Abstract
Meiotic drivers are selfish genetic elements that bias their own transmission, violating Mendel’s Law of Equal Segregation. It has long been recognized that sex chromosome–linked drivers present a paradox: Their success in transmission can severely distort populations’ sex ratio and lead to extinction. This paradox is typically solved by the presence of suppressors or fitness costs associated with the driver, limiting the propagation of the driver. Here, we show that Stellate (Ste) in Drosophila melanogaster represents a novel class of X chromosome–linked driver that operates with an inherent mechanism that weakens its drive strength. Ste protein asymmetrically segregates into Y-bearing cells during meiosis I, subsequently causing their death. Unexpectedly, Ste segregates asymmetrically again during meiosis II, sparing half of the Y-bearing spermatids from Ste-induced defects, thereby weakening the drive strength. Our findings reveal a mechanism by which sex chromosome drivers avoid suicidal success.
An intrinsically weak X chromosome–linked meiotic driver increases its transmission without exterminating Y chromosome.
INTRODUCTION
Meiotic drive is a phenomenon in which a genetic element (the meiotic driver) is transmitted to offspring at a rate higher than predicted by Mendel’s Law of Equal Segregation (1–4). Meiotic drivers are proposed to selfishly propagate within the population, even at the cost of the host’s fitness, and may exert a strong evolutionary force (2–4). Many drive systems are linked to sex chromosomes, leading to the non-Mendelian transmission of one sex chromosome over the other in the heterogametic sex, thereby distorting the sex ratio in the progeny (5). However, sex chromosome–linked drivers present a paradox: If a driver is successful and strongly skews the sex ratio in offspring, it would eventually drive the population to extinction, thus preventing its own transmission (6–8). This paradox was theorized by W. D. Hamilton, who used mathematical modeling to demonstrate that a strong sex ratio distortion rapidly leads to population extinction (9). There are several well-known mechanisms that solve this paradox. First, suppressors are known to many drivers that weaken the drive strength (4, 10–13). Second, many drivers are associated with fitness costs (either in the same or the opposite sex in which the drivers operate), limiting their propagation (14–18). Third, Fisher’s principle predicts a natural selection for a more balanced (1:1) sex ratio (9, 19). However, it remains unknown whether other mechanisms may exist to solve this paradox.
Stellate (Ste), an X chromosome–linked multicopy gene in Drosophila melanogaster, is normally repressed by Piwi-interacting RNAs (piRNAs) produced from the Y chromosome–linked Suppressor of Stellate [Su(Ste), also known as crystal (cry)] (Fig. 1A) (20–23). Ste is a suspected meiotic driver that biases transmission of the X chromosome in males (24, 25), yet previous studies found only weak sex ratio distortion (60 to 80% female) upon Ste derepression. In addition, a higher degree of Ste expression did not proportionally lead to increased transmission of the X chromosome, calling into question the identity of Ste as a meiotic driver (26–28). Here, we demonstrate that Ste is a meiotic driver; however, it operates through an inherent mechanism that weakens its drive strength. This mechanism may facilitate the propagation of the Ste-encoding X chromosome within the population while preventing extinction caused by an extremely distorted sex ratio. We propose that Ste represents a novel class of meiotic driver with an inherent mechanism that restrains drive strength, solving the paradox of sex chromosome drive.
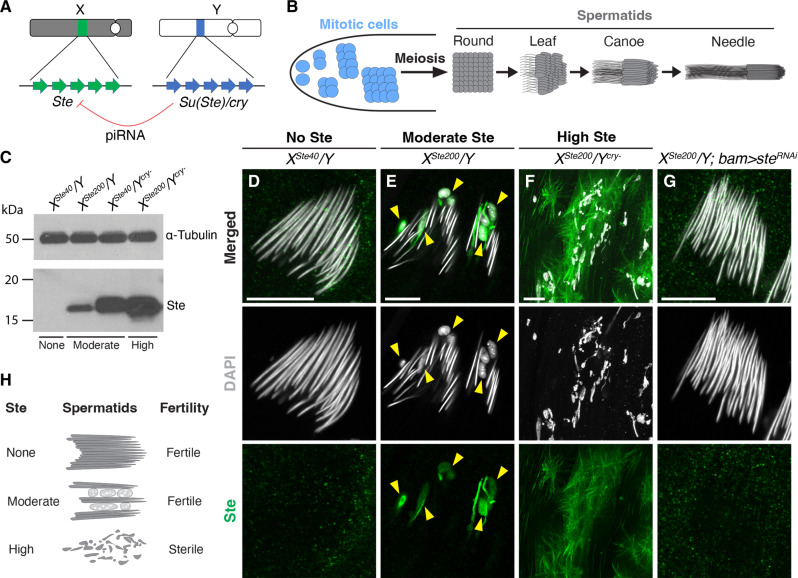
Fig. 1. Moderately expressed Ste leads to defective sperm nuclear compaction.
(A) Schematic showing the locations of Ste and Su(Ste)/cry on the X and Y chromosomes. Ste is typically silenced by piRNAs produced from Su(Ste). (B) Schematic of D. melanogaster male germ cell differentiation. (C) Western blotting of whole testis lysates from the indicated genotypes, probed with anti-α-Tubulin (loading control) and anti-Ste antibodies. (D to G) Immunofluorescence staining for Ste (green) in needle-stage spermatid cysts of XSte40/Y (D), XSte200/Y (E), XSte200/Ycry- (F), and XSte200/Y; bam > steRNAi (G). Yellow arrowheads indicate sperm nuclei with DNA compaction defects. Gray, DAPI. Scale bars, 10 μm. (H) Diagram showing phenotypes under different Ste-expressing conditions.
RESULTS
Ste impedes sperm nuclear DNA compaction
Previous studies have shown that Ste can be derepressed to varying degrees, depending on both Ste copy number and the activity of the piRNA pathway. In this study, similar to previous research (26, 29), we used combinations of methods to achieve varying levels of Ste derepression: (i) X chromosomes with different Ste copy numbers (XSte40 with ~40 copies and XSte200 with ~200 copies of Ste) (fig. S1A), (ii) Su(Ste)/cry-deleted Y chromosomes (fig. S1B), and (iii) knockdown of the piRNA pathway component Aubergine (Aub) (Fig. 1C and fig. S1C; see table S1 for a more detailed description). These manipulations resulted in conditions in which Ste was either not expressed, moderately expressed, or highly expressed. Under conditions without Ste (i.e., wild-type or equivalent), no cytological phenotype was observed, thus serving as a control (Fig. 1D and fig. S1D). Under high Ste conditions, because of catastrophic meiosis (26), no functional postmeiotic spermatids were produced and the males were completely sterile (Fig. 1F and fig. S1, H to J) (26, 30, 31). Under moderate Ste conditions, Ste was not expressed at levels high enough to cause sterility (fig. S1J), but it led to sex ratio distortion skewed toward female progeny (60 to 80% female) as previously reported (26, 28) (see below for the sex ratio assay). In this study, we used multiple genotypes (XSte200/Y, XSte40/Y; aubRNAi, and XSte40/Ycry-) that induce moderate Ste expression (table S1), and we investigated how Ste leads to meiotic drive.
