Abstract
Twenty-one atypical Shigella flexneri type 4 strains isolated from patients attending the Dhaka treatment center of the International Centre for Diarrhoeal Disease Research, Bangladesh, were extensively characterized and compared with S. flexneri serotypes 4a and 4b. The atypical strains agglutinated only with the type antigen factor 4 and did not agglutinate with any group factors, thereby excluding their characterization into serotype 4a or 4b. Of the 21 strains, 85.7% did not ferment mannitol but were able to ferment most of the sugars, whereas the remaining 14.3% strains fermented mannitol but were unable to ferment most of the sugars. Most of the strains were resistant to ampicillin, tetracycline, and trimethoprim-sulfomethoxazole. All of the strains harbored the 140-MDa plasmid, had the ipaH gene, had the sen gene (encoding Shigella enterotoxin 2), had the ability to bind Congo red, and were positive for keratoconjunctivitis in the guinea pig eye, attesting their invasive properties. All of the strains contained a middle-range plasmid (35 to 62 MDa) as well as a number of stable small plasmids, yielding mainly two plasmid profiles which were different from those of 4a and 4b strains. Conjugation and curing experiments suggested that the middle-range plasmids harbored a self-transferable multiple antibiotic resistance marker. Pulsed-field gel electrophoresis analysis of all of the tested strains yielded two types with numerous subtypes, whereas ribotyping yielded only two types which were completely different from those of types 4a and 4b. This study concluded that two different clones of atypical S. flexneri type 4 exist and strongly suggests that these are new subserotypes of S. flexneri that await further serological classification.
In developing countries like Bangladesh, bacillary dysentery is one of the major causes of morbidity and mortality, especially among children. The disease is caused by microorganisms belonging to the genus Shigella. The annual number of Shigella episodes throughout the world is estimated to be 164.7 million, of which 69% of all deaths are attributable to shigellosis involving children less than 5 years of age (19). Shigella is spread by direct fecal-hand-oral contact wherever personal hygiene is compromised (42). Clinical infection can be transmitted by as few as 10 Shigella organisms (7), even without neutralization of gastric acid. Recently, the World Health Organization has emphasized the need to understand the disease burden and epidemiology of Shigella infections in developing countries (44).
Shigellosis is caused by any of the four species of Shigella, namely, S. dysenteriae, S. flexneri, S. boydii, and S. sonnei. Except for S. sonnei, each species contains multiple serotypes based on the structure of the O antigen (34). Until recently, at least 47 serotypes of Shigella have been recognized, of which 15 belong to S. flexneri (44). The serotypes of S. flexneri (with the exception of serotype 6) have some degree of antigenic relatedness attributable to a common repeating tetrasaccharide unit, to which α-d-glucopyranosyl and O-acetyl groups are added, providing the basis for their type (i.e., I to VI) and group (i.e., 3.4, 6, and 7.8) antigenic factors (4, 8). Rabbit antisera raised against the specific type and group factors are routinely used in agglutination reactions to identify the S. flexneri serotypes (8). However, commercially available antisera and monoclonal antibodies specific for each type and group factor antigen currently used for typing the S. flexneri strains are not able to cover all possible epitopes of the O antigen. There probably are a multitude of epitopes in S. flexneri not covered by the typing scheme currently in use.
Indiscriminate use of the drugs and horizontal gene transfer has led to Shigella species becoming resistant to commonly used antibiotics. In this situation, development of a vaccine against shigellosis is an urgent requirement. However, several investigators have reported that immunity to Shigella is serotype specific, and vaccine protection will therefore depend on the representation of each serotype in the vaccine (11, 12, 25, 27, 28). The genetic variability between serotypes and emergence of atypical strains (38) accentuates the problems in development of an effective vaccine.
Isolation of uncommon serotypes and subserotypes of Shigella species, particularly of S. flexneri, is not a rare occurrence. A provisional serotype of S. flexneri, 1c, first identified in Bangladesh (41) and later isolated in rural Egypt (9), has yet to be fully characterized. Another provisional serotype of S. flexneri, designated 4c, not included in the typing scheme of Shigella was isolated in Russia (30). These also have atypical agglutination patterns with commercially available antisera. Recently in Bangladesh, an uncommon subserotype of S. flexneri type 4 was detected which showed a conflicting agglutination pattern with commercially available antisera as well as monoclonal antibodies by reacting strongly only with the serotype 4-specific antiserum and not with any other type- or group-specific antisera (38). Therefore, it was not possible to type these isolates by the present classification scheme for S. flexneri. Moreover, these strains were isolated from children and adults with severe dysentery, emphasizing the need to study these isolates in detail.
MATERIALS AND METHODS
Bacterial strains.
Twenty-one atypical clinical isolates of S. flexneri type 4 were isolated from patients attending the Dhaka treatment center operated by the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), between January 1997 and June 2001. These strains were isolated and identified in the clinical microbiology laboratory by standard microbiological and biochemical methods (43). The strains were grown in Trypticase soy broth with 0.3% yeast extract (TSBY) and stored at −70°C after addition of 15% glycerol. Reference strains of S. flexneri 4a (ATCC 12023), with the new antigenic determinant E1037, and 4b (NCTC 8522) were used for comparison purposes. S. flexneri 2a strain YSH6000 (32) and an Escherichia coli strain (ATCC 25922) lacking the 140-MDa invasive plasmid and sensitive to all antibiotics were used as positive and negative controls, respectively, in the Sereny test, Congo red binding ability test, and PCR assay for detection of the ipaH gene and the Shigella enterotoxin gene (set). E. coli strains PDK-9, V-517, Sa, RP4, and R1 were used as plasmid molecular weight standards (14). E. coli K-12 (lac+ F −), resistant to nalidixic acid, was used as the recipient in the conjugation experiments (14). The different strains representing the various subserotypes of S. flexneri (1a, 1b, 1c, 2a, 3a, 5a, 6, X, and Y) used in the ribotyping were obtained from our collection.
Serotyping.
Serotyping of the 21 S. flexneri type 4 strains was confirmed using two serotyping kits: (i) a commercially available antisera kit (Denka Seiken, Tokyo, Japan) specific for all type- and group-factor antigens and (ii) monoclonal antibody reagents specific for all S. flexneri type- and group-factor antigens (5). Strains were subcultured on MacConkey agar (Difco, Becton Dickinson and Company, Sparks, Md.) plates, and after about 18 h of incubation, serological reactions were performed by the slide agglutination test as described previously (38).
