Abstract
Background
Insular thyroid carcinoma is a rare subtype of thyroid cancer that constitutes an intermediate entity between differentiated (papillary & follicular) and undifferentiated (anaplastic) thyroid cancer.
Methods
This is a retrospective study that included all the patients with insular carcinoma of the thyroid gland who underwent surgical treatment in our department from January 2009 to December 2023. The epidemiological, clinical, and oncological data of the included patients were analyzed.
Results
A total of 1690 patients with thyroid cancer were screened. Twenty-four patients were included in the final analysis. The median time to recurrence (95% CI) was 24 months while the Restricted Mean Survival Time (RMST) at time point 24 months (95% CI) was 16.95. The median time to distant metastasis is 60 months while RMST at time point 24 months was 17.1. The median time to death was 55 months. There was a statistically significant difference in the RMST at 24 months for overall survival (OS) as regards older age, presence of comorbidity, multifocality, and lack of adjuvant RAI, but not sex. Male sex and lack of adjuvant RAI therapy were statistically significant independent predictors of the time to locoregional recurrence. There was no statistically significant difference in the time to distant metastasis as regards all the variables.
Conclusions
From our results, we can conclude that male sex, multifocality, and lack of RAI affect survival in patients with insular thyroid carcinoma. Adequate surgical resection of the thyroid gland and draining lymph nodes in addition to radioactive iodine remains the mainstay of treatment.
Keywords: Insular, Thyroid cancer, Survival, Prognosis, Staging
Introduction
Insular thyroid carcinoma is a rare subtype of thyroid cancer that constitutes an intermediate entity between differentiated (papillary & follicular) and undifferentiated (anaplastic) thyroid cancer. It carries an intermediate prognosis between both types, so early detection, optimal surgical intervention, and close follow-up are highly indicated in such groups of patients [1].
It was first described in 1984 and incorporated into the 2004 World Health Organization classification in the context of poorly differentiated carcinoma. While the diagnostic criteria are debatable, insular pattern (nests of follicular neoplastic cells), increased mitotic activity, and aggressive clinical behavior are needed to establish the diagnosis [1–3] Regarding its IHC pattern, insular carcinoma is usually positive for thyroglobulin, CEA and negative for calcitonin, synaptophysin, Chromogranin, and LCA to help distinguish it from medullary thyroid carcinoma and lymphoma consequently [4].
Despite having no unique clinical presentation, usually, the patients are presented with a long-standing thyroid swelling. Compression symptoms may be present as well. Some patients are presented with symptoms of nodal or distant spread [1, 3].
Insular thyroid carcinoma is best treated by aggressive surgical intervention trying to achieve complete resection of the tumor and extrathyroidal spread followed by radioactive iodine therapy in iodine avid tumors and molecular targeted therapy in iodine non-avid tumors [5].
In this manuscript, we present a 15-year outcome of a tertiary referral cancer center for patients with insular thyroid carcinoma. The epidemiological, clinical, and oncological outcomes were analyzed and presented to provide a picture of this rare disease aiming to improve its understanding of its nature and subsequently its management of such an aggressive disease.
Patients and methods
Source
This is a retrospective study that included all the patients with insular carcinoma of the thyroid gland who underwent surgical treatment in the surgical oncology department at the Mansoura University oncology center from January 2009 to December 2023. They were selected among a total of 1690 patients with thyroid cancer who underwent surgical management during this period. The epidemiological, clinical, and oncological data of the included patients were analyzed. IRB approval was obtained from the Institutional Research Board at the faulty of Medicine, Mansoura University under the number R.24.02.2499.
Variables
The following data were collected for every patient:
Epidemiological: Age, gender, medical comorbidities.
Clinical: complaint, site of the nodule, size, focality, laterality, associated lymph nodes, TIRADS, FNAC, type of surgery, and complications.
Oncological: pathological type, size, lymph node affection, stage, adjuvant treatment, recurrence, metastasis and survival.
Inclusion criteria
Any patient with pathologically proven insular thyroid carcinoma underwent surgical management during the study period.
Exclusion criteria
Missed or non-registered data.
No surgical management.
Non-confirmed diagnosis.
Pathology review
The pathology slides of the included patients were reviewed by an independent pathologist. Those with a different diagnosis were excluded from the study.