Consistent with earlier studies (26, 28), males with moderate Ste expression remained fertile (fig. S1J). Under this condition, spermatogenesis appeared mostly normal (fig. S1, E to G), with all stages of differentiating germ cells present in an apparently normal spatiotemporal order within the testis. However, abnormalities became apparent during postmeiotic spermatid development. In wild-type flies, postmeiotic sperm differentiation was accompanied by stereotypical morphological changes, resulting in highly compacted sperm nuclei (Fig. 1, B and D). In flies with moderate Ste expression, we observed Ste protein localizing to a subset of spermatids throughout the postmeiotic stages (fig. S2, B to E and G to J), which eventually exhibited nuclear DNA compaction defects (Fig. 1E and fig. S2, E and J). RNA interference (RNAi)–mediated knockdown of Ste rescued the nuclear DNA compaction defect (Fig. 1G and fig. S1K), confirming that this defect was caused by Ste expression. The observed nuclear DNA compaction defects resemble those induced by other known “sperm-killing” meiotic drivers, such as D. melanogaster Segregation Distorter (SD) (32, 33) and D. simulans Sex Ratio (SR) (12). Similar to SD (33), Ste-containing spermatids that failed to compact DNA also failed to incorporate protamines, such as Mst77F and ProtB, which are essential components of sperm chromatin (fig. S3, B to D) (34–36). These defective nuclei also lacked histones, indicating that histones were removed without undergoing the proper histone-to-protamine transition (fig. S3, A and B) (37). Together, we conclude that moderately expressed Ste protein localizes to a subset of differentiating spermatids, leading to defective sperm development and ultimately sperm death (Fig. 1H).
Ste preferentially harms Y-bearing sperm
Moderate Ste expression is known to mildly increase the proportion of females in the progeny, which originally led to the hypothesis that Ste is a meiotic driver (24, 25). To test whether Ste-mediated defects in sperm development preferentially affect Y-bearing sperm, we performed DNA fluorescence in situ hybridization (FISH) using probes specific to the X or Y chromosome [(TAGA)n for the X and (AATAAAC)n for the Y] (38). We found that 70 to 90% of the Ste-containing spermatids carried Y chromosomes (Fig. 2, A to C, and fig. S4, A and B), suggesting that Ste acts as a meiotic driver by preferentially killing Y-bearing spermatids. Moreover, we found that transgenic piRNA-resistant Ste (β-tubulin promoter-StepiRNA-resistant) also preferentially localized to Y chromosome–bearing spermatids and caused nuclear DNA compaction defects (fig. S5). Together with the finding that RNAi-mediated knockdown of Ste rescues the nuclear DNA compaction defect (Fig. 1G and fig. S1K), these results demonstrate that Ste is both necessary and sufficient to cause the biased killing of Y-bearing sperm.
Fig. 2. Ste preferentially segregates to Y-bearing spermatids through asymmetric segregation during meiosis I.
(A and B) Immunofluorescence staining for Ste (green) combined with DNA FISH for X- and Y-chromosome–specific satellite DNA sequences (X: Cy5-TCTA, blue; Y: Cy3-AATAAAC, magenta) in early (A) and late (B) spermatid cysts of XSte200/Y males. Nuclei of Ste-containing spermatids are indicated by yellow dotted lines. Gray, DAPI. Scale bars, 10 μm. (C) Percentage of X- or Y-bearing spermatids among Ste-containing spermatids in the indicated genotypes. The number of scored Ste-containing spermatids is shown above the bar graph. Statistical analysis was performed using two-sided Fisher’s exact tests (null hypothesis: Ste-containing spermatids have equal chances of carrying the X or Y chromosome). ****P < 0.0001. (D) Immunofluorescence staining for Ste (green) combined with phalloidin staining (F-actin, blue) in a telophase I cell (indicated by white dotted lines) of XSte200/Y; Ubi-GFP-Pav males (GFP-Pav, contractile ring, magenta). Gray, DAPI. Scale bar, 10 μm. (E) Percentage of telophase I cells displaying asymmetric segregation of Ste protein in the indicated genotypes. The number of scored telophase I cells is shown above the bar graph. (F) Immunofluorescence staining for Ste (green) and GFP-Pav (blue), combined with DNA FISH for the Y chromosome–specific satellite DNA sequence (Cy3-AATAAAC, magenta) in a telophase I cell (indicated by white dotted lines) of XSte200/Y; Ubi-GFP-Pav males. Gray, DAPI. Scale bar, 10 μm. (G) Percentage of telophase I cells with Ste cosegregating with the X or Y chromosome among cells with asymmetric Ste segregation in the indicated genotypes. The number of scored telophase I cells is shown above the bar graph. Statistical analysis was performed using two-sided Fisher’s exact tests (null hypothesis: Ste has equal chances of cosegregating with the X or Y chromosome during meiosis I). *P = 0.0121; **P = 0.0018 ****P < 0.0001.
Ste asymmetrically segregates with the Y chromosome in meiosis I
We next investigated how the Ste protein becomes concentrated in Y-bearing spermatids. Ste’s preferential localization in Y-bearing spermatids was evident immediately following meiosis (Fig. 2A and fig. S4A) and persisted throughout spermatid development (Fig. 2, A and B, and fig. S4, A to C). In contrast, Ste was expressed in all spermatocytes immediately before meiosis (fig. S2, A and F). These results suggest that Ste’s preferential localization to Y-bearing spermatids is established during meiosis. We observed that Ste protein was asymmetrically enriched in only one daughter cell in over 80% of meiotic telophase I cells (Fig. 2, D and E, and fig. S6, A and C). Notably, in 64 to 82% of the meiotic I cells that displayed Ste asymmetry, Ste and the Y chromosome cosegregated to the same side during telophase I (Fig. 2, F and G, and fig. S6, B and D). Although Ste protein is known to form amyloid-like aggregates (crystals) (30, 39, 40), we also noted a population of diffusely distributed Ste protein within the cell, which was also asymmetrically enriched in the same side as the Ste aggregates in telophase I cells (Fig. 2, D and F, and fig. S6). On the basis of these observations, we conclude that Ste preferentially localizes to Y-bearing spermatids because of its asymmetric segregation during meiosis I.
