Abstract
Saccharomyces cerevisiae is a yeast commonly used in baking and a frequent colonizer of human mucosal surfaces. It is considered relatively nonpathogenic in immunocompetent adults (J. N. Aucott, J. Fayan, H. Grossnicklas, A. Morrissey, M. M. Lederman, and R. A. Salata, Rev. Infect. Dis. 12:406-411, 1990). We present a case of S. cerevisiae fungemia and aortic graft infection in an immunocompetent adult. This is the first reported case of S. cerevisiae fungemia where the identity of the pathogen was confirmed by rRNA sequencing.
CASE REPORT
A 56-year-old man with a past medical history of hypertension, hyperlipidemia, and atherosclerosis had an aortic-bifemoral graft placed uneventfully in July 1998 for relief of intermittent claudication. In May 1999, the patient reported lancing a “cyst” that had developed in his right groin. Eight months later, the patient returned, reporting that the “cyst” had been spontaneously and intermittently draining since it had been lanced. Initial bacterial, mycobacterial, and fungal cultures of the draining material were without growth, and no organisms were seen with Gram staining. Subsequent abdominal computerized tomography showed fluid surrounding the graft. The patient was admitted to the hospital, two sets of (aerobic and anaerobic) blood cultures (ESP; Trek) were obtained, and intravenous cefoxitin and metronidazole were started. On hospital day eight, small, round yeasts were isolated from one of the initial aerobic blood cultures and amphotericin B was started. After receiving a total of 500 mg of amphotericin B, the patient underwent a repeat abdominal computerized tomography, which again showed fluid surrounding the graft. The patient then underwent surgery and was found to have an aortic-enteric fistula between the proximal end of the aorta-bifemoral graft and his proximal jejunum. The graft was replaced with cryopreserved aorta, and the adherent small bowel was resected. The patient's postoperative course was complicated by respiratory failure with acute lung injury, disseminated intravascular coagulation, anuria, and eventually circulatory collapse and death.
The blood culture isolate was identified as Saccharomyces by biochemical tests (API 20C AUX [Biomerieux] and Uni-Yeast-TEK [Remel]) and the presence of ascospores. Specifically, the isolate utilized glucose, galactose, α-methyl-d-glucoside, maltose, saccharose/sucrose, trehalose, and raffinose. Identification was confirmed by sequence analysis of the 18S rRNA gene (Applied Biosystems), which was compared to type strains of Saccharomyces cerevisiae (Fig. 1). Susceptibility testing was performed by the National Committee for Clinical Laboratory Standards macrodilution method (2). The isolate was sensitive to amphotericin B, ketoconazole, and fluconazole. Amphotericin B was replaced with fluconazole to lessen nephrotoxicity. Cultures of periaortic fluid obtained at surgery also grew yeast, which were subsequently identified as S. cerevisiae by the phenotypic methods previously described. Because it was phenotypically identical to the previous isolate, we did not repeat the rRNA sequencing; therefore, we cannot completely exclude the possibility that he was superinfected with a second species of Saccharomyces, but this is unlikely.
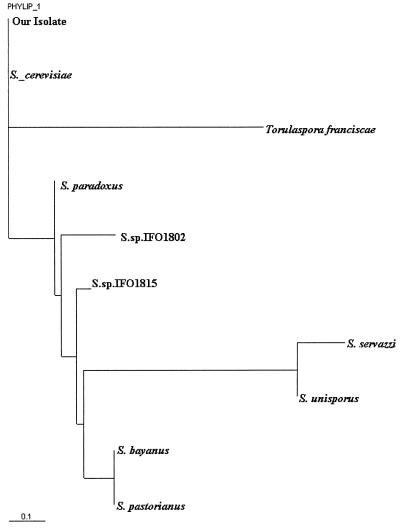
FIG. 1.
Phylogenetic dendrogram of 18S rRNA sequences showing that our isolate was identical to the ATCC strain of S. cerevisiae and differed from eight other closely related fungal species.