Biochemical characterization.
The biochemical reactions of the strains were determined by standard methods (43).
Antimicrobial susceptibility.
Bacterial susceptibility to antimicrobial agents was determined by the disk diffusion method as recommended by the National Committee for Clinical Laboratory Standards (24) with commercial antimicrobial disks (Oxoid, Basingstoke, United Kingdom). The antibiotic disks used in this study were ampicillin (10 μg), tetracycline (30 μg), mecillinum (25 μg), nalidixic acid (30 μg), trimethoprim-sulfomethoxazole (25 μg), and ciprofloxacin (5 μg). E. coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used as control strains for susceptibility studies.
Keratoconjunctivitis assay (Sereny test).
The Sereny test was performed by a procedure described elsewhere (20, 33). Briefly, an overnight culture of bacteria, suspended to a density of approximately 1010 viable cells in 20 μl of phosphate-buffered saline, was dropped into the conjunctival sac of the eye of a guinea pig. The other eye served as the control. The guinea pigs were observed daily for 72 h, and their inflammatory responses were graded.
Determination of Congo red binding ability.
TSBY with 1.5% agar and 0.01% Congo red (Sigma Chemical Co.) was used to study the pigment binding abilities of the test strains by a procedure described previously (31, 32).
Isolation of plasmid DNA.
Plasmid DNA was prepared according to the alkaline lysis method of Kado and Liu (17) with some modifications. An isolated colony of each strain was inoculated in 1.5 ml of TSBY and incubated overnight at 37°C. Cells were harvested by centrifugation. The cells were suspended in 100 μl of solution I (40 mM Tris-Na acetate, 2 mM EDTA, pH 7.4), and then 200 μl of solution II (3% sodium dodecyl sulfate, 50 mM Tris, pH 12.9) was added and incubated at 55°C for 1 h. After incubation, an equal volume of solution III (phenol-chloroform-isoamyl alcohol [25:24:1]) was added and mixed well, and plasmid DNA was collected by centrifugation. Plasmid DNA was separated by horizontal electrophoresis in a 0.7% agarose slab gel in Tris-borate-EDTA (TBE) buffer at room temperature at 100 V (50 mA) for 3 h. After electrophoresis, the gel was stained with ethidium bromide and video images were obtained by a gel documentation system. The molecular mass of the unknown plasmid DNA was assessed by comparison with the mobilities of plasmids with known molecular masses (14). Plasmids present in E. coli strains PDK-9 (140, 105, 2.7, and 2.1 MDa), R1 (62 MDa), RP4 (36 MDa), Sa (23 MDa), and V517 (35.8, 4.8, 3.7, 3.4, 3.1, 2.0, 1.8, and 1.4 MDa) were used as molecular mass standards.
Determination of resistance factor.
Conjugation experiments with multidrug-resistant (Ampr Sxtr Tetr) atypical S. flexneri type 4 donor strains (K-435 and K-584) and the E. coli K-12 recipient (Nalr Lac+ F−) was carried out by a method described previously (22). Transconjugant colonies were selected on MacConkey agar plates containing nalidixic acid (30 μg/ml) and ampicillin (50 μg/ml). Plasmid analysis and antimicrobial susceptibility tests of the transconjugant strains were carried out to determine the transfer of plasmids with antibiotic resistance. The transfer frequency of the resistance plasmid was calculated by a method described earlier (22).
PFGE.
Intact agarose-embedded chromosomal DNAs from clinical isolates of atypical S. flexneri type 4 were prepared, and pulsed-field gel electrophoresis (PFGE) was performed using the contour-clamped homogeneous electric field (CHEF-DRII) apparatus from Bio-Rad Laboratories (Richmond, Calif.) by procedures described earlier (1, 29, 37) but with different pulse times. Genomic DNA was digested with the NotI restriction enzyme (GIBCO-BRL, Gaithersburg, Md.) for 16 h at 37°C, and the restriction fragments were separated by using the CHEF-DRII system apparatus in 1% pulsed-field-certified agarose in 0.5× TBE buffer for 38 h at 200 V and 14°C with the following pulse times: 3 to 28 s for 8 h, 5 to 50 s for 8 h, 20 to 80 s for 11 h, and 60 to 120 s for 11 h. The gel was stained with ethidium bromide, destained, and photographed on a gel documentation system. The DNA size standards used were the bacteriophage lambda ladder ranging from 48.5 to 1,000 kb (Bio-Rad) and Saccharomyces cerevisiae chromosomal DNA ranging from 225 to 2,200 kb (Bio-Rad). Band patterns were established by criteria described previously (39).
Detection of Shigella enterotoxin genes (set1 and sen) and ipaH by PCR.
Detection of the set1 (encoding Shigella enterotoxin 1 [ShET-1]), sen (encoding ShET-2), and ipaH genes was performed by amplifying set1A, set1B, sen, and ipaH primers by PCR by procedures described previously (40). All of these primers were synthesized using an Oligo 1000 DNA Synthesizer (Beckman) in our laboratory at ICDDR,B.
Amplification of specific oligonucleotide primers for 16S rRNA conserved sequence by PCR.
Synthetic oligonucleotides 5′-GGA TTA GAT ACC CTG GTA GTC C-3′ (forward) and 5′-TCG TTG CGG GAC TTA ACC CAA C-3′ (reverse) from the highly conserved sequence of the 16S rRNA (18) were synthesized using an Oligo 1000 DNA Synthesizer (Beckman). They were then amplified by PCR using the purified DNA from S. flexneri as described previously. Briefly, 3 μg of template DNA and 1 μl of synthetic oligonucleotide was added in a total volume of 25 μl of reaction mixture consisting of 10× PCR buffer, 50 mM MgCl2, 2.5 mM deoxynucleoside triphosphates, and 1 U of Taq DNA polymerase enzyme (5 U/μl) (GIBCO-BRL). For PCR, the DNA was first denatured at 94°C for 5 min, followed by 30 cycles of 1 min each at 94°C (denaturation), 54°C (annealing), and 72°C (extension), followed by final extension at 72°C for 10 min. The PCR products were analyzed by horizontal gel electrophoresis with a 1% agarose gel in TBE buffer as described previously. The gel was stained with ethidium bromide (0.5 μg/ml) and visualized with a UV transilluminator.
