Abstract
Human caliciviruses (HuCVs) are classified into the Norwalk-like viruses (NLV) and Sapporo-like viruses (SLV) as genera within the family Caliciviridae. The NLV genus is further classified into genogroups I and II, based on sequence similarities. To study the antigenic determinants on the HuCV capsid protein and develop new diagnostic tools for field samples, we established and characterized monoclonal antibodies (MAbs) against baculovirus-expressed recombinant HuCV virus-like particles (VLPs). Hybrid clones producing MAbs were obtained from cultures of PAI myeloma cells fused with spleen or mesenteric lymph node cells from mice immunized orally with either a single type of recombinant Norwalk virus (rNV), Kashiwa 47 virus (rKAV), Snow Mountain agent (rSMA), or Sapporo virus (rSV) VLP or with mixtures of two types of VLPs from different genogroups. Twenty MAbs, obtained as mouse ascites, were characterized and classified into six groups according to their enzyme-linked immunosorbent assay (ELISA) and Western blotting (WB) cross-reactivity patterns to VLPs. Five groups of MAbs reacted by both WB and ELISA and were classified as follows: common cross-reactive MAbs for four genogroup I and six genogroup II VLPs (group A), genogroup I-specific MAbs (group B), genogroup II-specific MAbs (group C), and strain-specific MAbs (groups D and E). One MAb group (group F) reacted only by ELISA. The group A MAbs, which showed broad cross-reactivity with VLPs of both NLV genogroups, were obtained from mice immunized orally with a single type of VLP (either rNV or rKAV). Two MAbs, which were obtained from mice immunized with rSV, reacted with rSV but not with any NLV VLP. These are the first MAbs to be reported for any SLV. These strain-, genogroup-, and genus-reactive MAbs will be useful tools for further study of the antigenic and structural topography of the HuCV virion and for diagnostic assays for HuCVs.
Viruses in the Caliciviridae family are important causes of acute, epidemic nonbacterial gastroenteritis in humans (2, 12). Despite the clear association of these viruses with gastroenteritis and the initial description of the virus by use of immune electron microscopy (27), they remained unclassified until 1990 due to the lack of a cell culture system or a small animal model. Cloning and sequencing of the Norwalk virus (NV) genome (22, 26) and the Southampton virus (33) showed these viruses possess an ∼7.7-kb genome of single-stranded positive-sense RNA which is predicted to encode three primary open reading frames (ORFs). The capsid protein of NV spontaneously assembles into empty virus-like particles (VLPs) when expressed in insect cells infected with a recombinant baculovirus containing the capsid protein gene (25). These recombinant NV (rNV) VLPs resemble native NVs morphologically and antigenically (13, 25). The NV capsid protein, encoded by the second ORF (ORF2), is 530 amino acids in length and has an apparent molecular weight of 58,000 (5, 14, 25).
Human caliciviruses (HuCVs) are classified into two genera, Norwalk-like viruses (NLVs) and Sapporo-like viruses (SLVs) (10). The NLV genus has been further divided into two genogroups (I and II) on the basis of the genetic diversity of ORF2 or of the viral polymerase (10). Representative genogroup I viruses include the prototype NV, Southampton virus (33), Desert Shield virus (34), and Chiba virus (41), while representative genogroup II viruses include the prototype Snow Mountain agent (7, 28), Grimsby virus (15), Camberwell virus (40), Mexico virus (23), Hawaii virus (35), and others. (1, 10, 47; reviewed in references 6 and 12). Baculovirus-expressed VLPs from NV, Grimsby virus, Mexico virus, and Hawaii virus have been successfully used as antigens in the detection of antibody to NLVs (reviewed in reference 2). These studies have shown that infections with NLVs are common in all age groups and that children acquire antibodies to NLVs at an earlier age than previously believed (8, 37, 38, 39). The NLVs also play a predominant role as agents of epidemic gastroenteritis in nursing homes for the elderly (11). Enzyme-linked immunosorbent assays (ELISAs) for detection of viral antigen in stools also have been developed by using sera that are hyperimmune to the recombinant VLPs (7, 21). However, the efficiency of these antigen ELISAs has been relatively low due to the antigenic diversity among the NLVs and the relatively high specificity of the antisera raised for VLPs. Recent reports have suggested that NLVs can be further separated into at least 15 clusters, consisting of 7 genogroup I and 8 genogroup II clusters, based on the nucleotide sequence of approximately 300 bases in the capsid protein (9, 24; N. Takeda, unpublished data). Clear relationships between these genetic clusters and antigenicity has not yet been determined due to the lack of a cell culture system that would permit neutralization studies and distinction of viral serotypes. Little information is available on whether specific regions of the capsid protein are important for serotype specificity and whether type-specific and/or cross-reactive epitopes are present in HuCVs.
Hardy et al. (18) and Hale et al. (16) published the first reports of antigenic mapping of the rNV, using 10 monoclonal antibodies (MAbs) to begin to investigate how many epitopes exist on human caliciviruses. rNV VLPs are highly immunogenic when administered orally, in the absence of adjuvant, to mice and to human volunteers (3, 4), and oral immunization of mice with rNV VLPs results in high yields of MAbs (T. Tanaka, N. Kitamoto, X. Jiang, and M. K. Estes, unpublished data). This paper reports the establishment and characterization of new hybridoma cell lines from mice immunized orally with a single type of type of HuCV VLP, either rNV, rKashiwa 47 virus (rKAV), or rSapporo virus (rSV), or with mixtures of two types of VLPs from different genogroups. Previously unrecognized cross-reactive epitopes, shared among genogroup I and II NLVs, are detected by a subset of these new MAbs. In addition, we report the first MAbs for viruses in the SLV genus.