Saccharomyces cerevisiae fungemia is rare but has been reported in association with odontic procedures, use of Saccharomyces boulardi as a probiotic, pancreatic cancer, burns, renal failure, and prosthetic valve endocarditis (1, 3, 5, 6, 8, 10). In this case, it is likely that S. cerevisiae infected the graft after the aorta-enteric fistula developed, as S. cerevisiae is a common colonizer of duodenal mucosa (1). Previous cases of S. cerevisiae fungemia have been successfully treated with amphotericin B or ketoconazole, but as with our patient, antifungal treatment often fails (1). Failure is often due to comorbidities. Although our patient was immunocompetent, infected aorta-enteric fistulas from any cause have up to a 74% mortality (4). This case represents a postsurgical complication and further broadens the spectrum of infection caused by S. cerevisiae. The low virulence of S. cerevisiae could account for the prolonged course of his infection without systemic symptoms of inflammation.
Historically, phenotypic identification of Saccharomyces has been unreliable because standard commercial mycology test kits do not discriminate between the different species of Saccharomyces (9). Recently, different genotypic techniques such as ribosomal DNA sequencing, random amplified polymorphic DNA, δ elements, DNA chromosomal profiles, and mitochondrial DNA restriction analysis have been used to successfully identify isolates of Saccharomyces to the species level (11). We compared the 18S rRNA sequence of our isolate with sequences already published for Saccharomyces in the GenBank database and found that our isolate was identical to S. cerevisiae (GenBank accession no. AY007889) and differed fromeight other closely related species (Fig. 1) (7). To our knowledge, this is the first reported case of S. cerevisiae fungemia where the identity of the pathogen was confirmed by rRNA sequencing.
REFERENCES
- 1.Aucott, J. N., J. Fayan, H. Grossnicklas, A. Morrissey, M. M. Lederman, and R. A. Salata. 1990. Invasive infection with Saccharomyces cerevisiae: report of three cases and review. Rev. Infect. Dis. 12:406-411. [DOI] [PubMed] [Google Scholar]
- 2.Barchiesi, F., A. L. Colombo, D. A. McGough, and M. G. Rinaldi. 1994. Comparative study of broth macrodilution and microdilution techniques for in vitro antifungal susceptibility testing of yeasts by using the National Committee for Clinical Laboratory Standards' proposed standard. J. Clin. Microbiol. 32:2494-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassetti, S., R. Frei, and W. Zimmerli. 1998. Fungemia with Saccharomyces cerevisiae after treatment with Saccharomyces boulardii. Am. J. Med. 105:71-72. [DOI] [PubMed] [Google Scholar]
- 4.Bernhard, V. M. 1998. Aortoenteric fistulae, p. 611-618. In R. B. Rutherford (ed.), Vascular surgery, 4th ed., vol. 1. W. B. Saunders Company, Philadelphia, Pa.
- 5.Chertow, G. M., M. D. Marcantonio, and R. G. Wells. 1991. Saccharomyces cerevisiae empyema in a patient with esophago-pleural fistula complicating variceal sclerotherapy. Chest 99:1518-1519. [DOI] [PubMed] [Google Scholar]
- 6.Debelian, G. J., I. Olsen, and L. Tronstead. 1997. Observation of Saccharomyces cerevisiae in blood of patient undergoing root canal treatment. Int. Endod. J. 30:313-317. [DOI] [PubMed] [Google Scholar]
- 7.Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package), version 3.5c. Department of Genetics, University of Washington, Seattle, Washington.
- 8.Fredenucci, I., M. Chomarat, C. Boucaud, and J. P. Flandrois. 1998. Saccharomyces boulardii fungemia in a patient receiving ultra-leuvre therapy. Clin. Infect. Dis. 27:222-223. [DOI] [PubMed] [Google Scholar]
- 9.McCullough, M. J., K. V. Clemons, J. H. McCusker, and D. A. Stevens. 1998. Species identification and virulence attributes of Saccharomyces boulardii (nom. inval.). J. Clin. Microbiol. 36:2613-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niault, M., F. Thomas, J. Prost, F. Hojjat Ansari, and P. Kalfon. 1999. Fungemia due to Saccharomyces species in a patient treated with enteral Saccharomyces boulardii. Clin. Infect. Dis. 28:930.. [DOI] [PubMed] [Google Scholar]
- 11.Perapoch, J., A. M. Planes, A. Querol, V. López, I. Martínez-Bendayán, R. Tormo, F. Fernández, G. Peguero, and S. Salcedo. 2000. Fungemia with Saccharomyces cerevisiae in two newborns, only one of whom had been treated with ultra-levura. Eur. J. Clin. Microbiol. Infect. Dis. 19:468-470. [DOI] [PubMed] [Google Scholar]