Abstract
The demand for rice (Oryza sativa L.) as a staple food continues to grow, but rising temperatures due to climate change pose a significant threat to its production. This study addresses the challenge by employing endophytic bacteria and fertilizer to mitigate the adverse effects of high temperatures on rice plants. Seedlings were evaluated for growth parameters, comparing outcomes with non-inoculated counterparts under normal and 40 to 45 °C heat shock conditions. Isolates underwent thorough DNA extraction and 16 S rRNA gene sequencing for identification and were scrutinized for their plant growth-promoting (PGP) traits. The effects of fertilizer and thermotolerant bacteria on rice plants were investigated in controlled chambers at 25 °C for 14 days, succeeded by exposure to 40 °C for 10 days. A consecutive soil pot experiment extended over 150 days, exposing plants to growth chambers set at 35 °C for 60 days, followed by a rapid increase to 40 °C for 30 days and a subsequent reduction to 35 °C for an additional 60 days. Inoculating with the isolates resulted in panicle development and increased plant biomass and length, with fresh grain weights showing a 50% improvement when using bacterial strain W (B. paralicheniformis). Additionally, dry grain weights per panicle rose by 113% with strain W, 83% with strain N (B. pumilus), and 87% with strain D (B. paranthracis) compared to the control. Bacterial strain W exhibited the most pronounced effect on rice yield under heat stress. The results demonstrated a decrease in malondialdehyde (MDA) levels after 150 days of heat stress and half-dose of the recommended fertilizer. Bacterial inoculation increased proline, salicylate, and abscisic acid content, suggesting the alleviation of osmotic stress effects. This highlights the role of endophytic bacteria in stimulating biologically active responses within rice plant cells. Notably, bacterial strains W, N, and D show potential for enhancing plant growth and mitigating heat stress when used in conjunction with NPK50.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12284-025-00781-9.
Keywords: Bacterial endophytes, Fertilizer interaction, Growth promotion, Heat stress mitigation, High temperature, Rice (Oryza sativa)
Introduction
Rice (Oryza sativa L.), a vital staple crop, is globally in high demand, surpassing that of other crops. Serving as a fundamental dietary source for half of the world’s population, rice is indispensable for sustenance (Fukagawa and Ziska 2019). Contributing 35–75% of the caloric intake for over 3 billion people (Prakash et al. 2018), rice production faces significant challenges in meeting global demand (Fahad et al. 2019). In many African countries, inadequate yields force the import of 50 to 99% of rice (Gibson 2019), with approximately 33.6% of the 11.6 million tons consumed annually being imported (Gomez et al. 2019). This highlights the urgent need to overcome biotic and abiotic stressors to achieve self-sufficiency and enhance global food security (Ding et al. 2020).
One of the primary ecological challenges is rising temperatures (Ahmad et al. 2023; Ekwueme and Agunwamba 2021), which profoundly impact rice growth worldwide (Chen et al. 2020). Climate change has led to universal temperature increases, making agriculture vulnerable (Li et al. 2025). Projected temperature rises of 3 to 4 °C are expected to reduce agricultural output by 15–35% in Asia and Africa and 25–35% in the Middle East (Heikonen et al. 2025; Malhi et al. 2021). While the optimal temperature for rice cultivation is 25 °C (Pathak et al. 2020), temperatures above 35 °C severely limit growth. These temperature fluctuations, interconnected with weather patterns and plant phenology, significantly impact plant productivity (Kreyling et al. 2017; Sullivan et al. 2023).
Amidst these challenges, endophytic bacteria play a crucial role in enhancing Oryza sativa’s resistance to biotic and abiotic stresses (Ortiz et al. 2021). These bacteria colonize plant tissues, improving yield, suppressing pathogens, and enhancing plant development (El-Saadony et al. 2022). By degrading 1-aminocyclopropane-1-carboxylate (ACC) and enhancing nitrogen provisioning (Kaur et al. 2022), endophytes foster plant vitality (Muhammad et al. 2024). The interplay of microorganisms and nutrients is crucial for soil fertility and plant growth (Malik et al. 2020), with soil wetting post-prolonged high temperatures inducing metabolic activation and microbial diversity shifts (Luan et al. 2023).
While plant growth-promoting endophytic bacteria (PGPEB) have demonstrated benefits in various plants (Guo et al. 2025), their interaction under high temperatures remains unclear (Sena et al. 2024). This study investigates the response of endophytic bacteria to heat stress and their role in enhancing rice (Oryza sativa L.) resilience. The novelty lies in exploring the combined effects of bacterial endophytes and fertilizer under high temperatures, hypothesizing that their application will improve plant growth, biomass, and yield.
The study has several key objectives. First, it examines the interactive effects of endophytic bacteria and fertilizer on rice growth under normal and heat-stress conditions (40–45 °C). Second, it identifies and characterizes bacterial strains using DNA extraction and 16 S rRNA gene sequencing. Third, it evaluates the plant growth-promoting (PGP) traits of these strains. Fourth, it assesses the impact of fertilizer and thermotolerant bacteria on rice through controlled chamber and soil pot experiments. Fifth, it analyzes physiological and biochemical changes in rice under heat stress. Finally, it determines the potential of specific bacterial strains combined with NPK50 to enhance growth and mitigate heat stress. These objectives provide a comprehensive framework for understanding the synergistic effects of endophytic bacteria and fertilizer in improving rice resilience and productivity under heat stress.
Methodology
Isolation and Screening of Thermotolerant Endophytic Bacterial Isolates
Thermotolerant rice seeds (Oryza sativa L., Tainan No. 11) were selected for their resilience to high temperatures (Hsuan et al. 2019). The bacterial isolation process was conducted at the Environmental Microbiology and Biotechnology Laboratory, National Chung Hsing University, approximately 24.1130° N, 120.6745° E. Surface sterilization was performed by soaking seeds in 1.25% sodium hypochlorite for 30 min, followed by three washes with sterile distilled water. Endophytic bacteria were extracted by crushing seeds in 2 mL of sterile distilled water. The resulting solution was serially diluted (10− 1 to 10− 8), and 100 µL of each dilution was plated onto nutrient agar (NA; HiMedia). Plates were incubated at 40 °C and 45 °C for 5 days to screen for thermotolerant isolates. Selected isolates were stored at -80 °C (triplicate) and − 20 °C (duplicate) for further analysis.
Effect of Endophytic Bacteria on Rice Growth Under Heat Shock
Thermotolerant endophytic isolates were tested in petri dishes. Surface-sterilized rice seeds (1.25% sodium hypochlorite, followed by sterile water rinses) were soaked for 24 h. Ten seeds per dish (five replicates, 50 seedlings per isolate) were grown at 25 °C for 5 days, subjected to a 5-hour heat shock (40–45 °C), and then grown for an additional 5 days at 25 °C. Controls included growth at 25 °C without heat shock and heat shock without bacterial inoculation. Root and shoot lengths and fresh weights were measured.
Identification and Phylogenetic Analysis Via 16 S rRNA Gene Sequencing
Top-performing isolates from petri dish tests underwent 16 S rRNA gene sequencing (Patel and Archana 2017). Genomic DNA was extracted using the UltraClean Microbial DNA Isolation Kit, and PCR amplified using primers 27 F and 1492R (Willems and Collins 1993). Amplification conditions included 95 °C for 2 min, 35 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 90 s, and a final extension at 72 °C for 7 min (Verhelst et al. 2004). Sequences were identified via BLAST search and EzTaxon server, with phylogenetic analysis performed using the neighbor-joining method and 2000 bootstrap replications.(Honda et al. 1999).
Evaluation of Thermotolerant Endophytic Bacteria Plant Growth-Promoting Traits
Selected strains were assessed for nitrogen fixation and IAA production. Nitrogen fixation was evaluated using the acetylene reduction assay, with gas chromatography analysis (HITACHI Model 163) (Montes-Luz et al. 2023). IAA production was quantified by culturing strains in tryptophan-supplemented nutrient broth, followed by Salkowski reagent reaction and absorbance measurement at 530 nm (Alemneh et al. 2021).
Experimental Treatments
Fertilizer Application
Following recommendations from agricultural authorities in Taiwan, specifically the Ministry of Agriculture, and in consultation with relevant research findings, nitrogen, phosphorus, and potassium were applied as chemical fertilizer at ratios of 100:50:90 kg ha− 1 using urea, superphosphate, and potassium chloride (Lyu et al. 2021).
Soil Pot Experiment Over 24 Days
Forty plastic pots (600 g soil each) were prepared under a 12-hour photoperiod (153–179 µmole photons s⁻¹ m⁻¹). Rice seeds were surface-sterilized, treated with bacterial suspensions (A600 = 0.8), and incubated at 25 °C for 4 days. Treatments included five bacterial isolates, full-dose fertilizer, half-dose fertilization (urea, superphosphate, potassium chloride), and controls (no bacteria or no fertilizer). Half-dose and full-dose fertilizer was applied based on established practices (Pati et al. 2016). Pots were maintained at 25 °C for 14 days, then 40 °C for 10 days.
