Abstract
An outbreak of infection with Mycobacterium chelonae subsp. abscessus after the injection of penicillin in 86 patients attending a factory hospital is reported. The bacterium was isolated both from lids and from the soil where the drug was stored. Molecular analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of whole-cell proteins and plasmids revealed a pattern identical to that of the strains isolated from the wounds. The source of the infections was soil contamination of the vial lids and was caused by improper use and sterilization of penicillin vials.
Mycobacterium chelonae subsp. abscessus, a rapidly growing atypical mycobacterium, is part of the normal flora in the respiratory tract and the digestive tract of healthy human beings and animals and is widely distributed in soil, wastewater, and other materials. As a human pathogen, it seldom infects the lungs, but it often causes infections of skin and soft tissues, especially local infections and abscesses after surgery or intramuscular injection (1-3). Here we describe infection on a large scale caused by M. chelonae subsp. abscessus after the injection of penicillin, the investigation of the reason for the infection, and the development of prevention measures.
From January 1997 to July 1998, a factory hospital in Chongqing, China, received 86 patients with local infections at injection sites, on the right or left buttock or both buttocks. Among them, 31 were male and 55 were female, the oldest was 80 years old and the youngest was merely 1 year 1 month old, 55 (64%) were more than 55 years old and 9 (10.5%) were less than 6 years old, and 22 (25.5%) were between 55 and 6 years old. Initially, the skin of the injection sites of the patients became red and swollen, and then scleroma appeared and an abscess formed. The pus was thin and did not give off any extraordinary odor. After the necrotic tissue was resected and the pus was drained, the inflammation still could not be controlled, and the wound would not heal. The infection spread along the interstitial spaces and caused inguinal lymphadenitis through lymphatic vessels. However, general symptoms, such as a sensation of chills or fever, and lung infection did not occur.
Samples from the pus, curettage deep in the abscess, and swollen lymph nodes of all patients were examined. The bacteriologic examination was carried out by microscopy of paraffin sections of these materials stained by the Gram stain method. Many gram-positive bacilli were arranged in V or L shapes in the interstitial spaces, with deeply stained granules in the bodies of the bacilli (Fig. 1). After being cultured on blood agar plates for 3 days at 35°C, the organism developed into smooth, mucoid, round, thin, yellow colonies without hemolysis. At that time, according to the shape and staining characteristics, the organism would have been identified as Corynebacterium. Examination with the API Coryne autoanalytical system (Biomerieux, Marcy l'Etoile, France) indicated that the organism was most likely Corynebacterium equi. The pathological diagnosis was based on the granulomatous inflammation seen with curettage deep in the abscess and tuberculoid necrosis with swollen lymph nodes (Fig. 2). Drug susceptibility testing revealed that the organism was sensitive to amikacin, kanamycin, gentamicin, and vancomycin. On the basis of the results described above, the necrotic tissues were ablated, the pus was drained repeatedly, and amikacin, kanamycin, gentamicin, and vancomycin were applied locally and systematically on an alternating basis. All patients were cured in 6 months to 1 year, without any complications.
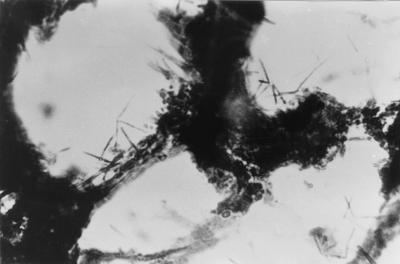
FIG. 1.
Bacteria in a pathogenic slice from an infectious focus in a patient. The specimen shows many gram-positive bacilli arranged in V or L shapes with observable dark granules within the tissue spaces. Gram stain. Oil immersion. Magnification, ×1,000.
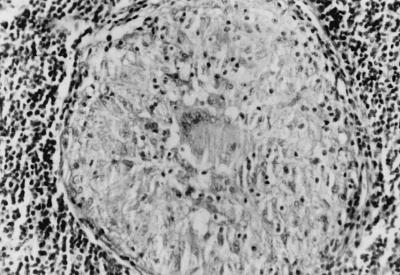
FIG. 2.
Swollen lymph nodes in a patient. The specimen shows granulomatous inflammation and tuberculoid necrosis. Hematoxylin-eosin stain. Magnification, ×200.
When we examined the history of the infection, we found that all the patients had had penicillin G injected in the buttocks at the outpatient department of the hospital and that the abscesses occurred at the injection sites. During this period, a total of 850 patients were injected with the same batch of drug, and the incidence of infection was 10.1%. The incidences of infection were 33.5% (55 of 164) for patients 55 years old or older, 15.8% (9 of 57) for patients 6 years old or younger, and 3.5% (22 of 629) for patients between 6 and 55 years old. A total of 24 patients (27.9%) became symptomatic within 1 month, 16 (18.6%) became symptomatic within 6 months, and 46 (53.5%) became symptomatic within 20 to 30 days (average, 1.85 months). Drug in the remaining bottles of the same batch and residual drug in the used bottles were repeatedly examined by the local pharmaceutical bureau, and no bacterium was found.
