Abstract
Concentrated growth factor (CGF) is widely applied in clinical practice, but whether it has bone promoting effects and its mechanism of action are still the focus of discussion. In this study, in vitro experiments demonstrate that CGF can promote the expression of Arg-1 in BMDM cells, facilitating their polarization towards the M2 macrophages and encouraging the secretion of IL-10 and VEGF-A. CGF modulates M1 macrophages by reducing the expression of iNOS, while enhancing Arg-1 expression, thereby converting them to M2 macrophages. This is accompanied by a decrease in the secretion of TNF- and IL-1β, and an increase in the secretion of IL-10 and VEGF-A. Mechanistically, CGF promotes the phosphorylation of STAT3, which in turn induces M2 macrophage polarization, suggesting that the function of CGF-mediated macrophages may be associated with the STAT3 signaling pathway. Moreover, CGF-mediated macrophages were found to enhance osteoblast activity, increasing the expression of ALP, RUNX2, and BMP-2, and improving cell migration capabilities. In vivo experiments showed that CGF could early recruit M2 macrophages to the bone defect site, promoting the expression of bone formation-related proteins such as ALP and BMP-2, and accelerating bone tissue regeneration. In summary, our study demonstrates that CGF can induce bone repair and regeneration by promoting immune modulation and macrophage polarization.
Keywords: CGF, Macrophages, Bone regeneration, JAK/STAT
1. Introduction
Bone regeneration remains a significant clinical challenge, and considerable research has focused on ways to promote bone regeneration [1]. Concentrated growth factor (CGF) has gained popularity in the tissue repair engineering due to its wide availability, non-immunogenicity, degradability, and good biocompatibility[[2], [3], [4], [5]].
CGF is a biomimetic scaffold rich in growth factors and cytokines [6]. It is obtained through gradient centrifugation of autologous blood, and compared to PRF, it has better viscoelasticity and tensile strength, with a denser fibrin structure [7]. Furthermore, CGF contains a high concentration of growth factors such as transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) [8]. Based on its structure and biological characteristics, CGF has great potential in promoting angiogenesis, cell migration and adhesion, as well as stem cell proliferation and differentiation, and can effectively promote tissue repair and regeneration [9,10]. Studies have shown that CGF exhibits excellent osteoinductive activity on human bone marrow-derived mesenchymal stem cells (hBMSCs) and upregulates osteogenic markers such as osteocalcin (OCN), alkaline phosphatase (ALP), and type I collagen (COL-1), inducing osteoblast differentiation and promoting bone formation[[11], [12], [13]].
As is well known, osteogenesis is also influenced by the immune microenvironment, and macrophages play an important role in immune regulation[[14], [15], [16]]. Many studies have shown that osteogenesis is also regulated by macrophages, which can differentiate into different phenotypes under different microenvironment stimuli. M1 macrophages induce osteoclast formation, promote bone resorption, initiate bone remodeling, and M2 macrophages promote bone tissue regeneration [17]. Recently, there have been studies reporting that autologous concentrated platelet derivatives have immunomodulatory effects on macrophages, inducing polarization of human macrophages [18]. This also suggests the impact of autologous concentrated platelet derivatives on immune regulation.Recent studies have indicated that autologous concentrated platelet derivatives have immunomodulatory effects on macrophages [19,20], inducing polarization of human macrophages. These findings suggest that CGF plays a significant role in immunomodulation.
Despite the promising potential of CGF in bone regeneration, its exact osteogenic mechanisms remain unclear. The interactions between CGF, macrophages, and osteoblasts require further investigation. In this study, we aim to explore the immunomodulatory effects of CGF on macrophages and its osteoinductive effects on osteoblasts, thereby enriching the theoretical foundation for the clinical application of CGF in bone regeneration.
2. Materials and methods
2.1. Cell experiments
2.1.1. Preparation of CGF conditioned medium
Male SD rats (purchased from Chengdu Dossy Experimental Animals Co., Ltd) were anesthetized with 1 % pentobarbital sodium, and 10 ml of blood was drawn from the abdominal aorta and centrifuged using a Medifuge centrifuge (Silfradent, Italy). The CGF layer, along with the red portion at its junction, was ground to a homogeneous state. The CGF extract was then mixed with Dulbecco's Modified Eagle Medium (DMEM, Thermo Scientific, MA, USA) containing 10 % fetal bovine serum (FBS, Thermo Scientific) and 1 % (v/v) penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) to prepare CGF conditioned medium (CCM). The CCM was named 0 %, 5 %, 10 %, 20 %, and 50 % CCM according to the concentration of the CGF extract in the medium. (For example, 10 ml 5 % CCM contains 1 ml FBS 、0.1 ml bispecific antibody 、 0.5 ml CGF extract 、8.4 ml of DMEM).
2.1.2. Cell proliferation assay
BMDM macrophage cell lines (Procell Life Science & Technology Co., Ltd.) were used for in vitro studies. The cells were cultured in 0 %, 5 %, 10 %, 20 %, and 50 % CCM conditioned media for 24 or 48 h. Cell proliferation was assessed using a Cell Counting Kit-8 (Vazyme, China) according to the kit instructions. The cells were incubated for 3 h and the absorbance at 450 nm was measured using an ELISA reader.
2.1.3. Establish macrophage polarization model
Log-phase BMDM cells (obtained from the Wuhan Pricella Biotechnology Co., Ltd.) were adjusted to a concentration of 1 × 10ˆ5 cells/well and inoculated evenly into 6-well plates.Add DMEM complete culture medium containing 1 μg/ml LPS to induce the formation of M1 macrophages for 24 h, and add DMEM complete culture medium containing 20 ng/ml IL-4 to induce the formation of M2 macrophages for 24 h. Wait for the cells to grow to about 70 % of the bottom area of the culture dish for later use.
2.1.4. Exploring the impact and mechanism of CGF on macrophage polarization
2.1.4.1. Observation of macrophage morphology under inverted phase contrast microscope
The macrophages in log phase were carefully resuspended by pipetting up and down and centrifuged at 1000 rpm for 4 min. The supernatant was discarded, fresh medium was added to resuspend the cells by pipetting up and down and adjusted to a density of 1 × 10ˆ4 cells/ml. 1 ml of the cell suspension was inoculated into a 24-well plate and cultured for 12 h in an incubator. The cell morphology was observed and photographed using an inverted microscope.
