Abstract
Current zoonotic visceral leishmaniasis (ZVL) control programs in Brazil include the culling of Leishmania infantum-infected reservoir dogs, a strategy that has failed to prevent a rise of canine and human ZVL cases over the past decade. One of the main reasons this strategy has failed is because of a long delay between sample collection, sample analysis, and control implementation. A rapid, sensitive, and specific diagnostic tool would be highly desirable, because it would allow control interventions to be implemented in situ. We compared an immunochromatographic dipstick test to enzyme-linked immunosorbent assay (ELISA) and PCR for detecting L. infantum infections in dogs from an area of ZVL endemicity in Brazil. The dipstick test was shown to have 61 to 75% specificity and 72 to 77% sensitivity, compared to 100% specificity for both ELISA and PCR and 71 to 88% and 51 to 64% sensitivity for ELISA and PCR, respectively. Of the field samples tested, 92 of 175 (53%), 65 of 175 (37%), and 47 of 175 (27%) were positive by dipstick, ELISA, and PCR, respectively. The positive and negative predictive values for the tested dipstick were 58 to 77% and 75%, respectively. Efforts should be made to develop a more specific dipstick test for diagnosis of leishmaniasis, because they may ultimately prove more cost-effective than currently used diagnostic tests when used in mass-screening surveys.
Domestic dogs (Canis familiaris) are established reservoir hosts of zoonotic visceral leishmaniasis (ZVL) caused by Leishmania infantum. Hence, one of the approaches to reduce the incidence of human ZVL (also known as kala-azar) is to cull infected dogs. The impact of such dog-culling programs on incidence of human and canine ZVL has been doubted on both theoretical and practical grounds (10), and the results of controlled intervention trials are equivocal (3, 9). Despite the culling of an average of 19,500 dogs per year since 1989, the incidence of human ZVL in Brazil has increased steadily during the same period (26). One of the reasons why such control campaigns have failed is because of the long delay between sample collection, sample analysis, and control implementation (i.e., culling of infected dogs). This delay typically is 30 days long, but can be as long as 80 days, with infected dogs remaining infectious to sandfly vectors during this period and transmitting ZVL to susceptible dogs and humans. In a study in Brazil, it was shown that whereas a standard culling strategy implemented 80 days postsample collection resulted in only a 9% decrease in dog seroprevalence, culling implemented 7 days postsample collection resulted in a 27% decrease in seroprevalence (6). Current diagnostic methods used for Leishmania mass-screening surveys (mainly enzyme-linked immunosorbent assay [ELISA], immunofluorescence antibody test, or direct agglutination test) lack sensitivity or specificity, require technological expertise and specialized laboratory equipment, and can be labor-intensive and time-consuming. Hence, a rapid, sensitive and specific diagnostic test would be extremely valuable in mass-screening surveys and intervention campaigns, because results could be read immediately and control measures could be implemented in situ. Implementation coverage rates would be improved (e.g., dog owners often hide their dogs from culling personnel), and the control intervention would be more effective.
Immunochromatographic dipstick tests for Leishmania diagnosis have recently been developed and are all based on recombinant K39 (rK39), a protein predominant in Leishmania infantum and Leishmania donovani tissue amastigotes (7). rK39 dipstick tests have been shown to be quite sensitive (reported sensitivities, 67 to 100%) and very specific (reported specificities, 97 to 100%) when tested on kala-azar patients (5, 8, 13, 25, 30), with results similar to those of rK39-ELISAs (1, 4, 7, 12, 14, 17, 18, 24, 28, 29). Although rK39-ELISAs have been used to detect ZVL infection in dogs (4, 17, 22, 23, 28), there are no published reports on the use of the rK39 dipstick to detect ZVL in dogs.
We report a study in which the sensitivity and specificity of a commercially available immunochromatographic rK39 dipstick test were compared to serological and molecular diagnostic tests (ELISA and PCR) used in canine leishmaniasis diagnosis. Epidemiological and control intervention implications are discussed.