MATERIALS AND METHODS
Recombinant VLPs.
The expression and purification of several calicivirus VLPs were performed as previously described (15, 19, 25, 29-31, 36, 43; N. Takeda, unpublished data). These VLPs were used as immunogens for mice and as antigens for immunological tests. The recombinant VLPs of genogroup I NLVs used were from NV (rNV; sequence accession number M87661 [26]), Seto 124 virus (rSeV; AB031013 [this strain resembles KY89 virus] [31]), Chiba 407 virus (rCV; AB022679 [41]), and Funabashi 258 virus (rFUV; AB078335 [this strain resembles Southampton virus] [43]). The VLPs of genogroup II viruses were from Snow Mountain agent (rSMA; U70059 [17]), Grimsby virus (rGV; AJ004864 [15]), Kashiwa 47 virus (rKAV; AB078334 [unpublished data]), Chitta 76 virus [rCHV; AB032758 [this strain resembles Hawaii virus] [30]), Ueno 7K virus (rUEV; AB078337 [43]), and Narita 104 virus (rNAV; AB078336 [this strain resembles Camberwell virus] [unpublished data]). The recombinant VLPs of the SLVs were Sapporo virus (rSV; U95643 [20, 36]). The rNV, rSMA, rGV, and rSV VLPs were produced in the Division of Molecular Virology, Baylor College of Medicine. The recombinant rCV, rUEV, rSeV, rCHV, rFUV, rKAV, and rNAV VLPs were produced in the Department of Virology II, National Institute of Infectious Diseases (29-31, 43; N. Takeda, unpublished data). Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, Calif.), with bovine serum albumin as the protein standard.
Preparation of MAbs.
The PAI myeloma cell line (42), kindly provided by M. Kotani (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan), was cultured in Dulbecco's modified Eagle's medium with 15% fetal calf serum. The MAbs were prepared essentially as previously described (32), with minor modifications. Briefly, BALB/c mice were primed orally with 10 μg of purified VLPs (rNV, rKAV, rSMA, or rSV) per mouse, in the absence of adjuvant. In some cases, a mixture of 10 μg of rNV and 10 μg of rSMA was used for immunization. Mice received booster inoculations orally 4 times at 1-week intervals. The animals received a final intravenous injection of antigen. Three or 4 days later, the animals were sacrificed and cells from the spleen or mesenteric lymph nodes were fused with the myeloma cells. The culture medium of hybridomas which resulted from successful fusions was screened for reactivity by ELISA using plates coated with VLPs as described below. Positive hybridomas were cloned by limiting dilution, and antibody-producing clones were grown and stored in liquid nitrogen until used for further tests. Finally, ascites fluid was prepared by injection of hybridomas into pristane-primed mice and used for MAbs in this study. The isotype and subclass of each MAb were determined by Ouchterlony agar gel diffusion using anti-mouse subtype MAbs (Cappel Laboratories, West Chester, Pa.). Three previously described MAbs, NV3912, NV2461, and NV834 (16, 18), were also used in this study.
ELISA.
An ELISA employing antigen coating as described previously (7) was used with slight modification for the screening and the characterization of MAbs. Briefly, 96-well plates (Sumitomo Bakelite Co. Ltd., Tokyo, Japan) were coated with 100 ng of VLPs/well in 50 μl of 0.01 M phosphate-buffered saline (PBS) (pH 7.2) for 4 h at room temperature. Each well was washed with PBS-0.05% Tween 20 and blocked with 5% skim milk in PBS overnight at 4°C. MAbs (50 μl) were added as the first antibody and incubated for 2 h at 37°C. After washing with PBS-0.05% Tween 20, 50 μl of a 1:5,000 dilution of horseradish peroxidase (HRPO)-conjugated goat anti-mouse immunoglobulin G (IgG), IgM, or IgA (Cappel Laboratories) as the second antibody was reacted for 2 h at 37°C. After washing, 50 μl of ABTS-H2O2 [0.5 mg of 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS)/ml, 0.002% H2O2, 0.1 M citrate-phosphate buffer (pH 4.3)] as a substrate was developed for 20 min and the optical density at 405 nm and 630 nm was determined with a Microplate Reader (Bio-Rad Co., Richmond, Calif.).
Immunoblotting.
The VLPs were suspended in electrophoresis sample buffer containing 1% sodium dodecyl sulfate (SDS), 10% 2-mercaptoethanol, 50 mM Tris-HCl (pH 6.8), 0.0025% phenol red, and 10% glycerol. Samples were boiled for 2 min or not boiled (18) and were subjected to Western blotting (WB) analysis by the method of Towbin et al. (44), with slight modification. Briefly, SDS-gel electrophoresis was conducted in 10% polyacrylamide slab gel (catalog no. EMP-8020; 1 mm thick, 8 cm long, 7 cm wide; Iwaki). Lysed VLP protein (1 or 2 μg per track) was applied on the gel. After electrophoresis, the gel was transferred electrophoretically to a nitrocellulose sheet (0.45-μm pore size; Millipore) in a semidry transfer (EPM-8460; Iwaki) at a constant current of 70 mA for 3 h. The strips were then incubated overnight at room temperature with MAbs (ascites fluid) at a dilution of 1:1,000. The blots were incubated with a 1:2,000 dilution of HRPO-conjugated goat anti-mouse IgG, IgM, and IgA (Bio-Rad Co.) for 1 h at 37°C. The blots were then soaked in a solution of CNP (0.5 mg/ml; 4-chloro-1-naphthol, 0.001% H2O2, 0.1 M citrate-phosphate buffer, pH 6.0) to detect the antigen-antibody complexes on the strips.