2.1.4.2. Effect of CGF on macrophage polarization observed by immunofluorescence staining
BMDM cells were cultured in the presence or absence of 10 % CGF medium for 48 h. The medium was discarded, and the cells were washed with PBS and observed under an inverted microscope. The cells were then fixed in 4 % formaldehyde, permeabilized with 0.5 % Triton X-100 solution, stained with TRITC-labeled phalloidin, and counterstained with DAPI solution (100 nmol/L) for nuclear staining. Fluorescence was observed under a fluorescence microscope.
2.1.4.3. Enzyme linked immunosorbent assay for measuring cytokine changes
ELISA was used to detect changes in pro-inflammatory cytokine (IL-1β, TNF-) and anti-inflammatory cytokine (VEGF-A, IL-10) in M1 and M2 macrophages cultured with 10 % CCM. Supernatants were collected from BMDM cells cultured in DMEM complete medium, 10 % CGF, 1 μg/ml LPS, or 20 ng/ml IL-4 for 48 h, and centrifuged at 2–8 °C, 1000 g for 20 min (ThermoFisher, USA). Supernatants were collected again as the sample to be tested. The target antibody was coated in the 48-well microplate, to which was added the standard or the sample and then was added horseradish peroxidase-labeled antibody. After the unconjugated antibody was washed, TMB was added to it again for chromogenic reaction. The absorbance (OD value) at 450 nm was measured with an ELISA reader to determine the sample concentration.
2.1.4.4. Western blot method for detecting surface markers of macrophages
BMDM cells and M1 macrophages were cultured in 10 % CCM conditioned media. Cells were lysed in buffer with protease and phosphatase inhibitors for 30 min, precooled to 4 °C in the centrifuge, and centrifuged at 12000 g for 5 min. Supernatants were collected and quantitatively measured with a BCA kit to determine the protein concentration; the protein was denatured by boiling at 100 °C for 10 min. Equal amounts of protein samples were added onto SDS-PAGE gels, transferred to PVDF membranes, and blocked with 5 % skim milk powder in TBST at normal temperature for 1 h; primary antibodies were diluted at specific dilution rate, placed on a shaker and incubated overnight at 4 °C; after washed with TBST, the secondary antibodies were incubated with at normal temperature for 1 h. After washing with TBST, the PVDF membranes were quantitatively analyzed for band intensity with Image J. In this study, primary antibodies were used for the following proteins: iNOS (Huabio, China, dilution rate 1:1000), Arg-1 (Huabio, China, dilution rate 1:2000), and β-Actin (Affinity, Austria, dilution rate 1:10,000).
2.1.4.5. Preliminary study on the mechanism of CGF regulating macrophage polarization
The steps are the same as 1.1.4.4.In this study, primary antibodies were used for the following proteins: STAT 3 (Huabio, China, dilution rate 1:1000), P-STAT 3 (Huabio, China, dilution rate 1:2000), and β-Actin (Affinity, Austria, dilution rate 1:10,000).
2.1.5. Exploring the effect of conditioned medium induced macrophage polarization by CGF on osteogenic protein in MC3T3-E1 cells
2.1.5.1. Preparation of conditioned medium for CGF induced macrophage polarization
Cells were divided into 5 groups according to different treatments, M0、M0+CGF、M0+IL-4、M1、M1+CGF. The processing method is the same as 1.1.3. Collect the supernatant of each group of culture media for later use, and name the supernatant S-M0 、S-M0+CGF、S-M0+IL-4、S-M1、S-M1+CGF.
2.1.5.2. Exploring the effects of different culture media on osteogenic proteins in MC3T3-E1 cells
MC3T3-E1 cells (obtained from the Wuhan Pricella Biotechnology Co., Ltd.)were adjusted to a density of 2 × 10ˆ5 cells/well and inoculated into 6-well plates, adding 2 ml α-MEM complete medium per well. Upon reaching 70 % of the cell concentration, the medium was discarded with 2 ml of osteogenic induction differentiation medium being added and incubated for 3 days. The medium was then discarded again, adding S-M0 、S-M0+CGF、S-M0+IL-4、S-M1、S-M1+CGF to each group separately, along with osteogenic induction components: 50 μg/ml ascorbic acid, 10 mM β-sodium glycerophosphate, and 10 nM dexamethasone. The cells were incubated at 37 °C and 5 % CO2 for 24 h.The steps are the same as 1.1.4.4.In this study, primary antibodies were used for the following proteins: BMP2 (Huabio, China, dilution rate 1:1000), RUNX 2 (Huabio, China, dilution rate 1:2000), and GAPDH (Affinity, Austria, dilution rate 1:10,000).
2.1.5.3. Exploring the effects of different culture media on the migration ability of MC3T3-E1 cells
To analyze the effect of CGF-mediated macrophages on MC3T3-E1 cell behavior, MC3T3-E1 cells were adjusted to a density of 1 × 10ˆ5 cells/well and inoculated into 24-well plates with α-MEM (2 % PBS) medium. When cells reached over 90 % confluence, a vertical scratch was made on the bottom of 24-well plates using a 200 μL pipette tip. The wells were washed with PBS three times to remove residual cells. The scratch area was photographed under a microscope. Subsequently, S-M0 、S-M0+CGF、S-M0+IL-4、S-M1、S-M1+CGF medium was added separately, and the cells were incubated in a 37 °C, 5 % CO2 cell culture incubator for 24 h before being photographed again under a microscope to record the area of scratch healing.
2.2. Animal experiment
2.2.1. Model establishment and sample collection
36 Male SD rats in total (36,8–10 weeks, 350–450 g) were purchased from Chengdu Dossy Experimental Animals Co., Ltd. After one week of acclimatization, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (350 mg/kg−1). The scalp hair was shaved and the unhaired area was disinfected. A 1.5 cm incision was made along the cranial midline to expose the bone, and two symmetric 5 mm bone defects were created using a trephine. The control group had untreated skull defects (n = 18), while the CGF group had both holes covered with centrifuged CGF, approximately 0.5 ml (n = 18). Incisions were sutured with 4-0 absorbable sutures. 4–5 rats were euthanized at 1, 2, 4, and 8 weeks after surgery, and skull specimens containing membranes were taken.The method of execution is intraperitoneal injection of 1 % pentobarbital sodium solution (0.2 ml/100 g per rat).
2.2.2. Micro-CT measurement and analysis
Cranial specimens were scanned and measured using Micro-CT (Bruker Skyscan 1276) at 70 kV, 200 μA, and 10 μm spatial resolution. X-ray images were reconstructed using NRecon software to determine the bone volume fraction (BV/TV) and trabecular number (Tb.N).