MATERIALS AND METHODS
Sampling.
Blood samples (2 to 10 ml) were taken from 148 dogs in the municipality of Capitão Eneas (16°30′S, 44°00′W), an area of L. infantum endemicity in Minas Gerais, Brazil. Twenty-seven dogs were sampled again after 5 months. Samples were taken by venipuncture and put into sterile, EDTA-coated 10-ml polypropylene tubes and processed 4 to 10 h after collection. The blood was centrifuged at 800 × g for 20 min, and the buffy coat layer and sera were separated and stored at −20°C. Dog age was estimated by tooth wear and by interviewing dog owners. The mean age of dogs was 34 months (range, 2 to 180 months); 57 of the dogs sampled were female, and 91 were male. No transmission of Leishmania (Viannia) spp. or Trypanosoma cruzi was reported in the area.
PCR.
DNA from buffy coat samples was extracted by using the DNeasy DNA extraction kit (Qiagen, Crawley, United Kingdom) according to the manufacturer's protocol. All samples were amplified with L. donovani complex-specific AJS31 (5′-GGGGTTGGTGTAAAATAGGGCC-3′) and DBY (5′-CCAGTTTCCCGCCCCGGAG-3′) primers according to previously published conditions (20). Amplification products were analyzed by electrophoresis on 1.5% agarose gels in 1× TAE buffer (40 mM Tris, 40 mM acetic acid, 1 mM EDTA [pH 8.3]). To evaluate sample degradation or PCR inhibition, sample DNA was also amplified for a canine housekeeping gene, an acidic ribosomal phosphoprotein fragment, by using primers PO3 (5′-GGAGAAGGGGGAGATGTT-3′) and PO5 (5′-TCATTGTGGGAGCAGACA-3′) (2). When samples did not yield amplification with PO3 and PO5 primers, they were extracted again, until a positive amplification was obtained. Each amplification cycle included negative (no DNA, DNA from uninfected dog) and positive (water-lysate mixtures of reference strain cultures) controls. PCR-grade H2O was used throughout. To avoid cross-contamination, separate areas were used for DNA extraction, PCR sample preparation, and amplification.
Hybridization.
Agarose gels were processed by standard procedures—i.e., in denaturation and neutralization buffer for 20 min each and Southern blotted onto a nylon membrane (Boehringer Mannheim, Basel, Switzerland)—and DNA was fixed by UV cross-linking. Membranes were prehybridized at 42°C and hybridized with a [γ-32P]ATP-labeled B4RsaB probe (5′-GACCTGAAACCCTGGGTCCTGGGCGT-3′) for 8 to 12 h (20) and then washed at 65°C twice for 15 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS) and in 0.1× SSC-0.1% SDS, before being exposed for autoradiography for 36 and 72 h at −70°C.
ELISA.
Log-phase L. donovani promastigotes (MHOM/ET/67/L82) were harvested at a concentration of ca. 2.5 × 109 cells/ml, centrifuged at 5,000 rpm, washed in phosphate-buffered saline (PBS), and frozen at −20°C. Cells were freeze-thawed and briefly sonicated. Antigen (105 promastigotes per well) was added to polysterene microtiter plates (Immunolon 2; Thermo LabSystems, Ashford, United Kingdom) in 100 μl of carbonate coating buffer (pH 9.6) and incubated overnight at 4°C. Plates were washed three times with PBS and blocked with 100 μl of 2% milk powder per well in coating buffer for 2 h at 37°C. They were then washed three times with PBS, and serum samples were added at dilutions of 1/100, 1/400, and 1/800 in 100 μl of incubation buffer (PBS-0.05% Tween 20-2% milk powder) and again incubated for 2 h at 37°C. After washing six times with PBS-0.05% Tween 20, peroxidase-conjugated, affinity-purified rabbit anti-dog immunoglobulin G (Sigma, Poole, United Kingdom) was added at 1/1,500 in 100 μl of incubation buffer, and plates were incubated for 2 h at 37°C. Plates were washed six times with PBS-0.05% Tween 20, and then 100 μl of substrate solution (O-phenylenediamine dihydrochloride in phosphate-citrate buffer [pH 5.5]) was added. The reaction was stopped with 50 μl of 2 M H2SO4, and plates were read at 490 nm in an ELISA plate reader.
