Abstract
Beauveria spp. are ubiquitous fungal entomopathogens that are commercially distributed as biological insecticides worldwide. In this paper we describe the clinical manifestation, diagnosis, and therapy of the first documented human deep tissue infection with an entomopathogenic Beauveria species in a patient receiving immunosuppressive therapy and describe the morphological and molecular characterization of the mold.
CASE REPORT
A 38-year-old woman had suffered from continuous pain in her left ear for 4 weeks. Computed tomography (CT) scan showed a tumor in her left mastoid. Histopathology of a biopsy specimen revealed myeloid blasts with a morphology of M2 blasts, according to French-American-British cooperation group classification (2, 15). Radionucleic imaging of the skeleton showed additional enhancement in the left sixth ventral rib, in the left sternoclavicular joint, and in the right proximal humerus. No further bone lesions or tumors were detected on X ray. Bone marrow puncture revealed no excess of myeloid blasts.
The patient was therefore diagnosed with extramedullary acute myeloid leukemia and was treated with four cycles of an intensive chemotherapy regimen according to the protocol of the South German Hemoblastoses Study Group (AML Trial 1996) (21). After each cycle the patient suffered from the expected leucopenia, lasting about 10 days. However, during the last two cycles the dosage was decreased because of prolonged thrombocytopenia.
The last stage of therapy showed complete regression of the tumor in the left mastoid. No lesions were detected by radionucleic imaging of the skeleton, and blasts were less than 5% in the bone marrow.
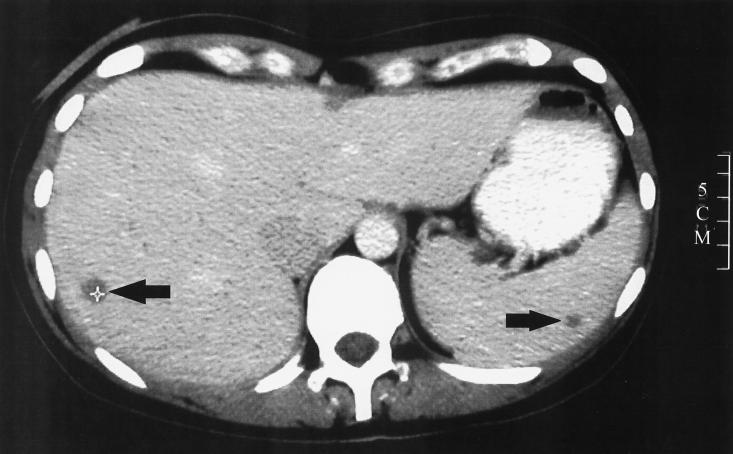
Two weeks after discharge, the patient was readmitted because of severe dyspnea, dry cough, pain in the right upper abdomen, and a fever of 38.5°C. In contrast to results from her previous lung function test, a mild restriction (forced vital capacity, 3.15 liters; ratio of forced expiratory volume/forced vital capacity, 87% predicted; total lung capacity, 84.1%) and a moderate reduction of the diffusion capacity (transfer factor for carbon monoxide a single breath corrected for hemoglobin, 66.7%) were now detected. CT scan of the thorax revealed a discrete interstitial infiltrate. Allergic alveolitis was histologically confirmed by transbronchial lung biopsy. On the day of admission an ultrasonography of the abdomen was performed that revealed multiple lesions in the liver and spleen, suggesting systemic fungal infection. CT scans of the abdomen confirmed several focal hypodense lesions in the liver and in the spleen (Fig. 1) with reduced uptake of dye and with a maximum diameter of 1.8 cm. As repetitive testing of serum samples did not confirm systemic hepatolienal candidiasis or invasive aspergillosis, a CT-guided needle aspiration of one of the hypodense liver lesions was performed on the third day after admission and the tissue was histopathologically and microbiologically examined. Hematoxylin-eosin-stained sections of specimen obtained by the CT-aided liver biopsy showed vital liver tissue in the circumference of the focus. However, in the center of the lesion the liver tissue was damaged and characterized by extensive focal necrosis. Little cellular reaction and cell debris and moderate numbers of erythrocytes were detected. Grocott Gomori methenamine silver stain (Fig. 2) and calcofluor white stain from a specimen obtained from the center of the focus revealed infiltrating hyphae. In areas of tissue destruction the mycelia consisted of slightly pleomorphic, nonmelanized, hyaline septate hyphae branching at angles of 45° to 90°.