2.2.3. Immunohistochemistry
Cranial specimens were decalcified in 15 % (w/v) EDTA-2Na at 25–30 °C, with decalcification solution being changed every 3 days for 4 weeks until complete decalcification was confirmed by ease of needle penetration into the bone tissue (non-target area). The completely decalcified specimens were embedded in paraffin, sectioned longitudinally at 4 μm in thickness, and subjected to immunohistochemical staining. Sections were incubated in citrate buffer, treated with hydrogen peroxide to quench endogenous peroxidase activity, and blocked with goat serum. Primary antibodies (BMP-2, ALP, RUNX2) were incubated overnight at 4 °C, followed by adding HRP-conjugated secondary antibodies and incubating at 37 °C for 45 min. Finally, DAB (DAB4033) staining and hematoxylin (BP022) counterstaining was performed.
2.2.4. Immunofluorescence
Decalcified, embedded cranial specimens of SD rats were sectioned as previously described. Sections were incubated overnight with primary antibodies iNOS (Abcam, UK) (1:200) and Arg-1 (PTG, USA) (1:100). Rabbit secondary antibodies conjugated to a fluorophore were incubated for 45 min, followed by DAPI staining. Fluorescent images were acquired using an automatic fluorescence microscope (Olympus Corporation, USA).
2.3. Statistical analysis
Images were processed using Image J. Statistical analyses were performed using GraphPad Prism 9.3.1. Data were expressed as mean ± standard deviation. Normality and homogeneity of variances were tested. One-way ANOVA with Tukey's multiple comparison test was used for multiple groups, and independent t-tests were used for comparisons between two groups. A P-value <0.05 was considered statistically significant.
3. Results
3.1. Surface characteristics of CGF and its effect on macrophage proliferation in vitro
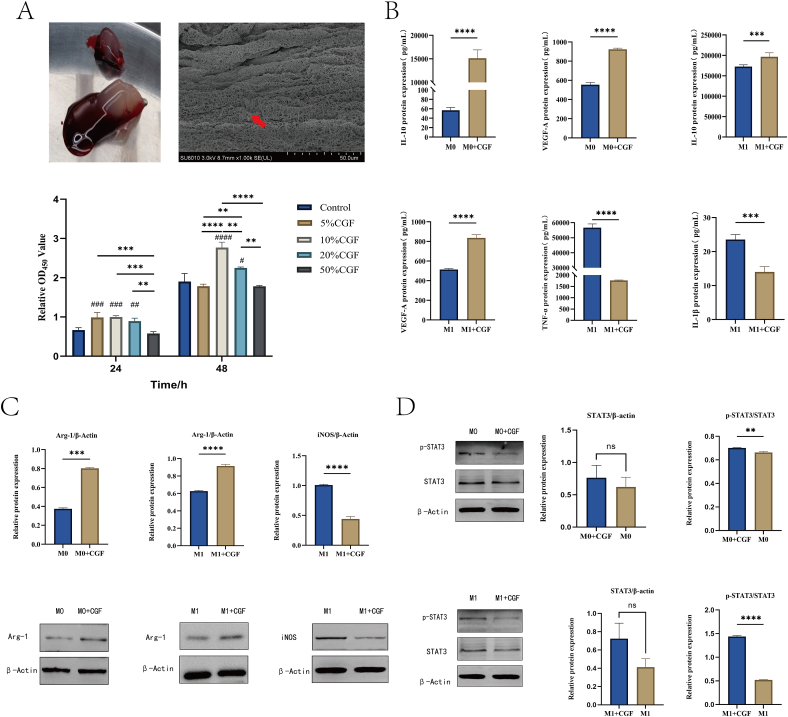
After the fresh blood from SD rats was centrifuged according to a predetermined protocol, it was separated into three distinct layers. The middle layer is semi transparent and light yellow gel shaped, and the middle layer is connected with its red part, which is the CGF extracted. CGF, displayed a fibrin network composed of collagen fibers under a scanning electron microscope, presenting a three-dimensional structure (Fig. 1A).
Fig. 1.
Surface characteristics of C GF and its effect on macrophages. A: CGF and its three-dimensional structure under electron microscopy; The proliferation of BMDM treated with different concentrations of CGF; B: ELISA method is used to detect the secretion of IL-1 、TNF-、VEGF-A、IL-10 after CGF acts on M0 and M1 macrophages; C: Western blot detection of protein expression of Arg-1 and iNOS after CGF acts on M0 and M1 macrophages; D: Western blot detection of JAK/STAT pathway related protein expression after CGF acts on M0 and M1 macrophages, respectively (∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001. #, P < 0.05; ##, P < 0.01; ###, P < 0.001; ####, P < 0.0001. # compared with the control group, ns, P > 0.05,one-way ANOVA).
Testing the effects of CGF in different concentration gradients on macrophage proliferation, BMDM cells showed significantly enhanced proliferation activity after 24 h of culture with 5 %, 10 %, and 20 % CGF compared with the control group (P < 0.05). However, cells cultured with 50 % CGF displayed lower activity than the control group, with no significant differences observed among the 5 %, 10 %, and 20 % CGF groups. After 48 h, macrophage proliferation activity was markedly increased in the 10 % CGF group, significantly higher than the other four groups (P < 0.05). These results indicate that 10 % CGF has the most notable effect on macrophage proliferation, consistent with previous research findings [13]. Thus, 10 % CGF was selected for subsequent cell experiments (Fig. 1A).
