Abstract
Adolescence is a developmental period that is associated with physical, cognitive, and affective maturation and a time when sex biases in multiple psychiatric diseases emerge. While puberty onset marks the initiation of adolescence, it is unclear whether the pubertal rise in gonadal hormones generates sex differences in approach-avoidance behaviors that may impact psychiatric vulnerability. To examine the influence of pubertal development on adult behavior, we removed the gonads or performed sham surgery in male and female mice just prior to puberty onset and assessed performance in an odor-guided foraging task and anxiety-related behaviors in adulthood. We observed no significant sex differences in foraging or anxiety-related behaviors between intact adult male and female mice but found significant differences between adult male and female mice that had been gonadectomized (GDX) prior to puberty. GDX males failed to acquire the odor-guided foraging task, showed reduced locomotion, and exhibited increased anxiety-like behavior, while GDX females showed the opposite pattern of behavior. These data suggest that puberty may minimize rather than drive differences in approach-avoidance phenotypes in male and female mice.
Introduction:
Across species, puberty initiates a cascade of physiological processes that induce sexual maturation and the emergence of sex differences in brain and behavior (Malina et al., 1988; Patton and Viner, 2007; Spear, 2000). Adolescent changes in affective and cognitive domains may be coordinated to mediate the transition to independence (Blakemore and Choudhury, 2006; Crone and Dahl, 2012; Huizinga et al., 2006; Johnson and Wilbrecht, 2011). In particular, puberty-dependent changes in the relative drive to approach novel environments versus avoid potential threats, described as „approach-avoidance conflict’ may shift to promote dispersal from the natal environment and attainment of adult roles (Palanza et al., 2001; Piekarski et al., 2017b). Understanding the influence of puberty on approach-avoidance behavior will provide insight into normative development as well as potential mechanisms that underlie the enhanced psychiatric vulnerability post-puberty (Casey et al., 2008; Steinberg, 2005).
Many psychiatric diseases show sex differences that often emerge during adolescence (Altemus et al., 2014; Angold et al., 1998; Paus et al., 2008). Notably, anxiety-related disorders are more common in females than males after puberty, and in girls, earlier pubertal timing has been associated with worse mental health outcomes (Angold et al., 1998; Deardorff et al., 2007; Graber, 2013; Graber et al., 2004; Hayward et al., 1992; Reardon et al., 2009; Whittle et al., 2015). Several studies in humans have linked developmental changes in affective behavior and cognitive task performance to pubertal status and/or gonadal hormone concentration (Braams et al., 2015; Cardoos et al., 2017; Laube et al., 2017; Master et al., 2019; Tyborowska et al., 2016). However, because adolescence is also a period of increased independence and shifting social roles (Dahl et al., 2018), it is difficult to disentangle the relative contributions of puberty and gonadal hormones from psychosocial changes that are secondary to pubertal development (Graber, 2013; Skoog and Stattin, 2014).
Here we turned to prepubertal gonadectomy in mouse models to understand how the rise in gonadal hormones at puberty influences approach-avoidance behavior in the context of a cognitively-demanding foraging task and anxiety-related behaviors in adult mice. We compared both male and female mice to determine whether puberty exerts different effects in males and females. In mice, as in humans, gonadal hormone production and release increases at the time of puberty onset after a quiescent period that begins shortly after birth. Increases in pulsatile release of gonadotropin releasing hormone (GnRH) from the hypothalamus stimulate pituitary gonadotropin release, which in turn promotes gonadal maturation and hormone production, increasing levels of testosterone, estradiol, and progesterone. In animal models, gonadal hormones can be manipulated via surgical removal of the gonads, or gonadectomy (GDX). By performing GDX prior to the onset of puberty, we can examine the influence of pubertal development on later adult behaviors (Boivin et al., 2017; Piekarski et al., 2017a; Schulz and Sisk, 2016; Vetter-O’Hagen and Spear, 2012).
In the current study, we sought to determine whether gonadal pubertal development influences adult performance in tasks that generate approach-avoidance conflict. To this end, we performed sham surgeries or gonadectomies on male and female C57Bl/6 mice before the onset of puberty and trained them as adults in an odor-guided foraging task followed by testing in the elevated plus maze (EPM) and open field to assess locomotion and anxiety-related behavior.
To interpret our data we considered three possible outcomes: A) If the sex-specific hormonal milieus at peripuberty generate sex differences in adult approach-avoidance behaviors, we would predict that males and females that were exposed to gonadal hormones at puberty will exhibit greater behavioral differences in adulthood compared to males and females that underwent prepubertal GDX; B) It has also been shown that the sex-specific hormonal milieu can act on sexually differentiated brain circuits to minimize phenotypic differences between males and females (De Vries, 2004), suggesting that prepubertal GDX may result in adult sex differences that are absent when mice are exposed to gonadal hormones during puberty C) Finally, we considered the possibility that puberty does not play a significant role in either male or female development in our selected tasks, and in this case, outcomes should be comparable between mice that underwent prepubertal GDX and same sex sham controls.
Materials & Methods:
Animals:
Male and female C57BL/6NCR mice (Charles River) were bred in-house. All mice were weaned on postnatal day (P)21 and housed in groups of 2–3 same-sex siblings on a 12:12 hr reversed light:dark cycle (lights on at 2200 h). All procedures were approved by the Animal Care and Use Committee of the University of California, Berkeley and conformed to principles outlined by the NIH Guide for the Care and Use of Laboratory Animals.
Gonadectomy:
To eliminate gonadal hormone exposure during and after puberty, gonadectomies (GDX) were performed before puberty onset at P25. In mice, vaginal opening is the earliest outward marker of puberty that occurs when estradiol causes the dissolution of a layer of cells that covers the vagina. Our lab has observed that vaginal opening in C57/Bl6 females occurs at a mean age of P29 (Piekarski et al., 2017b). In mice, first estrus occurs with a delay following vaginal opening at a mean age of P41 (Piekarski et al., 2017b). Prior to ovariectomy surgery, all female mice were visually inspected to confirm that they had not undergone vaginal opening. In males, the first outward sign of puberty appears later than in females, with the separation of the prepupice from the glans penis at approximately P36 (Deboer and Li, 2011). Prior to surgery, mice were injected with 0.05 mg/kg buprenorphine and 10 mg/kg meloxicam subcutaneously and were anesthetized with 1–2% isoflurane during surgery. The incision area was shaved and scrubbed with ethanol and betadine. Ophthalmic ointment was placed over the eyes to prevent drying. A 1 cm incision was made with a scalpel in the lower abdomen across the midline to access the abdominal cavity. The ovaries or testes were clamped off from the uterine horn or spermatic cord, respectively, with locking forceps and ligated with sterile sutures. After ligation, the gonads were excised with a scalpel. The muscle and skin layers were sutured, and wound clips were placed over the incision for 7–10 days to allow the incision to heal. An additional injection of 10 mg/kg meloxicam was given 12–24 h after surgery. Sham control surgeries were identical to gonadectomies except that the gonads were simply visualized and were not clamped, ligated, or excised. Mice were allowed to recover on a heating pad until ambulatory and were post-surgically monitored for 7–10 days to check for normal weight gain and signs of discomfort/distress. Mice were co-housed with 1–2 siblings who received the same surgical treatment. Subjects in this study included 15 intact male, 13 castrated male, 18 intact female, and 15 OVX female mice. To confirm the success of prepubertal ovariectomies, necropsy was performed on a subset of adult sham and ovariectomized mice to confirm that the uteri of OVX mice were underdeveloped compared to age-matched sham females (data not shown).
