Abstract
Introduction
Low-grade appendiceal mucinous neoplasms (LAMNs) are rare entities that can present significant challenges when discovered incidentally by general surgeons during surgery or through postoperative pathology. These lesions may mimic common abdominal conditions and are often not suspected preoperatively.
Methods
We present a case series of five patients in whom appendiceal mucoceles were incidentally identified either intraoperatively or on postoperative pathological examination. The patients ranged from 36 to 79 years old and presented with symptoms such as right lower quadrant pain, initially attributed to appendicitis, ovarian torsion, or other gynecological conditions. Intraoperative findings varied from dilated appendices with mucinous content to large cystic masses involving adjacent structures.
Results
In each case, the general surgeon had to make immediate decisions regarding management. Surgical interventions included laparoscopic appendectomy and open right hemicolectomy, with an emphasis on careful handling to prevent rupture and spillage of mucin. Postoperative pathology confirmed LAMNs, with tumor stages ranging from pTis to pT4a. Some patients required additional procedures, such as cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), due to the presence of acellular mucin or peritoneal involvement.
The discussion focuses on practical guidance for general surgeons when faced with an incidental appendiceal mucocele. Key recommendations include avoiding intraoperative rupture by gentle handling, assessing the need for extended resection based on intraoperative findings, and ensuring thorough communication with pathology for accurate staging. Postoperative management should involve reviewing pathology reports carefully, considering referral to a multidisciplinary team for higher-stage tumors, and implementing long-term surveillance protocols due to the risk of recurrence.
Conclusion
General surgeons play a critical role in the initial management of incidentally discovered appendiceal mucoceles. Prompt recognition and appropriate intraoperative decision-making are essential to optimize patient outcomes. By adhering to careful surgical techniques and collaborating with multidisciplinary teams, surgeons can effectively manage these unexpected findings and mitigate potential complications associated with LAMNs.
Keywords: Appendiceal neoplasm, Mucocele, Pseudomyxoma peritonei, Appendectomy, Hemicolectomy, Colectomy
Highlights
-
•
Maintain a high index of suspicion for appendiceal mucocele in patients presenting with right lower quadrant pain.
-
•
Gentle intraoperative handling of the appendix is crucial to prevent rupture and mucin spillage.
-
•
Assessing the extent of the neoplasm during surgery is essential; extended resections may be necessary for clear margins.
-
•
Collaboration with a multidisciplinary team ensures accurate staging and management based on pathological findings.
-
•
Establishing comprehensive long-term surveillance protocols are vital for early detection of recurrence.
1. Introduction
Low-grade appendiceal mucinous neoplasm (LAMN) is a rare but clinically significant entity that arises from the epithelial lining of the appendix [1]. The incidence of appendiceal tumors in appendectomy specimens is between 0.9 and 1.7 % with approximately one half of them being LAMNs [6]. These tumors are characterized by the production of mucin, leading to the formation of a mucocele, which can present a wide range of clinical manifestations, from asymptomatic incidental findings to acute abdominal pain mimicking appendicitis [2]. LAMNs are typically slow-growing and have a relatively favorable prognosis, but they have the potential for serious complications such as pseudomyxoma peritonei when mucin spreads into the peritoneal cavity [3].
The pathophysiology of LAMNs remains an area of active investigation, but their clinical course is closely tied to the presence of acellular mucin, and whether there is spillage of mucin into the abdominal cavity [4]. Most LAMNs are detected incidentally on imaging studies performed for other conditions, or during evaluation for right lower quadrant (RLQ) pain [5]. However, due to their insidious nature, LAMNs can be mistaken for more common abdominal conditions such as appendicitis or gynecological pathology such as pelvic inflammatory disease, complex cysts, ovarian torsion or even ectopic pregnancy [6].