FIG. 1.
CT scan of the abdomen with intravenously and orally administered dye. Reduced dye uptake is seen as dark lesions, in liver (1 cm) and spleen (0.6 cm) (arrows), 1 day after admission. In total, nine lesions where found in the liver and two more were found in the spleen (pictures not shown). A CT-guided puncture of a focal lesion in the liver was performed on the following day. A 5-cm scale is shown on the right side.
FIG. 2.
Grocott Gomori methenamine silver stain of a homogenized specimen obtained from the center of a liver lesion by CT-guided needle aspiration. Infiltrating branched, slightly pleomorphic, nonmelanized, hyaline septate hyphae were detected in areas of tissue destruction.
The specimen was homogenized under sterile conditions and was cultured for fungal growth on Sabouraud glucose agar (SGA) (Merck, Darmstadt, Germany), malt extract agar (MEA) (Oxoid, Wesel, Germany), and Sabouraud glucose broth (10 g of peptone/liter and 40 g of glucose/liter, pH 5.6) and was incubated at 37 and 26°C. More than 10 CFU of a hyphomycete was obtained on all SGA and MEA plates incubated at 26°C; no growth was obtained at 37°C. Subcultures of the Sabouraud glucose broth were performed on SGA and MEA and were incubated at 37 and 26°C. The isolate grew moderately rapidly at 26°C, forming a procumbent to flocculent mycelium with a powdery surface, which was white at first and later was pale yellow. The fungus was tentatively identified as Beauveria bassiana. The germination rate of conidia on 2% glucose-1% peptone-0.2% yeast extract-1.5% agar at 35°C was 3% after 24 h and was 6.5% after 72 h. In contrast, at this temperature no germination was observed with a control B. bassiana strain (B. bassiana reference number 252; Agricultural Research Station Collection of Entomopathogenic Fungi [ARSEF], U.S. Department of Agriculture, Ithaca, N.Y.). Neither the clinical isolate nor the control strain B. bassiana ARSEF No. 252 grew at 37°C. Conidiogenous cells of the clinical isolate were aggregated in moderately dense clusters alongside 1.5- to 3.0-μm wide hyphae and were subspherical to ampulliform with a peg-like, narrow, denticulate rachides that elongated in a zigzag manner. Conidia were smooth-walled, single, spherical to subspherical, and 2 to 3 μm in length (Fig. 3). The clinical isolate grew as an extremely floccose and velvety colony producing a low number of conidia, which is atypical for B. bassiana. Approximately 1 × 106 to 5 × 106 conidia could be washed off per culture plate (9 cm diameter), in contrast to 1 × 108 to 5 × 108 conidia per plate for the reference strain ARSEF No. 252. On the basis of the snowball-like appearance of conidiogenous cells and conidia, the strain was attributed to the genus Beauveria, close to the generic type species, B. bassiana, and to its relative, Beauveria brongniartii. The conidiogenous clusters typical for B. bassiana were less prominent in the clinical isolate than in the reference strain. The clinical isolate was deposited in the culture collection of the Centraalbureau voor Schimmelcultures (CBS; Utrecht, The Netherlands) as CBS 100544.
FIG. 3.
EM scan of conidiogenous cells of Beauveria sp. strain CBS 100544. Magnification, ×3,000. Bar, 10 μm.