4 choice odor-guided foraging task:
Sham or GDX mice were tested in an odor-guided foraging task as young adults (P60-P70). The 4 choice odor-guided foraging task used has previously been described in detail (Johnson and Wilbrecht, 2011; Johnson et al., 2016), and within this task only the odor cue is predictive, and spatial or egocentric information is irrelevant. This task holds ethological relevance because mice use odor information to locate food sources (Howard et al., 1968). Briefly, mice were food restricted to ~85% body weight by the discrimination phase. On day 1, mice were habituated to the testing arena, on day 2 were taught to dig for cheerio reward in a pot filled with unscented wood shavings, on day 3 underwent a 4 choice odor discrimination, and finally on day 4 were tested on recall of the previously learned odor discrimination immediately followed by a reversal phase. During the discrimination phase of the task, mice learned to discriminate among four pots with different scented wood shavings (anise, clove, litsea and thyme). All four pots were sham-baited with cheerio (under wire mesh at bottom) but only one pot was rewarded (anise). The pots of scented shavings were placed in each corner of an acrylic arena (12”, 12”, 9”) divided into four quadrants. Mice were placed in a cylinder in the center of the arena, and a trial started when the cylinder was lifted. Mice were then free to explore the arena and indicate their choice by making a bi-manual dig in one of the four pots of wood shavings. The cylinder was lowered as soon as a choice was made. If the choice was incorrect, the trial was terminated and the mouse was gently encouraged back into the start cylinder. Trials in which no choice was made within 3 minutes were considered omissions. If mice omitted for two consecutive trials, they received a reminder: a baited pot of unscented wood shavings was placed in the center cylinder and mice dug for the “free” reward. Mice were disqualified if they committed four pairs of omissions. The location of the four odor scented pots was shuffled on each trial, and criterion was met when the mouse completed 8 out of 10 consecutive trials correctly. 24 hours after completing discrimination, mice were tested for recall of the initial odor discrimination to criterion and immediately after reaching criterion proceeded to the reversal phase in which the previously rewarded odor (anise) was no longer rewarded, and a previously unrewarded odor (clove) now became rewarded. During the reversal phase, Odor 4 (thyme) was replaced by a novel odor (eucalyptus) that was unrewarded. Again, mice were run until they reached a criterion of 8 out of 10 consecutive correct trials.
To compare foraging task performance across groups, trials to criterion and errors (incorrect choices) were compared for each phase of the task (discrimination, recall, and reversal). In addition, for the reversal phase we separated errors in which mice chose the odor that was rewarded during discrimination (Odor 1) into two types: 1) perseverative errors occurred when Odor 1 was chosen prior to the first correct trial and 2) regressive errors occurred when Odor 1 was chosen after the first correct trial during the reversal phase. To compare the relative proportion of these error types within mice, we calculated reversal error bias as (perseverative – regressive errors)/(perseverative + regressive errors). Therefore, a value > 1 indicates a bias for perseverative errors whereas a value < 1 indicates a bias for regressive errors. Finally, we examined how quickly mice accumulated rewards after the first correct trial during the reversal phase by aligning trial histories to the first correct trial and summing rewarded trials across the subsequent 8 trials. Data were fit by linear regression for each group and the slope of the lines compared to determine whether groups significantly differed in their rate of reward accumulation.
Anxiety testing:
Mice underwent anxiety testing >1 week after completing the odor-guided reversal task and returned to ad libitum feeding. All testing took place during the last 1–2 hours of the light phase (0800–1000 h) and mice were allowed to habituate to the testing room in their home cages for 30 minutes prior to testing. Mice were sequentially run in the elevated plus maze immediately followed by the open field test.
Elevated plus maze:
The mouse was placed in the center of an elevated plus maze and allowed to explore freely for 10 min. The EPM was made out of opaque white acrylic consisting of 2 open arms (30 cm long by 6 cm wide), 2 closed arms (30 cm long by 6 cm wide, with 20.5 cm high walls on the sides and end of each arm), and a center square (6 cm by 6 cm). The closed arms of the EPM were attached to a stable platform raised 66 cm from the floor. The total time spent in each zone (center, closed, open) was analyzed using EthoVision software (Noldus; Sacramento, CA). The chamber was cleaned with 70% ethanol and allowed to dry between tests of mice. The EPM was performed with room lights on (260 lx).
Open field:
Immediately after completing EPM testing, the mouse was transferred to a clear acrylic open field arena (42 cm by 42 cm floor dimensions, with four walls that were each 30.5 cm high, and no ceiling) for 15 min. The acrylic open field arena was located inside a sound-attenuated chamber (Med Associates; Fairfax, VT) with lights on (40 lx inside the chamber). Locomotion was monitored using infrared beam breaks (Versamax, AccuScan Instruments; Columbus, OH). Total distance covered and percent of time spent in the center (defined as > 7.875 cm from the edges of the chamber, i.e. 3 grid squares in Versamax analysis software), as well as vertical rears were analyzed. Data suggest that unsupported vertical rears are an exploratory movement that is sensitive to environmental context and stressors (Sturman et al., 2018). As described in Sturman et al., we quantified supported and unsupported rears based on the zone in which they occurred (outer zone vs. center zone, respectively). The chamber was cleaned with 70% ethanol and allowed to dry between testing of individual mice. Mice were returned to the home cage immediately after the open field test.