Ste segregates asymmetrically in meiosis II
Our findings thus far support that Ste is a meiotic driver that biases transmission of the X chromosome to offspring by harming sperm DNA compaction of Y-bearing spermatids. However, questions remain unanswered as to why Ste causes only a mildly increased female-to-male ratio and why the frequency of X-bearing sperm (i.e., female offspring) does not increase proportionally with increasing amount of Ste, as noted decades ago (26–28).
To our surprise, we found that Ste undergoes asymmetric segregation during meiosis II as well (Fig. 3, A and B, and fig. S7, A and C). During telophase of meiosis II, ~80% of Ste-containing cells exhibited asymmetric segregation of Ste (Fig. 3B). Thus, even if a cell inherited Ste protein at the end of meiosis I, meiosis II would result in one spermatid with Ste and the other without it (Fig. 3D). We noted that the asymmetric segregation pattern during meiosis II was independent of the sex chromosomes: Whether cells were segregating X-X or Y-Y sister chromatids, Ste was asymmetrically segregated in ~80% of cases (fig. S7E).
Fig. 3. Ste exhibits asymmetric segregation during meiosis II.
(A) Immunofluorescence staining for Ste (green) combined with phalloidin staining (F-actin, blue) in a telophase II cell (indicated by white dotted lines) of XSte200/Y; Ubi-GFP-Pav males (GFP-Pav, magenta). Gray, DAPI. Scale bar, 10 μm. (B) Percentage of Ste-containing telophase II cells displaying asymmetric segregation of Ste protein in the indicated genotypes. The number of scored telophase II cells is shown above the bar graph. (C) Immunofluorescence staining for Ste (green) and GFP-Pav (blue), combined with DNA FISH for the Y chromosome–specific satellite DNA sequence (Cy3-AATAAAC, magenta) in a telophase II cell (indicated by white dotted lines) of XSte200/Y; Ubi-GFP-Pav males. Gray, DAPI. Scale bar, 10 μm. (D) Model of asymmetric segregation of Ste during meiosis I and II, producing two X-bearing sperm and one Y-bearing sperm. Note that the frequency of asymmetry is not 100% and this model represents the scenario with the highest probability. (E) Ratio of X- to Y-bearing sperm produced by males of the indicated genotypes (calculated by the ratio of female to male progeny). Each dot in the graph represents a single male. Ten males were assayed for each genotype. Data are means ± SD. Dashed line indicates the expected 1:1 ratio. Statistical analysis was performed using two-sided unpaired t tests. **P = 0.0086; ***P = 0.0001.
This asymmetric segregation during meiosis II has a notable implication: Although Ste protein is preferentially segregated with the Y chromosome during meiosis I, about half of the Y-bearing spermatids are spared from inheriting Ste due to the asymmetric segregation in meiosis II (Fig. 3C and fig. S7, B and D), allowing for the survival of roughly half of the Y-bearing spermatids (Fig. 3D). This explains why earlier studies observed only a mild female-biased sex ratio and why the female frequency did not increase proportionally with increasing levels of Ste expression (26–28). We recapitulated this mild female-biased sex ratio in the progeny of XSte200/Y males (Fig. 3E). RNAi-mediated depletion of Ste rescued the sex ratio distortion (Fig. 3E), confirming that Ste is responsible for the skewed sex ratio. In conclusion, we demonstrate that Ste is a meiotic driver; however, its asymmetric segregation during meiosis II results in only a weakly skewed sex ratio.
Weak drive avoids extinction
It has long been recognized that a sex chromosome driver runs a risk of population extinction by skewing the sex ratio (6–8). Through mathematical modeling, Hamilton demonstrated that complete sex chromosome drive (100% transmission of the driving chromosome) would result in population extinction due to a severely skewed sex ratio (9). Propagation of strong drivers can be limited by the presence of suppressors that weaken the drive outcome (4, 10–13). Alternatively, fitness costs associated with the driver may also restrict the propagation of the driver within the population (14–18). Our results described thus far suggest that the asymmetric segregation of Ste during meiosis II may serve as a mechanism to prevent Ste from becoming a complete X chromosome driver, in addition to the presence of suppressor [Su(Ste)].
To explore how weak X chromosome drivers affect population dynamics, we conducted mathematical modeling. It should be noted that this modeling is blind to the mechanism by which the drive strength is weakened. Using parameters similar to Hamilton’s model, we first recapitulated his results for 100% drive strength (complete drive) (Fig. 4, A and C): This strong drive caused rapid population extinction after a brief period of expansion, as shown previously (9). Modulation of the drive strength (defined as the frequency of X-sperm produced by males; see Materials and Methods) considerably influenced population outcomes. Drive strengths of 90 or 80% also led to extinction but at a slower rate than the 100% drive (Fig. 4A). In contrast, we found that lower drive strengths (70 and 60%) did not cause extinction, instead allowing continued population expansion (Fig. 4A). We found that there is a threshold drive strength that determines whether the population will eventually face extinction or not (Fig. 4B). When other parameters were kept consistent with Hamilton’s model (Materials and Methods), this threshold was 75% (Fig. 4B). Drivers stronger than 75% would shift from the positive growth phase (population change rate > 1) to the negative growth phase (population change rate < 1) after certain generations, ultimately leading to extinction (Fig. 4, B and C). Drivers with a strength of exactly 75% would reach a steady state (population change rate = 1) (Fig. 4, B and D). Any drivers with a strength between 50 and 75% would remain in the positive growth phase (Fig. 4B), resulting in continued population expansion (Fig. 4E). In contrast to a strong driver (strength = 100%) that rapidly leads to an extremely low male/female ratio in the population (Fig. 4C), weaker drivers that avoid extinction (strength = 75 and 60%) eventually reach a stabilized and less skewed sex ratio after many generations (Fig. 4, D and E). Notably, however, weak drivers do not compromise their ability to eventually fix themselves as the sole X chromosome in the population, although they do so at a slower rate than strong drivers (fig. S8A).
Fig. 4. Weak sex chromosome drive avoids population extinction.
(A) Population size across generations with varying degrees of drive strength (defined as the frequency of X-sperm produced by males). (B) (a) Population change rate over generations with varying degrees of drive strength. The transition line, where the population change rate is one (no population growth), is indicated by the white arrow. (b) Two-dimensional projection of (a), showing that the transition line separates the positive growth phase (rate > 1) from the negative growth phase (rate < 1). A driver with a strength of 75% will reach a steady state (no population growth) after a certain number of generations. (C to E) Simulation showing the male/female ratio (black line), number of females (blue line), and number of males (red line) across generations when the drive strength is 100% (C), 75% (D), and 60% (E).