Extraction, purification, and preparation of 16S rRNA gene probe.
The 320-bp PCR product specific for the 16S rRNA conserved sequence was excised from the gel, placed in a dialysis bag, and eluted by electrophoresis using the method described by Maniatis et al. (21). The DNA was then purified by phenol-chloroform extraction followed by ethanol precipitation as described above. The purified DNA probe specific for 16S ribosomal DNA (rDNA) was labeled with digoxigenin (DIG)-dUTP (Boehringer GmbH, Mannheim, Germany) by using a random primed DNA labeling kit (Boehringer) according to the instructions of the manufacturer. The DIG-labeled probe was stored at −20°C until used. Immediately prior to use, the probe was denatured to single-stranded DNA by boiling for 10 min and then chilling on ice to prevent renaturation.
DNA isolation, restriction enzyme digestion, and separation of restriction fragments.
Chromosomal DNAs of S. flexneri isolates were extracted and purified by the method described by Maniatis et al. (21) with some modifications. Briefly, from overnight-grown culture, cells were harvested by centrifugation and treated with TES (10 mM Tris [pH 8.0], 10 mM EDTA, 100 mM NaCl) and 10% sodium dodecyl sulfate at 65°C for 10 min. After proteinase K treatment at 45°C for 5 h, DNA was extracted with phenol-chloroform-isoamyl alcohol (25:24:1). DNA was purified by ethanol precipitation, dried, and dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). RNase treatment was performed at 37°C for 1 to 2 h, and final purification was done by ethanol precipitation. The purified DNA was dissolved in TE buffer and stored at −20°C. Three micrograms of chromosomal DNA was digested with HindIII restriction enzyme overnight at 37°C according to the instructions of the manufacturer (GIBCO-BRL) and separated by gel electrophoresis according to procedures described elsewhere (2).
Southern hybridization.
The gel in which the DNA fragments were separated by electrophoresis was depurinated, denatured, and neutralized according to the instruction manual from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom). The DNA fragments were transferred into a Hybond-N+ positively charged nylon membrane (Amersham Pharmacia Biotech) by using a vacuum transfer apparatus according to the instruction manual. Hybridization of the membrane with the probe for 18 h at 68°C and development of the membrane with anti-DIG-alkaline phosphatase were performed according to the instructions provided in the DIG DNA labeling and detection kit (Boehringer). Results were documented by taking photographs of the filter.
RESULTS
Serological typing.
None of the 21 S. flexneri isolates could be definitively serotyped with the commercially available kit (Denka Seiken). The strains displayed conflicting agglutination patterns, reacting strongly only with serotype 4-specific antisera and not with any group-specific antisera. All of the strains were then examined using a panel of monoclonal antibodies against S. flexneri (MASF). Each strain reacted strongly with the serogroup B-specific antibody, MASF B, confirming that all were S. flexneri (Table 1). All strains reacted strongly with the serotype 4-specific antibody, MASF IV-2, suggesting that all were serotype 4. However, none of the strains agglutinated with the MASF reagents in a pattern specific for subserotypes 4a, 4b, and 4c or for the new provisional subserotype 1c. All of the strains agglutinated with the provisional antigen MASF IV-1 specific for a new antigenic determinant (E1037) of S. flexneri.
TABLE 1.
Agglutination reactions of atypical strains of S. flexneri type 4 and the reference strains tested with the MASF
| Strain(s) | Reaction with MASF
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type antigen specific
|
Group antigen specific
|
|||||||||
| I | II | IV-2 | V | VI | B | Y-5 | 6 | 7.8 | IV-1 | |
| S. flexneri ATCC 4a | − | − | + | − | − | + | + | − | − | + |
| S. flexneri NCTC 4b | − | − | + | − | − | + | − | + | − | − |
| Atypical strains of S. flexneri type 4 | − | − | + | − | − | + | − | − | − | + |
Biochemical characterization.
All 21 strains examined exhibited biochemical characteristics typical of the genus Shigella and of the species S. flexneri (8). Two different biotypes (1 and 2) were found based on mannitol fermentation (Table 2). Of these, 85.7% of the isolates (biotype 1) did not ferment mannitol, produced indole, and were able to utilize sodium acetate, maltose, xylose, mannose, trehalose, sorbitol, and rhamnose. In contrast, 14.3% of the isolates (biotype 2) were able to ferment mannitol but unable to utilize sodium acetate, maltose, xylose, mannose, trehalose, sorbitol, and rhamnose and were negative for indole production. However, reference strains (S. flexneri ATCC 4a and NCTC 4b) showed differences from the two biotypes of the test strains in some tests (Table 2).
TABLE 2.
Biochemical profiles of mannitol-fermenting and non-mannitol-fermenting strains of atypical S. flexneri type 4 compared with reference strains
| Major typea | Strain no. | Reactionb
|
Bio- type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indole production | Arginine decarboxylase | Glucose (acid) | Sorbitol | Arabinose | Raffinose | Rhamnose | Trehalose | Sodium acetate | Maltose | Xylose | Mannose | |||
| a | K-147 | − | − | + | − | (+) | − | − | (+) | − | − | − | − | 2 |
| K-565 | − | − | + | − | (+) | − | − | (+) | − | − | − | − | 2 | |
| K-716 | − | − | + | − | (+) | − | − | (+) | − | − | − | − | 2 | |
| b | K-235 | + | + | + | (+) | + | − | (+) | + | + | + | + | + | 1e |
| K-282 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-311 | + | − | + | (+) | + | − | (+) | (+) | + | + | + | + | 1a | |
| K-342 | + | − | + | (+) | + | − | − | (+) | + | + | (+) | + | 1b | |
| K-360 | + | − | + | (+) | + | − | (+) | + | + | + | (+) | + | 1a | |
| K-361 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-435 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-441 | + | − | + | − | + | − | (+) | + | + | + | + | + | 1c | |
| K-472 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-557 | + | − | + | − | + | − | − | + | + | + | + | + | 1d | |
| K-584 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-594 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-615 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-772 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-818 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-820 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-1494 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| K-1571 | + | − | + | (+) | + | − | (+) | + | + | + | + | + | 1a | |
| c | ATCC 4a | + | − | + | (+) | + | − | − | + | + | − | + | − | 3 |
| NCTC 4b | + | − | + | − | + | − | − | + | − | − | − | + | 4 | |
a, mannitol-fermenting isolates; b, non-mannitol-fermenting isolates; c, reference strains.
