Abstract
Background
Acute myeloid leukemia (AML) is a highly aggressive hematologic malignancy with poor prognosis and high relapse rates. While the TNFAIP8 gene family (TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3) is implicated in cancer and immune regulation, its role in AML remains unclear. This study utilized bioinformatics analyses to investigate their expression, prognostic significance, genetic alterations, and immune associations in AML.
Methods
The expression levels and clinical significance of TNFAIP8 family genes in AML were evaluated using UCSC XENA databases. Kaplan–Meier survival analysis was performed to assess overall survival (OS) differences, and receiver operating characteristic (ROC) curves were utilized to evaluate the prognostic predictive abilities of these genes. Genetic alterations were analyzed using the cBioPortal platform, while immune infiltration was examined through ssGSEA and Spearman correlation analysis. Functional enrichment analysis of co-expressed genes was conducted using the KEGG and GO databases.
Results
TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3 were significantly overexpressed in AML tissues compared to normal tissues (P < 0.001). However, Kaplan–Meier survival analysis revealed no significant association between their expression levels and OS in AML patients. ROC curve analysis showed that TNFAIP8L2 had the highest predictive accuracy (AUC = 1.000) among the family members, followed by TNFAIP8L1 (AUC = 0.728), TNFAIP8 (AUC = 0.709), and TNFAIP8L3 (AUC = 0.629). Clinicopathological analysis indicated that TNFAIP8 and TNFAIP8L1 expressions were associated with poor cytogenetic risk, while TNFAIP8L3 expression correlated strongly with elevated bone marrow blasts (P < 0.001). Mutation analysis revealed a low frequency of genetic alterations, with TNFAIP8L1 being the only gene with mutations in 0.53% of cases. Immune infiltration analysis demonstrated that TNFAIP8 and TNFAIP8L3 were positively correlated with myeloid-derived suppressor cells (MDSCs), while TNFAIP8L1 expression was associated with natural killer (NK) cell enrichment.
Conclusion
TNFAIP8 family genes play distinct roles in AML pathogenesis and immune regulation. TNFAIP8L2 shows promise as a prognostic biomarker, while TNFAIP8 and TNFAIP8L1 may indicate adverse cytogenetic risk. The study highlights their potential as therapeutic targets in AML.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-02511-5.
Keywords: Acute Myeloid Leukemia (AML), TNFAIP8 Family, Survival Analysis, Receiver Operating Characteristic (ROC) Analysis, Biomarkers, Functional Analysis
Introduction
Acute myeloid leukemia (AML) is a highly heterogeneous and aggressive hematologic malignancy characterized by the abnormal proliferation of myeloid progenitor cells in the bone marrow, which impairs normal hematopoiesis [1, 2]. Despite recent advances in molecular targeted therapy and immunotherapy, which have significantly improved the prognosis for some patients, the majority of AML patients still face poor survival rates and high relapse rates [2, 3]. Therefore, it is urgent to uncover the molecular mechanisms underlying AML pathogenesis and identify new biomarkers and potential therapeutic targets.
The TNFAIP8 (tumor necrosis factor-induced protein 8) gene family comprises a group of members closely associated with cell survival, proliferation, apoptosis, and immune evasion, including TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3 [4, 5]. These family members have been found to be involved in the development and progression of various cancers, such as pancreatic, ovarian, and lung cancer [4, 6, 7]. Altough, the role of TNFAIP8 family genes in AML remains unclear. Existing studies suggest that the TNFAIP8 family genes may influence the tumor microenvironment and tumor cell survival by regulating inflammation and immune responses [4–6, 8]. Therefore, investigating the potential functions of this gene family in AML could deepen our understanding of the molecular mechanisms of AML and provide new insights into its diagnosis and treatment.
In recent years, bioinformatics analyses have gained increasing prominence in oncology research, providing valuable insights into diverse human malignancies and playing a pivotal role in advancing our understanding of cancer biology [4, 9, 10]. This study focuses on the TNFAIP8 family based on its important roles in various cancers and its potential to influence the biological behavior of AML by modulating the tumor immune microenvironment. Through bioinformatics analysis, we aim to systematically explore the differential expression, genetic mutations, and prognostic relevance of TNFAIP8 family genes in AML, further investigating their association with the immune microenvironment. This research not only helps elucidate the functional role of TNFAIP8 family genes in AML but may also provide new potential biomarkers and therapeutic targets for personalized treatment strategies in AML.
Methods
Expression profile analysis, survival analysis, and clinicopathological features of the TNFAIP8 family in AML
This study utilized the UCSC XENA database (https://xenabrowser.net) to investigate the expression patterns of TNFAIP8 family genes across multiple datasets, such as The Cancer Genome Atlas (TCGA) [11, 12]. The expression levels were depicted graphically using the"ggplot2"package in R, a widely used tool for generating detailed and flexible visual representations. Kaplan–Meier survival analysis was conducted to assess the association between TNFAIP8 gene expression and patient prognosis [13]. This method estimates survival rates over time and compares them between different patient groups. Hazard ratios (HR) and P-values were calculated to evaluate the significance and strength of these associations. The relationship between TNFAIP8 mRNA levels and clinicopathological features in AML was also examined and visualized via"ggplot2."The ROC curve analysis was applied to assess the performance of a binary classification model, with the curve illustrating sensitivity (true positive rate) against 1-specificity (false positive rate). The AUC (Area Under the Curve) was computed to evaluate model accuracy, with values nearing 1 indicating superior performance. The pROC package in R was utilized to create and analyze these ROC curves [14].
cBioPortal analysis of TNFAIP8 family genes in AML
The cBioPortal platform (https://www.cbioportal.org/) was employed to analyze genetic alterations in TNFAIP8 family genes within the TCGA dataset [15, 16]. All four members of TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3 were investigated across the AML cohort of the TCGA pan-cancer atlas. The gene names were entered into relevant fields, and alterations were retrieved using the"OncoPrint"and"Cancer Types Summary"tools available on cBioPortal.
Immune-related analysis of TNFAIP8 family genes in AML
The infiltration of 24 immune cell types within AML tissue samples was evaluated using the ssGSEA algorithm [17]. Correlations between the expressions of TNFAIP8 family genes and immune cells were examined via Spearman correlation analysis. These associations were visualized using both the"ggplot2"and"pheatmap"packages. Additionally, the Wilcoxon rank-sum test was performed to investigate the enrichment of immune cells in AML patients with varying levels of TNFAIP8 gene expression.
Functional analysis of biological processes
Gene expression data for AML patients, measured in TPM, were obtained from the TCGA. Co-expression analysis was performed using Pearson correlation coefficients (P < 0.05, |R|> 0.4) to identify genes co-expressed with TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3. Venn diagrams were used to display overlaps among significantly related genes. Functional enrichment analysis, including the Kyoto Encyclopedia of Genes and Genomes (KEGG) [18] and Gene Ontology (GO) [19] analyses, was conducted using the"clusterProfiler"package.
Statistical analysis
Normally distributed data were expressed as means ± standard deviation. The Wilcoxon rank-sum and Wilcoxon signed-rank tests in SPSS 23.0 software were used to compare TNFAIP8 family gene expression (TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3) between normal and AML tissues. The association between TNFAIP8 expression and clinicopathological variables was analyzed via chi-square tests. A P-value of < 0.05 was considered statistically significant.
Results
Expressions of TNFAIP8 family in AML
The expression patterns of TNFAIP8 family members in AML were analyzed using data from the UCSC XENA database, which included 70 normal samples and 173 tumor samples (supplementary 1 provides details on sample sizes and gene expressions). As shown in Fig. 1, the expression levels of TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3 were significantly elevated in AML tissues compared to normal tissues (P < 0.001).
Fig. 1.