Surgical management remains the cornerstone of treatment for LAMNs, with the goal of complete resection while minimizing the risk of mucin spillage [7]. Laparoscopic appendectomy is the preferred approach for smaller lesions, while right hemicolectomy may be required for larger or more invasive tumors [8]. Pathological evaluation of the surgical specimen is critical for determining the tumor stage, which informs the need for long-term surveillance [9]. In particular, cases with extension of mucin to the serosal surface or beyond (pT3, pT4) carry a higher risk of recurrence and require vigilant follow-up [10].
This case series specifically aims to provide general surgeons with practical guidance on the management of incidentally discovered appendiceal mucoceles, whether identified intraoperatively or through postoperative pathology. By detailing five diverse patient cases, we illustrate the critical steps surgeons should take upon such unexpected findings, including the importance of gentle handling to avoid rupture and mucin spillage, criteria for determining the need for extended resection, and the value of multidisciplinary collaboration for optimal patient outcomes. Additionally, we emphasize the necessity of thorough pathological assessment and the establishment of long-term surveillance protocols to monitor for potential recurrence. Through these documented experiences, this series seeks to present the pathology and enhance the surgical management of this rare but significant condition.
2. Methods
We present a case series of five patients in whom appendiceal mucoceles were incidentally identified either intraoperatively or on postoperative pathological examination at a community hospital performed by general surgeons with a combined experience of 25 years. The patients ranged from 36 to 79 years old and presented with symptoms such as right lower quadrant pain, initially attributed to appendicitis, ovarian torsion, or other gynecological conditions. Intraoperative findings varied from dilated appendices with mucinous content to large cystic masses involving adjacent structures (see Table 1).
Table 1.
Summary of key information for patients in case series.
| Age | Gender | Imaging studies | Final pathology | Outcome |
|---|---|---|---|---|
| 60 | Female | Initial and follow-up CT abdomen/pelvis, CT chest, CTA pulmonary, PET CT skull to thigh | Moderately differentiated adenocarcinoma of the appendix with no lymphovascular invasion, no perforation | No reoccurrence on surveillance |
| 76 | Male | Initial and follow-up CT abdomen/pelvis | Low-grade appendiceal mucinous neoplasm with extensive mucin, dissection of the appendiceal wall, and perforation | Hyperthermic intraperitoneal chemotherapy, no reoccurrence on surveillance |
| 79 | Male | Initial and follow-up CT abdomen/pelvis, CT chest, CTA pulmonary | 13.2 cm low-grade appendiceal mucinous neoplasm extending to subserosa, negative margins, no nodal involvement | No reoccurrence on surveillance |
| 36 | Female | Initial and follow-up CT abdomen/pelvis | Low-grade appendiceal mucinous neoplasm, with possible acellular mucin deposits in subserosal adipose tissue | No reoccurrence on surveillance |
| 55 | Female | CT abdomen/pelvis | Low-grade appendiceal mucinous neoplasm with mucin involving the muscularis propria | No reoccurrence on surveillance |
3. Case series presentation
3.1. Case 1
A 60-year-old female was referred for evaluation of a symptomatic right lower quadrant (RLQ) mass. She had a history of mild, sharp, non-migratory RLQ pain but denied fever, nausea, vomiting, or bowel habit changes. A computed tomography (CT) scan showed a 3.9 cm tubular structure, suggestive of an appendiceal neoplasm (Fig. 1). Past medical history included hypertension and depression. Surgical history included a laparoscopic cholecystectomy without complications. Screening colonoscopy one year prior was significant for mucoid hemorrhagic discharge from the appendiceal orifice. A right hemicolectomy was performed, revealing a moderately differentiated adenocarcinoma of the appendix (1.5 cm), with no lymphovascular invasion, perforation, or metastasis. The patient recovered well postoperatively, and surveillance imaging revealed no recurrence.
Fig. 1.
CT abdomen and pelvis with IV contrast (axial view panel A, coronal view panel B) showing a cystic structure suggestive of an appendiceal neoplasm (red boxes).