+, positive reaction within 1 or 2 days; (+), positive reaction after 3 or more days; −, negative reaction.
Antibiotic susceptibility test.
Of the 21 isolates, 81% were resistant to trimethoprim-sulfomethoxazole, 71.4% were resistant to tetracycline, and 52.4% were resistant to ampicillin. Resistance to ciprofloxacin, mecillinum, and nalidixic acid was not detected. Multiple resistance to ampicillin, tetracycline, and trimethoprim-sulfomethoxazole was 47.6%. Fourteen percent of the strains were sensitive to all six antibiotics. The remaining isolates were resistant to one or two antibiotics.
Test for invasiveness.
All 21 strains harbored the 140-MDa invasive plasmid, had the ability to bind Congo red, were positive for the ipaH gene, and were positive for keratoconjunctivitis in the guinea pig eye, attesting their invasive trait.
Plasmid profile analysis.
Analysis of plasmid DNA revealed that all of the strains contained multiple plasmids whose sizes ranged from 140 to 0.7 MDa, forming a number of unique banding patterns (Table 3). All of the strains contained the 140-MDa invasive plasmid. Two different plasmid patterns, designated P1 and P2, were found among the 21 tested strains (Table 3). Three strains (14.3%) had pattern P2 while the remaining 18 strains (85.7%) had pattern P1. The plasmid profiles of S. flexneri ATCC 4a and NCTC 4b were different from patterns P1 and P2.
TABLE 3.
Plasmid profile analysis of atypical strains of S. flexneri type 4 along with S. flexneri ATCC 4a and NCTC 4b
| No. of strains or strain no. | Presence of plasmid with molecular mass (MDa) of:
|
Plasmid pattern | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 140 | 80-30 | 5.9 | 4.3 | 3.9 | 4.0 | 2.7 | 2.1 | 1.0 | 0.7 | ||
| 8 | + | + | + | + | P1a | ||||||
| 2 | + | + | + | + | + | P1b | |||||
| 4 | + | + (62a) | + | + | + | P1c | |||||
| 4 | + | + (35a) | + | + | + | + | P1d | ||||
| 3 | + | + | + | + | + | + | + | P2 | |||
| ATCC 4a | + | + | + | + | P3 | ||||||
| NCTC 4b | + | + | + | P4 | |||||||
Actual size of the plasmid present.
Determination of resistance factor.
Two strains, K-584 (plasmid pattern P1c) and K-435 (plasmid pattern P1d) (Table 3), having the same resistance pattern (Ampr Tetr Sxtr) were selected for conjugation experiments with E. coli K-12. In the case of K-584, a 62-MDa plasmid was transferred, whereas in the case of K-435, a 35-MDa plasmid was transferred (Table 4), with the complete spectrum of drug resistance (Ampr Tetr Sxtr). The transfer frequencies were almost same for both strains. Both transconjugants were cured by loss of the plasmid and were sensitive to all antibiotics (Table 4).
TABLE 4.
Transfer of resistance plasmid to E. coli K-12 by conjugation
| Strain no. | Parent strain
|
Transconjugant
|
Transfer frequency of R plasmid | Cured strain
|
|||
|---|---|---|---|---|---|---|---|
| Resistance pattern | Plasmid profile (MDa) | Resistance pattern | Plasmid profile (MDa) | Resistance pattern | Plasmid profile (MDa) | ||
| K-435 | Ampr Sxtr Tetr | 140, 35, 5.9, 4.0, 2.7, 0.7 | Ampr Sxtr Tetr | 35 | 5.0 × 10−4 | Nal | No plasmid |
| K-584 | Ampr Sxtr Tetr | 140, 62, 5.9, 4.0, 2.7 | Ampr Sxtr Tetr | 62 | 4.3 × 10−4 | Nal | No plasmid |
Detection of Shigella enterotoxin genes (set1 and sen) by PCR assays.
The Shigella enterotoxin 1 gene (set1) was absent in all of the strains, while the Shigella enterotoxin 2 gene (sen) was present in all of the strains.
Ribotyping.
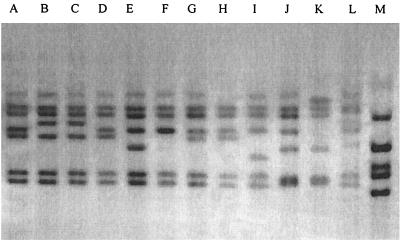
Two different reproducible rRNA gene restriction patterns, ribotypes R1 and R2, were observed among the atypical strains of S. flexneri type 4 (Fig. 1). The sizes of the bands ranged from 15 to 5 kb in all patterns, and the size distribution was optimum for the discrimination of the strains. Of the 21 isolates, 18 (85.7%) belonged to ribotype R1, and the remaining 3 (14.3%) belonged to ribotype R2 (Table 5). S. flexneri ATCC 4a and NCTC 4b showed ribotyping patterns different from those of the tested strains (Fig. 1). Moreover, the ribotypes of the other serotypes of S. flexneri were completely different from those of the S. flexneri type 4 strains (Fig. 1).
FIG. 1.
Ribotyping patterns of new subserotype of S. flexneri type 4 along with different serotypes of S. flexneri. Lanes: A, serotype 1a (strain K-647); B, serotype 1b (K-817); C, serotype 1c (K-212); D, serotype 2a (K-453); E, serotype 3a (K-452); F, S. flexneri ATCC 4a; G, S. flexneri NCTC 4b; H, S. flexneri type 4 (pattern R2); I, S. flexneri type 4 (pattern R1); J, serotype 5a (Y-787); K, serotype 6 (K-301); L, serotype Y (K-155); M, serotype X (K-608).
TABLE 5.