Primary outcome
The disease-free survival.
Secondary outcome
The development of metastases and overall survival.
Statistical analysis
Data were entered and analyzed using MedCalc® Statistical Software version 20.215 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2023).
Qualitative data were expressed N (%) while quantitative data were initially tested by Shapiro-Wilk’s test and the boxplots and were expressed as mean (SD) if normally distributed or median (Q1-Q3) if not. Intraclass correlation coefficient (ICC) and Bland-Altman plot were used to assess the agreement between the two methods of measurement. The Kaplan-Meier method was used to estimate the probability of survival past given time points. The survival distributions of two or more groups of a between-subjects factor were compared for equality using the log-rank test. Cox regression was used to investigate the effect of several variables on the time a specified event takes to happen. The hazard ratio associated with a predictor variable is given by the exponent of its coefficient; with a 95% confidence interval. The restricted mean survival time (RMST) was reported with its 95% confidence interval at a 24-month time point. Differences in RMST between groups were reported as P-values. For any of the used tests, results were considered statistically significant if p-value ≤ 0.050. Appropriate charts were used to graphically present the results whenever needed.
Results
A total of 1690 patients with thyroid cancer who underwent surgical management at our department were screened. Forty-four patients were assessed for inclusion in the study. After excluding 20 patients with incomplete data, lost follow-up, and non-confirmed diagnosis after pathology review, 24 patients were included in the final analysis. The epidemiological and clinical data of the included patients are summarized in Table 1.
Table 1.
Epidemiological and clinical variables
| Characteristic | N | % |
|---|---|---|
| Age (years) | 54.5 (SD:13) | |
| Sex | ||
| Male | 9 | 37.5 |
| Female | 15 | 62.5 |
| Comorbidity | 13 | 54.2 |
| Diabetes | 9 | 37.5 |
| Hypertension | 8 | 33.3 |
| Complaint | ||
| Neck swelling | 22 | 91.7 |
| Pain | 2 | 8.3 |
| Compression symptoms | 13 | 54.2 |
| Hoarseness of voice | 4 | 16.7 |
| Site | ||
| Right lobe | 3 | 12.5 |
| Left lobe | 7 | 29.2 |
| Isthmus | 13 | 54.4 |
| Both lobes | 1 | 4.2 |
| Focality | ||
| Unifocal | 13 | 54.2 |
| Multifocal | 11 | 45.8 |
| Largest tumor diameter (cm) | ||
| Radiological | 4.25 (2.58–6.38) | |
| Indirect Laryngoscopy | ||
| Not registered | 10 | 41.7 |
| Unilateral / Fixed | 2 | 8.3 |
| Bilateral / Mobile | 11 | 45.8 |
| Bilateral / Fixed | 1 | 4.2 |
| TI-RADs (n = 10) | ||
| TI-RAD 4 | 6 | 60 |
| TI-RAD 5 | 4 | 40 |
| Preoperative FNAC (Bethesda system) n = 19 | ||
| Category I (nondiagnostic) | 1 | 5.3 |
| Category II (benign) | 2 | 10.5 |
| Category III (atypia of undetermined significance) | 5 | 26.3 |
| Category IV (suspicious for follicular neoplasm) | 5 | 26.3 |
| Category V (suspicious for malignancy) | 5 | 26.3 |
| Category VI (malignant) | 1 | 5.3 |
| Suspicious LN | ||
| Laterality of suspicious LN | 13 | 54.2 |
| Ipsilateral | 5 | 38.5 |
| Bilateral | 8 | 61.5 |
Twenty-two of the included patients underwent total thyroidectomy (with no gross residual tumor tissue left in 16 patients) while two had irresectable tumors and underwent isthmusectomy & tracheostomy. Eleven patients underwent lateral neck dissection. While fifteen patients needed to receive adjuvant radioactive iodine, receiving RAI by nine patients was not confirmed (two patients died postoperatively, one patient received radiotherapy only and six patients had inconsistent adjuvant treatment data). Ten patients developed distant metastasis during the follow-up period. The surgical and oncological outcomes are summarized in Table 2.
Table 2.