RESULTS
Seventeen MAbs (tested as ascites) obtained from mice immunized orally with rNV, rKAV, or rSV or with mixtures of rNV and rSMA and three MAbs from mice immunized intraperitoneally with rNV were classified into six groups according to their ELISA and WB reactivity patterns with several VLPs (Table 1). The cross-reactivities of the MAbs observed by ELISA with different VLPs were confirmed by using hybridoma culture supernatants (data not shown). The MAb groups were as follows: common cross-reactive MAbs for genogroup I and II VLPs (group A), genogroup I-specific MAbs (group B), genogroup II-specific MAbs (group C), strain-specific MAbs (groups D and E), and ELISA-positive-WB-negative MAbs that could not be further classified (group F).
TABLE 1.
Reactivities of MAbs with various VLPs in WB and ELISA
| Group | MAb | Routeb | Organc | Immunogend | Isotype | Reactivity in WB
|
ELISA titer (103)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G-I
|
G-II
|
SLVs | G-I | G-II
|
SLVs | ||||||||||||||||
| NV | SeV | CV | FUV | SMA | GV | KAV | NAV | CHV | UEV | SV | NV | SMA | GV | KAV | SV | ||||||
| A | NV23 | po | SP | NV | IgG1 | + | + | + | + | + | + | + | + | + | + | − | >64 | >64 | >64 | >64 | <1 |
| NV37 | po | SP | NV | IgG1 | + | + | + | + | + | + | + | + | + | + | − | >64 | 64 | 32 | 64 | <1 | |
| NV3 | po | SP | NV | IgG1 | + | + | + | NTg | + | + | + | NT | NT | NT | − | >64 | NT | NT | NT | NT | |
| F8 | po | SP | KAV | IgG1 | + | + | + | + | + | + | + | + | + | + | − | >64 | 32 | >64 | >64 | <1 | |
| F120 | po | SP | KAV | IgG1 | + | + | + | + | + | + | + | + | + | + | − | 64 | 16 | 64 | 64 | <1 | |
| B | NV51 | po | LN | NV | IgA | + | + | + | + | − | − | − | − | − | − | − | >64 | <1 | <1 | <1 | <1 |
| NV138 | po | LN | NV | IgA | + | + | + | NT | − | − | − | NT | NT | NT | − | >64 | NT | NT | NT | NT | |
| NV172 | po | LN | NV | IgA | + | + | + | NT | − | − | − | NT | NT | NT | − | >64 | NT | NT | NT | NT | |
| NV3912e | ip | SP | NV | IgG1 | + | + | + | + | − | − | − | − | − | − | − | >64 | <1 | <1 | <1 | <1 | |
| NV2461e | ip | SP | NV | IgG1 | + | + | + | + | − | − | − | − | − | − | − | >64 | NT | NT | NT | NT | |
| C | NS14 | po | SP | NxS | IgG1 | − | − | − | − | + | + | + | + | + | + | − | 8 | >64 | >64 | >64 | <1 |
| NS28 | po | SP | NxS | IgG1 | − | − | − | − | + | + | + | + | + | + | − | 2 | >64 | >64 | >64 | <1 | |
| NS46 | po | SP | NxS | IgG1 | − | − | − | − | + | + | + | + | + | + | − | <1 | >64 | >64 | >64 | <1 | |
| D | NV5610 | po | LN | NV | IgA | +f | +f | − | − | − | − | − | − | − | − | − | >64 | <1 | <1 | <1 | <1 |
| NV5620 | po | LN | NV | IgA | +f | +f | − | − | − | − | − | NT | NT | NT | − | >64 | NT | NT | NT | NT | |
| NV834e | ip | SP | NV | IgG1 | +f | +f | − | − | − | − | − | − | − | − | − | >64 | <1 | <1 | <1 | <1 | |
| E | SV68 | po | SP | SV | IgG1 | − | − | − | − | − | − | − | − | − | − | + | <1 | <1 | <1 | <1 | >64 |
| SV137 | po | SP | SV | IgG1 | − | − | − | − | − | − | − | − | − | − | + | <1 | <1 | <1 | <1 | >64 | |
| F | F44 | po | SP | KAV | IgM | − | − | − | − | − | − | − | − | − | − | − | 2 | 8 | 2 | 32 | 2 |
| F74 | po | SP | KAV | IgM | − | − | − | − | − | − | − | − | − | − | − | 8 | 32 | 8 | >64 | 2 | |
Abbreviations: G-I and G-II, genogroups I and II of the genus Norwalk-like viruses; SLVs, the genus Sapporo-like viruses; NV, Norwalk virus VLPs; SeV, Seto 124 virus; CV, Chiba 407 virus; FUV, Funabashi 258 virus; SMA, Snow Mountain agent; GV, Grimsby virus; KAV, Kashiwa 47 virus; NAV, Narita 104 virus; CHV, Chitta 76 virus; UEV, Ueno 7K virus; SV, Sapporo virus.
Obtained from mice immunized peroraly (po) or intraperitoneally (ip).
Obtained from cells of spleen (SP) or mesenteric lymph nodes (LN) of mice.
Immunized with NV, KAV, or SV or mixtures of rNV and rSMA (NxS).