Characteristic patterns of atypical strains of S. flexneri type 4
| Strain no. | Biotype | Antibiotic susceptibility pattern | Plasmid type | PFGE type | Ribotype |
|---|---|---|---|---|---|
| K-235 | 1e | IV | P1b | A10 | R1 |
| K-282 | 1a | III | P1a | A1 | R1 |
| K-311 | 1a | II | P1a | A8 | R1 |
| K-342 | 1b | II | P1a | A1 | R1 |
| K-360 | 1a | II | P1a | A3 | R1 |
| K-361 | 1a | I | P1c | A1 | R1 |
| K-435 | 1a | I | P1d | A7 | R1 |
| K-441 | 1c | II | P1a | A1 | R1 |
| K-472 | 1a | III | P1a | A6 | R1 |
| K-557 | 1d | I | P1c | A4 | R1 |
| K-584 | 1a | I | P1c | A2 | R1 |
| K-594 | 1a | I | P1d | A2 | R1 |
| K-615 | 1a | I | P1d | A9 | R1 |
| K-772 | 1a | II | P1c | A1 | R1 |
| K-818 | 1a | III | P1a | A5 | R1 |
| K-820 | 1a | III | P1a | A1 | R1 |
| K-1494 | 1a | IV | P1b | A1 | R1 |
| K-1571 | 1a | I | P1d | A1 | R1 |
| K-147 | 2 | I | P2 | B | R2 |
| K-565 | 2 | I | P2 | B | R2 |
| K-716 | 2 | I | P2 | B | R2 |
| ATCC 4a | 3 | P3 | C | R3 | |
| NCTC 4b | 4 | P4 | D | R4 |
PFGE.
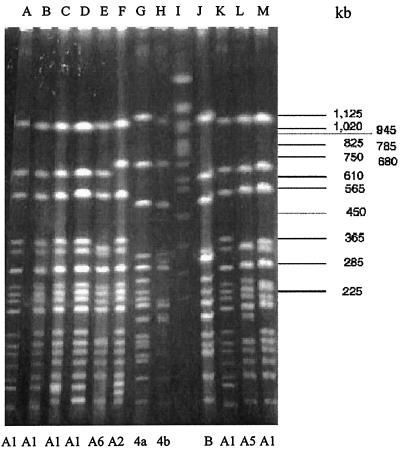
PFGE analysis of NotI-digested chromosomal DNAs of the atypical S. flexneri type 4 strains yielded 16 to 19 reproducible DNA fragments ranging in size from approximately 20 to 1,050 kb (Fig. 2). Two major PFGE patterns, designated A and B, were observed among the 21 strains, of which 18 (85.7%) strains belonged to type A and the remaining 3 isolates (14.3%) belonged to type B. Type A was further subdivided into 10 subtypes, A1 to A10 (Table 5). Pattern A1 (38%) was most prevalent among the different patterns of type A. The PFGE patterns of S. flexneri ATCC 4a and NCTC 4b were completely different from those of S. flexneri type 4, and hence these were placed into two different types, C and D.
FIG. 2.
PFGE patterns of NotI-digested chromosomal DNAs from representative strains of atypical S. flexneri type 4 and reference strains. Lanes: A, K-282 (type A1); B, K-342 (type A1); C, K-361 (type A1); D, K-441 (type A1); E, K-472 (type A6); F, K-584 (type A2); G, ATCC 4a (type C); H, NCTC 4b (type D); I, molecular size marker (S. cerevisiae); J: K-716 (type B); K, K-772 (type A1); L, K-818 (type A5); M, K-820 (type A1).
DISCUSSION
The close relatedness between E. coli and Shigella spp. makes serological identification a crucial step in the diagnosis of Shigella infection (6). According to recent reports, S. flexneri has eight serotypes, of which serotypes 1 to 5 are further classified into 12 subserotypes. However, this classification scheme for S. flexneri is not comprehensive, because atypical strains or newer subserotypes are being isolated from different parts of the world, including Bangladesh (9, 5, 30, 38). In this study, a total of 21 S. flexneri isolates were identified, primarily by using the standard biochemical and serological methods. However, none of the isolates could be definitively serotyped using the commercially available reagents (Denka Seiken). These serologically atypical strains displayed conflicting agglutination patterns, reacting strongly only with serotype 4-specific antisera and not with any of the group-specific antisera that enable subserotype classification as S. flexneri 4a or 4b. These equivocal results reflect the limitations of commercial antibody reagents for reliable detection of the full range of serological variants of S. flexneri. The panel of monoclonal antibodies specific for different type- and group-specific O-antigenic determinants of S. flexneri lipopolysaccharide (SBL, Stockholm, Sweden) also could not identify the serotypes of these isolates. The serologically atypical results allowed us to conclude that these strains might be a new subserotype of S. flexneri type 4. An extensive phenotypic and genotypic study was therefore performed to establish the standing of these strains in the classification scheme of S. flexneri.
On the basis of biochemical tests, 18 of the 21 isolates (85.7%) were mannitol negative but utilized sodium acetate. The remaining 3 (14.3%) were mannitol positive but did not utilize sodium acetate (Table 2). The S. flexneri subgroup is characteristically mannitol positive, but variants in each serotype that do not utilize mannitol have been reported. S. flexneri serotypes 4 and 6 appear to be the most common among the mannitol-negative varieties of S. flexneri (8), but apparently these do not occur as frequently as their mannitol-positive counterparts. Utilization of sodium acetate by the isolates was in accordance with the standard results for S. flexneri serotype 4. Mannitol-negative serobiotypes of S. flexneri 4a are able to utilize sodium acetate, whereas their mannitol-positive counterparts rarely utilize sodium acetate (8). On the other hand, S. flexneri 4b never utilizes sodium acetate. Edwards and Ewing (8) have shown that 43% of the mannitol-negative and around 8% of the mannitol-positive 4a strains were weakly positive in reaction with sodium acetate upon 2 to 7 days of incubation. In our study we did not get any positive reactions for mannitol-fermenting strains, and all of the non-mannitol-fermenting strains showed strong positive reactions within 48 h. All of the mannitol-negative isolates in the present study (biotype 1) were able to utilize xylose, mannose, and maltose, while the mannitol-positive isolates (biotype 2) were not able to utilize these sugars. Another important distinction between these two biotypes was that biotype 1 was able to produce indole within 24 h whereas strains of biotype 2 failed to produce indole. Arabinose was utilized by all of the strains, but again a slight variation was observed between the two biotypes in terms of incubation time. The mannitol-negative isolates showed a positive reaction after overnight incubation, but the mannitol-positive isolates had to be incubated for more than 3 days for utilization of arabinose. Detailed biochemical studies, particularly of the utilization of mannitol, sodium acetate, and xylose and production of indole, confirmed that all of the isolates belonged to serotype 4 of S. flexneri, but grouping at the subserotype level based on biochemical tests was not possible due to variable reactions. According to Edwards and Ewing (8), 82% of the mannitol-positive and 3% of the mannitol-negative strains of S. flexneri 4a are able to ferment raffinose, but none of the strains in this study showed a positive reaction in raffinose fermentation. An identical pattern was observed among strains of mannitol-positive biotype, which was designated biotype 2. The overall criteria for this biotype did not agree completely with those for any subserotypes of S. flexneri type 4. Among the mannitol-negative strains (biotype 1), variations were observed in some biochemical reactions, which divided them into five subbiotypes, designated 1a to 1e. However, the common characteristics of these biotypes did not correlate with those of any of the subserotypes of S. flexneri type 4.