3.2. Case 2
A 76-year-old male presented with epigastric and right upper quadrant (RUQ) pain, initially diagnosed as acute cholecystitis. Imaging revealed a cystic mass near the appendix (Fig. 2). The patient underwent a laparoscopic cholecystectomy and appendectomy. Pathological evaluation revealed a low-grade appendiceal mucinous neoplasm (LAMN) with extensive mucin, dissection of the appendiceal wall, and perforation. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) was performed to address peritoneal involvement. Postoperative follow-up showed no evidence of disease recurrence, and the patient remained stable with no further intervention.
Fig. 2.
CT abdomen and pelvis with IV contrast (axial view) demonstrating a cystic mass near the appendix concerning for an appendiceal neoplasm (red box).
3.3. Case 3
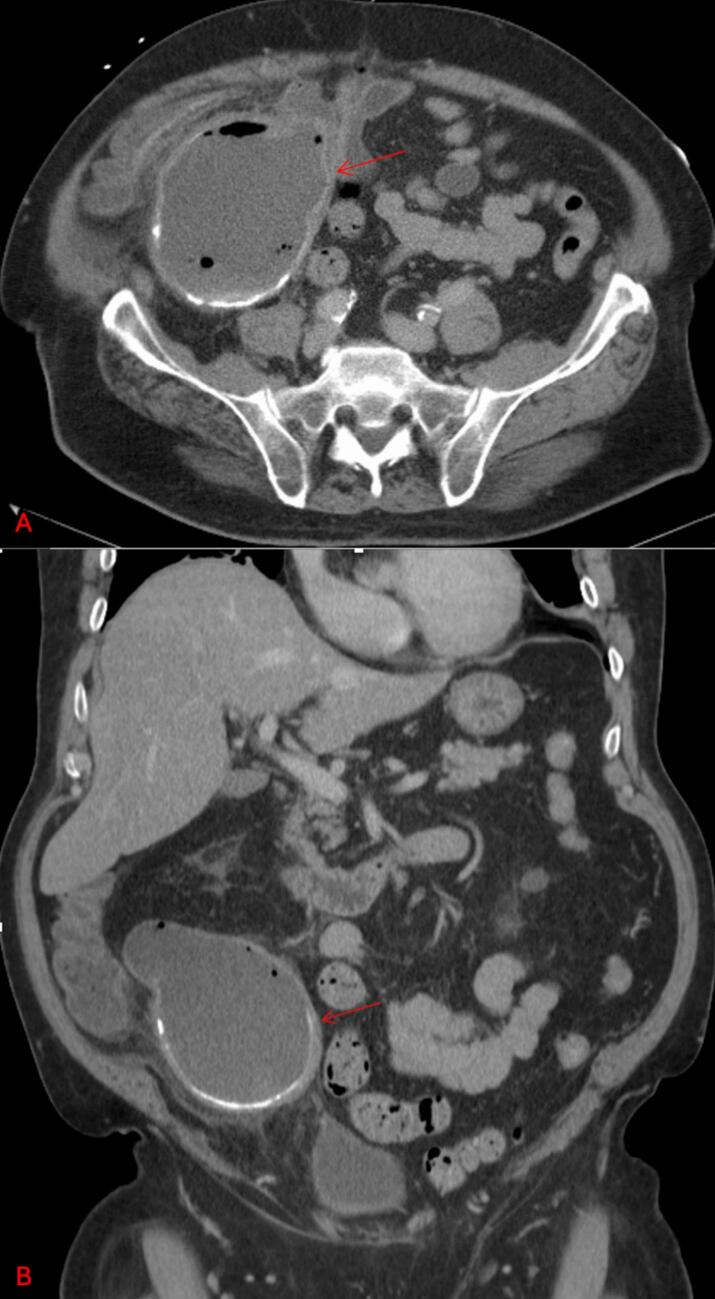
A 79-year-old male presented with RLQ pain, weight loss, and anorexia. History includes normal colonoscopy two years prior. Imaging demonstrated a large cystic mass in the right lower abdomen, likely arising from the appendix (Fig. 3). A right hemicolectomy was performed, revealing a 13.2 cm low-grade appendiceal mucinous neoplasm. The tumor extended into the subserosa, but all margins were negative, and no nodal involvement was identified. The patient had an uneventful recovery and remained under close surveillance, with no signs of recurrence.