Statistical analysis:
For comparisons between 2 groups, a t-test was used when data were normally distributed, and Welch’s correction was applied when variance was unequal. The D’Agostino & Pearson test was used to test for normality. Groups that were not normally distributed were compared using a Mann Whitney U test. When 4 sex by surgery groups were considered simultaneously and data was normally distributed, a 2-way ANOVA was performed with sex and surgery as factors. For experiments in which only 3 groups were compared, a one-way ANOVA was performed. Post-hoc comparisons were then performed as described above (i.e. using a t-test or Mann Whitney U test). Effect sizes were calculated as eta squared (η2) for main effects and interactions in two way ANOVAs, or as Cohen’s d for pair-wise comparisons. For the analysis shown in Fig. 2 and Figs. S1-3, planned comparisons were performed to address two a priori questions: 1) Do adult intact males and females differ in behavior? 2) Does prepubertal GDX alter performance compared to intact mice of the same sex? As these were separate a priori questions, multiple comparisons corrections were not applied to these tests.
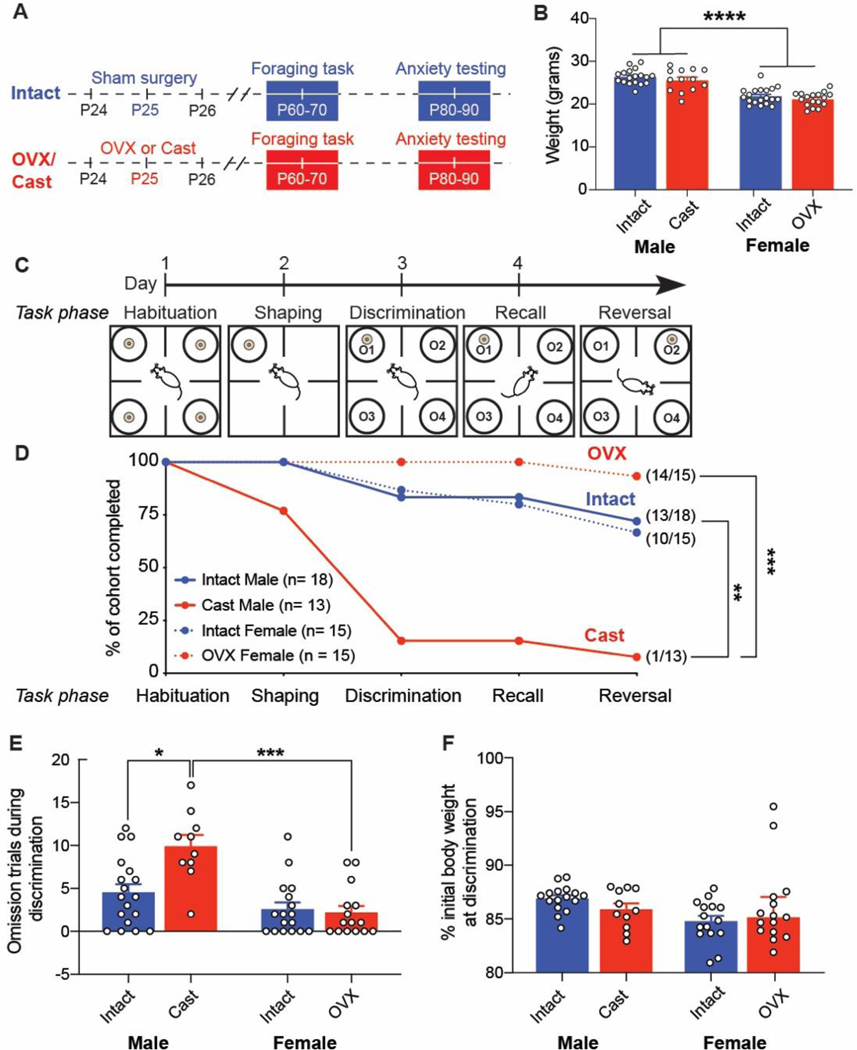
Figure 2. Prepubertal ovariectomy reduces bias for perseverative errors following reversal compared to intact females.
A. Overview of discrimination phase when mice learn that Odor 1 is rewarded. B. All cohorts exhibit similar trials to criterion (TTC) to complete discrimination. C. All cohorts made similar number of errors during the discrimination phase. D. Overview of reversal phase when mice learn that Odor 1 is no longer rewarded, and now Odor 2 is rewarded. E. All cohorts exhibited similar trials to criterion (TTC) to complete reversal. F. All cohorts exhibited similar total number of errors during the reversal phase. G. Intact female mice exhibited greater bias towards perseverative vs. regressive errors compared to OVX females (p= 0.0375, uncorrected Mann-Whitney U test; d= 0.74). Error bias was calculated for each mouse as (perseverative – regressive errors) / (perseverative + regressive errors). H. After the first rewarded trial in reversal, intact females accumulated rewards at a higher rate than OVX females (p< 0.0001, extra sum-of-squares F test). Data in A-F presented as mean ± SEM. Data in G presented as boxplot with median line and whiskers indicating minimum and maximum.
Results:
Prepubertal castration but not ovariectomy reduces likelihood of completing 4 choice odor-guided foraging task
We performed sham surgery or gonadectomy (GDX) on male and female mice at P25, generating four behavioral groups: intact males, intact females, castrated (Cast) males, and ovariectomized (OVX) females that were trained in an odor-guided foraging task (between P60–70) followed by open field and elevated plus maze testing (between P80–90) (Figure 1A). At the initiation of training, we compared body weights across groups and found a main effect of sex [F(1,63)= 86.37, p<0.0001; η2= 0.57] but no main effect of surgery [F(1,63)= 2.487, p= 0.1198; η2= 0.02] (Figure 1B). To motivate performance in the odor-guided foraging task, mice were food restricted to ~85% of their initial body weight and trained across four days, including 1) habituation, 2) shaping, 3) discrimination, and 4) recall and reversal task phases (Figure 1C, see methods for more detail). Over the course of training, we found that only (1/13) Cast males successfully completed the reversal phase of the task, with (11/13) mice dropping out before or during the discrimination phase (Figure 1D). While there was no significant difference in the proportion of intact males (13/18) vs. intact females (10/15) that completed the task (p > 0.99 Fisher’s exact test), there was a significant difference in the proportion of Cast males (1/13) vs. OVX females (14/15) that completed the task (p<0.0001, Fisher’s exact test) (Figure 1D). The proportion of Cast males that completed the task was also significantly lower compared to intact males (p= 0.0007, Fisher’s exact test), whereas OVX and intact females did not significantly differ (p= 0.17, Fisher’s exact test). Dropout among Cast males was driven by 1) a failure to complete the shaping phase within the allotted 3 hours (3/13) or 2) disqualification during the discrimination phase due to omission trials (4 pairs of omissions) (9/13) (Figure 1D). There was a significant interaction between sex and surgery on the number of omissions made during discrimination [F(1, 56) = 9.33, p= 0.0034; η2= 0.09]. Cast males made significantly more omissions compared to intact males (p= 0.0018, Tukey’s multiple comparisons test; d= 1.32) and OVX females (p<0.0001, Tukey’s multiple comparisons test) (Figure 1E). Meanwhile, intact males and females did not differ from each other in the number of omissions made during discrimination testing (p= 0.36, Tukey’s multiple comparisons test; d= 0.54) (Figure 1E). We next asked whether differences in body weight loss explained drop out among Cast males, reasoning that diminished weight loss could contribute to lower task motivation. However, we found no significant effect of surgery on percent initial body weight at discrimination phase [F(1,53) = 0.065, p= 0.80; η2= 0.001] (Figure 1F) indicating that Cast males lost a similar percentage of their body weight compared to other groups. Therefore, we were unable to further examine 4 choice odor-guided foraging task behavior in Cast males, including reversal learning.
