Abstract
The World Health Organization (WHO-5) and International Consensus Classification (ICC) acknowledge the poor prognosis of TP53-mutated (TP53mut) myeloid neoplasm (MN). However, there are substantial differences between the two classifications that may lead to under- or overestimation of the prognostic risk. We retrospectively applied WHO-5 and ICC to 603 MN cases harboring TP53mut (variant allele frequency, VAF ≥ 2%). WHO-5 and ICC would not classify 64% and 20% of these cases as TP53mut MN, respectively. Moreover, of those classified, 67.5% would be classified discrepantly. Primary drivers of discrepancies included: (i) prognostic importance of TP53mut acute myeloid leukemia (AML), (ii) interaction of the blast percentage and allelic status, (iii) 17p.13.1 deletion detected by cytogenetics, (iv) complex karyotype (CK) as multi-hit equivalent, and (v) TP53mut VAF threshold, we analyzed survival outcomes of each of these groups with an aim to provide clarity. TP53mut AML was associated with significantly poor survival compared to TP53-wild type TP53wt AML, myelodysplasia-related (AML, MR 4.7 vs. 18.3 months; P < 0.0001), supporting its inclusion within TP53mut MN as a distinct subentity. Secondly, the survival of TP53mut with blast 10–19% was poor regardless of the allelic status. Thirdly, for cases with a single TP53mut with VAF < 50%, 17p13.1 del or CK serve as practical surrogates of biallelic inactivation, obviating the need for an additional copy number analysis. Finally, TP53mut AML, MDS multi-hit/multi-hit equivalent with VAF < 10% had significantly poorer survival compared to TP53mut MDS VAF < 10% without CK and 17p del, and were comparable to those with VAF ≥ 10% (14.1 vs. 48.8 vs.7.8 months, P < 0.0001). Collectively, these findings address key areas of contention and provide valuable insights that will guide future revisions of the WHO and ICC classifications.
Subject terms: Acute myeloid leukaemia, Cancer genomics, Myelodysplastic syndrome
Introduction
TP53 mutations (TP53mut) are observed in 5–13% of de novo myelodysplastic syndrome (MDS) [1–6] and 8–11% of acute myeloid leukemia (AML) cases [7, 8]. These mutations are particularly enriched in myeloid neoplasm (MN) following cytotoxic therapies (30–35%) [9, 10], and older patients with MDS and AML (20–40%) [11, 12], being associated with extremely poor survival with limited therapeutic options in contemporary clinical practice [7, 13, 14]. Recognizing the poor prognosis, the World Health Organization (WHO) classifications (5th edition, hereafter referred to as WHO-5) [15] and the International Consensus Classification (ICC) [16] recognized TP53mut MDS and MN as distinct entities, respectively.
Both classification systems define an MDS biallelic TP53 alteration as a distinct subgroup. However, there are notable differences between the two classifications (Table 1). For example, the ICC recognizes TP53mut AML as a distinct entity, whereas WHO-5 does not. Both classifications acknowledge the poor prognosis associated with MDS with TP53 biallelic inactivation; however, they differ in their emphasis on blast percentage. The ICC prioritizes allelic status and blast percentage in risk stratification, while WHO-5 defines biallelic TP53 inactivation as a homogenous category with poor survival regardless of the blast percentage (0–19%). The ICC requires a VAF of ≥10% for TP53mut, whereas the WHO-5 does not specify a VAF threshold. In cases with a single TP53mut with VAF < 50%, complex karyotype (CK) is considered as multi-hit equivalent by ICC but not WHO-5.
Table 1.
Areas of discrepancy between the 5th edition of the World Health Organization (WHO-5) and the International Consensus Classifications (ICC).
| No. | Area of discrepancy | WHO-5 (MDS with biallelic TP53 inactivation) | ICC (Myeloid neoplasms with mutated TP53) | Evidence presented | Conclusion |
|---|---|---|---|---|---|
| 1 | Acute myeloid leukemia (AML) | No separate category, most cases would be classified as AML-MR | Separate category (BM/PB blasts ≥20%), includes AEL | Survival of TP53mut AML is significantly poorer compared to TP53wt AML-MR (4.7 vs. 18.3 months; P < 0.0001). | TP53mut AML should be included in TP53mut MN |
| 2 | Interaction of blast percentage category with allelic status | Requires demonstration of biallelic loss for 0–19% blasts | Requires demonstration of biallelic loss for 0–9% blasts | Survival of monoallelic TP53mut MDS with 10–19% blasts is comparable to biallelic loss. | Monoallelic TP53mut MDS with 10–19% blasts should be considered TP53mut MN |
| 3 | Confirmation of 17p13.1 deletion detected on karyotype by CNV analysis | Requires confirmation of loss of the TP53 locus detected on the karyotype with an additional CNA method | Does not require confirmation of 17p13.1 deletion |
Cases with single TP53mut (VAF < 50%) with 17p13.1 deletion on karyotype: CNV analysis verified the LOH across the TP53 locus in 94% of evaluable cases. Cases with single TP53mut (VAF < 50%) without 17p13.1 deletion on karyotype: • CNV analysis identified LOH/cnLOH in 26.9% of evaluable cases. • Importantly, all cases with LOH/cnLOH across the TP53 locus on CNV analysis had CK. • Conversely, no LOH/cnLOH was observed in cases with or without CK |
In a single TP53mut with VAF < 50%, confirmation of 17p deletion with an additional CNV is not necessary |
| 4 | CK as a multi-hit equivalent | CK is not considered a multi-hit equivalent | CK is considered a multi-hit equivalent | Survival of single TP53mut VAF < 50% with CK was comparable to those with 17p deletion on karyotype (10.4 vs. 11.0 months; P = 0.39) and poorer than monoallelic TP53mut without 17p loss or CK (33.4 months; P < 0.0001) | In single TP53mut with VAF < 50%, CK should be considered multi-hit equivalent |
| 5 | VAF threshold | None | VAF ≥ 10% | Survival of TP53mut ‘multi-hit’ VAF 2 to <10% was shorter compared to that of ‘single-hit’ TP53mut VAF < 10%, but comparable to VAF ≥ 10% (14.1 vs. 48.8 vs. 7.8 months, P < 0.0001). | ‘Multi-hit’ TP53mut with VAF 2 to <10% should be included in TP53mut MN |
AEL acute erythroid leukemia, AML acute myeloid leukemia, AML-MR AML with myelodysplasia-related changes, BM bone marrow, CK complex karyotype, CNA copy number analysis, ICC International Consensus Classification, MDS myelodysplastic syndrome, MN myeloid neoplasm, PB peripheral blast, t-MN therapy-related myeloid neoplasm, TP53mut TP53 mutated, TP53mut TP53 mutated, WHO-5 5th edition of the WHO classification, VAF variant allele frequency.