Treatment and follow-up variables
| Type of surgery | ||
| Isthmectomy plus tracheostomy | 2 | 8.3 |
| Total thyroidectomy | 22 | 91.7 |
| Lateral BND n = 11 | ||
| Modified | 1 | 9.1 |
| Selective | 10 | 90.9 |
| Operative time (hours) | 2.15 (2–4.25) | |
| Largest tumor diameter (cm) | ||
| Pathological | 5 (3–8.25) | |
| Extra-thyroidal extension | 11 | 45.8 |
| Lymphovascular emboli (LVE) | 10 | 41.7 |
| Lymph nodes | ||
| Number retrieved. | 11 (4–27) | |
| Number positive | 0.5 (0–9) | |
| Tumor staging | ||
| T | ||
| T2 | 12 | 50 |
| T3 | 4 | 16.7 |
| T4 | 8 | 33.3 |
| N1 | 10 | 41.7 |
| M1 | 7 | 29.1 |
| Stage | ||
| Stage 1 | 10 | 41.7 |
| Stage 2 | 7 | 292. |
| Stage 3 | 5 | 20.8 |
| Stage 4 | 2 | 8.3 |
| Postoperative complications | 8 | 33.3 |
| Postoperative Mortality | 2 | 8.3 |
| Adjuvant RAI therapy | 15 | 62.5 |
| Locoregional recurrence | 10 | 41.7 |
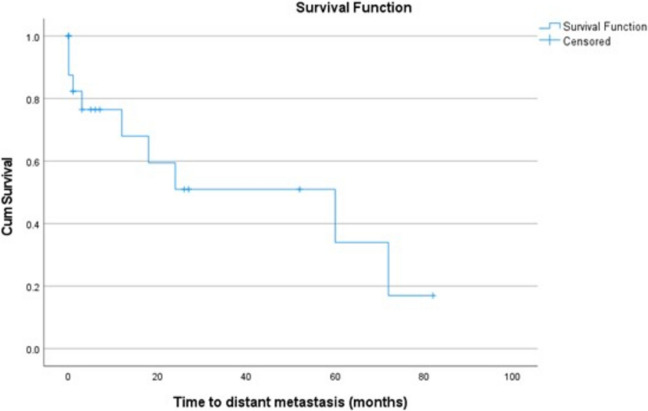
Regarding the primary and secondary outcomes, the median time to recurrence (95% CI) was 24 (0–65.2) months while the Restricted Mean Survival Time (RMST) at time point 24 months (95% CI) was 16.95 (12.8–21.1) (Fig. 1). The median time to distant metastasis (95% CI) is 60 (6.5–113.5) months while the Restricted Mean Survival Time (RMST) at time point 24 months (95% CI) was 17.1 (12.7–21.4) (Fig. 2). The median time to death (95% CI) is 55 (7.6–102.4) months while the Restricted Mean Survival Time (RMST) at time point 24 months (95% CI) is 15.6 (13.8–17.5) (Fig. 3).
Fig. 1.
Kaplan-Meyer curve for the time to recurrence
Fig. 2.
Kaplan-Meyer curve for the time to distant metastasis
Fig. 3.
Kaplan-Meyer curve for the time to death (overall mortality)
Univariate and multivariate analysis was conducted to evaluate factors affecting the recurrence, metastasis, and survival. Table 3 shows a statistically significant difference in the RMST at 24 months for OS as regards older age, presence of comorbidity, multifocality, and lack of adjuvant RAI, but not sex. Accordingly, a multivariable Cox regression was conducted (Table 4) to ascertain the effects of older age, presence of comorbidity, multifocality, and lack of adjuvant RAI on the time to death. The model was statistically significant (χ2 [4] = 9.595, p = .0478). The model has a good prediction performance with Harrell’s C-index (95% CI) of 0.806 (0.713–0.899). Of the 4 predictor variables, lack of adjuvant RAI therapy was the only statistically significant independent predictor of overall survival.
Table 3.