Figure 1. Prepubertal castration but not ovariectomy reduces likelihood of completing foraging task.
A. Male and female mice were weaned at postnatal day (P)21 and underwent sham or castration/ovariectomy at P25 before the onset of puberty. Mice were then trained in a 4 choice odor-guided foraging task in early adulthood (P60-P70) followed by anxiety testing from P80-P90. B. There was a significant main effect of sex on initial weight before food restriction began [F(1, 63) = 86.37, p<0.0001; η2= 0.57] but no significant effect of surgery (sham vs. GDX) on initial weight [F(1, 63) = 2.487, p = 0.1198; η2= 0.02]. C. Overview of 4 choice odor-guided foraging task. D. Completion rate of the four cohorts: Intact Male, Intact Female, castrated (Cast) Male, and ovariectomized (OVX) Female across the four days of behavioral training (aligned to panel C). There was a significant difference in the completion rate at reversal phase between Cast males and OVX females (p<0.001, Fisher’s exact test with Bonferroni correction) but no significant difference in completion rates between intact males and females (p>0.99, Fisher’s exact test with Bonferroni correction). Cast males also significantly differed from intact males (p<0.01, Fisher’s exact test with Bonferroni correction). E. Dropout by Cast males was largely driven by omission trials (no choice made within 3 min trial) during the discrimination phase. F. Weight loss at discrimination phase. Neither GDX group (Cast or OVX) significantly differed from intact groups, suggesting that differences in weight loss did not account for drop out by Cast males. All data presented as mean ± SEM.
Prepubertal ovariectomy reduces bias for perseverative errors during reversal compared to intact females
We next examined performance in the odor-guided foraging task across intact males, intact females, and OVX females. During the discrimination phase (Figure 2A), there was no significant difference among groups in trials to criterion (p= 0.85, Kruskal Wallis ANOVA) (Figure 2B) or total errors (p = 0.85, Kruskal Wallis ANOVA) (Figure 2C). The following day mice were tested for their recall of discrimination learning, and groups did not differ in performance (Figure S1). During a reversal phase that immediately followed the completion of the recall phase (Figure 2D), there was no significant difference in trials to criterion (p= 0.52, Kruskal Wallis ANOVA) (Figure 2E) or total errors (p= 0.28, Kruskal Wallis ANOVA) (Figure 2F) across groups. Next, we more closely examined the types of errors that mice made during reversal. All groups showed a strong bias for selecting the odor that was previously rewarded in the discrimination phase (Figure S1). We divided errors to the previously rewarded odor into 2 subtypes: perseverative (errors made before the first correct trial) and regressive (errors made after first correct trial) errors. Perseverative errors reflect a tendency to stick to a previously learned rule, while regressive errors reflect a failure to acquire or maintain the new rule. We found that groups did not significantly differ in the number of perseverative errors made (p= 0.60, Kruskal Wallis ANOVA), but OVX females made significantly more regressive errors than intact females (U= 36, p= 0.0459 uncorrected; d= −0.36) (Figure S1). We next examined the pattern of perseverative and regressive errors made by individual mice. While we did not find a difference in the pattern of reversal errors between intact males and females (U= 71.5, p= 0.3778; d= 0.82), we found that intact females exhibited a significantly higher bias for perseverative vs. regressive errors compared to OVX females (intact females vs. OVX females: U= 47.5, uncorrected p= 0.0375; d= −0.74) (Figure 2G). In addition, we observed that intact females accumulated rewards at a significantly higher rate after the first rewarded trial in reversal compared to OVX females (Figure 2H).
Prepubertal gonadectomy has opposing effects on anxiety-related behavior in approach-avoidance conflict tests in adult males and females
To determine whether prepubertal GDX affects „anxiety-like’ behavior in tasks that rely on approach-avoidance conflict, we tested mice from P80–90 in the elevated plus maze (EPM) and open field (Griebel and Holmes, 2013). In the EPM (Figure 3A), there was a significant interaction between sex and surgery on locomotion [F(1,53) = 14.84, p= 0.0003; η2= 0.16], such that differences in total distance travelled emerged between Cast males and OVX females (p<0.0001, Tukey’s multiple comparisons test; d= −2.4) that were not present between intact males and females (p=0.71, Tukey’s multiple comparisons test; d= −0.38). Cast males traveled significantly less distance compared to OVX females (Figure 3B, see Figure S2 for full post hoc comparisons). In addition, there was a significant interaction between sex and surgery on time spent in the open arms of the EPM [F(1, 53) = 6.95, p=0.011; η2= 0.10], with Cast males spending significantly less time in the open arms compared to OVX females (p=0.0039, Tukey’s multiple comparisons test; d= −1.32), while intact males and females did not differ from each other (p= 0.99, Tukey’s multiple comparisons test; d= −0.10) (Figure 3C,D, Figure S2). Immediately after completing EPM testing, mice were run in the open field test (Figure 3E). Similar to the EPM results, there was a significant interaction between sex and surgery on locomotion, and again, OVX females traveled greater distance compared to Cast males (Figure 3F, Figure S2). There was a significant interaction between sex and surgery on time spent in the center zone of the open field [F(1, 58) = 18.56, p<0.0001; η2= 0.22], with Cast males exhibiting more anxiety-like behavior (less time in center zone) compared to OVX females (p<0.001 Tukey’s multiple comparisons test; d= −1.76) (Figure 3G, Figure S2). In addition, Cast males made significantly fewer vertical rears in the open field compared to OVX females (p< 0.0001, Tukey’s multiple comparisons test; d= −2.43) (Figure 3H). We confirmed that across all animals, time spent in the open arms of the EPM and time spent in the center zone of the open field were significantly correlated (Figure S2). Finally, studies suggest that unsupported vertical movement, or rearing, is an exploratory movement that is sensitive to environmental context and stressors (Sturman et al., 2018). We found that Cast males made fewer unsupported rears compared to intact males (p= 0.0004; uncorrected Fisher’s LSD d= −1.28) but there was no significant difference between intact females and OVX females (Figure S3).
