Abstract
OBJECTIVES
To test the feasibility of sleep hygiene education and longitudinal wrist actigraph sleep metrics measurement alone versus in combination with telehealth-delivered cognitive behavioral therapy for insomnia (teleCBT-I) for people with prostate cancer (PC) receiving androgen deprivation therapy (ADT).
SAMPLE & SETTING
45 men with PC receiving ADT were recruited from a midwestern comprehensive cancer center.
METHODS & VARIABLES
Participants were provided with wrist actigraphs, their individual sleep metrics data, and sleep hygiene education. Half the sample was randomized to a four-week teleCBT-I intervention. Outcomes were collected at baseline, one month, and two months. Exit interviews were conducted to glean participants’ feedback about the study.
RESULTS
Feasibility was demonstrated. Physical function, sleep efficiency, fatigue, and health-related quality of life improved for participants receiving teleCBT-I.
IMPLICATIONS FOR NURSING
Assessment of sleep disturbance, access to sleep hygiene education, and teleCBT-I may benefit people with PC receiving ADT.
Keywords: prostate cancer, sleep disturbance, insomnia, cognitive behavioral therapy, sleep hygiene
Cancer and cancer therapy are associated with a cluster of long-term symptoms. Cancer-related fatigue (CRF) is one of the most common and distressing long-term symptoms experienced by cancer survivors (Bower et al., 2014). CRF commonly occurs in conjunction with sleep disturbance, distress (e.g., anxiety, depression), and cancer-/cancer treatment–related cognitive impairment (CRCI) (Sanford et al., 2014; Xie et al., 2023). Cognitive domains typically affected by CRCI include short-term memory, attention and concentration, processing speed (i.e., reaction time), visuospatial ability, and executive function (Ahles et al., 2012). This cluster of symptoms shares common mechanisms (e.g., increased production of inflammatory cytokines and related biomarkers) and has a significant negative impact on health-related quality of life (HRQOL) (Ahles et al., 2012; Dodd et al., 2001; Myers, 2008; Shahinian et al., 2006; Wilding et al., 2019).
Androgen deprivation therapy (ADT) is known to be associated with sleep disturbance, CRF, CRCI, and distress (Green et al., 2002). ADT is used to treat about 50% of biologic males with prostate cancer (PC) to reduce the risk of recurrence or to slow the progression of advanced disease (Lonergan et al., 2022). ADT-related sleep disturbance is further exacerbated by hot flashes, night sweats, and nocturia (Gonzalez et al., 2018). As in other populations, fatigue and sleep disturbance may exacerbate CRCI for biologic males with PC (Ahles et al., 2012). Given the fact that PC is one of the most diagnosed malignancies in the United States and new cases diagnosed in 2024 were estimated to surpass 299,000 (American Cancer Society, 2024), assessment and effective interventions to address these symptoms are critical for providing appropriate cancer survivorship care. Circadian (24-hour) factors and sleep metrics, such as sleep timing, duration, and efficiency (total sleep time/time in bed), have a strong influence on fatigue (Fowler et al., 2023). Evidence indicates that mitigation of sleep disturbance may significantly affect CRF, CRCI, distress, and overall HRQOL (National Comprehensive Cancer Network, 2024).
Current guidelines by the American Academy of Sleep Medicine and the National Comprehensive Cancer Network state that cognitive behavioral therapy for insomnia (CBT-I) is preferred for first-line treatment of chronic insomnia compared to pharmacologic interventions (Edinger et al., 2021; National Comprehensive Cancer Network, 2025). Components of CBT-I interventions vary but typically include sleep hygiene education, stimulus control, and sleep restriction, along with strategy guidance to manage negative thoughts, emotions, and behaviors associated with poor sleep. Some interventions also include relaxation and/or mindfulness strategies. CBT-I interventions are delivered by mental health professionals and other healthcare providers, such as advanced practice nurses, who have received specialized training. Despite these guidelines, most communities have limited access to CBT-I because of a shortage of available clinicians (Garland et al., 2021). Lack of insurance coverage is also a barrier (Garland et al., 2021). Similar to CRCI-related research, the majority of research conducted to evaluate CBT-I for the management of fatigue and sleep disturbance in cancer survivors has been conducted only in women with breast cancer (Squires et al., 2022). In a systematic review of 22 randomized controlled trials investigating CBT-I for improvement of insomnia severity, 7 studies were conducted in women with breast cancer (Squires et al., 2022). Of the 15 studies conducted with mixed tumor types, only 4 reported the inclusion of men with PC. In these four studies, the numbers of people with PC were 1 of 38, 3 of 41, 12 of 111, and 33 of 150 (Casault et al., 2015; Espie et al., 2008; Garland et al., 2014; Mercier et al., 2018).
Given the impact of ADT on sleep disturbance, CRF, CRCI, and distress for men with PC, more research is needed to address the best way to assess and intervene for this symptom cluster. The current authors completed a single-arm pilot study to test the feasibility of remote assessment of objective and subjective cognitive function, sleep disturbance, and a telehealth-delivered CBT-I intervention (teleCBT-I) (Myers et al., 2023). Feasibility was demonstrated for recruitment (N = 15) and adherence (100%), and significant improvement in sleep quality was achieved (p < 0.001). Information gleaned from semistructured exit interviews indicated that the participants found the neurocognitive testing burdensome and would have preferred a more contemporary device than the traditional actigraphy used during the study, specifically one that would provide them with real-time data on their sleep.
Of note, objective assessment of CRF and CRCI has proven to be challenging. Most CRF studies have included only subjective fatigue measures (National Comprehensive Cancer Network, 2025). Validated and objective fatigue assessments have yet to be widely incorporated into healthcare settings. The lack of congruence between cancer survivors’ self-report of CRCI and objective neurocognitive test performance makes clinical assessment and determination of efficacy for interventional research difficult. Accurate, accessible, and acceptable objective measures for CRF and CRCI are needed for the prospective assessment of symptom burden and evaluation of interventional efficacy. Sensitive objective measures to predict CRF and CRCI related to sleep metrics would have great potential for assessing interventional effectiveness and clinical application in the cancer survivor population.
Validated noninvasive actigraphy-based algorithms are available to longitudinally predict fatigue and cognitive performance (Hursh et al., 2004). The Sleep Activity and Fatigue Task Effectiveness (SAFTE®) model algorithm was developed by the U.S. Department of Defense and is well validated as a predictor of sleep restriction on subjective fatigue ratings and objectively measured cognitive performance (Van Dongen, 2004). This model depicts the relationship among the sleep reservoir (defined as the sleep-dependent processes governing the capacity to perform cognitive work), the depletion of the sleep reservoir during waking hours and replenishment during sleep, the impact of circadian processes and sleep debt on sleep intensity, and the impact of sleep fragmentation on physical and cognitive performance effectiveness (e.g., reaction time/processing speed). The ReadiWatch™ is a wrist-worn actigraph measure, which has been found to have greater than 93% accuracy with laboratory polysomnography results (Russell et al., 2000). This device contains an accelerometer that tracks wrist movements and consolidates those data to accurately determine sleep–wake and sleep metrics (e.g., quantity, timing, efficiency) based on the SAFTE model (Morgenthaler et al., 2007). Circadian rhythms estimate fatigue and cognitive performance effectiveness based on sleep metrics and daily activity over time. This information is conveyed to the participant through a user-friendly application (app). The app provides real-time feedback, including an hour-by-hour ReadiScore between 0 and 100. The ReadiScore represents physical and cognitive effectiveness based on fatigue and cumulative sleep metrics from three days of consecutive wear time. ReadiScores lower than 70 indicate fatigue and decreased cognitive performance effectiveness (such as reaction time) consistent with a blood alcohol level of 0.08%. The app also provides educational content for the sleep hygiene elements of CBT-I. Higher scores indicate better physical and cognitive performance effectiveness and less fatigue.
The use of the app allows participants to interact with and modify their sleep behavior in response to real-time feedback from the ReadiWatch. Input from the ReadiWatch, as well as education about the sleep hygiene elements of CBT-I, has been effective in improving sleep metrics and fatigue (Chung et al., 2018; Fowler et al., 2023; Tipps et al., 2019) in populations of patients without cancer. Except for one study abstract reporting the successful feasibility of assessing sleep disturbance and CRF for patients with high-grade glioblastoma (Tipps et al., 2019), measures of fatigue and cognitive function and the impact of real-time feedback on sleep disturbance have not yet been studied in cancer survivors. Additional research is needed to determine whether ReadiWatch sleep metrics data correlate with subjective reports of CRF and CRCI. Research is also needed to assess the impact of patient access to ReadiWatch sleep metrics data and sleep hygiene education modules on improving sleep disturbance for cancer survivors.