Since antibiotic resistance is a major phenotypic trait, particularly for clinical isolates, it can potentially be informative in exploring the characteristics of an untypeable Shigella strain. None of the isolates were found to be resistant to mecillinum, nalidixic acid, or ciprofloxacin. Although ciprofloxacin-resistant strains of S. flexneri have not yet been detected, nalidixic acid- and mecillinum-resistant strains of S. flexneri are frequently isolated in Bangladesh (15). Interestingly, 20% of the strains were found to be sensitive to all of the antibiotics commonly used for the treatment of shigellosis. The overall susceptibility patterns of the test strains focus on the fact that the strains were not frequently exposed to expanded- or broad-spectrum antibiotics. Multiple antimicrobial resistance among Shigella isolates is an important problem in developing countries, including Bangladesh. In the present study, about 48% of the strains were resistant to ampicillin, tetracycline, and co-trimoxazole.
Although there is a little information available on the association of plasmid profiles of S. flexneri strains and their serotypes, previously published reports have revealed a heterogeneous plasmid population in strains of S. flexneri, with most plasmids being smaller than 6 MDa (16, 36). The presence of additional plasmids in patterns related to particular serotypes suggests that plasmid profiles may be useful in distinguishing between serotypes of S. flexneri (14). It may also be possible to document the appearance of any new strain in a community by these patterns (14). In the present study, plasmid profiling distinguished the 21 isolates according to their major biotypes. Strains belonging to the mannitol-positive biotype (14.3%) showed an identical plasmid pattern (P2) which could be distinguished from that of the other strains (Table 3). On the other hand, four plasmids of approximately 140, 5.9, 4, and 2.7 MDa were commonly present in all mannitol-negative strains and appear to constitute a stable gene pool (Table 3). In addition, a middle-range plasmid approximately 35 to 62 MDa in size was found in 44.4% of strains with plasmid pattern P1. These additional plasmids along with common plasmids were used to arrange the strains in different plasmid patterns (P1a to P1d). However, plasmid profiles of both patterns of type 4 strains were different from those of the ATCC 4a and NCTC 4b strains of S. flexneri (Table 3). Plasmid profiles are useful tools to characterize multiple antibiotic resistance in different Shigella species. It appears from a previous study that the transferable resistance plasmid is the middle-range plasmid having a molecular mass of between 44 and 76 MDa (13). The present study showed that, 47.1% of the 21 strains were resistant to multiple antibiotics, of which 38% strains harbored the middle-range plasmid. The strong association observed between plasmid profiles and drug resistance patterns suggests that plasmids other than the common plasmids may have epidemiological significance and should be evaluated carefully. To confirm this, conjugation and curing experiments were carried out. Conjugal transfer of these plasmids to an E. coli K-12 strain followed by curing of the plasmid demonstrated that resistance against ampicillin, tetracycline, and trimethoprim-sulfomethoxazole was conferred by the plasmid having a molecular mass in the range between 35 and 62 MDa (Table 4). These plasmids were self-transferable. However, it is interesting that the same resistance pattern in different strains was transferred by plasmids of different molecular masses within the middle range.
Invasiveness is an important property of pathogenic Shigella species. The present study reviewed the invasive characteristics of all of the strains, since these were isolated from clinical cases. All isolates were invasive. Although the cardinal feature in the pathogenesis of S. flexneri infection involves the invasion of epithelial cells, it nevertheless has been reported that S. flexneri also produces an enterotoxin of mainly two types, ShET-1 and ShET-2. In our study, we found that the sen gene (which encodes ShET-2) was present in all of the strains but that the set1 gene (which encodes ShET-1) was absent in all. These findings were essentially similar to the report of Noriega et al. (26) in which the set1 gene was shown to be found almost exclusively in S. flexneri 2a. It is now well documented that the sen gene is located on the 140-MDa invasive plasmid and is present in all strains of S. flexneri which harbor this plasmid (23).
The ribotyping procedure identifies and compares restriction fragments of the chromosomal rDNA region, which includes DNA carrying rRNA genes grouped as operons and flanking DNA regions, after hybridization with rRNA or rDNA probes. rDNA probes are usually developed from a recombinant plasmid in which rrn (rRNA) DNA has been inserted, or in some cases commercially available rDNA probes are used. In this experiment a different procedure was followed, where a synthetic oligonucleotide was prepared from the highly conserved sequence of the 16S rRNA. It was then amplified (320 bp) by PCR and used as probe. Based on the rRNA gene restriction patterns, two different ribotypes (R1 and R2) were found among the 21 atypical strains, which indicated a good correlation with the results of other typing methods described earlier. In fact, the strains belonging to ribotype R2 were the same strains grouped in the mannitol-positive biotype and having an identical plasmid pattern, P2. Strains belonging to ribotype R1 are those grouped in the mannitol-negative biotype and included in plasmid pattern P1 (Table 5). Strains of the mannitol-negative biotype were further divided into several subtypes through biotyping and plasmid profiling, but this subclassification was not reflected in ribotyping. However, the reference strains ATCC 4a and NCTC 4b showed rRNA gene restriction patterns completely different from those of the atypical S. flexneri type 4 strains. Comparison of serotypes and ribotypes showed that different subserotypes belonged to the same ribotype (Fig. 1). It is possible for different strains to have differences in portions of their genomes that encode serotype-specific antigens but to have similarities in other portions of their genomes, e.g., highly conserved rRNA genes. Similar relationships between serotypes and ribotypes have previously been reported for S. flexneri strains isolated in Bangladesh (10). The occurrence of isolates with the same ribotypes but different serotypes can be explained by the fact that the rRNA genes of strains originating from the same ancestral clone were conserved while genes for serotype-specific antigens have undergone changes, since surface characteristics could be under the control of environmental influences (3, 35).