3.2. Morphological observation of CGF on macrophage polarization
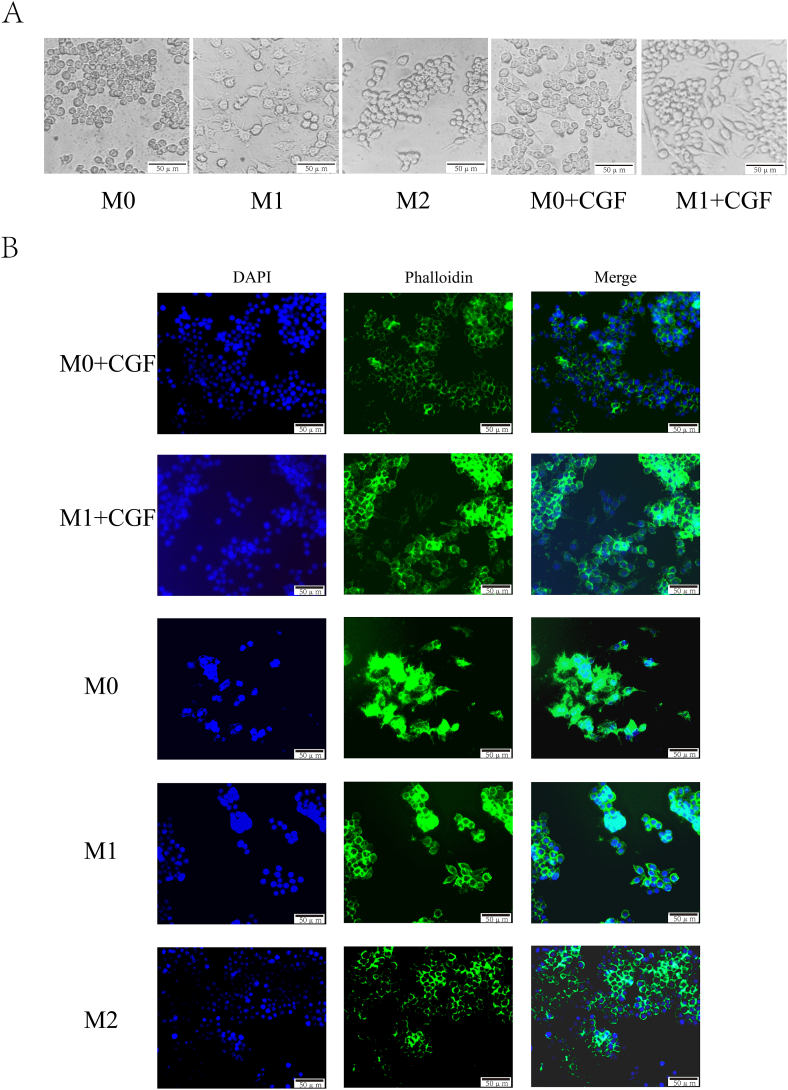
The morphology of macrophages is closely related to their polarization phenotypes [21]. M0 and M1 macrophages were respectively cultured with 10 % CGF for 48 h, forming the (M0+CGF) and (M1+CGF) groups. Their cellular morphology was observed under an inverted microscope: unstimulated M0 macrophages exhibited a round or oval shape with abundant cytoplasm, and a diameter of approximately 20 μm. When stimulated by LPS, M0 macrophages polarized towards M1 macrophages, resulting in morphological changes characterized by an increase in filopodia and a transition from a round to a fried-egg shape, with a slight increase in cell diameter. In contrast, M0 macrophages stimulated by IL-4 polarized towards M2 macrophages, with an increase in pseudopods, fewer than those in M1 macrophages, and a change in morphology to a spindle shape. When stimulated with CGF, the morphology of both M0 and M1 macrophages tended to change towards a spindle-like shape, becoming more similar to M2 macrophages morphologically (Fig. 2A).
Fig. 2.
Morphological observation of CGF on macrophage polarization. A: Morphological changes of macrophages under inverted microscope; B: Observation of Morphological Changes of Macrophages by Immunofluorescence Method (the scale represents 50 μm).
In cellular immunofluorescence staining, DAPI stains the nucleus, and phalloidin colors the cytoskeleton. Immunofluorescence observations revealed: After the addition of CGF, M1 macrophages exhibited morphological changes compared with their previous state, with a reduction in pseudopods and a transition from a fried-egg shape to a spindle shape, while the cell body remained large. The proliferative and differentiated macrophages appeared round. After the addition of CGF, some M0 macrophages transitioned from a round to a spindle shape, similar to the morphology of IL-4-induced M2 macrophages (Fig. 2B).
3.3. The effect of CGF on the secretion of pro-inflammatory and anti-inflammatory factors by macrophages
According to established methods, M1 macrophages were obtained by inducing BMDM cells with 1 μg/ml LPS [22]. M0 and M1 macrophages were cultured in complete medium containing 10 % CGF for 48 h. ELISA testing revealed that after culturing M0 macrophages in 10 % CCM (M0+CGF), the secretion levels of growth factors VEGF-A and IL-10 were significantly higher than those in the control group (P < 0.05). For M1 macrophages cultured in 10 % CCM, CGF markedly increased VEGF-A and IL-10 while significantly reducing the secretion of inflammatory factors TNF-α and IL-1β(P < 0.05) (Fig. 1B).
3.4. Detecting the effect of CGF induced macrophage polarization on cell surface markers
The Western blot experiment results showed that after co culturing M0 macrophages with 10 % CCM for 48 h, the expression of Arg-1 protein in M0 and M1 macrophages significantly increased (P < 0.05); The expression of iNOS protein in M1 macrophages was significantly decreased compared to the control group (P < 0.05). Arg-1 is often considered a surface marker for M2 macrophages, while iNOS is often considered a surface marker for M1 macrophages. These results indicate that M0 and M1 macrophages have a tendency to transform into M2 macrophages under CGF induction (Fig. 1C).
3.5. Preliminary study on the mechanism of CGF regulating macrophage polarization
To further investigate the deeper effects of CGF on macrophage polarization, we studied possible signaling pathways [23,24]. The Western blot experiment results showed that there was no significant difference in the total protein expression of STAT3 between M0 and M1 macrophages after co culturing with 10 % CCM for 48 h; Compared with the control group, the expression of phosphorylated STAT3 protein (P-STAT3) increased (P < 0.05), suggesting that CGF regulation of M0 and M1 macrophage polarization towards M2 may be closely related to the JAK/STAT pathway (Fig. 1D).