As both classifications are increasingly being used to govern clinical practice, the differences between the two classifications can lead to either under- or overestimation of the prognostic risk, which may translate into inconsistencies in treatment decisions. The lack of consensus likely stems from limited studies that integrate morphological, allelic status, blood and bone marrow blast percentage and genetic features.
In this study, we validated both classifications in our large international cohort with well-annotated clinical data and analyzed how the WHO-5 and ICC criteria impact classification and clinical practice. We also evaluated the factors contributing to the differences between these two classification systems. Finally, we provide evidence that can be used for the revision of both classifications and/or developing a consensus uniform classification system.
Methods
We analyzed TP53mut (VAF ≥ 2%, n = 603) MDS and AML patients managed at the Mayo Clinic (USA) and South Australia Health Network (Australia) between February 2002 and August 2024. Data were obtained with informed consent or appropriate consent waiver, in accordance with the Declaration of Helsinki and approved by the relevant Ethics Committee (HREC/15/RAH/496 for South Australia Health Network and IRB# 19-007595/19-007568 for Mayo Clinic).
TP53mut was identified by next-generation sequencing (NGS) panels that covered at least exons 4–11. TP53mut MDS and AML were retrospectively classified using the ICC [16] and WHO-5 [15] classifications (please refer to supplementary section for details), and their outcome was compared with TP53 wild type (TP53wt) MN (n = 600).
In the WHO-5, MDS with biallelic TP53 loss is defined by the presence of [1] two TP53mut; [2] the presence of a single TP53mut VAF < 50% with a 17p loss or copy-neutral loss of heterozygosity (cnLOH) verified by CNV analysis. One TP53 mutation with VAF ≥ 50%, without 17p loss or cnLOH, is considered presumptive evidence of biallelic TP53 inactivation. Conversely, a single TP53mut with VAF < 50% without 17p loss or cnLOH is defined as monoallelic TP53 inactivation. WHO-5 mandates the confirmation of 17p loss detected on metaphase karyotype by copy number variation (CNV) analysis, such as fluorescence in situ hybridization (FISH) and/or array techniques (single-nucleotide polymorphism arrays) or NGS.
According to the ICC, multi-hit TP53 inactivation for MDS is defined as [1] two TP53mut (each with VAF ≥ 10%), or [2] single TP53mut (VAF 10%) in combination with either (i) deletion of 17p13.1 causing loss of heterozygosity (LOH), (ii) confirmed copy neutral LOH (cnLOH), (iii) presumed cnLOH based on mutation VAF ≥ 50%, or (iv) a complex karyotype. Single hit status is defined as the presence of a deleterious TP53mut not meeting the multi-hit criteria. The ICC mandates multi-hit TP53 inactivation for MDS 0–9% blasts for inclusion as a TP53mut MN, while TP53mut MDS/AML (10–19% blasts) or AML (≥20% blasts) are included regardless of the hit-status.
Statistical methods
Comparisons were performed using the Mann–Whitney U-test for non-normally distributed variables. Fisher’s exact test was used to determine associations between categorical variables. Wilcoxon rank-sum test or Student’s t test was used to compare continuous variables. All statistical tests were two-sided. Overall survival (OS) was calculated from the date of MN diagnosis to the last follow-up or the date of death. Patients alive at the last follow-up date were censored. Kaplan–Meier estimations were used with comparisons using log-rank tests. P values < 0.05 were considered statistically significant. All statistical analyses were conducted using the GraphPad and R statistical platform (https://www.r-project.org/) v.4.1.1.
Results
Study population and characteristics
The majority of the TP53mut MN (n = 603) were MDS (n = 374, 62.0%) followed by AML (n = 229, 38.0%). The median age at diagnosis was 69.0 years (interquartile range, IQR 62.0, 75.0) and 63.4% (n = 382) were male (Table 2).
Table 2.
Clinical characteristics of TP53mut MDS and AML cohort.
| Variables | Overall (n = 603) |
|---|---|
| Female/male | 221 (36.7%)/382 (63.3%) |
| Age at MN diagnosis, median [IQR] | 68.60 [62.00, 75.00] |
| Blood counts and marrow blast, median [IQR] | |
| Hemoglobin, g/L | 9.00 [7.90, 10.50] |
| WBC | 3.00 [1.80, 5.10] |
| Platelets | 55.00 [30.00, 106.00] |
| PB blasts % | 1.00 [0.00, 7.00] |
| BM blasts % | 8.90 [2.50, 27.25] |
| Disease phenotype (%) | |
| AML | 229 (38%) |
| MDS | 374 (62%) |
| Cytogenetic changes, n (%)a | |
| Complex karyotype | 484 (80.7%) |
| Monosomal karyotype | 437 (72.8%) |
| Deletion 5q | 441 (73.9%) |
| Deletion 7q | 306 (51.3%) |
| Deletion 17p | 240 (40.2%) |
| TP53 mutation characteristics | |
| TP53mut VAF (median, IQR) | 36.80% (19.6, 59.0) |
| TP53mut VAF ≥ 2% | 603 (100%) |
| TP53mut VAF ≥ 10% | 520 (86.2%) |
| TP53mut VAF < 10% | 83 (13.8%) |
| Somatic co-mutations on NGS, n (%) | |
| TET2 | 66 (10.9%) |
| DNMT3A | 63 (10.4%) |
| ASXL1 | 48 (8.0%) |
| SF3B1 | 33 (5.5%) |
| RUNX1 | 29 (4.8%) |
| SRSF2 | 29 (4.8%) |
| JAK2 | 27 (4.5%) |
| U2AF1 | 25 (4.1%) |
| RAS | 20 (3.3%) |
| EZH2 | 16 (2.7%) |
| BCOR | 15 (2.5%) |
| IDH2 | 12 (2.0%) |
| PTPN11 | 11 (1.8%) |
| CBL | 7 (1.2%) |
| CEBPAb | 7 (1.2%) |
| ZRSR2 | 6 (1.0%) |
| First line therapy n (%)c | |
| Hypomethylating agents | 214 (36.4%) |
| Venetoclax-based therapies | 111 (18.9%) |
| Allogeneic stem cell transplantation | 99 (16.4%) |
| Intensive chemotherapy | 96 (16.3%) |
| Lenalidomide | 20 (3.4%) |
| Erythropoietin/Erythroid differentiation agents | 19 (3.2%) |
| Other therapies | 9 (1.5%) |
| Supportive therapy | 111 (18.9%) |
AML acute myeloid leukemia, BM bone marrow, MDS myelodysplastic syndrome, MN myeloid neoplasm, NGS next generation sequencing, PB peripheral blood, VAF variant allele frequency, WBC white blood cell.
aCytogenetics data was not available in three patients.
bCEBPA mutations were not screened for in five patients.
cFirst-line treatment data were not available in 15 patients.