ELISA standardization.
The method used for ELISA standardization was performed according to Quinnell et al. (19). Briefly, on each plate, a positive control serum was titrated twofold from 1/20 to 1/327,680. The positive control serum was assigned an arbitrary number of units per milliliter (81,920/ml), which was defined as the reciprocal of the highest dilution at which absorbance was greater than the mean + 3 standard deviations of background (no antibody) wells. Absorbance was calculated as observed absorbance − mean background absorbance. A standard line was fitted over the range 1/80 to 1/81,920 to the positive control absorbance values by log-logit transformation (19). The absorbances of the dilutions of the three test sera were expressed as antibody units per milliliter by using the standard line, from which the test sample's geometric mean number of antibody units per milliliter was calculated. Where the dilution curve for any test serum was noticeably nonparallel to the standard, test sera were repeated at dilutions of 1/100, 1/720, and 1/4,320. Samples were considered positive when their antibody level was greater than the arithmetic mean of antibody units per milliliter + 3 standard deviations of negative controls.
Dipstick.
The dipstick test (Leishmania RAPYDTEST; Intersep, Wokingham, United Kingdom) was carried out according to the manufacturer's instructions. The dipsticks were briefly placed into 50 μl of serum. After 5 to 8 min, a red control line and, if positive, a second line appeared on the test field. The test is based on a combination of protein A-colloidal gold conjugate and rK39 Leishmania antigen to detect anti-Leishmania antibody in serum or plasma.
Negative and positive controls.
Three groups of uninfected dog sera were used as negative controls for all diagnostic tests. The sera came from (i) dogs of various ages and breeds, which had attended a veterinary clinic in Lima, Peru (n = 17); (ii) mongrel dogs from Belém, Brazil (n = 12); and (iii) dogs of various ages and breeds that had attended a veterinary clinic in Cambridge, United Kingdom (n = 11). The positive standard control serum as well as nine other high-titer-positive control sera came from dogs with confirmed L. infantum infection (either by culture, microscopy, or xenodiagnosis) from Marajó, Brazil (19, 20).
Data analysis.
Sensitivity, specificity, and positive, and negative predictive values (PPV and NPV, respectively) for each diagnostic test were calculated according to the method of Fleiss (11). The PPV of a diagnostic test is the proportion of total positive test results that are true positives and the NPV of a diagnostic test is the proportion of total negative results that are true negatives.
RESULTS
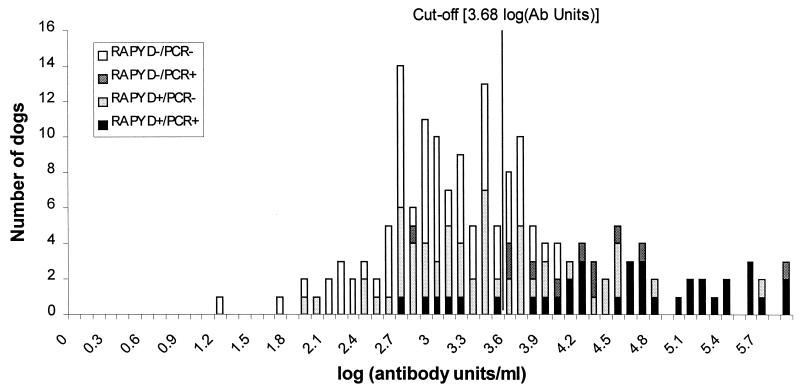
Table 1 summarizes the dipstick, ELISA, and PCR results from field samples and negative and positive controls, as well as the frequency distribution of log units of anti-Leishmania antibody units per milliliter depending on dipstick or PCR positivity is represented in Fig. 1. The mean antibody levels for the three groups of negative controls were 2,562 (standard deviation = 558 [Lima]), 1,099 (standard deviation = 689 [Marajó]), and 1,854 (standard deviation = 783 [Cambridge]) U/ml. The mean number of antibody units of all negative controls was 1,831 (standard deviation = 978) U/ml; hence, the cutoff for positivity was 4,765 U/ml (i.e., 3.68 log antibody units/ml). The mean for positive control sera was 170,237 (standard deviation = 97,281) U/ml.