PFGE has been employed to successfully discriminate strains of a variety of bacteria, including S. dysenteriae type 1 (38). A number of previous studies (20, 38) showed that NotI gave the best discrimination among the strains, since it has a long-range DNA cutting site and cuts the DNA infrequently. Hence, this endonuclease was used for typing of all isolates in the present study. Of the 21 atypical strains of S. flexneri type 4, two different types of PFGE patterns (A and B) were obtained. PFGE pattern A included the larger number of strains (85.7%) and corresponded to strains belonging to ribotype R1, which were further divided into 10 closely related subtypes (A1 to A10). PFGE pattern B comprised the remaining three strains (14.3%), with an identical banding pattern, and corresponded to ribotype R2. The most prevalent PFGE pattern was A1, which was observed in 38% of the total isolates. The PFGE banding patterns of the reference strains (i.e., ATCC 4a and NCTC 4b) were completely different from those shown by the atypical strains, suggesting that these are a new subserotype of S. flexneri. Based on the extensive phenotypic and genotypic studies, we suggest that these newly characterized strains of S. flexneri be considered a new subserotype of serotype 4 of S. flexneri.
Acknowledgments
This study was funded by the U.S. Agency for International Development (USAID) under Cooperative Agreement no. HRN-A-00-96-90005-00 and by the ICDDR,B, Centre for Health and Population Research, which is supported by countries and agencies which share its concern for the health problems of developing countries. Current donors providing unrestricted support include the aid agencies of the Governments of Australia, Bangladesh, Belgium, Canada, Japan, The Netherlands, Saudi Arabia, Sweden, Sri Lanka, Switzerland, the United Kingdom, and the United States; international organizations include the United Nations Children's Fund.
REFERENCES
- 1.Albert, M. J., N. A. Bhuiyan, K. A. Talukder, A. S. G. Faruque, S. Nahar, S. M. Faruque, M. Ansaruzzaman, and M. Rahman. 1997. Phenotypic and genotypic changes in Vibrio cholerae 0139 Bengal. J. Clin. Microbiol. 35:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1990. Current protocols in molecular biology, vol. 1. Greene Publishing Associates, New York, N.Y.
- 3.Bradbury, W. C., A. D. Pearson, M. A. Marko, R. V. Congi, and J. L. Penner. 1984. Investigation of a Campylobacter jejuni outbreak by serotyping and chromosomal restriction endonuclease analysis. J. Clin. Microbiol. 19:342-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlin, N. I. A., A. A. Lindberg, K. Bock, and D. R. Bundle. 1984. The Shigella flexneri O-antigenic polysaccharide chain. Nature of the natural repeating unit. Eur. J. Biochem. 139:189-194. [DOI] [PubMed] [Google Scholar]
- 5.Carlin, N. I. A., M. Rahman, D. A. Sack, A. Zaman, B. A. Kay, and A. A. Lindberg. 1989. Use of monoclonal antibodies to type Shigella flexneri in Bangladesh. J. Clin. Microbiol. 27:1163-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coimbra, R. S., P. Lenormand, F. Grimont, P. Bouvet, S. Matsushita, and P. A. D. Grimont. 2001. Molecular and phenotypic characterization of potentially new Shigella dysenteriae serotype. J. Clin. Microbiol. 39:618-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuPont, H. L., M. M. Levine, R. B. Hornick, and S. B. Formal. 1989. Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 159:1126-1128. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, P. R., and W. H. Ewing. 1972. Identification of Enterobacteriaceae, p. 126-131. Burgess Publishing Company, Minneapolis, Minn.
- 9.El-Gendy, A., N. El-Ghorab, E. M. Lane, R. A. Elyazeed, N. I. A. Carlin, M. M. Mitry, B. A. Kay, S. J. Savarino, and L. F. Peruski, Jr. 1999. Identification of Shigella flexneri subserotype 1c in rural Egypt. J. Clin. Microbiol. 37:873-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faruque, S. M., K. Haider, M. M. Rahman, A. R. M. Alim, Q. S. Ahmad, M. J. Albert, and R. B. Sack. 1992. Differentiation of Shigella flexneri strains by rRNA gene restriction patterns. J. Clin. Microbiol. 30:2996-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontaine, A., J. Arondel, and P. J. Sansonetti. 1990. Construction and evaluation of live attenuated vaccine strains of Shigella flexneri and Shigella dysenteriae 1. Res. Microbiol. 141:907-912. [DOI] [PubMed] [Google Scholar]
- 12.Formal, S. B., and E. E. Baker. 1953. Quantitative studies of cross-reactions between Shigella flexneri types 1a, 1b, and 3. J. Immunol. 70:260-266. [PubMed] [Google Scholar]
- 13.Haider, K., M. I. Huq, A. R. Samadi, and K. Ahmed. 1985. Plasmid characterization of Shigella species isolated from children with shigellosis and asymptomatic excretors. J. Antmicrob. Chemother. 16:691-698. [DOI] [PubMed] [Google Scholar]
- 14.Haider, K., M. I. Huq, K. A. Talukder, and Q. S. Ahmed. 1989. Electropherotyping of plasmid DNA of different serotypes of Shigella flexneri isolated in Bangladesh. Epidemiol. Infect. 102:421-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossain, M. A., M. Rahman, Q. S. Ahmed, M. A. Malek, R. B. Sack, and M. J. Albert. 1998. Increasing frequency of mecillinum-resistant Shigella isolates in urban Dhaka and rural Matlab, Bangladesh: a 6 year observation. J. Antimicrob. Chemother. 42:99-102. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson, A. F., D. A. Bremner, P. L. Bergquist, and H. E. Lane. 1979. Characterization of plasmids from antibiotic-resistant Shigella isolates by agarose gel electrophoresis. J. Gen. Microbiol. 113:73-81. [DOI] [PubMed] [Google Scholar]