Soil Pot Experiment Over 150 Days
Three top-performing isolates were tested in larger pots (4.3 kg soil) over 150 days. Seeds were inoculated with 10 mL bacterial suspension (A600 = 0.4), and fertilizer was applied during planting stage and 30 days post-planting. A second bacterial inoculation (0.5 mL, A600 = 0.4) was performed at 30 days. Growth chamber conditions were 35 °C for 60 days, 40 °C for 30 days, and 35 °C for 60 days.
Measurement of Plant Parameters
Growth Metrics and Chlorophyll Content
Roots and shoots were aseptically detached using scissors in both petri dish and soil pot experiments. For the petri dish test, 50 germinants (hypocotyls and radicles) per isolate were measured after 10 days and 5 h of growth. In the 24-day soil pot experiment, 50 seedlings (shoots and roots) per isolate were evaluated, while the 150-day experiment involved 19 plants per isolate. Root and shoot lengths were measured using a ruler, and fresh weights were recorded with an analytical balance. Dry weights were determined after oven-drying at 70 °C for 3 days. Leaf and root numbers were manually counted, with soil washing for accuracy. Chlorophyll content was assessed using a SPAD 502Plus meter (KONICA MINOLTA). Grain yield was measured by harvesting mature panicles, air-drying, threshing, and weighing grains.
Proline Content and Lipid Peroxidation (MDA)
Proline content was assessed using an ethanolic extraction method (Carillo and Gibon 2011). Fresh plant tissue was homogenized with stainless-steel beads and extracted with 1% ninhydrin in 60% acetic acid + 20% ethanol. The mixture was vortexed for 10 min, heated at 95 °C for 20 min, and absorbance was measured at 520 nm (U-3010 spectrophotometer, (Tiwari et al. 2016).
Lipid peroxidation was evaluated by analyzing malondialdehyde (MDA) levels. Plant tissues were vortexed with 80% ethanol, centrifuged, and treated with thiobarbituric acid (TBA). Absorbance was measured at 440, 532, and 600 nm (Tukozkan et al. 2006). MDA concentration (nmol/mL) was calculated using established formulas (Du and Bramlage 1992; Onyango 2020) presented below:
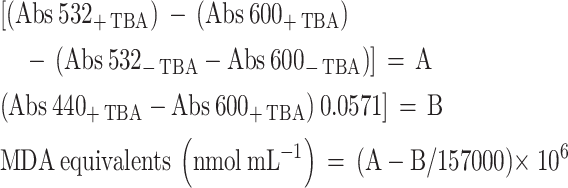 |
Rice Plant Endogenous Phytohormone Quantification
Salicylic acid (SA) was quantified using HPLC with fluorimetric detection (excitation: 305 nm, emission: 407 nm). Rice leaves were homogenized with stainless-steel beads in 70% ethanol, centrifuged, and the supernatant was analyzed (Allasia et al. 2018). Abscisic acid (ABA) was quantified using HPLC with a UV detector (261 nm). Leaves were homogenized in 80% methanol containing 0.1% butylated hydroxytoluene and 0.02 M sulfuric acid, centrifuged, and the supernatant was analyzed (Williams and De Mallorca 1982). Concentrations of SA and ABA were calculated using standard curves.
Statistical Analysis
The analysis of variance using a general linear model (Multivariate) was performed at a significance level of p-value of < 0.05 by the least significant different post-hoc comparison test (IBM SPSS Statistic version 25) and equal variances assumed LSD (Chang et al. 2023).
Results
Petri Dish Trial
In the Petri dish trial, specific isolates exhibited significant enhancements in hypocotyl and radicle lengths and fresh weights under elevated temperatures of 40 ℃ and 45 ℃ (Table 1). To ensure robustness, only the top 5 isolates demonstrating substantial improvements were retained for further analysis. Normal growth conditions showed that hypocotyl and radicle lengths were 5.65 cm and 10.39 cm, respectively. Under heat shock conditions, uninoculated seeds exhibited reductions in these lengths: 2.97 cm (hypocotyl) and 4.79 cm (radicle) at 45 °C and 2.29 cm (hypocotyl) and 3.34 cm (radicle) at 40 °C. Under a 40 °C heat shock, isolates N and D independently doubled the hypocotyl lengths and achieved similar outcomes in radicle lengths. Isolates N and D also increased hypocotyl fresh weight by 28.40 mg and 21.91 mg per plant, respectively. The same isolates resulted in increases in radicle fresh weight by 21.48 mg and 10.5 mg per plant, respectively. Advancing to a 45 °C heat shock scenario, strains W, K, and Y displayed noteworthy improvements in both radicle and hypocotyl lengths, exceeding 100%. The increase in fresh weights for both hypocotyl and radicle by these isolates signifies their robust growth-promoting effects. This observation also suggests that the effects of 45 °C heat shock surpass those of 40 °C, as demonstrated by the notable alterations in hypocotyl and radicle fresh weights and lengths.
Table 1.
Thermotolerant endophytes’ impact on rice seed germination bioassay after 10 days at 25℃ with 5-h heat shock
| Isolate | Heat shock |
Length (cm) Hypocotyl radicle |
Fresh weight (mg /plant) Hypocotyl radicle |
||
|---|---|---|---|---|---|
| BK-45 | 45°C | 2.68 ± 0.12d | 5.79 ± 0.28e | 12.49 ± 0.86d | 4.99 ± 0.48e |
| W | 45°C | 5.83 ± 0.16b*** | 10.67 ± 0.44c*** | 36.66 ± 1.25b*** | 36.13 ± 1.73a*** |
| K | 45°C | 5.97 ± 0.11b*** | 12.69 ± 0.29b*** | 36.39 ± 6.47b*** | 22.07 ± 1.77 cd*** |
| Y | 45°C | 5.96 ± 0.21b*** | 13.86 ± 0.36a*** | 35.67 ± 2.54b*** | 29.99 ± 1.39b*** |
| BK-40 | 40°C | 3.36 ± 0.14c | 7.05 ± 0.48d | 18.90 ± 1.27d | 14.17 ± 1.29d |
| N | 40°C | 6.70 ± 0.09a*** | 13.95 ± 0.31a*** | 47.39 ± 1.15a*** | 35.65 ± 1.51a*** |
| D | 40°C | 7.58 ± 0.18a*** | 13.80 ± 0.35a*** | 40.81 ± 1.00b | 24.67 ± 1.09c** |
| NG | 5.65 ± 0.16bc | 10.39 ± 0.31c*** | 25.06 ± 0.84c* | 18.07 ± 0.72d | |
aBK means no inoculation; NG means no heat shock and no inoculation. b All the values are expressed as mean ± standard deviation calculated from 50 germinated seeds of 5 independent replicates. c Different letters indicate significant differences in each growth parameter. d Asterisks indicate statistically significant differences compared to the control groups (W, K, Y: compared to BK-45; N, D, NG: compared to BK-40) at each time point (*P < 0.05, **P < 0.01, ***P < 0.001)
Genomic Identification of Heat-Tolerant Endophytic Bacteria in Oryza sativa
We performed amplification and sequencing of the 16 S rRNA genes of endophytic bacterial strains. The obtained sequences were cross-referenced with known sequences using BLAST analysis, revealing a significant similarity of over 97% to established counterparts (Rosselli et al. 2016). Identification at the genus level relied on a 97 to 99% match, while < 97% indicated a potentially new bacterial species (Drancourt et al. 2000; Stevanović et al. 2023). Analysis of the 16 S rDNA sequences suggests that isolate K corresponds to Bacillus tequilensis, isolate W to B. paralicheniformis, isolate N to B. pumilus, isolate Y to B. coagulans, and isolate D to B. paranthracis. These findings (Fig. 1 and Table 2) led to the deposition of these newly identified endophytic bacterial strains (K, W, N, Y, and D) obtained through 16 S rDNA sequences into the GenBank database. Accession numbers MZ144274, MZ340514, MZ144277, MZ340553, and MZ340638 respectively denote these sequences. Our genetic analysis successfully confirmed the identity of these five endophytic bacterial strains with striking similarity to well-characterized species, indicating potential for future agricultural applications and necessitating further exploration.
Fig. 1.
A Phylogenetic tree based on 16 S rRNA sequences of endophytic bacterial strains isolated from seeds of thermotolerant Taiwan Oryza sativa L. hybrid, Tainan No. 11, was constructed. The number of each node indicates statistical confidence fractions after 2,000 repeats in the bootstrap analysis, and the length of each line represents the genetic distance between sequences
Table 2.
16 S rRNA gene sequence-based identification and important PGP traits of rice endophytic bacterial isolates
| Name | Closest strain match | Similarity % | Variation (Δ bp) | Nitrogen Fixation (pmol/s) | Indole-3-acetic acid (mg/L) |
||
|---|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 45 °C | |||||
| W | Bacillus paralicheniformis | 99.64 | 1368/1373 | 5.56 | 54 | 14 | 37 |
| N | Bacillus pumilus | 99.72 | 1420/1424 | 66.7 | 15 | 14 | 9 |
| Y | Bacillus coagulans | 98.73 | 1402/1420 | 36.1 | 29 | 40 | 24 |
| D | Bacillus paranthracis | 99.93 | 1420/1421 | 2.78 | 7 | 13 | 34 |
Data is expressed as the mean of at least 3 independent replicates
Microbial Activities Evaluation
This section explores the plant growth-promoting (PGP) traits observed in endophytic bacterial isolates under heat shock conditions (Table 2). These traits, including enhanced nutrient availability, nitrogen fixation, and indole-3-acetic acid production, play crucial roles in improving plant growth and stress tolerance (Bruno et al. 2020; Duca and Glick 2020). Other studies have highlighted the significance of these traits in enhancing plant resilience to stress (Koskey et al. 2021; Prakash and Mishra 2022). The 5 isolates displayed positive results when evaluated for nitrogen fixation, and indole-3-acetic acid production. Nitrogen-fixing activity varied from 2.78 to 66.7 pmol/s, with isolate N exhibiting the highest activity (Table 2). Other isolates also showed positive results for nitrogen fixation. IAA production was detected in all isolates, ranging from 4 to 54.04 mg/L, with isolate W exhibiting the highest production.