Figure 3. Prepubertal gonadectomy produces sex differences in adult anxiety-related behavior not present in intact males and females.
A. Elevated plus maze (EPM) apparatus. B. There was a significant interaction between sex and surgery on total distance travelled during the EPM test [F(1,53)= 14.84, p= 0.0003; η2= 0.16]. C. There was a significant interaction between sex and surgery on fraction of time spent in the open arms during the EPM test [F(1, 53)= 10.67, p= 0.0019; η2= 0.15]. D. Representative heat maps showing time spent in open and closed arms for Intact female, OVX female, Intact male, and Cast male groups. E. Open field. F. There was a significant interaction between sex and surgery on total distance travelled during the open field test [F(1, 58)= 11.89, p= 0.0011; η2= 0.12]. G. There was a significant interaction between sex and surgery on time spent in the center of the open field [F(1, 58)= 18.56, p< 0.0001; η2= 0.22]. H. There was a significant interaction between sex and surgery on vertical rears made in the open field [F(1, 58)= 30.20, p< 0.0001; η2= 0.27]. **p< 0.01, ***p< 0.001, ****p< 0.0001 Tukey’s multiple comparisons test. All data presented as mean ± SEM. See Figure S2 for full post hoc comparisons.
Anxiety-like behavior in open field is associated with foraging task drop out in males but not females
Finally, we examined the relationship between behavior in the odor-guided foraging task and behavior in the EPM and open field. The foraging task, like the anxiety-related tests, may generate approach-avoidance conflict by requiring mice to explore an open arena and approach novel stimuli (scented odor pots), in order to consume a novel food (cheerio). We therefore hypothesized that the inability to complete the foraging task may be accompanied by decreased exploratory behavior in the anxiety-related tests. Indeed, we observed that Cast male group exhibited the highest measures of anxiety-like behavior and completed the foraging task at the lowest rate, while OVX female group exhibited the lowest anxiety-like behavior (in EPM) and completed the foraging task at the highest rate. To more directly test this relationship, we compared anxiety-related measures between mice that completed the odor-guided foraging task (“completers”) and those that were disqualified due to omissions (“drop-outs”). In males (Cast and sham combined), we found that the time spent in the center zone of the open field was significantly lower in “drop-outs” compared to “completers” (p= 0.0003, Sidak’s multiple comparisons test; d= 1.55) (Figure 4A). This effect was not purely driven by Cast males, as it was present when intact males were examined separately (Figure S4). One potential confound is that mice who completed the odor-guided foraging tasks experienced more handling and spent more time in the foraging task arena, which shares some characteristics with the open field. This may also explain why we did not also find a difference in time spent in the closed arms of the EPM between male drop-outs and completers.
Figure 4. Drop out in foraging task is associated with anxiety-like behavior in the open field for males but not females.
A. Open field. B. There was a significant interaction between sex and foraging task outcome (disqualified or completed) on fraction time in the outer zone [F(1, 48)= 5.98, p= 0.018; η2= 0.10]. Males who dropped out of the foraging task spent significantly more time in the outer zone of the open field compared to males who completed the task (p= 0.0003, Sidak’s multiple comparisons test; d= 1.55). C. Elevated plus maze. D. There was no main effect of reversal task outcome on time spent in the closed arms of the EPM. E. Fraction time in the outer zone of the open field showed no significant relationship with perseverative errors in the reversal phase. E. Fraction time in the closed arms of the EPM showed no significant relationship with perseverative errors in the reversal phase of the odor-guided foraging task. All data presented as mean ± SEM.
In females (OVX and sham combined), we found no significant difference in anxiety-related measures between drop-outs and completers, and time spent in the closed arms of the EPM did not differ between intact male or female mice that dropped-out or completed the task (Figure 4B). There are two important caveats to this finding: 1) our statistical power to detect differences between drop-outs and completers in the female group was reduced compared to males because fewer females overall (intact + OVX) were disqualified and 2) we did not control for phase of the estrous cycle when females were tested in the foraging task vs. anxiety-related tests. Previous data have shown that anxiety-related behavior fluctuates along the estrous cycle (Walf et al., 2009), so intact females may not have been in the same state between testing conditions.
Discussion:
It is well established that the presence or absence of the gonads at puberty has critical effects on a variety of sexual and social behaviors (Bell et al., 2013; Hlinak, 1985; Juraska et al., 2013; Kercmar et al., 2014; Schulz et al., 2006; Schulz et al., 2004), yet far less is known about how puberty affects cognitive and affective development. Standard models of gonadal hormone activity suggest the rise in gonadal hormones at puberty could drive the emergence of sex differences in cognitive and affective domains. Alternate models suggest that pubertal development could also prevent behavioral differences from arising between males and females (De Vries, 2004). Also, many aspects of development may occur at the same time as puberty but transpire independent of pubertal hormone changes (Ho et al., 2012; Paul et al., 2018).
In the current study, we used gonadectomy to test the role of gonadal pubertal development in one cognitive and two anxiety-related tasks that generate approach-avoidance conflict. We were particularly interested in these approach-avoidance related tasks because adolescence is a period during which enhanced approach is likely adaptive for gaining independence, and previous studies have shown that exploration of novel environments increases across adolescence in male and female mice (Adriani et al., 1998; Hefner and Holmes, 2007; Macri et al., 2002). It has been hypothesized that pubertal gonadal hormones may alter brain function to promote dispersal from the natal environment and to facilitate behaviors such as foraging that require exploration (Palanza et al., 2001; Piekarski et al., 2017a), but see (Holekamp et al., 1984). We previously showed that prepubertal gonadectomy increased anxiety-like behavior in males (but not females) during adolescence (Boivin et al., 2017). Our current data provide further support for the role of peripubertal testicular hormones in tuning approach-avoidance behavior: Cast males exhibited a lack of engagement in the foraging task and displayed reduced exploratory locomotion and increased anxiety-like behavior in the open field, all consistent with reduced approach behavior and/or greater avoidance. These data may parallel findings in humans: in boys (14–17 years old), lower testosterone levels were associated with increased anxiety and depressive symptoms (Granger et al., 2003). It is interesting to note that juvenile male mice, who secrete testicular hormones at much lower levels than adults, readily acquire the odor-based foraging task and exhibit more efficient reversal learning when compared to adult males (Piekarski et al., 2017b). Therefore, in male mice the behavioral requirement for testicular hormones may change in an age-dependent manner.
