Significance
G protein-coupled receptors (GPCRs), nature’s largest and most versatile class of signaling receptors, produce physiological responses by increasing the activity of heterotrimeric G proteins on cellular membranes. Many GPCRs initiate signaling from the plasma membrane and then produce a second increase of G protein activity on endosomes. The present understanding holds that this second signaling phase is communicated by the trafficking of receptors to endosomes and requires the presence of activated receptors in the endosome membrane. We provide evidence for a distinct cellular mechanism, in which endosomal G protein activity does not require internalized receptors. These results are fundamentally significant for understanding compartmentalized GPCR signaling and the emerging concept of location-biased therapeutics.
Keywords: GPCR, G protein, endosome, opioid
Abstract
Many GPCRs initiate a second phase of G protein-mediated signaling from endosomes. This inherently requires the GPCR to increase cognate G protein activity on the endosome surface. Gs-coupled GPCRs are thought to achieve this by internalizing and mediating a second round of allosteric coupling to G proteins on the endosome membrane. Here, we provide evidence that the μ-opioid receptor (MOR), a Gi-coupled GPCR, is able to increase endosomal G protein activity in a different way. Leveraging conformational biosensors, we show that MOR activation triggers a transient increase of active-state Gi/o on the plasma membrane that is followed by a prolonged increase on endosomes. Contrary to the Gs-coupled GPCR paradigm, however, we show that the MOR-induced increase of active-state Gi/o on endosomes requires neither internalization of MOR nor the presence of activated MOR in the endosome membrane. We propose a distinct and additional cellular mechanism of endosomal signaling by Gi/o that is communicated through trafficking of the activated G protein rather than its activating GPCR.
G protein-coupled receptors (GPCRs) comprise the largest family of signaling receptors and integral membrane proteins in animals. In response to a biologically relevant stimulus, GPCRs increase the activity of heterotrimeric GTP-binding proteins (G proteins) that act as transducers to relay biological information downstream of the activated receptor. Individual GPCRs differentially elevate the activity of homologous G protein subclasses defined by the α subunit (Gs/olf, Gi/o, Gq/11, and G12/13), and these differ, in turn, in their functional regulation of downstream effector systems in the cell (1, 2).
Physiological signaling through this biochemical framework is now recognized to be organized across multiple subcellular compartments (3, 4). In particular, GPCR signaling initiated from endosomes has been linked to distinct outcomes compared to signaling initiated from the plasma membrane (5, 6). Such compartmentalized signaling fundamentally requires an activated GPCR to trigger an increase in G protein activity at the appropriate membrane location. G proteins are activated through an allosteric coupling reaction that requires the membrane-tethered G protein to transiently contact its activating GPCR embedded in the membrane bilayer (7, 8). While this activation mechanism has been well described at the plasma membrane for all G protein subclasses, it is less clear how G protein activity is increased on the endosome membrane. The present understanding has been developed largely through the study of Gs-coupled GPCRs. Here, G protein activation on the endosome membrane requires activated receptors to internalize and mediate a second round of allosteric coupling to G proteins on the endosome limiting membrane. This model is supported by experiments demonstrating that the endosomal signal requires internalization of activated GPCRs (9–15) and that its strength depends on the degree or duration of receptor activation in the endosome membrane (16–21).
Through studying endosomal G protein activation mediated by the μ-opioid receptor (MOR), a Gi-coupled GPCR, we found evidence supporting a distinct and additional cellular mechanism for increasing endosomal G protein activity without requiring GPCR endocytosis.
Results
Endosomal Localization of Gi/o Increases Following Activation by MOR.
As a first step toward delineating the spatiotemporal organization of G protein activation by MOR, we examined the subcellular localization of fluorescently labeled Gi/o-subclass isoforms by live-cell confocal microscopy. mNeonGreen was inserted into the αb-αc loop of Gi1α, a tagging strategy previously demonstrated to preserve protein function (22). Gi1-mNeonGreen, an endosome marker (FYVE-mApple), and FLAG-tagged MOR (SSF-MOR) were coexpressed in HEK293 cells in which all endogenous Gi/o α-subunit genes (GNAI1, GNAI2, GNAI3, GNAO, GNAZ, and GNAT) were knocked out using CRISPR-Cas9 (Gi-KO cells) (23). Receptors present in the plasma membrane were labeled by preincubation with an anti-FLAG antibody conjugated to AlexaFluor 647 (M1-647). In cells not exposed to opioid agonist, Gi1-mNeonGreen localized primarily to the plasma membrane but was also observed on internal puncta, many of which were identified as endosomes by colocalization with FYVE-mApple (Fig. 1A). Upon application of the opioid full agonist DAMGO we observed a qualitative increase in Gi1-mNeonGreen fluorescence in SSF-MOR-containing endosomes (Fig. 1A). Similar results were observed using mNeonGreen-labeled versions of Gi2, Gi3, GoA, GoB, and Gz (SI Appendix, Fig. S1). To confirm this increase and quantify relocalization to endosomes across a large number of cells, we used a luciferase protein complementation assay (NanoBiT). In this assay, MOR or Gi1 were fused to LgBit (MOR-LgBiT or Gi1-LgBiT), and accumulation on endosomes was measured by protein complementation with an endofin-derived FYVE domain fused to SmBiT (FYVE-mApple-SmBiT) that localizes on the endosome limiting membrane. Application of DAMGO promoted MOR accumulation in endosomes (Fig. 1B) with the expected time course based on receptor internalization measured previously using other methods (24). DAMGO also promoted Gi1-LgBiT accumulation on endosomes (Fig. 1C), consistent with the microscopy results (Fig. 1A). Interestingly, this assay revealed that Gi1-LgBiT accumulation on endosomes occurs with distinct and generally faster kinetics than endosomal accumulation of MOR-LgBiT.
Fig. 1.
Gi1 relocalizes to endosomes upon activation by MOR. (A) Live imaging by confocal microscopy of Gi-KO HEK293A cells expressing Gi1-mNeonGreen, the endosome marker FYVE-mApple, and SSF-MOR labeled with ⍺-FLAG M1-647. Images depict frames before and after 20-min treatment with 10 µM DAMGO. Magenta arrows indicate endosomes positive for Gi1-mNeonGreen. Representative images from 3 independent experiments. (Scale bar, 10 µm.) (B) NanoBiT luminescence signal over time for cells expressing SSF-MOR-LgBiT + FYVE-mApple-SmBiT upon addition of 1 µM DAMGO. (C) NanoBiT luminescence signal over time for cells expressing Gi1-LgBiT + FYVE-mApple-SmBiT upon addition of 1 µM DAMGO. For NanoBiT assays, N = 3 independent experiments. Error bars represent ± SEM.
MOR and Gi/o Are Sequentially Activated on the Plasma Membrane Followed by Endosomes.
