Abstract

The agglomeration of micrometer-sized platinum particles prepared by chemical liquid-phase reduction results in inadequate dispersion, a wide particle size distribution, and low tap density, thereby impacting the application of the platinum powder. In this study, CaO and platinum powder were homogeneously mixed through ball milling and subsequently subjected to heat treatment at temperatures ranging from 700 to 1300 °C. Following treatment at 1100 °C, the particle size distribution of platinum became narrower with SD (D90-D10) decreasing from 14.5 to 3.23 μm, while the tap density increased by 76% compared to pretreatment levels. These findings suggest that barrier-heat treatment effectively prevents sintering and agglomeration of platinum powder. During heat treatment, atomic and grain boundary diffusion facilitated the dispersion of the platinum powder within the CaO matrix, leading to grain coarsening, densification of particle structure, improved dispersion properties, and increased tap density with a narrowed particle size distribution. This paper presents a novel method for preparing a high-performance platinum powder.
1. Introduction
Platinum powder exhibits high melting point, exceptional catalytic activity, excellent electrical and thermal conductivity, remarkable corrosion resistance, and outstanding resistance to high-temperature oxidation. It serves as an indispensable fundamental material in various fields including electronic information, energy conversion, automotive industry, water treatment sector, atmospheric purification applications, and biomedical research.1−9 Physical, chemical, and biological methodologies are commonly employed in the synthesis of precious metal powders, such as platinum powder.10−12 The physical method exhibits drawbacks, such as high reliance on equipment, substantial energy consumption, stringent preparation conditions, and low production efficiency. On the other hand, the biological method encounters challenges including an extended preparation period, difficulties in separation and particle size control, and low purity. In comparison to both physical and biological methods, chemical methods—particularly the chemical liquid-phase reduction method—offer simplicity and efficiency in controlling powder morphology and particle size by adjusting process parameters such as concentration, temperature, and pH of the precursor and reducing agent under ambient conditions. Consequently, they have emerged as the primary technique for preparing precious metal powders such as platinum.1,13−21
The single platinum nanocrystal exists in a metastable state and possesses the characteristics of small volume, large specific surface area, high surface energy, and high chemical reactivity. In chemical liquid-phase reduction media, nanocrystalline particles tend to aggregate and exhibit poor dispersibility due to intermolecular electrostatic forces, hydrogen bonding, and van der Waals interactions, especially on contact and collision.16,22−24 Consequently, the prepared platinum powder comprises agglomerated micron and submicron polycrystalline structures formed by nanocrystals with an average particle size of approximately 10 μm.17,25 Due to the presence of dispersants and adsorbed gases on the surface of microcrystalline grains, agglomerates form porous and loosely packed platinum micrometer-sized powders with low tap density. The process of preparing slurry sintering poses a series of technical challenges, including high shrinkage rate, low adhesion, high contact resistance, poor catalytic activity, limited oxygen sensitivity, slow response speed, and short service life.17 It is of paramount significance to address the agglomeration of platinum powder fabricated by chemical liquid-phase reduction and to enhance its dispersion. Currently, surface modification reagents, such as polyvinylpyrrolidone (PVP), gum arabic, and sodium alginate, are prevalently employed to augment the dispersion of the powder.17,26,27 However, surfactants featuring low surface energy are hard to be removed from the surface of platinum particles, which has an impact on their reaction activity and the purity of platinum powder.28
In this study, micrometer-sized platinum powder was fabricated via the chemical liquid reduction method. CaO, which possesses thermal stability, was utilized as a barrier agent and mixed with micrometer-sized platinum powder and a dispersed liquid. Subsequently, grinding was carried out for dispersion, followed by heat treatment. The influences of the heat treatment temperature on the phase structure, surface morphology, dispersibility, particle size distribution, and tap density of platinum powder under the action of CaO were examined. The preparation of high-performance ultrafine platinum powder and its application in the domain of electronic pastes are presented herein.
2. Experimental Section
2.1. Preparation and Heat Treatment of Platinum Powder
Micron-sized platinum powder was synthesized through chemical liquid-phase reduction at room temperature and atmospheric pressure using Na2PtCl6 (self-prepared) as the precursor (1 wt % aqueous solution of platinum) and N2H4·H2O (Chengdu Kelong Chemical Co., Ltd.) as the reducing agent (1 wt %). Na2PtCl6 was added dropwise into N2H4·H2O at a drop rate of 4 mL/min. Subsequently, 10 g of platinum powder, 90 g of CaO (Chengdu Kelong Chemical Co., Ltd.) as a barrier agent with excellent thermal stability (weight loss <5% in the temperature range of 25–1500 °C, Figure 1), and 100 g of dispersion liquid (ethanol) (Chongqing Maoye Chemical Reagent Co., Ltd.) were introduced into an agate ball-milling jar for grinding purposes. The process was conducted at ambient temperature, with a grinding speed of 300 rpm (rpm) for a duration of 4 h, and the volume ratio of grinding balls to materials was maintained at 3:1.
