Abstract
Fragments of glycoprotein G (gG-2281-594His), comprising residues 281 to 594 of herpes simplex virus type 2 (HSV-2), glycoprotein G of HSV-1 (gG-1t26-189His), and glycoprotein D of HSV-1 (gD-11-313), were expressed in the baculovirus expression system to develop an assay for the detection of HSV-1 and HSV-2 type-specific antibodies. The expression of the gG-1t26-189His and gG-2281-594His fragments was analyzed by Western blotting using monoclonal antibodies LP10 and AP1, respectively. The molecular masses of the major products of gG-1t26-189His and the fragment of gG-2281-594His were 36 to 39 kDa and 64 to 72 kDa, respectively. Human sera positive for HSV-1 reacted with gG-1t26-189His, sera positive for HSV-2 reacted with the gG-2281-594His fragment, and sera positive for both types reacted with gG-1t26-189His and gG-2281-594His in Western blotting. The human sera recognized polypeptides of gG-2281-594His with molecular masses of 57 to 67 and 120 to 150 kDa and additional faint bands of 21, 29, and 45 kDa. The recombinant gG-1t26-189His and the recombinant gG-2281-594His fragment were used as type-specific antigens for the detection of HSV-1- and HSV-2-specific antibody responses in human sera, respectively. As type-common antigens, an extract of HSV-1-infected Vero cells and recombinant gD-11-313 were used. An enzyme-linked immunosorbent assay to detect type-specific antibodies was developed, and the sensitivity and specificity were evaluated by comparison with commercial tests by using sera obtained from different sources. The sensitivity and specificity were 91.5 and 95.5%, respectively, compared to the Gull assay. The gG-2281-594His fragment can be obtained in relatively large quantities at low cost.
Herpes simplex virus type 1 (HSV-1) and HSV-2 are well known as similar subtypes of HSV (38). HSV-2 is the main cause of recurrent genital infections (8). Most primary infections are asymptomatic, and people silently shed virus (14, 30, 51, 57, 58). After primary infection, the virus establishes latent infections in the local ganglia and is reactivated frequently, and antibody titers against HSV become detectable in serum samples. Many of the HSV-2 reactivations are asymptomatic and clinically not recognized (15, 30, 39, 40, 57). Most HSV infections are transmitted in the absence of lesions (17, 57, 58). Severe, generalized infections are seen particularly in neonates and immunocompromised (human immunodeficiency virus-infected) patients. Epidemiological studies indicate that in developed countries there is an ongoing HSV-2 epidemic with a significant rise in HSV-2 prevalence. Risk factors for acquiring HSV-2 infections are related to sexual life style, gender, race, and socioeconomic status. HSV type-specific antibody testing may be considered in a number of at-risk situations. Thus, identification of HSV-2-infected individuals may be important to reduce the risk of transmission in these groups.
Laboratory diagnosis of HSV infections is based on direct detection of the virus and on serology (2, 16). Virus detection is useful in the diagnosis of clinical cases, but it fails to detect latent infections. Serology is helpful to confirm clinical cases and to identify latently infected individuals. Type-specific serology can be used to identify asymptomatically and subclinically HSV-2-infected individuals (2, 18). Most polypeptides of HSV-1 and HSV-2 show a high degree of similarity (20), which will result, when using these antigens in serological assays, in the detection of cross-reactive antibodies (2, 4). The most reliable typing of HSV antibodies is by serological tests using glycoprotein G (gG). Tran et al. (56) described the possible involvement of gG in efficient entry into polarized epithelial cells. gG consists of 238 amino acids in HSV-1 (gG-1) and of 699 amino acids in HSV-2 (gG-2) (6, 21, 45). gG-1 and gG-2 are not essential for virus replication. They have similar sequences at their amino termini. In addition, they have a segment of 153 amino acids with a high percentage of similarity at their carboxyl termini. This homologous sequence comprises the part adjacent to the transmembrane region and the cytoplasmic domain. gG-2 is cleaved during processing into an amino-terminal portion, which is secreted into the medium, and a cell-associated, highly O-glycosylated carboxyl-terminal portion (11, 12, 34, 36, 42, 52-54). Cleavage has been proposed to occur between residues 321 and 322 and between residues 342 and 343 (33, 35). The N-terminal part of the cell-associated gG-2 is unique for type 2 and contains most of the type-specific epitopes so far identified (24, 31, 32, 35, 37).
For the detection of type 2-specific antibodies, different assays have been developed. Western blotting using extracts of infected cells is the “gold standard” (7). Other tests are based on the type-specific gG antigens. Purified gG-2 isolated from HSV-2-infected cells by either immunoaffinity chromatography (7, 19, 28) or lectin chromatography was used (43, 55). The immunoaffinity-purified gG-2 was applied in an immunodot assay, and the lectin chromatography-purified gG-2 was used in an indirect enzyme-linked immunosorbent assay (ELISA). In addition, a capture ELISA (27) and a monoclonal blocking assay (23, 50) were developed. Recombinant truncated gG produced in the baculovirus expression system was also used in an immunodot assay (46). Others use synthetic peptides identical to gG-2 sequences for type-specific serology (24, 31, 32, 37, 41). Several commercial assays, based on ELISAs and immunoblotting, using affinity-purified gG (9, 47), lectin-purified gG (5, 29), and recombinant gG produced in baculovirus (44, 59) have been developed.
We cloned the complete sequence of gG-2 and a truncated version of gG-2, resulting in recombinant baculoviruses that expressed very little gG-2. In addition, these viruses were not stable. Therefore, we decided to clone a smaller fragment of the gG-2 sequence, comprising residues 281 to 594. This fragment contains most of the known epitopes of gG-2. The fragment of gG-2 contains the proposed cleavage sites that allow processing. In order to improve expression, the gG-2 signal sequence was replaced by the honeybee melittin signal sequence. Six histidines were added to the C terminus to allow purification by affinity chromatography. In addition, gG-1 (residues 26 to 189 [gG-1t26-189His]), and gD-1 (residues 1 to 313 [gD-11-313]) were cloned in the baculovirus expression system. The production of recombinant gG-2281-594 and gG-1t26-189 was confirmed by Western blotting using type-specific monoclonal antibodies (MAbs) (AP1 and LP10, respectively) and specific human sera.
In this study, we used the gG-2281-594His fragment, gG-1t26-189His, and gD-11-313 in combination with an extract of HSV-1-infected Vero cells to develop an ELISA to detect type-specific antibodies. Sensitivity and specificity were determined by comparison with a frequently used commercial kit based on immunoaffinity-purified complete gG-2, using well-defined and culture-proven sera.