Risk factors for overall survival (time to death)
| Risk factor | n/N (%) | Median survival (95% CI) | RMST at 24-months | Log rank test | HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | p-value | χ2 | p-value | ||||
| Age (years) | < 0.001 | 2.048 | 0.153 | ||||
| < 60 | 7/14 (50) | 76 (10–82) | 19.7 (18.1–21.2) | r (1) | |||
| ≥ 60 | 9/10 (90) | 1 (0–76) | 10 (6.9–13) | 2.2 (0.74–6.8) | |||
| Sex | 0.563 | 1.840 | 0.175 | ||||
| Female | 10/15 (66.7) | 76 (0–82) | 16.1 (13.6–18.7) | r (1) | |||
| Male | 6/9 (66.7) | 43 (0–55) | 15.1 (12.7–17.5) | 2.4 (0.67–8.9) | |||
| Comorbidity | 0.007 | 0.679 | 0.410 | ||||
| No | 5/11 (45.5) | 76 (0–78) | 18.2 (15.7–20.7) | r (1) | |||
| Yes | 11/13 (84.6) | 7 (1–76) | 13.3 (10.8–15.9) | 1.6 (0.53–4.8) | |||
| Focality | < 0.001 | 1.431 | 0.232 | ||||
| Unifocal | 6/13 (46.2) | 76 (43–82) | 20.4 (18.3–22.4) | r (1) | |||
| Multifocal | 10/11 (90.9) | 7 (0–76) | 10.2 (7.4–13) | 1.9 (0.66–5.7) | |||
| Adjuvant RAI | < 0.001 | 8.617 | 0.009 | ||||
| Yes | 8/15 (53.3) | 76 (43–78) | 21.4 (20.1–22.6) | r (1) | |||
| No | 8/9 (88.9) | 1 (0–7) | 5.6 (2.8–8.4) | 5.3 (1.5–18.5) | |||
n/N Frequency of events / number of cases, RMST Restricted mean survival time, HR Hazard ratio, χ2 Chi-square, r (1) Reference category
Table 4.
Cox proportional-hazards regression model for predicting overall survival
| Predictor | b | SE | Wald | Sig. | AHR | 95% CI of AHR |
|---|---|---|---|---|---|---|
| Age > 60 years | 0.7376 | 0.6948 | 1.1271 | 0.2884 | 2.0910 | 0.5357 to 8.1619 |
| Presence of Comorbidity | −0.1156 | 0.6372 | 0.03291 | 0.8560 | 0.8908 | 0.2555 to 3.1057 |
| Multifocality | 0.5661 | 0.6947 | 0.6641 | 0.4151 | 1.7615 | 0.4514 to 6.8743 |
| Lack of adjuvant RAI therapy | 1.6883 | 0.6607 | 6.5299 | 0.0106 | 5.4102 | 1.4819 to 19.7516 |
SE Standard error, Sig. p-value, AHR Adjusted hazard ratio, CI Confidence interval
Table 5 shows a statistically significant difference in the RMST at 24 months for locoregional recurrence as regards male sex, and a statistically significant difference in survival distributions (log-rank test) for locoregional recurrence as regards sex and lack of adjuvant RAI. Accordingly, a multivariable Cox regression (Table 6) was conducted to ascertain the effects of male sex, multifocality, and lack of adjuvant RAI on the time to locoregional recurrence. The model was statistically significant (χ2 [3] = 12.676, p = .0054). The model has a good prediction performance with a Harrell’s C-index of 0.864. Of the 3 predictor variables, male sex and lack of adjuvant RAI therapy were statistically significant independent predictors of the time to locoregional recurrence.
Table 5.
Risk factors for time to Locoregional recurrence
| Risk factor | n/N (%) | Median survival (95% CI) | RMST at 24-months | Log rank test | HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | p-value | χ2 | p-value | ||||
| Age (years) | 0.369 | 0.568 | 0.451 | ||||
| ≥ 60 | 3/10 (30) | 24 (7–84) | 19.8 (12.5–27) | r (1) | |||
| < 60 | 7/14 (50) | 18 (9–72) | 15.7 (10.8–20.7) | 1.7 (0.42–7.1) | |||
| Sex | 0.025 | 4.097 | 0.043 | ||||
| Female | 5/15 (33.3) | 72 (9–84) | 20.6 (16.5–24.7) | r (1) | |||
| Male | 5/9 (55.6) | 9 (1–18) | 11.9 (5.5–18.4) | 5 (1.05–23.4) | |||
| Comorbidity | 0.690 | 0.062 | 0.804 | ||||
| No | 4/11 (36.4) | 24 (2–24) | 17.8 (12.2–23.4) | r (1) | |||
| Yes | 6/13 (46.2) | 18 (7–84) | 16.2 (10.2–22.1) | 1.2 (0.29–4.9) | |||
| Focality | 0.075 | 2.379 | 0.123 | ||||
| Unifocal | 4/13 (30.8) | 72 (-) | 19.9 (15.8–23.9) | r (1) | |||
| Multifocal | 6/11 (54.6) | 12 (1–84) | 12.2 (4.8–19.6) | 3.1 (0.74–12.9) | |||
| Adjuvant RAIa | 0.205 | 8.255 | 0.004 | ||||
| Yes | 7/15 (46.7) | 72 (9–84) | 8.5 (7.4–9.5) | r (1) | |||
| No | 3/9 (33.3) | 7 (1–9) | 6.6 (3.9–9.3) | 39.7 (3.2–490) | |||
n/N Frequency of events / number of cases, RMST Restricted mean survival time, HR Hazard ratio, c2: Chi-square, r (1): Reference category
aRMST was readjusted at time point 9 months
Table 6.