Purpose
The purpose of this randomized, two-arm pilot study was to test the feasibility of sleep hygiene education and ReadiWatch longitudinal wrist actigraph sleep metrics measurement alone (ReadiWatch alone group/group 1) versus in combination with teleCBT-I (teleCBT-I combination group/group 2) for people with PC receiving ADT. The study aims were as follows:
■ Aim 1: Achieve successful accrual (N = 40) and adherence (85%) for two months of actigraph wear time (both groups) and participation in the four-week teleCBT-I intervention (teleCBT-I combination group only).
■ Aim 2: Examine and compare between-group pre-/postintervention changes in ReadiScores (SAFTE model objective estimate of fatigue and cognitive performance effectiveness), sleep metrics (quantity and efficiency), and patient-reported outcomes (PROs) for sleep disturbance, CRF, CRCI, distress, and HRQOL.
■ Aim 3: Explore the correlation of changes in ReadiScores with changes in PROs.
Methods
Conceptual Models
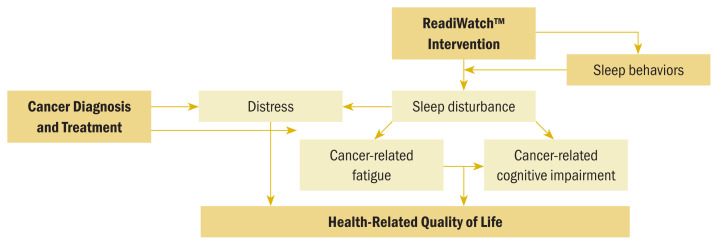
The conceptual model providing the framework for this study (see Figure 1) was developed based on extensive literature review, the SAFTE model algorithm for objective estimations of fatigue and cognitive performance effectiveness (Hursh et al., 2004), and the authors’ previous observational and interventional work related to sleep and fatigue (Fowler et al., 2023; Klinefelter et al., 2023; Myers et al., 2023). The conceptual model depicts the relationships among a diagnosis of cancer and related treatment and distress, sleep disturbance, CRF, and CRCI. These sequalae have a negative impact on HRQOL. The model also depicts the authors’ hypothesis that providing patients with sleep hygiene education and access to sleep metrics data, with or without CBT-I, will result in a positive change in sleep behaviors, ultimately leading to a reduction in sleep disturbance, CRF, and CRCI, and improving HRQOL. The teleCBT-I intervention was based on the Behavioral Model of Insomnia developed by Spielman et al. (1987) and is designed to address the predisposing, precipitating, and perpetuating factors associated with insomnia (Perlis et al., 2008; Spielman et al., 1987).
FIGURE 1.
Conceptual Framework
Patient Advocacy Feedback
Feedback on the study design was obtained from the University of Kansas Cancer Center’s Patient and Investigator Voices Organizing Together (PIVOT) program. PIVOT provides researchers with access to a panel of research advocates consisting of cancer survivors and caregivers. The study purpose, aims, and methodology were presented during a PIVOT Rapid Reactor Panel. This service is provided to facilitate the development of a feasible research design from the patient perspective. The PIVOT panel’s advice informed the selection of study instruments and was key to ensuring acceptability, minimizing participant burden, and reflecting the patient voice.
PRO Study Questionnaires
The study questionnaires were administered electronically via REDCap (Harris et al., 2009, 2019). Completion of the study questionnaires was estimated to take about 20 minutes. Demographic information was collected at baseline for age, race, ethnicity, years of education, relationship, and employment status. Other questionnaires are described in the following sections.
Insomnia Severity Index
The seven-item Insomnia Severity Index (ISI) is a well-validated (Cronbach’s alpha = 0.74) Likert-type scale ranging from 0 to 4 that is used to measure insomnia severity (0 = none, 4 = very), interference (0 = not at all, 4 = very much interfering), and related distress (0 = not at all, 4 = very much) (Bastien et al., 2001). Total scores range from 0 to 28, with higher scores indicating greater insomnia severity. Total scores lower than 8 indicate no clinically significant insomnia, scores between 8 and 14 indicate subthreshold insomnia, and scores of 15 or higher indicate clinical insomnia (scores of 15–21 = moderate and scores of 22–28 = severe). Patients with a total score of 8 or higher (subthreshold for insomnia) were eligible for the study.
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) is a well-validated instrument commonly used to measure sleep quality in cancer-related studies. A study conducted with 474 breast cancer survivors demonstrated strong internal consistency (Cronbach’s alpha = 0.7), test–retest reliability (intraclass correlation coefficient = 0.76), and strong correlation with subjective sleep complaints (r ≥ 0.6) (Hunter & Liao, 1995). The PSQI includes seven component scores calculated from 18 items and yields a global PSQI score wherein scores greater than 5 are considered poor sleep quality.
PROs Measurement Information System–29
The PROs Measurement Information System–29 (PROMIS-29) is a National Institutes of Health–supported suite of instruments designed to measure PROs across multiple domains (Cella et al., 2010). The PROMIS-29 includes 29 Likert-type items, with multiple-item scales across seven domains (depression, anxiety, physical function, pain interference, fatigue, sleep disturbance, and ability to participate in social roles and activities). Raw scores for all PROMIS-29 instruments are rescaled into T scores with a mean of 50 and an SD of 10. The PROMIS 29+2 Profile v2.1 (PROMIS Preference) includes two additional cognitive function abilities items used to calculate a preference score, indicating an overall summary of HRQOL on a common metric (0 = as bad as dead, 1 = perfect or ideal health). PROMIS-29 scoring is designed so that higher scores indicate higher ratings for the domain being measured (i.e., higher physical function scores indicate better physical function and higher fatigue scores indicate greater fatigue).
PROMIS Short Form–Cognitive Function
The eight-item PROMIS Short Form–Cognitive Function was derived from the Functional Assessment of Cancer Therapy–Cognition item bank and specifically designed to elicit self-report of cognitive function from cancer survivors. Version 2 is the most current version, and higher scores reflect better cognitive function. Items are ranked on a Likert-type scale ranging from 1 (very often/several times a day) to 5 (never). Test–retest analyses from the current authors’ pilot research indicated high reliability ratings (Cronbach’s alpha = 0.946) (Myers et al., 2022).
Sleep log
An abbreviated three-item electronic sleep log included the following items: (a) time of lights-out to go to sleep, (b) narrative description of anything unusual interfering with sleep, and (c) a Likert-type ranking (scale ranging from 0 [very good] to 3 [very bad]) of sleep quality.
With the exception of the demographic questionnaire, all questionnaires were administered at baseline and at the completion of study participation (two months). The ISI and PSQI were also administered at one month. The abbreviated electronic sleep log was administered daily to supplement the sleep metrics data obtained from the ReadiWatch. At the conclusion of the study, all participants were invited to take part in an exit interview and encouraged to share feedback about their experience with study participation. Semistructured interview questions were employed to solicit feedback about aspects of the study that participants found helpful or burdensome and any recommendations for modification. Participants were asked about their experience downloading the ReadiOne app, wearing the ReadiWatch actigraph, and accessing the sleep hygiene education modules and their individual sleep history/trends, as well as their experience with the daily sleep log, what they learned about their sleep patterns, and any strategies they planned to employ in the future after participation in the study. The exit interviews were audio recorded. The audio recordings were de-identified and stored on a Health Insurance Portability and Accountability Act (HIPAA)–secure, password-protected research drive. Only members of the study team had access to the audio recordings.
Sleep Metrics
Data were collected daily for total sleep quantity, awakenings per hour, minutes awake after sleep onset, sleep latency (i.e., time to fall asleep in minutes), and sleep efficiency (i.e., time asleep/time in bed). Data were collected daily for ReadiScores. Mean ReadiScores were calculated for seven days of consecutive wear time at baseline (T1/first seven days of wear time), one month (T2), and two months (T3).
Sample and Setting
Eligible participants included adults aged 18 years or older who were diagnosed with any stage PC and receiving ADT. Participants were required to be able to speak and read English and to score an 8 or higher on the ISI. Patients were excluded if they were diagnosed with Alzheimer disease or related dementias, other neurologic conditions involving impaired cognitive function (e.g., Parkinson disease, multiple sclerosis), sleep apnea without the use of or adherence to a continuous positive airway pressure device, or uncontrolled restless legs syndrome. Participation in another sleep-related interventional study was exclusionary. Participants using hypnotics or other sleep aids, such as melatonin, were not excluded.