Fig. 3.
CT abdomen and pelvis with IV contrast (axial view panel A, coronal view panel B) demonstrating a large cystic mass in the right lower quadrant likely arising from the appendix (red arrows).
3.4. Case 4
A 36-year-old female presented with RLQ and flank pain, initially thought to be related to a gynecological issue. Imaging showed a large cystic lesion in the right adnexa (Fig. 4). Transvaginal ultrasound ruled out ovarian torsion, and the patient was taken to surgery. Intraoperative findings revealed a dilated appendix, and a laparoscopic appendectomy was performed. Pathology confirmed a low-grade appendiceal mucinous neoplasm, with possible acellular mucin deposits in subserosal adipose tissue. She was placed on a long-term surveillance plan, with no signs of recurrence after follow-up imaging and tumor markers.
Fig. 4.
CT abdomen and pelvis with IV contrast (axial view) demonstrating a large cystic lesion near the right adnexa (red arrow).
3.5. Case 5
A 55-year-old female presented with a 2-day history of severe RLQ pain, nausea, and decreased appetite. Imaging revealed a massively dilated appendix with surrounding inflammation and free fluid (Fig. 5, Fig. 6). She underwent a laparoscopic appendectomy, and intraoperative findings showed gangrenous appendicitis due to volvulus of an appendiceal mucocele. Pathology confirmed a low-grade appendiceal mucinous neoplasm with mucin involving the muscularis propria. The patient recovered well postoperatively, with no complications, and continued surveillance showed no recurrence.
Fig. 5.
CT abdomen and pelvis with IV contrast (axial view) revealing a massively dilated appendix with surrounding inflammation and free fluid (red box).
Fig. 6.
Picture of postoperative specimen demonstrating massively dilated appendix and associated appendiceal mucocele.
4. Discussion
Low-grade appendiceal mucinous neoplasms (LAMNs) are rare epithelial tumors characterized by the production of mucin within the appendix, leading to distention and potential rupture [11]. Clinically, LAMNs often present with nonspecific symptoms that mimic other abdominal or gynecological conditions. In this series, patients ranged from 36 to 79 years old and presented with symptoms such as right lower quadrant (RLQ) pain, initially attributed to appendicitis, ovarian torsion, or adnexal masses. One patient was a young female whose symptoms led to an initial misdiagnosis of a gynecological mass, emphasizing that LAMNs can affect a wide demographic and present atypically. Imaging studies, including computed tomography (CT) scans and ultrasounds, played a crucial role in identifying appendiceal abnormalities. However, imaging alone was sometimes insufficient for definitive diagnosis, necessitating surgical exploration.
Surgical intervention remains the cornerstone of LAMN management. The primary goal is complete resection of the tumor while preventing intraoperative rupture and spillage of mucinous contents, which can lead to pseudomyxoma peritonei—a condition characterized by the accumulation of mucinous material in the peritoneal cavity [12]. In this series, most patients underwent laparoscopic appendectomy, which is appropriate for localized disease confined to the appendix. Two patients required open right hemicolectomy due to tumor size, involvement of adjacent structures, or suspicion of malignancy. The decision between appendectomy and more extensive resection depends on factors such as tumor size, location, involvement of the appendiceal base, and intraoperative findings.