MATERIALS AND METHODS
Cells and viruses.
Virus stocks of HSV-1 strain McIntyre and HSV-2 strain 333 were produced in Vero cells maintained in medium 199 supplemented with 10% fetal calf serum and 2.5 μg of gentamicin per ml (Invitrogen, Breda, The Netherlands). Spodoptera frugiperda (Sf21) cells grown in Insect-Xpress medium (BioWhittaker, Verviers, Belgium) supplemented with 2.5 μg of gentamicin per ml were used for infections of wild-type and recombinant Autographa californica nuclear polyhedrosis viruses (baculoviruses).
Construction of recombinant baculoviruses.
Recombinant baculovirus expressing gG-2281-594His was constructed in several steps. First, the open reading frame encoding a fragment of gG-2 (amino acid residues 281 to 640) was amplified by PCR from DNA isolated from HSV-2 (strain 333)-infected Vero cells. The synthetic oligonucleotide primers used were forward primer 1 (5′ CGCAGATCTATTCGGATGGCA 3′) and reverse primer 2 (3′ GTGGGGGGATCGCGGGGCCTAGGTA 5′). The PCR protocol was as follows: 97°C for 2 min (1 cycle); 40°C for 2 min (1 cycle); 97°C for 15 s, 40°C for 30 s, and 72°C for 1 min (10 cycles); 97°C for 15 s, 42°C for 30 s, and 72°C for 1 min (15 cycles); 97°C for 15 s, 45°C for 30 s, and 72°C for 1 min (10 cycles); and 72°C for 5 min (1 cycle). A Hybaid HB-TR1 thermal reactor and Taq polymerase (HT Biotechnology Ltd., Cambs, United Kingdom) were used. The amplified fragment of gG-2 was cloned into pGEMT (Promega, Leiden, The Netherlands) (pGEMT-gG-2281-640).
In order to achieve high levels of expression and secretion of the protein, a recombinant baculovirus producing gG-2 containing the honeybee melittin signal sequence was constructed (49). The plasmid pMelBac (Invitrogen) was digested with EcoRV and BamHI, and the honeybee melittin signal sequence was isolated and ligated upstream of the multiple cloning site of pFastBac (Invitrogen), producing pFastMelBac. The pGEMT-gG-2281-640 construct was used as template DNA for the following PCR. The synthetic oligonucleotide primers used were forward primer 3 (5′ TCGGCCACCGCCATGGCACC 3′) and reverse primer 4 (3′ CGTCAGAGCTCCGTACGTGGGTGGTAGTGGTAGTGGTATTCGAACAG 5′) containing the sequence encoding six histidines. The PCR protocol was as follows: 40°C for 2 min (1 cycle); 96°C for 5 min (1 cycle); 64/66°C for 3 min (1 cycle); 96°C for 1 min and 66°C for 1 min (40 cycles); and 96°C for 1 min, 66°C for 1 min, and 72°C for 10 min (1 cycle). The PCR product coding for residues 281 to 594 of gG-2 with a His tail at the C terminus was cloned into pFastMelBac (pFastMelBac-gG-2281-594). Isolated DNA was transposed and transfected according to the instructions of the manufacturer (Invitrogen). The recombinant baculovirus was designated gG-2281-594His.
In addition, a recombinant baculovirus coding for gG-1t26-189His was constructed by using similar procedures. The open reading frame encoding a truncated gG-1 (amino acid residues 26 to 189, without transmembrane region and signal sequence) was amplified by PCR from DNA isolated from HSV-1 (strain McIntyre)-infected Vero cells. The synthetic oligonucleotide primers forward primer 5 (5′ GATCGATTCATGTCGCAGGGC 3′) and reverse primer 6 (3′ GGAGGGGGCGGGACCTGCCATGGGT 5′) were used. The PCR protocol was as follows: 40°C for 2 min (1 cycle); 97°C for 2 min, 48°C for 2 min, and 72°C for 5 min (40 cycles); 53°C for 1 min (1 cycle); and 72°C for 1.5 min and 97°C for 1 min (1 cycle). A 500-bp DNA of gG-1t26-189His was ligated into plasmid pGEMT (pGEMT-gG-1t26-189His). The pGEMT-gG-1t26-189His was digested with the restriction enzymes ClaI and KpnI and ligated into pFastMelBac. Isolated DNA was transposed and transfected according to the instructions of the manufacturer (Invitrogen).
Glycoprotein D derived from HSV-1 (gD-1, amino acid residues 1 to 313) was also cloned by using the Bac-to-Bac expression system. To amplify the DNA of gD-11-313, we used the primers forward primer 7 (5′ GTGGTGCGGGATCCTATGGGGGGGACTGCCGCC 3′) and reverse primer 8 (3′ GCGATCAGGGATCCGTTGTTCGG 5′). The cloning of gD-11-313 was performed according to the protocol of the manufacturer (Invitrogen). The recombinant baculovirus was designated gD-11-313.
Antigens.
An extract of HSV-1-infected Vero cells was produced by infecting Vero cells at a multiplicity of infection of 10 PFU of HSV-1 per cell. After 18 h of infection, cells were collected and centrifuged at low speed. Pelleted cells were resuspended in the same volume of phosphate-buffered saline (PBS) as the cell pellet volume. An identical volume of 1.5% Nonidet P-40 in PBS was added to the cell suspension. The cell suspension was incubated for 1 h on ice, and subsequently cell debris was removed by centrifugation. The supernatant was stored in aliquots at −80°C and was used as extract of HSV-1-infected Vero cell antigen.
Culture medium from infected Sf21 cells was used as an antigen source for gD-11-313, gG-1t26-189His, and gG-2281-594His. To this end, Sf21 cells were infected with recombinant baculoviruses, i.e., baculovirus-gD-11-313, baculovirus-gG-1t26-189His, and baculovirus-gG-2281-594His, at multiplicities of 0.1. After 3 days of infection, the culture medium was collected. Low-speed centrifugation was used to spin down cell debris. The supernatant was dialyzed against demineralized water. The dialyzed culture media containing either gD-11-313, gG-1t26-189His, or gG-2281-594His were concentrated by lyophilization.
For the ELISAs, lyophilized gD-11-313, lyophilized gG-1t26-189His, and lyophilized gG-2281-594His, originating from 50-ml cultures, were reconstituted in 2,500, 1,500, and 5,000 μl of demineralized water, respectively. Aliquots of reconstituted gD-11-313, gG-1t26-189His, and gG-2281-594His were frozen at −80°C and kept as antigen stocks for the ELISA and Western blotting.
ELISA.
