Abstract
Calicivirus infection of dogs was epidemiologically investigated by using canine calicivirus (CaCV) strain 48 as a reference. Similar RNA polymerase gene sequences and neutralizing antibodies against CaCV were detected in 1.7% of clinical specimens and 57% of serum samples, respectively, suggesting a high prevalence of CaCV in dog populations.
Caliciviruses are important pathogens in both veterinary and human medicine (1, 6) and recently have been classified into four genera (Lagovirus, “Norwalk-like viruses,” “Sapporo-like viruses,” and Vesivirus) in the Caliciviridae family (7). Classical animal caliciviruses such as Feline calicivirus (FCV), Vesicular exanthema of swine virus, and San Miguel sea lion virus belong to the genus Vesivirus. In veterinary clinics, FCV has been an agent of great importance because it causes serious respiratory disease in cats and remains a difficult problem despite intensive vaccination practices (3).
In contrast, very little is known about calicivirus infection in dogs. Occasionally, dogs in the field have been found to be infected with caliciviruses, and most of these are considered to be FCV, transmitted from cats (2, 4, 5, 14, 16). However, two studies have described a candidate as a true canine calicivirus (CaCV) (13, 18). In 1985, a calicivirus was isolated from the feces of a dog with diarrhea in the United States (18). It appeared to be CaCV, but no further characterization was performed. Another candidate was found in a Japanese dog in 1990 (13): the virus, designated strain 48, was isolated from a 2-month-old dog that died showing intermittent watery diarrhea. Subsequent studies revealed that this virus has characteristics sufficiently unique and distinct that it can be regarded as CaCV (10-12, 15, 17). However, no epidemiological information is available for CaCV infection in dogs.
During the period from January 2000 to September 2001, 105 rectal and 14 oral swab samples were obtained from dogs at private animal hospitals located in various parts of Japan. The samples were submitted for general virus examination, and swab extracts were stored at −80°C until use. Swab extracts were tested for calicivirus by reverse transcriptase PCR (RT-PCR) using a recently described broadly reactive primer pair (p289-p290) (9). This amplifies the RNA polymerase regions of various caliciviruses, and the sizes of expected PCR products are 319 bp for Norwalk-like viruses and 331 bp for both Sapporo-like viruses and vesivirus (8). Methods for RT-PCR and sequence analysis of the PCR product were as described previously (8, 15).
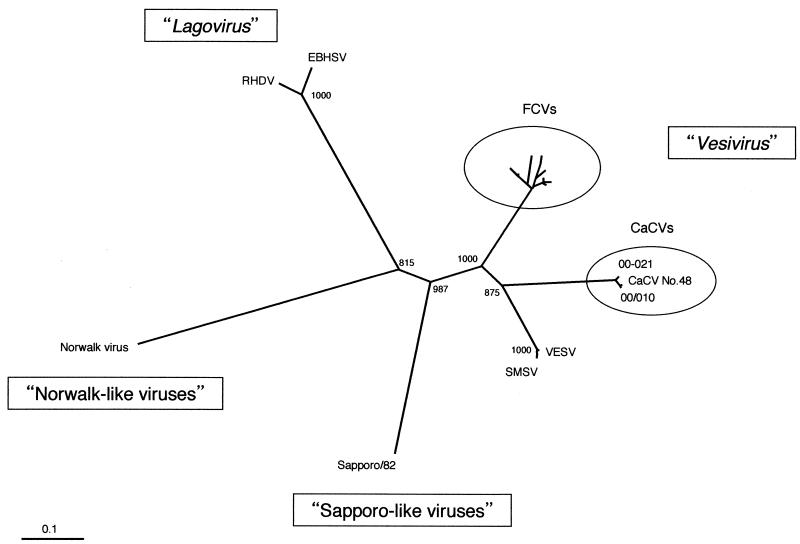
Two samples were shown to be positive; one was a tonsil swab (designated 00/010) of a 60-day-old puppy showing upper respiratory illness and diarrhea, and the other was a rectal swab (designated 00-021) of a 50-day-old puppy with diarrhea. Pathogenic agents such as canine distemper virus, canine parvovirus type 2, canine coronavirus, canine adenovirus, and rotavirus were not detected in either specimen. For both specimens, the size of the PCR product was 331 bp (data not shown), suggesting either Sapporo-like viruses or vesivirus. The deduced amino acid sequences were both 95 residues and showed the highest degree of homology to the sequence of strain 48 (97.9 to 98.9%). Figure 1 shows the inferred phylogenetic relationships between different caliciviruses on the basis of the alignment of the amino acids of the partial RNA polymerase region. The two newly found gene sequences formed a tight cluster with strain 48, making a new clade of caliciviruses, as suggested previously (15).
FIG. 1.