Physiochemical Properties of the Experimental Soil
The soil collected was tested for physicochemical properties (Table 3). The physicochemical properties of the soil were analyzed to characterize the soil conditions before the initiation of the soil pot experiment over 24 and 150 days. These analyses aimed to provide essential information on soil fertility, ensuring a comprehensive understanding of the growth environment for the subsequent rice planting experiments. Results showed that the soil’s pH and electrical conductivity (EC) were 7.45 and 23.83 mS/m, respectively.
Table 3.
Physico-chemical properties of soil used
| Parameters | Mean | SD |
|---|---|---|
| EC (mS/m) | 23.83 | 6.95 |
| pH | 7.45 | 0.36 |
| Total Nitrogen (g/kg) | 0.04 | 0.02 |
| Available Phosphorus (mg/kg) | 171.39 | 14.26 |
| Available Potassium (mg/kg) | 196.32 | 14.69 |
| Available Calcium (mg/kg) | 2671.69 | 36.75 |
| Available Magnesium (mg/kg) | 320.02 | 1.55 |
| Available Iron (mg/kg) | 535.40 | 2.71 |
| Available Manganese (mg/kg) | 45.65 | 3.47 |
| Available Copper (mg/kg) | 0.96 | 0.14 |
| Available Zinc (mg/kg) | 25.82 | 2.23 |
| Available Boron (mg/kg) | 3.98 | 0.01 |
| Total Organic Carbon (mg/g) | 14.2 | 2.6 |
aEach data is expressed as a mean of at least 4 independent replicates
The pH of 7.45 suggests that the soil is nearly neutral, making it suitable for a wide range of crops (Penn and Camberato 2019). The moderate EC of 23.83 mS/m indicates good solubility of essential nutrients in the soil. The available nitrogen (N) was 0.04 g/kg, and the total organic content was 14.2 mg/g; the soil contains moderate organic matter, which is vital for enhancing soil structure, water retention, and nutrient availability. Available phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), and boron (B) were 171.4, 196.32, 2641.69, 320.02, 535.4, 45.65, 0.96, 25.82 and 3.98 mg kg− 1, correspondingly. The low available N content of 0.04% signifies that the soil might require additional nitrogen supplementation. In contrast, the substantial levels of available P, K, Ca, Mg, Fe, Mn, and other micronutrients can benefit plant growth. The presence of essential micronutrients such as Cu, Zn, and B in the soil at reasonable levels is crucial for overall plant health and productivity.
Fertilizer-Endophyte Interaction in 24-Day Soil Pot Experiment
In comparison with the control (BK-40), which were rice plants without inoculation and no fertilizer, the results indicated that heat stress affected the growth of rice plants (Fig. 2). However, fertilizer application greatly improved the thermotolerance of rice plants, as shown through the differences in shoot and root weights and lengths and the number of roots and shoots.
Fig. 2.
Interactive effects of PGP bacterial isolates and fertilizer on rice plant growth under40 ℃ heat stress. (a) Shoot length, (b) Root Length, (c) Dry Weight, and (d) Shoot and Root number. Each data point represents the mean of 50 rice plants from 5 independent replicates. Different letters indicate significant differences in each growth parameter
In shoot length measurements taken at 11 days, N + NPK50 showed the highest increase of 39%, followed by D + NPK50 at 34%. (Fig. 2a). After 14 days, D + NPK50 and N + NPK50 continued to exhibit the highest increases through 21 and 24 days, followed by Y + NPK50 and W + NPK50. Root length after 24 days showed that N + NPK50, W + NPK50, and Y + NPK50 displayed the best results with an 84% increment, followed by K + NPK50, D + NPK50, NPK50, and NPK100 with 76%, 74%, 38%, and 17%, respectively, when compared with BK-40 (Fig. 2b). The data highlights the positive impact of fertilizer, particularly NPK50 and NPK100, on shoot and root dry weight (Fig. 2c). Shoot and root numbers were highest in D + NPK50, K + NPK50 and N + NPK50 (Fig. 2d). Notably, certain isolates, such as D + NPK50 and N + NPK50, showcased the most promising results, resulting in increased shoot and root lengths, numbers and weights (Fig. 2). In heat stress conditions, the combined application of select endophytic bacterial isolates and fertilizers has a substantial mitigating effect on rice plant growth.
Fertilizer-Endophyte Interaction in 150-Day Soil Pot Experiment
Shoot Length
A significant effect of 3 PGP endophytic bacterial isolates W, N, and D with fertilizer on rice plants was seen throughout the 150-day experiment (Fig. 3) when compared to NPK50. A significant increase in shoot lengths (cm/plant) was observed in W (56%) after 30 days, followed by D (41%) and N (36%), as seen in Fig. 3a. Subsequently, after 39 days, a good increase was observed in W (59%) followed by N (58%) and D (42%). After 46 days, a significant increase was observed in N (54%), W (48%), and D (23%); subsequently 60 days, a significant increase was observed in N (49%), W (44%) and D (9%), and 90 days the notable increase was in the order N (64%), W (57%) and D (23%).
Fig. 3.
Effect of heat stress and inoculation on rice plant (a) Shoot length, (b) fresh weight, (c) dry weight, and (d) leaf number at various time points (60 days 35 ℃, 30 days 40 ℃, and 60 days 35 ℃). Each data point represents the mean of 19 rice plants from 4 independent replicates. Different letters on the bars signify significant differences (P < 0.05) between groups at each time point, determined by the LSD test
Increasing the temperature from 35 ℃ after 60 days to 40 ℃ for 30 days resulted in a 2.85% decrease in uninoculated leaf numbers per plant due to a high extreme temperature that burned shoot buds (Fig. 3a, d). The damage was significantly observed after 90 days in control. In all inoculated plants, there was no decrease in shoot lengths throughout the experiment. Percentage growth was slow in plants exposed to a high temperature of 40 ℃ when compared to the previous temperature of 35 ℃, and altering the temperature from 40 ℃ to 35 ℃ resulted in a growth percentage increase. Rice was harvested after 150 days, and all inoculated rice plants displayed significantly higher shoot lengths than the control plants (NPK50). The control was 47 cm, while inoculated plants under W + NPK50, N + NPK50, and D + NPK50 displayed 63.85, 66.5, and 65.85 cm, respectively. The highest increase in shoot length was 41%, which was recorded under seedlings inoculated with N + NPK50, followed by 40% under D + NPK50. Throughout the experiment, leaf numbers under inoculated plants were higher in all inoculated plants than in uninoculated plants, and this can be seen under 30, 60, 90, and 150 days. The highest shoot numbers were observed in plants inoculated with isolate D, which resulted in 12 leaves per plant shoot after 90 days and 18 leaves per plant shoot after 150 days (Fig. 3d).
Plant Weights and Leaf
Inoculation caused a significant increase in plant fresh and dry weights after 30 days and 150 days (Fig. 3b). Shoots fresh weights under control (NPK50) after 30 and 150 days were 192.9 mg and 2490.5 mg, respectively. Plants inoculated with W, N, and D displayed an increase of 442.5 mg, 357 mg, and 274 mg, respectively, after 30 days. Later, in 150 days, an increase in fresh weights of plant shoots inoculated with W, N, and D was 3617 mg, 2629.5 mg, and 3094.5 mg, respectively. Inoculation with N isolate showed an insignificant percentage increase of 3.86% in root fresh weights compared to the control. In other inoculated plants, the fresh weights of the roots were significantly higher than NPK50 plants after 30 days and 150 days. Subsequently, 30 days of uninoculated plants recorded an average of 42.12 mg root fresh weights, while plants inoculated with W, N, and D recorded fresh weights of 93.36 mg, 94.23 mg, and 91.71 mg, correspondingly. After 150 days, the root fresh weights for uninoculated plants were 2 741.5 mg, while plants inoculated with W, N, and D recorded mean root fresh weights of 4 420 mg, 2 849.5 mg, and 3 909.5 mg, correspondingly. The highest increase in shoot fresh weight after 30 days was 129%, and it was seen under plants inoculated with isolate W, followed by 85% seen under rice plants inoculated with N. After 150 days, the highest increase in shoot fresh weight was 45% still under plants inoculated with W; however, it was now followed by plants inoculated by D (24%) as opposed to previous plants inoculated with N under 30 days. In root fresh weights, all plants inoculated with the three isolates showed about 120% (W, N, and D were 122, 124, and 118%, respectively) increase after 30 days. After 150 days, the highest increase in root fresh weights was seen under plants inoculated with W (61%), followed by root fresh weights under plants inoculated with D (43%).