The study was conducted at the University of Kansas Cancer Center in Kansas City, Missouri, the Masonic Cancer Alliance in Fairway, Kansas, and the University of South Carolina School of Medicine Greenville and Prisma Health Upstate following approvals by the institutional review boards of the study sites. The planned sample size of 40 (n = 20 participants in each group) allowed for 10% attrition with 80% power for a two-sided t test at alpha = 0.05 to detect a large between-group effect size (Cohen’s d = 0.85).
Recruitment and Informed Consent
Following institutional review board approval, the Curated Cancer Clinical Outcomes Database was used to identify potentially eligible patients receiving care at the University of Kansas Cancer Center. Study synopses and flyers were shared with members of the healthcare teams at all participating institutions. The study team received referrals from medical and radiation oncologists, urologists, advanced practice nurses, and other advanced practice providers. Additional recruitment strategies were presenting to local support groups, including study information on institutional social media platforms, and seeking referrals from external healthcare facilities serving the target population. During the informed consent discussion (conducted in person or via telephone or HIPAA-secure videoconferencing), the study team administered the five-item ISI to confirm eligibility (score of 8 or higher). The study team then assessed participants’ comfort level and previous experience with electronic questionnaires, wearable devices, smart devices, and app downloads through a series of screening questions. The study team also assessed participants’ available devices and internet access to determine their capacity for telehealth conferencing and the need for provision of study tablets. Study tablets were available for participants without access to a mobile smart device or a computer capable of downloading the ReadiOne app.
Intervention Procedures
The study schema for the ReadiWatch alone and the teleCBT-I combination groups is outlined in Figure 2. All participants were provided with a ReadiWatch actigraph (see Figure 3). All participants were instructed to wear the ReadiWatch 24/7 (except when bathing and/or swimming) for two months. After receiving the device, participants received instructions on how to use the ReadiWatch and the ReadiOne app to access their sleep metrics (e.g., sleep time, fragmentation, efficiency), ReadiScore (hour-by-hour predictions of fatigue and cognitive impairment), and sleep hygiene education modules. Technical support was provided via telephone, secure videoconferencing, or in person as needed to ensure participant access. The ReadiOne app provided 24-hour data, as well as 7- and 30-day trends for sleep quantity, patterns, and ReadiScores. App-provided sleep hygiene educational content included two modules on the importance of sleep and seven modules with instructions for how to get better sleep (see Figure 4). At one month, participants were randomized (1:1) to the ReadiWatch alone or the teleCBT-I combination group.
FIGURE 2.
Study Schema
teleCBT-I—telehealth-delivered cognitive behavioral therapy for insomnia
FIGURE 3.
ReadiWatch™
Note. Image courtesy of Fatigue Science. Used with permission.
FIGURE 4.
Sleep Hygiene Education Modules
During the exit interview, the principal investigator (J.S.M.) reviewed individual data with each ReadiWatch group participant and provided individualized recommendations for additional strategies to improve sleep. Additional content related to strategies to reduce or manage hot flashes and nocturia was provided as needed.
Participants randomized to the teleCBT-I combination group received a four-week teleCBT-I intervention in addition to the previously described components administered to participants in the ReadiWatch alone group. The teleCBT-I intervention was initiated after one month of access to ReadiWatch. As in the authors’ previous pilot study (Myers et al., 2023), the teleCBT-I intervention was delivered individually (1:1) by an advanced practice nurse certified as a CBT-I clinician who had completed a 19-hour CBT-I training workshop in addition to individual training and supervision by the director of the Sleep, Health, and Wellness Laboratory at the University of Kansas Medical Center and diplomate in behavioral sleep medicine.
The four-week teleCBT-I intervention was based on the Spielman Behavioral Model of Insomnia (Spielman et al., 1987) and a standard manual implemented in prior and ongoing clinical trials (R01AG058530) (Alshehri et al., 2021; Perlis et al., 2008; Siengsukon, Alshehri, et al., 2020; Siengsukon, Nelson, et al., 2020). A detailed outline of this four-week virtually delivered intervention has been published previously (Myers et al., 2023). In brief, a baseline assessment was conducted to assess bedtime routines, sleep environment, timing of fluid and food intake, and review of specific sleep disturbance issues. This assessment was followed by weekly goal setting related to individual prescribed sleep restrictions and stimulus control, as well as the incorporation of structured deep breathing, mindfulness, and progressive muscle relaxation strategies.
The teleCBT-I component of the intervention has been customized previously (Myers et al., 2023) to address issues that have a negative impact on sleep (e.g., hot flashes, nocturia, distress associated with the cancer diagnosis and treatment) and are common to biologic males with PC receiving ADT (Carroll et al., 2019; Wilding et al., 2019). To address these concerns, behavioral strategies to reduce hot flashes and nocturia were provided and further emphasis was placed on mindfulness and relaxation techniques known to reduce related patient-reported distress (Stefanopoulou et al., 2015).
The four weekly teleCBT-I sessions were conducted via HIPPA-secure videoconferencing and lasted about 60 minutes. The content included didactic and interactive information. Individualized goals were mutually set with the participant each week. The ReadiWatch sleep metrics data were reviewed by the principal investigator (J.S.M.) prior to each session and supplemented with the abbreviated electronic daily sleep log collected via REDCap (e.g., time of lights-out to sleep, description of unusual occurrences interfering with sleep, sleep quality rating). The final session included a discussion of participants’ intentions for continuation of the strategies that were implemented.
After completion of the study questionnaires (at baseline, one month, and two months), participant payments and postage-paid return of the ReadiWatch, charging cable, and study tablet (if provided) were provided. Payments for the completion of baseline and one-month questionnaires were $25 each. Payment for the completion of the two-month questionnaires and return of the study equipment was $50.
Data Access and Analyses
The ReadiWatch actigraphs and licenses for the ReadiOne app were obtained for the study from Fatigue Science. The research team had password-protected access to participants’ sleep metrics, ReadiWatch battery charge level, and app synchronization history throughout the course of the study. Data were extracted from the password-protected database by the principal investigator (J.S.M.) and de-identified for analyses.
Descriptive statistics (frequencies, percentages, means, and SDs) were used to summarize results for the feasibility measures (aim 1). The primary analysis for aim 2 was to estimate means (or medians) and 95% confidence intervals of the within-group changes and between-group differences of changes in all the outcomes. For missing data, last observation (baseline) carried forward was used for imputation for the intent-to-treat analysis. One-sample t tests or signed-rank tests (if normality was violated) were used to test the within-group changes, and two-sample t tests or Wilcoxon rank-sum tests were used to test the between-group differences from baseline for all variables at two months. No control for multiplicity was considered for secondary outcomes. Pearson correlation coefficients were used to explore the changes in ReadiScores with changes in the PROs (aim 3).
Expected outcomes for feasibility included the following: 95% recruitment of desired sample of 40, 85% adherence to ReadiWatch wear time and study questionnaire completion between baseline and two months for both groups, and 85% adherence to the four-week teleCBT-I intervention in group 2 (aim 1); both groups would demonstrate improvement in ReadiScores and PROs (aim 2); and ReadiScores would correlate inversely with CRF, CRCI, and distress (anxiety and depression) and positively with HRQOL (aim 3).
Audio-recorded data obtained during the exit interviews regarding participants’ perceptions of study participation were reviewed and discussed weekly by the research team throughout the study. The primary purpose for gathering this qualitative feedback was to continually inform the study team about participants’ level of satisfaction with study participation and to quickly identify and resolve challenges as they arose. Therefore, all data were reviewed and analyzed without regard to determination of saturation. These data were organized by group and used in an iterative manner to refine the study procedures. Group-specific data organization also was maintained to facilitate later supplementation of the within- and between-group comparisons planned for the quantitative analyses, if needed. Deductive qualitative content analysis was used to systematically organize and code the data (Elo & Kyngäs, 2008). The initial categories for organization of the exit interview data were determined a priori. This methodology was selected as the most pragmatic qualitative approach to broadly describe and condense participants’ responses in an iterative manner to inform the current study design as well as future research. The qualitative analyses were led by the principal investigator, and consensus for further categorization and coding was achieved during the weekly team meetings. Software was not required for these analyses.