The identity of the clinical fungal isolate (CBS 100544) was further studied by analyzing the nucleotide sequences of the internal transcribed spacer regions (ITS1 and ITS2) of the nuclear ribosomal DNA. These sequences evolve relatively quickly and therefore are attractive chronometers for determining the relationship of genotypically closely related species. Extraction of total DNA, PCR, and direct sequencing were done as described by White et al. (29). Sequencing data of CBS 100544 (EMBL accession numbers AJ457169 [ITS1] and AJ457170 [ITS2]) were compared to sequences from Beauveria spp. obtained by Shih et al. (24) as well as sequences obtained from the EMBL database. In addition, the ITS regions of B. brongniartii CBS 410.34 and CBS 112.42 were sequenced and analyzed (27) (EMBL accession numbers for CBS 410.34 are AJ457167 [ITS1] AJ457168 [ITS2] and for CBS 112.42 are AJ457165 [ITS1] and AJ457166 [ITS2]). Alignment of 36 strains was performed with the BioNumerics package (Applied Maths, Kortrijk, Belgium), and distance trees were generated with Treecon (28) (data not shown). Strain CBS 100544 was found to be identical, except for a single mutation in the ITS2 sequence, to three strains deposited in GenBank as Beauveria tenella: U18962, U35287, and Z54107. B. tenella is a synonym of B. brongniartii (3).
To assess the virulence of the clinical strain (CBS 100544) in its natural host, insects, bioassays using larvae of the Colorado potato beetle (Leptinotarsa decemlineata) were conducted on potato leaf disks in a method similar to that described previously (5, 14). Briefly, the surface of the leaf disks (diameter, 8 mm), laying on water agar, was treated with 10 μl of 106 conidia ml−1 in a first experiment and 5 × 106 conidia ml−1 in 0.1% Tween 80 in a second experiment. Thirty-one instar larvae of L. decemlineata were put on the treated leaves for 2 days at 25°C (16 h light, 8 h dark each day) and then were transferred to untreated potato leaves. Control larvae were fed with leaf disks treated with 0.1% Tween 80 only. Larvae were observed for 10 days. In a control assay, the highly insect-virulent culture collection strain B. bassiana ARSEF No. 252 was used as reference.
The bioassays showed that the clinical isolate was extremely virulent, with a rapid killing efficacy compared to that of the reference strain. At 106 conidia ml−1, the mortality for the larvae was 13% after 3 days and was 80% after 7 days. The corresponding data at 5 × 106 were 50 and 97%, respectively. The mortality of the reference strain B. bassiana ARSEF No. 252 at 106 conidia ml−1 was 0 and 60% after 3 and 7 days, respectively. In all bioassays the mortality of the control larvae was less than 3%.
The susceptibility of the strain to itraconazole was determined in a microdilution assay as described by Seibold and Werner (23). Briefly, a serial twofold dilution of itraconazole in RPMI broth supplemented with 2% glucose was inoculated with 104 conidia per ml. After 72 h the MIC was determined spectrophotometrically at 0.031 μg/ml.
Starting on the day of admission, the patient was treated with steroids (initially, 75 mg of prednisone/day). After 28 days the dose was reduced to 7.5 mg/day. Clinically, the patient improved slightly during the first week. Following microbiological and pathological findings, antifungal therapy was initiated with 200 mg of itraconazole orally twice daily 1 week after admission. Drug monitoring was performed to ensure a satisfactory plasma level of itraconazole (0.25 to 1.6 μg/ml) (11). Three weeks after the start of therapy the patient recovered. Seven weeks later no fungal lesions could be detected by CT scan and ultrasound. The pulmonary function test values had returned to normal. Consequently, the antifungal therapy was stopped after 3 months.
Recently we observed an increasing frequency and an enlarging spectrum of opportunistic, systemic mycosis in immunocompromised patients. So far, about 400 of the 73,000 accepted fungal species have been reported to cause human mycoses (4). Few species represent obligatory pathogens and are exclusively found in mammals. A considerable amount of fungi are well adapted to a nonhuman environmental niche but are able to cause mycoses when they are coincidentally implemented in the human host. These fungi apparently possess effective vitality factors that enable them to survive in host tissue. Invasive disseminated systemic infections may result when the innate cellular immune response is impaired under conditions such as steroid treatment, prolonged granulocytopenia following chemotherapy, hematological malignancies, or bone marrow transplantation.
