Abstract
Background
Traditional statistical methods have dominated research on peripartum depression (PPD), but innovative approaches may provide deeper insights. This study aims to predict the impact factors of PPD using elastic net regression (ENR) combined with machine learning (ML) model.
Methods
This longitudinal study was conducted from June 2020 to May 2023, involving healthy pregnant women in the first trimester, followed up until the completion of the assessment in the second trimester. PPD symptoms were assessed using the Edinburgh Postnatal Depression Scale (EPDS). Features with p <.05 from logistic regression were selected and refined using ENR. These features were then used to build six ML models to identify the best-performing one. SHapley Additive exPlanations (SHAP) analysis was employed to enhance model interpretability by visualizing its decision-making process.
Results
A total of 608 participants were followed, resulting in 384 valid questionnaires. After excluding incomplete or incorrect baseline data, 325 participants were ultimately included in the study. Among these, 130 were classified as having mild depression, and 32 were classified with major depression. Nineteen features were initially identified as being associated with PPD, with 14 retained after ENR refinement. The random forest (RF) model outperformed the other ML models. SHAP analysis identified the top five predictors of PPD: magnesium (Mg), remnant cholesterol (RC), calcium (Ca), mean corpuscular hemoglobin concentration (MCHc), and potassium (K). Mg, Ca, MCHc, and K were negatively correlated with PPD, while RC showed a positive correlation.
Conclusions
The RF model effectively identified associations between exposure factors and PPD. Mg, Ca, MCHc, and K were found to be protective factors, while RC emerged as a potential risk factor, highlighting its potential as a novel biomarker for PPD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-025-07656-3.
Keywords: Peripartum depression, Remnant cholesterol, Elastic net regression, Machine learning, Random forests
Introduction
Peripartum depression (PPD) is a major depressive disorder with perinatal onset, encompassing both depression during pregnancy and the postpartum period, as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, Text Revision (DSM-5-TR) [1]. The global prevalence of PPD is approximately 11.9% [2], posing significant risks to maternal health and potentially adversely affecting fetal neurodevelopment and behavior by altering hormone and neurotransmitter levels [3, 4]. Identifying risk factors for PPD is therefore essential.
While many studies have explored the factors influencing PPD, such as dietary habits and lipid parameters [5–7], most have employed traditional statistical methods. Newer analytical approaches may offer more accurate identification of PPD risk factors. Analyzing the relationship between PPD and multiple exposures in datasets with high dimensionality and multicollinearity is particularly challenging. Elastic net regression (ENR) addresses this issue by integrating L1 and L2 regularization, facilitating sparse solutions while managing datasets with numerous features and potential collinearity. Furthermore, traditional statistical methods often require extensive data preparation and high-quality, structured datasets, leading to the loss of valuable unstructured data [8]. In contrast, machine learning (ML) algorithms, often considered “black-box” models, require fewer predefined standards and less data preparation, enhancing the ability to analyze large, complex datasets. These methods provide valuable insights for disease diagnosis and the early identification of risk factors.
The research on the relationship between traditional lipid indicators and depression has yielded inconsistent results [9], limiting the clinical application of lipid biomarkers. Remnant cholesterol (RC), also known as triglyceride-rich lipoprotein cholesterol, comprises very low-density lipoprotein, intermediate-density lipoprotein, and chylomicron remnants in both fasting and non-fasting states [10]. Compared to traditional lipid parameters, RC may offer greater pathological significance as a depression biomarker. Its association with inflammation is well-documented; elevated serum levels of RC can permeate arterial walls, where macrophages are involved in foam cell formation [11]. A causal relationship between RC and low-grade chronic inflammation has been established [12], suggesting that RC may contribute to the development and progression of depression through inflammatory mechanisms.
This study aims to construct a predictive model using ENR combined with ML techniques to investigate the association between RC and PPD. Additionally, the SHapley Additive exPlanations (SHAP) technique will be used to quantify the contribution of each exposure factor to PPD prediction, providing a basis for early intervention.
Materials and methods
Data sources
This study recruited 608 pregnant women between June 2020 and May 2023. The study protocol was approved by the Ethics Review Committee of the Second Affiliated Hospital of Xinjiang Medical University (Approval Numbers: 20200531-13, KY2023112109). All participants were informed about the study’s purpose and significance and provided written informed consent.
The inclusion criteria for the study were as follows: (1) age between 20 and 45 years; (2) singleton pregnancy with an estimated gestational age of 6 to 13 weeks; (3) regular prenatal check-ups at the Second Affiliated Hospital of Xinjiang Medical University during pregnancy and completion of the questionnaire. The exclusion criteria included: (1) a history of depression; (2) diagnosis of type 1 or type 2 diabetes before pregnancy; (3) abnormal screening results for hypertension or Down syndrome during pregnancy; (4) diagnosed infectious diseases such as hepatitis B, hepatitis C, syphilis, or others; (5) metabolic diseases such as hyperthyroidism or hypothyroidism; (6) disabilities or organic mental disorders; (7) use of assisted reproductive technologies for conception; (8) missing early pregnancy biochemical data; (9) inability to provide informed consent or poor adherence to the study protocol.
Study design
This longitudinal study initially collected baseline data and fasting blood biochemical test results from participants in early pregnancy (6–13 weeks of gestation). Participants were then followed up during the second trimester (24–27 weeks of gestation), at which time they completed a depression scale assessment. Prior to the study’s initiation, all researchers underwent standardized and rigorous training. Data collection was conducted using electronic questionnaires, and data entry was verified independently by two researchers to ensure accuracy.
Sample size calculation
The sample size was calculated using PASS 15.0 software. With an estimated average incidence of PPD of 11.9%, the following parameters were set: α = 0.05 (two-sided), confidence level (1-α) = 0.95, and power (1-β) = 0.9. The calculation yielded a required sample size of 162 participants. Considering a 40% attrition rate due to follow-up requirements, a minimum of 227 participants was determined to be necessary for the study.
Questionnaire quality
Depression during the second trimester was assessed using the Edinburgh Postnatal Depression Scale (EPDS), a 10-item instrument with each item scored from 0 to 3. Higher total scores (maximum of 30) indicate more severe depressive symptoms. Based on clinical research and experience, EPDS scores are typically categorized as follows:
Normal: 0–9 points.
Mild depression: 10–12 points.
Moderate to severe depressive symptoms: 13–30 points [13].
For this study, an EPDS score of ≥ 10 was used as the cutoff for a positive depression screening result, consistent with the sensitivity (92%) and specificity (77%) reported in a recent meta-analysis [14]. The Chinese version of the EPDS has demonstrated high reliability and validity, with a Cronbach’s α of 0.87 [15].