There was an increase in shoot dry weights of all inoculated plants after 30 days and 150 days (Fig. 3c). Inoculating plants with strains W, N, and D resulted in an increase of 48%, 58%, and 93% shoot dry weights after 30 days, respectively. The shoot dry weight of plants inoculated with endophytic bacteria isolate W, N, and D increased by 44%, 5%, and 30%, respectively, after 150 days. After 30 and 150 days, no significant difference in root dry weights was observed between inoculated and uninoculated plants, except for those inoculated with isolate W after 150 days. Inoculation with isolate W led to a notable 112% increase in root dry weights after 150 days.
Inoculated plants consistently exhibit higher root fresh weights compared to uninoculated plants, and this effect is sustained over the 30-day and 150-day periods. This is particularly significant because robust root development is crucial for nutrient and water uptake, contributing to overall plant health. The study demonstrates that the growth-promoting effects of bacterial isolates W, N, and D with fertilizer are time-dependent.
SPAD Chlorophyll Analysis
The Single Photon Avalanche Diode (SPAD) chlorophyll values indicated that heat stress significantly reduced the chlorophyll content in rice plants (Fig. 4a). Significant effects of the three PGP endophytic bacterial isolates W, N, and D with fertilizer under heat stress (60 days at 35 ℃, 30 days at 40 ℃, and 60 days at 35 ℃) were observed in the chlorophyll contents of rice plants throughout the 150-day experiment. Chlorophyll content was determined at various time points: 30 days (35 ℃), 39 days (35 ℃), 46 days (35 ℃), 60 days (35 ℃), 76 days (40 ℃), 90 days (40 ℃), and 150 days (35 ℃). After 30 days, a significant increase in SPAD values was observed in plants inoculated with isolate W (47%), followed by N (39%) and D (32%). This increase correlated with the observed increases in shoot lengths and shoot numbers (Fig. 3a and d). At 39 days, SPAD values showed significant increases in W (51%), D (49%), and N (45%). At 46 days, improvements were in W (33%), N (30%), and D (25%). At 60 days, the increases were N (20%), W (8%), and D (3%). At 76 days, the increases were W (30%), N (16%), and D (4%). Subsequently, at 90 days, the significant increases were in W (80%), N (77%), and D (65%). After 150 days, inoculated rice plants exhibited higher average chlorophyll contents than the control group (NPK50). Plants inoculated with W, N, and D showed SPAD values of 31, 31, and 29, respectively, compared to the control group’s value of 20. Before harvest, plants inoculated with isolate W displayed the highest increase of 61%, followed by plants inoculated with N (57%) and D (46%). These results suggest that heat stress affects rice plant growth (Fig. 4b) and chlorophyll content (Fig. 4a). The interactive effect of the fertilizer NPK50 and the three PGP endophytic bacterial isolates W, N, and D significantly increased chlorophyll contents in all inoculated plants under heat stress.
Fig. 4.
The impact of heat stress and inoculation of endophytic bacteria on chlorophyll levels and rice plant parameters. (a) Chlorophyll content for 150 days (60 days at 35 ℃, 30 days at 40 ℃, and 60 days at 35 ℃), and (b) plant growth. The 150-day data represent the mean of 19 rice plants from 4 independent replicates. The letters on the bars denote the difference at a p-value of 0.05 in the LSD test within the same day
Rice Crop Grain Yield
Fresh grain weights per panicle for plants under NPK-50, NPK50-W, NPK50-N, and NPK50-D were 143.5 mg, 215 mg, 142 mg, and 180.05 mg, respectively (Fig. 5a). There was a significant improvement in fresh grain weight per panicle for plants inoculated with endophytic bacterial isolates N when compared to uninoculated plants. There was a noteworthy improvement in fresh grain weight per panicle for plants inoculated with W and D. The highest fresh grain weight improvement of 50% was seen under plants inoculated with W, followed by plants under D, which displayed a 26% increase in grain fresh weights. Dry grain weights per panicle for plants under control, W, N, and D were 66.84 mg, 142.52 mg, 122.86 mg, and 125.46 mg, respectively. There was an improvement in dry grain weights per panicle caused by inoculation with endophytic bacterial isolates W, N, and D under NPK50 treatment during heat stress conditions. Endophytic bacterial isolates W caused the highest improvement of 113%, followed by isolate D with 88%, and finally, isolate N with an 84% increase in rice grain dry weights.
Fig. 5.
Effects of heat stress and inoculation of endophytic bacteria on rice yield after 150 days (60 days at 35 ℃, 30 days at 40 ℃, and 60 days at 35 ℃), (a) fresh and dry grain yields, (b) panicle lengths, and (c) grain counts. Data points are averages of 19 rice plants from 4 independent replicates. Distinct letters on the bars denote significant differences at a p-value of 0.05 in the LSD test within the same growth parameter.21
Noteworthy panicle development was displayed under all inoculated rice plants compared to 5.7 cm length under uninoculated plants. The order from highest to lowest increase under endophytic bacterial isolates D (9.2 cm), W (7.9 cm), and N (7.5 cm) was 60%, 38%, and 30%, respectively (Fig. 5b). Inoculation of endophytic bacterial isolates W, N, and D resulted in a significant improvement in the number of grains per panicle and overall plant growth before harvest (Fig. 5). These results are also supported by the chlorophyll content and plant growth data (Figs. 3 and 4a-b). Rice plants under control recorded an average of 11 grains per panicle while inoculation of endophytic bacterial isolates W, N, and D resulted in average grain numbers of 18, 14, and 19, respectively. In expressions of percentage increase in the number of grains per panicle under inoculation of endophytic bacterial isolates D, W, and N, the increase was 66%, 56%, and 21%.
Physicochemical Processes in Rice Plants Under Heat Stress
Osmoprotectant Proline Content
Inoculated plants in the 150-day experiment displayed variation in proline content, showing increases at 30, 60, 90, and 150 days (Fig. 6a). Subsequently, after 30 days in 35 ℃ the plants inoculated with W + NPK50, N + NPK50, and D + NPK50 displayed 1.32, 1.38 and 1.38 µmole/g, correspondingly. BK plants displayed a proline content of 0.83 µmole/g after 30 days. The results of BK, W + NPK50, N + NPK50, and D + NPK50 proline content after 60 days were 0.70, 0.88, 0.72, and 1.38 µmole/g, respectively. After 90 days, the outcomes were 2.99, 3.92, 4.40 and 4.54 µmole/g, separately. Lastly, for 150 days, the outcomes were correspondingly 0.35, 0.42, 0.68, and 1.11 µmole/g. Under heat-stress conditions, proline content was significantly higher in the 150-d experiment, especially before harvest. A significant difference was seen under inoculated plants compared to uninoculated rice plants, with the highest increase of 214% seen under plants inoculated with isolate D in 50% fertilizer input.
Fig. 6.
The effect of fertilizer and thermotolerance PGPB on rice plants under heat stress (60 days 35 ℃, 30 days 40 ℃, and 60 days 35 ℃), focusing on the content of (a) proline, (b) MDA, (c) SA, and (d) ABA. Error bars represent standard errors calculated from at least 4 replicates. Distinct letters on the bars denote significant differences at a p-value of 0.05 in the LSD test within the same day of each growth parameter
The results displayed that increasing the temperature from 35 ℃ after 60 days to 40 ℃ for 30 days resulted in more than double upsurge of proline under all plants when compared to the previous 35 ℃ and 35 ℃ after this condition (Fig. 6a). The outcomes suggest that with an increase in heat stress, there will be an increase of proline production which will be even more in inoculated plants. The highest improvement (52%) after 90 days was seen under plants inoculated with isolate D compared to the control.
Lipid Peroxidation
Lower MDA levels in plants treated with endophytic bacterial isolates suggest protection against oxidative damage to cellular membranes under heat stress conditions (Fig. 6b). Combined application of these isolates and fertilizer notably reduces MDA content, indicating enhanced heat stress resilience. This finding underscores the potential of endophytic bacteria and fertilizer to mitigate lipid peroxidation and enhance plant resistance to high temperatures. The MDA content was found to be low in all inoculated plants under 30, 60, 90, and 150 days in 35 ℃ and 40 ℃ heat stress. Plants under NPK50, W + NPK50, N + NPK50, and D + NPK50 displayed MDA content of 46847, 10259, 7663, and 21361 µmole /g, correspondingly in 35 ℃. After 60 days, still under 35 ℃ the proline content plants in NPK50, W + NPK50, N + NPK50, and D + NPK50 were found to be 12236, 7917, 1750, and 4829 µmole /g, similarly. The results displayed a pattern decrease in MDA content under the same stress condition for double increase durations, with plants under control (NPK50) still showing the highest amount of MDA content. Increasing the temperature from 35 ℃ to 40 ℃ after 60 days for 30 days, making 90 days, caused an increase in MDA content. Even after further increasing temperature by 5 ℃, MDA content under control remains high when compared to inoculated plants. After 90 days, the MDA amount NPK50, W + NPK50, N + NPK50, and D + NPK50 were 29567, 21044, 23039, and 25429 µmole /g, correspondingly. Dropping the temperature from 40 ℃ to 35 ℃ after 90 days for 60 days resulted in 38029, 29280, 14355, and 18382 µmole /g MDA content under plants inoculated with isolate NPK50, W + NPK50, N + NPK50, and D + NPK50, respectively. Overall, in the 150-day interactive effect experiment under heat stress, MDA content was found to be low throughout 150 days when compared to control in plants inoculated plants, and this is because MDA functions as a way of a gauge of the degree of lipid peroxidation in plants. In short, the changes brought by the interactive effects of endophytic bacteria and NPK50 caused plants to resist heat stress. In comparison with the control, the highest percentage decrease in MDA was displayed by plants inoculated with N (84%), followed by plants under W (78%), and finally, plants under D (54%). After 60 days, plants displayed the highest decrease in N (86%. Subsequently, in 90 days, the highest decrease was seen under plants inoculated with W (28%). Finally, before harvest (150 d), the uppermost decrease from highest to lowest MDA content was in the order N (62%), D (52%), and W (235), respectively.