Comparison of expression levels of TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3 in AML tissues versus normal tissues. ***P < 0.001
Survival analysis of TNFAIP8 family in AML
This research evaluated the association between TNFAIP8 family expression levels and patient survival outcomes using Kaplan–Meier curves, derived from TCGA data, with supplementary 2.1–2.4 providing relevant sample sizes and survival information. The analysis focused on determining the overall survival (OS) characteristics of AML patients (Fig. 2). No statistically significant differences were identified between high and low expression levels of TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3 in terms of OS (P = 0.564, P = 0.829, P = 0.055, P = 0.628, respectively). Then, We used GEPIA2 (http://gepia2.cancer-pku.cn/#analysis) to analyze the disease-free survival (DFS) significance map data (setting Group cutoff = median) of TNFAIP8 family genes in LAML. We also found that there were no statistically significant differences between high and low levels of TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3 in DFS (Supplementary Fig. 1).
Fig. 2.
Survival analysis in AML patients based on TNFAIP8 family gene expression. The survival curves display overall survival (OS) contrasting patients with high versus low expression levels of TNFAIP8 family genes (A–D)
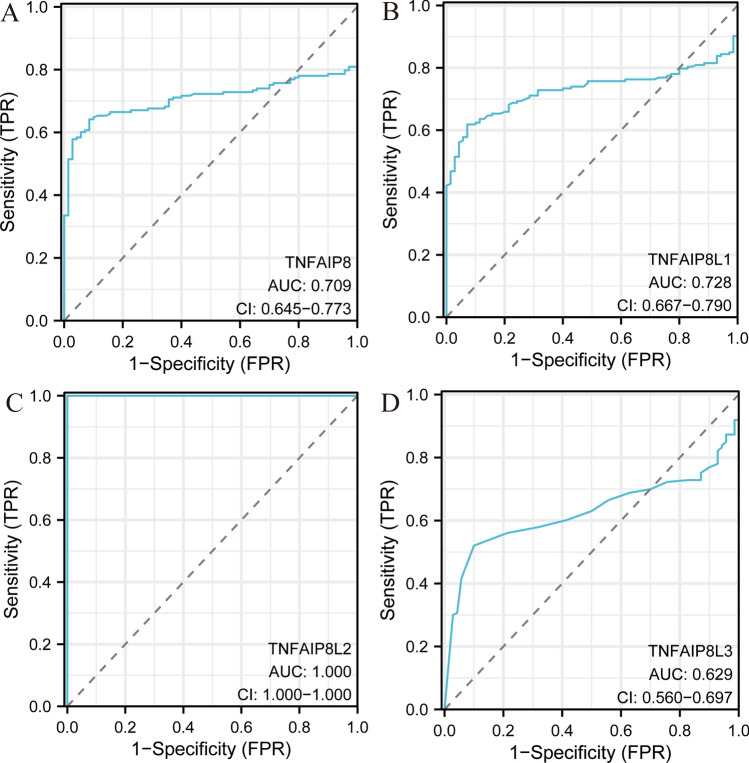
Receiver operating characteristic (ROC) analysis of TNFAIP8 family in AML
ROC curve analysis was performed to enhance understanding of the prognostic predictive capabilities of TNFAIP8 family members. Supplementary 3.1–3.4 provide details on the sample sizes used for this analysis. Results indicated that TNFAIP8 (AUC = 0.709), TNFAIP8L1 (AUC = 0.728), TNFAIP8L2 (AUC = 1.000), and TNFAIP8L3 (AUC = 0.629) demonstrated notable predictive accuracy for AML (Fig. 3).
Fig. 3.
Receiver operating characteristic (ROC) curve analysis for TNFAIP8 family members in AML, indicating the area under the curve (AUC) values (A–D)
Clinicopathological features of TNFAIP8 family of AML patients
In Table 1, the clinical characteristics of patients with low and high expression levels of TNFAIP8 family members (TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3) are compared. Each characteristic, such as gender, race, age, WBC count, and BM blasts, is assessed for statistical significance using P-values to indicate whether there are meaningful differences between the two expression groups. Across all TNFAIP8 family members, the analysis shows no significant gender distribution differences between the low and high expression groups (male vs. female). Additionally, race distribution does not exhibit significant differences for any TNFAIP8 family member, as indicated by P-values exceeding 0.5, reflecting similar racial composition regardless of expression levels. Age distribution also remains consistent across low and high expression groups for all TNFAIP8 family members, with P-values greater than 0.1. However, TNFAIP8 shows a marginally significant difference (P = 0.059), implying that higher WBC counts might be associated with elevated TNFAIP8 expression, though the other members do not display such associations. TNFAIP8L3, however, presents a highly significant association (P < 0.001), suggesting that elevated BM blast counts are strongly linked to high TNFAIP8L3 expression. No significant associations were observed between PB blasts and TNFAIP8 family members. Cytogenetic risk analysis reveals that both TNFAIP8 and TNFAIP8L1 display significant differences (P < 0.001), indicating that patients categorized with poor cytogenetic risk may exhibit higher expression of these genes. TNFAIP8L2 (P = 0.009) and TNFAIP8L3 (P = 0.002) also show significant differences concerning FAB classifications, suggesting certain FAB subtypes, such as M0 and M4, might be linked to higher expression. Mutations in FLT3, IDH1, and RAS do not show significant differences across expression levels for any TNFAIP8 family member. TNFAIP8L1, however, shows a notable difference (P = 0.033), with the NPM1 mutation being more common in the high-expression group. No differences in overall survival (OS) were observed between the low and high expression groups for any of the TNFAIP8 family members. However, significant differences in cytogenetic abnormalities, such as + 8, del(5), and complex karyotypes, were found for TNFAIP8 and TNFAIP8L1, indicating that these abnormalities are associated with higher gene expression. Overall, these findings suggest that certain clinical characteristics, including BM blasts, cytogenetic risk, FAB classifications, and specific cytogenetic abnormalities, are significantly linked to the expression of TNFAIP8 family members. TNFAIP8L3, in particular, demonstrates a strong correlation with BM blasts, while TNFAIP8 and TNFAIP8L1 show associations with cytogenetic risk, underscoring their potential role in prognostic assessments for hematological malignancies.
Table 1.