Compared to TP53 wild type (TP53wt), the median OS of TP53mut MN was poor independent of CK and blast categories (Supplementary Fig. 1A). In TP53mut cohort, allogeneic stem cell transplant was associated with longer OS compared to patients treated with intensive chemotherapy or hypomethylating agents with or without venetoclax (Supplementary Fig. 1B).
The majority of MDS and AML harboring TP53mut are NOT acknowledged as TP53mut MN by the WHO-5 classification
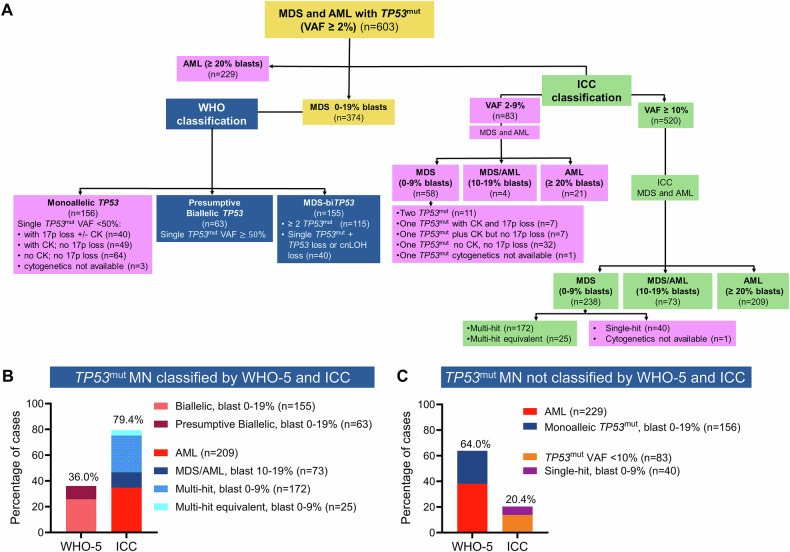
According to WHO-5 criteria, 155 MDS (25.7%) were classified as MDS-biTP53 as they harbored 2 TP53mut (n = 115) or 1 TP53mut plus confirmed LOH or cnLOH of the TP53 locus (n = 40). Additionally, 63 (10.4%) TP53mut MDS with VAF 50% were classified as presumptive MDS-biTP53 (Fig. 1A and Supplementary Fig. 2). In MDS with a single TP53mut with VAF < 50%, verification of loss of heterozygosity (LOH) or copy-neutral LOH (cnLOH) of the TP53 locus by CNV analysis is mandatory for defining MDS-biTP53 inactivation. In our cohort, 135 (22.4%) cases with single TP53mut with VAF 50% did not have CNV analysis and thus could not be classified as MDS-biTP53. Moreover, WHO-5 does not classify TP53mut AML as a distinct entity. Hence, 229 (38.0%) AML with TP53mut were grouped with other AML. In summary, applying WHO-5 would classify only 217 (36%) of 603 as TP53mut MN, while 386 (64.0%) were excluded (Fig. 1A–C).
Fig. 1. TP53-mutated (TP53mut) myeloid neoplasm (MN) classified by World Health Organization 5th edition (WHO-5) and International Consensus Classification (ICC).
A Consort diagram summarizing the classification of TP53mut MN by WHO-5 (blue) and ICC (green) criteria, along with disease subcategories presently excluded from each classification system, respectively (purple). B Percentage of MN with TP53mut that can be classified, and C not classified using WHO-5 and ICC criteria.
The majority of MDS and AML harboring TP53mut are included in the ICC classification of TP53mut myeloid neoplasm
We next performed a similar analysis using the ICC criteria. ICC mandates TP53mut VAF 10% and classifies TP53mut MN (n = 520) into three subcategories: multi-hit TP53mut MDS with BM and blood blast 0–9% (n = 238) as well as MDS/AML (BM or blood blasts 10–19%; n = 73), or AML (BM or blood blast 20%; n = 209) regardless of their allelic status. In the absence of comprehensive CNV analysis, CK is considered a multi-hit equivalent. Collectively, 479 (79.4%) MDS and AML with TP53mut were classified as “TP53-mutated MN”. One-hundred twenty-three (20.4%) cases with TP53mut VAF < 10% (n = 83) or single-hit MDS with blast 0–9% (n = 40) were classified along with other myeloid neoplasms (Fig. 1A–C and Supplementary Fig. 3A, B).
TP53mut MDS and AML cases classified differently by WHO-5 and ICC
We next focused on TP53mut MDS and AML, that are classified differently by the two classifications. TP53mut AML with VAF 10% (n = 209) were included in the ICC but not in WHO-5 as a distinct entity. Furthermore, 188 (50.4%) of 373 TP53mut MDS cases were classified differently by ICC and WHO-5. Collectively, 407 (67.5%) cases were classified discrepantly between the two classifications (Fig. 2A).
Fig. 2. Concordance and divergence in the WHO-5 and ICC classification of TP53-mutated (TP53mut) myeloid neoplasm (MN).
A Sankey plot depicting the divergences between the two classifications of TP53mut MN. B heterogeneity in the overall survival (OS) of WHO-5 classified monoallelic TP53 when reclassified using ICC criteria. C According to WHO-5, 90.9% of TP53mut acute myeloid leukemia (AML) were classified as AML-myelodysplasia related (MR), followed by acute erythroid leukemia (AEL, 7%). D OS of AML with TP53mut with variant allele frequency (VAF) ≥ 10% was significantly poorer compared to AML TP53mut with VAF < 10%; and E OS of TP53mut AML was significantly worse than TP53 wild type (TP53wt) AML-MR.