Cox proportional-hazards regression model for predicting time to locoregional recurrence
| Predictor | b | SE | Wald | Sig. | AHR | 95% CI of AHR |
|---|---|---|---|---|---|---|
| Male sex | 8229 | 0.8908 | 4.1877 | 0.0407 | 6.1898 | 1.0800 to 35.4755 |
| Multifocality | 3227 | 0.8679 | 2.3224 | 0.1275 | 3.7535 | 0.6849 to 20.5707 |
| Lack of Adjuvant RAI therapy | 2.4405 | 1.1849 | 4.2418 | 0.0394 | 11.4784 | 1.1252 to 117.0914 |
SE Standard error, Sig. p-value, AHR Adjusted hazard ratio, CI Confidence interval
Finally, Table 7 shows no statistically significant difference in the time to distant metastasis as regards all 5 variables (age, sex, comorbidity, focality, and adjuvant RAI).
Table 7.
Risk factors for time to distant metastasis
| Risk factor | n/N (%) | Median survival (95% CI) | RMST at 24-months | Log rank test | HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | p-value | χ2 | p-value | ||||
| Age (years) | 0.788 | 0.644 | 0.422 | ||||
| < 60 | 6/14 (42.9) | 72 (3–72) | 16.9 (11.8–22.1) | r (1) | |||
| ≥ 60 | 4/10 (40) | 24 (0–60) | 18.2 (10.9–25.4) | 1.8 (0.43–7.6) | |||
| Sex | 0.181 | 1.392 | 0.238 | ||||
| Female | 6/15 (40) | 60 (12–72) | 19.3 (14.6–24) | r (1) | |||
| Male | 4/9 (44.4) | 18 (0–18) | 13.4 (6.1–20.7) | 2.5 (0.54–12) | |||
| Comorbidity | 0.440 | 0.617 | 0.432 | ||||
| No | 5/11 (45.5) | 24 (0–24) | 15.5 (8.9–22) | r (1) | |||
| Yes | 5/13 (38.5) | 60 (1–72) | 18.8 (13.6–24) | 0.57 (0.14–2.3) | |||
| Focality | 0.779 | 0.136 | 0.712 | ||||
| Unifocal | 6/13 (46.2) | 72 (0–72) | 15.8 (9.8–21.8) | r (1) | |||
| Multifocal | 4/11 (36.4) | 24 (3–60) | 17.2 (9.8–24.5) | 1.3 (0.33–5.2) | |||
| Adjuvant RAI | 0.145 | 1.888 | 0.169 | ||||
| Yes | 9/15 (60) | 24 (1–72) | 16 (10.7–21.2) | r (1) | |||
| No | 1/9 (11.1) | - (-) | 21.3 (16.4–26.3) | 0.36 (0.08–1.5) | |||
This table shows no statistically significant difference in the time to distant metastasis as regards all 5 variables (age, sex, comorbidity, focality, and adjuvant RAI)
n/N Frequency of events/number of cases, RMST Restricted mean survival time, HR Hazard ratio, χ 2 : Chi-square, r (1): Reference category
Discussion
Insular thyroid carcinoma, which constitutes 1–10% of all thyroid cancers, is a variant of poorly differentiated thyroid carcinoma which was first named in 1984 as an independent thyroid cancer subtype that arises from follicular cells and is presented as an intermediate biological and morphological category between differentiated thyroid cancers (papillary and follicular) and anaplastic thyroid carcinoma. It behaves aggressively more than differentiated thyroid cancer but is not as lethal as anaplastic thyroid cancer [3, 6, 7].