Ninety-six-well ELISA plates (Greiner Labortechnik, Alphen aan den Rijn, The Netherlands) were coated with antigen (see above) that was diluted (1,000 times dilution of stocks) in coating buffer (50 mM sodium carbonate-bicarbonate buffer, pH 9.6) by incubation at 4°C overnight. Plates were washed three times with washing buffer (0.3% Tween 20 and 1 M NaCl in PBS). Sera were serially diluted, starting at 50 times, with dilution buffer (0.3% Tween 20 and 0.2 M NaCl in PBS), and 100 μl of the diluted sera was added to each well. Thereafter, the plates were incubated at 37°C for 1 h and subsequently washed three times with washing buffer. Then, 100 μl of peroxidase-conjugated rabbit anti-immunoglobulin G (IgG) (Dako, Glostrup, Denmark) diluted 2,000 times in dilution buffer was added to each well and the plates were incubated at 37°C for 1 h. After incubation, the plates were washed three times with washing buffer, and 100 μl of o-phenylenediamine dihydrochloride (0.5 mg/ml) in substrate buffer (0.05 M sodium phosphate-citrate buffer, pH 5.0) containing 40 μl of 30% hydrogen peroxide per 100 ml of substrate buffer was added. After 30 min, the reaction was stopped by adding 50 μl of 4 N H2SO4 per well, and the absorbance at 490 nm was measured. The mean absorbance value of HSV-negative sera at 490 nm plus three standard deviations was used as the cutoff value.
Sera.
MAbs AP1 and LP10 were from A. C. Minson, University of Cambridge, Cambridge, England (36, 45). Human sera from different sources were screened for the presence of HSV type-specific antibodies. A total of 211 sera from medical students of the University of Groningen, Groningen, The Netherlands; 64 sera provided by S. M. Bruisten, Municipal Health Service Amsterdam (GG/GD), Amsterdam, The Netherlands; 64 sera from the University of Göteborg, Göteborg, Sweden; and 20 sera from Erasmus Medical Center, Rotterdam, The Netherlands, were used in this study. The sera of medical students were collected when the students attended the practical course of medical microbiology in 1998. The sera from the GG/GD and from the University of Göteborg were culture proven for HSV-2. The sera from the GG/GD were collected in 1998. The sera from the Erasmus Medical Center were tested with three different HSV-2 type-specific tests (25) and gave discordant results with one of the three assays. The latter sera were tested in our laboratory in Groningen with our ELISA in a coded way (blind).
Deglycosylation.
Reconstituted lyophilized culture medium containing baculovirus-expressed gG-2281-594His was digested with peptide:N-glycosidase F (PNGase F) (New England BioLabs, Frankfurt am Main, Germany) and endoglycosidase H (endo H) (New England BioLabs) to cleave N-linked oligosaccharides and with O-glycosidase (Roche Diagnostics, Mijdrecht, The Netherlands) to cleave O-linked oligosaccharides. Deglycosylation was performed according to the manufacturer's instructions. The deglycosylated samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Western blot (immunoblot) analysis.
The gG-1 and gG-2 polypeptides, which were used as antigens in the ELISAs, were analyzed by SDS-PAGE. The polypeptides were preheated in SDS sample buffer for 4 min and separated on SDS-12% polyacrylamide gels. For Western blotting, polypeptides were transferred to polyvinylidene difluoride (PVDF) membranes (Schleicher & Schuell, Dassel, Germany). The PVDF membranes were blocked in PBS containing 0.3% Tween 20 and 4% skim milk (Oxoid, Haarlem, The Netherlands) overnight. All MAbs and human sera were preadsorbed. For this, the culture medium of Sf21 cells infected with wild-type baculovirus had been coupled to CNBr-activated Sepharose 4B beads (Amersham Pharmacia Biotech, Roosendaal, The Netherlands). These beads were added to the antibody dilutions, and the mixture was incubated by rotation at 4°C overnight. MAbs were diluted 200 times and human sera were diluted 100 times in dilution buffer (0.3% Tween 20 and 4% skim milk in PBS). The PVDF membranes were placed in a multiscreen apparatus, and after 1 h of incubation on a rocking table, the membranes were washed three times for 10 min each with washing buffer (0.3% Tween 20 in PBS). For the human sera, peroxidase-conjugated rabbit anti-human IgG (Dako) was diluted 2,000 times in dilution buffer and added to the membranes. For the MAbs, peroxidase-conjugated rabbit anti-mouse immunoglobulins (Dako) were diluted 1,000 times in the dilution buffer and added to the membranes. After 1 h of incubation, the membranes were washed five times for 10 min each with washing buffer as before. Finally, the membranes were analyzed by enhanced chemiluminescence (ECL) according to the instructions of the manufacturer (Amersham Pharmacia Biotech).
Commercially available kits.
We compared our ELISA with the HSV-2-specific gG-2-based enzyme immunoassay (EIA) (Gull Laboratories, Meridian Bioscience, Inc., Cincinnati, Ohio). The Gull EIA referred to in this paper was originally developed by Gull. This kit is no longer available.
RESULTS
Expression and production of gG-1t26-189His and gG-2281-594His in the baculovirus expression system.