Clinicopathological factors and expressions of the TNFAIP8 family in AML
| Characteristics | TNFAIP8 | P | TNFAIP8L1 | P | TNFAIP8L2 | P | TNFAIP8L3 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low expression | High expression | Low expression | High expression | Low expression | High expression | Low expression | High expression | |||||
| n | 75 | 75 | 75 | 75 | 75 | 75 | 75 | 75 | ||||
| Gender, n (%) | 0.250286334 | 0.411542861 | 0.139373842 | 0.622218264 | ||||||||
| Female | 30 (20%) | 37 (24.7%) | 31 (20.7%) | 36 (24%) | 29 (19.3%) | 38 (25.3%) | 35 (23.3%) | 32 (21.3%) | ||||
| Male | 45 (30%) | 38 (25.3%) | 44 (29.3%) | 39 (26%) | 46 (30.7%) | 37 (24.7%) | 40 (26.7%) | 43 (28.7%) | ||||
| Race, n (%) | 0.978952357 | 0.978952357 | 0.556565905 | 0.978952357 | ||||||||
| Asian & Black or African American | 7 (4.7%) | 7 (4.7%) | 7 (4.7%) | 7 (4.7%) | 8 (5.4%) | 6 (4%) | 7 (4.7%) | 7 (4.7%) | ||||
| White | 68 (45.6%) | 67 (45%) | 68 (45.6%) | 67 (45%) | 66 (44.3%) | 69 (46.3%) | 67 (45%) | 68 (45.6%) | ||||
| Age, n (%) | 0.136520288 | 0.868605105 | 0.246858368 | 0.246858368 | ||||||||
| ≤ 60 | 39 (26%) | 48 (32%) | 44 (29.3%) | 43 (28.7%) | 47 (31.3%) | 40 (26.7%) | 47 (31.3%) | 40 (26.7%) | ||||
| > 60 | 36 (24%) | 27 (18%) | 31 (20.7%) | 32 (21.3%) | 28 (18.7%) | 35 (23.3%) | 28 (18.7%) | 35 (23.3%) | ||||
| WBC count(× 10^9/L), n (%) | 0.059699274 | 0.459831302 | 0.163119042 | 0.163119042 | ||||||||
| ≤ 20 | 44 (29.5%) | 32 (21.5%) | 36 (24.2%) | 40 (26.8%) | 42 (28.2%) | 34 (22.8%) | 34 (22.8%) | 42 (28.2%) | ||||
| > 20 | 31 (20.8%) | 42 (28.2%) | 39 (26.2%) | 34 (22.8%) | 32 (21.5%) | 41 (27.5%) | 41 (27.5%) | 32 (21.5%) | ||||
| BM blasts(%), n (%) | 0.241988929 | 0.616060514 | 0.403303631 | 2.93208E-05 | ||||||||
| ≤ 20 | 26 (17.3%) | 33 (22%) | 31 (20.7%) | 28 (18.7%) | 27 (18%) | 32 (21.3%) | 17 (11.3%) | 42 (28%) | ||||
| > 20 | 49 (32.7%) | 42 (28%) | 44 (29.3%) | 47 (31.3%) | 48 (32%) | 43 (28.7%) | 58 (38.7%) | 33 (22%) | ||||
| PB blasts(%), n (%) | 0.25232333 | 0.413551183 | 0.870099532 | 0.25232333 | ||||||||
| ≤ 70 | 32 (21.3%) | 39 (26%) | 33 (22%) | 38 (25.3%) | 35 (23.3%) | 36 (24%) | 32 (21.3%) | 39 (26%) | ||||
| > 70 | 43 (28.7%) | 36 (24%) | 42 (28%) | 37 (24.7%) | 40 (26.7%) | 39 (26%) | 43 (28.7%) | 36 (24%) | ||||
| Cytogenetic risk, n (%) | 0.00062161 | 2.96979E-05 | 0.802692805 | 0.151756838 | ||||||||
| Favorable | 13 (8.8%) | 17 (11.5%) | 7 (4.7%) | 23 (15.5%) | 16 (10.8%) | 14 (9.5%) | 11 (7.4%) | 19 (12.8%) | ||||
| Intermediate/normal | 33 (22.3%) | 49 (33.1%) | 55 (37.2%) | 27 (18.2%) | 39 (26.4%) | 43 (29.1%) | 46 (31.1%) | 36 (24.3%) | ||||
| Poor | 28 (18.9%) | 8 (5.4%) | 13 (8.8%) | 23 (15.5%) | 19 (12.8%) | 17 (11.5%) | 16 (10.8%) | 20 (13.5%) | ||||
| FAB classifications, n (%) | 0.051839421 | 0.414802309 | 0.00945366 | 0.002332763 | ||||||||
| M0 | 11 (7.4%) | 4 (2.7%) | 6 (4%) | 9 (6%) | 10 (6.7%) | 5 (3.4%) | 10 (6.7%) | 5 (3.4%) | ||||
| M1 | 20 (13.4%) | 15 (10.1%) | 16 (10.7%) | 19 (12.8%) | 18 (12.1%) | 17 (11.4%) | 22 (14.8%) | 13 (8.7%) | ||||
| M2 | 19 (12.8%) | 19 (12.8%) | 19 (12.8%) | 19 (12.8%) | 24 (16.1%) | 14 (9.4%) | 21 (14.1%) | 17 (11.4%) | ||||
| M3 | 6 (4%) | 8 (5.4%) | 5 (3.4%) | 9 (6%) | 9 (6%) | 5 (3.4%) | 1 (0.7%) | 13 (8.7%) | ||||
| M4 | 8 (5.4%) | 21 (14.1%) | 19 (12.8%) | 10 (6.7%) | 7 (4.7%) | 22 (14.8%) | 16 (10.7%) | 13 (8.7%) | ||||
| M5&M6&M7 | 11 (7.4%) | 7 (4.7%) | 10 (6.7%) | 8 (5.4%) | 6 (4%) | 12 (8.1%) | 5 (3.4%) | 13 (8.7%) | ||||
| FLT3 mutation, n (%) | 0.13291061 | 0.209621652 | 0.163734626 | 0.252528306 | ||||||||
| Negative | 54 (37%) | 47 (32.2%) | 47 (32.2%) | 54 (37%) | 53 (36.3%) | 48 (32.9%) | 48 (32.9%) | 53 (36.3%) | ||||
| Positive | 18 (12.3%) | 27 (18.5%) | 26 (17.8%) | 19 (13%) | 18 (12.3%) | 27 (18.5%) | 26 (17.8%) | 19 (13%) | ||||
| IDH1 R132 mutation, n (%) | 0.161184829 | 0.771512451 | 0.383649685 | 0.771512451 | ||||||||
| Negative | 66 (44.6%) | 69 (46.6%) | 67 (45.3%) | 68 (45.9%) | 66 (44.6%) | 69 (46.6%) | 67 (45.3%) | 68 (45.9%) | ||||
| Positive | 9 (6.1%) | 4 (2.7%) | 7 (4.7%) | 6 (4.1%) | 8 (5.4%) | 5 (3.4%) | 7 (4.7%) | 6 (4.1%) | ||||
| IDH1 R140 mutation, n (%) | 0.546985617 | 0.078717486 | 0.579921079 | 0.546985617 | ||||||||
| Negative | 69 (46.6%) | 67 (45.3%) | 66 (44.6%) | 70 (47.3%) | 68 (45.9%) | 68 (45.9%) | 67 (45.3%) | 69 (46.6%) | ||||
| Positive | 5 (3.4%) | 7 (4.7%) | 9 (6.1%) | 3 (2%) | 5 (3.4%) | 7 (4.7%) | 7 (4.7%) | 5 (3.4%) | ||||
| IDH1 R172 mutation, n (%) | 1 | 0.46463152 | 1 | 1 | ||||||||
| Negative | 73 (49.3%) | 73 (49.3%) | 75 (50.7%) | 71 (48%) | 72 (48.6%) | 74 (50%) | 73 (49.3%) | 73 (49.3%) | ||||
| Positive | 1 (0.7%) | 1 (0.7%) | 0 (0%) | 2 (1.4%) | 1 (0.7%) | 1 (0.7%) | 1 (0.7%) | 1 (0.7%) | ||||
| RAS mutation, n (%) | 0.730892289 | 0.730892289 | 0.730892289 | 0.284239125 | ||||||||
| Negative | 71 (47.7%) | 70 (47%) | 70 (47%) | 71 (47.7%) | 71 (47.7%) | 70 (47%) | 72 (48.3%) | 69 (46.3%) | ||||
| Positive | 3 (2%) | 5 (3.4%) | 5 (3.4%) | 3 (2%) | 3 (2%) | 5 (3.4%) | 2 (1.3%) | 6 (4%) | ||||
| NPM1 mutation, n (%) | 0.083280281 | 0.033457236 | 0.877925846 | 0.154225412 | ||||||||
| Negative | 62 (41.6%) | 54 (36.2%) | 53 (35.6%) | 63 (42.3%) | 58 (38.9%) | 58 (38.9%) | 54 (36.2%) | 62 (41.6%) | ||||
| Positive | 12 (8.1%) | 21 (14.1%) | 22 (14.8%) | 11 (7.4%) | 16 (10.7%) | 17 (11.4%) | 20 (13.4%) | 13 (8.7%) | ||||
| OS event, n (%) | 0.608343053 | 0.23181619 | 0.23181619 | 0.864370526 | ||||||||
| Alive | 25 (16.7%) | 28 (18.7%) | 23 (15.3%) | 30 (20%) | 30 (20%) | 23 (15.3%) | 27 (18%) | 26 (17.3%) | ||||
| Dead | 50 (33.3%) | 47 (31.3%) | 52 (34.7%) | 45 (30%) | 45 (30%) | 52 (34.7%) | 48 (32%) | 49 (32.7%) | ||||
| Cytogenetics, n (%) | 0.004129799 | 0.000335193 | 0.876552136 | 0.114475127 | ||||||||
| Normal | 28 (20.9%) | 41 (30.6%) | 45 (33.6%) | 24 (17.9%) | 36 (26.9%) | 33 (24.6%) | 39 (29.1%) | 30 (22.4%) | ||||
| + 8&del(5)&del(7)&inv(16)&t(15;17)&t(8;21)&t(9;11) | 23 (17.2%) | 18 (13.4%) | 12 (9%) | 29 (21.6%) | 23 (17.2%) | 18 (13.4%) | 18 (13.4%) | 23 (17.2%) | ||||