Reported previously by Hardy et al. (13).
Positive reaction only when the proteins were not boiled before electrophoresis.
NT, not tested.
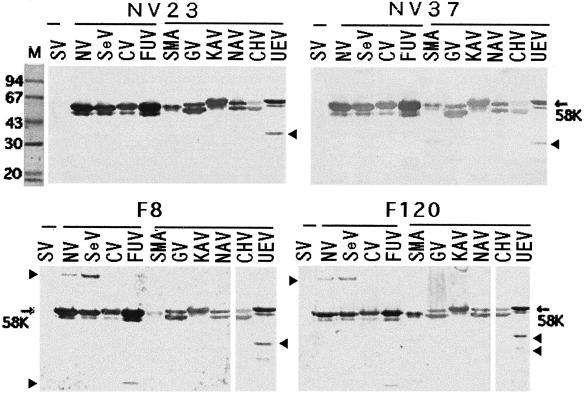
Five group A MAbs (NV23, NV37, NV3, F8, and F120) reacted with both genogroup I (rNV, rSeV, rCV, and rFUV) and genogroup II VLPs (rSMA, rGV, rKAV, rNAV, rCHV, and rUEV). MAb NV23, NV37, and NV3 clones were derived from mice immunized orally with the single rNV VLP, and the cross-reactive MAb F8 and F120 clones were obtained from mice immunized orally with the single rKAV VLP. All of these MAbs were isotype IgG1. The NV23, NV37, F8, and F120 MAbs reacted with all four genogroup I and six genogroup II VLPs by WB (Table 1), with a polypeptide band that was seen as a doublet with an apparent molecular weight of 58,000 to 62,000 (Fig. 1). In the case of rGV and rCHV VLPs, the lower band, which may be a translation product from an internal initiation codon present in the capsid protein gene, appeared to be of greater intensity than the 58,000-molecular-weight band (denoted by the arrow in the figure). One or two lower-molecular-weight bands of rCHV and rUEV VLPs and bands with MAb F8 and F120 (versus rNV, rSeV, and rFUV) other than the 58,000-molecular-weight doublet (some larger, some smaller) are likely oligomers or breakdown products of the capsid protein that appear on storage of the VLPs (16; unpublished data), because these bands were not detected when new, freshly prepared VLP samples were tested. The group A MAbs did not react with rSV by either ELISA or WB. The intensity of reactivity by WB of the MAbs with the polypeptide bands of each VLP type was consistent with the ELISA titer results.
FIG. 1.
Western blot of polypeptides from VLPs reacting with group A MAbs. The results shown are for MAbs NV23, NV37, F8, and F120 and VLPs rSV, rNV, rSeV, rCV, rFUV, rSMA, rGV, rKAV, rNAV, rCHV, and rUEV. Proteins were boiled for 2 min and lysed with sample buffer (about 1 to 2 μg/well), and SDS-polyacrylamide gel electrophoresis (PAGE) was conducted in 10% polyacrylamide gels. The separated proteins were transferred to nitrocellulose sheets, which were incubated overnight at room temperature with the indicated MAbs (at a 1:1,000 dilution of ascites) and then incubated with HRPO-conjugated goat anti-mouse IgG, IgM, and IgA for 1 h at 37°C and treated with substrate (4-chloro-1-naphthol). Calibration kits were used for molecular weight determination (lane M); numbers on the left side indicate the apparent molecular weights (103). These MAbs reacted with all VLPs of the NLV genus whose major polypeptide had an apparent molecular weight of 58,000 but not with rSV. Oligomers or possible breakdown bands (short arrows) were seen in some VLP preparations.
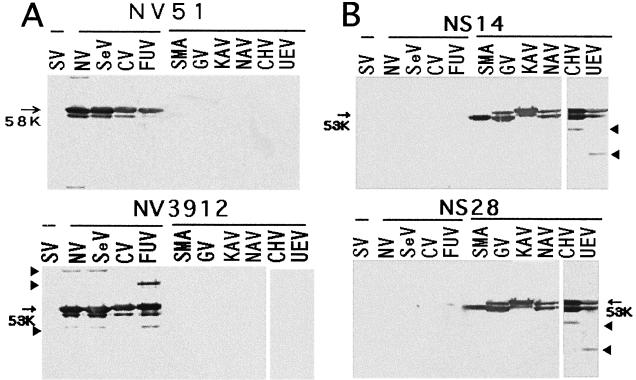
Three group B MAbs (NV51, NV138, and NV172) reacted by ELISA with only genogroup I VLPs. These MAbs were obtained from cells of mesenteric lymph nodes of mice immunized orally with only rNV and were isotype IgA (Table 1). By WB, these MAbs reacted with genogroup I VLPs but not with any VLP of genogroup II NLVs (Table 1) or rSV (Fig. 2A). In addition, the previously characterized MAbs NV3912 and NV2461, which were obtained from mice immunized intraperitoneally with rNV (18) and detect a range of genogroup I NLVs (16), showed the same reactivities as the NV51, NV138, and NV172 MAbs (Table 1). MAb 3912 appears to be more sensitive than MAb 51 at detecting additional forms of the capsid protein, in addition to the 58,000-molecular-weight doublet, present in the gels (Fig. 2A).
FIG. 2.