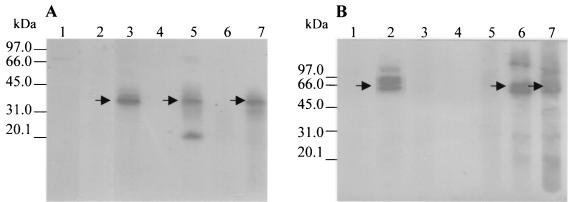
A baculovirus recombinant which expresses a gG-2 fragment containing most of the type 2-specific epitopes described so far was constructed. The open reading frames encoding gG-1t26-189His and gG-2281-594His were cloned into the pFastMelBac vector. Recombinant baculoviruses expressing gG-1t26-189His and gG-2281-594His were constructed. Cells were infected with the recombinant baculoviruses, and the culture medium was collected after 72 h of infection. The expressed gG-1t26-189His and gG-2281-594His were secreted into the medium due to the honeybee melittin signal sequence, and almost no gG-1t26-189His and gG-2281-594His could be detected in the insect cells. After the medium was dialyzed and concentrated by lyophilization, samples were prepared for SDS-PAGE and Western blotting. The presence of gG-1t26-189His and gG-2281-594His was detected with appropriate MAbs, AP1 for gG-2 and LP10 for gG-1. MAb LP10 reacted with gG-1t26-189His polypeptides with molecular masses of 36 to 39 kDa (Fig. 1A, lane 3) but did not react with polypeptides of insect cells infected with the recombinant baculovirus expressing gG-2281-594His (Fig. 1B, lane 3). MAb AP1, which is specific for type 2, reacted with polypeptides of 64 to 72 kDa and of approximately 120 to 150 kDa (Fig. 1B, lane 2) and not with polypeptides expressed in insect cells infected with recombinant baculovirus gG-1t26-189His (Fig. 1A, lane 2). In addition, the reactivity of the recombinant proteins with human sera was investigated. To reduce the background reaction with baculovirus products, all human sera were preadsorbed with CNBr-activated Sepharose 4B beads to which the proteins of Sf21 cells infected with wild-type baculovirus were coupled. The reactivities of human sera positive for HSV-1, for HSV-2, and for both types with the polypeptides expressed in insect cells infected with either recombinant baculovirus gG-1t26-189His or gG-2281-594His are shown in Fig. 1, lanes 4 to 7. Sera positive for HSV-1 (Fig. 1A, lanes 5 and 7) reacted with polypeptides of 36 to 39 kDa and with polypeptides with low molecular masses of 31 and 21 kDa. Sera positive for HSV-2 (Fig. 1B, lanes 6 and 7) reacted with polypeptides with high molecular masses of 120 to 150 kDa and with polypeptides with molecular masses in the range of 57 to 67 kDa. The latter are comparable to the bands found with MAb AP1. In addition some faint bands of polypeptides with lower molecular masses of 45, 29, and 21 kDa were present. An HSV-negative serum (Fig. 1, lanes 4) and MAb A16, directed against glycoprotein D (Fig. 1, lanes 1), did not react with gG-1- and gG-2-specific polypeptides. MAb A16 reacted with a very faint band between the 66- and 97-kDa markers. A similar very faint band is also present in Fig. 1A, lane 5. These bands probably represent aspecifc reactivities of the sera with residual insect cell proteins. These results showed that recombinant gG-1t26-189His and gG-2281-594His reacted with the MAbs and the human sera in the Western blotting in a type-specific way.
FIG. 1.
Reactivities of human sera and MAbs with concentrated medium of insect cells expressing gG-1t26-189His and gG-2281-594His. Insect cells were infected with recombinant baculovirus expressing gG-1t26-189His or gG-2281-594His. Medium of the infected cells was collected 3 days after infection, dialyzed against demineralized water, and lyophilized. Samples were heated prior to electrophoresis in SDS sample buffer. Electrophoresis of samples of reconstituted medium of insect cells expressing gG-1t26-189His (A) and gG-2281-594His (B) was performed on 12% polyacrylamide gels. The polypeptides were transferred to PVDF membranes and incubated with the antisera. Binding of antibodies was visualized by chemiluminescence (ECL). Lanes: 1, MAb A16, specific for gD; 2, MAb AP1, specific for gG-2; 3, MAb LP10, specific for gG-1; 4, HSV-negative human serum; 5, HSV-1-positive human serum; 6, HSV-2-positive human serum; 7, HSV-1- and HSV-2-positive human serum. Molecular mass markers are indicated.
gG-2281-594His has two potential N-linked glycosylation sites (54), which are at amino acid positions 437 and 512, and a highly O-glycosylated region between residues 355 and 550. Next, samples of gG-2281-594His expressed in the baculovirus expression system were deglycosylated and subsequently analyzed by Western blotting with MAb AP1 (data not shown). Deglycosylation of the gG-2 fragment by O-glycosidase reduced the size of the fragment by 17 kDa. No reduction in molecular mass was seen with endo H treatment.
Sensitivity and specificity.
A total 267 sera from different origins were selected to compare the results of the ELISA based on the recombinant proteins produced in the baculovirus expression system with those of a commercially available kit (Table 1). We used gG-1t26-189His and gG-2281-594His as type-specific antigens and an extract of HSV-1-infected Vero cells and gD-11-313-expressed baculovirus as type-common antigens. HSV-2 seropositivity is defined by the presence of antibodies against either the extract of HSV-1-infected Vero cells or gD-11-313 in combination with antibodies against gG-2281-594His. Reactivity of antibodies against gG-2281-594His without reactivity against gD-11-313 or the extract of HSV-1-infected Vero cells is regarded as false positive. Sera containing antibodies against gD-11-313 or HSV-1-infected Vero cell extract but not (yet) against gG-1t26-189His and gG-2281-594His were designated not typed. From other studies, it is known that antibodies against gG may appear later than antibodies against type-common HSV antigens (3, 6).
TABLE 1.
Reactivities of human sera in a gG-2281-594His-based ELISA compared with the Gull EIA IgG kit
| Gull EIA result | No. of samples with the indicated reactivity in the gG-2281-594His-based ELISAa
|
||||
|---|---|---|---|---|---|
| HSV-2 positive, positive | HSV-2 negative
|
HSV positive, not typed | Total | ||
| Only gG-2 positive | Negative | ||||
| Positive | 97 (28, 60, 7, 2) | 3 (0, 0, 1, 2) | 4 (2, 0, 1, 1) | 2 (1, 0, 1, 0) | 106 (31, 60, 10, 5) |
| Negative | 7 (3, 2, 2, 0) | 0 | 143 (25, 0, 8, 110) | 6 (3, 0, 0, 3) | 156 (31, 2, 10, 113) |
| Indeterminate | 3 (1, 2, 0, 0) | 0 | 2 (1, 0, 0, 1) | 0 | 5 (2, 2, 0, 1) |
| Total | 107 (32, 64, 9, 2) | 3 (0, 0, 1, 2) | 149 (28, 0, 9, 112) | 8 (4, 0, 1, 3) | 267 (64, 64, 20, 119) |
The numbers in parentheses indicate the number of samples from Amsterdam, Göteborg, Rotterdam, and Groningen, respectively. The sensitivity is 91.5% (97/106), and the specificity is 95.5% [(143 + 6)/156].
Sixty-four sera were from the University of Göteborg, Göteborg, Sweden, and were culture proven for HSV-2. Of the 64 sera, 35 samples were tested previously in Helix pomatia purified gG-2 assays (34). All 35 samples were positive in our ELISA and in the H. pomatia purified gG-2 assays. All 64 sera were HSV-2 positive in our ELISA. In the Gull EIA, 60 sera (93.8%) were positive, 2 (3.1%) were negative, and 2 (3.1%) gave repeated indeterminate results. In three of the four sera which gave indeterminate results or were negative for HSV-2 in the Gull EIA, antibodies against H. pomatia purified gG-2 were shown to be present. The fourth serum was not tested in the H. pomatia purified gG-2 assay. We also tested the sera for the presence of HSV-1 antibodies by using recombinant gG-1t26-189His. Of the 64 HSV-2-positive sera, 49 sera also had HSV-1 antibodies.