Insular thyroid carcinoma usually affects women more than men with the median age being the fifth decade [8]. It is usually presented as large neck swellings with a tendency to rapid growth and compression symptoms. It has no specific ultrasonographic features and its diagnosis by fine needle aspiration is a real challenge [6]. In our study the median age was 54 years, coping with that reported in the literature. The incidence was more in women than men as well.
Microscopic examination of insular thyroid carcinoma FNAC usually demonstrates high cellularity of round and small malignant cells arranged in insula (nests) which may be enveloped by a single layer of endothelial cells. The tumor cells usually have a high nucleocytoplasmic ratio, increased mitotic figures, and necrosis (less than ATC). By Immunocytochemically (IHC), insular thyroid carcinoma shows thyroglobulin and Bcl-2 positivity, high ki-67, and calcitonin negativity. These morphological features in addition to the IHC can help differentiate insular carcinoma from other primary thyroid tumors likely papillary, follicular, medullary, anaplastic carcinomas, and lymphoma, as well as metastasis to the thyroid gland [9].
The mainstay in the treatment of insular thyroid carcinoma is adequate surgical resection which includes total thyroidectomy and neck dissection of the involved lymph node groups. It is also advised to perform routine central neck dissection for all the patients preoperatively diagnosed with insular thyroid carcinoma. This should be followed by radioactive iodine for iodine-avid tumors which are the majority of tumors while radiotherapy, cytoreductive surgery, chemotherapy, bone-directed therapy, and molecular targeted therapy are valid options in iodine-resistant or non-iodine avid tumors [6–8, 10–13] The majority of patients in our cohort underwent total thyroidectomy (22 out of 24) with the incidence of postoperative mortality at 8.3%. This percentage may be considered high but actually, it is due to the small sample size. Only two patients died postoperatively due to a chest infection. Fifteen patients received RIA. Not receiving RAI was the most significant factor affecting the incidence of local recurrence and distant metastasis.
The prognosis of insular thyroid cancer is worse than differentiated thyroid cancers but better than anaplastic carcinoma. Insular thyroid carcinomas tend to be presented with larger tumors than differentiated carcinomas, more extrathyroidal extension, more tendency for nodal metastasis, and less possibility to achieve R0 resection. Bad prognostic factors of insular thyroid cancer include age older than 45 years, male gender, advanced stages, large-sized tumors, and incomplete resection [6, 12, 13] In our study, male sex and lack of RAI contributed to the incidence of local recurrence while the lack of RAI in addition to multifocality contributed to the incidence of distant metastasis.
Our study has strengths and limitations. The limitations include being a retrospective, single-center study, relatively low sample size making it difficult to draw solid conclusions, and missing data about radiation doses and details of imaging before RAI. It has strengths like presenting the experience of a tertiary referral center over a decade and a half and having the pathology of the included patients revised by an expert pathologist.
Conclusion
Insular thyroid carcinoma is a rare variant of poorly differentiated thyroid carcinoma with pathological and behavioral attitudes which are intermediate between differentiated and anaplastic thyroid carcinomas. Male sex, multifocality, and lack of RAI affect the patient’s survival. Adequate surgical resection of the thyroid gland and draining lymph nodes in addition to radioactive iodine remains the mainstay of treatment.
Acknowledgements
Not applicable.
Authors’ contributions
All authors have read and approved the manuscript. Data collection and revision: MHS, MAM. Conceptualization, writing: OH. Supervision and revision: SA, OH. Statistical analysis: AFE. Pathology part preparation & revision: AEE.
Funding
No funding was received for this research.
Data availability
All the clinical, radiological, and pathological data used in this manuscript are available on Mansoura University medical system (Ibn Sina Hospital Management System). http://srv137.mans.edu.eg/mus/newSystem/.
Declarations
Ethics approval and consent to participate
All procedures performed in the study involving human participants followed the ethical standards of the institutional research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. IRB approval was obtained from the Institutional Research Board at the Faculty of Medicine, Mansoura University under the number R.24.02.2499.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: "The author name Mohamed A Abdelfatth was corrected to Mohamed A Abdelfattah."