A total of 86 strains isolated from samples from the pus, curettage deep in the abscess, and swollen lymph nodes of the patients were further identified. The bacteria were gram-positive bacilli, but they were difficult to stain and were acid fast. They did not produce pigment, and they grew at 45°C. They grew on MacConkey medium but not on 5% NaCl medium, and they could utilize citrate at 28°C. The results of tests of iron absorption were positive, and the results of tests of nitrate reduction were negative. The contaminating bacteria were finally identified as M. chelonae subsp. abscessus in accordance with their characteristics.
In order to explore the course of the contamination, seven lids from bottles of penicillin G from the same batch as that used and stored at the outpatient department of the hospital were wiped clear with cotton swabs soaked in sterile normal saline. The organisms on the cotton swabs were washed and placed in 10 ml of bovine brain heart medium containing 200 μg of penicillin G/ml, 100 μg of cefoperazone/ml, and 250 μg of amphotericin B (Fungizone)/ml. After incubation for 3 days at 35°C, smears of 0.1 ml of culture fluid were placed on plates with bile salt agar containing the three above-mentioned antibacterial agents and cultured for 3 to 6 days at 35°C. A total of 25 soil samples were obtained randomly from the drug storage ground. One gram of soil from each sample was mixed with 9 ml of normal saline; the mixture was diluted to concentrations of 10−2, 10−3, 10−4, 10−5, and 10−6; and smears of 0.1 ml of each dilution were placed on plates as described above. The agar plates were cultured for 3 to 6 days at 35°C. A single colony was isolated from concentrations of 10−5 and 10−6. The M. chelonae subsp. abscessus strains isolated from the lids and soil samples were identified as described above. One strain of M. chelonae subsp. abscessus was obtained from one of the seven lids, and one strain was obtained from 1 of the 25 samples of soil.
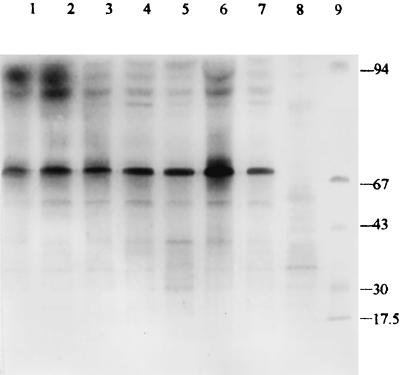
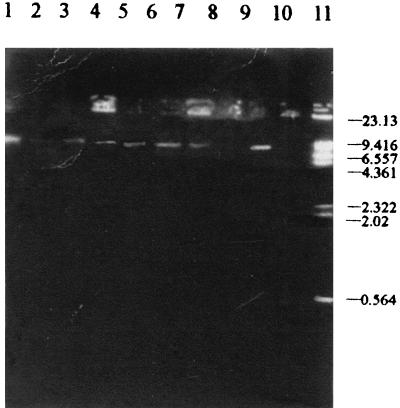
In order to evaluate whether the M. chelonae subsp. abscessus isolates were isogenic, sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis of whole-cell proteins and plasmids was carried out with 50 strains (36 strains were lost) isolated from the samples in the outbreak, the strain from the lids, and the strain from the soil samples. A reference strain of M. chelonae subsp. abscessus (93325) was obtained from the Pharmaceutical and Biological Certification Bureau of China, and a reference strain of C. equi (ATCC 7301) was a generous gift from Chongqing Health and Epidemic Prevention Institute. The preparation of protein samples and electrophoresis were carried out by the Laemmli method (4). Plasmids were extracted and electrophoresed by the method of Wallace et al. (8). The electrophoresis bands of the strains isolated from the lids and soil samples were similar to those of the strains isolated from the clinical samples and the reference strain of M. chelonae subsp. abscessus. All had major bands of 94, 91, 87, 85, 69, 59, 50, 35, and 32 kDa. The reference strain of C. equi did not have these bands but had bands of 91, 83, 60, 52, 45, and 33 kDa (Fig. 3). A large plasmid of 9.4 kb was found in the 50 strains isolated from the clinical samples, lids, and soil samples but not in the reference strain of M. chelonae subsp. abscessus. The plasmid in 18 randomly isolated strains could be eliminated by norfloxacin (5) (Fig. 4). When the plasmid was eliminated, the MIC of penicillin G was not changed.
FIG. 3.
Coomassie blue-stained SDS-PAGE profiles of whole-cell proteins from M. chelonae subsp. abscessus. Lanes: 1 to 4, strains isolated from clinical samples; 5, strain isolated from lids; 6, strain isolated from soil samples; 7, reference strain of M. chelonae subsp. abscessus; 8, C. equi reference strain; 9, molecular size markers (kilodaltons).