In adulthood, we observed no major differences in locomotion or anxiety-related behavior between gonadally intact adult male and female mice, consistent with previous data from our lab (Boivin et al. 2017) and others (Voikar et al., 2001). Notably, we did find highly significant differences in both locomotion and anxiety-like behavior when we compared prepubertally gonadectomized males (Cast) and females (OVX) (Figure 3). These data are consistent with less well-known models of hormonal action in which gonadal hormones act via different pathways in each sex to ultimately produce more similar behavioral phenotypes (De Vries, 2004). It is useful to point out that tests of anxiety-related behavior may not capture the same underlying processes in males and females; for example, it has been reported that the behavior of female rats in the EPM is driven more by locomotion than anxiety, whereas in males the reverse is true (Fernandes et al., 1999). We therefore interpret that the divergent phenotypes we observed in Cast males and OVX females could be driven by different mechanisms that shift behavior along an approach-avoidance continuum (Aupperle and Paulus, 2010). Future studies should be designed to address what underlying processes drive the different behavioral phenotypes we observed.
Given the link between puberty onset and the emergence of female bias for anxiety-related disorders, we were particularly interested to examine the role of gonadal pubertal development on approach-avoidance behaviors in females. We observed that as adults, females ovariectomized before puberty onset exhibited more exploratory behavior in the EPM (more time in the open arms) compared to intact females. Our group previously showed that OVX and sham females did not differ in EPM behavior at a mid-adolescent time point and that prepubertal ovarian hormone exposure (which advanced puberty onset) did not acutely increase anxiety-like behavior in juvenile female mice (Boivin et al., 2017). Taken together, these data suggest that peripubertal ovarian hormone exposure does not have acute anxiogenic effects but may have organizational or activational effects that tune approach-avoidance balance over a longer period of time. These data suggest ovarian hormones may play a role in anxiety-related behavior in females, but does not rule out the potential importance of modifiable factors such as environment and socialization (Graber 2013). In contrast, prepubertal castration affected anxiety-like behavior at both adolescent (Boivin et al., 2017) and adult time points (current study). This difference suggests that males and females exhibit different trajectories for the maturation of anxiety-related behaviors, and is consistent with testicular hormones having an activational effect on approach-avoidance behaviors (Aikey et al., 2002).
Previous studies indicate that in some cases male and female rodents utilize different decision making strategies (Grissom and Reyes, 2019), which may reflect ethologically relevant differences in how they use information in their environment (Jolles et al., 2015). Such reported sex differences in rodents include reduced risk preference in females compared to males (Ishii et al., 2018; Orsini et al., 2016; van den Bos et al., 2012), greater impulsivity in females under mild food restriction (Koot et al., 2009), and slower acquisition by female rats of a visual discrimination task (Izquierdo et al., 2016). While we did not find significant differences in performance between intact males and intact females in the odor-guided foraging task, we found that intact females exhibited a stronger bias for perseverative vs. regressive errors compared to OVX females, suggesting that pubertal development altered foraging strategy in females. This bias for perseverative errors reflects a difference in the strategy employed during reversal: intact females persisted in the old rule (Odor 1= rewarded) longer, but once they chose Odor 2 and were rewarded, they acquired the new rule faster and accumulated rewards at a higher rate compared to OVX females. This pattern of behavior by intact females may reflect reproductive pressure to exploit known available food sources, whereas OVX females exhibited more exploratory foraging strategy.
Our lab has previously shown that advancing puberty in juvenile female mice alters reversal performance in the same odor-guided foraging task (Piekarski et al., 2017a). Pubertal advancement increased the trials to criterion during reversal compared to age-matched prepubertal mice (Piekarski et al., 2017a), consistent with juveniles exhibiting faster reversal/greater flexibility compared to adults (Johnson and Wilbrecht, 2011). While the prepubertal OVX manipulation significantly altered reversal strategy in adult females, we did not observe any associated improvement in performance (i.e. a reduction in trials to criterion) suggesting that prepubertal OVX did not extend “juvenile-like” performance to P60 females. We previously showed that performance in the reversal task of the odor-guided foraging task is dependent on the dorsomedial prefrontal cortex (dmPFC) of mice (Johnson and Wilbrecht, 2011) and that prepubertal ovariectomy has long-lasting effects on inhibitory transmission in the dmPFC (Piekarski et al., 2017a). Future experiments will explore mechanisms by which puberty influences approach-avoidance behavior and its relationship to decision-making (Bariselli et al., 2018), with late-developing circuits of particular interest (Delevich et al., 2019).
Our current study has several limitations. First, we did not track the hormonal status of intact females. In part this was due to the foraging task design – because it occurs over 6 days and sessions are not repeated, we were unable to sample individual mice across the different phases of the estrous cycle. The fact that we were able to detect significant differences in behavior compared to OVX females in locomotion, anxiety-related behavior, and foraging strategy, suggests that these effects are robust to variance in ovarian hormone levels present in intact, cycling females. We compared the variance of behavior across groups for foraging task and anxiety-related test measures and did not find unstaged, intact females to be more variable compared to intact males or OVX females (Figure S5), consistent with other studies (Beery, 2018; Prendergast et al., 2014; Smarr et al., 2019; Smarr et al., 2017). It is important to highlight that our study design did not directly test the role of gonadal hormones or examine the effect of gonadectomy timing on behavior, and therefore we were unable to distinguish between activational and organizational effects of pubertal gonadal hormones. Future studies that control timing of gonadectomy and use hormone replacement are necessary to determine whether peripuberty is a sensitive period for the organization of anxiety-related behavior and foraging strategy. Regionally specific hormone replacement (Clemens et al., 2019) and cell-type specific manipulation of steroid hormone receptor expression (Bayless and Shah, 2016) could elucidate the neural mechanisms underpinning the effects of pubertal gonadal hormones on the behaviors examined here.
In rodent models, it is well known that species and strain differences, as well as environmental stressors during rearing, such as shipping (Laroche et al., 2009), can affect behavioral outcomes. Our current results conflict with reports in rats, where prepubertal castration reduced anxiety-like responses in adulthood (Brown et al., 2015; Primus and Kellogg, 1990) and increased ambulation (Brand and Slob, 1988). More generally, sex differences in anxiety-related behavior and locomotion appear more consistently in rats, with female rats ambulating more and spending more time in anxiogenic zone s compared to males (Donner and Lowry, 2013). However, in line with our findings, Kim and Spear observed sex-specific effects of prepubertal gonadectomy on social anxiety in rats: males displayed anxiety-like decreases in social preference whereas females displayed anxiolytic-like increases in social preference (Kim and Spear, 2016). In mice, other studies have found evidence for reduced anxiety-like behavior in intact males compared to intact females (An et al., 2011; Guilloux et al., 2011), and one study did not find an effect of prepubertal castration on anxiety-like behavior in the open field (McDermott et al., 2012). Therefore, the effects of species, strain, and rearing conditions should be taken into account when interpreting sex and gonadal hormone dependent differences in approach-avoidance behavior.