Figure 1.
TG curve of CaO.
The mixture was subjected to ball milling and dispersion for 4 h using a planetary ball mill, followed by drying at 80 °C for 20 h to remove ethanol. Subsequently, the platinum powder and barrier agent mixture underwent high-temperature treatment in an air atmosphere at temperatures ranging from 700 to 1300 °C for 2 h. The mixture was cooled to room temperature under an air atmosphere after heat treatment. The cooled mixture was dissolved in 5 wt % HCl (Chongqing Chuandong Chemical (Group) Co., Ltd.) aqueous solution to eliminate CaO, followed by repeated washing and precipitation with deionized water. Finally, it was tested with 1 wt % AgNO3 (Chongqing Chuandong Chemical (Group) Co., Ltd.) aqueous solution to confirm the removal of Cl–. On confirmation of the removal of Cl–, the powder was washed two to three times with anhydrous ethanol and dried at 80 °C under vacuum conditions for 20 h in order to obtain the heat-treated platinum powder. A schematic diagram is presented in Figure 2.
Figure 2.

Schematic diagram of the preparation and heat treatment of platinum powder.
2.2. Characterization Analysis
The microstructure of platinum powder was characterized by using scanning electron microscopy (SEM) (Zeiss EVO 10) and field-emission scanning electron microscopy (FESEM) (JSM-7800F). The morphology and crystal structure of platinum powder were examined using transmission electron microscopy (TEM) (FEI Talos F200X) after focused ion beam (FIB) preparation. X-ray diffraction (XRD) analysis was performed on the crystal structure of platinum powder using a PANalytical X’Pert3 powder diffractometer equipped with a Cu Kα radiation source, offering a resolution of 0.02° and a scanning speed of 8°·min–1. The tap density of platinum powder was determined by using a tap density tester (HY-100A). Laser diffraction particle size analysis was conducted on the particle distribution using a Micromeritics S3500 instrument, where the D90-D10 value represents the particle size distribution and it is referred to as SD. For the determination of tap density and particle distribution, three replicate samples were prepared for each specimen and the average of the test results was calculated.
3. Results and Discussion
3.1. Preparation of Micron-Sized Platinum Powder
Platinum powder was prepared by chemical liquid-phase reduction, where the Na2PtCl6 precursor solution was dropwise added to the N2H4·H2O solution, showing micron-sized spherical and quasi-spherical shapes (Figure 3a), with a relatively uniform particle distribution. The individual particle size ranged from 0.3 to 1.3 μm. There was obvious agglomeration among particles with several particles connected to each other, resulting in a wide particle size distribution, with D10 = 1.81 μm, D50 = 4.54 μm, and D90 = 16.31 μm (Figure 3b). FESEM analysis revealed that the surface of the micron-sized platinum particles was irregular, featuring prominent microprotrusions and pronounced intergranular pores (Figure 3c). This suggests that the micron-sized platinum powder prepared by chemical liquid-phase reduction was formed by the agglomeration of nanoscale microcrystals, and the presence of particle agglomeration led to a wide particle size distribution and a low tap density of only 5.9 g/mL.
Figure 3.
Properties of micrometer-sized platinum powder: (a) SEM, (c) FESEM inset in (a), and (b) particle size distribution.