Endogenous Phytohormones Salicylic Acid
The study shows the potential of salicylic acid as a key signaling molecule in plant stress responses, particularly under heat stress conditions. Manipulating SA levels via endophytic bacterial isolates and fertilizers could enhance plant resilience to heat stress, offering implications for sustainable agriculture. The effect of specific isolates and fertilizer in increasing SA content offers a promising strategy for bolstering crop plant resilience in extreme heat conditions. Overall, rice plants exposed to heat stress in the 150-day interactive effect of bacterial and fertilizer soil pot experiment indicated significantly increased SA content levels under a heat stress condition when compared to plants control throughout the experiment (Fig. 6c). Plants under NPK50, W + NPK50, N + NPK50 and D + NPK50 displayed SA content of 3.08,5.74, 4.96, and 4.98 ng/g, correspondingly after 30 days in 35 ℃. The SA content for the same treatment after 60 days in 35 ℃ was 1.69, 1.98, 2.99, and 2.19 ng/g, respectively. Increasing the temperature from 35 ℃ to 40 ℃ for an additional 30 days (90 d) caused a further increase in SA levels in all plants inoculated and uninoculated with those inoculated plants with significantly higher SA content. The results for plants under NPK50, W + NPK50, N + NPK50, and D + NPK50 were 3.34,9.10,4.96, and 8.09 ng/g, congruently. Subsequently, 60 more days in 35 ℃ heat stress caused SA content (150 days) to rise SA content in all inoculated plants significantly greater than in uninoculated plants. The 150 d outcomes for plants under NPK50, W + NPK50, N + NPK50, and D + NPK50 exhibited SA levels of 64.02, 102.60, 168.80, and 102.56 ng/g, congruently. The study demonstrates that rice plants subjected to heat stress conditions consistently exhibit elevated SA content levels throughout the 150-day experiment (Fig. 6c). This suggests that heat stress triggers a plant response that includes the production of SA as a defense mechanism. The effect of specific endophytic bacterial isolates and applying fertilizer (NPK50) results in a significant increase in SA content in inoculated plants.
Endogenous Phytohormones Abscisic Acid
Plants inoculated with endophytic bacteria isolate W, N, and D displayed higher amounts of ABA under heat stress of 35 ℃ condition after 30 days (Fig. 6d). Compared to the control (9474 ng/g), plants inoculated with the isolates W + NPK50 and N + NPK50 showed significant increases in ABA content by 3665 ng/g and 1576 ng/g, respectively, after 30 days. Inoculation with D + NPK50 resulted in an ABA level that was 363 ng/g higher than the control after 30 da ys. After 60 days of heat stress at 35 °C, the ABA level in control plants decreased from 9474 ng/g to 7206 ng/g. However, plants inoculated with isolates W + NPK50, N + NPK50, and D + NPK50 displayed significant increases in ABA content by 4385 ng/g, 4110 ng/g, and 13,067 ng/g, respectively, compared to the control after 60 days. Further increasing the temperature from 35 °C to 40 °C for 30 days following the initial 60 days of heat stress caused noteworthy increases in ABA levels: 3410 ng/g for W + NPK50, 3573 ng/g for N + NPK50, and 2423 ng/g for D + NPK50 compared to the control. The ABA level in control plants dropped substantially from 7206 ng/g to 1067 ng/g. Reducing the temperature from 40 °C to 35 °C for 60 days resulted in a decrease in the ABA content of all inoculated plants. Compared to NPK50 (1022 ng/g), plants inoculated with W + NPK50, N + NPK50, and D + NPK50 after 150 days displayed ABA levels of 3046 ng/g, 3757 ng/g, and 3944 ng/g, respectively.
Discussion
Petri Dish Trial and Genomic Identification of Heat-Tolerant Endophytic Bacteria
The significant improvements observed in hypocotyl and radicle lengths, as well as fresh weights, amidst the challenging conditions of 40 and 45 °C heat stress, underscore the remarkable potential of select endophytic bacterial strains in enhancing the thermotolerance of rice plants (Table 1). Heat stress negatively affected rice plant growth (controls: BK-40 and BK-45 compared with normal growth: NG); however, bacterial inoculation positively affected the early plant growth and biochemical parameters of Oryza sativa. This study advances previous findings by demonstrating superior thermotolerance under extreme heat stress, with strains W, K, Y, N, and D showing significantly higher growth metrics compared to controls and non-inoculated plants (Table 1). These results align with prior studies suggesting that bacterial inoculation alleviates abiotic stresses such as drought, chilling, salinity, and elevated temperatures (Morcillo and Manzanera 2021; Zia et al. 2021).
Despite plants’ inherent capacity for partial adaptation to temperature stress in temperate climates, overall growth and productivity typically decline under such conditions (Bita and Gerats 2013; Lippmann et al. 2019). The degree to which a plant can withstand such stress is intricately linked to metabolic adjustments (Arbona et al. 2017). Our findings suggest that thermotolerant endophytic bacterial strains can significantly enhance plant resilience to heat stress by modulating metabolism (Ahmad et al. 2022; Maitra et al. 2021). Seed inoculation with the strains used in this study markedly improved growth, development, and heat stress responses, demonstrating their potential for agricultural applications.
Using 16 S rRNA gene sequencing, we gained crucial insights into the microbial community associated with Oryza sativa L. under heat-stress conditions (Fig. 1 and Table 2). The genomic identification of bacterial endophytes, validated with > 99% accuracy, enhances the reliability of our findings and provides a robust foundation for future research. This aligns with previous studies (Mageshwaran 2024) while advancing the field by linking specific strains to measurable thermotolerance improvements. However, it is important to acknowledge the limitations of the 16 S rRNA gene in providing precise species-level identification, particularly within complex genera like Bacillus (Church et al. 2020). Future studies could explore advanced techniques, such as multilocus sequence typing (MLST), for more detailed taxonomic and functional insights (Uelze et al. 2020).
Microbial Activities, Nutrient Interactions, and their Impact on Plant Health and Yield
The observed plant growth-promoting traits, including nitrogen fixation, phosphate and potassium solubilization, indole-3-acetic acid production, and siderophore production (Table S1 and Table 2), are crucial mechanisms that facilitate enhanced growth and stress tolerance in rice plants (Zhang et al. 2021). Our study highlights the significant contributions of isolates K, W, N, Y, and D to plant growth promotion under heat-stress conditions, aligning with existing literature on the role of specific isolates in improving plant performance under environmental stress (Akhtar et al. 2020; Ayaz et al. 2022; Tsotetsi et al. 2022).
Endophytic bacteria enhance plant growth and stress tolerance through various mechanisms. Direct promotion involves resource acquisition, such as nitrogen fixation (Table 2) and the solubilization of essential nutrients like phosphorus and potassium (Table S1), which improve nutrient uptake (Hussain et al. 2023; Rai et al. 2023). Additionally, the production of IAA stimulates root growth and development (Table 2), enhancing water and nutrient absorption under stress conditions (Ahmed et al. 2021), while siderophore production facilitates iron sequestration (TableS1), making it more available for essential physiological processes (Ghosh et al. 2021). These mechanisms collectively underscore the versatility of endophytic bacteria in promoting plant resilience and productivity under adverse conditions.
The study also highlights the role of macronutrients (nitrogen, phosphate, potassium) in plant and bacterial growth dynamics under heat stress (Figs. 2, 3, 4, 5 and 6). The combination of endophytic isolates W, N, and D with NPK50 fertilizer significantly enhanced nutrient uptake and stress tolerance, aligning with previous studies (Kaur et al. 2023). However, the suboptimal performance of K + NPK50 (Fig. 2, and Fig. S2) suggests that certain isolates may not synergize well with fertilizers under heat stress, likely due to changes in water uptake and physicochemical properties (Sarker et al. 2021; Ullah et al. 2019).
Inoculation with W, N, D, and NPK50 significantly increased grain weights, panicle length, and grain number per panicle (Fig. 5), demonstrating their potential for improving rice yield in high-temperature environments. These improvements were attributed to multiple factors, including enhanced nutrient uptake, production of growth-promoting substances (TableS1, and Table 2), stress mitigation (Fig. S2a-b, Figs. 2 and 3, and Fig. 4b), improved photosynthesis (Fig. 4a), hormonal balance (Fig. 6), and robust root development (Fig. S2a, Figs. 2b and 3b-c). The integration of microbial inoculants with fertilization practices underscores their potential to optimize crop yield and resilience under stress (Fig. 5).