RC calculation
The RC value is obtained by subtracting the sum of high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) from total cholesterol (TC) [16].
Statistical analysis
To enhance statistical power and facilitate analysis, EPDS scores were categorized into three groups for univariate and differential analyses. For model development, EPDS was treated as a binary variable, with a threshold of EPDS ≥ 10 indicating a positive depression screen. Continuous variables were summarized as mean ± SD for normally distributed data or as median (interquartile range) for skewed distributions. Categorical variables were reported as frequencies and percentages.
Initially, logistic regression was used to examine the association between each feature and EPDS scores. Features with p <.05 in univariate analysis were included in the ENR model for further selection. The ENR model was constructed using RGui software (version 4.4.0) with the glmnetUtils package, and its performance was evaluated using the area under the curve (AUC) metric. Clinically, an AUC value between 0.7 and 1.0 is indicative of strong model discrimination, reliability, and validity [17].
After ENR model selection, the filtered dataset was divided into training (80%, n = 260) and testing (20%, n = 65) sets using JASP software (version 0.18.3.0) (available at https://jasp-stats.org/download/). Six ML models were employed to identify depression based on exposure features:
Random forest (RF).
Adaptive boosting (AdaBoost).
Support vector machine (SVM).
Decision tree (DT).
K-nearest neighbors (KNN).
Linear discriminant (LD).
The training dataset was used to develop each of the six ML models. Model performance was evaluated using a combination of metrics, including:
AUC [18].
Accuracy.
Precision.
Recall.
False positive rate (FPR).
F1 score.
Negative predictive value (NPV).
True negative rate.
False negative rate.
To interpret feature importance and visualize the decision-making process of the selected model, the SHAP tool was utilized within RGui software (version 4.4.0).
Results
Descriptive statistics
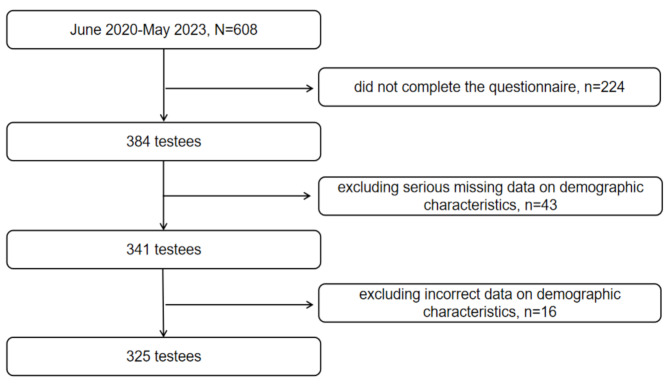
A total of 608 pregnant women were followed from early to mid-pregnancy, resulting in 384 valid questionnaires and a follow-up loss rate of 36.8%. After excluding incomplete or erroneous data, 325 pregnant women were included in the final analysis (Fig. 1).
Fig. 1.
Flowchart of study participant exclusion
The median age of the participants was 28 years (interquartile range: 27–31 years). Participants were grouped based on their EPDS scores, as summarized in Table 1. Among the 325 participants, 130 (40%) were categorized as having mild depression (10 ≤ EPDS ≤ 12), while 32 (9.8%) screened positive for major depression (EPDS ≥ 13).
Table 1.
The differences in EPDS score between groups
| Characteristics | Total (N = 325) |
Normal (n = 163, 50.2%) |
Mild depression (n = 130, 40%) |
Major depression (n = 32, 9.8%) |
F/χ2 | P value |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | 16.67 | .011a, * | ||||
| < 18.5 | 23(7.1%) | 10(6.1%) | 9(6.9%) | 4(12.5%) | ||
| 18.5–24.9 | 233(71.7%) | 107(65.6%) | 107(82.3%) | 19(59.4%) | ||
| 25-29.9 | 57(17.5%) | 38(23.3%) | 11(8.5%) | 8(25%) | ||
| ≥ 30 | 12(3.7%) | 8(4.9%) | 3(2.3%) | 1(3.1%) | ||
| Sleep behavior at noon | 10.15 | .006a, * | ||||
| no | 124(38.2%) | 66(40.5%) | 39(30%) | 19(59.4%) | ||
| yes | 201(61.8%) | 97(59.5%) | 91(70%) | 13(40.6%) | ||
| Frequency of eating breakfast | 15.50 | .004a, * | ||||
| every day | 250(77%) | 137(84%) | 86(66.2%) | 27(84.4%) | ||
| sometimes | 58(17.8%) | 22(13.5%) | 32(24.6%) | 4(12.5%) | ||
| rare | 17(5.2%) | 4(2.5%) | 12(9.2%) | 1(3.1%) | ||
| Eating habits | 15.39 | .017a, * | ||||
| drink milk or eat fruit regularly | 175(53.8%) | 99(60.7%) | 62(47.7%) | 14(43.8%) | ||
| eat green vegetables every time | 111(34.2%) | 44(27%) | 49(37.7%) | 18(56.3%) | ||
| often eat fish | 36(11.1%) | 18(11%) | 18(13.8%) | 0 | ||
| whole grains | 3(0.9%) | 2(1.2%) | 1(0.8%) | 0 | ||
| Daily meat and vegetable matching habits | 17.03 | .030a, * | ||||
| more meat and less vegetables | 51(15.7%) | 28(17.2%) | 23(17.7%) | 0 | ||
| less meat and more vegetables | 154(47.4%) | 71(43.6%) | 60(46.2%) | 23(71.9%) | ||
| as much meat as vegetables | 106(32.6%) | 60(36.8%) | 38(29.2%) | 8(25%) | ||
| all meat | 7(2.2%) | 1(0.6%) | 5(3.8%) | 1(3.1%) | ||
| all vegetables | 7(2.2%) | 3(1.8%) | 4(3.1%) | 0 | ||
| What are your dietary preferences | 14.65 | .023a, * | ||||
| bland taste | 191(58.8%) | 106(65%) | 63(48.5%) | 22(68.8%) | ||
| sweet | 42(12.9%) | 13(8%) | 24(18.5%) | 5(15.6%) | ||
| salty | 57(17.5%) | 2616%) | 29(22.3%) | 2(6.3%) | ||
| greasy | 35(10.8%) | 18(11%) | 14(10.8%) | 3(9.4%) | ||
| Oils commonly used at home | 24.57 | <.001a, * | ||||
| salad oil or blending oil | 34(10.5%) | 6(3.7%) | 26(20%) | 2(6.3%) | ||
| not fixed | 46(14.2%) | 20(12.3%) | 22(16.9%) | 4(12.5%) | ||
| vegetable oil | 245(75.4%) | 137(84%) | 82(63.1%) | 26(81.3%) | ||
| RBC (10^9/L) | 4.21(3.93,4.52) | 4.28(4.02,4.58) | 4.15(3.82,4.46) | 4.14(3.79,4.54) | 9.20 | .010b, * |