3.2. Investigation on Enhancing the Dispersion of Platinum Powder
3.2.1. Pressing and Sintering of Platinum Powder
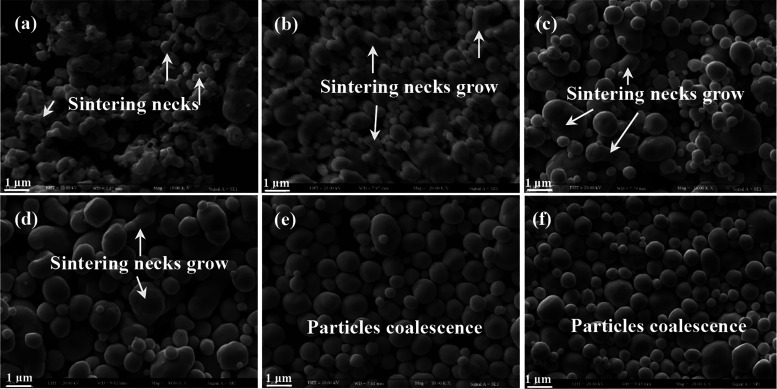
To facilitate the heat treatment process, the micrometer-sized platinum powder fabricated by chemical liquid-phase reduction was compressed under a pressure of 8 MPa for 30–40 s and subsequently subjected to heat treatment at temperatures ranging from 500 to 1300 °C for 2 h. The unsintered platinum powder is nearly spherical after compression, and the platinum particles on the surface are deformed due to the pressure effect, resulting in a distribution of smaller-sized platinum particles on the surface (Figure 4a). After 2 h of heat treatment at 500 °C, the particle size significantly decreased, and there was an obvious sintering neck between the particles, with the local connection being flaky (Figure 4b). When the temperature was further increased to 700 °C, the sintering neck formed by micrometer-sized platinum powder was connected to a sheet with obvious pores on the surface (Figure 4c). With the gradual increase in the heat treatment temperature (900–1300 °C), the micrometer-sized platinum particles fully merged into bulk materials, with a significant reduction in the number of pores and an increase in grain size (Figure 4d–f). Fine platinum particles have certain specific properties, such as small particle size, high chemical activity, and high surface energy. The high-temperature heat treatment process induces atomic oscillations and facilitates particle contact and diffusion. The sintering neck is gradually formed and gradually grows because of atomic diffusion and completely merges into larger particles, which reduces the surface energy of the platinum powder. The porosity decreases significantly with the increase of the grain size, and the grain size increases with the increase of temperature.29−31 This indicates that the direct heat treatment of micrometer-sized platinum powder leads to sintering and agglomeration between particles, resulting in the formation of bulk metallic materials, which fails to achieve the desired improvement in dispersion.
Figure 4.
SEM images of the pressing and sintering of platinum powder: (a) without sintering, (b) 500 °C, (c) 700 °C, (d) 900 °C, (e) 1100 °C, and (f) 1300 °C.
3.2.2. Barrier-Heat Treatment of Platinum Powder
CaO was employed as a barrier agent, while ethanol served as a dispersant. Subsequently, the mixture underwent grinding and dispersion with platinum powder, followed by thorough blending and subsequent drying. Finally, it underwent heat treatment at temperatures ranging from 700 to 1300 °C. The results demonstrate that on lower temperature heat treatment (700 °C) for 2 h, the platinum particles exhibited diverse shapes including spherical, quasi-spherical, and rod-like shapes with a loose structure. Notably observed were significant necking phenomena and interconnections between particles (Figure 5a). As the heat treatment temperature increased to 800–1000 °C, the surface of platinum particles became smoother. The irregular shapes transformed into quasi-spherical forms, accompanied by a decrease in rod-shaped particles and an increase in smaller particles. Additionally, it was observed that the connecting parts exhibited neck-like structures, with an enlargement in their dimensions (Figure 5b–d). The heat efficiency increased as the temperature continued to rise to 1100–1300 °C, resulting in an accelerated diffusion rate of atoms within the micron-sized particles. This led to the volatilization of adsorbed gases and a reduction in the porosity for nanoscale particles. Consequently, there was a decrease in the particle volume, leading to a denser platinum powder. Additionally, the disappearance of necking and the occurrence of particle merging resulted in a more uniform morphology primarily composed of spherical and quasi-spherical shapes, significantly improving particle dispersion. At 1300 °C, compared to 1100 °C, the higher heat treatment temperature caused neighboring micron-sized particles to melt and grow, resulting in larger particles (Figure 5e,f). These findings indicate that employing CaO as a barrier agent and increasing the heat treatment temperature can greatly enhance the uniformity and dispersion of platinum particles.
Figure 5.
SEM images of platinum powder heat-treated with CaO as a barrier agent: (a) 700 °C, (b) 800 °C, (c) 900 °C, (d) 1000 °C, (e) 1100 °C, and (f) 1300 °C.