- 17.Kado, C. L., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmid. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariyama, R., R. Mitsuhata, J. W. Chow, D. B. Clewell, and H. Kumon. 2000. Simple and reliable multiplex PCR assay for surveillance isolates of vancomycin-resistant enterococci. J. Clin. Microbiol. 38:3092-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: Implications for vaccine development and implementation of control strategies. Bull. W. H. O. 77:651-666. [PMC free article] [PubMed] [Google Scholar]
- 20.Mackel, D. C., L. F. Langley, and L. A. Venice. 1961. The use of guinea-pig conjunctivae as an experimental model for the study of virulence of Shigella organisms. Am. J. Hyg. 73:219-223. [DOI] [PubMed] [Google Scholar]
- 21.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 22.Munshi, M. H., D. A. Sack, K. Haider, Z. U. Ahmed, M. M. Rahman, and M. G. Morshed. 1987.. Plasmid mediated resistance to nalidixic acid in Shigella dysenteriae type 1. Lancet ii:419-421. [DOI] [PubMed] [Google Scholar]
- 23.Nataro, J. P., J. A. Serowatana, A. Fasano, D. R. Maneval, L. D. Guers, F. Noriega, F. Dubovsky, M. M. Levine, and J. G. Morris, Jr. 1995. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect. Immun. 63:4721-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 1990. Performance standards for antimicrobial disc susceptibility tests, 4th ed. Approved Standard M2-A5. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 25.Noriega, F. R., J. Y. Wang, G. Losonsky, D. R. Maneval, D. M. Hone, and M. M. Levine. 1994. Construction and characterization of attenuated ΔaroA ΔvirG Shigella flexneri 2a strain CVD 1203, a prototype live oral vaccine. Infect. Immun. 62:5168-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noriega, F. R., F. M. Liao, S. B. Formal, A. Fasano, and M. M. Levine. 1995. Prevalence of Shigella enterotoxin 1 among Shigella clinical isolates of diverse serotypes. J. Infect. Dis. 172:1408-1410. [DOI] [PubMed] [Google Scholar]
- 27.Noriega, F. R., G. Losonsky, C. Lauderbaugh, F. M. Liao, J. Y. Wang, and M. M. Levine. 1996. Engineered ΔguaBA ΔvirG Shigella flexneri 2a strain CVD 1205: construction, safety, immunogenicity, and potential efficacy as a mucosal vaccine. Infect. Immun. 64:3055-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noriega, F. R., F. M. Liao, D. R. Maneval, S. Ren, S. B. Formal, and M. M. Levine. 1999. Strategy for cross-protection among Shigella flexneri serotypes. Infect. Immun. 67:782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada, N., C. Sasakawa, T. Tobe, K. A. Talukder, K. Komatsu, and M. Yoshikawa. 1991. Construction of a physical map of the chromosome of Shigella flexneri 2a and the direct assignment of nine virulence-associated loci identified by Tn5 insertions. Mol. Microbiol. 5:2171-2180. [DOI] [PubMed] [Google Scholar]
- 30.Pryamukhina, N. S., and N. A. Khomenko. 1988. Suggestion to supplement Shigella flexneri classification scheme with the subserovar Shigella flexneri 4c: phenotypic characteristics of strains. J. Clin. Microbiol. 26:1147-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakai, T., C. Sasakawa, S. Makino, K. Kamata, and M. Yoshikawa. 1986. Molecular cloning of a genetic determinant for Congo red binding ability which is essential for the virulence of Shigella flexneri. Infect. Immun. 51:476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasakawa, C., K. Kamata, T. Sakai, S. Y. Murayama, S. Makino, and M. Yoshikawa. 1986. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect. Immun. 51:470-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sereny, B. 1957. Experimental keratoconjunctivitis Shigellosa. Acta Microbiol. Acad. Sci. Hung. 2:293-296. [PubMed] [Google Scholar]
- 34.Simmons, D. A., and E. Romanowska. 1987. Structure and biology of Shigella flexneri O antigens. J. Med. Microbiol. Rev. 23:289-302. [DOI] [PubMed] [Google Scholar]
- 35.Snipes, K. P., L. M. Hansen, and D. C. Hirsh. 1988. Plasma and iron-regulated expression of high molecular weight outer membrane proteins by Pasteurella multocida. Am. J. Vet. Res. 49:1336-1338. [PubMed] [Google Scholar]
- 36.Tacket, C. O., N. Shahid, A. R. Alim, and M. L. Cohen. 1984. Usefulness of plasmid profiles for differentiation of Shigella isolates in Bangladesh. J. Clin. Microbiol. 20:300-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talukder, K. A., D. K. Dutta, and M. J. Albert. 1999. Evaluation of pulsed-field gel electrophoresis for typing of Shigella dysenteriae type 1. J. Med. Microbiol. 48:781-784. [DOI] [PubMed] [Google Scholar]
- 38.Talukder, K. A., D K. Dutta, A. Safa, M. Ansaruzzaman, F. Hassan, K. Alam, K. M. N. Islam, N. I. A. Carlin, G. B. Nair, and D. A. Sack. 2001. Altering trends in the dominance of Shigella flexneri serotypes and emergence of serologically atypical S. flexneri strains in Dhaka, Bangladesh. J. Clin. Microbiol. 39:3757-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vargas, M., J. Gascon, M. T. Jimenez de Anta, and J. Vila. 1999. Prevalence of Shigella enterotoxin 1 and 2 among Shigella strains isolated from patients with traveler's diarrhea. J. Clin. Microbiol. 37:3608-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehler, T., and N. I. A. Carlin. 1988. Structural and immunochemical studies of the lipopolysaccharide from a new provisional serotype of Shigella flexneri. Eur. J. Biochem. 176:471-476. [DOI] [PubMed] [Google Scholar]
- 42.Weissman, J. B., E. J. Gangorosa, A. Schemerler, R. L. Marier, and J. N. Lewis. 1975.. Shigellosis in day-care centres. Lancet i:88-90. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 1987. Programme for control of diarrhoeal disease, p. 9-20. In Manual for laboratory investigation of acute enteric infections. CDD/93.3, rev. 1. World Health Organization, Geneva, Switzerland.
- 44.World Health Organization. 1999. Generic protocol to estimate the burden of Shigella diarrhoea and dysenteric mortality. W. H. O./V and B/ 99:26. [Google Scholar]