3.6. The effect of CGF mediated macrophage polarization conditioned medium on osteogenic protein in MC3T3-E1 cells
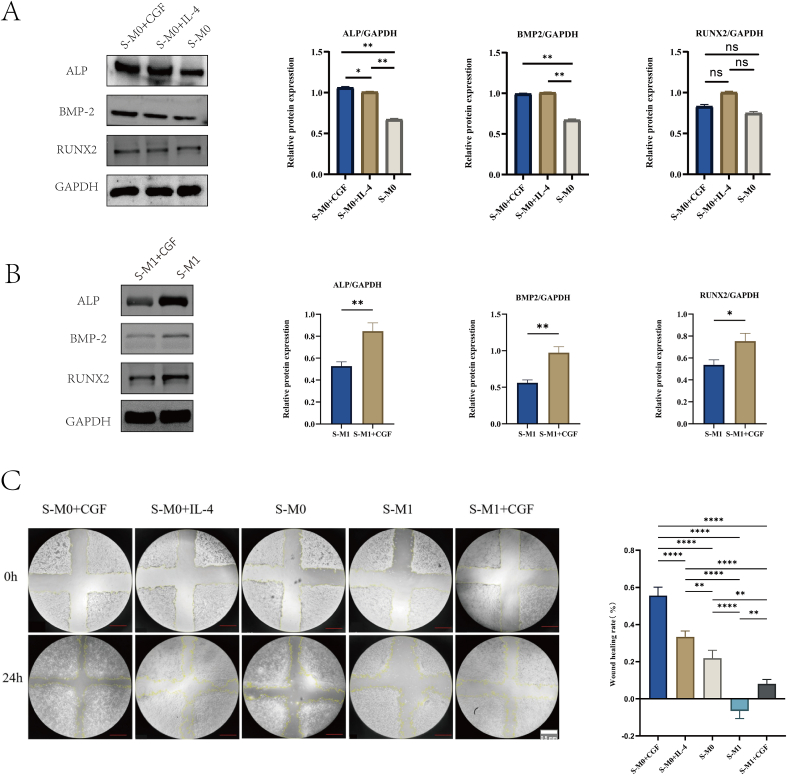
After incubating MC3T3-E1 cells in different conditioned media for 24 h, Western blot analysis was conducted for osteogenic proteins ALP, BMP-2, and Runx2 to observe their expression. The osteoblasts cultured with CGF-containing macrophage supernatant (S-M0+CGF) exhibited the most pronounced ALP expression, higher than the S-M0+IL-4 and S-M0 groups (P < 0.05). In terms of BMP-2 expression, both S-M0+CGF and S-M0+IL-4 groups exhibited higher BMP-2 levels than S-M0 group (P < 0.05), with no significant difference between S-M0+CGF and S-M0+IL-4 groups (Fig. 3A).
Fig. 3.
The effect of C GF induced macrophage polarization on osteoblasts. A: The effect of CGF mediated M0 macrophages on the expression of osteogenic related proteins in MC3T3-E1 cells; B: The effect of CGF mediated M1 macrophages on the expression of osteogenic related proteins in MC3T3-E1 cells; C: Scratch assay to detect the effect of CGF induced macrophage polarization on the migration ability of MC3T3-E1 cells. (∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.00005, ns, P > 0.05).
There was no noticeable difference in RUNX2 expression among the three groups. For MC3T3-E1 cells cultured in S-M1+CGF for 24 h, the expressions of BMP-2, ALP, and RUNX2 in the S-M1+CGF group was significantly higher than those in the S-M1 group (Fig. 3B).
3.7. The effect of conditioned medium mediated macrophage polarization on the migration ability of MC3T3-E1 cells
The results of the cell scratch test showed that the migration rate of the S-M0+CGF group was significantly higher than that of the S-M0, S-M0+IL-4, S-M1, and S-M1+CGF groups (P < 0.05). The migration rate of the S-M0+IL-4 group was significantly higher than that of the S-M0, S-M1, and S-M1+CGF groups (P < 0.05). The migration rate of the S-M0 group was significantly higher than that of the S-M1 and S-M1+CGF groups (P < 0.05), and the migration rate of the S-M1+CGF group was significantly higher than that of the S-M1 group (P < 0.05) (Fig. 3C).
3.8. In vivo experiments
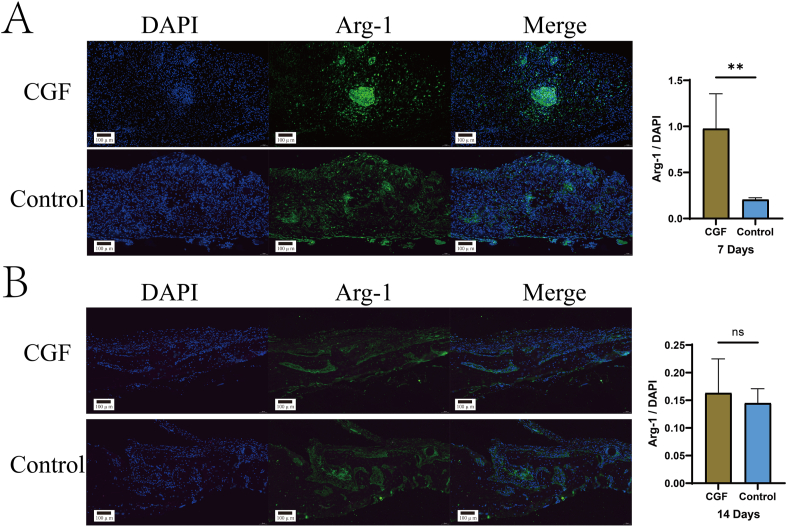
All animal studies evaluated the impact of CGF on macrophage polarization and osteogenic capacity. CGF was added to the rat cranial defect models and the rats were euthanized on days 7 and 14 for immunofluorescence staining (Fig. 4). Arg-1 positive M2 macrophages displayed green fluorescence, with the nucleus stained blue by DAPI. In the rat cranial defect models, the CGF group showed a significant increase in Arg-1 fluorescence intensity after 7 days (Fig. 4A), with different sizes of fluorescence clusters observed, unlike the scattered fluorescence distribution in the control group. After 14 days (Fig. 4B), the CGF group's fluorescence intensity decreased, with a few M2 macrophages observed on the periosteum and the emergence of new bone tissue. In contrast, the control group displayed more strong fluorescence clusters and scattered new bone formation after 14 days versus 7 days. These results indicate that, in the early stages of bone defects, CGF more rapidly and abundantly promotes M2 macrophage aggregation, playing a crucial role in M2 macrophage generation.
Fig. 4.
Immunofluorescence images of rat skull bone defects at 7 and 14 days post injury. A: Arg-1 immunofluorescence image on the 7th day. B: Arg-1 immunofluorescence image on the 14th day (ns, P > 0.05, ∗∗P < 0.01) (the scale represents 100 μm).