3.3. Impact of Barrier-Heat Treatment on Size Distribution and Tap Density
The impact of heat treatment on the particle size distribution and tap density of platinum powder prepared through a chemical liquid-phase reduction was investigated in this study. The as-prepared platinum powder exhibited a wide particle size distribution with a high SD value of 14.5 μm. However, with increasing heat treatment temperature, the particle size distribution became more concentrated. Specifically, after heat treatment at 700 °C, the SD value decreased to 6.78 μm. Furthermore, at 1100 °C, the particle size distribution of platinum powder showed even higher concentration compared with that at 700 °C, with SD being only 48% of that observed at 700 °C (Figure 6a). The platinum powder prepared initially had a relatively low tap density (5.9 g/mL) due to significant particle agglomeration, large interparticle porosity, rough surface morphology, and high interparticle friction. The surface treatment of platinum powder, prepared via chemical liquid-phase reduction using a silane coupling agent, has a negligible effect on the tap density; it remains almost unchanged, with a value of 6.4 g/mL after the treatment. However, the tap density of the platinum powder generally increased after heat treatment, with the effect of temperature being significant. Treatment at 700 °C resulted in diverse particle morphologies and wide particle size distribution for the platinum powder, leading to a tap density of only 6.11 g/mL. As the heat treatment temperature increased to 800–1000 °C, the particle shape gradually transformed from irregular to quasi-spherical and spherical. Additionally, the particle size distribution became smaller (SD < 5 μm), while the tap density increased to 7.8–8.9 g/mL. After heat treatment at 1100 °C, the platinum powder exhibited enhanced density and a predominantly spherical shape, leading to improved uniformity and a smaller particle size distribution (SD = 3.23 μm). Furthermore, dispersion was enhanced along with achieving a maximum tap density of 10.4 g/mL. There was minimal change observed in the particle size distribution of platinum powder at temperatures ranging from 1200 to 1300 °C due to high heat treatment conditions, resulting in coalescence and growth phenomena that led to larger spherical particles with slightly reduced tap density (10.3–9.7 g/mL) (Figure 6b).
Figure 6.

Use of CaO as a barrier agent for heat treatment temperature: (a) particle size distribution (T0: without heat treatment) and (b) tap density (T0: without heat treatment).
3.4. Impact of Heat Treatment on the Crystal Structure
The FIB-TEM test results are in agreement with the SEM and FESEM test results, demonstrating pronounced agglomeration and inadequate dispersibility of micrometer-sized Pt particles synthesized via chemical liquid-phase reduction (Figure 7a). The HRTEM test reveals (Figure 7b) that each micrometer-sized particle is composed of numerous irregularly agglomerated nanocrystals, exhibiting distinct grain boundaries between them and possessing crystal sizes ranging from 9 to 24 nm. The diffraction pattern exhibits a circular shape, indicating a characteristic polycrystalline structure (Figure 7c).32
Figure 7.
TEM images and electron diffraction patterns of platinum powder: (a) TEM, (b) HRTEM, (c) diffraction patterns, and 700 °C heat treatment: (d) TEM, (e) HRTEM, and (f) diffraction patterns.
After being subjected to a 2 h heat treatment at 700 °C, CaO was employed as a barrier material. The results obtained from TEM and SEM spectroscopy exhibited certain similarities. The dispersibility of platinum particles improved, resulting in variations in their morphologies, including spherical, quasi-spherical, and rod-like shapes. During the heat treatment process, the effectiveness of the barrier was not ideal due to the formation of sintering necks between closely positioned particles and atomic diffusion occurring among them. Consequently, there was a tendency for particle growth. However, due to the relatively low heat treatment temperature and limited extent of diffusion, noticeable pore structures were observed within the newly grown particles (with intragranular pore sizes reaching up to 512 nm). This is illustrated in Figure 7d where the cross section of pores appears relatively complex with circular, elliptical, and irregular shapes present. Figure 7e showcases an individual particle exhibiting good barrier properties with a size of 320 nm and also displaying a polycrystalline structure. In comparison to untreated platinum particles, there is a significant decrease in the number of single crystals within each particle along with an increase in overall size up to 126 nm; however small-sized single crystals (measuring 24 nm) coexist alongside larger ones. The electron diffraction pattern shown in Figure 7f further confirms that this particular particle possesses a polycrystalline structure.
The dispersibility of Pt powder is significantly improved after further heat treatment at 1300 °C, resulting in predominantly spherical or quasi-spherical particle morphology and greater uniformity. Compared with 700 °C, atomic diffusion and volume contraction intensify in Pt particles at 1300 °C, leading to the persistence of hollow regions formed by sintering necks between adjacent particles. However, the intragranular pore size decreases to 253 nm (Figure 8a). The single particle with an excellent barrier effect (460 nm in size) undergoes a significant crystal structure change from polycrystal to monocrystal (as shown in Figure 8b,c). Larger particles (1955 nm in size) are formed due to a poor barrier effect, resulting in the sintering of multiple original platinum particles into spherical or quasi-spherical shapes during the heat treatment process, exhibiting polycrystalline characteristics (as shown in Figure 8d–f). Unlike the heat treatment at 700 °C, high-temperature heat treatment at 1300 °C clearly reveals crystal boundaries within the polycrystalline structure and increases relative grain size (1188 nm) while decreasing the number of grains with increasing characteristic grain size.33
Figure 8.