Monoallelic MDS are excluded from the WHO-5 category of TP53mut MDS, as their survival is considered comparable to TP53wt MN. We analyzed WHO-5 defined 155 monoallelic TP53mut using the ICC criteria and observed a significant spread: 49 (31.6%) had TP53mut VAF < 10% and did not meet the ICC criteria for inclusion as TP53mut MN; whereas 36 (23.2%), 22 (14.2%), 25 (16.1%), and 22 (14.2%) cases were classified as single-hit, multi-hit, multi-hit equivalent, and MDS/AML, respectively (Fig. 2A, B). Cytogenetic data were not available in one patient, limiting their accurate placement.
Importantly, there were significant differences in survival between these groups (P = 0.001, Fig. 2B), demonstrating heterogeneity in cases classified as monoallelic TP53 loss as defined by WHO-5. Implementing the WHO-5 definition of monoallelic TP53mut MDS would underestimate the poor prognosis of 69 (52.1%) of the 155 cases (11.4% of the entire cohort). Similarly, median OS of WHO-5 classified biallelic/presumptive biallelic MDS was significantly different when reclassified according to ICC criteria (Supplementary Fig. 4A).
Factors driving differences in the WHO-5 and ICC of TP53mut MDS and AML
As the discordance between the two classifications was driven by differences in the prognostic significance of: (i) TP53mut AML; (ii) blood and bone marrow blast cut-off in MDS; (iii) 17p.13.1 deletion detected by cytogenetics; (iv) CK detected on metaphase cytogenetics; (v) TP53mut VAF cut-off, we analyzed survival outcomes of each of these groups with an aim to provide clarity.
Prognostic significance of TP53mut AML
AML contributed to 38.0% (n = 229) of the cohort. Considering the extremely poor prognosis, ICC includes TP53mut AML with VAF 10% (n = 209), as a distinct entity in TP53mut MN, regardless of allelic status; whereas TP53mut AML with VAF < 10% (n = 20) were excluded from the ICC (Fig. 2C). Within our cohort, the median OS of TP53mut AML VAF ≥ 10% was 4.2 months compared to 14.4 months for AML with TP53mut VAF < 10% (Fig. 2D).
On the other hand, WHO-5 classifies TP53mut AML with other AML categories and suggests further evidence is required for determining that biTP53 status is per se as ‘AML-defining’. Hence, applying the WHO-5 criteria, 209 (90.9%) of TP53mut AML were classified as AML-myelodysplasia related (AML-MR), 16 (7%) as acute erythroid leukemia (AEL), and 2 (0.9%) as AML with maturation. Additionally, three cases were classified as AML without maturation (n = 1, 0.4%), AML with minimal differentiation (n = 1, 0.4%), and AML with KMT2A-rearrangement (n = 1, 0.4%) (Fig. 2A, C).
As the vast majority of TP53mut AML would be re-classified as AML-MR according to WHO-5, we compared the survival of TP53mut AML with TP53wt AML-MR. The median survival of TP53mut AML was significantly poorer compared to TP53wt AML-MR (4.7 vs. 18.3 months; P < 0.0001) (Fig. 2E). Collectively, these results indicate that TP53mut AML is a genetically defined subentity with extremely poor survival.
Prognostic significance of the blast percentage cutoffs in MDS
Another driver of divergence between the two classifications is the bone marrow and/or PB blasts cut-off. The underlying assumption by the WHO-5 is that the prognosis of biTP53mut MDS is homogenously poor irrespective of blast percentages between 0 and 19%. Conversely, the survival of monoallelic TP53mut MDS, regardless of the blast category, was considered comparable to TP53wt MDS and therefore, excluded from MDS with biTP53mut. In contrast, the ICC acknowledges the importance of the allelic status in the context of specific blast cut-offs. For example, it mandates multi-hit status for MDS with blast 0–9%, but not for MDS/AML and AML, for inclusion in TP53mut MN.
We evaluated whether the blast cut-off retains significance when the WHO-5 criteria of the allelic status were used. There was no significant OS difference between biallelic and presumptive biallelic TP53mut, hence the subgroups were combined during further analysis (Supplementary Fig. 4B). Median OS of biallelic and presumptive biallelic TP53-inactivation was significantly shorter for BM or blood blast 10–19% compared to 0–9% (7.4 vs. 12.1 months, P = 0.001) (Fig. 3A). Similarly, the median OS of monoallelic TP53mut was significantly poor in cases with BM or blood blast 10–19% compared to 0–9% (6.8 vs. 16.2 months, P = 0.01) (Fig. 3B), while median OS of monoallelic and biallelic inactivation was comparable in cases with blood or bone marrow blast 10–19% (6.8 vs. 7.4 months; P = 0.17) (Fig. 3C). However, we did observe significant survival difference between biallelic inactivation and monoallelic mutations in cases with blast 0–9% (12.1 vs. 16.2 months; P < 0.0001) (Fig. 3D). Collectively, the results provide compelling evidence that blast percentage remains an important predictor of poor outcome in addition to allelic status.
Fig. 3. Interaction between blast cut-off and WHO-5 allelic status of TP53-mutated myelodysplastic syndrome (MDS).
A Within the WHO-5 biallelic/presumptive biallelic inactivation group, the median overall survival (OS) of MDS with 10–19% blasts was significantly poorer compared to MDS 0–9% blasts. B Within the WHO-5 monoallelic group, the median OS of MDS 10–19% blasts was significantly poorer compared to MDS 0–9% blasts. C In the MDS 10–19% blast group, the median OS of biallelic/presumptive biallelic inactivation was comparable to that of monoallelic mutations. D While in the MDS 0–9% blast group, the median OS of biallelic/presumptive biallelic inactivation was significantly poorer compared to monoallelic mutations.