Western blot of polypeptides from VLPs reacting with group B (panel A) and C (panel B) MAbs. Proteins were boiled for 2 min and lysed (about 1 to 2 μg/well), and SDS-PAGE was conducted in 10% polyacrylamide gels. Each MAb (ascites) was diluted to a level of 1:1,000. (A) The group B NV51 and NV3912 MAbs reacted with all VLPs of genogroup I of genus NLVs and with the major polypeptide whose apparent molecular weight was 58,000 but not with genogroup II or rSV proteins. (B) The group C NS14 and NS28 MAbs reacted with all VLPs of genogroup II of genus NLVs and with the major polypeptide whose apparent molecular weight was 58,000 but not with genogroup I or rSV proteins. Oligomers or breakdown products (short arrows) were seen.
Three group C MAbs (NS14, NS28, and NS46) reacted strongly with genogroup II VLPs and weakly with genogroup I VLPs in ELISA (Table 1). By WB, these MAbs reacted only with genogroup II VLPs and did not react with any polypeptides of VLPs of genogroup I NLVs or rSV (Fig. 2B). These MAbs were obtained from mice immunized orally with mixed rNV and rSMA.
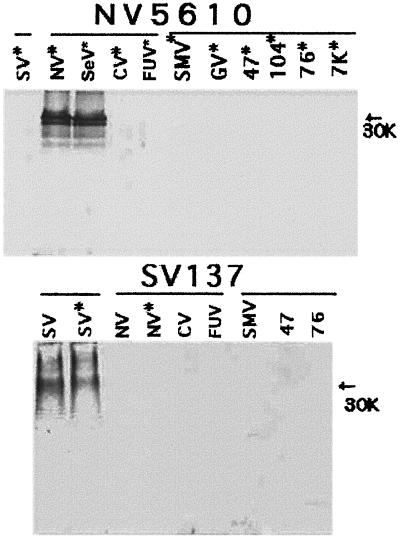
Two group D IgA MAbs (NV5610 and NV5620), which were created from mesenteric lymph node cells of mice immunized orally with the single rNV VLP, reacted by ELISA only with rNV (Table 1). These MAbs did not react with the capsid polypeptide of any VLPs when the VLP samples were boiled before electrophoresis (data not shown). However, when the protein was not boiled, these MAbs detected high-molecular-weight forms (over 63,000) of only rNV and rSeV (Fig. 3; lanes marked with an asterisk). In addition, MAb NV834, which was obtained from mice immunized intraperitoneally with rNV (16, 18), showed the same reactivities as MAbs NV5610 and NV5620.
FIG. 3.
Western blot of polypeptides from VLPs reacting with a subset of group D and E MAbs. The results for MAbs NV5610 and SV137 are shown. Proteins (about 1 to 2 μg/well) were boiled for 2 min or not boiled (lanes marked with asterisks). SDS-PAGE was conducted in 10% polyacrylamide gels. Each MAb (ascites) was diluted to a level of 1:1,000. The NV5610 MAb reacted only with rNV and rSeV of the NLV genus and detected higher-molecular-weight forms only when the protein was not boiled. The SV137 MAbs reacted only with rSV, detecting high-molecular-weight forms whether the protein was boiled or not boiled, but did not react with proteins of other VLPs.
Two group E MAbs (SV68 and SV137), obtained from mice immunized orally with rSV, reacted by ELISA and WB with rSV but not with any VLP of the NLV genus (Table 1). These MAbs detected high-molecular-weight forms of rSV, even when the protein was boiled before electrophoresis (Fig. 3). Similar bands were found when the protein was not boiled (Fig. 3; lane marked with an asterisk).
Two MAbs (F44 and F47) in group F were positive by ELISA but negative by WB, regardless of whether the protein was boiled or not (data not shown). Both of these MAbs were of the IgM isotype and were obtained from an animal immunized orally with rKAV VLPs, and both reacted strongly by ELISA with only rKAV and weakly with other VLPs (Table 1). These MAbs were not characterized further.
DISCUSSION
Oral immunization of rNV VLPs without adjuvant is known to induce humoral immune responses in mice (3, 4). We also found that oral immunization of mice, which resembles the route of natural infection of NV, resulted in high yields of MAbs (44). In this study, we established and characterized hybridoma cell lines from mice immunized orally with HuCV VLPs. The MAbs obtained showed genus-specific (genogroup I and II common) and genogroup-specific reactivities. Of interest, five group A MAbs (NV23, NV37, NV3, F8, and F120) reacted with both genogroup I and II NLVs. These broadly reactive MAbs were obtained from mice immunized orally with only a single strain of VLP, indicating that cross-reactive epitopes must be present on the capsid of caliciviruses in the two genogroups. The three group B MAbs (NV51, NV138, and NV172), which were obtained from mice immunized orally only with rNV, and 2 MAbs (NV3912 and NV2461), which were obtained from mice immunized intraperitoneally with rNV, reacted only with genogroup I NLVs, indicating that shared antigenic epitopes exist among genogroup I NLVs. Similarly, shared epitopes exist on strains within genogroup II NLVs, based on the characterization of the group C MAbs. Group C MAbs reacted only with genogroup II VLPs, although the antibodies were derived by immunizing mice with both rNV and rSMA. These MAbs might recognize relatively dominant epitopes of rSMA VLPs. We obtained several other types of MAbs from mice immunized intraperitoneally with both rNV and rSMA, for example, cross-reactive MAbs for genogroup I and II VLPs, genogroup I-specific MAbs, and one strain-specific MAb. Two potentially shared-genogroup IgM MAbs remain incompletely characterized, because they failed to react with the capsid protein by WB. Five IgA MAbs (NV51, NV138, NV172, NV5610, and NV5620) were obtained from fusions with mesenteric lymph node cells of mice. These MAbs were from animals immunized orally without adjuvant, suggesting that immunization by a natural route of infection may favor inducing IgA MAbs.