TEM images and electron diffraction patterns of platinum powder following heat treatment at 1300 °C: (a, b) TEM, (c) diffraction patterns, (d, e) HRTEM, and (f) diffraction pattern.
The XRD diffraction peak of platinum powder prepared through chemical liquid-phase reduction shows variations in both peak width and intensity. Following heat treatment, there is a decrease in the peak width, accompanied by a significant increase in intensity. Furthermore, this trend becomes more pronounced as heat treatment temperature increases (Figure 9a, b). The Scherrer formula34 includes variables such as D for grain size and K = 0.89 as the Scherrer constant along with other parameters such as λ for diffraction wavelength and FWHM for full width at half-maximum at an incident angle θ. It can be observed that under fixed conditions, a larger half-width corresponds to a smaller grain size.
| 1 |
Figure 9.

Comparison of XRD data for platinum powder treated at different temperatures: (a) whole and (b) local magnification.
The platinum powder, prepared through chemical liquid-phase reduction, undergoes polycrystalline formation by agglomerating nanocrystals. As a result, its XRD diffraction peaks exhibit reduced intensity and a broader width. With an increase in heat treatment temperature up to 700 °C, atom diffusion occurs within the particles, leading to a transition from nanocrystals to microcrystals, grain coarsening, and alteration in crystal structure. This results in an elevation of the diffraction peak intensity and a decrease in half-width. Subsequent heat treatment at 1300 °C completely transforms the small particles into either monocrystal or micron-sized polycrystals, thereby further enhancing the diffraction peak intensity (Figure 9).
3.5. Mechanisms for Improving the Performance of Platinum Powder
The internal structure of metal particles plays a crucial role in their application, particularly in the dense packing and deposition of conductive structures within electronic materials, which are closely associated with the crystal structure. Polycrystalline particles often exhibit lower sintering density and discontinuous regions due to ″intraparticle sintering”, leading to significant volume shrinkage and impaired conductivity. In contrast, particles with excellent crystallinity demonstrate slower sintering (only involving mass transfer between particles), smaller volume shrinkage, and the formation of dense and continuous high-conductivity structures.35 Reforming the polycrystalline structure of platinum particles through heat treatment is of paramount importance, as it facilitates grain growth, reduces grain boundary energy, and enhances stability.
The diffusion-driven growth of crystal nuclei initiates the formation of irregular nanocrystalline primary particles in the chemical liquid-phase reduction system, which subsequently undergo rapid agglomeration to yield polycrystalline micron-sized spherical particles.17,36−38 Furthermore, severe agglomeration occurs among micrometer-sized platinum particles, resulting in a relatively loose structure (Figure 10a). However, during the heat treatment process without a barrier effect, atomic diffusion between particles promotes the growth of sintering necks and merging of particles. This ultimately leads to bulk sintering (Figure 10b), which fails to enhance powder dispersion effectively. In order to improve the dispersion of platinum powder more efficiently, CaO is used as a thermally stable barrier that forms a homogeneous mixture with Pt particles. Through subsequent heat treatment, particle dispersion is improved, resulting in reduced particle size distribution and increased tap density while facilitating the formation of larger single crystals in micron-sized particles. The reason for this transformation lies in the heat treatment process during which monodispersed particles undergo atomic and grain boundary diffusion, resulting in a transition from a polycrystalline structure to a single crystal. As a result, gas adsorption at grain boundaries and interfaces is reduced, specific surface area decreases, grain boundary energy decreases, and powder surface energy diminishes. Consequently, stability improves and the structure becomes denser (Figure 10c). Furthermore, as the heat treatment temperature increases within a certain duration, atom diffusion intensifies and promotes the conversion from polycrystals to single crystals while exacerbating grain coarsening tendencies. This ultimately enhances powder dispersion (Figure 10d).
Figure 10.

Schematic diagram illustrating the microstructure changes of platinum powder during heat treatment: (a) micrometer-sized platinum powder, (b) platinum powder sintering, (c) platinum powder and CaO heat treatment at 700 °C, and (d) platinum powder and CaO heat treatment at 1300 °C.
Moreover, the morphology of the Pt powder is significantly influenced by barrier agents. Monodisperse platinum powder with effective barriers undergoes atom diffusion between nanocrystals and grain boundaries during heat treatment, resulting in an increase in the grain size. Higher temperatures lead to larger particle sizes and further transformation to monocrystal structures. In regions with poor barrier effects, heat treatment causes neighboring platinum particles to agglomerate, forming sintering necks and larger particles accompanied by noticeable pores within them. As the temperature increases, grains in these regions also coarsen but remain comparable in size to monodisperse particles composed of only a few monocrystals.