We next characterized the cytogenetic profile and outcome of TP53mut MN using ICC criteria. TP53mut with VAF ≥ 10% (n = 520) cases included 238 (39.5%) of MDS (BM blasts 0–9%), 73 MDS/AML (12.1%), and 209 AML (34.7%). Furthermore, the impact of blast percentage is also reflected in the corroborating genomic aberrations seen with the various subgroups, along with survival outcomes. For example, poor-risk cytogenetic features such as CK, MK, 17p del, and multi-hit status were enriched in MDS/AML and AML compared to MDS (BM blasts 0–9%) (Fig. 4A, B). Similarly, TP53mut VAF was higher MDS/AML and AML compared to MDS (Fig. 4C). The median OS of TP53mut MDS/AML and AML was significantly poorer compared to TP53mut MDS (BM blasts 0–9%) (4.2 vs. 7.4 vs. 12.3 months; P < 0.0001) (Fig. 4D). Consistent with prior reports, the median OS of multi-hit MDS (BM blasts 0–9%) was significantly poorer compared to single hit (11.6 vs. 25.4 months, P = 0.007, Fig. 4E), whilst the survival of TP53mut AML was poor regardless of allelic status (Fig. 4F). Furthermore, across the blast percentage categories, survival was comparable between multi-hit equivalent and those with multi-hit status (Fig. 4E–G). Collectively, multi-hit or multi-hit equivalent MN had poor survival in cases with TP53mut VAF ≥ 10%.
Fig. 4. Interactions between blast percentage, TP53 mutation (TP53mut) variant allele frequency (VAF), and allelic status based on the ICC criteria and survival of TP53mut myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
A Adverse risk cytogenetic features. BTP53mut multi-hit and multi-hit equivalent. C Higher TP53mut VAF were more prevalent in TP53mut MDS 10–19% blasts and AML compared to MDS 0–9% blasts. D overall survival (OS) of TP53mut MDS 10–19% blast and AML was significantly poorer compared to MDS 0–9% blasts. Overall survival of patients with a marrow/blood blast count of E 0–9%, F ≥20%, and G 10–19% according to allelic status.
Verification of 17p13.1 loss detected on cytogenetics by CNV analysis
In cases with a single TP53mut with VAF < 50%, WHO-5 mandates confirmation of 17p13.1 deletion by an additional CNV analysis method (e.g., FISH, SNP, or NGS), and mere detection of 17p13.1 deletion on karyotype is not sufficient to demonstrate inactivation of the other allele [15]. However, in the absence of confirmatory testing, no explicit guidelines surrounding the further classification of these cases have been provided. Conversely, the ICC does not mandate such confirmation, and both classifications do not elaborate on the need for CNV analysis in the context of a single TP53mut with VAF < 50% without 17p13.1 deletion on karyotype.
Metaphase karyotype was available in 600 (99.5%) and TP53 CNV data were available in 138 (22.9%) cases. Conventional karyotype detected loss at the TP53 locus in 240 (40.2%) cases, and 83 (34.6%) of these had CNV analysis, which confirmed LOH in 78 (94%) of the evaluable cases. Furthermore, CNV analysis detected LOH or copy neutral LOH (cnLOH) in 14 (26.9%) of 52 evaluable cases without 17p deletion on karyotype (Fig. 5A). Importantly, in cases without 17p13.1 deletion on karyotype, striking enrichment of LOH/cnLOH (by CNV analysis) was detected in cases with CK compared to cases without CK (44.4% vs. 0%; P = 0.0007) (Fig. 5B). At the same time, all 14 cases with LOH/cnLOH across TP53 locus on CNV, without 17p del on karyotype, had CK.
Fig. 5. Validation and prognostic significance of 17p13.1 deletion and complex karyotype (CK) in TP53-mutated (TP53mut) myeloid neoplasm (MN).
A Copy number variation (CNV) analysis confirmed 17p13.1 deletion in 94% of cases where it was observed on metaphase cytogenetics. Additionally, CNV analysis identified loss of heterozygosity (LOH) or copy-neutral LOH (CnLOH) across the TP53 locus in 26.9% of cases without 17p13.1 deletion on metaphase cytogenetics. B In cases without 17p13.1 deletion detected on karyotype, LOH/CnLOH was significantly enriched in the presence of CK compared to those without CK. C Median overall survival (OS) of patients with a single TP53mut with variant allele frequency (VAF) < 50% with either 17p13.1 deletion or CK was comparable to cases with biallelic/presumptive biallelic TP53 inactivation.
The sensitivity, specificity, positive and negative predictive value of metaphase cytogenetics to detect loss across the TP53 locus were 84.8% (95% CI 75.8–91.4%), 88.4% (74.9–96.1%), 94.0%, and 73.1%, respectively. Collectively, these results suggest that validation of LOH across the TP53 locus by CNV analysis may not be required in the majority of cases with 17p13.1 deletion detected on metaphase karyotype, except complex rearrangements including derivative chromosomes. In cases without 17p del on karyotype, CK can be a reasonable surrogate marker, as none of the cases without CK and 44.4% cases with CK had LOH/CnLOH across the TP53 locus.
Next, we assessed the number of biallelic inactivations that would be underestimated in our MDS cohort in the absence of comprehensive CNV analysis. Of the 374 MDS cases, 218 (58.2%) cases had 2 TP53mut with each VAF 2% (n = 115), single TP53mut plus LOH or cnLOH across TP53 locus (n = 40) or one mutation with VAF 50% (n = 63) and were hence able to be classified as biallelic or presumptive biallelic inactivation, respectively (Supplementary Fig. 2). The remaining 178 (47.6%) MDS had single TP53mut with VAF < 50%. Fifty-nine cases had 17p loss on metaphase karyotype, and CNV method confirmed LOH across the TP53 locus in 91% (n = 20) of 22 evaluable cases. Of the 116 (31.1%) cases with single TP53mut VAF < 50% without 17p13.1 loss on metaphase karyotype, CNV information was available in 20 cases, and 3 (15%) cases had LOH (n = 1) or cnLOH (n = 2). Importantly, LOH/cnLOH detected by CNV analysis was enriched in cases with single TP53mut VAF < 50% with CK without 17p13.1 loss on karyotype compared to their counterparts without CK (33.3% vs. 0%; P = 0.07). In summary, in the absence of comprehensive CNV data, biallelic inactivation would have been missed in 14 cases with single TP53mut VAF < 50% with CK but without 17p loss, which accounts for 4.0% of the total 374 MDS cases. However, the absence of this information is less likely to underestimate the poor prognosis of these cases, as shown below, as the median OS of single TP53mut VAF < 50% with CK is poor and comparable to biallelic inactivation.