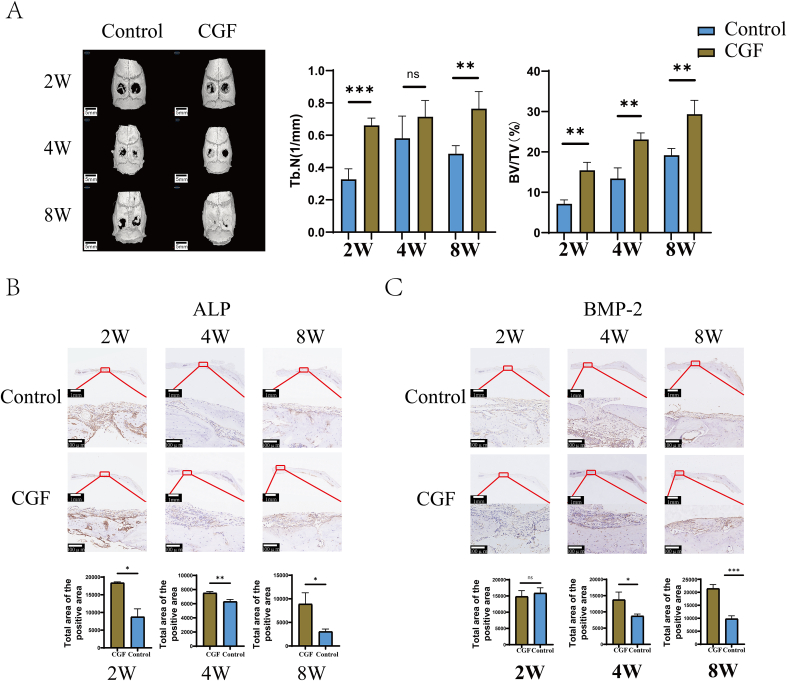
Micro-computed tomography (Micro-CT) 3D reconstruction images were acquired at 2, 4, and 8 weeks post-operatively (Fig. 5A). At 2 weeks, both the CGF group and the control group showed a small amount of new bone formation. By 4 weeks, distinct new bone formation was observed in both groups, with higher bone volume and density in the CGF group. In contrast, the control group had less, scattered bone at the defect and the new bone formation was mainly concentrated on the edge of bone defect. By 8 weeks, the CGF group showed extensive bone regeneration, almost filling the defect space, whereas the control group showed notably less new bone formation with the defect outline being still visible. BV/TV and Tb.N analyses indicated significantly higher values in the CGF group at 2/4 and 8 weeks.
Fig. 5.
Micro CT and immunohistochemical images of rat skull bone defects at 2 W, 4 W, and 8 W. A: Statistical analysis of Micro CT images and BV/TV, Tb. N values at 2 W, 4 W, and 8 W after skull bone defect in rats; B: Immunohistochemical staining images of ALP after 2 W, 4 W, and 8 W of skull bone defect in rats; C: Immunohistochemical staining images of BMP-2 after 2 W, 4 W, and 8 W of skull bone defect in rats; (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, ns, P > 0.05) (the scales represent 1 mm and 100 μm, respectively).
Decalcified specimens subjected to immunohistochemistry supported the Micro-CT results. The expression activity of osteogenic proteins ALP and BMP-2 in newly formed bone tissue was analyzed to explore CGF's osteogenic properties. Positive DAB staining appeared deep brown, with blue-stained nuclei by hematoxylin. At 2 weeks, strong positive ALP expression was observed in CGF-treated rat cranial defect models (Fig. 5B), more prominent than in the control group. ALP expression weakened over the subsequent 4 and 8 weeks, but remained higher in the CGF group. No significant BMP-2 expression difference was observed between the two groups at 2 weeks (Fig. 5C). However, BMP-2 immunolabeling in newly formed bone tissue periosteum became apparent and stronger in the CGF group at 4 and 8 weeks compared with the control group.
4. Discussion
The promotion of bone repair and regeneration holds paramount significance in the field of oral implantology. More and more studies have focused on the potential of CGF in promoting bone regeneration. The process of bone regeneration is complex and involves multiple factors of intervention. Some scholars believe that CGF can stimulate the skeletal system to promote bone regeneration, but the mechanisms by which CGF promotes bone regeneration are multifaceted. Some scholars even believe that there is insufficient evidence to prove that CGF can induce osteogenic differentiation.Our research preliminarily establishes that CGF can foster bone regeneration by mediating immune modulation.
Macrophage polarization is contingent upon various environmental conditions, which may trigger an M1 macrophage pro-inflammatory response or an M2 immunoregulatory response [25]. M2 macrophages can secrete cytokines such as VEGF-A and IL-10, which play a significant role in tissue repair and regeneration, with arginase-1 (Arg-1) serving as a marker for M2 macrophages [26]. Conversely, IL-1β and TNF-α are pro-inflammatory factors secreted by M1 macrophages that are instrumental in eliminating bacteria, viruses, and other pathogens, with nitric oxide synthase (iNOS) acting as a marker of M1 macrophages [27]. It was found in our study that M0 and M1 macrophages change in their morphology and function under the action of CGF, accompanied by an increase in the expression of Arg-1 and their polarization toward M2 macrophages. After their phenotype changes, their biological functions are altered accordingly, with an upsurge in the secretion of cytokines like IL-10 and VEGF-A and a remarkable reduction in inflammatory factors TNF-α and IL-1β secreted by M1 macrophages. Macrophages exhibit a high degree of plasticity, facilitating their interconversion among various types. This plasticity is underpinned by the integration of microenvironmental signals and cytokines, leading to the synthesis of specific signaling molecules that reinitiate macrophage gene transcription. This re-transcription results in macrophages with distinct epigenetic characteristics [28,29]. Under lipopolysaccharide (LPS) stimulation, macrophages are activated into the inflammatory M1 macrophages, exhibiting high surface expression of iNOS, CD80, and CD86. Conversely, IL-4 stimulates macrophages to adopt M2 macrophages with high surface expression of CD206 and Arg-1, to secrete growth factors like IL-10 that facilitate tissue repair. Aligning with our research results and previous findings, we propose that CGF, as a concentrated blood extract rich in various cytokines, can bind to macrophages, steering their phenotype towards M2 macrophage polarization and thereby promoting bone regeneration.
Further exploring the interplay between CGF and macrophage polarization, we observed a close association between M2 macrophage polarization and the JAK/STAT signaling pathway32. Phosphorylation of STAT3 contributes significantly to macrophage polarization towards M2 macrophages [23,24,30]. Additionally, certain immunomodulatory molecules, like PepOprimed, have been found to inhibit M2 polarization by downregulating IL-10 expression and suppressing JAK2/STAT3 phosphorylation [31]. Given our observation that CGF induces IL-10 secretion in macrophages, we investigated the relationship between CGF-induced macrophage polarization and STAT3 phosphorylation. Our findings indicate that CGF augments STAT3 phosphorylation levels during M0 and M1 macrophage polarization towards M2 macrophages, with no significant variance observed in total STAT levels. The phosphorylation of the STAT3 pathway thus provides a plausible mechanism for CGF-induced macrophage polarization toward M2 macrophages.