TABLE 1.
Comparative diagnosis of L. infantum in dog blood
| ELISA/PCR result | No. of samples with result
|
||||
|---|---|---|---|---|---|
| Negative controlsa (n = 40)
|
Positive controlsa (RAPYD+ [n = 10]) | Field samples (n = 175)
|
|||
| RAPYD+ | RAPYD− | RAPYD+ | RAPYD− | ||
| ELISA+/PCR+ | 8 | 30 | 8 | ||
| ELISA−/PCR+ | 6 | 3 | |||
| ELISA+/PCR− | 2 | 17 | 10 | ||
| ELISA−/PCR− | 10 | 30 | 39 | 62 | |
PCRs of positive and negative Marajó controls were carried out with sera, not buffy coat.
FIG. 1.
Frequency distribution of anti-Leishmania antibody (log units per milliliter) in field samples tested.
None of the 40 negative control samples was positive by ELISA or PCR (Table 1), but 10 out of 40 negative control sera were positive by using the dipstick. Thus, the specificities of the PCR, ELISA, and dipstick were 100, 100, and 75%, respectively. The 10 negative control dogs that were positive by the dipstick test came from Cambridge (n = 4), Peru (n = 2), and Brazil (n = 4) (mean number of antibody units per milliliter = 1,851, standard deviation = 1,230). All 10 positive controls were positive by both ELISA and dipstick (i.e., 100% sensitivity), while 8 of 10 were positive by PCR. The proportions of the field samples positive by each diagnostic technique were 53% (92 of 175), 37% (65 of 175), and 27% (47 of 175) for dipstick, ELISA, and PCR, respectively.
To estimate the sensitivity and specificity of each test in the field samples, the true number of infected dogs must be estimated. Two approaches were used to estimate the number of infected and uninfected field dogs. In approach 1, we used the estimates of 100% specificity for ELISA and PCR from the control samples (i.e., all ELISA and/or PCR positives are true positives) and assumed that all ELISA-negative, PCR-negative samples were true negatives (i.e., all 39 RAPYD-positive, but ELISA-negative, PCR-negative samples were false positives). Approach 1 gives an estimated 74 positives and 101 negatives. In approach 2, we again used the estimated 100% specificity for ELISA and PCR, but also uded the estimated 75% dipstick specificity. Thus, only 21 (62/3) of the 39 RAPYD-positive, ELISA-negative, PCR-negative samples are false positives, and 18 are true positives. Approach 2 gives a total of 92 positives and 83 negatives. Table 2 summarizes the sensitivity, specificity, PPV, and NPV of each test by each approach. With approach 1, the sensitivity and specificity of the dipstick test were 72 and 61%, respectively; approach 2 increases the estimated sensitivity to 77%. The respective sensitivities of ELISA and PCR were 88 and 64% by approach 1 and 71 and 51% by approach 2 (Table 2).
TABLE 2.
Sensitivity, specificity, PPV, and NPV for the diagnostic tests used in this studya
| Approach | % PPV | % NPV | % Sensitivity | % Specificity |
|---|---|---|---|---|
| PCR | ||||
| 1 | 100 (47/47) | 79 (101/128) | 64 (47/74) | 100 (101/101) |
| 2 | 100 (47/47) | 65 (83/128) | 51 (47/92) | 100 (83/83) |
| ELISA | ||||
| 1 | 100 (65/65) | 92 (101/110) | 88 (65/74) | 100 (101/101) |
| 2 | 100 (65/65) | 75 (83/110) | 71 (65/92) | 100 (83/83) |
| RAPYDTEST | ||||
| 1 | 58 (53/92) | 75 (62/83) | 72 (53/74) | 61 (62/101) |
| 2 | 77 (71/92) | 75 (62/83) | 77 (71/92) | 75 (62/83) |
See the text for specific details of the approaches used to estimate the PPV, NPV, sensitivity, and specificity of each diagnostic test. The different assumptions made for each approach are in italic. Values in parentheses represent the number with result/number tested.