| Complex | 19 (14.2%) | 5 (3.7%) | 8 (6%) | 16 (11.9%) | 12 (9%) | 12 (9%) | 8 (6%) | 16 (11.9%) | ||||
Mutation and correlation analysis of TNFAIP8 family in AML
Epigenetic modifications are pivotal in the early stages of malignancy development. An investigation was conducted using the cBioPortal online platform to explore alterations, such as mutations and copy number changes, within the TNFAIP8 family in AML. The genetic variants and their associated alteration frequencies are depicted in Fig. 4A. Notably, mutations were identified exclusively in TNFAIP8L1 (0.53%), which included amplification. These genetic alterations in the TNFAIP8 family affected 0.53% of the 190 AML patients, as shown in Fig. 4B. Additionally, a Pearson correlation analysis was carried out to evaluate the relationships between the TNFAIP8 family members. The results, illustrated in Fig. 4C, demonstrate statistically significant positive correlations between TNFAIP8 and both TNFAIP8L2 and TNFAIP8L3 (P < 0.05). In contrast, a significant negative correlation was observed between TNFAIP8 and TNFAIP8L1 (P < 0.05). No notable correlation was found between TNFAIP8L1 and either TNFAIP8L2 or TNFAIP8L3 (P > 0.05).
Fig. 4.
Genetic alterations in the TNFAIP8 family and their prognostic associations in AML patients. This includes a summary of the differentially expressed TNFAIP8 family genes in AML (A, B) and their interrelationships (C)
Correlation between expressions of TNFAIP8 family and tumor immunity in AML
The relationships between the relative abundance of 24 immune cell types and TNFAIP8 family gene expression in AML were assessed using the ssGSEA algorithm (Fig. 5). Supplementary data 4.1–4.4 provide detailed information on the expression of TNFAIP8 family genes and the immune cell types. TNFAIP8 expression was positively linked with multiple immune cells, such as Neutrophils, Eosinophils, Macrophages, Tem, iDC, DC, and Th17 cells. On the contrary, a significant negative association was noted between TNFAIP8 expression and immune cells like NK CD56 dim cells, CD8 T cells, TFH, Th1 cells, NK cells, and NK CD56bright cells (Fig. 5A). TNFAIP8L1 expression was observed to have a positive correlation with nine immune cell types, including NK cells, Mast cells, NK CD56bright cells, pDC, T cells, CD8 T cells, NK CD56 dim cells, Cytotoxic cells, and B cells (Fig. 5B). However, TNFAIP8L1 expression displayed a negative relationship with Neutrophils, DC, and Th2 cells. Differential expression patterns of TNFAIP8L2 were found across different immune cells, such as iDC, Neutrophils, Th17 cells, Tem, Eosinophils, DC, and TReg. A negative correlation was discovered between TNFAIP8L2 expression and CD8 T cells, Tcm, and T helper cells (Fig. 5C). Regarding TNFAIP8L3, there was a positive correlation between its expression and immune cells like Macrophages, iDC, Tem, Th1 cells, Neutrophils, Th17 cells, Cytotoxic cells, NK CD56 dim cells, and T cells. aDC showed a negative correlation with TNFAIP8L3 expression (Fig. 5D).
Fig. 5.
TNFAIP8 family expression and its association with tumor immunity in AML. The bar graph presents the relationship between TNFAIP8 (A), TNFAIP8L1 (B), TNFAIP8L2 (C), and TNFAIP8L3 (D) expression levels and the infiltration of 24 distinct immune cells in AML. *P < 0.05, **P < 0.01, ***P < 0.001
The Wilcoxon Rank-Sum test was applied to evaluate immune cell presence in AML patients (Fig. 6). Supplementary data 5.1–5.4 contain details on enriched immune cells in the high and low expression groups of TNFAIP8 family genes. Significantly enriched immune cells, such as Eosinophils, iDC, Macrophages, Neutrophils, and Tcm, were found in the TNFAIP8 high expression group when compared to the low expression group (Fig. 6A). Conversely, immune cells like NK CD56bright cells, NK cells, and TH1 cells were negatively enriched in the TNFAIP8 high expression group. A higher enrichment of CD8 T cells, NK CD56bright cells, and NK cells was seen in the TNFAIP8L1 high expression group compared to the low expression group (Fig. 6B). Elevated enrichment levels of iDC, Macrophages, Neutrophils, and Tem were observed in the TNFAIP8L2 high expression group compared to the low group (Fig. 6C). Similarly, iDC, Macrophages, Neutrophils, Tem, TFH, and Th1 cells were significantly enriched in the TNFAIP8L3 high expression group compared to the low expression group (Fig. 6D).
Fig. 6.
Evaluation of immune cell infiltration in relation to TNFAIP8 family gene expression in AML. The plots depict groups with high and low expression of TNFAIP8 family members (A–D) in connection with immune cell enrichment. *P < 0.05, **P < 0.01, ***P < 0.001
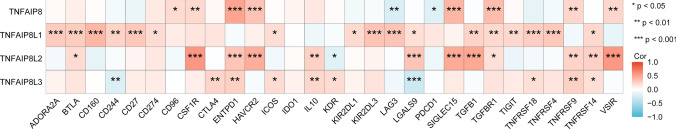
To further detect the association between TNFAIP8 family genes and immune regulation, we do an extensive correlation analysis between TNFAIP8 family genes levels and immune checkpoint genes in LAML. The correlation analysis between TNFAIP8 family genes levels and immune checkpoints in LAML are shown in supplementary 6. As shown in the Fig. 7, a significant positive correlation between TNFAIP8 and CD96, SCF1R, ENTPD1. HAVCR2, SIGLEC15, TGFBR1, TNFRSF9 and VSIR, while a significant negative association was found in LAG3 and PDCD1. TNFAIP8L1 exhibited a consistent positive correlation with 17 checkpoint genes, including ASORA2 A, BTLA, CD160, CD244, CD27, CD274, ICOS, KIR2DL1, KIR2DL3, LAG3, LGALS9, TGFB1, TGFBR1, TIGIT, TNFRSF18, TNFRSF4 and TNFRSF14. Similarly, a significantly positive association was found between TNFAIP8L2 and 12 check points genes, including BTLA, CSF1R, ENTPD1, HAVCR2, IL10, LGALS9, SIGLEC15, TGFBR1, TNFRSF9, TNFRSF14 and VSIR. While, TNFAIP8L2 and KDR showed a negative correaltion in LAML. Moreover, TNFAIP8L3 expression positively correlates with CTLA4, ENTPD1, ICOS, IL10, KDR, TNFRSF18, TNFRSF9 and TNFRSF14. Conversely, negative correlations were observed bentween TNFAIP8L3 and CD244 and LGALS9.