Having observed endosomal accumulation of Gi/o triggered by agonist-induced activation of MOR, we next tested for the presence of active-state MOR and Gi/o at each membrane location. To detect active-state MOR, we utilized a receptor-binding nanobody (Nb33) validated previously as an active-state biosensor by fluorescence microscopy (25) and adapted it for NanoBiT assays by fusion to SmBiT (Fig. 2A). We measured recruitment of Nb33-SmBiT to LgBiT tethered to the plasma membrane (LgBiT-CAAX) or to the endosome membrane (FYVE-LgBiT). In HEK293 cells expressing SSF-MOR, DAMGO promoted sequential phases of Nb33-SmBiT recruitment, first to the plasma membrane and then to endosomes (Fig. 2 A and B), with a similar dependence on agonist concentration at each location (Fig. 2C). To detect active-state Gi/o, we chose an engineered Gi/o effector domain derived from RAP1GAP that was shown previously to specifically detect active-state Gi/o in intact cells [Gi-ED, (26)], and we similarly adapted this protein for the NanoBiT assay. Gi-ED-SmBiT detected sequential DAMGO-induced phases of endogenous Gi/o activation on the plasma membrane followed by endosomes. Verifying specificity for Gi/o-subclass proteins, both signals were blocked by pretreatment of cells with pertussis toxin (Fig. 2 D and E, orange points). Each phase of DAMGO-induced Gi/o activation had a similar dependence on agonist concentration at each location (Fig. 2F) and the concentration dependence of Gi/o activation was left-shifted relative to MOR activation at both locations (Fig. 2 C and F), consistent with signal amplification. Further verifying the location-specificity of biosensor detection, no recruitment signal was observed using either sensor in control experiments in which LgBiT was targeted to the mitochondrial outer membrane (SI Appendix, Fig. S2).
Fig. 2.
Sequential phases of MOR and Gi/o activation on the plasma membrane and endosomes. (A) Nb33-SmBiT recruitment to the plasma membrane labeled with LgBiT-CAAX following addition of 1 µM DAMGO. (B) Nb33-SmBiT recruitment to endosomes labeled with FYVE-LgBiT following addition of 1 µM DAMGO. (C) Concentration–response curves for Nb33-SmBit recruitment to each LgBiT construct. EC50 values are noted in parentheses. (D) Gi-ED-SmBiT recruitment to the plasma membrane labeled with LgBiT-CAAX following addition of 1 µM DAMGO. (E) Gi-ED-SmBiT recruitment to endosomes labeled with FYVE-LgBiT following addition of 1 µM DAMGO. In panels D and E, orange curves represent cells pretreated with 100 ng/mL pertussis toxin (PTX). (F) Concentration–response curves for Gi-ED-SmBiT recruitment to each LgBiT construct. EC50 values are noted in parentheses. N = 3 independent experiments. Error bars represent ± SEM.
We additionally confirmed specificity of Gi-ED-SmBiT in detecting activation of endogenous Gi/o by demonstrating that DAMGO-induced activation of SSF-MOR failed to produce any detectable recruitment at either the plasma membrane or endosomes in Gi-KO cells (SI Appendix, Fig. S3, mock condition). Moreover, the recruitment signal was rescued at both locations by reexpression of Gi/o constructs used in Fig. 1 and SI Appendix, Fig. S1. Gi-ED-SmBiT detected activation at both the plasma membrane and endosomes in Gi-KO cells upon rescue with Gi1, Gi2, Gi3, GoA, or GoB (SI Appendix, Fig. S3). Together, these data indicate that the observed sequential elevation of active-state Gi/o on the plasma membrane followed by endosomes is specific and widespread across Gi/o isoforms.
We also noted that the increase of active-conformation Gi/o on endosomes, detected by Gi-ED-SmBiT, occurred more rapidly than the accumulation of active-conformation MOR detected by Nb33-SmBiT (Tmax = 23 min for Nb33-SmBiT, Tmax = 8 min for Gi-ED-SmBiT, Fig. 2 B and E). This observation, together with the generally faster kinetics of G protein trafficking to endosomes relative to MOR (Fig. 1), motivated us to investigate the ligand-dependent regulation of endosomal G protein activity in more detail.
Endosomal Gi/o Activity Does Not Require MOR Activation in the Endosome Membrane.
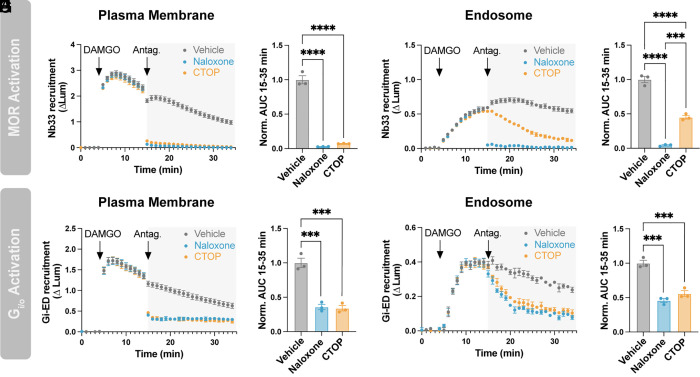
We began by examining the reversibility of MOR and Gi/o activation. We activated MOR by bath application of a moderate concentration of DAMGO (100 nM) and then reversed this by applying antagonist in excess (10 µM), using either naloxone, a membrane-permeant alkaloid antagonist, or CTOP, an antagonist peptide that is membrane-impermeant. Both antagonists fully reversed MOR activation at the plasma membrane, detected by Nb33-SmBiT, within 1 min (Fig. 3A), the temporal resolution limit of our assay. The effects of each antagonist on MOR inactivation were also indistinguishable in an area under the curve (AUC) analysis (Fig. 3B). Naloxone rapidly reversed the DAMGO-induced increase of active-state MOR in endosomes but CTOP, consistent with its membrane-impermeant nature, reversed the endosomal activation signal much more slowly (Fig. 3C). This resulted in a significant difference when assessed by AUC analysis (Fig. 3D). The slower time course of the CTOP-induced loss of active-state MOR in endosomes (t½ = 9.4 min) is consistent with the kinetics of MOR recycling (27), so we interpret the slow decrease in the Nb33-SmBiT signal produced by CTOP as a result of receptor exit from endosomes for the recycling pathway.
Fig. 3.