Although the exact threshold for drive strength is influenced by parameters such as the number of females a male can fertilize and the number of offspring a female can produce, changing these parameters still resulted in the presence of the threshold, which ranged >75% (fig. S8B and Materials and Methods). Thus, drivers with a strength below 75% reside in a “safe zone” that does not cause extinction. The observed drive strength for Ste (the frequency of X-sperm produced by Ste-expressing males) in this study and a previous work (26) ranges from 56 to 82% with an average of 73% (fig. S8C and table S2). These results indicate that Ste’s drive strength is likely below the threshold, allowing it to avoid extinction and resolving the paradox of sex chromosome meiotic drive.
DISCUSSION
Sex chromosome meiotic drive is believed to be an important evolutionary force (2, 5); however, it runs the risk of extinction due to an extremely skewed sex ratio that interferes with successful breeding (6, 7, 9). Although there are known mechanisms by which strong drivers are restricted, such as suppressors and fitness costs (4, 10–18), we propose that Stellate represents a novel class of meiotic drivers with a built-in mechanism that weakens the drive strength, which allows them to avoid the fate of extinction. The preferential segregation of Ste protein to the Y chromosome–bearing side during meiosis I provides the foundation for drive, whereas the asymmetric segregation during meiosis II serves as the mechanism that dampens the strength of drive. Our mathematical modeling shows that extinction is not the inevitable outcome of any X chromosome–linked drivers; only those exceeding a certain strength threshold lead to extinction. We propose that the asymmetric segregation of Ste during meiosis II weakens the drive strength below this critical threshold, allowing Ste to avoid the fate of extinction and resolving the paradox of sex chromosome drivers. It is worth noting that, whereas the rise of suppressors at distinct genetic loci is generally thought to counteract meiotic drivers (3, 4), suppressors can be separated from the driver through breeding, making the fate of a driver reliant on the probability to be with suppressors. In contrast, Ste uses a mechanism that makes the driver inherently weak even in the absence of a suppressor. This is not to say that the suppressing mechanism, i.e., Su(Ste), is unnecessary. The asymmetric segregation of Ste during meiosis II cannot prevent the meiotic failure caused by the high-level expression of Ste, thus requiring the action of Su(Ste).
Meiotic drivers in females often use the inherent asymmetry of female meiosis by preferentially segregating into the egg and avoiding the nontransmissive polar bodies (gonotaxis), thus ensuring their transmission to the offspring (3, 41–43). In contrast, in males and fungi, where meiosis symmetrically produces four viable gametes from a diploid germ cell, meiotic drivers are often assumed to operate during the postmeiotic stage by sperm or spore killing (3, 4). This is because cytological defects typically appear in the postmeiotic stages (12, 32, 33, 44–46). However, it has been speculated that postmeiotic cytological defects may be caused by earlier events (4, 47, 48). Our study provides an example where sperm killing can be seeded during meiosis, through the asymmetric segregation of a driver-encoded protein, which later kills sperm in the postmeiotic stages. It remains unclear whether the asymmetric segregation of Ste during meiosis is an implication of any unknown inherent asymmetry of male meiosis, similar to female meiosis. It also remains elusive how Ste cosegregates with the Y chromosome during meiosis I, which is the foundation of its drive. Although Ste might have an affinity for the Y chromosome, the fact that Ste segregates asymmetrically during meiosis II, when identical sister chromatids are segregating away from each other, suggests that Ste’s asymmetry may not be entirely chromosome dependent.
In summary, we propose that Ste is a sex chromosome–linked meiotic driver with a built-in mechanism that “self-restrains” its drive strength, allowing it to avoid the fate of extinction without necessarily relying on suppressors. This type of driver with a built-in weakness may have been overlooked and understudied due to its incomplete drive.
MATERIALS AND METHODS
Fly husbandry and strains used
All D. melanogaster strains were raised on standard Bloomington medium at 25°C. The following strains were used: the standard lab wild-type strain y w (y1w1, used as XSte40), the double balancer strain XSte200/Y; Sp/CyO;TM2/TM6B, Ycry- (BScry1Yy+, a gift from M. P. Bozzetti) (26, 28), nos-gal4ΔVP16 (49), nos-gal4:VP16 (50), bam-gal4:VP16 [Bloomington Drosophila Stock Center (BDSC): 80579, a gift from D. McKearin] (51), steTRiP.HMJ30118 (BDSC: 63552) (52), aubTRiP.GL00076 (BDSC: 35201) (52), Ubi-GFP-Pav (BDSC: 81650, a gift from D. Glover) (53), Mst77F-EGFP (Kyoto Stock Center DGRC: 109174) (35), and ProtB-EGFP (BDSC: 58406) (35).
βTub-Ste transgene construction
The βTub-StepiRNA-resistant transgenic strain was generated via phiC31 site-directed integration into the D. melanogaster genome. The piRNA-resistant Ste cDNA was designed by introducing silent mutations throughout the entire CDS (sequence provided in table S3). We designed the CDS by adopting the consensus sequence from the 13 annotated Ste genes on FlyBase (Ste:CG33236, Ste:CG33237, Ste:CG33238, Ste:CG33239, Ste:CG33240, Ste:CG33241, Ste:CG33-242, Ste:CG33243, Ste:CG33244, Ste:CG33245, Ste:CG33246, Ste:CG33247, and SteXh:CG42398). The piRNA-resistant cDNA was synthetized by Thermo Fisher Scientific (GeneArt Gene Synthesis) and inserted into the pattB vector along with the β2-tubulin (βTub) promoter (generously provided by P. Chen and A. Aravin). The pattB-βTub-StepiRNA-resistant construct was inserted into the attP18 integration site on the X chromosome. The transgenic line was generated by BestGene Inc.
Western blots
Testes (25 pairs per sample) from 2- to 3-day-old males were dissected, rinsed with 0.1 M phosphate-buffered saline (1x PBS) at pH 7.2, snap frozen, and stored at −80°C until use. The testes were homogenized in 150 μl of 1x PBS supplemented with cOmplete protease inhibitor (EDTA-free, Roche) and mixed with 150 μl of 2× Laemmli Sample Buffer (Bio-Rad), supplemented with 2-mercaptoethanol. Cleared lysates were denatured at 95°C for 3 to 5 min, separated on a 14% Tris-glycine gel (Thermo Fisher Scientific), and transferred onto a polyvinylidene fluoride membrane (iBlot2, Invitrogen). Mouse anti-α-Tubulin (AA 4.3; 1:3000, obtained from the Developmental Studies Hybridoma Bank) and guinea pig anti-Ste (54) (used at 1:10,000) were used as primary antibodies. Horseradish peroxidase–conjugated goat anti-mouse IgG (115-035-003; 1:10,000; Jackson ImmunoResearch Laboratories) and anti-guinea pig IgG (106-035-003; 1:10,000; Jackson ImmunoResearch Laboratories) were used as secondary antibodies. Signals were detected using the Pierce ECL Western Blotting Substrate enhanced chemiluminescence system (Thermo Fisher Scientific).