Fig. 7.
The correlation analysis between TNFAIP8 family genes levels and immune checkpoints in LAML. *P < 0.05, **P < 0.01, ***P < 0.001
Genes co-expressed with TNFAIP8 family in AML and enrichment analysis
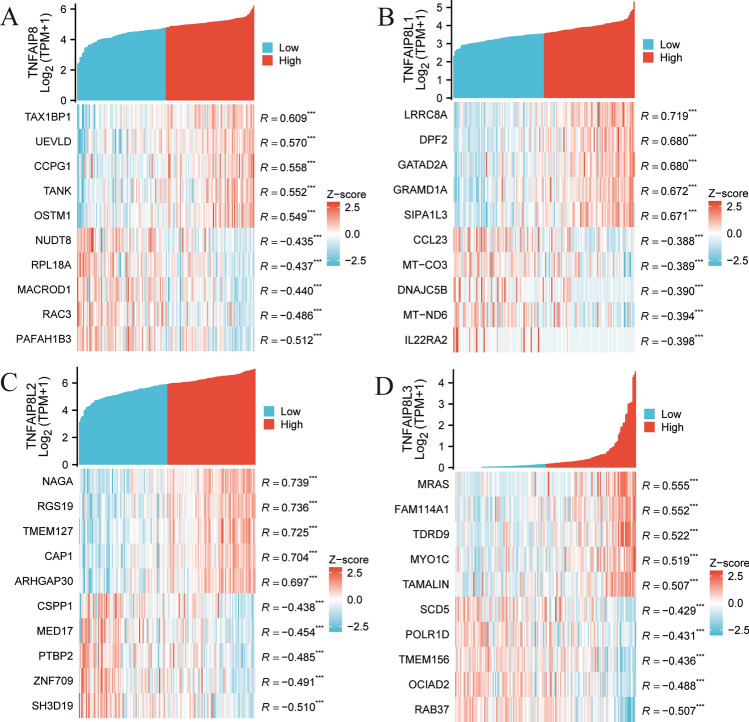
Analysis of TCGA AML transcriptome data showed that TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3 were significantly co-expressed with 366, 9433, 1209, and 94 genes, respectively, using criteria of |R|> 0.4 and P < 0.05 (sample sizes are provided in supplementary 7.1–7.4). Figure 8A–D illustrate the top ten co-expressed genes (five positively correlated and five negatively correlated) with each TNFAIP8 family member (sample sizes are provided in supplementary 8.1–8.4). In Fig. 8A, TNFAIP8 displayed significant positive correlations with genes such as TAX1BP1, UEVLD, CCPG1, TANK, and OSTM1, and negative correlations with NUDT8, RPL18 A, MACROD1, RAC3, and PAFAH1B3. In Fig. 8B, TNFAIP8L1 was positively correlated with LRRC8 A, DPF2, GATAD2 A, GRAMD1 A, and SIPA1L3, while negatively correlated with CCL23, MT-CO3, DNAJC5B, MT-ND6, and IL22RA2. For TNFAIP8L2, significant positive correlations were found with NAGA, RGS19, TMEM127, CAP1, and ARHGAP30, and negative correlations with CSPP1, MED17, PTBP2, ZNF709, and SH3D19 (Fig. 8C). Lastly, TNFAIP8L3 was positively correlated with MRAS, FAM114 A1, TDRD9, MYO1 C, and TAMALIN, and negatively correlated with SCD5, POLR1D, TMEM156, OCIAD2, and RAB37 (Fig. 8D).
Fig. 8.
The top 5 genes that are positively and negatively correlated with the TNFAIP8 (A), TNFAIP8L1 (B), TNFAIP8L2 (C), and TNFAIP8L3 (D) in AML, respectively. *P < 0.05, **P < 0.01, ***P < 0.001
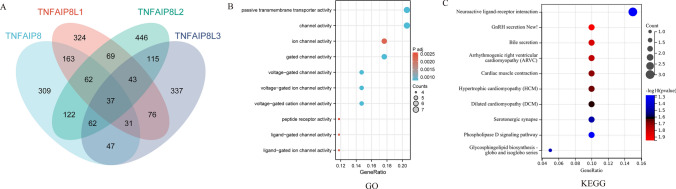
A Venn diagram (Fig. 9A) displays the 37 intersecting genes co-expressed with TNFAIP8 family members, including CACNA2D2, GAL, and GUCY2 C (sample sizes provided in Supplementary 9). To further explore the functional roles of the TNFAIP8 family in AML, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were conducted on these co-expressed genes. The GO analysis results indicated that the TNFAIP8 family co-expressed genes were involved in processes such as passive transmembrane transporter activity, channel activity, and ion channel activity (Fig. 9B). KEGG analysis revealed that these co-expressed genes played roles in Neuroactive ligand-receptor interaction, GnRH secretion, bile secretion, among others (Fig. 9C).
Fig. 9.
Co-expression analysis of TNFAIP8 family genes in AML, including Gene Ontology (GO) and KEGG enrichment analysis. The Venn diagram shows the overlap of co-expressing genes (A), while bubble charts represent GO (B) and KEGG (C) enrichment terms
Discussion
Acute myeloid leukemia (AML), a fast-developing cancer of the blood and bone marrow, is the most prevalent type of acute leukemia found in adults [20]. An accurate evaluation of prognosis is essential for guiding early therapeutic decisions and improving patient outcomes. The use of biomarkers in cancer treatment has the potential to enhance prognostic accuracy in certain malignancies. This study performed an in-depth analysis of four TNFAIP8 family molecules in AML, utilizing self-developed indicators to evaluate their expression patterns, prognostic significance, immune involvement, and other relevant factors. It is the first research exploring the expression and prognostic roles of different TNFAIP8 family members in AML. The findings are expected to contribute to advancements in current understanding.
The upregulation of TNFAIP8 family members (TNFAIP8, TNFAIP8L1, TNFAIP8L2, and TNFAIP8L3) observed in AML tissues compared to normal tissues highlights their potential role in AML pathogenesis. This differential expression, statistically significant across all family members (P < 0.001), suggests that the TNFAIP8 family may contribute to the oncogenic processes in AML. The substantial overexpression in tumor samples aligns with previous findings where dysregulation of TNFAIP8 family genes has been linked to tumor development and progression in other malignancies [4, 6, 21, 22]. Given that aberrant expression patterns in oncogenes or tumor suppressors often correlate with disease initiation or progression, further studies on the mechanistic contributions of these genes in AML could help clarify their specific oncogenic roles.
Survival analysis revealed no statistically significant differences in overall survival (OS) between high and low expression groups for TNFAIP8 family members in AML. While TNFAIP8L2 approached significance (P = 0.055), the results for the other members (TNFAIP8, TNFAIP8L1, and TNFAIP8L3) showed no strong survival associations (P > 0.5). This finding indicates that while these genes may be upregulated, their expression levels might not directly influence patient survival outcomes. However, this lack of association with OS does not rule out the possibility of these genes contributing to other aspects of AML prognosis, such as disease progression, resistance to therapy, or relapse potential [23]. Further investigation into the molecular pathways regulated by TNFAIP8 family genes could shed light on their role in clinical outcomes beyond survival.
ROC analysis showed that TNFAIP8L2 had an exceptional AUC value of 1.000, indicating perfect accuracy in predicting AML, while TNFAIP8, TNFAIP8L1, and TNFAIP8L3 also demonstrated moderate predictive abilities (AUC values of 0.709, 0.728, and 0.629, respectively). These findings suggest that TNFAIP8 family genes, particularly TNFAIP8L2, may serve as valuable biomarkers for diagnosing AML. Given the high accuracy of TNFAIP8L2 in distinguishing between normal and tumor samples, it could be explored as a potential diagnostic marker, warranting further validation in clinical settings.