Endosomal Gi/o activation does not require active MOR in the endosome membrane. (A) Nb33-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) with addition of 100 nM DAMGO at 5 min followed by addition of 10 µM antagonist (naloxone or CTOP) at 15 min. (B) Quantification of data in panel A. AUC was calculated from data collected after the antagonist addition (15 to 35 min). (C) Nb33-SmBiT recruitment to endosomes (FYVE-LgBiT) with addition of 100 nM DAMGO at 5 min followed by addition of 10 µM antagonist at 15 min. (D) Quantification of data in panel C. AUC was calculated from data collected after the antagonist addition (15 to 35 min). (E) Gi-ED-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) with addition of 100 nM DAMGO at 5 min followed by addition of 10 µM antagonist at 15 min. (F) Quantification of data in panel E. AUC was calculated from data collected after the antagonist addition (15 to 35 min). (G) Gi-ED-SmBiT recruitment to endosomes (FYVE-LgBiT) with addition of 100 nM DAMGO at 5 min followed by addition of 10 µM antagonist at 15 min. (H) Quantification of data in panel G. AUC was calculated from data collected after the antagonist addition (15 to 35 min). One-way ANOVA with Tukey’s multiple comparisons, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. N = 3 independent experiments. Error bars represent ± SEM.
Both antagonists rapidly reversed the DAMGO-induced increase of active-state Gi/o at the plasma membrane, detected by Gi-ED-SmBiT (Fig. 3 E and F). Based on the present model of endosomal G protein activation, and the results described above, we expected naloxone to reverse the DAMGO-induced increase of active-state Gi/o on endosomes more quickly than CTOP. Surprisingly, this was not the case. Rather, naloxone reversed Gi/o activity on endosomes more slowly than on the plasma membrane (t½ = 2.8 min, Fig. 3G). Further, the rate of endosomal Gi/o activity reversal produced by naloxone was not significantly different from that produced by CTOP (t½ = 2.8 vs 3.3 min, respectively, SI Appendix, Fig. S4A). Naloxone and CTOP were also indistinguishable by AUC analysis (Fig. 3H). If endosomal Gi/o activation requires direct receptor–G protein coupling on endosomes, we predicted that the loss of active-state Gi/o on endosomes would occur no faster than loss of active-state MOR. Contrary to this, CTOP reversed Gi/o activity on endosomes more rapidly than it reversed endosomal MOR activity (t½ = 3.3 vs 9.4 min, respectively, SI Appendix, Fig. S4B). To summarize, we found that endosomal Gi/o activity 1) decreases slowly in response to a membrane-permeant antagonist, 2) decreases at the same rate irrespective of antagonist permeability, and 3) decreases more rapidly than endosomal MOR activity after CTOP application. Together, these observations suggested that the elevation of active-state Gi/o on endosomes may not require the presence of active-state MOR in the endosome membrane.
Local Inhibition of MOR Activity on Endosomes Does Not Affect Endosomal Gi/o Activation.
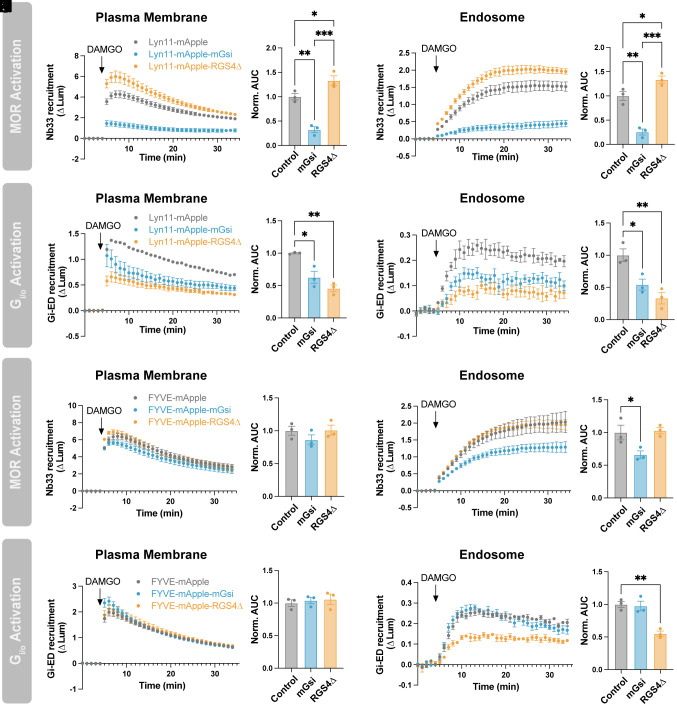
To further investigate this possibility, we sought to selectively inhibit MOR activity on endosomes without affecting plasma membrane receptors. To do this, we created an endosome-targeted mini-Gsi (FYVE-mApple-mGsi). mGsi is an engineered G protein fragment that engages and stabilizes active-state Gi-coupled receptors (28–30). Consistent with this, mini-G proteins occlude the binding of endogenous G proteins and have been shown to disrupt downstream signaling (31). As a separate approach to regulate Gi/o activity, we also targeted a truncated form of regulator of G protein signaling 4 (RGS4) to endosomes (FYVE-mApple-RGS4Δ) to accelerate G protein inactivation. In this construct, we omitted the first 33 residues of RGS4, which promote plasma membrane localization (32), and instead relied on the FYVE domain to retarget RGS4Δ to endosomal membranes. A similar approach has been used previously to inhibit Gi/o activity at discrete plasma membrane domains (33). As additional controls, we created plasma membrane-targeted constructs (Lyn11-mApple-mGsi and Lyn11-mApple-RGS4Δ).
We confirmed proper localization of these targeted constructs by confocal microscopy (SI Appendix, Fig. S5) and tested their effect on MOR and Gi/o activation using our NanoBiT approach. mGsi targeted to the plasma membrane (Lyn11-mApple-mGsi) strongly inhibited plasma membrane Nb33-SmBiT recruitment (Fig. 4 A and B), confirming that mGsi binds competitively to active-state MOR and occludes Nb33 binding. Lyn11-mApple-mGsi also inhibited Nb33-SmBiT recruitment to endosomes (Fig. 4 C and D), likely due to the ability of mini-G proteins to inhibit MOR internalization (31).
Fig. 4.
Local inhibition of MOR in the endosome membrane does not affect endosomal Gi/o activation. (A and B) Nb33-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or (C and D) to endosomes (FYVE-LgBiT) by 1 µM DAMGO in cells expressing Lyn11-mApple, Lyn11-mApple-mGsi, or Lyn11-mApple-RGS4Δ. (B and D) Quantification of AUC in A and C, respectively. (E and F) Gi-ED-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or (G and H) to endosomes (FYVE-LgBiT) by 1 µM DAMGO in cells expressing Lyn11-mApple, Lyn11-mApple-mGsi, or Lyn11-mApple-RGS4Δ. (F and H) Quantification of AUC in E and G, respectively. (I and J) Nb33-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or (K and L) to endosomes (FYVE-LgBiT) by 1 µM DAMGO in cells expressing of FYVE-mApple, FYVE-mApple-mGsi, or FYVE-mApple-RGS4Δ. (J and L) Quantification of AUC in I and K, respectively. (M and N) Gi-ED-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or (O and P) to endosomes (FYVE-LgBiT) by 1 µM DAMGO in cells expressing of FYVE-mApple, FYVE-mApple-mGsi, or FYVE-mApple-RGS4Δ. (N and P) Quantification of AUC in M and O, respectively. One-way ANOVA with Tukey’s multiple comparisons, *P < 0.05, **P < 0.01, and ***P < 0.001. N = 3 independent experiments. Error bars represent ± SEM.