4. Conclusions
The micrometer-sized platinum powder prepared via chemical liquid-phase reduction exhibits significant agglomeration, with a tap density of only 5.9 g/mL and a wide particle size distribution (SD of 14.5 μm). Direct sintering of unmixed CaO promotes the formation of sintering necks between powder particles, enhancing interparticle bonding. When the sintering temperature exceeds 700 °C, metallization of platinum powder occurs, leading to bulk material formation. However, ball milling followed by heat treatment for uniform mixing of CaO with platinum powder results in an improved dispersion of platinum particles, increased tap density, and a narrower particle size distribution. For instance, after heat treatment at 1100 °C, the particle size distribution narrows significantly (SD = 3.23 μm), while the tap density increases to 10.4 g/cm3. The addition of CaO effectively inhibits sintering and agglomeration during heat treatment, resulting in an increased grain size and coarsening of platinum particles. Consequently, this leads to densification and decreased surface energy, ultimately enhancing the stability and dispersion of platinum powder, narrowing its particle size distribution and increasing tap density. This research will establish a robust theoretical framework and provide comprehensive technical guidance for preparing high-dispersion, high-tap-density micrometer-sized platinum powder for electronic pastes.
Acknowledgments
The authors acknowledge financial support provided by the Chongqing Postdoctoral Science Foundation Project (No. CSTB2023NSCQ-BHX0071) and the Chongqing Technology Innovation and Application Development Special Key Project (No. CSTB2022TIAD-KPX0026) for this research.
The authors declare no competing financial interest.
References
- Jeyaraj M.; Gurunathan S.; Qasim M.; Kang M.-H.; Kim J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9 (12), 1719. 10.3390/nano9121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.; Yu X.; Hou J.; Xiang Z. Secondary reduction strategy synthesis of Pt–Co nanoparticle catalysts towards boosting the activity of proton exchange membrane fuel cells. Particuology 2023, 79, 18–26. 10.1016/j.partic.2022.11.010. [DOI] [Google Scholar]
- Quinson J.; Inaba M.; Neumann S.; Swane A. A.; Bucher J.; Simonsen S. B.; Theil Kuhn L.; Kirkensgaard J. J. K.; Jensen K. M. Ø.; Oezaslan M.; et al. Investigating Particle Size Effects in Catalysis by Applying a Size-Controlled and Surfactant-Free Synthesis of Colloidal Nanoparticles in Alkaline Ethylene Glycol: Case Study of the Oxygen Reduction Reaction on Pt. ACS Catal. 2018, 8 (7), 6627–6635. 10.1021/acscatal.8b00694. [DOI] [Google Scholar]
- Quinson J.; Neumann S.; Wannmacher T.; Kacenauskaite L.; Inaba M.; Bucher J.; Bizzotto F.; Simonsen S. B.; Theil Kuhn L.; Bujak D.; et al. Colloids for Catalysts: A Concept for the Preparation of Superior Catalysts of Industrial Relevance. Angew. Chem., Int. Ed. 2018, 57 (38), 12338–12341. 10.1002/anie.201807450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux R. M.; Song H.; Grass M.; Habas S.; Niesz K.; Hoefelmeyer J. D.; Yang P.; Somorjai G. A. Monodisperse platinum nanoparticles of well-defined shape: synthesis, characterization, catalytic properties and future prospects. Top. Catal. 2006, 39 (3–4), 167–174. 10.1007/s11244-006-0053-2. [DOI] [Google Scholar]
- Azharuddin M.; Zhu G. H.; Das D.; Ozgur E.; Uzun L.; Turner A. P. F.; Patra H. K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55 (49), 6964–6996. 10.1039/C9CC01741K. [DOI] [PubMed] [Google Scholar]
- Li D.; Wang C.; Tripkovic D.; Sun S.; Markovic N. M.; Stamenkovic V. R. Surfactant Removal for Colloidal Nanoparticles from Solution Synthesis: The Effect on Catalytic Performance. ACS Catal. 2012, 2 (7), 1358–1362. 10.1021/cs300219j. [DOI] [Google Scholar]