As our patient showed signs of alveolitis, confirmed by lung function tests and a histopathology of lung specimen and manifested lever and spleen lesions caused by Beauveria sp. at the same time, we speculate that there is an airborne route of infection. The respiratory symptoms of our patient resembled the clinical manifestations of type III and type IV hypersensitivity reactions observed in the lungs of rats and mice (farmers' lung-like or extrinsic allergic alveolitis-like lesions) that were experimentally exposed to B. bassiana conidia (25). Although the source of infection could not be definitely identified, the patient reported extensive walks through forests and fields. The fact that histopathology of liver samples revealed invasive fungal mycelia within damaged liver tissue seems to refute the claim that the isolate might be a laboratory or specimen collection contaminant that overgrew the real pathogen.
The identification of the fungus at hand is problematic. The macro- and micromorphological data for the fungus suggest a degenerate strain of B. bassiana or B. brongniartii. The loose clusters of conidiogenous cells and the ellipsoidal conidia favor attribution to B. brongniartii. The high virulence against larvae of L. decemlineata, however, suggests that this isolate belongs to B. bassiana (30). In our strain the ITS sequences were found to be highly homologous to several strains deposited in GenBank as B. tenella. This species was regarded as a synonym of B. brongniartii by de Hoog in 1971 (3). It should be noted, however, that the found molecular variability within Beauveria was larger than that anticipated on the basis of present taxonomy; a revision of the genus is necessary.
Infections with B. bassiana or B. brongniartii in cold-blooded vertebrate animals have been described in the literature (4, 7-10). However, no systemic infections in humans caused by fungi of the genus Beauveria are known (4, 12, 17, 19, 26). Freour et al. (6) attributed a case of pulmonary mycotic infection to B. bassiana, but this isolate was probably a misidentified strain of Acrodontium crateriforme (4). So far, the only documented human infection with B. bassiana is a case of keratitis reported by Sachs et al. (20). In 1932, Kuru reported on a human abscess caused by Isaria shiotae (16), which was listed as a synonym of B. bassiana by de Hoog (3). This synonymy was found to be incorrect on the basis of ITS sequencing data (4) and, thus, this record appears not to apply to B. bassiana. A fungal endocarditis published by Augustinsky et al. (1) was ascribed to Engyodontium album, a species that was originally included in the genus Beauveria. At the time of publication, however, the new genus, Engyodontium, had already been created for this species because of marked morphological differences from Beauveria (8). The molecular distance between the two genera was confirmed by de Hoog et al. (4).
Our report on the first case of a human deep tissue infection by a member of the soil-inhabiting entomopathogenic genus Beauveria sheds new light upon the potential hazards of these species. So far, B. bassiana was attributed biosafety level 1, while the remaining members of the genus are unclassified (4). B. bassiana and B. brongniartii are insect pathogens that are ubiquitous in nature, commercially distributed (e.g., B. bassiana is distributed as Conidia by Life Systems Technology S.A., Sante Fe de Bogota, Colombia, as Boverol by Fytovita, Ostrozská Lhota, Czech Republic, and as Ostrinil by Natural Plant Protection, Pau, France; B. brongniartii is distributed [only for Scarab control] as Beauveria Schweizer by Eric Schweizer Samen AG, Thun, Switzerland, as Engerlingspilz by Andermatt Biocontrol AG, Grossdietwil, Switzerland, and as Melocont Pilzgerste by Agrifutur srl, Alfianello [Brescia], Italy, distributed by Joh. Kwizda GmbH, Wien, Austria), and used as biological control agents against various insect pests world wide (13, 17, 26, 30). These bioinsecticides have been regarded as absolutely safe for humans (17, 30). The case report by Freour et al. (6), frequently cited in connection with possible hazards caused by bioinsecticides, is based on the misidentification mentioned above. In our bioassays using first-instar larvae of L. decemlineata we observed a high virulence characterizing the clinical strain. Strain-dependent variation of virulence of B. bassiana strains was also reported by Neuvéglise et al. (18), who found characteristic and unique 28S ribosomal DNA group I intron patterns in strains virulent to Hoplochelus marginalis (a sugar cane pest) compared to those of avirulent isolates. It is noteworthy that although germination of the clinical isolate was observed at higher temperatures (35°C) than those at which the reference strain germinated, the former isolate did not grow at 37°C in vitro. This seems to contradict systemic human infection. On the other hand, Alternaria, Ulocladium, and Engyodontium strains were identified as infecting agents from body sites such as the brain and subcutis in immunosuppressed patients. These fungi did not proliferate at 37°C in vitro but were able to grow in the tissue at body temperature (22).