Lyn11-mApple-mGsi suppressed Gi/o activity on the plasma membrane detected by Gi-ED-SmBiT (Fig. 4 E and F), consistent with the ability of mGsi to inhibit MOR-Gi/o coupling, and the degree of inhibition was comparable to that produced by Lyn11-mApple-RGS4Δ (Fig. 4 E and F). Both Lyn11-mApple-mGsi and Lyn11-mApple-RGS4Δ also decreased Gi/o activity on endosomes (Fig. 4 G and H). The effect of Lyn11-mApple-RGS4Δ suggests that Gi/o activation at the plasma membrane is a prerequisite for elevation of Gi/o activity on endosomes. However, because Lyn11-mApple-mGsi inhibited MOR internalization as well as coupling on the plasma membrane, we could not determine from these results if MOR-Gi/o coupling on the endosome membrane is required for endosomal Gi/o activation.
To specifically investigate the requirement for MOR-Gi/o coupling at this location, we next tested the effect of targeting mGsi to endosomes. FYVE-mApple-mGsi did not affect Nb33-SmBiT recruitment at the plasma membrane (Fig. 4 I and J) but it inhibited recruitment at endosomes (Fig. 4 K and L). This confirmed the ability of FYVE-mApple-mGsi to selectively engage endosomal MORs without affecting plasma membrane-localized MORs. Also consistent with proper targeting, neither FYVE-mApple-mGsi nor FYVE-mApple-RGS4Δ affected Gi/o activity on the plasma membrane detected by Gi-ED-SmBiT recruitment (Fig. 4 M and N). If MOR-Gi/o coupling on the endosomal membrane contributes to endosomal Gi/o activation, we expected FYVE-mApple-mGsi to decrease Gi/o activity on endosomes. Instead, FYVE-mApple-mGsi had no effect on endosomal Gi/o activity, despite its proper localization (Fig. 4 O and P and SI Appendix, Fig. S5), whereas FYVE-mApple-RGS4Δ inhibited Gi/o activity on endosomes as expected (Fig. 4 O and P). Together, these results are consistent with the hypothesis that endosomal Gi/o activation does not require coupling to active-state MOR in the endosome membrane.
Endosomal Activation of Gi/o Does Not Require MOR Internalization.
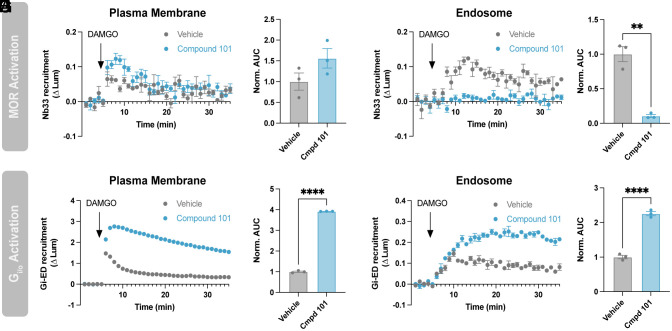
If this is the case, we predicted that the MOR-triggered production of active-conformation Gi/o on endosomes would also not be inhibited by a different manipulation that suppresses MOR internalization. We tested this using compound 101, a chemical inhibitor of GRK2/3 activity that suppresses MOR internalization by preventing phosphorylation of key residues in the receptor’s cytoplasmic tail (34). Verifying this endocytic inhibition, compound 101 suppressed the DAMGO-induced accumulation of active-state MOR in endosomes as detected by Nb33-SmBiT (Fig. 5 C and D). Importantly, in contrast, compound 101 had no detectable effect on the elevation of active-state Gi/o on endosomes as assessed by Gi-ED-SmBiT (Fig. 5 G and H).
Fig. 5.
Endosomal Gi/o activation does not require MOR internalization. (A and B) Nb33-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or (C and D) to endosomes (FYVE-LgBiT) by 1 µM DAMGO with and without 20-min pretreatment with 30 µM Compound 101. (B and D) Quantification of AUC in A and C, respectively. (E and F) Gi-ED-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or (G and H) to endosomes (FYVE-LgBiT) by 1 µM DAMGO with and without pretreatment with 30 µM Compound 101. (F and H) Quantification of AUC in E and G, respectively. (I and J) Nb33-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or K and L) to endosomes (FYVE-LgBiT) by 1 µM DAMGO, morphine, or PZM21. (J and L) Quantification of AUC in I and K, respectively. (M and N) Gi-ED-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or (O and P) to endosomes (FYVE-LgBiT) by 1 µM DAMGO, morphine, or PZM21. (N and P) Quantification of AUC in M and O, respectively. One-way ANOVA with Tukey’s multiple comparisons, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. N = 3 to 5 independent experiments. Error bars represent ± SEM.
To further test the role of MOR internalization, we compared the effects of three chemically distinct agonists that differ in relative endocytic activity: DAMGO, a full agonist that robustly internalizes MOR; morphine, a partial agonist that weakly internalizes MOR; and PZM21, a less efficacious and G protein-biased partial agonist with minimal endocytic activity (35, 36). The reduced agonist efficacies of morphine and PZM21 were reflected in differences in the level of Nb33-SmBiT recruitment that they produced on the plasma membrane at saturating concentration (Fig. 5 I and J). Also as expected, based on partial agonists stimulating MOR internalization poorly, morphine and PZM21 produced much less accumulation of active-state MOR in endosomes than DAMGO (Fig. 5 K and L). Each agonist elevated active-state Gi/o on the plasma membrane and endosomes to comparable levels (Fig. 5 M–P). Because PZM21 promoted endosomal Gi/o activation to a similar degree as DAMGO, despite it elevating MOR activity on endosomes relatively poorly, this suggested that MOR internalization may not be required for Gi/o activation on endosomes.
Endogenous MOR Triggers Endosomal Gi/o Activation Specifically From the Plasma Membrane.
The ability of PZM21 to produce maximal Gi/o activity at the plasma membrane indicated high receptor reserve in HEK293 cells expressing recombinant receptors. This could potentially make it difficult to detect a requirement for endosomal MOR activity in this system, especially if only a small number of endosomal receptors are needed. Therefore, we next sought to assess the role of MOR internalization in a cell model that expresses opioid receptors endogenously and at a lower level.