In terms of resectability criteria for appendiceal mucoceles, the decision to perform an appendectomy versus a more extensive procedure, such as right hemicolectomy, is guided by several key factors. Studies have shown that tumors confined to the distal appendix without cecal involvement can be managed with a simple appendectomy, while those extending to the appendiceal base or demonstrating high-grade features require a right hemicolectomy to achieve clear margins and reduce recurrence [13]. Additional evidence indicates that involvement of the mesoappendix or adjacent structures increases the risk of mucin spillage and subsequent pseudomyxoma peritonei, warranting more aggressive surgical intervention [13,14]. These criteria, along with tumor size and the possibility of lymphatic spread, although rare in low-grade lesions, are central to intraoperative decision-making [15]. By carefully assessing these factors, general surgeons can determine the most appropriate extent of resection and thereby optimize patient outcomes.
Pathological examination is essential for accurate diagnosis and staging. LAMNs are characterized by villous or flat adenomatous epithelium with low-grade cytologic atypia and mucin production (Fig. 7, Fig. 8). The presence of acellular mucin beyond the muscularis propria or invasion into the subserosa or serosa upstages the tumor and has prognostic implications [16]. In this series, several patients had tumors staged as pTis (tumor in situ) or pT3, indicating confinement to the muscularis propria or extension into the subserosa, respectively. Two patients had tumors staged as pT4a due to acellular mucin invasion of the serosal surface, which is associated with a higher risk of recurrence and requires vigilant postoperative surveillance [17].
Fig. 7.
H&E stained histopathology at 10× magnification shows epithelium with apical mucin, elongated nuclei and low-grade nuclear atypia. The muscularis mucosae is partially effaced.
Fig. 8.
H&E stained histopathology at 10× magnification shows acellular mucin in the subserosa.
The management of LAMNs with peritoneal dissemination poses significant challenges. One patient in the series underwent cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) after acellular mucin deposits were found in the peritoneum. HIPEC involves the circulation of heated chemotherapy agents within the peritoneal cavity to eradicate microscopic residual disease and has shown promise in reducing recurrence rates and improving survival in selected patients [18]. However, its use is typically reserved for patients with evidence of peritoneal spread and should be considered on a case-by-case basis.
Long-term follow-up is crucial due to the potential for late recurrence, even in cases where the initial resection margins are clear. Surveillance strategies often include periodic imaging studies, such as CT scans, and monitoring of tumor markers like carcinoembryonic antigen (CEA), CA 19-9, and CA 125 [19]. The frequency and duration of follow-up depend on the tumor stage and individual patient risk factors [20]. In this series, patients were followed for varying lengths of time, with some under surveillance for up to ten years without evidence of recurrence.
From these cases, several teaching points emerge. First, clinicians should maintain a broad differential diagnosis when evaluating patients with abdominal pain, especially when imaging reveals unusual findings such as cystic masses in the RLQ. Early involvement of a multidisciplinary team, including general surgeons, gynecologists, radiologists, and pathologists, can facilitate accurate diagnosis and appropriate management. Second, careful surgical technique is paramount. The avoidance of intraoperative rupture of the appendix or mucocele is essential to prevent peritoneal seeding and the development of pseudomyxoma peritonei, which significantly worsens the prognosis [21]. Third, the pathological assessment should include a thorough evaluation of the tumor's histologic features and extent of spread, as this information guides staging and follow-up recommendations [4].
The literature corroborates the findings from this case series. LAMNs are rare, accounting for less than 1 % of all gastrointestinal tumors, but their incidence may be underestimated due to misdiagnosis or underreporting [6]. Studies have shown that LAMNs often present in the sixth decade of life, with no significant gender predilection, although some reports suggest a slight female predominance [22]. The nonspecific nature of symptoms contributes to diagnostic delays, reinforcing the need for awareness among clinicians.
Surgical resection remains the definitive treatment for LAMNs. Appendectomy is considered adequate for tumors confined to the appendix without perforation or involvement of the base [23]. Right hemicolectomy is generally reserved for cases with positive margins, involvement of the cecum, or high-grade histological features suggestive of invasive carcinoma. The role of lymphadenectomy is limited, as lymph node metastasis is rare in LAMNs, but lymph node evaluation can be considered in cases with suspicious features [24].