Another group of 64 HSV-2 culture-proven sera was obtained from the GG/GD. Among these 64 sera, we found 32 sera positive for HSV-2 in our ELISA, of which 25 had also antibodies against gG-1. Nineteen were only HSV-1 positive, four had antibodies against HSV but not against gG-1 and gG-2, and nine were still HSV negative. In total, 31 were HSV-2 positive in the Gull EIA, 2 were indeterminate, and 31 were HSV-2 negative. Concordant results were found for 53 (28 HSV-2 positive and 25 HSV-2 negative) of the 64 sera. Four discordant sera that were positive for HSV-2 in our ELISA were HSV-2 negative (three sera) or indeterminate (one serum) in the Gull EIA. Of the three Gull EIA-positive discordant sera, one could not be typed and two were HSV-2 negative in our ELISA. The IgM test of the Gull EIA (5) was also applied to the 64 sera. Sixteen sera were HSV-2 IgM positive, and four were indeterminate. Nine of IgM-positive sera belonged to the group of 28 concordant sera and were already IgG positive for HSV-2. The remaining seven sera were all negative in the Gull EIA IgG test. In our ELISA these seven sera were either HSV-2 IgG negative (three sera; one serum was only HSV-1 positive and two sera were HSV negative), not typed (three sera), or HSV-2 positive (one serum, which was HSV-1 negative). Of the four indeterminate IgM sera, two were HSV-2 IgG positive in both assays, one was negative in both assays, and the fourth was negative in the Gull EIA IgG test and positive in our ELISA.
Twenty sera were from a study in which different commercially immunoassays had been compared (25, 26). The sera were not culture proven and were collected at the outpatient clinic for sexually transmitted diseases of the Erasmus Medical Center, Rotterdam, The Netherlands. The 20 sera gave discordant results in at least one of the assays. The numbers of sera positive for HSV-2 were 10, 10, and 8 when tested in the Gull HSV-2 IgG EIA (Gull Laboratories), the Chiron rapid immunoblot assay (RIBA; Chiron Corporation, Amsterdam, The Netherlands), and the Captia Select HSV-2-G EIA (Centocor, Malvern, Pa.), respectively. With our ELISA we found nine sera to be HSV-2 seropositive. Comparison showed that the Centocor EIA gave concordant results for 7 sera (2 positive and 5 negative), the Gull EIA gave concordant results for 15 sera (7 positive and 8 negative), and the Chiron RIBA gave concordant results for 15 sera (7 positive and 8 negative), with our ELISA. In the Chiron RIBA HSV-1 antibodies also were determined. Eighteen of the 20 samples (13 HSV-1 positive and 5 HSV-1 negative) gave concordant results. Of the two discordant results, one sample was not typed in our ELISA, and this sample was weakly HSV-1 positive in the Chiron RIBA; the other sample was HSV-1 positive in our ELISA and negative in the Chiron RIBA. The latter sample was also discordant for HSV-2 in our ELISA and the Chiron RIBA.
Finally, our ELISA was used to investigate the HSV seroprevalence in a group of 211 second-year medical students at the University of Groningen, Groningen, The Netherlands. Seventy six (36.0%) of the 211 were HSV-1 positive and 2 (0.9%) were HSV-2 positive in our assay. To compare our ELISA and the Gull EIA, we selected 119 sera out of 211. Predominantly, HSV-negative and only HSV-2-positive serum samples were selected. Among the 119 sera, 110 were HSV-2 seronegative and 2 were seropositive in both assays. Of the remaining seven sera, three were negative in the Gull EIA, and these three were not typed in our ELISA. One serum was indeterminate in the Gull EIA, and it was negative in our assay. Three sera were positive in the Gull EIA, and of these, one was HSV-2 negative and two had only gG-2 antibodies in our assay. The latter two were regarded as false positive by our criteria.
The Gull EIA was evaluated by others and was found to compare reasonably well with Western blotting (9). The Gull EIA was the only assay that was used for the detection of antibodies to HSV-2 in all 267 sera, next to our ELISA. When the results of our assay for IgG are compared with those obtained with the Gull EIA, the sensitivity and specificity are 91.5 and 95.5%, respectively (Table 1).
DISCUSSION
Recombinant gG-1t26-189His and gG-2281-594His.
The sequences of fragments of gG-1 and gG-2 were cloned in the baculovirus expression system. The identities of the fragments were confirmed by reactivity with gG-1- and gG-2-specific MAbs AP1 and LP10 in Western blotting. The sizes of truncated gG-1 produced in the baculovirus expression system were 36 to 39 kDa. This is in agreement with previously reported data (22). The sizes of the major gG-2 fragments produced in the baculovirus expression system were 64 to 72 kDa. This is higher than the calculated molecular mass of 25 kDa of the backbone. Others have also reported an abnormal molecular mass for mature gG-2. During synthesis of gG-2 produced in mammalian cells, the gG-2 precursor of 104 kDa is cleaved to generate the 31- and 72-kDa intermediates. The 31-kDa product is the N-terminal portion, and the 72-kDa product is the C-terminal cell-associated part of the precursor gG-2. The 31-kDa product is glycosylated to 34 kDa and secreted into the medium. The 72-kDa product is processed to a polypeptide with a molecular mass of 108 kDa, which is the mature gG-2 present in infected cells. The high molecular mass of mature cell-associated gG-2, comprising residues 343 to 699 of the precursor, is speculated to be partly due to highly O-linked glycosylated regions and to the relatively high proline content of the protein. Others also found high molecular masses, ranging from 92 to 115 kDa, for mature gG-2 (13, 28, 35). Taking into account that our fragment lacks 100 amino acids of the C-terminal part compared to the mature gG-2 described by others, a molecular mass of 64 to 72 kDa for gG-2281-594His could very well be in agreement with the molecular masses of 92 to 108 kDa of the mature cell-associated gG-2. Some HSV-2-specific sera reacted with, in addition to the 64- to 72-kDa bands, bands of high molecular masses of 120 to 150 kDa. Whether these bands represent gG-2 aggregates or dimers or whether they originate from insect cell proteins is not yet known.
The gG-2281-594His fragment contains the highly O-linked glycosylated part. When it was treated with O-glycosidase, the molecular mass of gG-2281-594His was reduced by 17 kDa. Also, this gG-2281-594His fragment has two N-linked glycosylation sites, both of which are glycosylated (54). To remove N-linked sugars, PNGase F and endo H were used. Neither PNGase F nor endo H treatment reduced the size of the gG-2281-594His fragment. As a control, gD1-313 was used, and upon endo H treatment, the size of gD1-313 was reduced by 2 kDa (data not shown). It has been reported earlier that fully processed cell-associated gG-2 is insensitive to endo H digestion (10). One explanation could be that the N-linked glycosylation sites are hidden by the highly O-linked glycosylated part. Another possibility is that the size reduction by endo H and PNGase F treatment in this expression system was too small to be detected by Western blotting.