DISCUSSION
To our knowledge, this is the first study to use a dipstick test to detect Leishmania infection in dogs. Our study indicates that the dipstick test has a comparable sensitivity to ELISA, but that its specificity is very low (61 to 75%). Thus, use of the dipstick tests would lead to a high proportion of dogs being misdiagnosed as false positives (up to 39 out of 92 positive field samples; Table 1). The reason why this was the case is unknown, but it could include test cross-reactivity to some factor present in dog blood, because tested rK39 dipsticks were highly specific when tested on blood from kala-azar patients. Previous studies using the rK39-ELISA assay reported responsiveness to rK39 in (i) 2 of 33 Chinese toxoplasmosis patients (although the authors reported that the two responsive patients may have had subclinical ZVL) (24), (ii) 2 of 61 healthy Sudanese controls from a region of endemicity (29), (iii) 1 of 10 Turkish malaria patients (17), and (iv) 6 of 83 Turkish cutaneous leishmaniasis patients (17). The rK39 antigen is not known to cross-react with Leishmania braziliensis or T. cruzi (4, 7, 8, 18). Also, rK39 responsiveness appears to be restricted to active kala-azar infections, as opposed to asymptomatic, self-healing, cured, or treatment-resistant patients (4, 14, 24) or dogs (22, 23), although other studies failed to show such an association (29, 30).
Five reported studies used an rK39-ELISA to detect Leishmania infection in dogs (4, 17, 22, 23, 28). The rK39-ELISA was 100% sensitive in 90 parasitologically confirmed, high-antibody-titer dogs in Brazil (4) and in 37 parasitologically confirmed dogs in Venezuela (negative controls not included in either study) (28). In a Turkish study, 18 of 494 dogs were positive by rK39-ELISA; sensitivity and specificity were reported to be 93% and 100%, respectively (17). In a large epidemiological survey in Italy, rK39-ELISA sensitivity and specificity were 97 and 99%, respectively (23). Finally, in a Moroccan study, the rK39-ELISA was 100% sensitive in detecting 11 parasitologically confirmed, clinically symptomatic dogs, but failed to detect ZVL infection in 9 parasitologically confirmed, clinically asymptomatic dogs (22). Variability in dipstick performance will depend on factors such as the type of diagnostic antigen and conjugate used. Previous experience with malaria dipstick tests show that these tests can be highly variable in terms of sensitivity and specificity (27). False-positive rates for malaria dipsticks can be as high as 28%, which may, for example, be due to cross-reactivity to rheumatoid factor (16).
A number of PCR protocols to detect L. infantum have been developed, and PCR has been shown to be a sensitive and highly specific technique for the detection of symptomatic or parasitologically proven infections (2, 20, 28). Evidence suggests that PCR is less sensitive in detecting asymptomatic dogs (2, 20). The results presented here confirm this observation, because PCR detected only 79% of ELISA-positive and 39% of dipstick-positive field samples, with PCR positivity being associated with ELISA antibody units (linear regression after arc-sine transformation of data: df = 39, r = 0.84, P < 0.001). The sensitivity and specificity of the PCR assay depend on several factors, including PCR primers, DNA extraction protocol, and source of biopsy material (15, 21). The advantage of using blood (buffy coat) is that the sampling is less invasive than bone marrow, spleen, or lymph node aspirates, and samples can be processed readily. On the other hand, the parasite load in blood tends to be lower than those in bone marrow, spleen, or lymph node aspirates, and blood may contain a number of PCR inhibitors (e.g., heme) that may affect PCR assay sensitivity.