We focused on SH-SY5Y neuroblastoma cells because this neuronally derived cell line expresses endogenous MOR in much lower amount [~100 fmol/mg (37)] than typically achieved by recombinant expression in HEK293 cells [> 1 pmol/mg (38, 39)]. We found both Nb33-SmBiT and Gi-ED-SmBiT sufficiently sensitive to detect sequential phases of endogenous receptor and G protein activation, respectively, in this native cell model (Fig. 6, gray curves). DAMGO-induced recruitment of Nb33-SmBiT was considerably weaker than observed in HEK293 cells, consistent with a much lower level of endogenous receptor expression in SH-SY5Y cells compared to transfected HEK293 cells (Fig. 6A). However, Nb33-SmBiT recruitment was sufficiently strong to detect a clear signal corresponding to active-state MOR in endosomes after application of DAMGO (Fig. 6C). Gi-ED-SmBiT recruitment clearly detected Gi/o activation signals at both locations, consistent with amplification at the level of MOR-Gi/o coupling (Fig. 6 E and G). We also noted that the concentration dependence of Gi-ED-SmBiT recruitment, both to the plasma membrane and endosomes, was strongly right-shifted (1.5 to 2 log) relative to that measured in HEK293 cells, further supporting a considerably reduced level of receptor reserve for Gi/o activation at both locations in SH-SY5Y cells (SI Appendix, Fig. S6).
Fig. 6.
Surface MORs drive Gi/o activity on endosomes in SH-SY5Y cells. (A and B) Nb33-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or (C and D) to endosomes (FYVE-LgBiT) by 1 µM DAMGO with and without pretreatment with 30 µM compound 101. (B and D) Quantification of AUC in A and C, respectively. (E and F) Gi-ED-SmBiT recruitment to the plasma membrane (LgBiT-CAAX) or (G and H) to endosomes (FYVE-LgBiT) by 1 µM DAMGO with and without pretreatment with 30 µM compound 101. (F and H) Quantification of AUC in E and G, respectively. One-way ANOVA with Tukey’s multiple comparisons, **P < 0.01 and ****P < 0.0001. N = 3 independent experiments. Error bars represent ± SEM.
We next examined the effect of compound 101 in this cell system. Compound 101 had little effect on MOR activation at the plasma membrane, as measured by Nb33-SmBiT recruitment, but it almost completely blocked the recruitment signal at endosomes (Fig. 6 A–D, compare blue and gray curves). This is consistent with its effects in HEK293 cells, as well as the known ability of compound 101 to suppress MOR endocytosis in multiple systems (40). In addition, compound 101 increased and prolonged DAMGO-induced Gi/o activity on the plasma membrane, detected by Gi-ED-SmBiT. This is also consistent with suppressed desensitization and endocytosis of MOR (Fig. 6 E and F). We did not observe such an effect in HEK293 cells (Fig. 5), presumably due to high receptor reserve that saturates the endogenous G protein pool. Based on this, we expected SH-SY5Y cells to be a more sensitive system for probing the location at which MOR activity is required for endosomal Gi/o activity.
If endosomal Gi/o activity requires the presence of active MOR in endosomes, we anticipated compound 101 to reduce the endosomal Gi-ED-SmBiT recruitment signal in SH-SY5Y cells. However, this was not the case and, remarkably, we actually observed the opposite effect: compound 101 markedly enhanced the DAMGO-induced accumulation of active-state Gi/o on endosomes (Fig. 6 G and H). This observation is not consistent with endosomal Gi/o activation requiring the presence of active-state MOR in the endosome limiting membrane. Rather, in our opinion, it provides strong support for the existence of a distinct cellular mechanism for endosomal Gi/o activation triggered from the plasma membrane and not requiring coupling to MOR on endosomes.
To further test this interpretation, we compared the effects of the three MOR agonists in SH-SY5Y cells. Only DAMGO produced detectable accumulation of active-state MOR on the plasma membrane and endosomes (SI Appendix, Fig. S7 A–D), but all three agonists detectably increased the level of active-state Gi/o at both locations (SI Appendix, Fig. S7 E–H). The initial level of G protein activity produced on the plasma membrane by morphine and PZM21 was lower than that produced by DAMGO and only the DAMGO-induced increase rapidly desensitized (SI Appendix, Fig. S7 E and F). In contrast, all three agonists increased Gi/o activity on endosomes to a comparable level (SI Appendix, Fig. S7 G and H). These data provide additional support for the interpretation that MOR increases cognate G protein activity on endosomes irrespective of the presence of activated MOR in the endosome membrane, and they suggest that the process producing active-state Gi/o on endosomes is more saturable than on the plasma membrane.
Discussion
Over the past two decades, the cell biological understanding of GPCR signaling has evolved to include a second phase of G protein activity on endosomes. However, the present mechanistic understanding of the endosomal activation phase has been elucidated largely through the study of Gs-coupled GPCRs. Gi-coupled GPCRs are also thought to promote signaling from endosomes (25, 41–43), and recent advances in biosensor development have made it possible to spatiotemporally resolve both active-state Gi-coupled GPCRs (25) and their cognate G proteins (26, 44) in intact cells. Here, we apply these tools to delineate the subcellular landscape of cognate G protein activation triggered by MOR, a Gi-coupled GPCR prototype. By doing so, we provide evidence for a distinct and additional cellular mechanism of receptor-triggered elevation of cognate G protein activity on endosomes. Further, we demonstrate that this mechanism functions in cells with endogenous expression of both receptor and G protein.
A number of Gs-coupled GPCRs increase endosomal G protein activity by mediating a second round of allosteric G protein activation on the endosome membrane. This model is supported by multiple lines of evidence, including that endosomal G protein-mediated signaling requires receptors to internalize (9–15) and depends on the presence of activated receptors in the endosome membrane (16–21). There is particularly strong support for this model for receptors that form a stable complex containing receptor, Gs, and β-arrestin in endosomes (19, 45, 46). Here, we show that MOR triggers an elevation of active-state Gi/o-subclass G proteins on the endosome membrane, but that neither MOR internalization nor the presence of activated MOR in endosomes is required. This is not compatible with the current understanding and suggests the operation of a fundamentally different cellular mechanism for elevating endosomal G protein activity by a Gi-coupled GPCR. In this second mechanism, we propose that allosteric coupling on the plasma membrane is sufficient to drive an elevation of G protein activity on endosomes without requiring additional allosteric coupling on the endosome. Essentially, the proposed model utilizes trafficking of the activated G protein, rather than its activating GPCR, to communicate ligand-dependent activation to endosomes (Fig. 7).
Fig. 7.
Two distinct models of endosomal G protein activation by GPCRs. In model A endosomal G protein activity is increased by trafficking of the activated receptor (R*) to endosomes followed by allosteric coupling (~) on the endosome membrane to generate active-conformation G protein (G*). This model is supported by experimental evidence from Gs-coupled GPCRs. In model B supported by the present study, active-conformation G protein (G*) arrives on the endosome membrane following allosteric coupling (~) with active receptor (R*) on the plasma membrane. These two models differ fundamentally according to 1) whether information is “carried” to endosomes by the GPCR or G protein and 2) the location where allosteric coupling is required.