Immunofluorescence staining
Testes from 0- to 3-day-old males were dissected in 1x PBS and fixed in 4% formaldehyde in 1x PBS for 30 min. Fixed testes were then washed in 1x PBST (PBS containing 0.1% Triton X-100) for at least 2 hours, followed by incubation with primary antibodies diluted in 1x PBST containing 3% bovine serum albumin (BSA) at 4°C overnight. Samples were washed three times in 1x PBST for 30 min each and then incubated with secondary antibodies in 1x PBST with 3% BSA at 4°C overnight. After a similar washing procedure, samples were mounted in VECTASHIELD with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Labs). Images were acquired using a Leica Stellaris 8 confocal microscope with a 63x oil immersion objective lens (numerical aperture 1.4) and processed with Fiji (ImageJ) software. The primary antibodies used were anti-Ste (1:200; guinea pig) (54), anti-GFP (1:100; rabbit; Abcam, ab290), anti-histone H3 (1:200; rabbit; Abcam, ab1791), anti-Mst77F (1:100; guinea pig) (55), anti-Pavarotti (Pav) (1:100; rabbit; a gift from D. Glover) (56), and anti-ATP5a (1:1000; mouse; Abcam, ab14748). Phalloidin–Alexa Fluor 568 (1:200; Thermo Fisher Scientific, A12380) was used to stain F-actin. Alexa Fluor–conjugated secondary antibodies (Life Technologies) were used at a 1:200 dilution.
DNA fluorescence in situ hybridization
Testes from 0- to 3-day-old males were dissected as described above, and an optional immunofluorescence staining protocol (modified by adding 1 mM EDTA to formaldehyde, PBST, and BSA) was performed first when necessary. Subsequently, samples were postfixed with 4% formaldehyde + 1 mM EDTA for 30 min and washed in 1x PBST + 1 mM EDTA for 30 min. Fixed samples were incubated with RNase A solution (2 mg/ml; in PBST) at 37°C for 10 min, followed by washing with 1x PBST + 1 mM EDTA for 5 to 10 min. Samples were then rinsed in 2x SSC + 1 mM EDTA + 0.1% Tween 20 and washed in 2x SSC + 0.1% Tween 20 with increasing formamide concentrations (20, 40, and 50%) for 15 min each, followed by a final 30-min wash in 2x SSC + 0.1% Tween 20 + 50% formamide. Hybridization mix (50% formamide, 10% dextran sulfate, 2x SSC, 1 mM EDTA, and 1 mM probe) was added to the washed samples. Samples were denatured at 91°C for 2 min and then incubated overnight at 37°C. After hybridization, samples were washed three times in 2x SSC + 1 mM EDTA + 0.1% Tween 20 for 20 min each and mounted in VECTASHIELD with DAPI (Vector Labs). The following satellite DNA probes were used: Cy5-(TCTA)8 and Cy5-359 for the X chromosome, and Cy3-(AATAAAC)6 for the Y chromosome (38). The sequence of the Cy5-359 probe is AGGATTTAGGGAAATTAATTTTTGGATCAATTTTCGCATTTTTTGTAAG.
Droplet digital PCR
Genomic DNA was extracted from individual 0- to 3-day-old males and virgin females using a modified protocol of the DNeasy Blood and Tissue DNA extraction kit (Qiagen). Briefly, individual flies were homogenized in 200 μl of Buffer ATL containing proteinase K using a pipette tip in PCR tubes, followed by vortexing for 15 s and incubation at 56°C for 1.5 hours. The samples were then transferred to 1.5-ml Eppendorf tubes and processed according to the manufacturer’s instructions. DNA samples were then quantified and checked for purity using a NanoDrop One spectrophotometer (Thermo Fisher Scientific). For droplet digital polymerase chain reaction (ddPCR), 30 ng of DNA was used per 20-μl reaction for control genes (RpL and Upf1), 3 ng for Ste, and 0.3 ng for Su(Ste). The primers and probes for control reactions were as described in our previous studies (57, 58), whereas those for Ste and Su(Ste) were designed by Bio-Rad. The specificities of Ste and Su(Ste) primers and probes were validated in fig. S1 (A and B). ddPCR reactions were prepared according to the manufacturer’s protocol (Bio-Rad). In short, master mixes containing ddPCR Supermix for Probes (No dUTP) (Bio-Rad), DNA samples, and primer/probe mixes were assembled in PCR tubes and incubated at room temperature for 15 min to allow for restriction enzyme digestion. For Ste and Su(Ste) ddPCR, the Hae III restriction enzyme (New England Biolabs) was used to digest repetitive DNA into single units. ddPCR droplets were generated from samples using the QX200 Droplet Generator (Bio-Rad) and underwent complete PCR cycling on a C100 deep-well thermocycler (Bio-Rad). Droplet fluorescence was read using the QX200 Droplet Reader (Bio-Rad). Sample copy numbers were determined using Quantasoft software (Bio-Rad). Ste and Su(Ste) copy numbers were calculated based on the copy numbers of reference genes RpL and Upf1. The copy number values determined by each control gene was averaged to determine the final copy number for each sample. Six flies were analyzed per genotype.
Fertility and sex ratio assay
For each genotype, 10 males were assayed as follows. Individual 0- to 1-day-old males were crossed with two 1- to 4-day-old virgin y w females for 5 days at 25°C, and all F1 progenies were counted for fig. S1J. The raw data for the fertility assay are provided in table S4. For the sex ratio assay, individual 0- to 1-day-old males were crossed with two 1- to 4-day-old virgin y w females in a vial for 5 days at 25°C. After 5 days of mating, females were discarded, and the same males were transferred to new vials to mate with two new 1- to 4-day-old virgin y w females for another 5 days at 25°C. This mating scheme was repeated five times. The number and sex of progenies from each cross were recorded. The total female and male progenies from all five mating periods were used to calculate the X:Y (female:male) ratio for each male in Fig. 3E (table S5). The percentage of X-bearing sperm for each genotype was calculated based on the total number of female and male progenies from all 10 assayed males and is presented in fig. S8C, along with sex ratio data from previous study (26) (table S2). Notably, we observed an increase in the female/male progeny ratio as the male aged.