Results
Quantitative Results
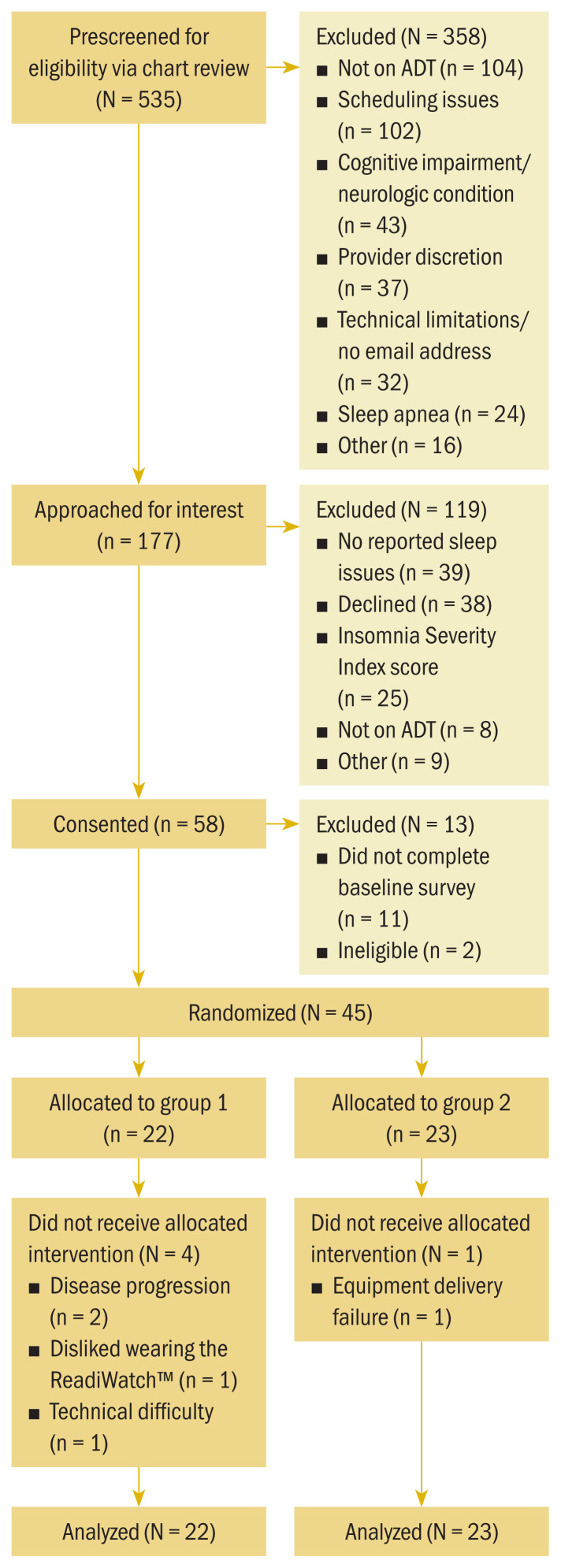
In total, 58 men consented to participate in the study (see Figure 5). Of those, 2 participants were deemed ineligible and 11 did not complete baseline assessment. Overall, 45 participants were randomized, with 22 participants to the ReadiWatch alone group (group 1) and 23 participants to the teleCBT-I combination group (group 2). Participants ranged in age from 47 to 87 years, with a mean age of 69.09 years (see Table 1). Participants primarily were White (n = 36), retired (n = 33), and married (n = 29), with a mean of 15.57 years of education. Mean time on ADT was 44.84 months. The majority of participants had received radiation therapy (n = 31), and 18 participants had received chemotherapy. Demographic characteristics were similar for both groups with one exception. Participants in group 1 had been receiving ADT longer than participants in group 2 (a mean of 62.77 months compared to a mean of 28 months, respectively, p = 0.038).
FIGURE 5.
CONSORT Flow Diagram for Sample
ADT—androgen deprivation therapy; CONSORT—Consolidated Standards of Reporting Trials
TABLE 1.
Participant Demographic Characteristics
| Characteristic | Total (N = 45) | Group 1 (N = 22) | Group 2 (N = 23) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| X̄ | Range | X̄ | Range | X̄ | Range | |
| Age (years) | 69.09 | 47–87 | 68.36 | 56–81 | 69.91 | 47–87 |
| Years of education | 15.57 | 9–20 | 14.86 | 9–20 | 16.22 | 12–20 |
| Time on androgen deprivation therapy (months)a | 44.84 | 2–228 | 62.77 | 2–228 | 28 | 3–156 |
| ReadiWatch™ wear time (%) | 94.14 | 73.08–100 | 95.87 | 73.08–100 | 92.98 | 74–100 |
|
| ||||||
| Characteristic | n | n | n | |||
|
| ||||||
| Received chemotherapy | 18 | 9 | 9 | |||
| Received radiation therapy | 31 | 18 | 13 | |||
| Receiving sleeping medication | 10 | 4 | 6 | |||
|
| ||||||
| Current wearable device (N = 8) | ||||||
|
| ||||||
| Fitbit | 5 | 3 | 2 | |||
| Apple Watch | 3 | – | 3 | |||
|
| ||||||
| Race | ||||||
|
| ||||||
| Black | 5 | 4 | 1 | |||
| Hawaiian or Pacific Islander | 1 | – | 1 | |||
| White | 36 | 16 | 20 | |||
| Prefer not to answer | 3 | 2 | 1 | |||
|
| ||||||
| Ethnicity | ||||||
|
| ||||||
| Not Hispanic or Latino | 40 | 18 | 22 | |||
| Prefer not to answer | 5 | 4 | 1 | |||
|
| ||||||
| Employment status | ||||||
|
| ||||||
| Retired | 33 | 15 | 18 | |||
| Full-time | 8 | 6 | 2 | |||
| Part-time | 3 | – | 3 | |||
| Medical leave | 1 | 1 | – | |||
|
| ||||||
| Relationship status | ||||||
|
| ||||||
| Married | 29 | 12 | 17 | |||
| Not in a relationship | 7 | 5 | 2 | |||
| In a relationship | 3 | 2 | 1 | |||
| Widowed | 3 | 1 | 2 | |||
| Divorced | 2 | 1 | 1 | |||
| Prefer not to answer | 1 | 1 | – | |||
|
| ||||||
| Cancer stage | ||||||
|
| ||||||
| I | 5 | 4 | 1 | |||
| II | 1 | 1 | – | |||
| III | 3 | 2 | 1 | |||
| IV | 31 | 15 | 16 | |||
| Unknown | 5 | – | 5 | |||
Significant between-group difference at baseline (p = 0.038)
Feasibility was demonstrated; 100% of the recruitment goal, 94% ReadiWatch wear time (range = 73%–100%), and 100% adherence to the teleCBT-I sessions were achieved. Attrition was 18% for group 1 (n = 4) and 4% for group 2 (n = 1).
Insomnia ratings for participants in both groups improved from moderate insomnia (ISI scores between 15 and 21) to subthreshold insomnia (scores between 8 and 14) (see Table 2). Several significant between-group differences were noted. Men in group 2 reported better physical function and demonstrated greater sleep efficiency (p < 0.05) at T3 (two months) compared to participants in group 1 (see Table 3). Moderate to large between-group effect sizes were noted in favor of group 2 for fatigue, anxiety, HRQOL, physical function, and cognitive abilities. Comparison of mean change scores demonstrated greater reduction in patient-reported fatigue (–4.173, p < 0.05) and greater improvement in HRQOL (0.088, p < 0.01) at T3 (two months) for men in group 2 (see Table 4). Moderate within-group effect sizes (Cohen’s d > 0.5) were detected for change from baseline to two months for report of cognitive abilities in group 1 and improvement in insomnia severity and HRQOL for group 2 (see Table 5). Within-group effect sizes greater than 0.4 were detected for participants in both groups from baseline to two months for improvement in fatigue and reduction in sleep disturbance, anxiety, and pain interference for group 2.
TABLE 2.