We believe that our proposed model is consistent with all of the present observations, but acknowledge that we cannot fully exclude some role of Gi/o coupling to activated MOR on endosomes. Indeed, we do observe the presence of active-state MOR in the endosome membrane, as reported previously (25), and we now demonstrate this with endogenously expressed receptors. Therefore, it is conceivable that endosomal receptor activation influences Gi/o activity on endosomes to some degree that we could not detect with our experimental manipulations. Nevertheless, the present results provide several lines of evidence suggesting that endosomal MOR activation is not strictly required and thus support the existence of a second cellular mechanism of endosomal G protein activation.
As our study is limited to MOR, it remains to be determined whether the present observations will generalize across other Gi-coupled GPCRs or more broadly to other receptor and G protein subclasses. In the latter regard, we note that Gq-coupled GPCRs increase G protein activity on endosomes by a mechanism that is dependent on both receptor internalization and the presence of activated receptors in the endosome membrane, consistent with the Gs-coupled GPCR paradigm. Interestingly, a small amount of endosomal Gq activity remained when receptor internalization was suppressed, suggesting that this dependence may not be absolute (47). The present results provide explicit evidence for endosomal G protein activation not detectably dependent either on GPCR internalization or activation in endosomes.
Our results raise several additional questions for future investigation. Foremost among these, what is the mechanistic basis for active-state Gi/o accumulation on endosomes? Heterotrimeric G proteins are well known to dynamically redistribute in cells (48–50), but it remains unknown whether Gi/o transits directly to endosomes from the plasma membrane or arrives indirectly from a different subcellular location. It is also unclear how G protein activity is sustained on endosomes in the absence of local activation by a GPCR. Our antagonist reversal experiments indicate that the active-state lifetime of Gi/o is longer on endosomes than on the plasma membrane, as reversal of the Gi/o activation signal occurred more slowly on endosomes than the plasma membrane after application of either antagonist (Fig. 3 E and G). We anticipate that this difference likely involves location-specific differences in RGS proteins that accelerate Gi/o inactivation. However, the presently estimated active-state lifetime on endosomes exceeds that measured using purified G proteins in the absence of any RGS activity (51). This suggests the existence of additional differences in the cell biology of G proteins on endomembranes that enables the local concentration of active-state G proteins to be elevated for an extended time period.
One possibility is that endosomal Gi/o activity is prolonged as a consequence of rapid and iterative trafficking of Gi/o through endomembranes. This is plausible in light of the rapid kinetics of active-state Gi/o accumulation on endosomes that we demonstrate and the apparent saturability of this process. Another possibility could be that the local biochemical environment of endosomes extends the active-state lifetime of individual Gi/o α-subunits beyond that presently known from in vitro studies, or that MOR activation in the plasma membrane triggers a downstream signaling pathway that results in Gi/o reactivation. Clearly, an important direction for future investigation is to elucidate the biochemical basis for the presently established cell biological observations.
A second, broader question regards the functional consequences of MOR-mediated Gi/o activity on endosomes. Previous studies indicate that endocytic inhibitors suppress cellular cAMP regulation by Gi-coupled GPCRs, including opioid receptors, implying the presence of Gi/o-sensitive adenylyl cyclase(s) on the endosome membrane (25, 43). Gi/o-subclass G proteins can also regulate many other effectors, in addition to adenylyl cyclase, reviewed in (52). We anticipate that identifying specific Gi/o-sensitive effectors on endosomes will contribute to a more comprehensive understanding of GPCR signaling selectivity.
In closing, we propose a second cellular mechanism mediating endosomal G protein activation triggered by Gi-coupled GPCRs. As Gi-coupled GPCRs comprise the largest GPCR subfamily in mammals (26, 53), we anticipate that these findings will prove broadly relevant to understanding GPCR physiology and help to inform the future development of location-biased therapeutics.
Materials and Methods
Cell Culture.
HEK293 (ATCC CRL-1573) and Gi-KO HEK239A cells (23) were cultured in DMEM (Gibco 1196511) supplemented with 10% FBS (UCSF Cell Culture Facility). Gi-KO HEK293A were grown and plated on dishes coated with collagen (Corning 354236). SH-SY5Y cells were cultured in DMEM:F12 (Gibco 11320082) supplemented with 10% FBS.
Drugs.
Chemicals were purchased from commercial sources as follows: DAMGO (Sigma-Aldrich, E7384), morphine sulfate (Sigma-Aldrich, 1448005), naloxone (Tocris, 05-991-00), CTOP (Tocris, 1578), compound 101 (HelloBio, H2840), and pertussis toxin (Sigma-Aldrich, P7208). PZM21 was generously provided by Aashish Manglik (UCSF).
cDNA Constructs.
Constructs for pCAGGS-SE-SSF-MOR (25), pcDNA3-SSF-MOR-LgBiT (36), LgBiT-CAAX (54), and FYVE-LgBiT (54) were previously published. DNA encoding human Gαi/o/z subunits with the internal linker SGGGGTRSGTGGGGS containing restriction sites for KpnI and MluI were synthesized by Twist Bio and cloned into pCAGGS-SE at the KpnI site (site destroyed) by In Fusion cloning (Takara 638946). The insertion sites for each subtype can be found in SI Appendix, Table S1. DNA encoding mNeonGreen (Twist Bio) or LgBiT (amplified from LgBiT-CAAX) was further subcloned at the KpnI and MluI sites by In Fusion to create pCAGGS-SE-Gα-mNeonGreen constructs and pCAGGS-SE-Gi1-LgBiT, respectively. Nb33-SmBiT and Gi-ED-SmBiT were formed by substituting Nb33 or Gi-ED for miniGs in pcDNA3.1-miniGs-SmBiT (15) at BamHI/HindIII sites. Nb33 was amplified from Nb33-EGFP (25) while Gi-ED was synthesized by Twist Bio. Gi-ED is the first 442 amino acids of human RAP1GAP1 with residues S437, S439, and S441 mutated to alanine as described in (26). Mitochondria-target LgBiT was constructed by fusing the first 35 amino acids of human Tom20 plus a flexible linker upstream of LgBiT (synthesized by Twist Bio). FYVE-mApple-SmBiT was constructed by inserting SmBiT into pmApple-N1 at the C-terminus of mApple using the Q5 site-directed mutagenesis kit (NEB M0492 and M0054) and then inserting the FYVE domain from FYVE-LgBiT at the N-terminus of mApple. pcDNA3-FYVE-mApple was formed by amplifying FYVE-mApple from FYVE-mApple-SmBiT and moving to pcDNA3 at the BamHI site. pcDNA3-FYVE-mApple-mGsi was made by amplifying mGsi from NES-Venus-mGsi (29) and inserting into pcDNA3-FYVE-mApple at the BamHI site with the linker GGGGSGSGS between mApple and mGsi. pcDNA3-FYVE-mApple-RGS4Δ was created by substituting mGsi for DNA encoding rat RGS4 lacking residues 1-33 (Twist Bio). pcDNA3-Lyn11-mApple-mGsi and -RGS4Δ were created using the same approach by insertion into pcDNA3-Lyn11-mApple, which contains the plasma membrane-targeting sequence MGCIKSKGKDS from Lyn11.