Conclusion
The current study sought to determine whether pubertal development influences performance in a cognitively demanding foraging task and anxiety-related tests in a sex-specific manner. We found that prepubertal gonadectomy in mice revealed significant differences in these behaviors that were not present between intact male and female mice suggesting that similar phenotypes are achieved in male and female mice via mechanisms downstream of gonadal pubertal development.
Supplementary Material
Highlights:
Adult male and female C57Bl/6 mice exhibit similar approach-avoidance behavior
Gonadectomy (GDX) prior to puberty produces sex differences in behavior
GDX males exhibit increased anxiety-like behavior compared to intact males
GDX females exhibit decreased anxiety-like behavior compared to intact females
Sham and OVX females utilize different foraging strategies during reversal
Acknowledgments
We thank Kenechukwu Okwuosa and Linnea Sepe-Forest for assistance with mouse behavior testing. We thank Dr. George Prounis, Dr. Josiah Boivin, Dr. Lance Kriegsfeld, and Dr. Irv Zucker for their feedback on the manuscript.
Funding and Disclosures
The authors have nothing to disclose. This project was supported in part by a National Science Foundation SLCN1640885 grant and an NIMH F32MH110184 fellowship (K.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Adriani W, Chiarotti F, Laviola G, 1998. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behavioral Neuroscience 112, 1152–1166. [DOI] [PubMed] [Google Scholar]
- Aikey JL, Nyby JG, Anmuth DM, James PJ, 2002. Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm Behav 42, 448–460. [DOI] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, Neill Epperson C, 2014. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 35, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An XL, Zou JX, Wu RY, Yang Y, Tai FD, Zeng SY, Jia R, Zhang X, Liu EQ, Broders H, 2011. Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Exp Anim 60, 111–123. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM, 1998. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med 28, 51–61. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Paulus MP, 2010. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin Neurosci 12, 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariselli S, Fobbs WC, Creed MC, Kravitz AV, 2018. A competitive model for striatal action selection. Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless DW, Shah NM, 2016. Genetic dissection of neural circuits underlying sexually dimorphic social behaviours. Philos Trans R Soc Lond B Biol Sci 371, 20150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, 2018. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci 23, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Meerts SH, Sisk CL, 2013. Adolescent brain maturation is necessary for adult-typical mesocorticolimbic responses to a rewarding social cue. Dev Neurobiol 73, 856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S, 2006. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry 47, 296–312. [DOI] [PubMed] [Google Scholar]
- Boivin JR, Piekarski DJ, Wahlberg JK, Wilbrecht L, 2017. Age, sex, and gonadal hormones differently influence anxiety- and depression-related behavior during puberty in mice. Psychoneuroendocrinology 85, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde AC, Peper JS, Crone EA, 2015. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J Neurosci 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand T, Slob AK, 1988. Peripubertal castration of male rats, adult open field ambulation and partner preference behavior. Behav Brain Res 30, 111–117. [DOI] [PubMed] [Google Scholar]
- Brown GR, Kulbarsh KD, Spencer KA, Duval C, 2015. Peri-pubertal exposure to testicular hormones organizes response to novel environments and social behaviour in adult male rats. Horm Behav 73, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoos SL, Ballonoff Suleiman A, Johnson M, van den Bos W, Hinshaw SP, Dahl RE, 2017. Social status strategy in early adolescent girls: Testosterone and value-based decision making. Psychoneuroendocrinology 81, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA, 2008. The adolescent brain. Ann N Y Acad Sci 1124, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens AM, Lenschow C, Beed P, Li L, Sammons R, Naumann RK, Wang H, Schmitz D, Brecht M, 2019. Estrus-Cycle Regulation of Cortical Inhibition. Curr Biol 29, 605–615 e606. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE, 2012. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci 13, 636–650. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Allen NB, Wilbrecht L, Suleiman AB, 2018. Importance of investing in adolescence from a developmental science perspective. Nature 554, 441–450. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, 2004. Minireview: Sex differences in adult and developing brains: Compensation, compensation, compensation. Endocrinology 145, 1063–1068. [DOI] [PubMed] [Google Scholar]
- Deardorff J, Hayward C, Wilson KA, Bryson S, Hammer LD, Agras S, 2007. Puberty and gender interact to predict social anxiety symptoms in early adolescence. J Adolesc Health 41, 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer MD, Li Y, 2011. Puberty is delayed in male mice with dextran sodium sulfate colitis out of proportion to changes in food intake, body weight, and serum levels of leptin. Pediatr Res 69, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevich K, Thomas AW, Wilbrecht L, 2019. Adolescence and “Late Blooming” Synapses of the Prefrontal Cortex. Cold Spring Harb Symp Quant Biol. [DOI] [PubMed] [Google Scholar]
- Donner NC, Lowry CA, 2013. Sex differences in anxiety and emotional behavior. Pflugers Arch 465, 601–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, Gonzalez MI, Wilson CA, File SE, 1999. Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav 64, 731–738. [DOI] [PubMed] [Google Scholar]
- Graber JA, 2013. Pubertal timing and the development of psychopathology in adolescence and beyond. Horm Behav 64, 262–269. [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM, 2004. Is pubertal timing associated with psychopathology in young adulthood. J Am Acad Child Adolesc Psychiatry 43, 718–726. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P, 2003. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Dev Psychopathol 15, 431–449. [PubMed] [Google Scholar]
- Griebel G, Holmes A, 2013. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov 12, 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom NM, Reyes TM, 2019. Let’s call the whole thing off: evaluating gender and sex differences in executive function. Neuropsychopharmacology 44, 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Seney M, Edgar N, Sibille E, 2011. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods 197, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Hammer LD, Litt IF, Wilson DM, Simmonds B, Taylor CB, 1992. Pubertal stage and panic attack history in sixth- and seventh-grade girls. Am J Psychiatry 149, 1239–1243. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A, 2007. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res 176, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlinak Z, 1985. Oestradiol and progesterone treatment and precopulatory behaviour in female rats ovariectomized at different ages. Physiol Bohemoslov 34, 373–380. [PubMed] [Google Scholar]
