Abstract
Enterococcus hirae is a rare isolate in clinical specimens. We describe a case of native aortic-valve endocarditis that was caused by Enterococcus hirae in a 72-year-old man. This is the first reported case of endocarditis due to this organism.
CASE REPORT
A 72-year old man presented with a 1-month history of fever, chills, progressive malaise, and generalized weakness. During this period, the patient received several short courses of antibiotics, including trimethoprim-sulfamethoxazole, levofloxacin, and pristinamycin. He had a history of coronary artery disease and, 7 years previously, had undergone percutaneous transluminal coronary angioplasty. On arrival, he was found to have an oral temperature of 36.5°C and a blood pressure of 122/64 mm Hg. Cardiac examination revealed 2/6 systolic and 2/6 diastolic murmurs. Physical examination revealed no evidence of local or systemic infection and no peripheral embolic or immunologic stigmata of infectious endocarditis. Laboratory evaluation showed the following values: white blood cells, 11.6 × 109/liter with 86% neutrophils; hemoglobin, 9.2 g/dl; and platelets, 256 × 109/liter. Examination of cardiac enzymes showed no evidence of myocardial injury. Blood samples of four sets of culture were all positive and yielded an Enterococcus sp. A transesophageal echocardiography showed vegetations involving the left and right aortic-valve leaflets with aortic insufficiency.
The isolated strain was identified by phenotypic methods and genetic analysis. In phenotypic analysis, the strain hydrolyzed esculin in the presence of 4% bile and reacted with Lancefield group D antiserum. It was not possible to identify this strain to the species level with the API 20 Strep system (bio-Mérieux, Marcy l'Etoile, France). With the rapid ID 32 Strep system (bio-Mérieux), which has greater reliability in the identification of enterococci (3), the strain was misidentified as Enterococcus durans. The strain was correctly identified to the species level as Enterococcus hirae by genetic methods using sodAint gene sequencing (7). Sequence analysis of the strains yielded 99.5 and 85.6% identities with the sequences of the type strains of E. hirae (accession no. AJ387916) and E. durans (accession no. AJ387911), respectively. The strain was sensitive to ampicillin (MIC, 0.75 mg/liter; measured by E-test), vancomycin, teicoplanin, and chloramphenicol and resistant to clindamycin, erythromycin, rifampin, and tetracycline and exhibited low-level resistance to streptomycin, kanamycin, and gentamicin.
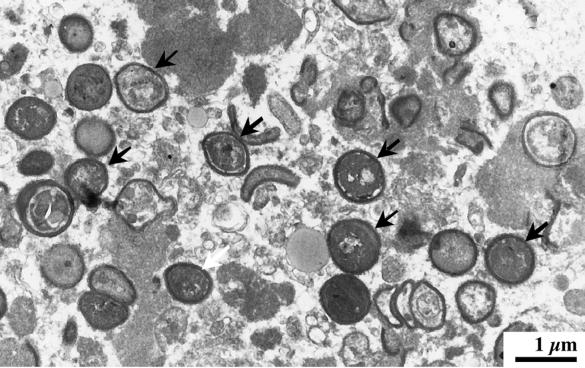
Intravenous ampicillin (200 mg per kg of body weight per day) and gentamicin (3 mg/kg/day) were administered for 4 weeks. Rifampin (25 mg/kg/day) was added 2 weeks after the initiation of antimicrobial treatment for 15 days. The patient improved clinically; and blood cultures performed 1 month later were negative. Five weeks after admission, the patient was discharged from the hospital in good condition and continued to receive oral ampicillin and rifampin for 3 weeks. The patient was readmitted for control 3 months later. He was afebrile, but six sets of blood cultures drawn over a course of 3 days grew the same strain of E. hirae, as determined by pulsed-field gel electrophoresis analysis as previously described (data not shown) (6). A second transesophageal echocardiography showed an increase in size of the aortic vegetations and severe aortic insufficiency. Intravenous administration of vancomycin (60 mg/kg/day) and gentamicin (3 mg/kg/day) was started. Two days after initiation of antimicrobial therapy, blood cultures were sterile and remained negative during the follow-up. Ten days later, the patient underwent aortic valve replacement with a homograft. Pathological examination of the native valve revealed focal acute endocarditis. Transmission electron microscopy demonstrated the presence of numerous cocci (Fig. 1), and cultures yielded E. hirae. Intravenous therapy with vancomycin and gentamicin was continued during 6 weeks, followed by oral amoxicillin (6 g per day) for a total of 8 weeks. The patient remains clinically well 6 months after therapy.