Recent studies on endophytic bacterial diversity and their ecological roles further support our findings, emphasizing the potential of these microorganisms for agricultural applications (Hussain et al. 2023; Rai et al. 2023). For instance, research has demonstrated that endophytic bacteria can enhance plant growth and stress tolerance through the production of growth-promoting substances and the solubilization of essential nutrients (Afzal et al. 2019; Kamran et al. 2022). These insights underscore the importance of integrating microbial-based solutions and soil management practices into agricultural systems to promote sustainable crop production.
Our findings, supported by data from Table 1, Table S1, Table 2; Figs. 2, 3 and 4, and Fig. 5, highlight the potential of developing tailored biocontrol and growth-promoting agents to advance sustainable agriculture practices and ensure global food security. Future research should focus on elucidating the underlying mechanisms driving plant-microbe-soil interactions while exploring novel strategies to optimize agricultural sustainability across diverse agroecosystems.
Nutrient-Endophyte Interaction: Short-Term Soil Pot Trial
Heat stress significantly threatens rice cultivation, detrimentally impacting growth and yield (Kumar et al. 2020). Our investigation reveals that applying bacterial endophytes and fertilizer enhances plant growth under heat-stress conditions (Fig. S1, and Fig. 2), highlighting the importance of understanding microbial-nutrient interactions (Chen et al. 2021).
Endophytic bacteria play pivotal roles in plant growth promotion through direct and indirect mechanisms (Vandana et al. 2021). Direct promotion involves resource acquisition such as nitrogen, phosphorus, and iron and hormone modulation like auxin, cytokinin, ethylene, while indirect promotion includes stress mitigation through antibiotic production and induced systemic resistance (Eid et al. 2021; Pandey et al. 2019). These mechanisms underscore the versatility of bacterial endophytes in enhancing plant resilience and productivity (Shah et al. 2021). Recent studies on gene mutants of nitrogen-fixing endophytes such as glycosyltransferase (gumD), glutathione reductase (GR), superoxide dismutase (SOD), have elucidated their role in biofilm formation, colonization, and plant growth promotion (Dudeja et al. 2021; Santoyo et al. 2016), supporting our findings and emphasizing the importance of genetic analyses in understanding plant-microbe interactions.
Endophyte Interaction in Long-Term Soil Pot Experiments: Growth Metrics and Biomass
The study demonstrates that endophytic bacterial isolates, combined with fertilizer, significantly enhance rice plant growth, biomass, and chlorophyll content over 150 days under heat stress (Fig. 3, and Fig. 4). This synergy between microbial inoculants and NPK50 fertilizer improved chlorophyll content (Fig. 4a) and bolstered plant resilience, highlighting the potential of microbial-nutrient interactions in optimizing crop productivity under stress (Anas et al. 2025).
Temporal dynamics in growth-promoting outcomes were observed, emphasizing the nuanced interplay between biological and environmental factors (Ku et al. 2024; Nizamani et al. 2024). These time-dependent effects underscore the importance of precise timing in agricultural interventions for maximal efficacy. Notably, isolates W, N, and D exhibited remarkable potential in augmenting crop yields under high-temperature stress (Fig. 3, and Fig. 4), offering valuable insights for enhancing agricultural productivity in climate-challenged regions.
Physicochemical Processes in Rice Plant Responses to Heat Stress
Under heat stress, rice plants exhibited increased proline production (Fig. 6a), which acts as an osmoprotectant and stabilizes proteins and membranes (Ghosh et al. 2022). The interactive effect of bacterial isolates and fertilizer further elevated proline content, enhancing plant resilience to heat stress (Pathania et al. 2020). This aligns with previous studies on Bacillus pumilus and SN13 under salt stress (Khan et al. 2020) and highlights the potential of microbial symbionts in boosting plant defenses (Pathania et al. 2020).
MDA levels decreased significantly in plants inoculated with bacterial isolates under heat stress (Fig. 6b), indicating reduced oxidative damage to cellular membranes (Jahan et al. 2019). This protective role underscores the potential of microbial symbionts and nutrient supplementation in mitigating lipid peroxidation and enhancing plant resilience (Jangra et al. 2024).
Heat stress induced SA production, with bacterial inoculation and fertilizer further increasing SA levels (Fig. 6c), suggesting enhanced heat stress tolerance (Sena et al. 2024; Yang et al. 2023). Interestingly, SA levels remained relatively stable at 35 °C and 40 °C during earlier intervals (30, 60, and 90 days) but increased significantly after 150 days (Fig. 6c). This pattern suggests that the observed SA increase may not be solely due to temperature but could also result from the cumulative effects of bacterial inoculation and fertilizer application over time (Urban et al. 2022; Wani et al. 2017). Additionally, the significant rise in SA at 150 days may coincide with a critical growth stage such as flowering or grain filling, where plants naturally produce higher levels of stress-related hormones to cope with developmental and environmental stresses (Bhattacharya and Bhattacharya 2021).
Similarly, ABA levels increased under stress but declined at 40 °C, indicating a critical threshold for ABA synthesis (Fig. 6d). The partial recovery of ABA levels in inoculated plants highlights the role of endophytic bacteria in mitigating severe heat stress effects (Mal and Panchal 2024). This phenomenon may reflect a shift in the plant’s stress-response strategy, where other mechanisms like proline accumulation or SA production become prioritized under prolonged or extreme stress conditions (Balasubramaniam et al. 2023).
Conclusion
The study demonstrates the potential of specific endophytic bacterial isolates and fertilizer to enhance rice thermotolerance, offering promising solutions for agriculture in challenging environments. The synergistic relationship between microbial inoculants and nutrients significantly improves plant biomass, chlorophyll content, and overall health under stress. This research contributes to sustainable agricultural practices, emphasizing the importance of microbial-nutrient interactions for enhanced crop productivity.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge Dr. Wei-An Lai for his technical support and expertise.
Author Contributions
Authorship Contribution StatementWonder Nathi Dlamini: Conceptualization, methodology, data collection, data curation, investigation, formal analysis, writing– original draft.Fo-Ting Shen: Project administration, supervision, funding acquisition, resources, data curation, formal analysis, validation, writing– review & editing.Wen-Ching Chen: Supervision, data curation, formal analysis, validation, writing– review & editing.Kuo-Pin Yu: Validation, writing– original draft, writing– review & editing.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing Interests
The authors declare no competing interests.
Generative AI in Scientific Writing
During the preparation of this work, the authors did not use generative AI.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kuo-Pin Yu, Email: kpyu03@nycu.edu.tw.
Wen-Ching Chen, Email: julychen@dragon.nchu.edu.tw.
Fo-Ting Shen, Email: ftshen@dragon.nchu.edu.tw.