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/19/2025
A Correction to this paper has been published: 10.1186/s12902-025-01956-7
References
- 1.Abdellaoui W, Assarrar I, Benyakhlef S, Tahri A, Messaoudi N, Haloui A, et al. Insular thyroid carcinoma in a young Moroccan man: case report and review of the literature. Ann Med Surg. 2022;77:103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31(8):1256–64. [DOI] [PubMed] [Google Scholar]
- 3.Carcangiu ML, Zampi G, Rosai J. Poorly differentiated (insular) thyroid carcinoma. A reinterpretation of langhans’ wuchernde struma. Am J Surg Pathol. 1984;8(9):655–68. [DOI] [PubMed] [Google Scholar]
- 4.Cornetta AJ, Burchard AE, Pribitkin EA, O’Reilly RC, Palazzo JP, Keane WM. Insular carcinoma of the thyroid. Ear Nose Throat J. 2003;82(5):384–9. [PubMed] [Google Scholar]
- 5.Uçmak G, Demirel BB. In: Özülker T, Adaş M, Günay S, editors. The clinical management of a patient with insular thyroid carcinoma BT - thyroid and parathyroid diseases: a case-based guide. Cham: Springer International Publishing; 2019. p. 365–70. 10.1007/978-3-319-78476-2_58. [Google Scholar]
- 6.Fat I, Kulaga M, Regina, Carling T, Theoharis C, Rennert NJ. Insular variant of poorly differentiated thyroid carcinoma. Endocrine Practice. 2011;17(1):115–21 https://www.sciencedirect.com/science/article/pii/S1530891X20408651. [DOI] [PubMed] [Google Scholar]
- 7.Yin L, Hou S, Hou LL, Pu CC. Clinical characteristics and prognostic nomogram for patients with insular thyroid carcinoma: a population-based analysis. Endocrine. 2023;79(2):331–41. 10.1007/s12020-022-03200-x. [DOI] [PubMed] [Google Scholar]
- 8.Hod R, Bachar G, Sternov Y, Shvero J. Insular thyroid carcinoma: a retrospective clinicopathologic study. Am J Otolaryngol. 2013;34(4):292–5. Available from https://www.sciencedirect.com/science/article/pii/S0196070913000021. [DOI] [PubMed] [Google Scholar]
- 9.Kini H, Nirupama M, Rau AR, Gupta S, Augustine A. Poorly differentiated (insular) thyroid carcinoma arising in a long-standing colloid goitre: a cytological dilemma. J Cytol. 2012;29(1):97–9. Available from: https://journals.lww.com/jocy/fulltext/2012/29010/poorly_differentiated__insular__thyroid_carcinoma.28.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu PC, Shi X, Ma B, Li CW, Tan LC, Hu WP et al. Treating Clinically Node-Negative Insular Thyroid Carcinoma without Prophylactic Central Compartment Neck Dissection Is Associated with Decreased Survival Regardless of T Staging and Administration of Radioactive Iodine Therapy: The First Evidence. Rosato L, editor. Int J Endocrinol. 2019;2019:3078012. 10.1155/2019/3078012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uçmak G, Demirel BB. The clinical management of a patient with insular thyroid carcinoma. In: Özülker T, Adaş M, Günay S, editors. Thyroid and parathyroid diseases: a case-based guide. Cham: Springer International Publishing; 2019. p. 365–70. 10.1007/978-3-319-78476-2_58. [Google Scholar]
- 12.Pezzi TA, Sandulache VC, Pezzi CM, Turkeltaub AE, Feng L, Cabanillas ME, et al. Treatment and survival of patients with insular thyroid carcinoma: 508 cases from the national cancer data base. Head Neck. 2016;38(6):906–12. 10.1002/hed.24342. [DOI] [PubMed] [Google Scholar]
- 13.Kazaure HS, Roman SA, Sosa JA. Insular thyroid cancer. Cancer. 2012;118(13):3260–7. 10.1002/cncr.26638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the clinical, radiological, and pathological data used in this manuscript are available on Mansoura University medical system (Ibn Sina Hospital Management System). http://srv137.mans.edu.eg/mus/newSystem/.