- Pradeep T.; Anshup Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 2009, 517 (24), 6441–6478. 10.1016/j.tsf.2009.03.195. [DOI] [Google Scholar]
- Han M.; Kani K.; Na J.; Kim J.; Bando Y.; Ahamad T.; Alshehri S. M.; Yamauchi Y. Retrospect and Prospect: Nanoarchitectonics of Platinum-Group-Metal-Based Materials. Adv. Funct. Mater. 2023, 33 (44), 2301831. 10.1002/adfm.202301831. [DOI] [Google Scholar]
- Dhand C.; Dwivedi N.; Loh X. J.; Jie Ying A. N.; Verma N. K.; Beuerman R. W.; Lakshminarayanan R.; Ramakrishna S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: a comprehensive overview. RSC Adv. 2015, 5 (127), 105003–105037. 10.1039/C5RA19388E. [DOI] [Google Scholar]
- Wan X.; Li J.; Li N.; Zhang J.; Gu Y.; Chen G.; Ju S. Preparation of Spherical Ultrafine Silver Particles Using Y-Type Microjet Reactor. Materials 2023, 16 (6), 2217. 10.3390/ma16062217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A. R.; Mamun M. S. A.; Ara M. H. Review on platinum nanoparticles: Synthesis, characterization, and applications. Microchemical Journal 2021, 171, 106840 10.1016/j.microc.2021.106840. [DOI] [Google Scholar]
- Danilenko M. V.; Guterman V. E.; Vetrova E. V.; Metelitsa A. V.; Paperzh K. O.; Pankov I. V.; Safronenko O. I. Nucleation/growth of the platinum nanoparticles under the liquid phase synthesis. Colloids Surf., A 2021, 630, 127525 10.1016/j.colsurfa.2021.127525. [DOI] [Google Scholar]
- Miyabayashi K.; Nakamura S.; Miyake M. Synthesis of Small Platinum Cube with Less Than 3 nm by the Control of Growth Kinetics. Cryst. Growth Des. 2011, 11 (10), 4292–4295. 10.1021/cg200937u. [DOI] [Google Scholar]
- Thanh N. T. K.; Maclean N.; Mahiddine S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114 (15), 7610–7630. 10.1021/cr400544s. [DOI] [PubMed] [Google Scholar]
- Zeng J.; Lee J. Y.; Zhou W. Activities of Pt/C catalysts prepared by low temperature chemical reduction methods. Applied Catalysis A: General 2006, 308, 99–104. 10.1016/j.apcata.2006.04.019. [DOI] [Google Scholar]
- Farrell B. P.; Sevonkaev I. V.; Goia D. V. Preparation of Dispersed Spherical Platinum Particles with Controlled Size and Internal Structure. Platinum Metals Review 2013, 57 (3), 160–168. 10.1595/147106713X667605. [DOI] [Google Scholar]
- Lai Y.; Guo Z.; Huang H.; Wang S.; Zhang H. Synthesis of high tap density spherical micro-size silver particles by liquid-phase reduction using response surface methodology. Journal of Materials Science: Materials in Electronics 2014, 25 (4), 1893–1900. 10.1007/s10854-014-1817-2. [DOI] [Google Scholar]
- Rodrigues T. S.; Zhao M.; Yang T.; Gilroy K. D.; da Silva A. G. M.; Camargo P. H. C.; Xia Y. Synthesis of Colloidal Metal Nanocrystals: A Comprehensive Review on the Reductants. Chem. - Eur. J. 2018, 24 (64), 16944–16963. 10.1002/chem.201802194. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yang H.; Yu X.; Hu C.; Hu J.; Li R.; Zhang Y. Functional metal powders: Design, properties, applications, and prospects. Materials Science and Engineering: B 2022, 280, 115708 10.1016/j.mseb.2022.115708. [DOI] [Google Scholar]
- Gu S.; Wang W.; Wang H.; Tan F.; Qiao X.; Chen J. Effect of aqueous ammonia addition on the morphology and size of silver particles reduced by ascorbic acid. Powder Technol. 2013, 233, 91–95. 10.1016/j.powtec.2012.08.036. [DOI] [Google Scholar]
- Masuda H. Dry dispersion of fine particles in gaseous phase. Advanced Powder Technology 2009, 20 (2), 113–122. 10.1016/j.apt.2009.02.001. [DOI] [Google Scholar]
- Calvert G.; Hassanpour A.; Ghadiri M. Mechanistic analysis and computer simulation of the aerodynamic dispersion of loose aggregates. Chem. Eng. Res. Des. 2011, 89 (5), 519–525. 10.1016/j.cherd.2010.08.013. [DOI] [Google Scholar]