FIG. 4.
Plasmid profiles of M. chelonae subsp. abscessus. Lanes: 1 to 5 and 9, strains isolated from clinical samples; 6, strain isolated from lids; 7, strain isolated from soil samples; 8, strain with plasmids eliminated; 10, reference strain of M. chelonae subsp. abscessus; 11, molecular size markers (kilobases).
In order to evaluate the cause of the contamination, the resistance of 18 strains randomly isolated from the samples to 2% iodine tincture and 75% ethanol was tested by the method of Mei et al. (6). The MIC of penicillin G for the bacterium was determined as described by Swenson et al. (7). We found that 105 CFU/ml of bacteria could be killed by iodine tincture and ethanol in 30 min and that the MIC of penicillin G was >4 mg/ml.
M. chelonae is a common soil contaminant and is widely distributed in soil. Although there might have been small differences in the minor bands in the SDS-PAGE profiles of whole-cell proteins among the strains from the lids, the soil where the drug was stored, and the wounds (and a few polymorphisms could have been differentiated by DNA analysis), all the strains isolated from the clinical samples, lids, and soil samples had an identical pattern, in particular, the same plasmid; therefore, it was suggested that all the strains came from the same source, i.e., the soil. Field observations showed that the drug was stored on the humid ground of the basement; the packages were damaged, and the bottles were scattered on the ground. Therefore, the drug bottles were contaminated by M. chelonae from soil.
During injection, the nurses often arranged dozens of bottles in a line and pried up the center of the aluminum shields of the bottles beforehand. Then, they wiped the rubber lids with only one cotton swab dipped in 2% tincture of iodine successively and rapidly from the first to the last lid and repeated this procedure once more with a cotton swab containing 75% ethanol. When they sterilized the last lid, the residual tincture of iodine or ethanol on the cotton swab was small. With this sterilization method, the organisms on the lids could not be killed; instead, the bacteria contaminated the aluminum shields beside the rubber lids. For injection, about 2 ml of normal saline was placed into the bottle to dissolve the pink penicillin G (8 or 16 mg/bottle; 4 or 8 mg/ml); when the drug solution was taken out of the bottle, it became contaminated by bacteria. Because the organism was resistant to penicillin G, it grew slowly in the focus where the drug was injected and caused infection.
Another piece of convincing evidence was that in other departments of the same hospital, when nurses found bottles from the same batch adherent to soil, they washed the bottles and dried them. Before injecting the drug, they sterilized every rubber lid with a cotton swab dipped in 2% iodine tincture from inside to outside and then repeated the procedure with a cotton swab containing 75% ethanol. No infection occurred. It was clear that the reason for the outbreak of infection was not that the bacteria produced resistance to iodine and ethanol but that the sterilization method was improper.
Although infections caused by M. chelonae subsp. abscessus are well known, an infection on such a large scale had not yet been reported. This case provides a warning that bottles must be kept clean and strict sterilization measures must be carried out, so that iatrogenic infection caused by M. chelonae subsp. abscessus can be prevented.
Acknowledgments
We extend sincere thanks to the doctors and nurses at Jianshe Hospital of Chongqing, Jianshe Industrial Company, who provided the prevalence data and the materials for the experiments in our study.
REFERENCES
- 1.Borghans, J. G. A., and L. Stanford.1973. Mycobacterium chelonei in abscesses after injection of diphtheria-pertussis-tetanus-polio vaccine. Am. Rev. Respir. Dis. 107:1-8. [DOI] [PubMed] [Google Scholar]
- 2.Covert, T. C., M. R. Rodgers, A. L. Reyes, et al.1999. Occurrence of nontuberculous mycobacteria in environmental samples. Environ. Microbiol. 65:2492-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inman, P. M., A. Beck, A. E. Brown, and J. L. Stanford.1969. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch. Dermatol. 100:141-147. [PubMed] [Google Scholar]
- 4.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 5.Li, L. G., Z. W. Wang, and W. Z. Hu.1994.. Elimination of drug resistance plasmids from Shigella by norfloxacin and berberine. Chin. J. Infect. Dis. 12: 1-7.
- 6.Mei, J. M., S. Pan, Z. T. Yuan, et al. 1991. Study on resistance of Campylobacter pylori to physical and chemical agents.Chin. J. Disinfect. 8:147-150. [Google Scholar]
- 7.Swenson, J. M., C. Thornsberry, V. A. Silcox.1982. Rapidly growing mycobacteria: testing of susceptibility to 34 antimicrobial agents by broth microdilution. Antimicrob. Agents Chemother. 22:186-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace, R. J., S. I. Hull, et al.1985. Mutational resistance as the mechanism of acquired drug resistance to aminoglycosides and antibacterial agents in Mycobacterium fortuitum and Mycobacterium chelonei.Am. Rev. Respir. Dis. 132:409-416. [DOI] [PubMed] [Google Scholar]