We also obtained strain-specific MAbs. Two group D MAbs (NV5610 and NV5620) reacted only with rNV and rSeV VLPs, which represent viruses that share 89% ORF2 sequence identity (31). rSeV showed high sequence identity with KY89 (97%), followed by rNV (89%), rCV (66%), rFUV (62%), and genogroup II rSMA (52%) (31). We also obtained many strain-specific MAbs against rNV, rSV, or rMexico virus in other studies (data not shown). In addition, several clones producing rSV-specific MAbs from mice immunized with rSV reacted by ELISA and WB with only rSV.
In this study, we could not establish any clones that cross-reacted with both NLVs and SLVs. However, several MAbs (e.g., F44 and F74) showed weak cross-reactivity by ELISA with VLPs of the genogroup I and II NLVs and SV, but they did not react by WB with polypeptides of any VLP. These results suggest that a common epitope(s) is present among NLVs and SLVs, but further work is required to confirm this hypothesis.
Characteristics of these MAbs confirm and extend results of antigenic mapping of the NV capsid initiated with our previous MAbs (16, 18). The previous MAbs identified strain-specific and genogroup-specific epitopes which have been confirmed by the group B and group D MAbs in the present study. Characterization of the reactivity of the previous ten MAbs to rNV VLPs showed seven of the MAbs (NV834, NV142, NV101, NV813, NV7411, NV812, and NV8301) recognize discontinuous epitopes, whereas the other three MAbs (NV3901, NV3912, and NV2461) recognize continuous epitopes located in the C terminus of the capsid protein. In this study, MAbs NV5610 and NV5620, because of their similar reactivities, may have the same character as MAb NV834. In addition, MAbs NV51, NV138, and NV172 may have the same character as the NV3912 and NV2461 MAbs because of their similar reactivities. Competition assays or molecular mapping will be needed to confirm whether the epitopes of the new group B and D MAbs represent new, similar, or overlapping epitopes compared to those detected by the previously characterized MAbs.
The 58,000-molecular-weight rNV protein is cleaved specifically by trypsin at amino acid residue 227, yielding a 32,000-molecular-weight cleavage product that contains the C-terminal half of the capsid protein (18, 19). The NV3912, NV3901, and NV2461 MAbs detected the 32,000-molecular-weight cleavage product when the protein was treated with trypsin prior to electrophoresis. Preliminary results indicated that several of the genogroup-specific MAbs (NV51, NV138, NS14, and NS28) detected the 32,000-molecular-weight cleavage product of NV and/or several different polypeptides among the different VLPs when tested by WB for reactivity with products cleaved by trypsin (data not shown). These results suggest that the capsid protein may assume a slightly different conformation that exposes epitopes on different forms of the protein in different types of VLPs. In contrast, even when the protein was treated by trypsin, the cross-reactive MAbs examined so far (NV23, F8, and SV137) reacted only with the full-length VLP capsid proteins and did not detect any cleavage products, suggesting that these MAbs can recognize the relatively conserved and stable N-terminal region of the capsid protein, which is not detected after cleavage by trypsin. Another possibility is that the cross-reactive MAbs that do not react with the 32,000-molecular-weight cleavage product can detect an epitope that spans the trypsin cleavage site, and this epitope may be destroyed in the process of generating the 32,000-molecular- weight protein. Further study using these MAbs is necessary to understand the molecular location of these epitopes.
Several MAbs (NV5610, SV137, etc.) react with a higher-molecular-weight form (>80,000-molecular weight) of the full-length capsid protein, regardless of whether or not the protein is denatured by boiling prior to electrophoresis. These results suggest that these MAbs recognize epitopes that are discontinuous and require the capsid protein to be properly folded. The composition of the 80,000-molecular-weight protein is unclear, as the apparent molecular weight is less than that of a dimer. This aberrant migration is likely a result of incomplete denaturation of the protein (18). The group A MAbs characterized in this paper are clearly of interest, because they represent a new group of MAbs that recognize cross-reactive, genus-specific epitopes present on viruses from both genogroup I and II NLVs. Such MAbs have not been reported previously. Two MAbs (1B4 and B-1F6) that showed limited cross-reactivity by ELISA between one genogroup I and one genogroup II NLV strain were produced by immunizing mice with bacterially expressed peptides (46). These MAbs recognize linear epitopes in the N terminus of the capsid protein, yet they apparently do not detect nondenatured virions or a broad range of strains (even within genogroup II) that are closely related to the immunogen. Our group A MAbs, which show cross-reactivity between genogroup I and genogroup II virions, seem more useful. These MAbs are of particular interest as possible reagents for the development of simple, rapid, cross-reactive diagnostic assays for detection of NLVs in epidemiologic studies.
Acknowledgments
This work was supported in part by grants from the Ministry of Health and Welfare of Japan, the National Institutes of Health (AI46581), and the Environmental Protection Agency (CX 827430).