Descriptive Statistics for Patient-Reported Outcome Scores and Sleep Metrics
| Variable | Group 1 | Group 2 | T3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| T1 (N = 22) | T2 (N = 17) | T3 (N = 17) | T1 (N = 23) | T2 (N = 23) | T3 (N = 22) | ||||||||
|
|
|
|
|
|
|
|
|||||||
| X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | p | |
| Fatigue | 55.51 | 6.93 | – | – | 56.48 | 4.98 | 57.26 | 8.27 | – | – | 52.84 | 0.1 | 0.23 |
|
| |||||||||||||
| Anxiety | 52.25 | 8.99 | – | – | 52.85 | 9.52 | 53.89 | 7.31 | – | – | 51.59 | 7.72 | 0.52 |
|
| |||||||||||||
| Depression | 49.85 | 8.11 | – | – | 50.95 | 8.71 | 50.71 | 7.71 | – | – | 50.15 | 8.05 | 0.94 |
|
| |||||||||||||
| PROPr HRQOL | 0.31 | 0.15 | – | – | 0.3 | 0.15 | 0.31 | 0.18 | – | – | 0.41 | 0.2 | 0.05 |
|
| |||||||||||||
| PROMIS CF | 45.9 | 8.04 | – | – | 47.71 | 8.52 | 45.6 | 9.72 | – | – | 46.91 | 6.65 | 0.99 |
|
| |||||||||||||
| PROMIS CA | 52.28 | 5.97 | – | – | 49.42 | 10.07 | 50.14 | 7.52 | – | – | 51.81 | 6.42 | 0.48 |
|
| |||||||||||||
| ISI | 15.23 | 3.93 | 15.24 | 3.31 | 14.71 | 4.31 | 15.09 | 3.79 | 13.57 | 4.57 | 11.77 | 4.97 | 0.06 |
|
| |||||||||||||
| PSQI | 10.77 | 3.93 | 9.53 | 2.85 | 8.88 | 3.76 | 9.65 | 2.93 | 9.09 | 2.76 | 8.77 | 3.83 | 0.83 |
|
| |||||||||||||
| PROMIS PF | 41.04 | 6.48 | – | – | 40.38 | 5.68 | 42.97 | 0.09 | – | – | 45* | 8.6 | 0.03 |
|
| |||||||||||||
| PROMIS Sleep Disturbance | 56.85 | 5.76 | – | – | 54.88 | 5.81 | 56.1 | 5.63 | – | – | 52.16 | 7.22 | 0.21 |
|
| |||||||||||||
| PROMIS Social Roles | 45.65 | 6.22 | – | – | 47.45 | 7.7 | 46.85 | 8.14 | – | – | 49.96 | 9.23 | 0.42 |
|
| |||||||||||||
| PROMIS Pain Interfere | 57.14 | 8.53 | – | – | 57.06 | 8.62 | 55.3 | 8.73 | – | – | 51.95 | 8.8 | 0.58 |
|
| |||||||||||||
| Variable | Group 1 | Group 2 | T3 | ||||||||||
|
|
|
||||||||||||
| T1 (N = 16) | T2 (N = 16) | T3 (N = 15) | T1 (N = 22) | T2 (N = 22) | T3 (N = 22) | ||||||||
|
|
|
|
|
|
|
|
|||||||
| X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | p | |
|
| |||||||||||||
| Readi- Score | 79.17 | 18.48 | 81.64 | 17.33 | 81.6 | 21.16 | 82.14 | 16.24 | 83.59 | 8.69 | 81.32 | 13.78 | 0.51 |
|
| |||||||||||||
| Minutes asleep | 357 | 58.61 | 364.78 | 60.88 | 354.54 | 52.44 | 365.63 | 85.98 | 359.32 | 74.01 | 357.63 | 78.15 | 0.7 |
|
| |||||||||||||
| Sleep efficiency | 69.8 | 14.22 | 85.09 | 63.06 | 67.3 | 10.41 | 73.4 | 12.2 | 73.59 | 11.82 | 74.79* | 10.89 | 0.04 |
p < 0.05
Cohen’s d
CA—Cognitive Abilities; CF—Cognitive Function; HRQOL—health-related quality of life; Interfere—Interference; ISI—Insomnia Severity Index; PF—Physical Function; PROMIS—Patient-Reported Outcomes Measurement Information System; PROPr—PROMIS Preference; PSQI—Pittsburgh Sleep Quality Index; T—time
Note. T1 was baseline (first 7 days of wear time), T2 was 1 month, and T3 was 2 months.
TABLE 3.
Between-Group Effect Sizesa
| Variable | T2–T1 | T3–T2 | T3–T1 |
|---|---|---|---|
| Fatigue | – | – | 0.873 |
| Anxiety | – | – | 0.543 |
| Depression | – | – | 0.297 |
| PROMIS Preference HRQOL | – | – | 0.938 |
| PROMIS Cognitive Function | – | – | 0.084 |
| PROMIS Cognitive Abilities | – | – | 0.671 |
| Insomnia Severity Index | 0.467 | 0.361 | 0.643 |
| Pittsburgh Sleep Quality Index | 0.045 | 0.079 | 0.139 |
| PROMIS Physical Function | – | – | 0.594 |
| PROMIS Sleep Disturbance | – | - | 0.352 |
| PROMIS Social Roles | – | – | 0.178 |
| PROMIS Pain Interference | – | – | 0.475 |
| ReadiScore | – | 0.322 | 0.325 |
| Minutes asleep | – | 0.104 | 0.168 |
| Sleep efficiency | – | 0.439 | 0.362 |
Cohen’s d
HRQOL—health-related quality of life; PROMIS—Patient-Reported Outcomes Measurement Information System; T—time
Note. T1 was baseline (first 7 days of wear time), T2 was 1 month, and T3 was 2 months.
TABLE 4.
Mean Group Change Scores and 2-Sample T Test or Wilcoxon Rank-Sum Test Scores
| Variable | Group 1 Mean Change Score | Group 2 Mean Change Score | T3–T1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| T2–T1 (N = 17) | T3–T2 (N = 17) | T3–T1 (N = 17) | T2–T1 (N = 23) | T3–T2 (N = 22) | T3–T1 (N = 22) | ||||||||
|
|
|
|
|
|
|
|
|||||||
| X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | p | |
| Fatigue | – | – | – | – | 2.2 | 5.47 | – | – | – | – | −4.17* | 8.44 | 0.01 |
|
| |||||||||||||
| Anxiety | – | – | – | – | 1.52 | 0.43 | – | – | – | – | −2.57 | 5.67 | 0.13 |
|
| |||||||||||||
| Depression | – | – | – | – | 1.24 | 7.92 | – | – | – | – | −0.64 | 4.74 | 0.38 |
|
| |||||||||||||
| PROPr HRQOL | – | – | – | – | −0.031 | 0.13 | – | – | – | – | 0.088** | 0.14 | 0.009 |
|
| |||||||||||||
| PROMIS CF | – | – | – | – | 1.16 | 6.91 | – | – | – | – | 1.71 | 6.01 | 0.8 |
|
| |||||||||||||
| PROMIS CA | – | – | – | – | −4.48 | 8.34 | – | – | – | – | 1.55 | 9.43 | 0.06 |
|
| |||||||||||||
| ISI | 0.88 | 4.69 | −0.53 | 2.38 | 0.35 | 5.01 | −1.52 | 5.46 | −1.5 | 2.91 | −3.23 | 5.95 | 0.05 |
|
| |||||||||||||
| PSQI | −0.71 | 3.48 | −0.65 | 2.52 | −1.35 | 3.77 | −0.57 | 2.91 | −0.41 | 3.35 | −0.82 | 3.92 | 0.67 |
|
| |||||||||||||
| PROMIS PF | – | – | – | – | −1.06 | 3.77 | – | – | – | – | 1.11 | 3.54 | 0.074 |
|
| |||||||||||||
| PROMIS Sleep Disturbance | – | – | – | – | −1.29 | 5.55 | – | – | – | – | −3.94 | 8.71 | 0.28 |
|
| |||||||||||||
| PROMIS Social Roles | – | – | – | – | 1.49 | 6.62 | – | – | – | – | 2.75 | 7.37 | 0.73 |
|
| |||||||||||||
| PROMIS Pain Interfere | – | – | – | – | 0.68 | 8.25 | – | – | – | – | −3.08 | 7.64 | 0.15 |
|
| |||||||||||||
| Group 1 Mean Change Score | Group 2 Mean Change Score | T3–T1 | |||||||||||
|
|
|
||||||||||||
| T2–T1 (N = 16) | T3–T2 (N = 15) | T3–T1 (N = 15) | T2–T1 (N = 22) | T3–T2 (N = 22) | T3–T1 (N = 22) | ||||||||
|
|
|
|
|
|
|
|
|||||||
| Variable | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | p |
|
| |||||||||||||
| Readi- Score | 2.47 | 6.94 | 0.56 | 8.47 | 3.25 | 8.7 | 1.45 | 0.78 | −2.27 | 9.01 | −0.82 | 14.52 | 0.18 |
|
| |||||||||||||
| Minutes asleep | 7.78 | 71.97 | −6.7 | 44.81 | 3.39 | 76.7 | −6.3 | 52.91 | −1.69 | 50.61 | −7.99 | 60.77 | 0.62 |
|
| |||||||||||||
| Sleep efficiency | 15.28 | 61.36 | −17.51 | 67.03 | −1.4 | 8.56 | 0.19 | 7.15 | 1.21 | 6.28 | 1.4 | 7.09 | 0.29 |
p < 0.05;
p < 0.01
CA—Cognitive Abilities; CF—Cognitive Function; HRQOL—health-related quality of life; Interfere—Interference; ISI—Insomnia Severity Index; PF—Physical Function; PROMIS—Patient-Reported Outcomes Measurement Information System; PROPr—PROMIS Preference; PSQI—Pittsburgh Sleep Quality Index; T—time
Note. T1 was baseline (first 7 days of wear time), T2 was 1 month, and T3 was 2 months.