Our study also unveiled that CGF-induced macrophage polarization positively influences the expression of bone formation-related proteins BMP-2, ALP, and RUNX2 in osteoblast cultures. Notably, when osteoblasts were cultured in CGF-induced M1 macrophage supernatant, a significant increase in RUNX2 expression was observed compared with the control group. Some researches suggest that RUNX2 undergoes protease degradation upon oxidation by reactive oxygen species (ROS). However, the use of reducing agents to eliminate excess ROS mitigates RUNX2 degradation [32], leading us to hypothesize that CGF-induced macrophage polarization from M1 to M2 reduces inflammatory factor secretion and subsequent ROS production, thereby preventing RUNX2 degradation.
Given the early involvement of macrophages in bone repair and regeneration, we established a rat cranial defect model to more accurately simulate human bone defects and investigate CGF's impact on macrophage polarization and osteogenesis. Seven days after CGF application, distinct M2 macrophage aggregates were visible, and CGF had been fully degraded, with substantial M2 macrophage aggregation on the periosteum. By day 14, the aggregation was less pronounced, and significant new bone formation was evident. Numerous studies have underscored the pivotal role of macrophage phenotypes in maintaining bone homeostasis and regulating bone healing [33]. Macrophages are among the earliest immune cells to arrive at bone defects, where they are activated to M1 macrophages under pro-inflammatory microenvironment stimulation, secreting copious inflammatory factors to enhance inflammation, eradicate bacteria, and promote angiogenesis. Following acute inflammation resolution, macrophages transition from M1 to M2, secreting cytokines that facilitate tissue repair and regeneration. Thus, promoting the M1 to M2 macrophage transition is crucial in bone repair and regeneration [34]. CGF expedites early M2 macrophage polarization, shortening the inflammation period, and mitigating its adverse impact on bone regeneration. Throughout the 2, 4, and 8-week periods following CGF application, significant bone regeneration was observed, with elevated expression of osteogenic proteins ALP and BMP-2 compared with the control group. The rat cranial defect bone regeneration results corroborate our hypothesis that CGF accelerates M2 macrophage induction and subsequent osteoblast secretion of osteogenic proteins like ALP and BMP-2, thereby enhancing osteogenesis. On the one side, CGF's fibrin network provides macrophages with adhesion sites, promoting cellular adhesion and inducing macrophage polarization towards M2 phenotype, which in turn secretes growth factors to aid bone regeneration [35]. On the other side, CGF enhances the expression of osteogenic proteins ALP and BMP-2, promoting osteoblast differentiation, and thereby collaboratively expediting bone tissue repair and regeneration.
Although our study demonstrates CGF's capacity to modulate macrophage phenotypes and promote osteogenesis, with evidence suggesting this polarization mechanism may involve enhanced STAT3 phosphorylation, further research is necessary to comprehensively elucidate the mechanisms underlying CGF-induced macrophage polarization.
5. Conclusion
In summary, we investigated the immunomodulatory effect of CGF on macrophages and found that CGF can upregulate the expression level of p-STAT3 protein in the JAK/STAT pathway, regulate macrophage polarization towards M2 type, increase the secretion of anti-inflammatory factors VEGF-A and IL-10, reduce the secretion of pro-inflammatory factors TNF- and IL-1 , accelerate osteoblast migration speed, upregulate the expression of osteogenic related proteins BMP-2, ALP, and RUNX2, and recruit more M2 type macrophages around bone defects in the early stage to promote early osteogenesis.
Informed consent
Not applicable to this animal study.
Ethics approval ethics declarations
The Ethics Committee of the Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital approved the study protocol on March 30, 2023 [Ethics (Research), No. 112 in 2023].
The protocol complied with local and international recommendations for the care and use of laboratory animals.
The protocol are reported in accordance with ARRIVE guidelines.
Author contribution
Deng xin, Zheng Junwen, Zou Jiacheng wrote the manuscript text; Li peng, Liao juan designed the research; He Huanzong, Xun Qiongyu performed the research; Deng xin, Zheng Junwen analyzed the data; all authors reviewed the manuscript.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Funding
This work was supported by the Chinese National Natural Science Foundation (grant no. 82201108); the Sichuan Provincial Health Commission Foundation (grant no. 24CXTD19); Sichuan Province Health care Research Project 2025 (grant no. 2025-206) and the Science and Technology Plan Project of Sichuan Province, China (grant no. 2023YFS0137).
Declaration of competing interest
The authors declare no financial and non-financial conflicts.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Li peng, Email: lipengmazui@qq.com.
Liao juan, Email: 109497731@qq.com.