The clinical significance of TNFAIP8 family expression was further supported by the associations with specific clinicopathological features. TNFAIP8L3 expression was significantly correlated with higher bone marrow (BM) blast counts (P < 0.001), suggesting its potential role in disease severity. Similarly, both TNFAIP8 and TNFAIP8L1 were associated with poor cytogenetic risk (P < 0.001), indicating that their elevated expression may be linked to more aggressive disease forms. Additionally, TNFAIP8L2 and TNFAIP8L3 were significantly associated with specific FAB subtypes, hinting at their involvement in the differentiation states of AML cells. Interestingly, TNFAIP8L1 showed a significant association with NPM1 mutation, a common genetic alteration in AML, suggesting that its high expression could serve as a surrogate marker for this mutation. These findings highlight the potential utility of TNFAIP8 family genes in stratifying AML patients based on clinical characteristics, which could guide personalized therapeutic approaches.
Epigenetic alterations play a crucial role in the early stages of tumor development [24]. Genetic alterations in the TNFAIP8 family were minimal, with only TNFAIP8L1 showing amplification (0.53%), indicating that mutations in these genes are not a common feature in AML. However, the significant positive correlations between TNFAIP8 and TNFAIP8L2/TNFAIP8L3, and the negative correlation with TNFAIP8L1, suggest that these family members may interact or regulate common pathways. Understanding the functional interplay between these genes could provide insights into their collective roles in AML pathogenesis [25]. The absence of frequent mutations suggests that the TNFAIP8 family’s contribution to AML may occur primarily at the transcriptional or post-transcriptional levels, rather than through genomic alterations.
Tumor cells interact with the immune microenvironment, influencing both tumor initiation and progression [26]. The immune cells that infiltrate the tumor are essential in shaping this microenvironment [27]. The high expression of TNFAIP8 is positively correlated with various myeloid immune cells, including neutrophils, eosinophils, macrophages, and dendritic cells, while showing a negative correlation with adaptive immune cells such as CD8⁺ T cells, NK cells, TFH cells, and Th1 cells. This trend may reflect the myeloid cell-dominant characteristics of the AML microenvironment and could be associated with immune evasion mechanisms in AML. Previous studies have demonstrated that myeloid-derived suppressor cells (MDSCs) are highly enriched in AML and promote disease progression by secreting immunosuppressive cytokines such as TGF-β and IL-10, thereby inhibiting the antitumor activity of T cells and NK cells [28]. Furthermore, TNFAIP8 has been reported to facilitate tumor cell survival and immune evasion in certain cancer types, and its role in AML may involve the regulation of myeloid immune cell function to reinforce the immunosuppressive microenvironment. In contrast, TNFAIP8L1 exhibits a distinct expression pattern, with high expression positively correlated with effector cells such as NK cells and CD8⁺ T cells, suggesting a potentially different role from TNFAIP8 in AML antitumor immunity. However, TNFAIP8L1 overexpression is also accompanied by a reduction in neutrophils and dendritic cells, implying a dual role in modulating immune activation. Additionally, the expression of TNFAIP8L2 and TNFAIP8L3 is associated with specific pro-inflammatory and immunosuppressive cell types, further supporting the hypothesis that the TNFAIP8 family genes may play a complex regulatory role in the AML immune microenvironment.
These findings provide evidence that the TNFAIP8 family may influence AML progression by shaping the tumor immune microenvironment. Exploring these interactions could help in the development of immune-based therapies targeting TNFAIP8 family-mediated immune evasion mechanisms [29, 30]. The association between TNFAIP8 family genes and immune cell infiltration suggests that they may play a critical role in the immune evasion of AML and serve as potential therapeutic targets. Notably, the high expression of TNFAIP8 is correlated with a reduction in NK cells and CD8⁺ T cells, indicating that it may promote leukemia cell survival by shaping the immunosuppressive microenvironment of AML. Therefore, targeting TNFAIP8 inhibition could help restore antitumor immunity and enhance the responsiveness of AML patients to immunotherapy. Furthermore, the expression pattern of TNFAIP8L1 suggests that it may enhance anti-AML immune responses under specific conditions, warranting further investigation into its regulatory role in different immune states. Additionally, the expression characteristics of TNFAIP8L2 and TNFAIP8L3 indicate their potential involvement in modulating the inflammatory microenvironment of AML, offering new directions for the development of personalized immunotherapeutic strategies.
Our findings also presented here in illuminate the intricate interplay between TNFAIP8 family genes and immune checkpoint regulation in LAML, offering a novel perspective on the immunomodulatory landscape of this aggressive malignancy. The robust positive correlations observed between TNFAIP8 and key immune checkpoint genes such as CD96, CSF1R, ENTPD1, HAVCR2, SIGLEC15, TGFBR1, TNFRSF9, and VSIR suggest a potential role for TNFAIP8 in promoting an immunosuppressive tumor microenvironment (TME). This is particularly intriguing given the known functions of these checkpoint molecules in dampening anti-tumor immune responses. For instance, HAVCR2 (TIM-3) and SIGLEC15 are well-documented mediators of immune evasion in various cancers, including LAML [31, 32]. The negative correlation with LAG3 and PDCD1 (PD-1), however, hints at a more complex regulatory mechanism, possibly indicating a dual role of TNFAIP8 in immune modulation. The consistent positive correlation of TNFAIP8L1 with a broad spectrum of checkpoint genes, including CD274 (PD-L1), LAG3, and TGFB1, further underscores the potential involvement of this gene family in immune evasion strategies. TGFB1, in particular, is a critical player in the immunosuppressive TME, promoting regulatory T cell (Treg) differentiation and inhibiting cytotoxic T cell activity [33]. The association of TNFAIP8L1 with these genes suggests its potential as a therapeutic target to reverse immune suppression in LAML. Similarly, TNFAIP8L2's positive correlation with genes like CSF1R, HAVCR2, and TGFBR1 aligns with emerging evidence that these pathways are pivotal in myeloid-derived suppressor cell (MDSC) expansion and T cell exhaustion. The negative correlation with KDR (VEGFR2) is noteworthy, as VEGF signaling is known to contribute to immunosuppression and angiogenesis in LAML. This inverse relationship may imply a compensatory mechanism whereby TNFAIP8L2 expression modulates angiogenic and immune responses in the TME. TNFAIP8L3's positive correlation with CTLA4 and TNFRSF18 (GITR) is particularly compelling, as these checkpoints are central to T cell activation and tolerance [34]. The negative correlations with CD244 and LGALS9, however, suggest a divergent role for TNFAIP8L3 in immune regulation, potentially influencing natural killer (NK) cell and macrophage activity. These findings resonate with recent studies highlighting the heterogeneity of immune checkpoint expression in LAML and its impact on clinical outcomes. For instance, the upregulation of PD-L1 and TIM-3 has been associated with poor prognosis and resistance to immunotherapy in LAML patients [35]. The involvement of TNFAIP8 family genes in these pathways positions them as potential biomarkers for immune checkpoint blockade response and as targets for combination therapies. In the context of global research, these results align with the growing recognition of the TNFAIP8 family's role in cancer immunity. Studies in solid tumors have implicated TNFAIP8 in promoting tumor progression and immune evasion, but its role in hematologic malignancies remains underexplored. Our findings bridge this gap, providing a foundation for future investigations into the therapeutic potential of targeting TNFAIP8 family genes in LAML. Our study elucidates the complex relationship between TNFAIP8 family genes and immune checkpoint regulation in LAML, offering new insights into the molecular mechanisms underlying immune evasion. These results underscore the need for further functional studies to validate these associations and explore their therapeutic implications. The integration of these findings with existing knowledge on LAML immunology could pave the way for innovative treatment strategies that harness the immune system to combat this devastating disease.