| MCV (fL) | 91.30(88.03,93.90) | 91.20(88.20,93.90) | 90.50(85.50,92.90) | 92.60(89.20,97.10) | 8.66 | .013b, * |
| MCH (pg) | 30.40(28.90,31.25) | 30.40(29.10,31.50) | 29.90(28.33,30.69) | 31(29.40,32.30) | 18.07 | <.001b, * |
| MCHc (g/L) | 330(325,337) | 331(326,338) | 330(323,333) | 332(325,338) | 8.18 | .017b, * |
| FT4 (pmol/L) | 15.98(14.51,16.67) | 15.72(14.24,16.51) | 16.25(14.94,17.18) | 15.77(14.20,16.25) | 8.42 | .015b, * |
| K (mmol/L) | 3.92(3.73,4.16) | 3.95(3.78,4.36) | 3.86(3.59,4.06) | 3.94(3.74,4.13) | 14.48 | .001b, * |
| Mg (mg/dL) | 2.02(1.90,2.24) | 2.07(1.92,2.48) | 2.01(1.90,2.10) | 1.90(1.83,2.06) | 22.17 | <.001b, * |
| FPG (mmol/L) | 4.82(4.62,5.04) | 4.82(4.64,5.01) | 4.82(4.68,5.10) | 4.82(4.48,5.01) | 6.29 | .043b, * |
| TBA (µmol/L) | 1.80(1,3.20) | 1.90(1.15,3.25) | 1.40(0.73,2.50) | 2.30(1.40,3.25) | 12.52 | .002b, * |
| 5’-nucleotidase (U/L) | 2.24(1.42,3.40) | 2.40(1.50,3.50) | 1.72(1.30,2.80) | 2.89(2.20,3.90) | 13.83 | .001b, * |
| SOD (U/ml) | 199(179.10,202) | 202(180.75,203.50) | 191(176,202) | 202(173,206) | 13.26 | .001b, * |
| TC (mmol/L) | 4.13(3.72,4.51) | 4.01(3.69,4.49) | 4.21(3.76,4.45) | 4.85(3.73,5.40) | 11.95 | .003b, * |
| LDL-C (mmol/L) | 2.25(1.74,2.40) | 2.18(1.54,2.44) | 2.25(1.89,2.35) | 2.25(2.10,2.62) | 6.13 | .047b, * |
| RC (mmol/L) | 0.25(0.07,0.57) | 0.21(0.11,0.47) | 0.27(0,0.57) | 0.55(0.14,1.62) | 9.68 | .008b, * |
Note:The annotations for all abbreviated variables in Table 1 can be found in the supplementary section. The table only shows the features with statistically significant differences. a Comparison was tested by Chi-square test (n, %); b Comparison was tested by Kruskal-Wallis H test (Median, Q1, Q3); * p <.05
Analysis of body mass index (BMI) showed that 71.7% of participants had a BMI within the normal range (18.5 ≤ BMI < 24.9). Additionally, 61.8% reported regularly taking naps at noon. Significant differences (p <.05) were observed across 21 features between the EPDS score groups. These included BMI, sleep behavior at noon, breakfast frequency, eating habits, and RC levels.
Analysis for PPD
Univariate analysis for PPD
Logistic regression was performed on 62 exposure characteristics (Table 2) to identify features associated with PPD. The univariate analysis identified 19 features with statistically significant associations with depression (p <.05). These features included nationality, breakfast frequency, and RC levels. For RC specifically, participants with major depression were found to have 4.57 times higher odds of elevated RC levels compared to those without depression (OR = 4.57, 95% CI: 2.18–9.57).
Table 2.
Univariate logistic regression analysis of risk factors for PDD
| Characteristics | Mild depression (10 ≤ EPDS ≤ 12, n = 130) | Major depression (EPDS ≥ 13, n = 32) | ||||
|---|---|---|---|---|---|---|
| β | OR(95%CI) | P value | β | OR(95%CI) | P value | |
| Nationality | ||||||
| The Han nationality | -1.71 | 0.18(0.04,0.87) | 0.033* | 16.39 | -- | 0.998 |
| The Manchu | -19.81 | -- | 0.998 | -1.77 | -- | -- |
| The Hui nationality | -1.05 | 0.35(0.05,2.41) | 0.286 | 17.55 | -- | 0.997 |
| The Uighurs | -1.05 | 0.35(0.05,2.41) | 0.286 | 16.45 | -- | 0.998 |
| Other | Ref | Ref | Ref | Ref | Ref | Ref |
| Frequency of eating breakfast | ||||||
| every day | -1.56 | 0.21(0.07,0.67) | 0.008* | -0.24 | 0.79(0.09,7.33) | 0.834 |
| sometimes | -0.72 | 0.49(0.14,1.70) | 0.258 | -0.32 | 0.73(0.06,8.32) | 0.798 |
| rare | Ref | Ref | Ref | Ref | Ref | Ref |
| Picky eating behavior | ||||||
| yes | 0.41 | 1.5(0.64,3.51) | 0.349 | -0.46 | 0.63(0.13,3.02) | 0.564 |
| occasionally | 0.63 | 1.89(1.16,3.08) | 0.011* | -0.29 | 0.75(0.33,1.68) | 0.483 |
| no | Ref | Ref | Ref | Ref | Ref | Ref |
| Oils commonly used at home | ||||||
| salad oil or blending oil | 1.98 | 7.24(2.86,18.33) | < 0.001* | 0.56 | 1.76(0.34,9.19) | 0.505 |
| not fixed | 0.61 | 1.84(0.95,3.57) | 0.073 | 0.05 | 1.05(0.34,3.34) | 0.929 |
| vegetable oil | Ref | Ref | Ref | Ref | Ref | Ref |
| RBC (10^9/L) | -0.77 | 0.46(0.30,0.74) | 0.001* | -0.72 | 0.49(0.23,1.02) | 0.058 |
| MCV (fL) | -0.04 | 0.96(0.92,0.99) | 0.031* | 0.06 | 1.06(0.98,1.14) | 0.150 |
| MCH (pg) | -0.14 | 0.87(0.79,0.97) | 0.008* | 0.16 | 1.17(0.94,1.46) | 0.168 |
| MCHc (g/L) | -0.03 | 0.97(0.95,0.99) | 0.022* | -0.01 | 1(1,1.04) | 0.847 |
| K (mmol/L) | -1.43 | 0.24(0.12,0.46) | < 0.001* | -1.09 | 0.34(0.12,0.96) | 0.042* |
| Ca (mmol/L) | -2.68 | 0.07(0.01,0.38) | 0.002* | -2.86 | 0.06(0.04,0.81) | 0.034* |
| Mg (mg/dL) | -2.67 | 0.07(0.03,0.19) | < 0.001* | -3.58 | 0.03(0,0.19) | < 0.001* |
| FPG (mmol/L) | 0.84 | 2.32(1.10,4.91) | 0.027* | -0.4 | 0.67(0.20,2.26) | 0.520 |
| TBA (µmol/L) | -0.21 | 0.81(0.69,0.96) | 0.012* | 0.04 | 1.04(0.91,1.19) | 0.530 |
| 5’-nucleotidase (U/L) | -0.19 | 0.83(0.69,0.99) | 0.035* | 0.10 | 1.11(0.89,1.38) | 0.363 |
| SOD (U/ml) | -0.03 | 0.98(0.96,0.99) | < 0.001* | -0.01 | 0.99(0.97,1.01) | 0.280 |
| TG (mmol/L) | 0.11 | 1.12(0.72,1.74) | 0.630 | 0.76 | 2.15(1.27,3.64) | 0.005* |
| TC (mmol/L) | 0.12 | 1.13(0.80,1.59) | 0.478 | 1.05 | 2.84(1.64,4.92) | < 0.001* |
| LDL-C (mmol/L) | 0.27 | 1.31(0.85,2.02) | 0.217 | 0.76 | 2.14(1.09,4.17) | 0.026* |