Phylogenetic analysis of the amino acid sequences in the RNA polymerase region generated by RT-PCR using primer set p289-p290 (9). The tree was constructed by the method used previously (15). The tree topology was based on the neighbor-joining method. Bootstrap values indicate the number of times each branching was found in 1,000 bootstrap analyses. Branch lengths indicate phylogenetic distances calculated from distance matrices of deduced amino acid sequences. The sequences used in the alignment were retrieved from the DDBJ/EMBL/GenBank databases and are as follows (with accession numbers given in parentheses): CaCV strain 48 (AF053720), FCV Sapporo/283 (AF098932), FCV F2 (AF098929), FCV F14 (AF098928), FCV FC35 (AF098930), FCV FC61 (AF098931), FCV F4 (D31836), FCV F9 (Z11536), FCV CFI/68 (U13992), FCV Urbana (L40021), San Miguel sea lion virus (SMSV) 1 (M87481), SMSV 4 (M87482), vesicular exanthema of swine virus (VESV) A48 (NC_002551), rabbit hemorrhagic disease virus (RHDV) (M67473), European brown hare syndrome virus (EBHSV)-GD (NC_002615), Norwalk virus (M87661), and Sapporo/82 (S77903).
Virus isolation from the PCR-positive samples was attempted by using Madin-Darby canine kidney (MDCK) and Crandell feline kidney cells. However, no evidence for virus growth was obtained. This suggests that CaCVs are fastidious, as is generally accepted for many caliciviruses of human origin and some of animal origin (1, 6, 8), or possibly that only low levels of virus were present in the swab extracts.
Neutralizing antibodies against CaCV strain 48 were examined by a microneutralization test (MNT). Serum samples were collected from 244 dogs presenting at university animal hospitals located in Yamaguchi (western Japan), Tokyo (eastern Japan), and Sapporo (northern Japan) during the same period as the swab collection (Table 1). After serum samples were heat inactivated at 56°C for 30 min, they were serially diluted with Eagle's minimal essential medium containing 7.5% fetal bovine serum in wells of a 96-well flat-bottom plate. Fifty microliters of a virus suspension containing 100 50% tissue culture infective doses was added to each well. The plate was gently agitated for mixing and was then incubated at 37°C for 1 h. After that, 100 μl of an MDCK cell suspension containing 104 cells was added to each well and incubation was continued for 72 h. The MNT titer was defined as the reciprocal of the highest serum dilution at which virus cytopathic effect had been inhibited completely, and a titer of 1:4 or more was considered significant because none of 12 specific-pathogen-free beagle dogs had such titers. As shown in Table 1, more than half of the dogs at each location had neutralizing antibodies. In all, 57% of dogs were serologically positive, indicating previous exposure to caliciviruses antigenically the same as, or related to, strain 48. No significant differences in the antibody-positive rates and the geometric mean antibody titers were observed between locations. Although there is no comparable recent seroepidemiological literature, Mochizuki et al. (13), by using strain 48, reported high seroprevalence (positive rates, 72% of 50 dogs in 1985 and 94% of 50 dogs in 1991) among dogs in Kagoshima (southern Japan). Schaffer et al. (18) described similarly high seropositivity (76% of 125 dogs from 1976 to 1981) for their CaCV isolate among dogs in Tennessee; however, only 2 of 25 dogs in the United Kingdom had low anti-CaCV titers.
TABLE 1.
Serological survey of anti-CaCV antibodies in dog populations
| Antibody titera | No. of serum samples collected in:
|
||
|---|---|---|---|
| Sapporo | Tokyo | Yamaguchi | |
| <4 | 36 | 44 | 28 |
| 4 | 21 | 23 | 15 |
| 8 | 7 | 11 | 13 |
| 16 | 5 | 7 | 4 |
| 32 | 1 | 8 | 3 |
| 64 | 5 | 3 | 3 |
| 128 | 1 | 2 | 2 |
| 256 | 0 | 0 | 0 |
| 512 | 1 | 0 | 0 |
| 1,024 | 0 | 0 | 0 |
| 2,048 | 0 | 1 | 0 |
| Total no. of samples | 77 | 99 | 68 |
| Mean positive antibody titer | 1:9.6 | 1:11.0 | 1:9.8 |
| Antibody-positive rate (%) | 53.3 | 55.6 | 58.8 |
Serum samples collected from dogs for other purposes were stored frozen, and antibody titers were determined by an MNT using CaCV strain 48.
Studies of CaCV infections in dogs have just begun, but the evidence obtained in the present study indicates that caliciviruses genetically as well as antigenically related to CaCV strain 48 have been circulating widely among dogs in Japan. In addition, such viruses appear to be fastidious in vitro, if not uncultivable, because they have not been isolated during the past decade despite thousands of routine virus isolation attempts using canine cells in many diagnostic laboratories, including our own. The pathogenic potential of the CaCVs remains to be elucidated.