The co-expression analysis identified several genes significantly associated with TNFAIP8 family members, with each gene potentially contributing to the oncogenic processes in AML. TANK, as a crucial regulatory protein of TRAF (TNF receptor-associated factor), played a significant role in the modulation of the NF-κB signaling pathway and the Type I interferon (IFN-I) pathway [36]. TANK seems to be already studied in AML, so their positive correlation makes more sense according to other studies [37, 38] and our results. Enrichment analysis indicated that TNFAIP8 family co-expressed genes are involved in ion channel and transporter activity, as well as pathways related to neuroactive ligand-receptor interaction and bile secretion. These biological processes and pathways may provide novel insights into the molecular mechanisms underlying AML progression and offer potential therapeutic targets for future investigations.
The significant correlations between TNFAIP8 family expression and clinicopathological features, along with their immune interactions, underscore their potential as both diagnostic and prognostic biomarkers in AML. The high predictive accuracy of TNFAIP8L2, in particular, suggests that this gene could be incorporated into clinical diagnostic panels for early AML detection. Moreover, the associations with immune cell infiltration indicate that TNFAIP8 family members may serve as targets for immune-modulatory therapies. Given the evolving landscape of AML treatment, particularly with the rise of immune-based therapies, targeting TNFAIP8 family-mediated immune interactions could open new avenues for treatment strategies.
Limitations
Despite the comprehensive analysis of the TNFAIP8 family in AML, this study has several limitations that should be acknowledged: The study primarily relies on publicly available datasets, such as UCSC XENA and TCGA, which may introduce biases related to data collection, processing, and patient selection. The inclusion of additional independent cohorts would enhance the generalizability of the findings. The study is based solely on bioinformatics analyses without experimental validation. Functional assays, such as in vitro and in vivo experiments, are necessary to confirm the biological roles of TNFAIP8 family members in AML progression and immune interactions. The Kaplan–Meier survival analysis did not show statistically significant associations between TNFAIP8 family expression and overall survival (OS), and disease-free survival (DFS). This may be due to sample size limitations, patient heterogeneity, or the influence of other unmeasured prognostic factors. The importance of exploring alternative survival endpoints, such as event-free survival (EFS) and relapse-free survival (RFS), is better assess the prognostic significance of the TNFAIP8 family genes in AML. However, due to data availability constraints in publicly accessible databases, we were unable to retrieve reliable EFS and RFS data. The future studies should incorporate these additional survival endpoints. Our study provides a static snapshot of TNFAIP8 family expression in AML. Longitudinal studies tracking expression changes over the course of disease progression and treatment would provide more dynamic insights into their prognostic and therapeutic potential. So, future research integrating multi-omics data, experimental validation, and clinical trials is necessary to fully elucidate the role of TNFAIP8 family members in AML pathogenesis and their potential as therapeutic targets.
Conclusion
In summary, the TNFAIP8 family displays significant expression alterations, clinical associations, and immune interactions in AML. These findings highlight the potential clinical utility of the TNFAIP8 family as diagnostic biomarkers and therapeutic targets, particularly in modulating the immune microenvironment. Further studies are needed to validate these findings and explore the mechanistic pathways regulated by these genes in AML.
Supplementary Information
Supplementary 1. the details on sample sizes and gene expressions
Supplementary 2.1. relevant sample sizes and survival information of TNFAIP8
Supplementary 2.2 relevant sample sizes and survival information of TNFAIP8L1
Supplementary 2.3 relevant sample sizes and survival information of TNFAIP8L2
Supplementary 2.4 relevant sample sizes and survival information of TNFAIP8L3
Supplementary 3.1 the sample sizes used for ROC of TNFAIP8
Supplementary 3.2 the sample sizes used for ROC of TNFAIP8L1
Supplementary 3.3 the sample sizes used for ROC of TNFAIP8L2
Supplementary 3.4 the sample sizes used for ROC of TNFAIP8L3
Supplementary 4.1 the expression of TNFAIP8 and the immune cell types
Supplementary 4.2 the expression of TNFAIP8L1 and the immune cell types
Supplementary 4.3 the expression of TNFAIP8L2 and the immune cell types
Supplementary 4.4 the expression of TNFAIP8L3 and the immune cell types
Supplementary 5.1 enriched immune cells in the high and low expression groups of TNFAIP8
Supplementary 5.2 enriched immune cells in the high and low expression groups of TNFAIP8L1
Supplementary 5.3 enriched immune cells in the high and low expression groups of TNFAIP8L2
Supplementary 5.4 enriched immune cells in the high and low expression groups of TNFAIP8L3
Supplementary 6 the correlation analysis between TNFAIP8 family genes levels and immune checkpoints in LAML
Supplementary 7.1 the expression of genes co-expressed with TNFAIP8
Supplementary 7.2 the expression of genes co-expressed with TNFAIP8L1
Supplementary 7.3 the expression of genes co-expressed with TNFAIP8L2
Supplementary 7.4 the expression of genes co-expressed with TNFAIP8L3
Supplementary 8.1 the association of top ten co-expressed genes and TNFAIP8
Supplementary 8.2 the association of top ten co-expressed genes and TNFAIP8L1
Supplementary 8.3 the association of top ten co-expressed genes and TNFAIP8L2
Supplementary 8.4 the association of top ten co-expressed genes and TNFAIP8L3
Supplementary 9 a Venn diagram of genes co-expressed with TNFAIP8 family members
Supplementary Fig. 1. Disease-free survivalof TNFAIP8, TNFAIP8L1, TNFAIP8L2and TNFAIP8L3in LAML
Author contributions
Xuezhong Zhang and Tonggang Liu proposed the study idea. Xuezhong Zhang, Min Qu, Lei Bi and Xiaolei Wang collected and analyzed the data. Xuezhong Zhang drafted the manuscript. Tonggang Liu critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
No Funding.
Data availability
Research data are shown in the supplements files. Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
The datasets utilized in this study are accessible through public repositories. The datasets employed in this study are available through public repositories.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuezhong Zhang, Min Qu, Lei Bi share first authorship. Correspondence: Xiaolei Wang, Email: zxyybjk413@163.com; Tonggang Liu, liutonggang123@126.com
Contributor Information
Xiaolei Wang, Email: zxyybjk413@163.com.
Tonggang Liu, Email: liutonggang123@126.com.