| RC (mmol/L) | -0.05 | 0.95(0.52,1.72) | 0.863 | 1.52 | 4.57(2.18,9.57) | < 0.001* |
Note: The annotations for all abbreviated variables in Table 2 can be found in the supplementary section. The table only shows the characteristics that affect the outcome variables. In the multiple logistic regression analysis, Normal (0 < EPDS ≤ 9, n = 163) was set as the control group. Ref, means reference. “--” Indicates an invalid value, which is caused by the number of observation units in the reference category being 0 and the sample size being too small. However, based on the authenticity of the data, this cannot be changed. * p <.05
ENR screens out important features of PPD
The ENR model employs cross-validation to optimize parameters (α and λ) and minimize prediction error. Ten-fold cross-validation was applied as a model evaluation and selection strategy to assess the model’s generalization capability and identify the best hyperparameters. The process involves:
Data segmentation: Dividing the dataset into training and validation subsets.
Model training and validation: Training the model on the training subset and validating it on the validation subset.
Loop iteration: Repeating the process across multiple folds.
Average validation error: Calculating the mean validation error across all folds.
Optimal parameter selection: Choosing parameters that yield the lowest average validation error.
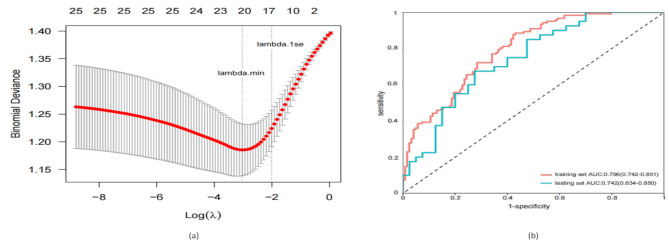
Using ten-fold cross-validation, the ENR model determined the optimal parameters as α = 0.18 and λ = 0.13. The “lambda.1se” criterion was used to screen 19 features, resulting in the selection of 14 important feature variables (Fig. 2a). Receiver operating characteristic (ROC) curve analysis validated the predictive performance of the ENR model. As illustrated in Fig. 2b, the model demonstrated strong accuracy, with an AUC of 0.796 (95% CI: 0.742–0.851) in the training set and 0.742 (95% CI: 0.634–0.850) in the testing set.
Fig. 2.
Elastic net regression completes the prognostic model variable screening. (a) Quantitative display of important features filtered out by elastic net regression. (b) Superposition of ROC curves of training set and test set in elastic net regression model
Abbreviations: ROC, receiver operating characteristic; AUC, area under the curve
Note: All categorical variables in the model have been one-hot encoded
The results indicated that lower levels of red blood cell count (RBC), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHc), potassium (K), magnesium (Mg), calcium (Ca), total bile acids (TBA), and superoxide dismutase (SOD), alongside higher levels of RC and triglycerides (TG), were significantly associated with an increased risk of PPD. The regression coefficients for these variables were as follows: RBC (-0.124), MCH (-0.008), MCHc (-0.007), K (-0.201), Mg (-1.370), Ca (-0.796), TBA (-0.034), SOD (-0.005), TG (0.054), and RC (0.196) (Table 3).
Table 3.
Elastic net regularized regression screens the predictive features of PPD
| Characteristics | Non-zero coefficient (β) | Characteristics | Non-zero coefficient (β) |
|---|---|---|---|
| Nationality(years) | RBC (10^9/L) | -0.124 | |
| The Han nationality | Ref | MCV (fL) | NA |
| The Manchu | -0.407 | MCH (pg) | -0.008 |
| The Hui nationality | 0.259 | MCHc (g/L) | -0.007 |
| The Uighurs | 0 | K (mmol/L) | -0.201 |
| Other | 0.869 | Mg(mg/dL) | -1.370 |
| Frequency of eating breakfast | Ca (mmol/L) | -0.796 | |
| every day | Ref | FPG (mmol/L) | NA |
| sometimes | 0.166 | TBA (µmol/L) | -0.034 |
| rare | 0.412 | 5’-nucleotidase (U/L) | 0 |
| Are you picky eaters | SOD (U/ml) | -0.005 | |
| yes | Ref | TG (mmol/L) | 0.054 |
| occasionally | 0 | TC (mmol/L) | 0 |
| no | -0.176 | LDL-C (mmol/L) | 0 |
| Oils commonly used at home | RC (mmol/L) | 0.196 | |
| salad oil or blending oil | Ref | ||
| not fixed | 0 | ||
| vegetable oil | -0.427 |
Abbreviations: RBC, red blood cell count; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHc, mean corpuscular hemoglobin concentration; K, serum potassium; Mg, serum magnesium; Ca, serum calcium; FPG, fasting plasma glucose; TBA, total bile acids; SOD, superoxide dismutase; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; RC, remnant cholesterol
Note: Ref, means reference
Performance of six ML models in identifying PPD
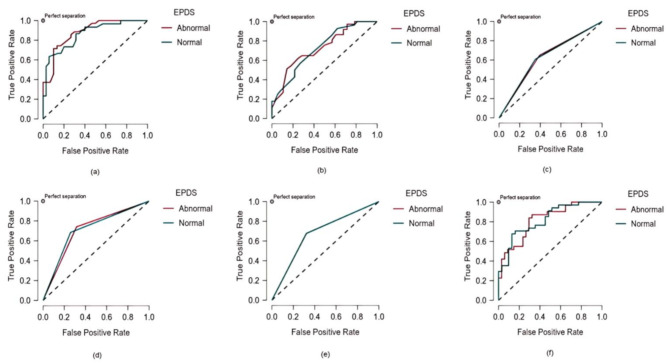
To analyze the 14 features identified by the ENR model, six ML models were employed: RF, DT, AdaBoost, SVM, KNN, and LD. Table 4 provides a summary of their performance metrics. Among the models, the RF approach showed the highest average precision (0.754), reflecting superior discrimination capability. Both RF and DT models demonstrated similar performance in accuracy, precision, recall, FPR, NPV, and F1 score. Additionally, the RF model achieved the highest AUC value (0.850), significantly outperforming the other models (Fig. 3). Comprehensive feature-based analysis confirmed that the RF model exhibited the best precision and robustness for identifying PPD, making it the most effective method among the six evaluated approaches.