With a conservative cutoff (i.e., mean + 3 standard deviations) (19, 20), ELISA was 100% sensitive in detecting culture-positive dogs and 84% sensitive to detect parasitologically confirmed (PCR) field dogs. Interestingly, four of nine PCR-positive but ELISA-negative samples were from dogs that had recovered serologically by the time the second samples were taken. This demonstrates that the sensitivity of a diagnostic technique can change with the course of infection (20) and that these dogs appear to have developed an immune response that controls infection. The sensitivity and specificity of ELISA depend on the type of antigen used (e.g., parasite species, promastigotes, or amastigotes) and changes to the standard experimental protocol (e.g., incubation time or type of microtiter plates used).
We conclude that research into developing a more specific Leishmania dipstick test should be pursued, because there are many potential advantages of such a tool with respect to other diagnostic methods. With dipsticks, a large number of samples can be processed quickly and with minimum effort. Compared to microscopy, ELISA, or PCR, the technological expertise (i.e., training of personnel) necessary to perform the dipstick tests is minimal, as is the requirement for specialized laboratory equipment. Another advantage of dipstick tests is that patients (in our case, dog owners) can see the results for themselves, which will contribute to a better working relationship between local communities and people carrying out the surveys and would increase compliance rates. From an epidemiological point of view, a dipstick test allows interventions to be implemented in situ. The outcome should be to significantly reduce the mean duration of infectiousness of dogs that have become infected, thereby significantly enhancing the impact of the intervention on the basic reproductive number, Ro (10).
Immunochromatographic dipstick tests are comparatively expensive, but considering the data presented above, a sensitive and specific dipstick test could provide very cost-effective alternatives to currently available diagnostic tests, especially when used in mass-screening surveys.
Acknowledgments
We thank the staff at the Fundação Nacional de Saúde in Capitão Eneas for logistical help in dog blood collection, David Sargan (Cambridge University, Cambridge, United Kingdom) and Manuel Diaz (Centro Antirábico, Lima, Peru) for providing negative control sera, and Paul Coleman and Diarmid Campbell-Lendrum (LSHTM, United Kingdom) for comments on the manuscript.
This study was funded by the Sir Halley Stewart Trust and the Sir Patrick Manson Bequest Fund.
REFERENCES
- 1.Altintas, N., A. Yolasigmaz, N. Sakru, S. Yazar, S. Ertug, and Y. Ozbel. 1998. A sero-epidemiological study of visceral leishmaniosis in Izmir District, Turkey. J. Egypt. Soc. Parasitol. 28:389-394. [PubMed] [Google Scholar]
- 2.Ashford, D. A., M. Bozza, M. Freire, J. C. Miranda, I. Sherlock, C. Eulalio, U. Lopes, O. Fernandes, W. Degrave, R. H. Baker, R. Badaró, and J. R. David. 1995. Comparison of the polymerase chain reaction and serology for the detection of canine visceral leishmaniasis. Am. J. Trop. Med. Hyg. 53:251-255. [DOI] [PubMed] [Google Scholar]
- 3.Ashford, D. A., J. R. David, M. Freire, R. David, I. Sherlock, M. C. Eulalio, D. P. Sampaio, and R. Badaró. 1998. Studies on control of visceral leishmaniasis: impact of dog control on canine and human visceral leishmaniasis in Jacobina, Bahia, Brazil. Am. J. Trop. Med. Hyg. 59:53-57. [DOI] [PubMed] [Google Scholar]
