cerebrotendinous xanthomatosis (CTX): a modified Delphi study. Orphanet J Rare Dis. 2021;16(1):353. doi: 10.1186/s13023-021-01980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkhof F., Verrips A., Wesseling P., van Der Knaap M.S., van Engelen B.G., Gabreëls F.J., et al. Cerebrotendinous xanthomatosis: the spectrum of imaging findings and the correlation with neuropathologic findings. Radiology. 2000;217(3):869–876. doi: 10.1148/radiology.217.3.r00dc03869. PMID: 11110956. [DOI] [PubMed] [Google Scholar]
- 13.Heinzen EL, et al.. Rare deletions at 16p13.11 predispose to a diverse spectrum of sporadic epilepsy syndromes. Am J Hum Genet. 2010 May 14;86(5):707-18. doi: 10.1016/j.ajhg.2010.03.018. Epub 2010 Apr 15. PMID: 20398883; PMCID: PMC2869004. [DOI] [PMC free article] [PubMed]
- 14.Granata P, Cocciadiferro D, Zito A, Pessina C, Bassani A, Zambonin F, Novelli A, Fasano M, Casalone R. Whole Exome Sequencing in 16p13.11 Microdeletion Patients Reveals New Variants Through Deductive and Systems Medicine Approaches. Front Genet. 2022 Mar 15;13:798607. doi: 10.3389/fgene.2022.798607. PMID: 35368691; PMCID: PMC8965081. [DOI] [PMC free article] [PubMed]
- 15.Saadeldin I.Y., Matlik H.N. Coexistence of fixation-off sensitivity and inverted fixation-off sensitivity in a female child with Panayiotopoulos syndrome: Video-electroencephalography documentation. Epilepsy Behav Case Rep. 2015;28(4):1–5. doi: 10.1016/j.ebcr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poleon S., Szaflarski J.P. Photosensitivity in generalized epilepsies. Epilepsy Behav. 2017;68:225–233. doi: 10.1016/j.yebeh.2016.10.040. Epub 2017 Feb 16 PMID: 28215998. [DOI] [PubMed] [Google Scholar]
- 17.Panayiotopoulos C.P. Fixation-off, scotosensitive, and other visual-related epilepsies. Adv Neurol. 1998;75:139–157. PMID: 9385419. [PubMed] [Google Scholar]
- 18.Zawar I., Pestana Knight E.M. An Overview of the Electroencephalographic (EEG) Features of Epilepsy with Eyelid Myoclonia (Jeavons Syndrome) Neurodiagn J. 2020;60(2):113–127. doi: 10.1080/21646821.2020.1750879. Epub 2020 May 5 PMID: 32369428. [DOI] [PubMed] [Google Scholar]