References
- 1.Heng B.C., Bai Y., Li X., Lim L.W., Li W., Ge Z., et al. Electroactive biomaterials for facilitating bone defect repair under pathological conditions. Adv Sci. 2023;10 doi: 10.1002/advs.202204502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeulen S., Tahmasebi Birgani Z., Habibovic P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials. 2022;283 doi: 10.1016/j.biomaterials.2022.121431. [DOI] [PubMed] [Google Scholar]
- 3.Lesage C., Lafont M., Guihard P., Weiss P., Guicheux J., Delplace V. Material-assisted strategies for osteochondral defect repair. Adv Sci. 2022;9 doi: 10.1002/advs.202200050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu F., Qiao L., Zhao Y., Chen W., Hong S., Pan J., et al. The potential application of concentrated growth factor in pulp regeneration: an in vitro and in vivo study. Stem Cell Res Ther. 2019;10:134. doi: 10.1186/s13287-019-1247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo X., Wan Q., Cheng L., Xu R. Mechanisms of bone remodeling and therapeutic strategies in chronic apical periodontitis. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.908859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miron R.J., Fujioka-Kobayashi M., Sculean A., Zhang Y. Optimization of platelet-rich fibrin. Periodontology. 2000 2024;94:79–91. doi: 10.1111/prd.12521. [DOI] [PubMed] [Google Scholar]
- 7.Malcangi G., Patano A., Palmieri G., Di Pede C., Latini G., Inchingolo A.D., et al. Maxillary sinus augmentation using autologous platelet concentrates (Platelet-Rich plasma, platelet-rich fibrin, and concentrated growth factor) combined with bone graft: a systematic review. Cells. 2023;12:1797. doi: 10.3390/cells12131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanca E., Calabriso N., Giannotti L., Nitti P., Damiano F., Stanca B.D.C., et al. Analysis of CGF biomolecules, structure and cell population: characterization of the stemness features of CGF cells and osteogenic potential. Int J Mol Sci. 2021;22:8867. doi: 10.3390/ijms22168867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L., Huang C., Zhong Y., Chen Y., Zhang H., Zheng Z., et al. Multifunctional sponge scaffold loaded with concentrated growth factors for promoting wound healing. iScience. 2023;26 doi: 10.1016/j.isci.2022.105835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabir MdA., Hirakawa A., Zhu B., Yokozeki K., Shakya M., Huang B., et al. Mechanical properties of human concentrated growth factor (CGF) membrane and the CGF graft with bone morphogenetic protein-2 (BMP-2) onto periosteum of the skull of nude mice. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222111331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochira A., Siculella L., Damiano F., Palermo A., Ferrante F., Carluccio M.A., et al. Concentrated growth factors (CGF) induce osteogenic differentiation in human bone marrow stem cells. Biology. 2020;9:370. doi: 10.3390/biology9110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang F., Zhang R., Xu J., Du J., Leng S., Zhang L., et al. Comparative effects of concentrated growth factors on the biological characteristics of periodontal ligament cells and stem cells from apical papilla. J Endod. 2022;48:1029–1037. doi: 10.1016/j.joen.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Liao Y., Fang Y., Zhu H., Huang Y., Zou G., Dai B., et al. Concentrated growth factors promote hBMSCs osteogenic differentiation in a Co-culture system with HUVECs. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.837295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blin-Wakkach C., De Vries T.J. Editorial: advances in osteoimmunology. Front Immunol. 2019;10:2595. doi: 10.3389/fimmu.2019.02595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humbert P., Brennan M.Á., Davison N., Rosset P., Trichet V., Blanchard F., et al. Immune modulation by transplanted calcium phosphate biomaterials and human mesenchymal stromal cells in bone regeneration. Front Immunol. 2019;10:663. doi: 10.3389/fimmu.2019.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miron R.J., Bohner M., Zhang Y., Bosshardt D.D. Osteoinduction and osteoimmunology: emerging concepts. Periodontol. 2000 doi: 10.1111/prd.12519. 2023:prd. [DOI] [PubMed] [Google Scholar]
- 17.Wasnik S., Rundle C.H., Baylink D.J., Yazdi M.S., Carreon E.E., Xu Y., et al. 1,25-Dihydroxyvitamin D suppresses M1 macrophages and promotes M2 differentiation at bone injury sites. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo H., Liu W., Zhou Y., Jiang X., Liu Y., Yang Q., et al. Concentrated growth factor regulates the macrophage-mediated immune response. Regen Biomater. 2021;8 doi: 10.1093/rb/rbab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodella L.F., Favero G., Boninsegna R., Buffoli B., Labanca M., Scarì G., et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech. 2011;74:772–777. doi: 10.1002/jemt.20968. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J., Yin C., Zhao Q., Zhao Z., Wang J., Miron R.J., et al. Anti-inflammation effects of injectable platelet-rich fibrin via macrophages and dendritic cells. J Biomed Mater Res, Part A. 2020;108:61–68. doi: 10.1002/jbm.a.36792. [DOI] [PubMed] [Google Scholar]
- 21.He L., Jhong J.-H., Chen Q., Huang K.-Y., Strittmatter K., Kreuzer J., et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida K., Nagatake T., Saika A., Kawai S., Node E., Hosomi K., et al. Induction of unique macrophage subset by simultaneous stimulation with LPS and IL-4. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L., Wang Z., Sun X., Yu J., Li T., Zhao H., et al. STAT3/Mitophagy Axis coordinates macrophage NLRP3 inflammasome activation and inflammatory bone loss. J Bone Miner Res. 2020;38:335–353. doi: 10.1002/jbmr.4756. [DOI] [PubMed] [Google Scholar]
- 24.Abaricia J.O., Shah A.H., Chaubal M., Hotchkiss K.M., Olivares-Navarrete R. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials. 2020;243 doi: 10.1016/j.biomaterials.2020.119920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S., Saeed A.F.U.H., Liu Q., Jiang Q., Xu H., Xiao G.G., et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Targeted Ther. 2023;8:207. doi: 10.1038/s41392-023-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sha W., Zhao B., Wei H., Yang Y., Yin H., Gao J., et al. Astragalus polysaccharide ameliorates vascular endothelial dysfunction by stimulating macrophage M2 polarization via potentiating Nrf2/HO-1 signaling pathway. Phytomedicine. 2023;112 doi: 10.1016/j.phymed.2023.154667. [DOI] [PubMed] [Google Scholar]
- 27.Kadomoto S., Izumi K., Mizokami A. Macrophage polarity and disease control. Indian J Manag Sci. 2021;23:144. doi: 10.3390/ijms23010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locati M., Curtale G., Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natoli G. Maintaining cell identity through global control of genomic organization. Immunity. 2010;33:12–24. doi: 10.1016/j.immuni.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Wang L., Li S., Zhang T., Chen C., Hu J., et al. Mechanical stimulation improves rotator cuff tendon-bone healing via activating IL-4/JAK/STAT signaling pathway mediated macrophage M2 polarization. J Orthop Trans. 2022;37:78–88. doi: 10.1016/j.jot.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B., Huang J., Xiao J., Xu W., Zhang H., Yuan Y., et al. The Streptococcus virulence protein PepO triggers anti-tumor immune responses by reprograming tumor-associated macrophages in a mouse triple negative breast cancer model. Cell Biosci. 2023;13:198. doi: 10.1186/s13578-023-01153-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu G., Yu Y., Sharma D., Pruett-Miller S.M., Ren Y., Zhang G.-F., et al. Glutathione limits RUNX2 oxidation and degradation to regulate bone formation. JCI Insight. 2023;8 doi: 10.1172/jci.insight.166888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X., Wan Z., Yang L., Song S., Fu Z., Tang K., et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J Nanobiotechnol. 2022;20:110. doi: 10.1186/s12951-022-01314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow S.K.-H., Wong C.H.-W., Cui C., Li M.M.-C., Wong R.M.Y., Cheung W.-H. Modulating macrophage polarization for the enhancement of fracture healing, a systematic review. J Orthop Trans. 2022;36:83–90. doi: 10.1016/j.jot.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z., Liu L., Wang L., Song D. The effects and potential applications of concentrated growth factor in dentin–pulp complex regeneration. Stem Cell Res Ther. 2021;12:357. doi: 10.1186/s13287-021-02446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.