Acknowledgments
Dog serum samples were kindly provided by Akira Hashimoto, Yumi Une, and Toshifumi Ohnishi, of Hokkaido, Azabu, and Yamaguchi Universities, respectively.
REFERENCES
- 1.Clarke, I. N., and P. R. Lambden. 1997. Viral zoonoses and food of animal origin: caliciviruses and human disease. Arch. Virol. Suppl. 13:141-152. [DOI] [PubMed] [Google Scholar]
- 2.Crandell, R. A. 1988. Isolation and characterization of caliciviruses from dogs with vesicular genital disease. Arch. Virol. 98:65-71. [DOI] [PubMed] [Google Scholar]
- 3.Dawson, S., and K. Willoughby. 1999. Feline infectious upper respiratory tract disease—an update. In Practice 21:232-237. [Google Scholar]
- 4.Evermann, J. F., G. M. Bryan, and A. J. McKeirnan. 1981. Isolation of a calicivirus from a case of canine glossitis. Canine Pract. 8:36-39. [Google Scholar]
- 5.Evermann, J. F., A. J. McKeirnan, A. W. Smith, D. E. Skilling, and R. L. Ott. 1985. Isolation and identification of caliciviruses from dogs with enteric infections. Am. J. Vet. Res. 46:218-220. [PubMed] [Google Scholar]
- 6.Green, K. Y. 1997. The role of human caliciviruses in epidemic gastroenteritis. Arch. Virol. Suppl. 13:153-165. [DOI] [PubMed] [Google Scholar]
- 7.Green, K. Y., T. Ando, M. S. Balayan, T. Berke, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Neill, M. J. Studdert, and H.-J. Thiel. 2000.. Taxonomy of the caliciviruses. J. Infect. Dis. 181(Suppl. 2):S322-S330. [DOI] [PubMed]
- 8.Guo, M., J. F. Evermann, and L. J. Saif. 2001. Detection and molecular characterization of cultivable caliciviruses from clinically normal mink and enteric caliciviruses associated with diarrhea in mink. Arch. Virol. 146:479-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang, X., P. W. Huang, W. M. Zhong, T. Farkas, D. W. Cubitt, and D. O. Matson. 1999. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J. Virol. Methods 83:145-154. [DOI] [PubMed] [Google Scholar]
- 10.Matsuura, Y., Y. Tohya, M. Mochizuki, K. Takase, and T. Sugimura. 2001. Identification of conformational neutralizing epitopes on the capsid protein of canine calicivirus. J. Gen. Virol. 82:1695-1702. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura, Y., Y. Tohya, K. Nakamura, M. Shimojima, F. Roerink, M. Mochizuki, K. Takase, H. Akashi, and T. Sugimura. 2002. Complete nucleotide sequence, genome organization and phylogenic analysis of the canine calicivirus. Virus Genes 25:67-73. [DOI] [PubMed]
- 12.Matsuura, Y., Y. Tohya, M. Onuma, F. Roerink, M. Mochizuki, and T. Sugimura. 2000. Expression and processing of the canine calicivirus capsid precursor. J. Gen. Virol. 81:195-199. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki, M., A. Kawanishi, H. Sakamoto, S. Tashiro, R. Fujimoto, and M. Ohwaki. 1993. A calicivirus isolated from a dog with fatal diarrhoea. Vet. Rec. 132:221-222. [DOI] [PubMed] [Google Scholar]
- 14.Pratelli, A., G. Greco, M. Camero, M. Corrente, G. Normanno, and C. Buonavoglia. 2000. Isolation and identification of a calicivirus from a dog with diarrhoea. Microbiologica 23:257-260. [PubMed] [Google Scholar]
- 15.Roerink, F., M. Hashimoto, Y. Tohya, and M. Mochizuki. 1999. Organization of the canine calicivirus genome from the RNA polymerase gene to the poly(A) tail. J. Gen. Virol. 80:929-935. [DOI] [PubMed] [Google Scholar]
- 16.San Gabriel, M. C., Y. Tohya, and M. Mochizuki. 1996. Isolation of a calicivirus antigenically related to feline caliciviruses from feces of a dog with diarrhea. J. Vet. Med. Sci. 58:1041-1043. [DOI] [PubMed] [Google Scholar]
- 17.San Gabriel, M. C., Y. Tohya, T. Sugimura, T. Shimizu, S. Ishiguro, and M. Mochizuki. 1997. Identification of canine calicivirus capsid protein and its immunoreactivity in Western blotting. J. Vet. Med. Sci. 59:97-101. [DOI] [PubMed] [Google Scholar]
- 18.Schaffer, F. L., M. E. Soergel, J. W. Black, D. E. Skilling, A. W. Smith, and W. D. Cubitt. 1985. Characterization of a new calicivirus isolated from feces of a dog. Arch. Virol. 84:181-195. [DOI] [PubMed] [Google Scholar]