Live-Cell Confocal Microscopy.
Image series were acquired using a Nikon Ti inverted microscope controlled by NIS Elements HC v.5.21.03 (Nikon) and fitted with a CSU-22 spinning disk unit (Yokogawa), custom laser launch (100 mW at 405, 488, 561, and 640 nm, Coherent OBIS), Sutter emission filter wheel, Photometrics Evolve Delta EMCCD camera, and a temperature- and humidity-controlled chamber (Okolab).
Gi-KO HEK293A cells were plated on collagen-coated 35 mM glass-bottom dishes (Cellvis) and transfected 24 h prior to imaging with 1 µg SSF-MOR, 500 ng Gα-mNeonGreen, and 200 ng FYVE-mApple. To surface label SSF-MOR, cells were incubated with anti-FLAG M1 antibody (Millipore Sigma F3040) labeled with Alexa Fluor 647 (Thermo Fisher A20186) for 10 min at 37 °C and 5% CO2. Cells were imaged in imaging media (DMEM, no Phenol Red, 30 mM HEPES, pH 7.4) at 37 °C using an Apo TIRF ×100/1.49 numerical aperture oil objective (Nikon). Images were acquired at a 20 s frame rate over a 22-min period with 10 µM DAMGO added at 2 min. Images for figures were processed using FIJI software.
NanoBiT Luciferase Complementation Assays.
Cells grown in 6-well dishes were transfected with the appropriate constructs 24 h before the experiment using Lipofectamine 2000. For HEK293 and Gi-KO HEK293A cells, the amount transfected was as follows: 100 ng of CAAX-LgBiT, FYVE-LgBiT, or FYVE-mApple-SmBiT, 500 ng of Nb33-SmBiT or Gi-ED-SmBiT, 250 ng SSF-MOR, and 250 ng tagged Gα constructs where appropriate. For SH-SY5Y cells, the amount of LgBiT constructs was increased to 200 ng and SmBiT constructs to 1 µg. On the day of the assay, cells were lifted using TrypLE (Gibco 12604021) and centrifuged at 500×g for 3 min. Cells were resuspended in assay buffer (in mM: 135 NaCl, 5 KCl, 20 HEPES, 5 D-glucose, 1.8 CaCl2, and 0.4 MgCl2, pH 7.4) supplemented with 5 µM coelenterazine-H (Research Products International) to a concentration of 1 × 106. 100 µL of cells were plated into untreated white 96-well plates (Corning 3912). In experiments with PTX, cells were treated overnight with 100 ng/mL PTX prior to assay setup. In experiments with Compound 101 pretreatment, the assay buffer used to plate cells and drug solutions contained 30 µM Compound 101 or DMSO. Total pretreatment time for Compound101 was 20 min. Receptor ligands were diluted in assay buffer with 5 µM coelenterazine-H and 50 µL was added during the experiment.
Prior to the start of the assay, the plate was equilibrated for 10 min within a platereader warmed to 37 °C. Experiments in Fig. 2 were performed on a Synergy H4 platereader (Gen5 v.2.05, Biotek). All other experiments were performed on a Tecan Spark platereader. Luminescence values were acquired at 1-min intervals with an integration time of 500 ms. After a 5-min baseline, vehicle or drug was added and luminescence was read for an additional 30 min. For each well, luminescence was first normalized to the average luminescence during the baseline period. The average normalized luminescence for vehicle-treated wells was then subtracted from the drug-treated wells at each time point. Each condition was performed as 2 to 3 technical replicates, which were subsequently averaged. AUC was calculated as the sum of all averaged data points following the drug add.
Statistical Analysis.
All data points represent the mean ± SEM from at least three independent experiments. Statistical tests noted in the figure legends were performed using Prism (GraphPad, v10).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank A. Inoue and M. Bouvier for valuable discussions and A. Inoue for generously sharing the Gi-KO HEK293A cell line. We thank A. Manglik for sharing PZM21 and A. Inoue, N. Lambert, J. Li, B. Wysolmerski, and E. Blythe for sharing expression constructs. We thank all members of the von Zastrow laboratory for valuable discussion and feedback on the manuscript. Imaging experiments were carried out in the University of California San Francisco (UCSF) Center for Advanced Light Microscopy; we thank K. Herrington and S. Kim for their technical support and expertise. This study was supported by research grants from the NIH (DA012864, DA010711, and MH120212 to M.v.Z.). N. Fisher was supported by the UCSF Institutional Research and Academic Career Development Award program (GM081266).