Statistics and reproducibility
No statistical method was used to predetermine sample size due to the ample sample sizes afforded by the use of Drosophila. No data were excluded from the analyses. Data analysis was performed using Microsoft Excel and GraphPad Prism 10. All graphs, except those in Fig. 4 and fig. S8 (A and B), were generated using GraphPad Prism 10. The experiments were not randomized because this study did not involve treatment or exposure of animals to any agents. The investigators were not blinded for data collection and analyses because methods used for data acquisition (immunofluorescence, DNA FISH, Western blotting, fly number quantification, and ddPCR) are not influenced by the experimenter’s knowledge of fly genotype, and the analyses were performed with the same automated algorithms. Two-sided Fisher’s exact tests were used for analyzing Ste’s preferential localization in spermatids and Ste’s preferential segregation during meiosis I (tables S7 and S8). Note that a low frequency (0 to 4%) of nondisjunction events (XY or O spermatids) was observed (table S6) and was excluded from statistical analyses. Two-sided unpaired t tests were used to compare the sex ratios of progeny in Fig. 3E. The exact P values are provided in each figure legend.
Mathematical modeling
Mathematical modeling in Fig. 4 and fig. S8 (A and B) was performed using MATLAB. Code is deposited at https://github.com/xuefengmeng/Meng_et_al_2024.git. The code is also available as supporting files. ChatGPT (GPT-4-turbo) was used to polish the code by asking “how to make the following matlab code more efficient?” to generate the final version of the code.
We designate the driving X chromosome as X’ and normal sex chromosomes as X and Y. The following symbols are assigned for generation n: Totaln (total number of flies), Mn (number of males), Fn (number of females), An (number of X’Y males), Bn (number of XY males), Cn (number of X’X’ females), Dn (number of X’X females), En (number of XX females), Rn (frequency of X’ in males), and Kn (frequency of X’ in females). Drive strength t is defined as the frequency of X-bearing sperm produced by males, where 0.5 < t ≤ 1. Same as Hamilton’s modeling (9), we initiate the population with 1000 females and 1000 males, with the frequency of the driving X chromosome (X’) in both females and males set at 1/1000 (M0 = F0 = 1000, R0 = K0 = 1/1000).
To simulate the effect of the driving X’ chromosome on the population, we calculate population sizes in each generation using the following method. If flies in generation n produce N eggs, then the number of X’ eggs is N · Kn and the number of X eggs is N · (1 − Kn). Given that the frequency of X’Y males is Rn, the frequency of XY males is 1 − Rn, and the frequency of X’-sperm produced by X’Y males is t (whereas XY males produce X-sperm at a frequency of 0.5), the following equations are derived
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
Thus, we can derive
| (9) |
| (10) |
Thus,
| (11) |
Equations 9, 10, and 11 define the recursive functions for Rn and Kn, given the initial conditions R0 = K0 = 1/1000. These equations enable the calculation of Rn and Kn for any generation n. We found that, as n increases, both Rn and Kn will eventually reach 1 and the rate depends on the value of t.
We further define the reproduction index z as the number of offspring a female produces and the mating index w as the number of females a male can fertilize. The model requires z ≥ 2 because z = 1 leads to population decline even without a driver. Because Drosophila is a promiscuous species, we consider w ≥ 2 in our model. The parameter w is crucial in determining the population’s sensitivity to sex ratio distortion. In generation n, if w · Mn ≥ Fn, there would be enough males to fertilize all females (all females are able to reproduce). Thus, the number of fertilized eggs produced in generation n is N = z · Fn. In this scenario, the population will increase over generations as each generation produces more females than the previous generation. The female-to-male ratio will also increase over generations. By incorporating N = z · Fn into Eqs. 1 and 2, we get
| (12) |
| (13) |
However, as the female-to-male ratio in the population continues to increase, eventually w · Mn < Fn, meaning some females will not be fertilized and thus will not reproduce. This will lead to population decline, as shown in Hamilton’s modeling (9). In this scenario, w · Mn females are able to reproduce; thus, N = z · w · Mn. By incorporating this into Eqs. 1 and 2, we get
| (14) |
| (15) |
Using Eqs. 12, 13, 14, and 15, combined with the recursive functions for Rn and Kn and the initial conditions (M0 = F0 = 1000), we can calculate Mn, Fn, and Totaln for each generation, when given specific values of parameters t, z, and w. Population change rate Totaln+1/Totaln and the male-to-female ratio Mn/Fn can be calculated accordingly. Hamilton’s modeling (9) specifically addressed conditions where t = 1 (complete X chromosome drive), z = 2 (so that the population remains stable in the absence of driver), and w = 2. In this study, we explored how the population responds to a broader spectrum of t, z, and w values.
The threshold drive strength can be calculated as follows. As n increases, if population reaches a steady state, then Mn+1 = Mn, Fn+1 = Fn, and Rn = Kn = 1. This steady state cannot occur when w · Mn ≥ Fn as the population would continue to increase as discussed above. We can mathematically prove this: If w · Mn ≥ Fn, Eq. 13 yields tthreshold = 1/z. Because the model requires z ≥ 2, tthreshold = 1/z ≤ 0.5, which contradicts the premise that 0.5 < t ≤ 1. Therefore, a steady state is achievable only when w · Mn < Fn. In this scenario, Eq. 14 yields (fig. S8B). The minimum values of z and w (both equal to 2) yield the minimum tthreshold = 0.75 (fig. S8B).
Acknowledgments
We thank the members of the Yamashita lab for discussions and comments on the manuscript and help with experiments. We thank R. Lehmann, A. Jain, S. Hawley, A. Clark, D. Page, G. Fink, J. Mueller, and Christina. Lilliehook for insightful comments and suggestions. We thank former Yamashita lab members Z. G. Venkei, S. (Chloe) Choi, J. O. Nelson, G. J. Watase, M. Jagannathan, J. Flynn, and J. Park for insightful suggestions and help with experiments. We thank P. Chen and A. Aravin for providing the sequence of the βTub promoter. We thank the Lehmann lab members for discussing the project. We thank the Flybase, Bloomington Stock Center, Developmental Studies Hybridoma Bank, and Kyoto Stock Center for reagents and critical information. We acknowledge ChatGPT (GPT-4-turbo) (https://chat.openai.com/) for assistance with writing the code for mathematical modeling.
Funding: This work was supported by the Howard Hughes Medical Institute (Y.M.Y.) and Gordon and Betty Moore Foundation (#12261, Y.M.Y.).