- 4.Badaró, R., D. Benson, M. C. Eulálio, M. Freire, S. Cunha, E. M. Netto, D. Pedral-Sampaio, C. Madureira, J. M. Burns, R. L. Houghton, J. R. David, and S. G. Reed. 1996. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J. Infect. Dis. 173:758-761. [DOI] [PubMed] [Google Scholar]
- 5.Bern, C., S. N. Jha, A. B. Joshi, G. D. Thakur, and M. B. Bista. 2000. Use of the recombinant K39 dipstick test and the direct agglutination test in a setting endemic for visceral leishmaniasis in Nepal. Am. J. Trop. Med. Hyg. 63:153-157. [DOI] [PubMed] [Google Scholar]
- 6.Braga, M. D., I. C. Coelho, M. M. Pompeu, T. G. Evans, I. T. MacAullife, M. J. Teixeira, and J. W. Lima. 1998. Control of canine visceral leishmaniasis: comparison of results from a rapid elimination program of serum-reactive dogs using an immunoenzyme assay and slower elimination of serum-reactive dogs using filter paper elution indirect immunofluorescence. Rev. Soc. Bras. Med. Trop. 31:419-424. [DOI] [PubMed] [Google Scholar]
- 7.Burns, J. M., W. G. Shreffler, D. R. Benson, H. W. Ghalib, R. Badaró, and S. G. Reed. 1993. Molecular characterisation of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc. Natl. Acad. Sci. USA 90:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado, O., M. D. Feliciangeli, V. Coraspe, S. Silva, A. Perez, and J. Arias. 2001. Value of a dipstick based on recombinant RK39 antigen for differential diagnosis of American visceral leishmaniasis from other sympatric endemic diseases in Venezuela. Parasite 8:355-357. [DOI] [PubMed] [Google Scholar]
- 9.Dietze, R., G. B. Barros, L. Teixeira, J. Harris, K. Michelson, A. Falqueto, and R. Corey. 1997. Effect of eliminating seropositive canines on the transmission of visceral leishmaniasis in Brazil. Clin. Infect. Dis. 25:1240-1242. [DOI] [PubMed] [Google Scholar]
- 10.Dye, C. 1996. The logic of visceral leishmaniasis control. Am. J. Trop. Med. Hyg. 55:125-130. [DOI] [PubMed] [Google Scholar]
- 11.Fleiss, J. L. 1981. Statistical methods for rates and proportions, 2nd ed., p.212-236. John Wiley & Sons, New York, N.Y.
- 12.Ibrahim, M. E., B. Lambson, A. O. Yousif, N. S. Deifalla, D. A. Alnaiem, A. Ismail, H. Yousif, H. W. Ghalib, E. A. Khalil, A. Kadaro, D. C. Barker, and A. M. El Hassan. 1999. Kala-azar in a high transmission focus: an ethnic and geographic dimension. Am. J. Trop. Med. Hyg. 61:941-944. [DOI] [PubMed] [Google Scholar]
- 13.Jelinek, T., S. Eichenlaub, and T. Lõscher. 1999. Sensitivity and specificity of a rapid immunochromatographic test for diagnosis of visceral leishmaniasis. Eur. J. Clin. Microbiol. Infect. Dis. 18:669-670. [DOI] [PubMed] [Google Scholar]
- 14.Kumar, R., K. Pai, K. Pathak, and S. Sundar. 2001. Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 8:1220-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lachaud, L., E. Chabbert, P. Dubessay, J. Reynes, J. Lamothe, and P. Bastien. 2001. Comparison of various sample preparation methods for PCR diagnosis of visceral leishmaniasis using peripheral blood. J. Clin. Microbiol. 39:613-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laferi, H., K. Kandel, and H. Pichler. 1997. False positive dipstick test for malaria. N. Engl. J. Med. 337:1635-1636. [DOI] [PubMed] [Google Scholar]