TABLE 5.
Within-Group Effect Sizesa for Change Scores
| Variable | T1 Versus T2 | T2 Versus T3 | T1 Versus T3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | |
| Fatigue | – | – | – | – | 0.402 | 0.495 |
|
| ||||||
| Anxiety | – | – | – | – | 0.161 | 0.454 |
|
| ||||||
| Depression | – | – | – | – | 0.156 | 0.134 |
|
| ||||||
| PROMIS Preference HRQOL | – | – | – | – | 0.235 | 0.699 |
|
| ||||||
| PROMIS Cognitive Function | – | – | – | – | 0.168 | 0.281 |
|
| ||||||
| PROMIS Cognitive Abilities | – | – | – | – | 0.537 | 0.164 |
|
| ||||||
| Insomnia Severity Index | 0.188 | 0.279 | 0.223 | 0.516 | 0.07 | 0.542 |
|
| ||||||
| Pittsburgh Sleep Quality Index | 0.203 | 0.195 | 0.256 | 0.122 | 0.358 | 0.209 |
|
| ||||||
| PROMIS Physical Function | – | – | – | – | 0.281 | 0.312 |
|
| ||||||
| PROMIS Sleep Disturbance | – | – | – | – | 0.233 | 0.452 |
|
| ||||||
| PROMIS Social Rules | – | – | – | – | 0.225 | 0.372 |
|
| ||||||
| PROMIS Pain Interference | – | – | – | – | 0.082 | 0.404 |
|
| ||||||
| ReadiScore | 0.356 | 0.148 | 0.066 | 0.252 | 0.373 | 0.056 |
|
| ||||||
| Minutes asleep | 0.108 | 0.119 | 0.15 | 0.033 | 0.044 | 0.132 |
|
| ||||||
| Sleep efficiency | 0.249 | 0.027 | 0.261 | 0.192 | 0.163 | 0.197 |
Cohen’s d
HRQOL—health-related quality of life; PROMIS—Patient-Reported Outcomes Measurement Information System; T—time
Note. T1 was baseline (first 7 days of wear time), T2 was 1 month, and T3 was 2 months.
No significant between- or within-group changes were seen for ReadiScores. No significant correlations were noted between changes in ReadiScores and changes in fatigue or cognitive function (see Table 6). As expected, significant correlations were noted between changes in ReadiScores and changes in total sleep time for all three study time point comparisons (baseline to one month, one month to two months, and baseline to two months). Likewise, significant correlations were noted between changes in ReadiScores and changes in sleep efficiency from baseline to T2 (one month) and from baseline to T3 (two months).
TABLE 6.
Correlation Analyses Between Change in ReadiScores and Patient-Reported Outcome Scores
| Variable | Group 1 | Group 2 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| T2–T1 | T3–T2 | T3–T1 | T2–T1 | T3–T2 | T3–T1 | |
| Fatigue | – | – | −0.022 | – | – | −0.25 |
| Anxiety | – | – | 0.03 | – | – | 0.09 |
| Depression | – | – | −0.015 | – | – | −0.064 |
| PROMIS Preference HRQOL | – | – | −0.006 | – | – | 0.356 |
| PROMIS Cognitive Function | – | – | 0.34 | – | – | 0.327 |
| PROMIS Cognitive Abilities | – | – | −0.281 | – | – | 0.33 |
| PROMIS Physical Function | – | – | 0.226 | – | – | −0.277 |
| PROMIS Sleep Disturbance | – | – | −0.234 | – | – | −0.127 |
| PROMIS Social Rules | – | – | −0.048 | – | – | 0.352 |
| PROMIS Pain Interference | – | – | 0.126 | – | – | −0.014 |
| Insomnia Severity Index | 0.0298 | −0.116 | 0.213 | 0.101 | −0.648** | −0.261 |
| PSQI | 0.134 | −0.185 | 0.073 | 0.242 | −0.375 | −0.085 |
| Minutes asleep | 0.63* | 0.6* | 0.668** | 0.72*** | 0.748*** | 0.774*** |
| Sleep efficiency | 0.515* | 0.314 | 0.093 | 0.398 | 0.221 | 0.659** |
p < 0.05;
p < 0.01;
p < 0.001
HRQOL—health-related quality of life; PROMIS—Patient-Reported Outcomes Measurement Information System; PSQI—Pittsburgh Sleep Quality Index; T—time
Note. T1 was baseline (first 7 days of wear time), T2 was 1 month, and T3 was 2 months.
Qualitative Results
The initial deductive organization of the data was based on the three main questions posed during the semistructured interviews. These categories included the following: (a) helpful aspects of study participation, (b) burdensome/problematic aspects of study participation and/or suggestions for improvement, and (c) strategies learned and/or intentions for continuation. The qualitative content analyses and coding procedures resulted in refinement and further subdivision of the initial categories as described in the following sections (see Figure 6). Relevant samples of participant quotes are outlined in Figure 7.
FIGURE 6.
Perceptions of Participation
tech—technical
FIGURE 7.
Sampling of Participant Quotes From Qualitative Content Analyses
tech—technical
The helpful aspects category of study participation reflected what participants shared regarding learning about sleep from the patterns evident within their sleep metrics data. Overall, men in both groups expressed benefit from seeing their sleep metrics data. For some, this confirmed their assessment of poor sleep. For others, they realized they were “getting more sleep than they thought.”
The burdensome/problematic aspects of the study participation category was refined to challenging aspects and included subcategories for watch wear and technical issues. A few men expressed discomfort with wearing a watch or difficulties with the tightness of the watch band or the brightness of the watch screen during the night (based on movement). Based on the exit interview data and the study team’s observations, study procedures were refined during the course of the pilot. The written instructions for syncing the ReadiWatch with the participant’s smartphone were revised for clarity. The research team developed technology screening questions (administered after informed consent) to determine participants’ comfort level and/or previous experience with wearable devices and downloading apps so that orientation could be better tailored based on individual needs. The semistructured guide for the exit interviews was eventually revised to include a question about participants’ thoughts on the refinement of future study procedures to provide all participants with a study tablet that was pre-paired with the ReadiWatch device and used for all study communications, data collection (e.g., REDCap, study questionnaires), and videoconferencing to address some of the technical issues experienced by participants. The subcategory of technical solutions was added to capture these data. Responses to this question were mixed. Those who were unfamiliar with technology agreed this idea would have eased the study burden, whereas those participants who were savvy with technology found the idea to be unnecessary.
A third category, mixed value aspects, emerged from the data analyses because participants shared mixed responses to the value they perceived from their ReadiScores and the ReadiWatch sleep hygiene education modules. Participants were equally divided between those who perceived the ReadiScores as highly accurate representations of their sleep patterns and changes that occurred because of new sleep behaviors and those who felt the ReadiScore had no value. Similarly, some participants felt they “learned a lot” from the sleep hygiene education modules, whereas others felt the information provided was too general, or they did not access the modules during the study.
Many participants in group 2 (teleCBT-I combination) described the structured deep breathing strategy as particularly helpful for returning to sleep after awakening with hot flashes, nocturia, or perseverant thoughts. This strategy also was reviewed as appropriate during the exit interview with group 1 participants for whom wake after sleep onset and return to sleep were noted as components of their sleep disturbance. Of the strategies participants described as ones they planned to use going forward, the structured deep breathing was the most mentioned strategy. Other commonly reported sustainable strategies included restricting the frequency and timing of naps (earlier in the day and shorter duration), increasing physical activity, adjusting the timing of exercise and fluid and caffeine intake, and maintaining the positive changes made to the sleeping environment (e.g., room darkening, lower temperature, use of ceiling fan). The initial sustainable strategies category was ultimately recoded as an outcome category of the helpful and mixed value aspects of study participation.
Although a deductive approach was used for data organization and coding, following the completion of all data collection from the exit interviews, the final round of review and coding yielded the following overarching theme for the qualitative results: Thank you for caring about sleep. Regardless of group, participants reported being very grateful to be approached for a study designed to investigate an intervention to improve sleep. Participants were interested in receiving a summary of the study results once complete. They recognized the importance of sleep to all aspects of life and expressed concerns that sleep disturbance was not something that was addressed by their primary or oncology healthcare providers.