The prognosis for patients with LAMNs is generally favorable when the disease is confined to the appendix and completely resected [25]. However, the risk of recurrence and progression to pseudomyxoma peritonei increases with higher tumor stages and the presence of acellular mucin outside the appendix [12]. Studies indicate that patients with acellular mucin confined to the appendiceal wall (pTis) have a 5-year survival rate of nearly 100 %, while those with serosal involvement (pT4a) require close monitoring due to a higher risk of peritoneal dissemination [26]. For stage IV disease, the five-year overall survival rates are 56.7 % for well differentiated, 31.5 % for moderately differentiated, and 11.3 % for poorly differentiated [27].
In recent years, advancements in imaging techniques and surgical methods have improved the diagnosis and management of LAMNs. Minimally invasive surgical approaches, such as laparoscopy, offer the benefits of reduced postoperative pain, shorter hospital stays, and quicker recovery, provided that the principles of oncologic surgery are upheld [24]. Moreover, the development of consensus guidelines for the pathological classification and staging of appendiceal tumors has enhanced the standardization of care and facilitated more accurate prognostication [4].
General surgeons should consistently include appendiceal mucocele in the differential diagnosis when evaluating patients with appendicitis or right lower quadrant pain, as recognizing this entity can significantly alter management and outcomes—what the brain doesn't anticipate, the eye may miss. When imaging reveals unusual findings such as cystic masses in the RLQ, maintaining a broad differential is essential. Early collaboration with a multidisciplinary team, including gynecologists, radiologists, and pathologists, facilitates accurate diagnosis and appropriate management. Meticulous surgical technique is crucial; avoiding intraoperative rupture of the appendix or mucocele prevents peritoneal seeding and the development of pseudomyxoma peritonei, thereby preserving the patient's prognosis. Additionally, comprehensive pathological assessment should thoroughly evaluate the tumor's histologic features and extent of spread, guiding accurate staging and informed follow-up recommendations. By keeping appendiceal mucocele in mind and adhering to these best practices, general surgeons can enhance diagnostic accuracy, optimize surgical outcomes, and improve long-term prognosis for their patients.
5. Conclusion
General surgeons who incidentally discover an appendiceal mucocele during surgery or through postoperative pathology should handle the appendix carefully to prevent rupture and mucin spillage, thereby avoiding pseudomyxoma peritonei. They must assess the extent of the neoplasm intraoperatively and consider extended resections, such as a right hemicolectomy, to achieve clear margins and reduce the risk of recurrence.
Author contribution
Nathaniel Grabill, MD: Conceptualization, Investigation, Writing – Review & Editing, Visualization, Project Administration. Mena Louis, DO: Conceptualization, Formal Analysis, Writing – Original Draft. Jonathon Ray, MD: Investigation, Writing – Review & Editing, Visualization. Ana Tucker, MS: Writing – Review & Editing, Visualization, Investigation. Sumi So, MD: Writing – Review & Editing, Visualization, Investigation. Travelyan Walker, MD: Writing – Review & Editing, Visualization, Investigation. Daniel Eduardo Sarmiento Garzon, MD: Writing – Review & Editing, Visualization, Investigation. James Chambers, MD: Writing – Review & Editing, Visualization, Investigation.
Registration of research studies
NA.
Consent
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
This manuscript has been reported in line with the PROCESS criteria [28].
Ethical approval
This case series has IRB approval at Northeast Georgia Health System.
Written informed consent was obtained from the patients for publication and any accompanying images. A copy of the written consents are available for review by the Editor-in-Chief of this journal on request.
Guarantor
Nathaniel Grabill.
Funding
There are no sources of funding for this work.
Declaration of competing interest
All authors declare that there are no financial or personal relationships with other people or organizations that could inappropriately influence (bias) this work.