Author contributions: Conceptualization: X.M. and Y.M.Y. Methodology: X.M. and Y.M.Y. Software: X.M. Validation: X.M. and Y.M.Y. Formal Analysis: X.M. Investigation: X.M. Resources: Y.M.Y. Data curation: X.M. Writing—original draft: X.M. and Y.M.Y. Writing—review and editing: X.M. and Y.M.Y. Visualization: X.M. Supervision: Y.M.Y. Project administration: X.M. and Y.M.Y. Funding acquisition: Y.M.Y.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The MATLAB code for mathematical modeling is available in the Supplementary Materials and deposited at https://github.com/xuefengmeng/Meng_et_al_2024.git.
Supplementary Materials
The PDF file includes:
Figs. S1 to S8
Tables S1 to S8
Other Supplementary Material for this manuscript includes the following:
MATLAB code for the mathematical modeling
REFERENCES AND NOTES
- 1.Novitski E., Non-random disjunction in Drosophila. Genetics 36, 267–280 (1951). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandler L., Novitski E., Meiotic drive as an evolutionary force. Am. Nat. 91, 105–110 (1957). [Google Scholar]
- 3.Kruger A. N., Mueller J. L., Mechanisms of meiotic drive in symmetric and asymmetric meiosis. Cell. Mol. Life Sci. 78, 3205–3218 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courret C., Chang C.-H., Wei K. H.-C., Montchamp-Moreau C., Larracuente A. M., Meiotic drive mechanisms: Lessons from Drosophila. Proc. Biol. Sci. 286, 20191430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helleu Q., Gérard P. R., Montchamp-Moreau C., Sex chromosome drive. Cold Spring Harb. Perspect. Biol. 7, a017616 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novitski E., Genetic analysis of anomalous sex ratio condition in Drosophila affinis. Genetics 32, 526–534 (1947). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturtevant A. H., Dobzhansky T., Geographical distribution and cytology of “sex ratio” in Drosophila pseudoobscura and related species. Genetics 21, 473–490 (1936). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards A. W. F., The population genetics of “sex-ratio” in Drosophila pseudoobscura. Heredity 16, 291–304 (1961). [Google Scholar]
- 9.Hamilton W. D., Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science 156, 477–488 (1967). [DOI] [PubMed] [Google Scholar]
- 10.Núñez M. A. B., Lange J. J., Zanders S. E., A suppressor of a wtf poison-antidote meiotic driver acts via mimicry of the driver’s antidote. PLOS Genet. 14, e1007836 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vedanayagam J., Lin C.-J., Lai E. C., Rapid evolutionary dynamics of an expanding family of meiotic drive factors and their hpRNA suppressors. Nat. Ecol. Evol. 5, 1613–1623 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao Y., Masly J. P., Araripe L., Ke Y., Hartl D. L., A sex-ratio meiotic drive system in Drosophila simulans. I: An autosomal suppressor. PLOS Biol. 5, e292 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao Y., Hartl D. L., Laurie C. C., Sex-ratio segregation distortion associated with reproductive isolation in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 98, 13183–13188 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishman L., Saunders A., Centromere-associated female meiotic drive entails male fitness costs in monkeyflowers. Science 322, 1559–1562 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Wallace B., Studies on sex-ratio in Drosophila pseudoobscura; selection and sex-ratio. Evolution 2, 189–217 (1948). [PubMed] [Google Scholar]
- 16.Charlesworth B., Hartl D. L., Population dynamics of the segregation distorter polymorphism of Drosophila melanogaster. Genetics 89, 171–192 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlesworth B., The evolution of lethals in the t-haplotype system of the mouse. Proc. Biol. Sci. 258, 101–107 (1994). [DOI] [PubMed] [Google Scholar]
- 18.Dyer K. A., Charlesworth B., Jaenike J., Chromosome-wide linkage disequilibrium as a consequence of meiotic drive. Proc. Natl. Acad. Sci. U.S.A. 104, 1587–1592 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R. A. Fisher, The genetical theory of natural selection. Dover books on science (Dover Publications, New York, ed. 2d rev., 1958), p. 291. [Google Scholar]
- 20.Aravin A. A., Naumova N. M., Tulin A. V., Vagin V. V., Rozovsky Y. M., Gvozdev V. A., Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 11, 1017–1027 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Aravin A. A., Klenov M. S., Vagin V. V., Bantignies F., Cavalli G., Gvozdev V. A., Dissection of a natural RNA silencing process in the Drosophila melanogaster germ line. Mol. Cell. Biol. 24, 6742–6750 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vagin V. V., Sigova A., Li C., Seitz H., Gvozdev V., Zamore P. D., A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313, 320–324 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Nishida K. M., Saito K., Mori T., Kawamura Y., Nagami-Okada T., Inagaki S., Siomi H., Siomi M. C., Gene silencing mechanisms mediated by Aubergine piRNA complexes in Drosophila male gonad. RNA 13, 1911–1922 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurst L. D., Is Stellate a relict meiotic driver? Genetics 130, 229–230 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurst L. D., Further evidence consistent with Stellate’s involvement in meiotic drive. Genetics 142, 641–643 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palumbo G., Bonaccorsi S., Robbins L. G., Pimpinelli S., Genetic analysis of Stellate elements of Drosophila melanogaster. Genetics 138, 1181–1197 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins L. G., Palumbo G., Bonaccorsi S., Pimpinelli S., Measuring meiotic drive. Genetics 142, 645–647 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belloni M., Tritto P., Bozzetti M. P., Palumbo G., Robbins L. G., Does Stellate cause meiotic drive in Drosophila melanogaster? Genetics 161, 1551–1559 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt A., Palumbo G., Bozzetti M. P., Tritto P., Pimpinelli S., Schafer U., Genetic and molecular characterization of sting, a gene involved in crystal formation and meiotic drive in the male germ line of Drosophila melanogaster. Genetics 151, 749–760 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy R. W., Lindsley D. L., Livak K. J., Lewis B., Siversten A. L., Joslyn G. L., Edwards J., Bonaccorsi S., Cytogenetic analysis of a segment of the Y chromosome of Drosophila melanogaster. Genetics 107, 591–610 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak K. J., Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 107, 611–634 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokuyasu K. T., Peacock W. J., Hardy R. W., Dynamics of spermiogenesis in Drosophila melanogaster. VII. Effects of segregation distorter (SD) chromosome. J. Ultrastruct. Res. 58, 96–107 (1977). [DOI] [PubMed] [Google Scholar]