REFERENCES
- 1.Ando, T., S. S. Monroe, J. R. Gentsch, Q. Jin, D. C. Lewis, and R. I. Glass. 1995. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and Southern hybridization. J. Clin. Microbiol. 33:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atmar, R. L., and M. K. Estes. 2001. Diagnosis of noncultivatable gastroenteritis viruses, the human caliciviruses. Clin. Microbiol. Rev. 14:15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball, J. M., D. Y. Graham, A. R. Opekun, M. A. Gilger, R. A. Guerrero, and M. E. Estes. 1999. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology 117:40-48. [DOI] [PubMed] [Google Scholar]
- 4.Ball, J. M, M. E. Hardy, R. L. Atmar, M. E. Conner, and M. K. Estes. 1998. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J. Virol. 72:1345-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter, M. J., I. D. Milton, J. Meanger, M. Bennett, R. M. Gaskell, and P. C. Turner. 1992. The complete nucleotide sequence of a feline calicivirus. Virology 190:443-448. [DOI] [PubMed] [Google Scholar]
- 6.Estes, M. K., R. L. Atmar, and M. E. Hardy. 1997. Norwalk and related diarrhea viruses, p. 1073-1095. In D. D. Richmann, F. G. Whiteley, and F. G. Hayden (ed.), Clinical virology. Churchill Livingstone Inc., New York, N.Y.
- 7.Graham, D. Y., X. Jiang, T. Tanaka, A. R. Opekun, H. P. Madore, and M. K. Estes. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 170: 34-43. [DOI] [PubMed] [Google Scholar]
- 8.Gray, J. J., X, Jiang, P. Morgan-Capner, U. Desselberger, and M. K. Estes. 1992. Prevalence of antibodies to Norwalk virus in England: detection by enzyme-linked immunosorbent assay using baculovirus-expressed Norwalk virus capsid antigen. J. Clin. Microbiol. 31:1022-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green, J., J. Vinje, C. I. Gallimore, M. Koopmans, A. Hale, and D. W. Brown. 2000. Capsid protein diversity among Norwalk-like viruses. Virus Genes 20:227-236. [DOI] [PubMed] [Google Scholar]
- 10.Green, K. Y., T. Ando, M. S. Balayan, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Neill, M. J. Studdert, and H. J. Thiel. 2001. Caliciviridae, p. 725-735. In M. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. Carsten, M. K. Estes, S. M. Lemon, J. Maniloff, M. Mayo, D. McGeoch, C. R. Pringle, and R. Wickner (ed.), Virus taxonomy: 7th report of the International Committee on Taxonomy of Viruses. Academic Press, Orlando, Fla.
- 11.Green, K. Y., G. Belliot, J. L. Taylor, J. Valdesuso, J. F. Lew, A. Z. Kapikian, and F. Y. C. Lin. 2002. A predominant role for Norwalk-like viruses as agents of epidemic gastroenteritis in Maryland nursing homes of the elderly. J. Infect. Dis. 185: 133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, K. Y., R. M. Chanick, and A. Z. Kapikian. 2001. Human caliciviruses, p. 847-874. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott, Williams, and Wilkins, Philadelphia, Pa.
- 13.Green, K. Y., J. F. Lew, X. Jiang, A. Z. Kapikian, and M. K. Estes. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 31:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg, H. B., J. R. Valdesuso, A. R. Kalica, R. G. Wyatt, V. J. McAuliffe, A. Z. Kapikian, and R. M. Chanock. 1981. Proteins of Norwalk virus. J. Virol. 37:994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hale, A. D., S. E. Crawford, M. Ciarlet, J. Green, C. Gallimore, D. W. Brown, X. Jang, and M. K. Estes. 1999. Expression and self-assembly of Grimsby virus: antigenic distinction from Norwalk and Mexico viruses. Clin. Diagn. Lab. Immunol. 6:142-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hale, A. D., T. Tanaka, N. Kitamoto, M. Ciarlet, X. Jiang, N. Takeda, D. W. G. Brown, and M. K. Estes. 2000. Identification of an epitope common to genogroup 1 Norwalk-like viruses. J. Clin. Microbiol. 38:1656-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy, M., S. F. Kramer, J. J. Treanor, and M. K. Estes. 1997. Human calicivirus genogroup II capsid sequence diversity revealed by sequence analyses of the prototype Snow Mountain agent. Arch. Virol. 142:1469-1479. [DOI] [PubMed] [Google Scholar]
- 18.Hardy, M. E., T. Tanaka, N. Kitamoto, L. J. White, J. M. Ball, X. Jiang, and M. K. Estes. 1996. Antigenic mapping of the recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology 217:252-261. [DOI] [PubMed] [Google Scholar]
- 19.Hardy, M. E., L. J. White, J. M. Ball, and M. K. Estes. 1995. Specific proteolytic cleavage of recombinant Norwalk virus capsid protein. J. Virol. 69:1693-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, X., D. W. Cubitt, T. Berke, W. N. Zhong, X. Dai, S. Nakata, L. K. Pickering, and D. O. Matson. 1997. Sapporo-like human caliciviruses are genetically and antigenetically diverse. Arch. Virol. 142:1813-1827. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, X., D. Cubitt, J. Hu, X. Dai, J. Treanor, D. O. Matson, and L. K. Pickering. 1995. Development of an ELISA to detect MX virus, a human calicivirus in the Snow Mountain agent genogroup. J. Gen. Virol. 76:2739-2747. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, X., D. Y. Graham, K. N. Wang, and M. K. Estes. 1990. Norwalk virus genome cloning and characterization. Science 250:1580-1583. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, X., D. O. Matson, F. R. Velazquez, J. J. Calva, W. N. Zhong, J. Hu, G. M. Ruiz-Palacios, and L. K. Pickering. 1995. Study of Norwalk-related viruses in Mexican children. J. Med. Virol. 47:309-316. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, X., J. Wang, D. Y. Graham, and M. K. Estes. 1992. Detection of Norwalk virus in stool by polymerase chain reaction. J. Clin. Microbiol. 30:2529-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang, X., M. Wang, K. Wang, and M. K. Estes. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51-61. [DOI] [PubMed] [Google Scholar]