The reactions of human sera with gG-1t26-189His and gG-2281-594His showed multiple polypeptide bands derived from gG-1t26-189His and gG-2281-594His expressed in the baculovirus system. The number of bands which reacted with the human sera was different from the number that reacted with the MAbs. This suggests and confirms that human sera react with several different epitopes of gG-1t26-189His or gG-2281-594His (24, 31, 35).
Sensitivity and specificity.
Western blotting is considered the gold standard for the detection of type-specific antibodies (7) and can distinguish most reliably between type 1 and type 2 antibodies by their reactivities with separated polypeptide bands. For screening of large populations, Western blotting is time-consuming. Since ELISAs are more cost-effective, we have compared our ELISA based on the recombinant antigens gG-1t26-189His, gG-2281-594His, and gD-11-313 and the extract of HSV-1-infected cells with one of the established commercial tests. We characterized the sera as HSV positive when the sera reacted with at least one of two type-common antigens. The sera were typed as type 1, type 2, or positive for both types by demonstration of reactivity against the type-specific antigen gG-1t26-189His or gG-2281-594His. In some cases, sera reacted only with HSV type-common antigens and not with type-specific antigens. These sera were designated not typed. Probably these sera were taken early in the primary infection and already contained type-common antibodies but did not yet contain antibodies against the type-specific glycoproteins (gG-1 or gG-2). This is suggested by the results obtained with the sera of the Amsterdam GG/GD, a number of which were not typed by our IgG ELISA and were positive in the Gull IgM assay. Follow-up serum samples could be useful to diagnose the serological status of individuals whose serum samples reacted as not typed.
Our test has 100% concordant results with culture-proven serum samples from Sweden but not with culture-proven sera from the Amsterdam GG/GD. The serum samples in Amsterdam were collected during the period that the patient visited the clinic with symptoms. It was not known at the time of the visit whether these patients had primary, initial, or recurrent HSV-2 infections. In the case of primary and initial HSV-2 infections, this might have been too early to detect antibody titers against gG-2. Usually anti-gG antibodies appear later than antibodies against HSV type-common antigen. This also demonstrates the advantages of using type-common antigens together with type-specific antigens. Recently, it was described that, sporadically, sera of patients from whom HSV-2 mutants with frameshift mutations in the gG-2 gene were isolated (33) lacked antibodies against gG-2. It cannot be excluded, therefore, that some of the sera diagnosed as not typed have no detectable antibodies due to the presence of aberrant forms of gG-2. In addition, it has been reported (1, 48) that the absence of antibodies against gG-2 can be due to seroreversion or loss of antibodies.
Finally we used our assay to test sera that were screened in three different assays. The results of our assay with the sera that were discordant among three kits showed that our test had the highest agreement with the Gull EIA and the Chiron RIBA. Data on HSV-1 seropositivity obtained with a commercial kit were available only for the 20 serum samples from the Erasmus Medical Center, Rotterdam (Chiron RIBA). This number was too small to compare the sensitivity and specificity with those of our ELISA.
Our results demonstrated that a recombinant baculovirus expressing the fragment gG-2281-594His can be used to detect HSV-2 type-specific antibodies. This baculovirus recombinant expressing the gG-2281-594His fragment has the advantage over the recombinant baculovirus expressing truncated gG-21-610 that approximately 10 times more gG-2281-594His fragment was obtained. The sensitivity and specificity were compared to those of the Gull assay, which is based on affinity-purified complete gG-2. The sensitivity and specificity of our assay were slightly lower than those of the Gull assay. One difference besides the antigens used is the dilution of the serum samples. In the Gull EIA, lower serum dilutions than we used in our assay are recommended. Our assay, with sensitivity and specificity of 91.5 and 95.5%, respectively, compared to the Gull test offers the possibility of determining HSV-1 and HSV-2 prevalence within reliable limits with the gG-2281-594His fragment.
Acknowledgments
We thank Yasuhiro Oda and Gjalt W. Welling for helpful discussion and critical reading of the manuscript. We are grateful to Sylvia Bruisten for supplying the serum samples from the Municipal Health Service Amsterdam and for critical reading of the manuscript.
REFERENCES
- 1.Arvaja, M., M. Lehtinen, P. Koskela, M. Lappalainen, J. Paavonen, and T. Vesikari. 1999. Serological evaluation of herpes simplex virus type 1 and type 2 infections in pregnancy. Sex. Transm. Infect. 75:168-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley, R. L. 1993. Laboratory techniques in the diagnosis of herpes simplex infection. Genitourin. Med. 69:174-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley, R. L. 1998. Type-specific antibodies to HSV-1 and -2: review of methodology. Herpes 5:33-37. [Google Scholar]
- 4.Ashley, R. L. 2001. Sorting out the new HSV type specific antibody tests. Sex. Transm. Infect. 77:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley, R. L., and M. Eagleton. 1998. Evaluation of a novel point of care test for antibodies to herpes simplex virus type 2. Sex. Transm. Infect. 74:228-229. [PubMed] [Google Scholar]