Table 4.
Comparison of discrimination characteristics among six machine learning models
| Characteristics | RF | AdaBoost | SVM | DT | KNN | LD |
|---|---|---|---|---|---|---|
| Accuracy | 0.738 | 0.569 | 0.677 | 0.708 | 0.677 | 0.646 |
| Precision /Positive Predictive Value | 0.754 | 0.611 | 0.683 | 0.720 | 0.683 | 0.653 |
| Recall /True Positive Rate | 0.738 | 0.569 | 0.677 | 0.708 | 0.677 | 0.646 |
| FPR | 0.255 | 0.409 | 0.323 | 0.288 | 0.323 | 0.354 |
| F1 Score | 0.738 | 0.562 | 0.678 | 0.710 | 0.678 | 0.648 |
| AUC* | 0.850 | 0.655 | 0.677 | 0.712 | 0.677 | 0.777 |
| NPV | 0.749 | 0.598 | 0.674 | 0.706 | 0.674 | 0.643 |
| True Negative Rate | 0.745 | 0.591 | 0.677 | 0.712 | 0.677 | 0.646 |
| False Negative Rate | 0.255 | 0.409 | 0.323 | 0.288 | 0.323 | 0.354 |
Abbreviations: EPDS, Edinburgh Postnatal Depression Scale; RF, random forest; AdaBoost, adaptive boosting; SVM, support vector machine; DT, decision tree; KNN, k-nearest neighbors; LD, linear discriminant; FPR, false positive rate; AUC, area under curve; NPV, negative predictive value
Note: *The corresponding p-values and confidence intervals for the above values are not displayed in the JASP platform
Fig. 3.
The AUC comparison of six machine learning models. (a) RF model, AUC = 0.850; (b) AdaBoost model, AUC = 0.655; (c) SVM model, AUC = 0.677; (d) DT model, AUC = 0.712; (e) KNN model, AUC = 0.677; (f) LD, AUC = 0.777
Abbreviations: EPDS, Edinburgh Postnatal Depression Scale; AUC, area under the curve; RF, random forest; AdaBoost, adaptive boosting; SVM, support vector machine; DT, decision tree; KNN, k-nearest neighbors; LD, linear discriminant
Note: Normal (0 < EPDS ≤ 9), Abnormal (EPDS ≥ 10)
Visualization of feature importance
The RF model was trained using optimized hyperparameters to ensure maximum performance. Feature importance was evaluated and visualized, highlighting the model’s efficiency and stability. The performance metrics, illustrated by the ROC curve and out-of-bag error, indicated robust predictive capabilities. The training set achieved an AUC of 0.934 (95% CI: 0.905–0.963), while the testing set yielded an AUC of 0.824 (95% CI: 0.742–0.907).
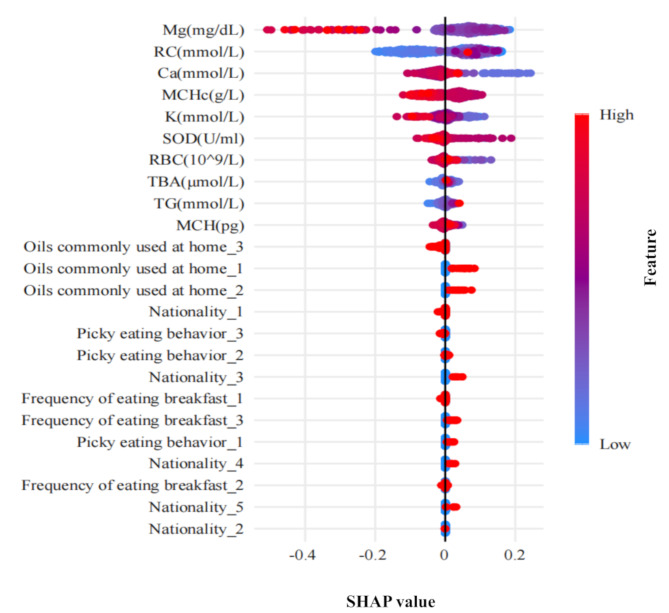
To further explore the model’s predictive insights, SHAP values were employed to graphically represent the impact of individual features on depression prediction (Fig. 4). The analysis identified Mg, RC, Ca, MCHc, and K as the top five most influential features. Notably, Mg, Ca, MCHc, and K demonstrated negative contributions, indicating their association with a reduced risk of PPD. In contrast, RC exhibited a positive contribution, linking it to an increased risk of PPD. The SHAP plot also revealed additional behavioral and dietary factors associated with a higher risk of PPD, including picky eating habits, infrequent breakfast consumption, and frequent use of salad dressing or blended oil in cooking.
Fig. 4.