Although a rare event, our data show that under immunosuppression systemic human infection with Beauveria is possible. Itraconazole seems to be an effective antimycotic drug against this fungus.
Acknowledgments
We thank A. Haas of the Pettenkofer Institute of the Ludwig-Maximilians University Munich for determining the itraconazole plasma levels, D. Harmsen for helping with the ITS sequence analysis, and M. Seibold of the Robert-Koch-Institute Berlin for performing microdilution susceptibility testing. R. C. Summerbell and B. K. Rubin are thanked for reviewing the manuscript.
REFERENCES
- 1.Augustinsky, J., P. Kammeyer, A. Husain, G. S. de Hoog, and C. R. Libertin. 1990. Engyodontium album endocarditis. J. Clin. Microbiol. 28:1479-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett, J. M. 1986. Classification of the myelodysplastic syndromes. Clin. Haematol. 15:909-923. [PubMed] [Google Scholar]
- 3.de Hoog, G. S. 1971. The genera Beauveria, Isaria, Tritirachium and Acrodontium gen. nov. Studies Mycol. 1:1-41. [Google Scholar]
- 4.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelculures/Universitat Rovira i Virgili, Utrecht/Reus, The Netherlands.
- 5.Dulmage, H. T. 1973. Assay and standardization of microbial insecticides. Ann. N.Y. Acad. Sci. 217:187-199. [DOI] [PubMed] [Google Scholar]
- 6.Freour, P., M. Lahourcade, and P. Chomy. 1966. [“Beauveria” fungi in human pathology. Apropos of a case of pulmonary localization]Les champignons “Beauveria”en pathologie humaine. A propos d'un cas a localisation pulmonaire. Presse Med. 74:2317-2320. [PubMed] [Google Scholar]
- 7.Fromtling, R. A., S. D. Kosanke, J. M. Jensen, and G. S. Bulmer. 1979. Fatal Beauveria bassiana infection in a captive American alligator. J. Am. Vet. Med. Assoc. 175:934-936. [PubMed] [Google Scholar]
- 8.Gams, W., G. S. de Hoog, and R. A. Samson. 1984. The hyphomycete genus Engyodontium, a link between Verticillium and Aphanocladium. Persoonia 12:135-147. [Google Scholar]
- 9.Georg, L. K. 1962. Mycotic pulmonary disease of captive giant tortoise due to Beauveria bassiana and Paecilomyces fumosoroseus. Sabouraudia 2:80-86. [Google Scholar]
- 10.Gonzalez-Cabo, J. F., S. J. Espejo, and M. C. Barcena-Asensio. 1995. Mycotic pulmonary disease by Beauveria bassiana in a captive tortoise. Mycoses 38:167-169. [DOI] [PubMed] [Google Scholar]
- 11.Haas, A., G. Anding, C. H. Grimm, and K. S. Boos. 1999. Determination of itraconazole and its metabolite hydroxy-itraconazole by direct injection of human plasma into a coupled column liquid chromatographic system with on-line solid-phase extraction. Mycoses 42:178. [Google Scholar]
- 12.Harrap, K. A. 1982. Assessment of the human and ecological hazards of microbial insecticides. Parasitology 84:269-296. [DOI] [PubMed] [Google Scholar]
- 13.Ignoffo, C. M. 1975. Entomopathogens as insecticides. Environ. Lett. 8:23-40. [DOI] [PubMed] [Google Scholar]