Prognostic significance of 17p loss and CK detected on metaphase cytogenetics
Of the 178 MDS with TP53mut VAF < 50%, 37 (20.8%) had 17p loss on karyotype, and the median OS of these cases was comparable to biallelic or presumed biallelic TP53 loss (11.0 vs. 9.9 months; P = 0.63) but significantly poorer than those with monoallelic TP53mut VAF < 50% without 17p loss and/or CK on metaphase cytogenetics (9.9 vs. 33.4 months; P < 0.0001) (Fig. 5C). Furthermore, 52 (29.2%) cases had CK without 17p loss on karyotype analysis, while 64 (40.0%) cases did not exhibit either abnormality. The median OS of single TP53mut VAF < 50% with CK without 17p loss (n = 52) was comparable to those with 17p13.1 deletion on karyotype (10.4 vs. 11.0 months; P = 0.39) but significantly poorer than those cases with monoallelic TP53mut without 17p loss or CK (10.4 vs. 33.4 months; P < 0.0001) (Fig. 5C). Collectively, these results indicate that in MDS cases with single TP53mut VAF < 50%, the presence of CK can be considered a practical surrogate for biallelic TP53 inactivation.
Differences in classification based on TP53mut VAF cut-off
The ICC mandates the inclusion of TP53mut with VAF 10% into the category of TP53mut MN, whereas the WHO-5 does not have a similar threshold. Within our cohort, 83 (13.8%) of 603 TP53mut MDS and AML had TP53mut VAF < 10% and were not classified as TP53mut MN by ICC (Fig. 1A). These 83 cases included AML (n = 21), MDS/AML (n = 4) and MDS with 0–9% blasts (n = 58), which are currently classified by ICC as AML-MR (n = 20), MDS-EB (n = 14), MDS not otherwise specified (NOS) multilineage dysplasia (MLD, n = 14), MDS NOS single lineage dysplasia (SLD, n = 9), MDS del 5q (n = 6), MDS SF3B1 (n = 7) and clonal cytopenia of undetermined significance (CCUS) (n = 6).
The median OS of TP53mut AML, MDS with 2 TP53mut or single TP53mut plus 17p13.1 deletion and/or CK (n = 50) was significantly shorter compared to that of single TP53mut VAF < 10% without CK and 17p loss (n = 33) and was comparable to TP53mut VAF ≥ 10% (14.1 vs. 48.8 vs. 7.8 months, P < 0.0001) (Fig. 6A), whereas the median OS of monoallelic TP53mut VAF < 10% without CK was comparable to TP53wt MDS (Fig. 6B).
Fig. 6. The median OS of biallelic or single TP53mut VAF < 10% plus CK was significantly shorter compared to that of single TP53mut VAF < 10% without CK and was comparable to TP53mut VAF ≥ 10%.

A The median OS of AML or multi-hit/multi-hit equivalent MDS was significantly worse compared to MDS cases with a single TP53mut VAF < 10% without 17p loss or CK, and was comparable to TP53mut VAF ≥ 10%. B In a TP53mut VAF < 10% MDS, the median OS of biallelic or monoallelic with CK was significantly poor. While the median OS of monoallelic TP53mut VAF < 10% without CK was comparable to TP53 wild type (TP53wt) MDS.
A recent study suggested that a TP53mut VAF 20% is associated with poor outcome and may be considered as presumptive biallelic [17]. In line with the literature, the median OS of TP53mut VAF 20% was significantly poorer compared to TP53mut VAF < 20% (7.6 vs. 14.4 months; P < 0.0001) (Supplementary Fig. 5A). However, VAF 20% should not be equated with biallelic equivalent due to the complex interaction of VAF, allelic status, blast percentage, and CK.
For cases with biallelic and monoallelic TP53mut plus CK, the median OS was poor regardless of whether the VAF was 20% or <20% (9.6 vs. 14.1 months; P = 0.01), although it was shorter in the VAF 20% group. In contrast, for monoallelic TP53mut without CK, the median OS was significantly longer regardless of VAF 20% vs. < 20% (33.4 vs. 37.3 months; P = 0.64) (Supplementary Fig. 5B). This highlights that biallelic status remains a key driver of poor prognosis, irrespective of VAF.
The poor prognosis of VAF 20% is primarily due to the enrichment of MDS-EB2, AML, CK, biallelic, and monoallelic inactivation with CK (Supplementary Fig. 5C–E). However, monoallelic TP53mut without CK and VAF 20% are associated with longer survival. Therefore, considering TP53mut VAF 20% as equivalent to biallelic inactivation could overestimate the poor prognosis of monoallelic mutation without CK.
Discussion
The classification of TP53mut MN is aimed at improving diagnostic and prognostic accuracy, guiding therapeutic decision-making, advancing research, and facilitating clinical trial design and enrollment. However, discrepancies between classifications can delay diagnosis, lead to divergent treatment approaches, and hinder drug development.
We systematically investigated discordances between the two classifications and provided novel evidence to refine subsequent iterations. The urgency of such an effort is evident given that 64% of TP53mut MN cases were not classified as such by WHO-5, compared to 20% by ICC. Additionally, 67.5% of TP53mut MN cases, including 50% of MDS, were classified differently by the two systems.
Our analysis highlights that the clinical significance of biallelic TP53 inactivation is highly context-dependent and influenced by blast percentage. The discrepancy between WHO-5 and ICC emphasizes this issue: the former considers MDS with 0–19% BM/PB blasts as a homogenous group, mandating the demonstration of biallelic TP53 inactivation regardless of blast percentage. In contrast, ICC mandates the evidence of multi-hit TP53 loss for cases with 0–9% blasts, but single TP53mut VAF ≥ 10% is sufficient to designate MDS/AML and AML as TP53mut MN. First, we show that the survival of the 10–19% blast subset was poor regardless of allelic status; whereas the survival of biallelic TP53mut MDS with 0–9% blasts was significantly poorer compared to the monoallelic cases. These findings follow recent reports from our [18] and other groups [3] that the 0–9% blast category is not homogenous and MDS-low blast (MDS-LB, <2% blood blast and <5% BM blast) exhibit distinct biological and clinical characteristics compared to MDS-excess blast 1 (MDS-EB1, 2–4% blood blast and 5–9% BM blast) [18] and the significance of biallelic inactivation was restricted to MDS-LB but not for MDS-EB1 or MDS-EB2. Furthermore, MDS-EB1 and -EB2 with VAF ≥ 10% had comparable survival outcomes, regardless of allelic status. Similarly, Stengel et al. also observed that MDS with <5% blasts differed biologically and prognostically from cases with ≥5% to <10% blasts, ≥10% to <20% blasts, and AML. Specifically, TP53 single-hit cases were more common in the blast <5% group, while TP53 double-hit cases dominated the >5% blast groups, with frequencies of 67%, 91% and 71% in the ≥5% to 9%, and ≥10% to 19%, and AML categories, respectively [3]. In contrast to their prior report, Bernard et al. [2] recently reported that the TP53mut cases can be further stratified by blast categories with a significant OS difference. In their cohort, the median OS was 1.4, 0.8, and 0.7 years in cases with 0–5%, 5–10%, and 10–20% bone marrow blasts, respectively [19]. However, the International Consortium for MDS and the TITAN study proposed a single group of biallelic TP53mut regardless of blast categories (bone marrow blast <20%) [20, 21]. In summary, the prognostic implication of blast percentage remains an area of active research.