- 6.Ashley, R. L., M. Eagleton, and N. Pfeiffer. 1999. Ability of a rapid serology test to detect seroconversion to herpes simplex virus type 2 glycoprotein G soon after infection. J. Clin. Microbiol. 37:1632-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashley, R. L., J. Militoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J. Clin. Microbiol. 26:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashley, R. L., and A. Wald. 1999. Genital herpes: review of the epidemic and potential use of type-specific serology. Clin. Microbiol. Rev. 12:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashley, R. L., L. Wu, J. W. Pickering, M. C. Tu, and L. Schnorenberg. 1998. Premarket evaluation of a commercial glycoprotein G-based enzyme immunoassay for herpes simplex virus type-specific antibodies. J. Clin. Microbiol. 36:294-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balachandran, N., and L. M. Hutt-Fletcher. 1985. Synthesis and processing of glycoprotein gG of herpes simplex virus type 2. J. Virol. 54:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balachandran, N., D. E. Oba, and L. M. Hutt-Fletcher. 1987. Antigenic cross-reactions among herpes simplex virus types 1 and 2, Epstein-Barr virus, and cytomegalovirus. J. Virol. 61:1125-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergström, T., and E. Trybala. 1996. Antigenic differences between HSV-1 and HSV-2 glycoproteins and their importance for type-specific serology. Intervirology 39:176-184. [DOI] [PubMed] [Google Scholar]
- 13.Boucher, F. D., L. L. Yasukawa, K. Kerns, M. Kastelein, A. M. Arvin, and C. G. Prober. 1993. Detection of antibodies to herpes simplex virus type 2 with a mammalian cell line expressing glycoprotein gG-2. Clin. Diagn. Virol. 1:29-38. [DOI] [PubMed] [Google Scholar]
- 14.Brown, Z. A., J. K. Benedetti, D. H. Watts, S. Selke, S. Berry, R. L. Ashley, and L. Corey. 1995. A comparison between detailed and simple histories in the diagnosis of genital herpes complicating pregnancy. Am. J. Obstet. Gynecol. 172:1299-1303. [DOI] [PubMed] [Google Scholar]
- 15.Brown, Z. A. 2000. HSV-2 specific serology should be offered routinely to antenatal patients. Rev. Med. Virol. 10:141-144. [DOI] [PubMed] [Google Scholar]
- 16.Bruisten, S. M., I. Cairo, H. Fennema, A. Pijl, M. Buimer, P. G. H. Peerbooms, E. van Dijk, A. Meijer, J. M. Ossewaarde, and G. J. J. van Doornum. 2001. Diagnosing genital ulcer disease in a clinic for sexually transmitted diseases in Amsterdam, The Netherlands. J. Clin. Microbiol. 39:601-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryson, Y., M. Dillon, D. I. Bernstein, J. Radolf, P. Zakowski, and E. Garratty. 1993. Risk of acquisition of genital herpes simplex virus type 2 in sex partners of persons with genital herpes: a prospective couple study. J. Infect. Dis. 167:942-946. [DOI] [PubMed] [Google Scholar]
- 18.Cowan, F. M. 2000. Testing for type-specific antibody to herpes simplex virus—implications for clinical practice. J. Antimicrob. Chemother. 45(Suppl.):9-13. [DOI] [PubMed] [Google Scholar]
- 19.Cusini, M., M. Cusan, C. Parolin, L. Scioccati, I. Decleva, C. Mengoli, B. Suligoi, and G. Palu. 2000. Seroprevalence of herpes simplex virus type 2 infection among attendees of a sexually transmitted disease clinic in Italy. Italian Herpes Forum. Sex. Transm. Dis. 27:292-295. [DOI] [PubMed] [Google Scholar]
- 20.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeoch. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 72:2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frame, M. C., H. S. Marsden, and D. J. McGeoch. 1986. Novel herpes simplex virus type 1 glycoproteins identified by antiserum against a synthetic oligopeptide from the predicted product of gene US4. J. Gen. Virol. 67:745-751. [DOI] [PubMed] [Google Scholar]
- 22.Ghiasi, H., R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1992. Baculovirus-expressed glycoprotein G of herpes simplex virus type 1 partially protects vaccinated mice against lethal HSV-1 challenge. Virology 190:233-239. [DOI] [PubMed] [Google Scholar]
- 23.Gopal, R., T. Gibbs, M. J. Slomka, J. Whitworth, L. M. Carpenter, A. Vyse, and D. W. Brown. 2000. A monoclonal blocking EIA for herpes simplex virus type 2 antibody: validation for seroepidemiological studies in Africa. J. Virol. Methods 87:71-80. [DOI] [PubMed] [Google Scholar]
- 24.Grabowska, A., C. Jameson, P. Laing, S. Jeansson, E. Sjogren-Jansson, J. Taylor, A. Cunningham, and W. L. Irving. 1999. Identification of type-specific domains within glycoprotein G of herpes simplex virus type 2 (HSV-2) recognized by the majority of patients infected with HSV-2, but not by those infected with HSV-1. J. Gen. Virol. 80:1789-1798. [DOI] [PubMed] [Google Scholar]
- 25.Groen, J., B. Hersmus, H. G. Niesters, W. Roest, G. van Dijk, W. van der Meijden, and A. D. Osterhaus. 1999. Evaluation of a fully automated glycoprotein G-2 based assay for the detection of HSV-2 specific IgG antibodies in serum and plasma. J. Clin. Virol. 12:193-200. [DOI] [PubMed] [Google Scholar]
- 26.Groen, J., G. van Dijk, H. G. Niesters, W. I. van der Meijden, and A. D. Osterhaus. 1998. Comparison of two enzyme-linked immunosorbent assays and one rapid immunoblot assay for detection of herpes simplex virus type 2-specific antibodies in serum. J. Clin. Microbiol. 36:845-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashido, M., F. K. Lee, S. Inouye, and T. Kawana. 1997. Detection of herpes simplex virus type-specific antibodies by an enzyme-linked immunosorbent assay based on glycoprotein G. J. Med. Virol. 53:319-323. [DOI] [PubMed] [Google Scholar]
- 28.Ho, D. W., P. R. Field, W. L. Irving, D. R. Packham, and A. L. Cunningham. 1993. Detection of immunoglobulin M antibodies to glycoprotein G-2 by Western blot (immunoblot) for diagnosis of initial herpes simplex virus type 2 genital infections. J. Clin. Microbiol. 31:3157-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinghorn, G. R. 1998. Type-specific serological testing for herpes simplex infection. Int. J. Sex. Transm. Dis. Acquir. Immune Defic. Syndr. 9:497-500. [DOI] [PubMed] [Google Scholar]
- 30.Koelle, D. M., and A. Wald. 2000. Herpes simplex virus: the importance of asymptomatic shedding. J. Antimicrob. Chemother. 45(Suppl.):1-8. [DOI] [PubMed] [Google Scholar]
- 31.Levi, M., U. Ruden, H. Carlberg, and B. Wahren. 1999. The use of peptides from glycoproteins G-2 and D-1 for detecting herpes simplex virus type 2 and type-common antibodies. J. Clin. Virol. 12:243-252. [DOI] [PubMed] [Google Scholar]
- 32.Levi, M., U. Ruden, and B. Wahren. 1996. Peptide sequences of glycoprotein G-2 discriminate between herpes simplex virus type 2 (HSV-2) and HSV-1 antibodies. Clin. Diagn. Lab. Immunol. 3:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liljeqvist, J. Å., B. Svennerholm, and T. Bergström. 1999. Herpes simplex virus type 2 glycoprotein G-negative clinical isolates are generated by single frameshift mutations. J. Virol. 73:9796-9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liljeqvist, J. Å., B. Svennerholm, and T. Bergström. 2000. Conservation of type-specific B-cell epitopes of glycoprotein G in clinical herpes simplex virus type 2 isolates. J. Clin. Microbiol. 38:4517-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liljeqvist, J. Å., E. Trybala, B. Svennerholm, S. Jeansson, E. Sjögren-Jansson, and T. Bergström. 1998. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J. Gen. Virol. 79:1215-1224. [DOI] [PubMed] [Google Scholar]