- 17.Ozensoy, S., Y. Ozbel, N. Turgay, M. Z. Alkan, K. Gul, A. Gilman-Sachs, K.-P. Chang, S. G. Reed, and M. A. Ozcel. 1998. Serodiagnosis and epidemiology of visceral leishmaniasis in Turkey. Am. J. Trop. Med. Hyg. 59:363-369. [DOI] [PubMed] [Google Scholar]
- 18.Qu, J. Q., L. Zhong, M. Masoom-Yasinzai, M. Rab, H. S. Z. Aksu, S. G. Reed, K.-P. Chang, and A. Gilman-Sachs. 1994. Serodiagnosis of Asian leishmaniasis with recombinant antigen from repetitive domain of a Leishmania kinesin. Trans. R. Soc. Trop. Med. Hyg. 88:543-545. [DOI] [PubMed] [Google Scholar]
- 19.Quinnell, R. J., O. Courtenay, L. Garcez, and C. Dye. 1997. The epidemiology of canine leishmaniasis: transmission rates estimated from a cohort study in Amazonian Brazil. Parasitol. 115:143-156. [DOI] [PubMed] [Google Scholar]
- 20.Quinnell, R. J., O. Courtenay, S. Davidson, L. Garcez, B. Lambson, P. Ramos, J. J. Shaw, M. A. Shaw, and C. Dye. 2001. Detection of Leishmania infantum by PCR, serology and immune response in a cohort study of Brazilian dogs. Parasitology 122:253-261. [DOI] [PubMed] [Google Scholar]
- 21.Reithinger, R., B. L. Lambson, D. C. Barker, and C. R. Davies. 2000. Use of PCR to detect Leishmania (Viannia) spp. in dog blood and bone marrow. J. Clin. Microbiol. 38:748-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhalem, A., H. Sahibi, N. Guessous-Idrissi, S. Lasri, A. Natami, M. Riyad, and B. Berrag. 1999. Immune response against Leishmania antigens in dogs naturally and experimentally infected with Leishmania infantum. Vet. Parasitol. 81:173-184. [DOI] [PubMed] [Google Scholar]
- 23.Scalone, A., R. De Luna, G. Oliva, L. Baldi, G. Satta, G. Vesco, W. Mignone, C. Turilli, R. R. Mondesire, D. Simpson, A. R. Donoghue, G. R. Frank, and L. Gradoni. 2002. Evaluation of the Leishmania recombinant K39 antigen as a diagnostic marker for canine leishmaniasis and validation of a standardized enzyme-linked immunosorbent assay. Vet. Parasitol. 104:275-285. [DOI] [PubMed] [Google Scholar]
- 24.Singh, S., A. Gilman-Sachs, K.-P. Chang, and S. G. Reed. 1995. Diagnostic and prognostic value of K39 recombinant antigen in Indian leishmaniasis. J. Parasitol. 81:1000-1003. [PubMed] [Google Scholar]
- 25.Sundar, S., S. G. Reed, V. P. Singh, P. S. K. Kumar, and H. W. Murray. 1998. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet 351:563-565. [DOI] [PubMed] [Google Scholar]
- 26.Vieira, J. B., and G. E. Coelho. 1998. Visceral leishmaniasis or kala-azar: the epidemiological and control aspects. Rev. Soc. Bras. Med. Trop. 31(Suppl. 2):85-92. [PubMed] [Google Scholar]
- 27.Wongsrichanalai, C. 2001. Rapid diagnostic techniques for malaria control. Parasitol. Today 17:307-309. [DOI] [PubMed] [Google Scholar]
- 28.Zerpa, O., M. Ulrich, E. Negrón, N. Rodríguez, M. Centeno, V. Rodríguez, R. M. Barrios, D. Belizario, S. Reed, and J. Convit. 2000. Canine visceral leishmaniasis on Margarita island (Nueva Esparta, Venezuela). Trans. R. Soc. Trop. Med. Hyg. 5:484-487. [DOI] [PubMed] [Google Scholar]
- 29.Zijlstra, E. E., N. S. Daifalla, P. A. Kager, E. A. G. Khalil, A. M. El-Hassan, S. G. Reed, and H. W. Ghalib. 1998. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin. Diagn. Lab. Immunol. 5:717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zijlstra, E. E., Y. Nur, P. Desjeux, E. A. G. Khalil, A. M. El-Hassan, and J. Groen. 2001. Diagnosing visceral leishmaniasis with the recombinant K39 strip tests: experience from the Sudan. Trop. Med. Int. Health 6:108-113. [DOI] [PubMed] [Google Scholar]