SHAP feature importance summary plot. Each feature corresponds to a specific shapley value and is sorted according to importance from large to small
Abbreviations: SHAP, SHapley Additive exPlanations. Mg, magnesium; RC, remnant cholesterol; Ca, calcium; MCHc, mean corpuscular hemoglobin concentration; K, potassium; SOD, superoxide dismutase; RBC, red blood cell count; TBA, total bile acids; TG, triglycerides; MCH, mean corpuscular hemoglobin; Nationality_1, The Han nationality; Nationality_2, The Manchu; Nationality_3, The Hui nationality; Nationality_4, The Uighurs; Nationality_5, Other; Oils commonly used at home_1, salad oil or blending oil; Oils commonly used at home_2, not fixed; Oils commonly used at home_3, vegetable oil; Picky eating behavior_1, yes; Picky eating behavior_2, occasionally; Picky eating behavior_3, no; Frequency of eating breakfast_1, every day; Frequency of eating breakfast_2,sometimes; Frequency of eating breakfast_3, rare
Note: All categorical variables in the RF model have been one-hot encoded
Discussion
This study represents a pioneering approach by combining ENR with six interpretable ML algorithms to explore exposure factors associated with PPD. After comprehensive performance evaluations, the RF model emerged as the most effective for identifying PPD. SHAP analysis further highlighted the five most influential features, with Mg, Ca, MCHc, and K showing protective effects against PPD. In contrast, RC, the second most influential feature, was positively associated with PPD, suggesting it as a potential risk factor.
In recent years, ML has become a prominent tool in disease prediction, offering unique advantages in PPD risk assessment. Its effectiveness depends on factors such as data quality, feature selection, and sample size. A review of ML applications in predicting postpartum depression found these methods to be effective tools for identifying individuals at risk, especially given the expanding computational data in psychiatric research [19]. In this study, all six ML models demonstrated good predictive reliability, with AUC values exceeding 0.7.
Among the models, the RF algorithm consistently outperformed others. By constructing multiple decision trees and combining their outputs through majority voting, RF minimizes the risk of overfitting seen in single models, enhancing generalization. Additionally, its robustness to outliers and noisy data makes RF particularly suitable for the heterogeneous datasets typical in PPD research, encompassing demographic, psychological, and biological variables. In comparison, other ML models, such as the SVM, require meticulous hyperparameter tuning and are computationally intensive, limiting their practicality in complex datasets. These advantages establish RF as a reliable and efficient tool for predicting PPD risk.
The link between RC and depression is not yet fully understood, but several plausible biological mechanisms have been proposed. Elevated RC levels have been associated with low-grade inflammation and endothelial dysfunction, which may impair brain microvascular function and contribute to depression [20–22]. Specifically, RC-induced inflammation promotes the production of mediators like interleukin-6 (IL-6) and tumor necrosis factor-alpha, leading to neuronal damage and neurotransmitter dysregulation, which disrupts emotional regulation [23]. Moreover, RC may compromise endothelial integrity, increasing vascular permeability and promoting leukocyte infiltration and platelet aggregation. These changes are hypothesized to result in microvascular dysfunction, reducing cerebral perfusion and impairing the delivery of essential nutrients and oxygen to neurons, thereby exacerbating depression’s neurobiological underpinnings [24].
Our findings linking RC to PPD are supported by recent studies [25]. One proposed mechanism involves RC’s role in activating the hypothalamic-pituitary-adrenal (HPA) axis, a pathway closely associated with depression. Elevated RC levels may enhance arterial wall penetration, where RC particles are more readily absorbed by macrophages than LDL-C, accelerating foam cell formation. Macrophage foam cells express IL-6 and circulating IL-6 stimulates the HPA axis [26]. Dysregulation of the HPA axis is extensively implicated in depression’s pathophysiology [27, 28].
Previous research has suggested a connection between trace element deficiencies and depression [29]. Our findings support this association, showing that individuals with PPD have lower serum levels of Mg, Ca, and K. Mg plays a vital role in the synthesis and release of neurotransmitters, and supplementation has shown potential in alleviating depressive symptoms [30]. Similarly, Ca is integral to nervous system functions such as nerve conduction and cellular signaling [31]. Ca deficiencies may lead to abnormal neuronal excitability and impaired nerve conduction, which could affect mood regulation and increase the risk of depression [32]. K is critical for maintaining ion balance within and between cells, ensuring proper neuromuscular function. Low K levels may disrupt nervous system function, adversely affecting mood and mental health [33].
Our study also revealed a negative correlation between MCHc and depression. Although the precise mechanism underlying this association remains unclear, existing research suggests that low MCHc levels may be linked to inflammation, oxidative stress, and insufficient oxygen delivery to the brain [34]. These factors can impair neurotransmitter function, thereby influencing emotional and psychological well-being [35].
This study has several limitations that should be considered. (1) PPD Identification: PPD was identified based on self-reported EPDS scores. While self-reports may be participant to information bias due to personal feelings and memory recall, the EPDS scale is a reliable and valid tool for PPD screening, as supported by numerous studies [36, 37]. Additionally, the EPDS threshold for diagnosing PPD varies from some guidelines, which could influence the study’s results. However, many clinical studies still use an EPDS score of ≥ 10 as the cut-off for identifying PPD [38–40]. It is important to note that investigating the impact of different EPDS cut-off values in other populations will be a focus of our future research. (2) Fasting Data: The analysis was based solely on fasting data, excluding non-fasting samples. While previous research suggests minimal differences in most lipid parameters between fasting and non-fasting states [41], future studies incorporating non-fasting data may provide additional insights. Non-fasting lipid samples have also shown comparable prognostic value to fasting samples for general risk assessment [42]. (3) Model Complexity: The complexity of the machine learning models used in this study may present challenges for reproducibility and real-world application. Simplifying the models or exploring alternative methods could improve their practical utility.
Conclusion
This study identified the RF model as an efficient and robust tool for analyzing the association between exposures and PPD. The findings suggest that Mg, Ca, MCHc, and K are protective factors, while RC serves as a risk factor for PPD. These results underscore the potential of RC as a biomarker for PPD screening. However, further validation through large cohort studies or clinical trials is essential to confirm these findings and clarify their causal relationships.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all participants for their willingness to participate in the study and the time that they devoted to the study.