- 36.Marsden, H. S., A. Buckmaster, J. W. Palfreyman, R. G. Hope, and A. C. Minson. 1984. Characterization of the 92,000-dalton glycoprotein induced by herpes simplex virus type 2. J. Virol. 50:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsden, H. S., K. MacAulay, J. Murray, and I. W. Smith. 1998. Identification of an immunodominant sequential epitope in glycoprotein G of herpes simplex virus type 2 that is useful for serotype-specific diagnosis. J. Med. Virol. 56:79-84. [DOI] [PubMed] [Google Scholar]
- 38.McGeoch, D. J., H. W. Moss, D. McNab, and M. C. Frame. 1987. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J. Gen. Virol. 68:19-38. [DOI] [PubMed] [Google Scholar]
- 39.Mertz, G. J., J. Benedetti, R. Ashley, S. A. Selke, and L. Corey. 1992. Risk factors for the sexual transmission of genital herpes. Ann. Intern. Med. 116:197-202. [DOI] [PubMed] [Google Scholar]
- 40.Mertz, G. J., R. W. Coombs, R. Ashley, J. Jourden, M. Remington, C. Winter, A. Fahnlander, M. Guinan, H. Ducey, and L. Corey. 1988. Transmission of genital herpes in couples with one symptomatic and one asymptomatic partner: a prospective study. J. Infect. Dis. 157:1169-1177. [DOI] [PubMed] [Google Scholar]
- 41.Oladepo, D. K., P. E. Klapper, and H. S. Marsden. 2000. Peptide based enzyme-linked immunoassays for detection of anti-HSV-2 IgG in human sera. J. Virol. Methods 87:63-70. [DOI] [PubMed] [Google Scholar]
- 42.Olofsson, S., M. Lundstrom, H. Marsden, S. Jeansson, and A. Vahlne. 1986. Characterization of a herpes simplex virus type 2-specified glycoprotein with affinity for N-acetylgalactosamine-specific lectins and its identification as g92K or gG. J. Gen. Virol. 67:737-744. [DOI] [PubMed] [Google Scholar]
- 43.Persson, K., A. Mansson, E. Jonsson, and E. Nordenfelt. 1995. Decline of herpes simplex virus type 2 and Chlamydia trachomatis infections from 1970 to 1993 indicated by a similar change in antibody pattern. Scand. J. Infect. Dis. 27:195-199. [DOI] [PubMed] [Google Scholar]
- 44.Prince, H. E., C. E. Ernst, and W. R. Hogrefe. 2000. Evaluation of an enzyme immunoassay system for measuring herpes simplex virus (HSV) type 1-specific and HSV type 2-specific IgG antibodies. J. Clin. Lab. Anal. 14:13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richman, D. D., A. Buckmaster, S. Bell, C. Hodgman, and A. C. Minson. 1986. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J. Virol. 57:647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sánchez-Martínez, D., D. S. Schmid, W. Whittington, D. Brown, W. C. Reeves, S. Chatterjee, R. J. Whitley, and P. E. Pellett. 1991. Evaluation of a test based on baculovirus-expressed glycoprotein G for detection of herpes simplex virus type-specific antibodies. J. Infect. Dis. 164:1196-1199. [DOI] [PubMed] [Google Scholar]
- 47.Saville, M., D. Brown, C. Burgess, K. Perry, S. Barton, F. Cowan, G. Palu, and C. Mengoli. 2000. An evaluation of near patient tests for detecting herpes simplex virus type-2 antibody. Sex. Transm. Infect. 76:381-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmid, D. S., D. R. Brown, R. Nisenbaum, R. L. Burke, D. Alexander, R. L. Ashley, P. E. Pellet, and W. C. Reeves. 1999. Limits in reliability of glycoprotein G-based type-specific serologic assays for herpes simplex virus type 1 and 2. J. Clin. Microbiol. 37:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sisk, W. P., J. D. Bradley, R. J. Leipold, A. M. Stoltzfus, D. L. Ponce, M. Hilf, C. Peng, G. H. Cohen, and R. J. Eisenberg. 1994. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 68:766-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slomka, M. J., R. L. Ashley, F. M. Cowan, A. Cross, and D. W. Brown. 1995. Monoclonal antibody blocking tests for the detection of HSV-1- and HSV-2-specific humoral responses: comparison with western blot assay. J. Virol. Methods 55:27-35. [DOI] [PubMed] [Google Scholar]
- 51.Stanberry, L. R. 2000. Asymptomatic herpes simplex virus shedding and Russian roulette. Clin. Infect. Dis. 30:268-269. [DOI] [PubMed] [Google Scholar]
- 52.Su, H. K., and R. J. Courtney. 1988. Inducible expression of herpes simplex virus type 2 glycoprotein gene gG-2 in a mammalian cell line. J. Virol. 62:3668-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su, H. K., R. Eberle, and R. J. Courtney. 1987. Processing of the herpes simplex virus type 2 glycoprotein gG-2 results in secretion of a 34,000-Mr cleavage product. J. Virol. 61:1735-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su, H. K., J. D. Fetherston, M. E. Smith, and R. J. Courtney. 1993. Orientation of the cleavage site of the herpes simplex virus glycoprotein G-2. J. Virol. 67:2954-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svennerholm, B., S. Olofsson, S. Jeansson, A. Vahlne, and E. Lycke. 1984. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with Helix pomatia lectin-purified antigens. J. Clin. Microbiol. 19:235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tran, L. C., J. M. Kissner, L. E. Westerman, and A. E. Sears. 2000. A herpes simplex virus 1 recombinant lacking the glycoprotein G coding sequences is defective in entry through apical surfaces of polarized epithelial cells in culture and in vivo. Proc. Natl. Acad. Sci. USA 97:1818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wald, A., J. Zeh, S. Selke, R. L. Ashley, and L. Corey. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 333:770-775. [DOI] [PubMed] [Google Scholar]
- 58.Wald, A., J. Zeh, S. Selke, T. Warren, A. J. Ryncarz, R. Ashley, J. N. Krieger, and L. Corey. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342:844-850. [DOI] [PubMed] [Google Scholar]
- 59.Wutzler, P., H. W. Doerr, I. Färber, U. Eichhorn, B. Helbig, A. Sauerbrei, A. Brandstädt, and H. F. Rabenau. 2000. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations—relevance for the incidence of genital herpes. J. Med. Virol. 61:201-207. [DOI] [PubMed] [Google Scholar]