References
- Afzal I, Shinwari ZK, Sikandar S, Shahzad S (2019) Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol Res 221:36–49 [DOI] [PubMed] [Google Scholar]
- Ahmad M, Imtiaz M, Shoib Nawaz M, Mubeen F, Imran A (2022) What did we learn from current progress in heat stress tolerance in plants? Can microbes be a solution? Front Plant Sci 13:794782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Waraich EA, Zulfiqar U, Ullah A, Farooq M (2023) Thiourea application increases seed and oil yields in camelina under heat stress by modulating the plant water relations and antioxidant defense system. J Soil Sci Plant Nutr 23:290–307 [Google Scholar]
- Ahmed B, Shahid M, Syed A, Rajput VD, Elgorban AM, Minkina T, Bahkali AH, Lee J (2021) Drought tolerant Enterobacter sp./leclercia adecarboxylata secretes indole-3-acetic acid and other biomolecules and enhances the biological attributes of vigna radiata (L.) R. Wilczek in water deficit conditions. Biology 10:1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar SS, Amby DB, Hegelund JN, Fimognari L, Großkinsky DK, Westergaard JC, Müller R, Moelbak L, Liu F, Roitsch T (2020) Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front Plant Sci 11:297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemneh AA, Cawthray GR, Zhou Y, Ryder MH, Denton MD (2021) Ability to produce Indole acetic acid is associated with improved phosphate solubilising activity of rhizobacteria. Arch Microbiol 203:3825–3837 [DOI] [PubMed] [Google Scholar]
- Allasia V, Ponchet M, Quentin M, Favery B, Keller H (2018) Quantification of Salicylic acid (SA) and SA-glucosides in Arabidopsis Thaliana. Bio-protocol 8:e2844–e2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anas M, Khalid A, Saleem MH, Ali Khan K, Ahmed Khattak W, Fahad S (2025) Symbiotic synergy: unveiling Plant-Microbe interactions in stress adaptation. J Crop Health 77:1–21 [Google Scholar]
- Arbona V, Manzi M, Zandalinas SI, Vives-Peris V, Pérez-Clemente RM, Gómez-Cadenas A (2017) Physiological, metabolic, and molecular responses of plants to abiotic stress. Stress Signal Plants Genom Proteomics Perspect 2:1–35
- Ayaz M, Ali Q, Jiang Q, Wang R, Wang Z, Mu G, Khan SA, Khan AR, Manghwar H, Wu H (2022) Salt tolerant Bacillus strains improve plant growth traits and regulation of phytohormones in wheat under salinity stress. Plants 11:2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam T, Shen G, Esmaeili N, Zhang H (2023) Plants’ response mechanisms to salinity stress. Plants 12:2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Bhattacharya A (2021) Role of plant growth hormones during soil water deficit: a review. In: Soil water deficit and physiological issues in plants, pp 489–583
- Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno LB, Karthik C, Ma Y, Kadirvelu K, Freitas H, Rajkumar M (2020) Amelioration of chromium and heat stresses in sorghum bicolor by Cr6 + reducing-thermotolerant plant growth promoting bacteria. Chemosphere 244:125521 [DOI] [PubMed] [Google Scholar]
- Carillo P, Gibon Y (2011) Protocol: extraction and determination of proline. PrometheusWiki 2011:1–5 [Google Scholar]
- Chang J, Shen F-T, Lai W-A, Liao C-S, Chen W-C (2023) Co-exposure of dimethomorph and Imidacloprid: effects on soil bacterial communities in vineyard soil. Front Microbiol 14:1249167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Li X, Liu X, Chen Y, Liang X, Leng J, Xu X, Liao W, Qiu Y, Wu Q (2020) Global projections of future urban land expansion under shared socioeconomic pathways. Nat Commun 11:537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-C, Ko C-H, Su Y-S, Lai W-A, Shen F-T (2021) Metabolic potential and community structure of bacteria in an organic tea plantation. Appl Soil Ecol 157:103762 [Google Scholar]
- Church DL, Cerutti L, Gürtler A, Griener T, Zelazny A, Emler S (2020) Performance and application of 16S rRNA gene cycle sequencing for routine identification of bacteria in the clinical microbiology laboratory. Clin Microbiol Rev 33. 10.1128/cmr.00053-00019 [DOI] [PMC free article] [PubMed]
- Ding Y, Shi Y, Yang S (2020) Molecular regulation of plant responses to environmental temperatures. Mol Plant 13:544–564 [DOI] [PubMed] [Google Scholar]
- Drancourt M, Bollet C, Carlioz A, Martelin R, Gayral J-P, Raoult D (2000) 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol 38:3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1566–1570 [Google Scholar]
- Duca DR, Glick BR (2020) Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl Microbiol Biotechnol 104:8607–8619 [DOI] [PubMed] [Google Scholar]
- Dudeja SS, Suneja-Madan P, Paul M, Maheswari R, Kothe E (2021) Bacterial endophytes: molecular interactions with their hosts. J Basic Microbiol 61:475–505 [DOI] [PubMed] [Google Scholar]
- Eid AM, Fouda A, Abdel-Rahman MA, Salem SS, Elsaied A, Oelmüller R, Hijri M, Bhowmik A, Elkelish A, Hassan SE-D (2021) Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants 10:935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwueme BN, Agunwamba JC (2021) Trend analysis and variability of air temperature and rainfall in regional river basins. Civil Eng J 7:816–826 [Google Scholar]
- El-Saadony MT, Saad AM, Soliman SM, Salem HM, Ahmed AI, Mahmood M, El-Tahan AM, Ebrahim AA, Negm SH (2022) Plant growth-promoting microorganisms as biocontrol agents of plant diseases: mechanisms, challenges and future perspectives. Front Plant Sci 13:923880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahad S, Adnan M, Noor M, Arif M, Alam M, Khan IA, Ullah H, Wahid F, Mian IA, Jamal Y (2019) Major constraints for global rice production. In: Advances in rice research for abiotic stress tolerance. Elsevier, pp 1–22
- Fukagawa NK, Ziska LH (2019) Rice: importance for global nutrition. J Nutri Sci Vitaminol 65:S2–S3 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Bhagwat T, Webster TJ (2021) Endophytic microbiomes and their plant growth-promoting attributes for plant health. Curr Trends Microb Biotechnol Sustainable Agric, 245–278
- Ghosh U, Islam M, Siddiqui M, Cao X, Khan M (2022) Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol 24:227–239 [DOI] [PubMed] [Google Scholar]
- Gibson J (2019) Rice import substitution and employment in Papua New Guinea
- Gomez D, Akuze A, Peterson S, Tetui M, Forsberg B, Hanson C (2019) The experience of farmers under the rice value chain programme in the Gambia. South Afr J Dev Res 1:55–73 [Google Scholar]
- Guo S, Pan R, Zhang Y, Gu Q, Shen Q, Yang J, Huang L, Shen Z, Li R (2025) Plant-microbe interactions influence plant performance via boosting beneficial root-endophytic bacteria. Environ Microbiome 20:18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikonen S, Heino M, Jalava M, Siebert S, Viviroli D, Kummu M (2025) Climate change threatens crop diversity at low latitudes. Nat Food, 1–12 [DOI] [PMC free article] [PubMed]
- Honda D, Yokota A, Sugiyama J (1999) Detection of seven major evolutionary lineages in cyanobacteria based on the 16S rRNA gene sequence analysis with new sequences of five marine Synechococcus strains. J Mol Evol 48:723–739 [DOI] [PubMed] [Google Scholar]
- Hsuan T-P, Jhuang P-R, Wu W-C, Lur H-S (2019) Thermotolerance evaluation of Taiwan Japonica type rice cultivars at the seedling stage. Bot Stud 60:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain K, Ahmad R, Nuñez MA, Dar TUH, Rashid I, Khuroo AA (2023) Plant invasion shifts soil Microbiome and physico-chemical attributes along an elevational gradient in Kashmir himalaya. Environ Sci Pollut Res 30:84283–84299 [DOI] [PubMed] [Google Scholar]
- Jahan MS, Wang Y, Shu S, Zhong M, Chen Z, Wu J, Sun J, Guo S (2019) Exogenous Salicylic acid increases the heat tolerance in tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci Hort 247:421–429 [Google Scholar]
- Jangra A, Kumar K, Maikhuri S, Bhandari MS, Pandey S, Singh H, Barthwal S (2024) Unveiling stress-adapted endophytic bacteria: characterizing plant growth-promoting traits and assessing cross-inoculation effects on Populus deltoides under abiotic stress. Plant Physiol Biochem 210:108610 [DOI] [PubMed] [Google Scholar]
- Kamran M, Imran QM, Ahmed MB, Falak N, Khatoon A, Yun B-W (2022) Endophyte-mediated stress tolerance in plants: A sustainable strategy to enhance resilience and assist crop improvement. Cells 11:3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Mudgal G, Chand K, Singh GB, Perveen K, Bukhari NA, Debnath S, Mohan TC, Charukesi R, Singh G (2022) An exopolysaccharide-producing novel Agrobacterium pusense strain JAS1 isolated from snake plant enhances plant growth and soil water retention. Sci Rep 12:21330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Devi R, Kumar S, Kour D, Yadav AN (2023) Plant growth promotion of Pearl millet (Pennisetum glaucum L.) by novel bacterial consortium with multifunctional attributes. Biologia 78:621–631 [Google Scholar]
- Khan MA, Asaf S, Khan AL, Jan R, Kang S-M, Kim K-M, Lee I-J (2020) Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol 20:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskey G, Mburu SW, Awino R, Njeru EM, Maingi JM (2021) Potential use of beneficial microorganisms for soil amelioration, phytopathogen Biocontrol, and sustainable crop production in smallholder agroecosystems. Front Sustainable Food Syst 130
- Kreyling J, Khan MAA, Sultana F, Babel W, Beierkuhnlein C, Foken T, Walter J, Jentsch AJE (2017) Drought effects in climate change manipulation experiments: quantifying the influence of ambient weather conditions and rain-out shelter artifacts. 20:301–315