- Calvert G.; Ghadiri M.; Dyson M.; Kippax P.; McNeil-Watson F. The flowability and aerodynamic dispersion of cohesive powders. Powder Technol. 2013, 240, 88–94. 10.1016/j.powtec.2012.07.003. [DOI] [Google Scholar]
- Robb D. T.; Halaciuga I.; Privman V.; Goia D. V.. Computational model for the formation of uniform silver spheres by aggregation of nanosize precursors. J. Chem. Phys. 2008, 129 ( (18), ), 10.1063/1.3009625. [DOI] [PubMed]
- Garcia-Gutierrez D. I.; Gutierrez-Wing C. E.; Giovanetti L.; Ramallo-Lopez J. M.; Requejo F. G.; Jose-Yacaman M. Temperature effect on the synthesis of Au-Pt bimetallic nanoparticles. journal of physical chemistry. B 2005, 109 (9), 3813–3821. 10.1021/jp048114a. [DOI] [PubMed] [Google Scholar]
- Tan Y.; Dai X.; Li Y.; Zhu D. Preparation of gold, platinum, palladium and silver nanoparticles by the reduction of their salts with a weak reductant–potassium bitartrate. J. Mater. Chem. 2003, 13 (5), 1069–1075. 10.1039/b211386d. [DOI] [Google Scholar]
- Mi J. L.; Clausen H. F.; Bremholm M.; Schmøkel M. S.; Hernández-Fernández P.; Becker J.; Iversen B. B. Pulsed-Flow Near-Critical and Supercritical Synthesis of Carbon-Supported Platinum Nanoparticles and In Situ X-ray Diffraction Study of Their Formation and Growth. Chem. Mater. 2015, 27 (2), 450–456. 10.1021/cm5033817. [DOI] [Google Scholar]
- Mazlan M. R.; Jamadon N. H.; Rajabi A.; Sulong A. B.; Mohamed I. F.; Yusof F.; Jamal N. A. Necking mechanism under various sintering process parameters – A review. Journal of Materials Research and Technology 2023, 23, 2189–2201. 10.1016/j.jmrt.2023.01.013. [DOI] [Google Scholar]
- Rahaman M. N.Kinetics and mechanisms of densification. Sintering of Advanced Materials; Woodhead Publishing; 2010, 33–64. [Google Scholar]
- Li B. Q.; Sun Z. Q.; Hou G. L.; Ding F.; Hu P.; Yuan F. L. The sintering behavior of quasi-spherical tungsten nanopowders. International Journal of Refractory Metals & Hard Materials 2016, 56, 44–50. 10.1016/j.ijrmhm.2015.10.007. [DOI] [Google Scholar]
- Wang H.; Wang L.; Sato T.; Sakamoto Y.; Tominaka S.; Miyasaka K.; Miyamoto N.; Nemoto Y.; Terasaki O.; Yamauchi Y. Synthesis of Mesoporous Pt Films with Tunable Pore Sizes from Aqueous Surfactant Solutions. Chem. Mater. 2012, 24 (9), 1591–1598. 10.1021/cm300054b. [DOI] [Google Scholar]
- German R. M. Coarsening in Sintering: Grain Shape Distribution, Grain Size Distribution, and Grain Growth Kinetics in Solid-Pore Systems. Critical Reviews in Solid State and Materials Sciences 2010, 35 (4), 263–305. 10.1080/10408436.2010.525197. [DOI] [Google Scholar]
- Pavlets A. S.; Alekseenko A. A.; Tabachkova N. Y.; Safronenko O. I.; Nikulin A. Y.; Alekseenko D. V.; Guterman V. E. A novel strategy for the synthesis of Pt–Cu uneven nanoparticles as an efficient electrocatalyst toward oxygen reduction. Int. J. Hydrogen Energy 2021, 46 (7), 5355–5368. 10.1016/j.ijhydene.2020.11.094. [DOI] [Google Scholar]
- Lu L.; Sevonkaev I.; Kumar A.; Goia D. V. Strategies for tailoring the properties of chemically precipitated metal powders. Powder Technol. 2014, 261, 87–97. 10.1016/j.powtec.2014.04.015. [DOI] [Google Scholar]
- Sevonkaev I., Privman V., Goia D.. Growth of highly crystalline nickel particles by diffusional capture of atoms. J. Chem. Phys. 2013, 138 ( (1), ), 10.1063/1.4772743. [DOI] [PubMed]
- Wang L.; Andreassen J.-P.; Ucar S. Precipitation of silver particles with controlled morphologies from aqueous solutions. CrystEngComm 2020, 22 (3), 478–486. 10.1039/C9CE01601E. [DOI] [Google Scholar]
- Halaciuga I.; Goia D. V. Preparation of silver spheres by aggregation of nanosize subunits. J. Mater. Res. 2008, 23 (6), 1776–1784. 10.1557/JMR.2008.0219. [DOI] [Google Scholar]