Abbreviations
- EPDS
Edinburgh Postnatal Depression Scale
- PPD
Peripartum depression
- ENR
Elastic net regression
- M
Machine learning
- SHAP
SHapley Additive exPlanations
- BMI
Body mass index
- WBC
White blood cell count
- RBC
Red blood cell count
- HGB
Hemoglobin
- PLT
Platelet count
- HCT
Hematocrit
- MCV
Mean corpuscular volume
- MCH
Mean corpuscular hemoglobin
- MCHc
Mean corpuscular hemoglobin concentration
- LYC
Lymphocyte count
- MONO
Number of monocytes
- NEUT
Neutrophils count
- Fbg
Fibrinogen
- FT4
Free thyroid
- TSH
Thyroid stimulating hormone
- K
Potassium
- Na
Sodium
- Cl
Chloride
- Ca
Calcium
- Mg
Magnesium
- P
Phosphorus
- Fe
Iron
- FPG
Fasting plasma glucose
- BUN
Blood urea nitrogen
- CR
Creatinine
- UA
Uric acid
- TBIL
Total bilirubin
- DBIL
Direct bilirubin
- IBIL
Indirect bilirubin
- TP
Total protein
- ALB
Albumin
- GLO
Globulin
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ALP
Alkaline phosphatase
- GGPT
Glutamyl-transpeptidase
- LDH
Lactate dehydrogenase
- TBA
Total bile acids
- SOD
Superoxide dismutase
- TG
Triglycerides
- TC
Total cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- RC
Remnant cholesterol
- RF
Random forest
- AdaBoost
Adaptive boosting
- SVM
Support vector machine
- DT
Decision tree
- KNN
k-nearest neighbors
- LD
Linear discriminant
- AUC
Area under the curve
- ROC
Receiver operating characteristic
- IL-6
Interleukin-6
- HPA
Hypothalamic-pituitary-adrenal
Author contributions
HC and FW designed the study. FW, TK, DW secured funding for the study. JS, BG, and CS collected the data. HC, TK, YW, and DM led the drafting of the manuscript. HC, GL, GC, and YN finished the statistical analyses and drew the graph. All authors approved the final manuscript for submission.
Funding
This work was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region [2023D01C119] and Xinjiang Medical University Youth Science and Technology Elite Talent Program [XYD2024Q09]. The funders had no role in the data collection, data analysis, or reporting of this study.
Data availability
As this study is only part of the subject matter, the datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Review Committee of the Second Affiliated Hospital of Xinjiang Medical University [approval number: 20200531-13, KY2023112109]. All participants were informed about the research purpose and significance of this investigation and provided written informed consent. In addition, this study strictly adhered to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongxu Chen and Denglan Wang contributed equally to this work.
Contributor Information
Tiantian Kong, Email: 123457417@qq.com.
Fan Wang, Email: FanWang@bjmu.edu.cn.
References
- 1.Vitte L, Nakić Radoš S, Lambregtse-van den Berg M, Devouche E, Apter G. Peripartum depression: what’s new?? Curr Psychiatry Rep. 2025;27(1):31–40. 10.1007/s11920-024-01573-6. [DOI] [PubMed] [Google Scholar]
- 2.Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86–92. 10.1016/j.jad.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Reissland N, Froggatt S, Reames E, Girkin J. Effects of maternal anxiety and depression on fetal neuro-development. J Affect Disord. 2018;241:469–74. 10.1016/j.jad.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry. 2012;25(2):141–8. 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segoviano-Mendoza M, Cárdenas-de la Cruz M, Salas-Pacheco J, Vázquez-Alaniz F, La Llave-León O, Castellanos-Juárez F, Méndez-Hernández J, Barraza-Salas M, Miranda-Morales E, Arias-Carrión O, Méndez-Hernández E. Hypocholesterolemia is an independent risk factor for depression disorder and suicide attempt in Northern Mexican population. BMC Psychiatry. 2018;18(1):7. 10.1186/s12888-018-1596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan Y, Zhao Y, Qu Y, Yue H, Shi Y, Chen Y, Liu X, Liu R, Lyu T, Jing A, Meng Y, Huang J, Jiang Y. Longitudinal association of maternal dietary patterns with antenatal depression: evidence from the Chinese pregnant women cohort study. J Affect Disord. 2022;308:587–95. 10.1016/j.jad.2022.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Davies-Kershaw H, Fahmida U, Htet MK, Kulkarni B, Faye B, Yanti D, Shinta D, Zahra NL, Angelin TC, Madhari R, Pullakhandam R, Palika R, Dasi T, Fernandez Rao S, Banjara SK, Selvaraj K, Palepu DP, Yadev D, Diouf S, Lopez-Sall P, Diallo B, Mouissi P, Fall S, Diallo I, Djigal A, Immerzeel TDV, Tairou F, Diop A, Pradeilles R, Strout S, Momo Kadia B, Tata DT, Jobarteh ML, Allen S, Walker A, Webster JP, Haggarty P, Heffernan C, Ferguson E. Anthropometric, biochemical, dietary, morbidity and well-being assessments in women and children in Indonesia, India and Senegal: a UKRI GCRF action against stunting hub protocol paper. BMJ Paediatr Open. 2024;8(Suppl 1):e001683. 10.1136/bmjpo-2022-001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Zhao Y, Zhang D, Kuang L, Huang H, Chen W, Fu X, Wu Y, Li T, Zhang J, Yuan L, Hu H, Liu Y, Zhang M, Hu F, Sun X, Hu D. Development of an interpretable machine learning model associated with heavy metals’ exposure to identify coronary heart disease among US adults via SHAP: findings of the US NHANES from 2003 to 2018. Chemosphere. 2023;311(Pt 1):137039. 10.1016/j.chemosphere.2022.137039. [DOI] [PubMed] [Google Scholar]
- 9.Cepeda MS, Kern DM, Blacketer C, Drevets WC. Low levels of cholesterol and the cholesterol type are not associated with depression: results of a cross-sectional NHANES study. J Clin Lipidol. 2020 Jul-Aug;14(4):515–21. 10.1016/j.jacl.2020.06.001. [DOI] [PubMed]
- 10.Ozkan J. Danish scientist wins prestigious prize for piecing together the facts about remnant cholesterol. Eur Heart J. 2022;43(34):3192–3. 10.1093/eurheartj/ehac429. [DOI] [PubMed] [Google Scholar]
- 11.Miller YI, Choi SH, Fang L, Tsimikas S. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell Biochem. 2010;51:229–51. 10.1007/978-90-481-8622-8_8. [DOI] [PubMed] [Google Scholar]
- 12.Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128(12):1298–309. 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 13.Ngai FW, Gao LL. Effect of couple-based interpersonal psychotherapy on postpartum depressive symptoms: A randomised controlled trial. Asian J Psychiatr. 2022;78:103274. 10.1016/j.ajp.2022.103274. [DOI] [PubMed] [Google Scholar]