Discussion
Insomnia ratings for participants in both groups improved from moderate insomnia to subthreshold insomnia. This result suggests that access to real-time sleep metrics data and sleep hygiene education is beneficial for this population. Although participants in both groups reported benefits to learning about their sleep patterns and having access to educational information about sleep hygiene, significantly greater improvements in physical function, sleep efficiency, fatigue, and HRQOL were demonstrated for participants in the teleCBT-I combination group. A contributing factor to this difference may be the additional structure and guidance associated with the four weekly teleCBT-I sessions. Weekly goal setting and discussion of sleep metrics may have had a greater influence on behavior change than the more self-directed, independent approach (i.e., being provided access to real-time sleep metrics data and written sleep hygiene education modules). Data gleaned from the qualitative semistructured interviews indicated that more men in group 2 reported specific strategies that they planned to carry forward related to improving their sleep. Additional contributing components of CBT-I not typically incorporated into sleep hygiene education are the prescriptions for sleep restriction, directions for stimulus control, and guidance to mitigate the perpetuation of negative thoughts and anxiety associated with trying to get a good night’s sleep. A related contributing factor also may be the benefits associated with the additional individual attention received by participants in group 2. In the authors’ previous interventional pilot studies with this patient population, which investigated education, prescribed aerobic and resistance training, and nutritional behavior changes to mitigate cardiovascular sequalae, men receiving treatment for PC reported greater appreciation for regular contact with the study team and access to healthcare professionals seeking methods to reduce the side effects of ADT (Myers et al., 2023, 2024).
Positive results noted for group 2 for insomnia, sleep efficiency, fatigue, and HRQOL were consistent with another study that was conducted to investigate the benefits of CBT-I for cancer survivors (Squires et al., 2022). As noted previously, much of the research conducted to date has focused on various formats for CBT-I delivery for women with breast cancer. To the authors’ knowledge, the current study and a previous pilot study (Myers et al., 2023) are the only two studies that have investigated sleep hygiene education and CBT-I specifically for men with PC receiving ADT.
The lack of correlation between ReadiScores and PROs for fatigue and cognitive function (aim 3) was disappointing. Given the extensive testing and validation of the SAFTE model across multiple military, transportation, and medical industries (Hursh et al., 2004; McCormick et al., 2012; Van Dongen, 2004), the authors expected participants’ ReadiScores to serve as an accurate predictor of patient-reported fatigue and cognitive performance effectiveness. One explanation may be that correlations were evaluated between mean ReadiScores for the seven-day period preceding and including the day the PROs were collected. Although ReadiScores are based on cumulative sleep metrics data from the past three days, given the potential for daily variation in sleep metrics data, the possibility exists that capturing real-time ReadiScores at the time of the survey completion would have provided a stronger correlation between these variables.
Limitations
The results from this pilot study are limited because of the small sample size and the fact that the study was powered only to detect a large effect size. Generalization of the findings is also limited to the racially homogeneous makeup of the sample; namely, 80% of the sample was White, and only 11% of the sample was Black. This racial disparity is congruent with the results reported from a previous systematic review and indicates the need for focused efforts to increase access to sleep disturbance interventional studies for members of minority populations (Li et al., 2023). One additional limitation is the dependence on self-report regarding whether participants accessed the sleep hygiene education modules because the data collected by the ReadiOne app did not reflect module usage.
Implications for Nursing
These study results provide further evidence that men with PC receiving ADT experience sleep disturbance and may benefit from access to data reflecting their sleep patterns, sleep hygiene education, and CBT-I. Additional research is needed, but nurses should consider regularly assessing patients with PC for sleep disturbance. Nurses also can consider discussing the potential benefits of wearable devices to track sleep metrics and explore resources for providing sleep hygiene education and opportunities for study participation related to CBT-I. In addition, awareness of local resources and practitioners providing CBT-I may be of benefit.
Conclusion
Feasibility was demonstrated for recruitment, retention, the use of wearable actigraphs to capture sleep metrics, and adherence to virtually delivered CBT-I for men with PC receiving ADT. Access to sleep metrics data (ReadiOne app) via wearable actigraphs (ReadiWatch) was viewed positively by men with PC receiving ADT, and participants reported feeling grateful that research was being conducted to improve sleep. Significant technical support was necessary to ensure participants’ ability to download and pair the ReadiOne app with the participants’ smartphones or study-provided, cellular data plan–enabled tablet. Improvements in sleep disturbance were significant when access to sleep metrics and sleep hygiene education were combined with individualized teleCBT-I. Further investigation is warranted. Future research should be conducted with a larger and more racially diverse sample and powered to detect a moderate effect size. Determination of the level of sleep disturbance severity and other phenotypic characteristics that may necessitate the addition of CBT-I to real-time sleep metrics data and sleep hygiene education access may be helpful to improve sleep disturbance in this population. A thorough assessment of participants’ technological experience is recommended to ensure appropriate support and the need for provision of smart devices that can be prepaired with the necessary sleep metrics app.
KNOWLEDGE TRANSLATION.
■ People with prostate cancer receiving androgen deprivation therapy may benefit from assessment of sleep disturbance, access to sleep metrics data, sleep hygiene education, and cognitive behavioral therapy for insomnia.
■ Oncology nurses should explore available resources for sleep hygiene education and cognitive behavioral therapy for insomnia for people with prostate cancer.
■ To investigate the effect of cognitive behavioral therapy for insomnia for people with prostate cancer, oncology nurses can encourage patients to participate in clinical trials.
Funding Statement
This research was funded, in part, by a National Cancer Institute Cancer Center support grant (P30 CA168524), by an Oncology Nursing Foundation endowment (2022 REO3), and through a contribution made to the Oncology Nursing Foundation from Merck.
Footnotes
The authors gratefully acknowledge David Streeter for use of the Curated Cancer Clinical Outcomes Database for participant recruitment and the advanced practice providers of the University of Kansas Cancer Center for recruitment referrals.
This research was funded, in part, by a National Cancer Institute Cancer Center support grant (P30 CA168524), by an Oncology Nursing Foundation endowment (2022 REO3), and through a contribution made to the Oncology Nursing Foundation from Merck.
Myers, Fowler, English, Stickler, He, Siengsukon, Parker, and Maliski contributed to the conceptualization and design. Myers, English, Stickler, Hooper, Penne Mays, Shen, and Heins completed the data collection. Humphrey-Sewell, Hooper, Kim, and He provided statistical support. Myers, Stickler, Kim, He, and Parker provided the analysis. Myers, Fowler, Stickler, He, Siengsukon, Wulff-Burchfield, Shen, Parker, and Maliski contributed to the manuscript preparation.
REFERENCES
- Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment–associated cognitive change: An update on the state of the science. Journal of Clinical Oncology. 2012;30(30):3675–3686. doi: 10.1200/jco.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri MM, Alenazi AM, Alothman SA, Rucker JL, Phadnis MA, Miles JM, Kluding PM. Using cognitive behavioral therapy for insomnia in people with type 2 diabetes, pilot RCT part I: Sleep and concomitant symptom. Behavioral Sleep Medicine. 2021;19(5):652–671. doi: 10.1080/15402002.2020.1831501. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts and figures, 2023. 2024. https://bit.ly/3E98ZeZ .