In the current form, the WHO-5 may underestimate the poor prognosis of monoallelic TP53mut cases with 10–19% blasts. Consequently, such patients may be denied potentially curative therapies, such as allogeneic stem cell transplant, as they are misclassified as having median OS comparable to TP53wt cases. It further follows that clinical trials evaluating novel therapeutics should continue to stratify patients based on blast percentage categories rather than handling them as a single group. For instance, achieving a median OS of 12–14 months, which may represent a substantial improvement for patients with 10–19% blasts, with current predicted survival of ~7.5 months, but not for patients with 0–9% blasts with predicted survival of ~12 months.
TP53mut AML is frequently associated with adverse cytogenetics and has extremely poor survival [13, 22, 23] and is recognized as a distinct subcategory in the ICC [16]. On the other hand, WHO-5 [24] classifies TP53mut AML within ‘other AML categories’, stating that further evidence is required to confirm biTP53 status as an ‘AML-defining abnormality’. Consequently, WHO-5 would instead classify >90% of TP53mut AML as AML-MR. We note that the survival of TP53mut AML was significantly poorer compared to TP53wt AML-MR. A similar observation was recently reported by Hart et al.. The clinical implication of these findings is that TP53mut AML may, inadvertently, be excluded from clinical trials of novel therapeutics if the WHO-5 criteria are followed. Secondly, when combined as a single entity, this misclassification could lead to prognostic overestimation and underestimation of TP53mut AML and TP53wt AML-MR, respectively.
In cases with one TP53mut VAF < 50%, demonstrating loss of the other copy of TP53 is critical to establish multi-hit loss. While the WHO-5 classification mandates verification of 17p13.1 deletion detected by metaphase cytogenetics using a confirmatory CNV analysis [15], the ICC does not [16]. Current practice reflects substantial limitations, with only ~20% of patients at our tertiary cytogenetics laboratories having undergone the confirmatory CNV analysis. We found that the confirmatory CNV analysis verified the LOH across the TP53 locus in 94% of evaluable cases with 17p13.1 deletion detected by metaphase cytogenetics. Notably, the survival of cases with single TP53mut VAF < 50% and concurrent 17p loss on karyotype was comparable to that of cases with biallelic TP53 inactivation. Additionally, CNV analysis identified LOH/cnLOH in 26.9% of evaluable cases without 17p deletion on karyotype, consistent with findings by Bernard et al. [2] ( ~ 25%). Strikingly, cases without 17p13.1 deletion on karyotype but with LOH/cnLOH on CNV coexisted with CK only. All cases with LOH/cnLOH across the TP53 locus on CNV analysis without 17p deletion on karyotype had CK. Conversely, no LOH/cnLOH was observed in cases with single TP53mut (VAF < 50%) without CK on karyotype. In the absence of comprehensive CNV data, biallelic inactivation would have been missed in approximately 4.0% of 374 MDS, primarily among those with single TP53mut VAF 2–50% and CK but without 17p loss. However, the prognostic impact of this omission is expected to be limited, as the median OS of single TP53mut VAF < 50% with CK is poor and comparable to biallelic inactivation. Our findings demonstrate that 17p13.1 deletion and/or CK detected by karyotype is associated with poor prognosis and may be considered “presumptive biallelic TP53 loss”. Validation in larger, independent cohorts will enhance the utility in clinical practice.
Finally, the ICC mandates a TP53mut VAF threshold of ≥10%, whereas WHO-5 does not specify a VAF cut-off. Our study provides compelling evidence that multiple TP53mut, each with VAF < 10%, as well as single TP53mut VAF < 10% in the context of 17p loss or CK, as well as TP53mut AML, are associated with poor survival. These findings suggest that the ICC-recommended TP53mut VAF cut-off of ≥10% warrants reconsideration, and cases with biallelic and monoallelic with CK with VAF 2 to 10% should be included in TP53mut MN.
The findings of our retrospective study, spanning over two decades, reflect the diagnostic workup, therapeutic sequencing, and clinical decision-making influenced by institutional guidelines of the time. This includes limited utilization of confirmatory CNV analysis. It is possible that workflow mandating confirmatory CNV analysis and/or wider utilization of whole genome sequencing may result in a higher proportion of cases being considered multi-hit [25]. Another limitation is the lack of bone marrow fibrosis, and hence, we could not evaluate the association between TP53mut MN and marrow fibrosis. Our study demonstrates a poor outcome of TP53mut MN with the currently available therapies. Although allogeneic SCT is associated with longer survival compared to other disease-modifying therapies, the median OS following allogeneic SCT is only ~1 year. The high relapse rate following allogenic SCT remains a pressing challenge, and our group has previously investigated the factors associated with this high relapse rate [26].
Despite these limitations, our analysis of the largest cohort of MN harboring TP53mut to date addresses key areas of contention and provides valuable insights that may guide future revisions of the WHO and ICC classifications. For the WHO-5 revision, we propose recognizing 17p13.1 deletion or a CK identified through conventional karyotyping as indicative of biallelic inactivation or its equivalent, without requiring confirmation by CNV analysis in the vast majority of cases. Furthermore, the poor prognosis associated with monoallelic TP53 inactivation in MDS cases with 10–19% blasts should be acknowledged, and TP53mut AML should be included in the TP53mut MN category. For the ICC, we propose revisiting the TP53mut VAF threshold of ≥10%, as multi-hit, multi-hit equivalent TP53mut MDS, and AML with VAF < 10% demonstrated survival outcomes comparable to those with VAF ≥ 10% (summarized in Table 1). This highlights the need for a more nuanced classification. Both the WHO-5 and ICC classifications should also reconsider the blast percentage cut-off and allelic status, as emerging data suggest that MDS-LB has distinct disease biology and outcomes compared to MDS with 5–9% blasts, 10–19% blasts, and AML.