- Ku YS, Liao YJ, Chiou SP, Lam HM, Chan C (2024) From trade-off to synergy: microbial insights into enhancing plant growth and immunity. Plant Biotechnol J 22:2461–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Singh S, Gaurav AK, Srivastava S, Verma JP (2020) Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Front Microbiol 11:1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao G, Wen J, Zhao N, Du G, Stanny M (2025) The measurement of agricultural disaster vulnerability in China and implications for land-supported agricultural resilience Building. Land Use Policy 148:107400 [Google Scholar]
- Lippmann R, Babben S, Menger A, Delker C, Quint M (2019) Development of wild and cultivated plants under global warming conditions. Curr Biol 29:R1326–R1338 [DOI] [PubMed] [Google Scholar]
- Luan L, Jiang Y, Dini-Andreote F, Crowther TW, Li P, Bahram M, Zheng J, Xu Q, Zhang X-X, Sun B (2023) Integrating pH into the metabolic theory of ecology to predict bacterial diversity in soil. Proc Nat Acad Sci 120:e2207832120 [DOI] [PMC free article] [PubMed]
- Lyu Y, Yang X, Pan H, Zhang X, Cao H, Ulgiati S, Wu J, Zhang Y, Wang G, Xiao Y (2021) Impact of fertilization schemes with different ratios of Urea to controlled release nitrogen fertilizer on environmental sustainability, nitrogen use efficiency and economic benefit of rice production: A study case from Southwest China. J Clean Prod 293:126198 [Google Scholar]
- Mageshwaran V (2024) New insights into the taxonomy of Bacillus and related genera in relevance to their antimicrobial peptides. In: Applications of Bacillus and Bacillus derived genera in agriculture, biotechnology and beyond. Springer, pp 253–265
- Maitra S, Pramanick B, Dey P, Bhadra P, Shankar T, Anand K (2021) Thermotolerant soil microbes and their role in mitigation of heat stress in plants. Functional Annotation, Soil Microbiomes for Sustainable Agriculture, pp 203–242 [Google Scholar]
- Mal S, Panchal S (2024) Drought and salt stress mitigation in crop plants using stress-tolerant auxin-producing endophytic bacteria: a futuristic approach towards sustainable agriculture. Front Plant Sci 15:1422504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Kaur M, Kaushik P (2021) Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 13:1318 [Google Scholar]
- Malik G, Singh N, Hooda S (2020) Nitrogen stress in plants and the role of phytomicrobiome. In: Phyto-microbiome in stress regulation. Springer, pp 295–322
- Montes-Luz B, Conrado AC, Ellingsen JK, Monteiro RA, de Souza EM, Stacey G (2023) Acetylene reduction assay: A measure of nitrogenase activity in plants and bacteria. Curr Protocols 3:e766 [DOI] [PubMed] [Google Scholar]
- Morcillo RJ, Manzanera M (2021) The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 11:337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad M, Wahab A, Waheed A, Mohamed HI, Hakeem KR, Li L, Li W-J (2024) Harnessing bacterial endophytes for environmental resilience and agricultural sustainability. J Environ Manage 368:122201 [DOI] [PubMed] [Google Scholar]
- Nizamani MM, Hughes AC, Qureshi S, Zhang Q, Tarafder E, Das D, Acharya K, Wang Y, Zhang Z-G (2024) Microbial biodiversity and plant functional trait interactions in multifunctional ecosystems. Appl Soil Ecol 201:105515 [Google Scholar]
- Onyango DA (2020) Physiological and molecular mechanisms of iron-toxicity tolerance in rice and implication for breeding. Maseno University
- Ortiz M, Leung PM, Shelley G, Jirapanjawat T, Nauer PA, Van Goethem MW, Bay SK, Islam ZF, Jordaan K, Vikram S (2021) Multiple energy sources and metabolic strategies sustain microbial diversity in Antarctic desert soils. Proc Nat Acad Sci 118: e2025322118 [DOI] [PMC free article] [PubMed]
- Pandey PK, Samanta R, Yadav RNS (2019) Inside the plant: addressing bacterial endophytes in biotic stress alleviation. Arch Microbiol 201:415–429 [DOI] [PubMed] [Google Scholar]
- Patel JK, Archana G (2017) Diverse culturable diazotrophic endophytic bacteria from Poaceae plants show cross-colonization and plant growth promotion in wheat. Plant Soil 417:99–116 [Google Scholar]
- Pathak H, Tripathi R, Jambhulkar N, Bisen J, Panda B (2020) Eco-regional-based rice farming for enhancing productivity, profitability and sustainability
- Pathania P, Rajta A, Singh PC, Bhatia R (2020) Role of plant growth-promoting bacteria in sustainable agriculture. Biocatal Agric Biotechnol 30:101842 [Google Scholar]
- Pati S, Pal B, Badole S, Hazra GC, Mandal B (2016) Effect of silicon fertilization on growth, yield, and nutrient uptake of rice. Commun Soil Sci Plant Anal 47:284–290 [Google Scholar]
- Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9:120 [Google Scholar]
- Prakash J, Mishra S (2022) Role of beneficial soil microbes in alleviating climatic stresses in plants. In: Microbiome under changing climate. Elsevier, pp 29–68
- Prakash HP, Verma O, Chaudhary AK, Amir M (2018) Correlation and path coefficient analysis in rice (Oryza sativa L.) for sodicity tolerance. Int J Curr Microbiol App Sci 7:177–187 [Google Scholar]
- Rai S, Omar AF, Rehan M, Al-Turki A, Sagar A, Ilyas N, Sayyed R, Hasanuzzaman M (2023) Crop microbiome: their role and advances in molecular and omic techniques for the sustenance of agriculture. Planta 257:27 [DOI] [PubMed] [Google Scholar]
- Rosselli R, Romoli O, Vitulo N, Vezzi A, Campanaro S, de Pascale F, Schiavon R, Tiarca M, Poletto F, Concheri G (2016) Direct 16S rRNA-seq from bacterial communities: a PCR-independent approach to simultaneously assess microbial diversity and functional activity potential of each taxon. Sci Rep 6:32165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoyo G, Moreno-Hagelsieb G, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99 [DOI] [PubMed] [Google Scholar]
- Sarker A, Ansary MWR, Hossain MN, Islam T (2021) Prospect and challenges for sustainable management of climate change-associated stresses to soil and plant health by beneficial rhizobacteria. Stresses 1:200–222 [Google Scholar]
- Sena L, Mica E, Valè G, Vaccino P, Pecchioni N (2024) Exploring the potential of endophyte-plant interactions for improving crop sustainable yields in a changing climate. Front Plant Sci 15:1349401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Nazari M, Antar M, Msimbira LA, Naamala J, Lyu D, Rabileh M, Zajonc J, Smith DL (2021) PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front Sustainable Food Syst 5:667546 [Google Scholar]
- Stevanović O, Milanov D, Prošić I, Gajdov V, Nedić D, Sladojević Ž, Radalj A (2023) Multilocus sequence analysis (MLSA) of a nocardia cyriacigeorgica strain causing severe bovine mastitis in Bosnia and Herzegovina. Acta Veterinaria Hungarica 71:65–70 [DOI] [PubMed] [Google Scholar]
- Sullivan MK, Fayolle A, Bush E, Ofosu-Bamfo B, Vleminckx J, Metz MR, Queenborough SA (2023) Cascading effects of climate change: new advances in drivers and shifts of tropical reproductive phenology. Plant Ecol, 1–13
- Tiwari S, Lata C, Chauhan PS, Nautiyal CSJPP and Biochemistry (2016) Pseudomonas Putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. 99:108–117 [DOI] [PubMed]
- Tsotetsi T, Nephali L, Malebe M, Tugizimana F (2022) Bacillus for plant growth promotion and stress resilience: what have we learned? Plants 11:2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukozkan N, Erdamar H, Seven I (2006) Measurement of total malondialdehyde in plasma and tissues by high-performance liquid chromatography and thiobarbituric acid assay. Firat Tip Dergisi 11:88–92 [Google Scholar]
- Uelze L, Grützke J, Borowiak M, Hammerl JA, Juraschek K, Deneke C, Tausch SH, Malorny B (2020) Typing methods based on whole genome sequencing data. One Health Outlook 2:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A, Nisar M, Ali H, Hazrat A, Hayat K, Keerio AA, Ihsan M, Laiq M, Ullah S, Fahad S (2019) Drought tolerance improvement in plants: an endophytic bacterial approach. Appl Microbiol Biotechnol 103:7385–7397 [DOI] [PubMed] [Google Scholar]
- Urban L, Lauri F, Ben Hdech D, Aarrouf J (2022) Prospects for increasing the efficacy of plant resistance inducers stimulating Salicylic acid. Agronomy 12:3151 [Google Scholar]
- Vandana UK, Rajkumari J, Singha LP, Satish L, Alavilli H, Sudheer PD, Chauhan S, Ratnala R, Satturu V, Mazumder PB (2021) The endophytic Microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 10:101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst R, Verstraelen H, Claeys G, Verschraegen G, Delanghe J, Van Simaey L, De Ganck C, Temmerman M (2004) and M. J. B. m. Vaneechoutte. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. 4:16 [DOI] [PMC free article] [PubMed]
- Wani AB, Chadar H, Wani AH, Singh S, Upadhyay N (2017) Salicylic acid to decrease plant stress. Environ Chem Lett 15:101–123 [Google Scholar]
- Willems A, Collins M (1993) Phylogenetic analysis of rhizobia and agrobacteria based on 16S rRNA gene sequences. Int J Syst Bacteriol 43:305–313 [DOI] [PubMed] [Google Scholar]
- Williams P, De Mallorca MS (1982) Abscisic acid and gibberellin-like substances in roots and root nodules of Glycine max. Plant Soil 65:19–26 [Google Scholar]
- Yang H, Fang R, Luo L, Yang W, Huang Q, Yang C, Hui W, Gong W, Wang J (2023) Uncovering the mechanisms of Salicylic acid-mediated abiotic stress tolerance in horticultural crops. Front Plant Sci 14:1226041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu T, Zhang X, Xie J, Wang Y, Yan R, Jiang Y, Zhu D (2021) Cultivable endophytic bacteria in seeds of Dongxiang wild rice and their role in plant-growth promotion. Diversity 13:665 [Google Scholar]
- Zia R, Nawaz MS, Yousaf S, Amin I, Hakim S, Mirza MS, Imran A (2021) Seed inoculation of desert-plant growth‐promoting rhizobacteria induce biochemical alterations and develop resistance against water stress in wheat. Physiol Plant 172:990–1006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.