Author contributions
N.M.F. and M.v.Z. designed research; N.M.F. performed research; N.M.F. analyzed data; and N.M.F. and M.v.Z. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Rosenbaum D. M., Rasmussen S. G. F., Kobilka B. K., The structure and function of G protein-coupled receptors. Nature 459, 356–363 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S., Anderson P. J., Rajagopal S., Lefkowitz R. J., Rockman H. A., G protein-coupled receptors: A century of research and discovery. Circ. Res. 135, 174–197 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eichel K., von Zastrow M., Subcellular organization of GPCR signaling. Trends Pharmacol. Sci. 39, 200–208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayser C., Melkes B., Derieux C., Bock A., Spatiotemporal GPCR signaling illuminated by genetically encoded fluorescent biosensors. Curr. Opin. Pharmacol. 71, 102384 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Tsvetanova N. G., Irannejad R., von Zastrow M., G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. J. Biol. Chem. 290, 6689–6696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flores-Espinoza E., Thomsen A. R. B., Beneath the surface: Endosomal GPCR signaling. Trends Biochem. Sci. 49, 520–531 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oldham W., Hamm H., Heterotrimeric G protein activation by G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 9, 60–71 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Weis W. I., Kobilka B. K., The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem. 87, 897–919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrandon S., et al. , Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 5, 734–742 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calebiro D., et al. , Persistent cAMP-signals triggered by internalized G protein-coupled receptors. PLoS Biol. 7, e1000172 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotowski S. J., Hopf F. W., Seif T., Bonci A., von Zastrow M., Endocytosis promotes rapid dopaminergic signaling. Neuron 71, 278–290 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irannejad R., et al. , Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyga S., et al. , Persistent cAMP signaling by internalized LH receptors in ovarian follicles. Endocrinology 157, 1613–1621 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Cajulao J. M. B., Hernandez E., von Zastrow M. E., Sanchez E. L., Glucagon receptor-mediated regulation of gluconeogenic gene transcription is endocytosis-dependent in primary hepatocytes. Mol. Biol. Cell 33, ar90 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blythe E. E., von Zastrow M., β-Arrestin-independent endosomal cAMP signaling by a polypeptide hormone GPCR. Nat. Chem. Biol. 20, 323–332 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinstein T. N., et al. , Retromer terminates the generation of cAMP by internalized PTH receptors. Nat. Chem. Biol. 7, 278–284 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinstein T. N., et al. , Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J. Biol. Chem. 288, 27849–27860 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gidon A., et al. , Endosomal GPCR signaling turned off by negative feedback actions of PKA and v-ATPase. Nat. Chem. Biol. 10, 707–709 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomsen A. R. B., et al. , GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell 166, 907–919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian X., et al. , The α-arrestin ARRDC3 regulates the endosomal residence time and intracellular signaling of the β2-adrenergic receptor. J. Biol. Chem. 291, 14510–14525 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varandas K. C., Irannejad R., von Zastrow M., Retromer endosome exit domains serve multiple trafficking destinations and regulate local G protein activation by GPCRs. Curr. Biol. 26, 3129–3142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson S. K., Gilman A. G., Gialpha and Gbeta subunits both define selectivity of G protein activation by alpha2-adrenergic receptors. Proc. Natl. Acad. Sci. U.S.A. 103, 212–217 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono Y., et al. , Generation of Gαi knock-out HEK293 cells illuminates Gαi-coupling diversity of GPCRs. Commun. Biol. 6, 112 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finn A. K., Whistler J. L., Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32, 829–839 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Stoeber M., et al. , A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 98, 963–976.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avet C., et al. , Effector membrane translocation biosensors reveal G protein and βarrestin coupling profiles of 100 therapeutically relevant GPCRs. eLife 11, e74101 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanowitz M., von Zastrow M., A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J. Biol. Chem. 278, 45978–45986 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Nehmé R., et al. , Mini-G proteins: Novel tools for studying GPCRs in their active conformation. PLoS ONE 12, e0175642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan Q., et al. , Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J. Biol. Chem. 293, 7466–7473 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoeber M., et al. , Agonist-selective recruitment of engineered protein probes and of GRK2 by opioid receptors in living cells. eLife 9, e54208 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manchanda Y., et al. , Engineered mini-G proteins block the internalization of cognate GPCRs and disrupt downstream intracellular signaling. Sci. Signal. 17, eabq7038 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Srinivasa S. P., Bernstein L. S., Blumer K. J., Linder M. E., Plasma membrane localization is required for RGS4 function in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 95, 5584–5589 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Neill P. R., Gautam N., Subcellular optogenetic inhibition of G proteins generates signaling gradients and cell migration. Mol. Biol. Cell 25, 2305–2314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miess E., et al. , Multisite phosphorylation is required for sustained interaction with GRKs and arrestins during rapid μ-opioid receptor desensitization. Sci. Signal. 11, eaas9609 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Manglik A., et al. , Structure-based discovery of opioid analgesics with reduced side effects. Nature 537, 185–190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J., Inoue A., Manglik A., von Zastrow M., Role of the GRK2/3 N-terminus in discriminating the endocytic effects of opioid agonist drugs. Mol. Pharmacol. 107, 100003 (2024), 10.1124/molpharm.124.000951. [DOI] [PubMed] [Google Scholar]
- 37.Kazmi S. M., Mishra R. K., Opioid receptors in human neuroblastoma SH-SY5Y cells: Evidence for distinct morphine (mu) and enkephalin (delta) binding sites. Biochem. Biophys. Res. Commun. 137, 813–820 (1986). [DOI] [PubMed] [Google Scholar]
- 38.Blake A. D., Bot G., Freeman J. C., Reisine T., Differential opioid agonist regulation of the mouse mu opioid receptor. J. Biol. Chem. 272, 782–790 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Tanowitz M., Hislop J. N., von Zastrow M., Alternative splicing determines the post-endocytic sorting fate of G protein-coupled receptors. J. Biol. Chem. 283, 35614–35621 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jullié D., et al. , A discrete presynaptic vesicle cycle for neuromodulator receptors. Neuron 105, 663–677.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullershausen F., et al. , Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 5, 428–434 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Jimenez-Vargas N. N., et al. , Endosomal signaling of delta opioid receptors is an endogenous mechanism and therapeutic target for relief from inflammatory pain. Proc. Natl. Acad. Sci. U.S.A. 117, 15281–15292 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eiger D. S., et al. , Location bias contributes to functionally selective responses of biased CXCR3 agonists. Nat. Commun. 13, 5846 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maziarz M., et al. , Revealing the activity of trimeric G proteins in live cells with a versatile biosensor design. Cell 182, 770–785.e16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wehbi V. L., et al. , Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gβγ complex. Proc. Natl. Acad. Sci. U.S.A. 110, 1530–1535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen A. H., et al. , Structure of an endosomal signaling GPCR-G protein-β-arrestin megacomplex. Nat. Struct. Mol. Biol. 26, 1123–1131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright S. C., et al. , BRET-based effector membrane translocation assay monitors GPCR-promoted and endocytosis-mediated Gq activation at early endosomes. Proc. Natl. Acad. Sci. U.S.A. 118, e2025846118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wedegaertner P. B., G protein trafficking. Subcell. Biochem. 63, 193–223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ajith Karunarathne W. K., O’Neill P. R., Martinez-Espinosa P. L., Kalyanaraman V., Gautam N., All G protein βγ complexes are capable of translocation on receptor activation. Biochem. Biophys. Res. Commun. 421, 605–611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jang W., Senarath K., Feinberg G., Lu S., Lambert N. A., Visualization of endogenous G proteins on endosomes and other organelles. Elife 13, RP97033 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linder M. E., Ewald D. A., Miller R. J., Gilman A. G., Purification and characterization of Go alpha and three types of Gi alpha after expression in Escherichia coli. J. Biol. Chem. 265, 8243–8251 (1990). [PubMed] [Google Scholar]
- 52.Syrovatkina V., Alegre K. O., Dey R., Huang X.-Y., Regulation, signaling, and physiological functions of G proteins. J. Mol. Biol. 428, 3850–3868 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue A., et al. , Illuminating G protein-coupling selectivity of GPCRs. Cell 177, 1933–1947.e25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Z., et al. , Structural basis of sphingosine-1-phosphate receptor 1 activation and biased agonism. Nat. Chem. Biol. 18, 281–288 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.