References
- 1.Lagunas-Rangel FA, Chávez-Valencia V, Gómez-Guijosa M, Cortes-Penagos C. Acute myeloid leukemia-genetic alterations and their clinical prognosis. Int J Hematol Oncol Stem Cell Res. 2017;11(4):328–39. [PMC free article] [PubMed] [Google Scholar]
- 2.Yao Y, Li F, Huang J, Jin J, Wang H. Leukemia stem cell-bone marrow microenvironment interplay in acute myeloid leukemia development. Exp Hematol Oncol. 2021;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazinet A, Kantarjian HM. Moving toward individualized target-based therapies in acute myeloid leukemia. Ann Oncol. 2023;34(2):141–51. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Zhang X, Liu T, Sha K. Comprehensive analysis of the prognostic and immunological signature of TNFAIP8 family genes in human glioma. Sci Rep. 2024;14(1):17875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordoloi D, Banik K, Shabnam B, Padmavathi G, Monisha J, Arfuso F, Dharmarajan A, Mao X, Lim LHK, Wang L, et al. TIPE family of proteins and its implications in different chronic diseases. Int J Mol Sci. 2018;19(10):2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padmavathi G, Banik K, Monisha J, Bordoloi D, Shabnam B, Arfuso F, Sethi G, Fan L, Kunnumakkara AB. Novel tumor necrosis factor-α induced protein eight (TNFAIP8/TIPE) family: Functions and downstream targets involved in cancer progression. Cancer Lett. 2018;432:260–71. [DOI] [PubMed] [Google Scholar]
- 7.Zhong M, Chen Z, Yan Y, Bahet A, Cai X, Chen H, Ran H, Qu K, Han Z, Zhuang G, et al. Expression of TIPE family members in human colorectal cancer. Oncol Lett. 2021;21(2):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Liu R, Luan YY, Yao YM. Tumor necrosis factor-α induced protein 8: pathophysiology, clinical significance, and regulatory mechanism. Int J Biol Sci. 2018;14(4):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Li N, Chu T, Zhao H, Liu T. Comprehensive pan-cancer analysis of ENOPH1 in human tumors. Discov Oncol. 2025;16(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Zhang X, Liu T, Sha K. A comprehensive pan-cancer analysis of RNF187 in human tumors. Discov Oncol. 2025;16(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Li D, Sha K, Zhang X, Liu T. Human pan-cancer analysis of the predictive biomarker for the CDKN3. Eur J Med Res. 2024;29(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee AV, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173(2):400-416.e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformat. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587-d592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behrmann L, Wellbrock J, Fiedler W. The bone marrow stromal niche: a therapeutic target of hematological myeloid malignancies. Expert Opin Ther Targets. 2020;24(5):451–62. [DOI] [PubMed] [Google Scholar]
- 21.Niture S, Lin M, Odera JO, Moore J, Zhe H, Chen X, Suy S, Collins SP, Kumar D. TNFAIP8 drives metabolic reprogramming to promote prostate cancer cell proliferation. Int J Biochem Cell Biol. 2021;130: 105885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao X, Xiong C, Wang D, Yang J, Jia W, Yang L, Wang X, Zhao Y. Association of TNFAIP8 gene polymorphisms with cancer risk in Chinese population. Nucleosides Nucleotides Nucleic Acids. 2022;41(5–6):555–65. [DOI] [PubMed] [Google Scholar]
- 23.Pasquer H, Tostain M, Kaci N, Roux B, Benajiba L. Descriptive and functional genomics in acute myeloid leukemia (AML): paving the road for a cure. Cancers. 2021;13(4):748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma M. The role of epigenomics in the study of cancer biomarkers and in the development of diagnostic tools. Adv Exp Med Biol. 2015;867:59–80. [DOI] [PubMed] [Google Scholar]
- 25.Kishtagari A, Levine RL. The role of somatic mutations in acute myeloid leukemia pathogenesis. Cold Spring Harb Perspect Med. 2021;11(4):a034975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Galen P, Hovestadt V, Wadsworth Ii MH, Hughes TK, Griffin GK, Battaglia S, Verga JA, Stephansky J, Pastika TJ, Lombardi Story J, et al. Single-cell RNA-Seq reveals AML hierarchies relevant to disease progression and immunity. Cell. 2019;176(6):1265-1281.e1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21(8):485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limongello R, Marra A, Mancusi A, Bonato S, Hoxha E, Ruggeri L, Hui S, Velardi A, Pierini A. Novel immune cell-based therapies to eradicate high-risk acute myeloid leukemia. Front Immunol. 2021;12: 695051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haddad F, Daver N. An update on immune based therapies in acute myeloid leukemia: 2021 and beyond! Adv Exp Med Biol. 2021;1342:273–95. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Yang D, Hao M, Liu H. Differential expression of HAVCR2 gene in pan-cancer: a potential biomarker for survival and immunotherapy. Front Genet. 2022;13: 972664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Zhang B, Wang X, Zeng Z, Huang Z, Zhang L, Wei F, Ren X, Yang L. Expression signature, prognosis value, and immune characteristics of Siglec-15 identified by pan-cancer analysis. Oncoimmunology. 2020;9(1):1807291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreau JM, Velegraki M, Bolyard C, Rosenblum MD, Li Z. Transforming growth factor-β1 in regulatory T cell biology. Sci Immunol. 2022;7(69):eabi4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. [DOI] [PubMed] [Google Scholar]
- 35.Astaneh M, Rezazadeh H, Hossein-Nataj H, Shekarriz R, Zaboli E, Shabani M, Asgarian-Omran H. Tim-3 and PD-1 blocking cannot restore the functional properties of natural killer cells in early clinical stages of chronic lymphocytic leukemia: An in vitro study. J Cancer Res Ther. 2022;18(3):704–11. [DOI] [PubMed] [Google Scholar]
- 36.Holicek P, Truxova I, Rakova J, Salek C, Hensler M, Kovar M, Reinis M, Mikyskova R, Pasulka J, Vosahlikova S, et al. Type I interferon signaling in malignant blasts contributes to treatment efficacy in AML patients. Cell Death Dis. 2023;14(3):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S, Ni M, Hu T, Gu Y, Feng C, Pan C, Zhang S, Wen S, Zhao N, Wang W, et al. TANK-binding kinase 1 inhibitor GSK8612 enhances daunorubicin sensitivity in acute myeloid leukemia cells via the AKT-CDK2 pathway. Am J Transl Res. 2021;13(12):13640–53. [PMC free article] [PubMed] [Google Scholar]
- 38.Youmaran A. Investigating the role of TANK-binding kinase 1 (TBK1) in MLL-AF9+ acute myeloid leukemia. 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1. the details on sample sizes and gene expressions
Supplementary 2.1. relevant sample sizes and survival information of TNFAIP8
Supplementary 2.2 relevant sample sizes and survival information of TNFAIP8L1
Supplementary 2.3 relevant sample sizes and survival information of TNFAIP8L2
Supplementary 2.4 relevant sample sizes and survival information of TNFAIP8L3
Supplementary 3.1 the sample sizes used for ROC of TNFAIP8
Supplementary 3.2 the sample sizes used for ROC of TNFAIP8L1
Supplementary 3.3 the sample sizes used for ROC of TNFAIP8L2
Supplementary 3.4 the sample sizes used for ROC of TNFAIP8L3
Supplementary 4.1 the expression of TNFAIP8 and the immune cell types
Supplementary 4.2 the expression of TNFAIP8L1 and the immune cell types
Supplementary 4.3 the expression of TNFAIP8L2 and the immune cell types
Supplementary 4.4 the expression of TNFAIP8L3 and the immune cell types
Supplementary 5.1 enriched immune cells in the high and low expression groups of TNFAIP8
Supplementary 5.2 enriched immune cells in the high and low expression groups of TNFAIP8L1
Supplementary 5.3 enriched immune cells in the high and low expression groups of TNFAIP8L2
Supplementary 5.4 enriched immune cells in the high and low expression groups of TNFAIP8L3
Supplementary 6 the correlation analysis between TNFAIP8 family genes levels and immune checkpoints in LAML
Supplementary 7.1 the expression of genes co-expressed with TNFAIP8
Supplementary 7.2 the expression of genes co-expressed with TNFAIP8L1
Supplementary 7.3 the expression of genes co-expressed with TNFAIP8L2
Supplementary 7.4 the expression of genes co-expressed with TNFAIP8L3
Supplementary 8.1 the association of top ten co-expressed genes and TNFAIP8
Supplementary 8.2 the association of top ten co-expressed genes and TNFAIP8L1
Supplementary 8.3 the association of top ten co-expressed genes and TNFAIP8L2
Supplementary 8.4 the association of top ten co-expressed genes and TNFAIP8L3
Supplementary 9 a Venn diagram of genes co-expressed with TNFAIP8 family members
Supplementary Fig. 1. Disease-free survivalof TNFAIP8, TNFAIP8L1, TNFAIP8L2and TNFAIP8L3in LAML
Data Availability Statement
Research data are shown in the supplements files. Data is provided within the manuscript or supplementary information files.