- 33.Herbette M., Wei X., Chang C.-H., Larracuente A. M., Loppin B., Dubruille R., Distinct spermiogenic phenotypes underlie sperm elimination in the Segregation Distorter meiotic drive system. PLOS Genet. 17, e1009662 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirmarche S., Kimura S., Sapey-Triomphe L., Sullivan W., Landmann F., Loppin B., Drosophila protamine-like Mst35Ba and Mst35Bb are required for proper sperm nuclear morphology but are dispensable for male fertility. G3 (Bethesda) 4, 2241–2245 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayaramaiah Raja S., Renkawitz-Pohl R., Replacement by Drosophila melanogaster protamines and Mst77F of histones during chromatin condensation in late spermatids and role of sesame in the removal of these proteins from the male pronucleus. Mol. Cell. Biol. 25, 6165–6177 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura S., Loppin B., The chromosomal protein Mst77F is processed to generate an essential component of mature sperm chromatin. Open Biol. 6, 160207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathke C., Baarends W. M., Jayaramaiah-Raja S., Bartkuhn M., Renkawitz R., Renkawitz-Pohl R., Transition from a nucleosome-based to a protamine-based chromatin configuration during spermiogenesis in Drosophila. J. Cell Sci. 120, 1689–1700 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Jagannathan M., Warsinger-Pepe N., Watase G. J., Yamashita Y. M., Comparative analysis of satellite DNA in the Drosophila melanogaster species complex. G3 (Bethesda) 7, 693–704 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bozzetti M. P., Massari S., Finelli P., Meggio F., Pinna L. A., Boldyreff B., Issinger O. G., Palumbo G., Ciriaco C., Bonaccorsi S., The Ste locus, a component of the parasitic cry-Ste system of Drosophila melanogaster, encodes a protein that forms crystals in primary spermatocytes and mimics properties of the beta subunit of casein kinase 2. Proc. Natl. Acad. Sci. U.S.A. 92, 6067–6071 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egorova K. S., Olenkina O. M., Kibanov M. V., Kalmykova A. I., Gvozdev V. A., Olenina L. V., Genetically derepressed nucleoplasmic stellate protein in spermatocytes of D. melanogaster interacts with the catalytic subunit of protein kinase 2 and carries histone-like lysine-methylated mark. J. Mol. Biol. 389, 895–906 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Akera T., Chmatal L., Trimm E., Yang K., Aonbangkhen C., Chenoweth D. M., Janke C., Schultz R. M., Lampson M. A., Spindle asymmetry drives non-Mendelian chromosome segregation. Science 358, 668–672 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu T., Lane S. I. R., Morgan S. L., Jones K. T., Spindle tubulin and MTOC asymmetries may explain meiotic drive in oocytes. Nat. Commun. 9, 2952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawe R. K., Lowry E. G., Gent J. I., Stitzer M. C., Swentowsky K. W., Higgins D. M., Ross-Ibarra J., Wallace J. G., Kanizay L. B., Alabady M., Qiu W., Tseng K. F., Wang N., Gao Z., Birchler J. A., Harkess A. E., Hodges A. L., Hiatt E. N., A kinesin-14 motor activates neocentromeres to promote meiotic drive in maize. Cell 173, 839–850.e18 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Dalstra H. J., Swart K., Debets A. J., Saupe S. J., Hoekstra R. F., Sexual transmission of the [Het-S] prion leads to meiotic drive in Podospora anserina. Proc. Natl. Acad. Sci. U.S.A. 100, 6616–6621 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herrmann B. G., Koschorz B., Wertz K., McLaughlin K. J., Kispert A., A protein kinase encoded by the t complex responder gene causes non-mendelian inheritance. Nature 402, 141–146 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Nuckolls N. L., Núñez M. A. B., Eickbush M. T., Young J. M., Lange J. J., Yu J. S., Smith G. R., Jaspersen S. L., Malik H. S., Zanders S. E., wtf genes are prolific dual poison-antidote meiotic drivers. eLife 6, e26033 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helleu Q., Gérard P. R., Dubruille R., Ogereau D., Prud’homme B., Loppin B., Montchamp-Moreau C., Rapid evolution of a Y-chromosome heterochromatin protein underlies sex chromosome meiotic drive. Proc. Natl. Acad. Sci. U.S.A. 113, 4110–4115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mange E. J., Temperature sensitivity of segregation-distortion in Drosophila melanogaster. Genetics 58, 399–413 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inaba M., Buszczak M., Yamashita Y. M., Nanotubes mediate niche-stem-cell signalling in the Drosophila testis. Nature 523, 329–332 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Doren M., Williamson A. L., Lehmann R., Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8, 243–246 (1998). [DOI] [PubMed] [Google Scholar]
- 51.Chen D., McKearin D. M., A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130, 1159–1170 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Perkins L. A., Holderbaum L., Tao R., Hu Y., Sopko R., Call K. M., Yang-Zhou D., Flockhart I., Binari R., Shim H.-S., Miller A., Housden A., Foos M., Randkelv S., Kelley C., Namgyal P., Villalta C., Liu L.-P., Jiang X., Huan-Huan Q., Wang X., Fujiyama A., Toyoda A., Ayers K., Blum A., Czech B., Neumuller R., Yan D., Cavallaro A., Hibbard K., Hall D., Cooley L., Hannon G. J., Lehmann R., Parks A., Mohr S. E., Ueda R., Kondo S., Ni J.-Q., Perrimon N., The Transgenic RNAi Project at Harvard Medical School: Resources and validation. Genetics 201, 843–852 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minestrini G., Máthé E., Glover D. M., Domains of the Pavarotti kinesin-like protein that direct its subcellular distribution: Effects of mislocalisation on the tubulin and actin cytoskeleton during Drosophila oogenesis. J. Cell Sci. 115, 725–736 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Venkei Z. G., Gainetdinov I., Bagci A., Starostik M. R., Choi C. P., Fingerhut J. M., Chen P., Balsara C., Whitfield T. W., Bell G. W., Feng S., Jacobsen S. E., Aravin A. A., Kim J. K., Zamore P. D., Yamashita Y. M., A maternally programmed intergenerational mechanism enables male offspring to make piRNAs from Y-linked precursor RNAs in Drosophila. Nat. Cell Biol. 25, 1495–1505 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park J. I., Bell G. W., Yamashita Y. M., Derepression of Y-linked multicopy protamine-like genes interferes with sperm nuclear compaction in D. melanogaster. Proc. Natl. Acad. Sci. U.S.A. 120, e2220576120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams R. R., Tavares A. A., Salzberg A., Bellen H. J., Glover D. M., pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12, 1483–1494 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelson J. O., Slicko A., Yamashita Y. M., The retrotransposon R2 maintains Drosophila ribosomal DNA repeats. Proc. Natl. Acad. Sci. U.S.A. 120, e2221613120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watase G. J., Nelson J. O., Yamashita Y. M., Nonrandom sister chromatid segregation mediates rDNA copy number maintenance in Drosophila. Sci. Adv. 8, eabo4443 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S8
Tables S1 to S8
MATLAB code for the mathematical modeling