- Ho A, Villacis AJ, Svirsky SE, Foilb AR, Romeo RD, 2012. The pubertal-related decline in cellular proliferation and neurogenesis in the dentate gyrus of male rats is independent of the pubertal rise in gonadal hormones. Dev Neurobiol 72, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp KE, Smale L, Simpson HB, Holekamp NA, 1984. Hormonal influences on natal dispersal in free-living Belding’s ground squirrels (Spermophilus beldingi). Horm Behav 18, 465–483. [DOI] [PubMed] [Google Scholar]
- Howard WE, Marsh RE, Cole RE, 1968. Food detection by deer mice using olfactory rather than visual cues. Anim Behav 16, 13–17. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW, 2006. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia 44, 2017–2036. [DOI] [PubMed] [Google Scholar]
- Ishii H, Onodera M, Ohara S, Tsutsui KI, Iijima T, 2018. Sex Differences in Risk Preference and c-Fos Expression in Paraventricular Thalamic Nucleus of Rats During Gambling Task. Front Behav Neurosci 12, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Pozos H, Torre Ade L, DeShields S, Cevallos J, Rodriguez J, Stolyarova A, 2016. Sex differences, learning flexibility, and striatal dopamine D1 and D2 following adolescent drug exposure in rats. Behav Brain Res 308, 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Wilbrecht L, 2011. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev Cogn Neurosci 1, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CM, Peckler H, Tai LH, Wilbrecht L, 2016. Rule learning enhances structural plasticity of long-range axons in frontal cortex. Nat Commun 7, 10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles JW, Boogert NJ, van den Bos R, 2015. Sex differences in risk-taking and associative learning in rats. R Soc Open Sci 2, 150485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Sisk CL, DonCarlos LL, 2013. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav 64, 203–210. [DOI] [PubMed] [Google Scholar]
- Kercmar J, Snoj T, Tobet SA, Majdic G, 2014. Gonadectomy prior to puberty decreases normal parental behavior in adult mice. Horm Behav 66, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EU, Spear LP, 2016. Sex-dependent consequences of pre-pubertal gonadectomy: Social behavior, stress and ethanol responsivity. Behav Brain Res 296, 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot S, van den Bos R, Adriani W, Laviola G, 2009. Gender differences in delay-discounting under mild food restriction. Behav Brain Res 200, 134–143. [DOI] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD, 2009. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology 150, 2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube C, Suleiman AB, Johnson M, Dahl RE, van den Bos W, 2017. Dissociable effects of age and testosterone on adolescent impatience. Psychoneuroendocrinology 80, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Adriani W, Chiarotti F, Laviola G, 2002. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Anim Behav 64, 541–546. [Google Scholar]
- Malina RM, Bouchard C, Beunen G, 1988. Human Growth - Selected Aspects of Current Research on Well-Nourished Children. Annu Rev Anthropol 17, 187–219. [Google Scholar]
- Master SL, Eckstein MK, Gotlieb N, Dahl R, Wilbrecht L, Collins AGE, 2019. Disentangling the systems contributing to changes in learning during adolescence. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, Liu D, Schrader LA, 2012. Role of gonadal hormones in anxiety and fear memory formation and inhibition in male mice. Physiol Behav 105, 1168–1174. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Willis ML, Gilbert RJ, Bizon JL, Setlow B, 2016. Sex differences in a rat model of risky decision making. Behav Neurosci 130, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Morley-Fletcher S, Laviola G, 2001. Novelty seeking in periadolescent mice: sex differences and influence of intrauterine position. Physiology & Behavior 72, 255–262. [DOI] [PubMed] [Google Scholar]
- Patton GC, Viner R, 2007. Pubertal transitions in health. Lancet 369, 1130–1139. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Probst CK, Brown LM, de Vries GJ, 2018. Dissociation of Puberty and Adolescent Social Development in a Seasonally Breeding Species. Curr Biol 28, 1116–1123 e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN, 2008. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski DJ, Boivin JR, Wilbrecht L, 2017a. Ovarian Hormones Organize the Maturation of Inhibitory Neurotransmission in the Frontal Cortex at Puberty Onset in Female Mice. Curr Biol 27, 1735–1745 e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski DJ, Johnson CM, Boivin JR, Thomas AW, Lin WC, Delevich K, E MG, Wilbrecht L, 2017b. Does puberty mark a transition in sensitive periods for plasticity in the associative neocortex? Brain Res 1654, 123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I, 2014. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40, 1–5. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK, 1990. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav 24, 311–323. [DOI] [PubMed] [Google Scholar]
- Reardon LE, Leen-Feldner EW, Hayward C, 2009. A critical review of the empirical literature on the relation between anxiety and puberty. Clin Psychol Rev 29, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL, 2006. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav 50, 477–483. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL, 2004. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav 45, 242–249. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL, 2016. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci Biobehav Rev 70, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog T, Stattin H, 2014. Why and Under What Contextual Conditions Do Early-Maturing Girls Develop Problem Behaviors? Child Dev Perspect 8, 158–162. [Google Scholar]
- Smarr B, Rowland NE, Zucker I, 2019. Male and female mice show equal variability in food intake across 4-day spans that encompass estrous cycles. PLoS One 14, e0218935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr BL, Grant AD, Zucker I, Prendergast BJ, Kriegsfeld LJ, 2017. Sex differences in variability across timescales in BALB/c mice. Biol Sex Differ 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav R 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Steinberg L, 2005. Cognitive and affective development in adolescence. Trends Cogn Sci 9, 69–74. [DOI] [PubMed] [Google Scholar]
- Sturman O, Germain PL, Bohacek J, 2018. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress 21, 443–452. [DOI] [PubMed] [Google Scholar]
- Tyborowska A, Volman I, Smeekens S, Toni I, Roelofs K, 2016. Testosterone during Puberty Shifts Emotional Control from Pulvinar to Anterior Prefrontal Cortex. J Neurosci 36, 6156–6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, Jolles J, van der Knaap L, Baars A, de Visser L, 2012. Male and female Wistar rats differ in decision-making performance in a rodent version of the Iowa Gambling Task. Behav Brain Res 234, 375–379. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP, 2012. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behavioural Brain Research 227, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H, 2001. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav 72, 271–281. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA, 2009. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res 196, 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Simmons JG, Byrne ML, Strikwerda-Brown C, Kerestes R, Seal ML, Olsson CA, Dudgeon P, Mundy LK, Patton GC, Allen NB, 2015. Associations between early adrenarche, affective brain function and mental health in children. Soc Cogn Affect Neurosci 10, 1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.