- 14.Levis B, Negeri Z, Sun Y, Benedetti A, Thombs BD, DEPRESsion Screening Data (DEPRESSD) EPDS Group. Accuracy of the Edinburgh postnatal depression scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ. 2020;371:m4022. 10.1136/bmj.m4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Gao R, Dai X, Liu H, Zhang J, Liu X, Si D, Deng T, Xia W. The association between symptoms of depression during pregnancy and low birth weight: a prospective study. BMC Pregnancy Childbirth. 2020;20(1):147. 10.1186/s12884-020-2842-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Huang S, Cao Y, Dong G, Chen Y, Zhu X, Yun W, Zhang M. Remnant cholesterol and mild cognitive impairment: A cross-sectional study. Front Aging Neurosci. 2023;15:1069076. 10.3389/fnagi.2023.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, Song Q. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun (Lond). 2020;40(7):301–12. 10.1002/cac2.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31. 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 19.Cellini P, Pigoni A, Delvecchio G, Moltrasio C, Brambilla P. Machine learning in the prediction of postpartum depression: A review. J Affect Disord. 2022;309:350–7. 10.1016/j.jad.2022.04.093. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Takano H, Umetani K, Kawabata K, Obata JE, Kitta Y, Kodama Y, Mende A, Ichigi Y, Fujioka D, Saito Y, Kugiyama K. Remnant lipoproteinemia is a risk factor for endothelial vasomotor dysfunction and coronary artery disease in metabolic syndrome. Atherosclerosis. 2005;181(2):321–7. 10.1016/j.atherosclerosis.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Lizano P, Pong S, Santarriaga S, Bannai D, Karmacharya R. Brain microvascular endothelial cells and blood-brain barrier dysfunction in psychotic disorders. Mol Psychiatry. 2023;28(9):3698–708. 10.1038/s41380-023-02255-0. [DOI] [PubMed] [Google Scholar]
- 22.van Agtmaal MJM, Houben AJHM, Pouwer F, Stehouwer CDA, Schram MT. Association of microvascular dysfunction with Late-Life depression: A systematic review and Meta-analysis. JAMA Psychiatry. 2017;74(7):729–39. 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: A meta-analysis. J Affect Disord. 2020;277:940–8. 10.1016/j.jad.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Matsuno H, Tsuchimine S, O’Hashi K, Sakai K, Hattori K, Hidese S, Nakajima S, Chiba S, Yoshimura A, Fukuzato N, Kando M, Tatsumi M, Ogawa S, Ichinohe N, Kunugi H, Sohya K. Association between vascular endothelial growth factor-mediated blood-brain barrier dysfunction and stress-induced depression. Mol Psychiatry. 2022;27(9):3822–32. 10.1038/s41380-022-01618-3. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Shen R. Association of remnant cholesterol with depression among US adults. BMC Psychiatry. 2023;23(1):259. 10.1186/s12888-023-04770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–14. 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 27.Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, Schatzberg AF. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22(4):527–36. 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35(9):1275–86. 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Quan Z, Li H, Quan Z, Qing H. Appropriate macronutrients or mineral elements are beneficial to improve depression and reduce the risk of depression. Int J Mol Sci. 2023;24(8):7098. 10.3390/ijms24087098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du J, Zhu M, Bao H, Li B, Dong Y, Xiao C, Zhang GY, Henter I, Rudorfer M, Vitiello B. The role of nutrients in protecting mitochondrial function and neurotransmitter signaling: implications for the treatment of depression, PTSD, and suicidal behaviors. Crit Rev Food Sci Nutr. 2016;56(15):2560–78. 10.1080/10408398.2013.876960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lezmy J, Arancibia-Cárcamo IL, Quintela-López T, Sherman DL, Brophy PJ, Attwell D. Astrocyte Ca2+-evoked ATP release regulates myelinated axon excitability and conduction speed. Science. 2021;374(6565):eabh2858. 10.1126/science.abh2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma A, Schray A, Bartolovic M, Roesch-Ely D, Aschenbrenner S, Weisbrod M. Relationship between serum calcium and neuropsychological performance might indicate etiological heterogeneity underlying cognitive deficits in schizophrenia and depression. Psychiatry Res. 2017;252:80–6. 10.1016/j.psychres.2017.01.101. [DOI] [PubMed] [Google Scholar]
- 33.Knuth B, Radtke V, Rocha P, da Silva KS, Dalsóglio F, Gazal M, Jansen K, Souza DO, Portela LV, Kaster M, Oses JP. Prevalence of depression symptoms and serum levels of interleukin-6 in Hemodialysis patients. Psychiatry Clin Neurosci. 2014;68(4):275–82. 10.1111/pcn.12130. [DOI] [PubMed] [Google Scholar]
- 34.Maes M, Van de Vyvere J, Vandoolaeghe E, Bril T, Demedts P, Wauters A, Neels H. Alterations in iron metabolism and the erythron in major depression: further evidence for a chronic inflammatory process. J Affect Disord. 1996;40(1–2):23–33. 10.1016/0165-0327(96)00038-9. [DOI] [PubMed] [Google Scholar]
- 35.Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–56. 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozinszky Z, Dudas RB. Validation studies of the Edinburgh postnatal depression scale for the antenatal period. J Affect Disord. 2015;176:95–105. 10.1016/j.jad.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 37.van der Zee AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, Reijneveld SA. Postpartum depression and anxiety: a community-based study on risk factors before, during and after pregnancy. J Affect Disord. 2021;286:158–65. 10.1016/j.jad.2021.02.062. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Deng CM, Zeng Y, Chen XZ, Li AY, Feng SW, Xu LL, Chen L, Yuan HM, Hu H, Yang T, Han T, Zhang HY, Jiang M, Sun XY, Guo HN, Sessler DI, Wang DX. Efficacy of a single low dose of Esketamine after childbirth for mothers with symptoms of prenatal depression: randomised clinical trial. BMJ. 2024;385:e078218. 10.1136/bmj-2023-078218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groer ME, Baumgartel K, Springer C, Mutka T, Postolache TT. Depression in pregnant Hispanic women: risk factors, pregnancy outcomes and plasma cytokines. Brain Behav Immun Health. 2024;38:100765. 10.1016/j.bbih.2024.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai Y, Zeng Z, Li X, Gong W. The predictive effect of mid-pregnancy sleep disorders on perinatal depression within women with or without depression in early pregnancy: A prospective cohort study. J Affect Disord. 2024;345:18–23. 10.1016/j.jad.2023.10.103. [DOI] [PubMed] [Google Scholar]
- 41.Sathiyakumar V, Park J, Golozar A, Lazo M, Quispe R, Guallar E, Blumenthal RS, Jones SR, Martin SS. Fasting versus nonfasting and Low-Density lipoprotein cholesterol accuracy. Circulation. 2018;137(1):10–9. 10.1161/CIRCULATIONAHA.117.030677. [DOI] [PubMed] [Google Scholar]
- 42.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O, ESC Scientific Document Group. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88. 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As this study is only part of the subject matter, the datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.