- 14.Ignoffo, C. M., C. Garcia, M. Kroha, A. Samsináková, and S. Kálalová. 1983. A leaf surface treatment bioassay for determining the activity of conidia of Beauveria bassiana against Leptinotarsa decemlineata. J. Invert. Pathol. 41:385-386. [Google Scholar]
- 15.Kouides, P. A., and J. M. Bennett. 1992. Morphology and classification of myelodysplastic syndromes. Hematol. Oncol. Clin. N. Am. 6:485-499. [PubMed] [Google Scholar]
- 16.Kuru, M. 1932. Über einen neuen pathogenen Schimmelpilz “Isaria shiotae, nov. spec.,” von einem pseudoxanthomatösen Herd des Menschen kultiviert. Jap. J. Med. Sci. 2:327-358. [Google Scholar]
- 17.Melnikova, E. A., and V. I. Murza. 1980. Investigation of the safety of industrial strains of microorganisms and microbial insecticides. J. Hyg. Epidemiol. Microbiol. Immunol. 24:425-431. [PubMed] [Google Scholar]
- 18.Neuvéglise, C., Y. Brygoo, and G. Riba. 1997. 28s rDNA group-I introns: a powerful tool for identifying strains of Beauveria brongniartii. Mol. Ecol. 6:373-381. [DOI] [PubMed] [Google Scholar]
- 19.Pore, R. S., N. L. Goodman, and H. W. Larsh. 1970. Pathogenic potential of fungal insecticides. Am. Rev. Respir. Dis. 101:627-628. [DOI] [PubMed] [Google Scholar]
- 20.Sachs, S. W., J. Baum, and C. Mies. 1985. Beauvaria bassiana keratitis. Br. J. Ophthalmol. 69:548-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaich, M., M. Ritter, T. Illmer, P. Lisske, C. Thiede, U. Schakel, B. Mohr, G. Ehninger, and A. Neubauer. 2001. Mutations in ras proto-oncogenes are associated with lower mdr1 gene expressionin adult acute myeloid leukaemia. Br. J. Haematol. 112:300-307. [DOI] [PubMed] [Google Scholar]
- 22.Seeliger, H. P. 1983. Infections of humans by opportunistic molds—their identification and nomenclature of their diseases. Mykosen 26:587-598. [PubMed] [Google Scholar]
- 23.Seibold, M., and E. Werner. 1995. Testing susceptibility of Candida species to fluconazole and itraconazole using the microdilution assay. Mycoses 38:443-448. [DOI] [PubMed] [Google Scholar]
- 24.Shih, H. L., C. P. Lin, R. F. Liou, and S. S. Tzean. 1995. Complete nucleotide sequence of Beauveria bassiana 5.8s rRNA coding gene and flanking internal transcribed spacers. DNA Seq. 5:381-383. [DOI] [PubMed] [Google Scholar]
- 25.Song, J. Y. 1989. Experimental study on farmer's lung-like lesions caused by Beauveria bassiana. Chung Hua Ping Li Hsueh Tsa Chih 18:111-114. [PubMed] [Google Scholar]
- 26.Strasser, H., T. M. Butt, and A. Vey. 2000. Are there any risks in using entomopathogenic fungi for pest control, with particular reference to the bioactive metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Sci. Technol. 10:717-735. [Google Scholar]
- 27.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]
- 29.White, T. I., T. Bruns, S. Lee, and T. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sni, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, Calif.
- 30.Zimmermann, G. 1998. Der entomopathogene Pilz Beauveria brongniartii (Sacc.) Petch und Erfahrungen bei seinem Einsatz zur biologischen Bekämpfung von Feld-und Waldmaikäfer. Nachrichtenbl. Deut. Pflanzenschutzd. 50:249-256. [Google Scholar]