FIG. 1.
Transmission electron microscopy of the aortic vegetation demonstrating the presence of numerous cocci (arrows).
Enterococci are gram-positive bacteria that are now established as major nosocomial pathogens and have become increasingly important in recent years due to the development and transmission of antibiotic resistance traits (5). The enterococci are the third most common cause of infective endocarditis, accounting for 5 to 15% of cases, and are associated with a mortality of 20 to 30% (5). The majority of enterococcal strains isolated from human specimens belong to the species Enterococcus faecalis (80%) and Enterococcus faecium (10%). Other enterococcal species are rarely isolated, and E. hirae accounts for less than 1% of enterococcal species in human clinical samples (8). This species is known to cause infections in a range of young farmed species and psittacine birds (1, 2) but is very rare in humans. The first description of E. hirae infection in humans, by Gilad et al. in 1998, reported a case of septicemia in a patient with end-stage renal disease undergoing hemodialysis (4). We describe a case of E. hirae native aortic-valve endocarditis in a 72-year-old man, which relapsed despite apparent efficacy of antibiotic treatment and finally required aortic valve replacement due to severe aortic insufficiency. To our knowledge, our report constitutes the first description of E. hirae endocarditis in a human.
E. durans and E. hirae differ only by two fermentation sugar tests: acid production by using raffinose and saccharose should be positive with E. hirae but negative with E. durans. The misidentification of the clinical isolate characterized in this study by the ID32 Strep system was due to the fact that it did not form acid with either sugar. Proper identification of this strain at the species level required the use of genetic methods. The difficulty in phenotypic identification of these strains confirms the need to use a molecular approach for identification of enterococci isolated in cultures with clinical relevance. Despite the fact that the strain was susceptible in vitro to ampicillin and exhibited low-level resistance to gentamicin, treatment with a combination of these two molecules was unable to sterilize the aortic vegetations within 12 weeks of treatment, which eventually led to the valve replacement. This grave prognosis shows that E. hirae may be a cause of severe endocarditis.
REFERENCES
- 1.Devriese, L. A., J. I. Cruz Colque, F. Haesebrouck, M. Desmidt, E. Uyttebroek, and R. Ducatelle. 1992. Enterococcus hirae in septicaemia of psittacine birds. Vet. Rec. 130:558-559. [DOI] [PubMed] [Google Scholar]
- 2.Devriese, L. A., and F. Haesebrouck. 1991. Enterococcus hirae in different animal species. Vet. Rec. 129:391-392. [DOI] [PubMed] [Google Scholar]
- 3.Facklam, R. R., and D. F. Sahm. 1995. Enterococcus, p. 308-314. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 4.Gilad, J., A. Borer, K. Riesenberg, N. Peled, A. Shnaider, and F. Schlaeffer. 1998. Enterococcus hirae septicemia in a patient with end-stage renal disease undergoing hemodialysis. Eur. J. Clin. Microbiol. Infect. Dis. 17:576-577. [DOI] [PubMed] [Google Scholar]
- 5.Murray, B. E. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poyart, C., C. Pierre, G. Quesne, B. Pron, P. Berche, and P. Trieu-Cuot. 1997. Emergence of vancomycin resistance in the genus Streptococcus: characterization of a vanB transferable determinant in Streptococcus bovis. Antimicrob. Agents Chemother. 41:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poyart, C., G. Quesne, and P. Trieu-Cuot. 2000. Sequencing the gene encoding manganese-dependent superoxide dismutase for rapid species identification of enterococci. J. Clin. Microbiol. 38:415-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandamme, P., E. Vercauteren, C. Lammens, N. Pensart, M. Ieven, B. Pot, R. Leclercq, and H. Goossens. 1996. Survey of enterococcal susceptibility patterns in Belgium. J. Clin. Microbiol. 34:2572-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]