- 27.Kapikian, A. Z., R. G. Wyatt, R. Dolin, T. S. Thornhill, A. R. Kalica, and R. M. Chanock. 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J. Virol. 10:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King, A. D., and K. Y. Green. 1997. Sequence analysis of the gene encoding the capsid protein of the Snow Mountain human calicivirus. Virus Genes 15:5-7. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi, S., K. Sakae, K. Natori, N. Takeda, T. Miyamura, and Y. Suzuki. 2000. Serotype-specific antigen ELISA for detection of Chiba virus in stools. J. Med. Virol. 62:233-238. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi, S., K. Sakae, Y. Suzuki, H. Ishiko, K. Kamata, K. Suzuki, K. Natori, T. Miyamura, and N. Takeda. 2000. Expression of recombinant capsid proteins of Chitta virus, a genogroup II Norwalk virus, and development of an ELISA to detect the viral antigen. Microbiol. Immunol. 44:687-693. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi, S., K. Sakae, Y. Suzuki, K. Shinozaki, M. Okada, H. Ishiko, K. Kamata, K. Suzuki, K. Natori, T. Miyamura, and N. Takeda. 2000. Molecular cloning, expression, and antigenicity of Seto virus belonging to genogroup I Norwalk-like viruses. J. Clin. Microbiol. 38:3492-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 33.Lambden, P. R., E. O. Caul, C. R. Ashley, and I. N. Clarke. 1993. Sequence and genome 7 organization of a human small round-structured (Norwalk-like) virus. Science 256:516-519. [DOI] [PubMed] [Google Scholar]
- 34.Lew, J. F., A, Z. Kapikian, X. Jiang, M. K. Estes, and K. Y. Green. 1994. Molecular characterization and expression of the capsid protein of a Norwalk-like virus recovered from a Desert Shield troop with gastroenteritis. Virology 200:319-325. [DOI] [PubMed] [Google Scholar]
- 35.Lew, J. F., A, Z. Kapikian, J. Valdesuso, and K. Y. Green. 1994. Molecular characterization of Hawaii virus and other Norwalk-like viruses: evidence for genetic polymorphism among human caliciviruses. J. Infect. Dis. 170: 535-542. [DOI] [PubMed] [Google Scholar]
- 36.Numata, K., M. E. Hardy, S. Nakata, S. Chiba, and M. K. Estes. 1997. Molecular characterization of morphologically typical human calicivirus Sapporo. Arch. Virol. 142:1537-1552. [DOI] [PubMed] [Google Scholar]
- 37.Numata, K., S. Nakata, X. Jiang, M. K. Estes, and S. Chiba. 1994. Epidemiological study of Norwalk virus infections in Japan and Southeast Asia by enzyme-linked immunosorbent assays with Norwalk virus capsid protein produced by the baculovirus expression system. J. Clin. Microbiol. 32:121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker, S. P., W. D. Cubitt, X. Jiang, and M. K. Estes. 1994. Seroprevalence studies using a recombinant Norwalk virus protein enzyme immunoassay. J. Med. Virol. 42:146-150. [DOI] [PubMed] [Google Scholar]
- 39.Parker, S. P., W. D. Cubitt, and X. Jiang. 1995. Enzyme immunoassay using baculovirus-expressed human calicivirus (Mexico) for the measurement of IgG responses and determining its seroprevalence in London, United Kingdom. J. Med. Virol. 46:194-200. [DOI] [PubMed] [Google Scholar]
- 40.Seah, E. L., J. A. Marshall, and P. J. Wright. 1999. Open reading frame 1 of the Norwalk-like virus Camberwell: completion of sequence and expression in mammalian cells. J. Virol. 73:10531-10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Someya, Y., N. Takeda, and T. Miyamura. 2000. Complete nucleotide sequence of the Chiba virus genome and functional expression of the 3C-like protease in Escherichia coli. Virology 278:490-500. [DOI] [PubMed] [Google Scholar]
- 42.Stocker, J. W., H. K. Forster, V. Miggiano, C. Stahli, G. Stalger, B. Takacs, and T. Staehelin. 1982. Generation of two new mouse myeloma cell lines “PAI” and “PAI-O” for hybridoma production. Res. Disclosure 217:155-157.
- 43.Tamura, M., K. Natori, M. Kobayashi, T. Miyamura, and N. Takeda. 2000. Interaction of recombinant Norwalk virus particles with the 105-kilodalton cellular binding protein, a candidate receptor molecule for virus attachment. J. Virol. 74:11589-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, J., X. Jiang, H. P. Madore, J. Gray, U. Desselberger, T. Ando, Y. Seto, I. Oishi, J. F. Lew, K. Y. Green, and M. K. Estes. 1994. Sequence diversity of small round-structured viruses in the Norwalk virus group. J. Virol. 68:5982-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoda, T., Y. Terano, Y. Suzuki, K. Yamazaki, I. Oishi, E. Utagawa, A. Shimada, S. Matsuura, M. Nakajima, and T. Shibata. 2000. Characterization of monoclonal antibodies generated against Norwalk virus GII capsid protein expressed in Escherichia coli. Microbiol. Immunol. 44:905-914. [DOI] [PubMed] [Google Scholar]