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bower JE, Bak K, Berger AM, Breitbart W, Escalante CP, Ganz PA, Jacobsen PB. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. Journal of Clinical Oncology. 2014;32(17):1840–1850. doi: 10.1200/jco.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Small BJ, Tometich DB, Zhai W, Zhou X, Luta G, Mandelblatt JS. Sleep disturbance and neurocognitive outcomes in older patients with breast cancer: Interaction with genotype. Cancer. 2019;125(24):4516–4524. doi: 10.1002/cncr.32489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casault L, Savard J, Ivers H, Savard M-H. A randomized-controlled trial of an early minimal cognitive-behavioural therapy for insomnia comorbid with cancer. Behaviour Research and Therapy. 2015;67:45–54. doi: 10.1016/j.brat.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Hays R. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K-F, Lee C-T, Yeung W-F, Chan M-S, Chung EW-Y, Lin W-L. Sleep hygiene education as a treatment of insomnia: A systematic review and meta-analysis. Family Practice. 2018;35(4):365–375. doi: 10.1093/fampra/cmx122. [DOI] [PubMed] [Google Scholar]
- Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncology Nursing Forum. 2001;28(3):465–470. [PubMed] [Google Scholar]
- Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL, Martin JL. Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine. 2021;17(2):255–262. doi: 10.5664/jcsm.8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo S, Kyngäs H. The qualitative content analysis process. Journal of Advanced Nursing. 2008;62(1):107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, Paul J. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. Journal of Clinical Oncology. 2008;26(28):4651–4658. doi: 10.1200/jco.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- Fowler LA, Hirsh EL, Klinefelter Z, Sulzbach M, Britt TW. Objective assessment of sleep and fatigue risk in emergency medicine physicians. Academic Emergency Medicine. 2023;30(3):166–171. doi: 10.1111/acem.14606. [DOI] [PubMed] [Google Scholar]
- Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. Journal of Clinical Oncology. 2014;32(5):449–457. doi: 10.1200/jco.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- Garland SN, Trevino K, Liou KT, Gehrman P, Spiguel E, MacLeod J, Mao JJ. Multi-stakeholder perspectives on managing insomnia in cancer survivors: Recommendations to reduce barriers and translate patient-centered research into practice. Journal of Cancer Survivorship. 2021;15(6):951–960. doi: 10.1007/s11764-021-01001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez BD, Small BJ, Cases MG, Williams NL, Fishman MN, Jacobsen PB, Jim HSL. Sleep disturbance in men receiving androgen deprivation therapy for prostate cancer: The role of hot flashes and nocturia. Cancer. 2018;124(3):499–506. doi: 10.1002/cncr.31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HJ, Pakenham KI, Headley BC, Yaxley J, Nicol DL, Mactaggart PN, Gardiner RA. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: A randomized controlled trial. BJU International. 2002;90(4):427–432. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, Duda SN. The REDCap Consortium: Building an international community of software platform partners. Journal of Biomedical Informatics. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MS, Liao KL. A psychological analysis of menopausal hot flushes. British Journal of Clinical Psychology. 1995;34(4):589–599. doi: 10.1111/j.2044-8260.1995.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Redmond DP, Johnson ML, Thorne DR, Belenky G, Balkin TJ, Eddy DR. Fatigue models for applied research in warfighting. Aviation, Space, and Environmental Medicine. 2004;75(3 Suppl):A44–A53. [PubMed] [Google Scholar]
- Klinefelter Z, Hirsh EL, Britt TW, George CL, Sulzbach M, Fowler LA. Shift happens: Emergency physician perspectives on fatigue and shift work. Clocks and Sleep. 2023;5(2):234–248. doi: 10.3390/clockssleep5020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Ma Y, Arditte Hall KA, Johnson C, Philpotts LL, Perez GK, Hall DL. Representation of race and ethnicity among cancer survivors in trials of cognitive behavioral therapy for insomnia (CBT-I): A systematic review. Supportive Care in Cancer. 2023;32(1):23. doi: 10.1007/s00520-023-08207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan PE, Washington SL, Cowan JE, Zhao S, Broering JM, Cooperberg MR, Carroll PR. Androgen deprivation therapy and the risk of dementia after treatment for prostate cancer. Journal of Urology. 2022;207(4):832–840. doi: 10.1097/JU.0000000000002335. [DOI] [PubMed] [Google Scholar]
- McCormick F, Kadzielski J, Landrigan CP, Evans B, Herndon JH, Rubash HE. Surgeon fatigue: A prospective analysis of the incidence, risk, and intervals of predicted fatigue-related impairment in residents. Archives of Surgery. 2012;147(5):430–435. doi: 10.1001/archsurg.2012.84. [DOI] [PubMed] [Google Scholar]
- Mercier J, Ivers H, Savard J. A non-inferiority randomized controlled trial comparing a home-based aerobic exercise program to a self-administered cognitive-behavioral therapy for insomnia in cancer patients. Sleep. 2018;41(10) doi: 10.1093/sleep/zsy149. [DOI] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Swick TJ. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Myers JS. Proinflammatory cytokines and sickness behavior: Implications for depression and cancer-related symptoms. Oncology Nursing Forum. 2008;35(5):802–807. doi: 10.1188/08.ONF.802-807. [DOI] [PubMed] [Google Scholar]
- Myers JS, Manson A, Billinger SA, Hamilton-Reeves J, Parker W, Maliski SL. Staying strong and healthy during androgen deprivation therapy. Cancer Nursing. 2024;47(1):43–55. doi: 10.1097/ncc.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JS, Shirazipour CH, Wertheimer JC, Asher A. Feasibility pilot study of a virtual intervention for survivors with decreased perceived cognitive function after cancer treatment. Oncology Nursing Forum. 2022;49(1):90–95. doi: 10.1188/22.ONF.90-95. [DOI] [PubMed] [Google Scholar]
- Myers JS, Siengsukon C, Sherman J, Shen X, Ptomey LT, Montgomery R, Maliski S. Androgen deprivation and sleep disturbance: A mixed methods pilot study of remote assessment and intervention. Cancer Nursing. 2023;46(4):259–269. doi: 10.1097/ncc.0000000000001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Survivorship [v.2.2024] 2024. https://www.nccn.org .
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Cancer-related fatigue [v.1.2025] 2025. https://www.nccn.org .
- Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive behavioral treatment of insomnia: A session-by-session guide. Springer; 2008. [Google Scholar]
- Russell C, Caldwell JA, Arand D, Myers L, Wubbels P, Downs H. Archinoetics. 2000. Validation of the Fatigue Science Readiband™ Actigraph and associated sleep/wake classification algorithms. [Google Scholar]
- Sanford SD, Beaumont JL, Butt Z, Sweet J, Cella D, Wagner LI. Prospective longitudinal evaluation of a symptom cluster in breast cancer. Journal of Pain and Symptom Management. 2014;47(4):721–730. doi: 10.1016/j.jpainsymman.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Archives of Internal Medicine. 2006;166(4):465–471. doi: 10.1001/archinte.166.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siengsukon CF, Alshehri M, Williams C, Drerup M, Lynch S. Feasibility and treatment effect of cognitive behavioral therapy for insomnia in individuals with multiple sclerosis: A pilot randomized controlled trial. Multiple Sclerosis and Related Disorders. 2020;40:101958. doi: 10.1016/j.msard.2020.101958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siengsukon CF, Nelson E, Williams-Cooke C, Ludwig R, Beck ES, Jr, Vidoni ED, Burns JM. Cognitive behavioral therapy for insomnia to enhance cognitive function and reduce the rate of Aβ deposition in older adults with symptoms of insomnia: A single-site randomized pilot clinical trial protocol. Contemporary Clinical Trials. 2020;99:106190. doi: 10.1016/j.cct.2020.106190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatric Clinics of North America. 1987;10(4):541–553. [PubMed] [Google Scholar]
- Squires LR, Rash JA, Fawcett J, Garland SN. Systematic review and meta-analysis of cognitive-behavioural therapy for insomnia on subjective and actigraphy-measured sleep and comorbid symptoms in cancer survivors. Sleep Medicine Reviews. 2022;63:101615. doi: 10.1016/j.smrv.2022.101615. [DOI] [PubMed] [Google Scholar]
- Stefanopoulou E, Yousaf O, Grunfeld EA, Hunter MS. A randomised controlled trial of a brief cognitive behavioural intervention for men who have hot flushes following prostate cancer treatment (MANCAN) Psycho-Oncology. 2015;24(9):1159–1166. doi: 10.1002/pon.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipps M, Hultman M, Banerji N, Jackson K, Liu M, Ellenberger A, Trusheim J. INNV-08. Validation of Readiband™ actigraph and associated sleep/wake classification algorithms in predicting cancer-related fatigue in high grade glioblastoma. Neuro-Oncology. 2019;21(Suppl 6):vi132. doi: 10.1093/neuonc/noz175.551. [DOI] [Google Scholar]
- Van Dongen HPA. Comparison of mathematical model predictions to experimental data of fatigue and performance. Aviation, Space, and Environmental Medicine. 2004;75(3 Suppl):A15–A36. [PubMed] [Google Scholar]
- Wilding S, Downing A, Wright P, Selby P, Watson E, Wagland R, Glaser AW. Cancer-related symptoms, mental well-being, and psychological distress in men diagnosed with prostate cancer treated with androgen deprivation therapy. Quality of Life Research. 2019;28(10):2741–2751. doi: 10.1007/s11136-019-02212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Ng DQ, Heshmatipour M, Acharya M, Coluzzi P, Guerrero N, Chan A. Electroacupuncture for the management of symptom clusters in cancer patients and survivors (EAST) BMC Complementary Medicine and Therapies. 2023;23(1):92. doi: 10.1186/s12906-023-03926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]