Supplementary information
Acknowledgements
The authors thank their patients and their families. The authors gratefully acknowledge the support of the South Australia Cancer Research Biobank (SACRB). M.V.S. is supported by a Clinician Career Development Award in Transplant Research Honoring Brigid Kiley; D.H. is supported by a National Health and Medical Research Council (NHMRC)/Medical Research Future Fund (MRFF) Investigator Grant (MRF1195517), and grants from Cancer Australia and the Leukemia Foundation Australia; S.K. is supported by an NHMRC Investigator Grant (GNT2007739); and D.T. is supported by a CSL Centenary Fellowship, Medical Research Futures Fund, and Leukemia-Lymphoma Translational Research Program funding.
Author contributions
M.V.S. and D.H. designed the study, contributed the patient data, analyzed the data, and wrote the paper; K.H., G.W., C.T., and M.K. collated the data, analyzed the data, and edited the paper; C.H.K. performed statistical analysis and edited the paper; A.B., D.L., H.S.C., and C.N.H. analyzed variant data and edited the paper; A.B., A.A.K., P.G., A.S., A.M., D.C., K.B., D.R., P.B., M.R.L., W.J.H., T.B., D.T.Y., M.M.P., J.M.F., R.H., N.G., C.Y.A.Y., H.A., A.A.M., A.O., D.A.A., M.H., and A.T. contributed patients and edited the paper; D.T. and S.K. edited the paper. All authors agreed to the final version of the paper.
Data availability
Additional methods and data can be found in the Supplementary Methods section. For original data, please contact devendra.hiwase@sa.gov.au or shah.mithun@mayo.edu.
Competing interests
MVS declares research funding to the institution from AbbVie, Astellas, Celgene, KURA Oncology, and Marker Therapeutics. D.H. is a member of the board of directors or advisory committees of AbbVie and Novartis. A.A.K. provides research support to Novartis and Astex. M.P. is a member of the board of directors or advisory committees of Stemline Therapeutics and receives research funding from Kura Oncology. P.G. is a member of the advisory board of AbbVie. N.G. has served on the Advisory Board for Agio and DISC Medicine. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mithun Vinod Shah, Email: shah.mithun@mayo.edu.
Devendra Hiwase, Email: devendra.hiwase@sa.gov.au.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-025-01290-0.
References
- 1.Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. New Engl J Med. 2017;376:536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26:1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stengel A, Meggendorfer M, Walter W, Baer C, Nadarajah N, Hutter S, et al. Interplay of TP53 allelic state, blast count, and complex karyotype on survival of patients with AML and MDS. Blood Adv. 2023;7:5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. New Engl J Med. 2011;364:2496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. New Engl J Med. 2016;374:2209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen D, Groves MJ, Burnett AK, Patel Y, Allen C, Green C, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia. 2009;23:203–6. [DOI] [PubMed] [Google Scholar]
- 9.Hiwase D, Hahn C, Tran ENH, Chhetri R, Baranwal A, Al-Kali A, et al. TP53 mutation in therapy-related myeloid neoplasm defines a distinct molecular subtype. Blood. 2023;141:1087–91. [DOI] [PubMed] [Google Scholar]
- 10.Shah MV, Tran ENH, Shah S, Chhetri R, Baranwal A, Ladon D, et al. TP53 mutation variant allele frequency of ≥10% is associated with poor prognosis in therapy-related myeloid neoplasms. Blood Cancer J. 2023;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadia TM, Jain P, Ravandi F, Garcia-Manero G, Andreef M, Takahashi K, et al. TP53 mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer. 2016;122:3484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbosa K, Li S, Adams PD, Deshpande AJ. The role of TP53 in acute myeloid leukemia: Challenges and opportunities. Genes Chromosomes Cancer. 2019;58:875–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grob T, Al Hinai ASA, Sanders MA, Kavelaars FG, Rijken M, Gradowska PL, et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2022;139:2347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Gorlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686–98. [DOI] [PubMed] [Google Scholar]
- 15.WHO Classification of Tumours Editorial Board. Haematolymphoid tumours: WHO classification of tumours. 5th ed. Vol. 11. Lyon: International Agency for Research on Cancer (IARC); 2022.
- 16.Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahaj W, Kewan T, Gurnari C, Durmaz A, Ponvilawan B, Pandit I, et al. Novel scheme for defining the clinical implications of TP53 mutations in myeloid neoplasia. J Hematol Oncol. 2023;16:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah MV, Hung K, Baranwal A, Kutyna MM, Al-Kali A, Toop C, et al. Evidence-based risk stratification of myeloid neoplasms harboring TP53 mutations. Blood Adv. (Accepted, 2025) 10.1182/bloodadvances.2024015238. [DOI] [PubMed]
- 19.Bernard E, Hasserjian RP, Greenberg PL, Arango Ossa JE, Creignou M, Tuechler H, et al. Molecular taxonomy of myelodysplastic syndromes and its clinical implications. Blood. 2024;144:1617–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komrokji RS, Lanino L, Ball S, Bewersdorf JP, Marchetti M, Maggioni G, et al. Data-driven, harmonised classification system for myelodysplastic syndromes: a consensus paper from the International Consortium for Myelodysplastic Syndromes. Lancet Haematol. 2024;11:e862–e72. [DOI] [PubMed] [Google Scholar]
- 21.Lanino L, D’Amico S, Maggioni G, Al Ali NH, Wang Y-H, Gurnari C, et al. Artificial-intelligence, data-driven, comprehensive classification of myeloid neoplasms based on genomic, morphological and histological features. Blood. 2024;144:1005 (Supplement 1). [Google Scholar]
- 22.Short NJ, Montalban-Bravo G, Hwang H, Ning J, Franquiz MJ, Kanagal-Shamanna R, et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Adv. 2020;4:5681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77. [DOI] [PubMed] [Google Scholar]
- 24.Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart SA, Lee LA, Seegmiller AC, Mason EF. Diagnosis of TP53-mutated myeloid disease by the ICC and WHO fifth edition classifications. Blood Adv.2025;9:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baranwal A, Langer K, Gannamani V, Rud D, Cibich A, Saygin C, et al. Factors associated with survival following allogeneic transplantation for myeloid neoplasms harboring TP53 mutations. Blood Adv. (Accepted, 2025) 10.1182/bloodadvances.2024015335. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional methods and data can be found in the Supplementary Methods section. For original data, please contact devendra.hiwase@sa.gov.au or shah.mithun@mayo.edu.