Acknowledgements
Alec Seaton, MD, Molly McName, MD, and Mary Hunter Benton, MD for providing patients to add to this series. Sumi So, MD for providing pathology images.
Contributor Information
Nathaniel Grabill, Email: Nathaniel.grabill@nghs.com.
Jonathan W. Ray, Email: jonathan.ray@nghs.com.
Ana Tucker, Email: ana.tucker@nghs.com.
Travelyan Walker, Email: travelyan.walker@nghs.com.
James Chambers, Email: james.chambers@nghs.com.
References
- 1.Wang A.S., Ismael H.N., Parikh J., Modesto V.L. Low-grade appendiceal mucinous neoplasm: a case series. Cureus. Sep 2022;14(9) doi: 10.7759/cureus.28755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fournier K., Rafeeq S., Taggart M., et al. Low-grade appendiceal mucinous neoplasm of uncertain malignant potential (LAMN-UMP): prognostic factors and implications for treatment and follow-up. Ann. Surg. Oncol. Jan 2017;24(1):187–193. doi: 10.1245/s10434-016-5588-2. [DOI] [PubMed] [Google Scholar]
- 3.Saim H.A., Chik I., Jaafar F.F., Zuhdi Z., Jarmin R., Azman A. A rare presentation of low-grade appendiceal mucinous neoplasm within an amyand’s hernia: a case report. Ann. Coloproctol. Apr 2023;39(2):183–187. doi: 10.3393/ac.2021.00430.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misdraji J., Yantiss R.K., Graeme-Cook F.M., Balis U.J., Young R.H. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am. J. Surg. Pathol. Aug 2003;27(8):1089–1103. doi: 10.1097/00000478-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Konnai K., Fujiwara H., Kitagawa M., Wakabayashi R., Onose R., Kato H. Low-grade appendiceal mucinous neoplasm encountered during risk-reducing salpingo-oophorectomy: a case of laparoscopic surgery. J. Obstet. Gynaecol. Res. Dec 2023;49(12):2975–2978. doi: 10.1111/jog.15802. [DOI] [PubMed] [Google Scholar]
- 6.Köhler F., Reese L., Hendricks A., et al. Low-grade mucinous neoplasms (LAMN) of the appendix in Germany between 2011 and 2018: a nationwide analysis based on data provided by the German Center for Cancer Registry Data (ZfKD) at the Robert Koch Institute (RKI) Langenbeck’s Arch. Surg. Dec 2022;407(8):3615–3622. doi: 10.1007/s00423-022-02639-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guner M., Aydın C. Low-grade appendiceal mucinous neoplasm: what is the best treatment? Cureus. Oct 2023;15(10) doi: 10.7759/cureus.46591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guaglio M., Sinukumar S., Kusamura S., et al. Clinical surveillance after macroscopically complete surgery for low-grade appendiceal mucinous neoplasms (LAMN) with or without limited peritoneal spread: long-term results in a prospective series. Ann. Surg. Oncol. Apr 2018;25(4):878–884. doi: 10.1245/s10434-018-6341-9. [DOI] [PubMed] [Google Scholar]
- 9.Makino A., Okumura T., Yamashita K., Isogaki J., Kawabe A. A case of low-grade appendiceal mucinous neoplasm that led to surgery after 12 years of no treatment. Cureus. Aug 2023;15(8) doi: 10.7759/cureus.43024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald J.R., O’Dwyer S.T., Rout S., et al. Classification of and cytoreductive surgery for low-grade appendiceal mucinous neoplasms. Br. J. Surg. Jul 2012;99(7):987–992. doi: 10.1002/bjs.8739. [DOI] [PubMed] [Google Scholar]
- 11.Wong M., Barrows B., Gangi A., Kim S., Mertens R.B., Dhall D. Low-grade appendiceal mucinous neoplasms: a single institution experience of 64 cases with clinical follow-up and correlation with the current (eighth edition) AJCC staging. Int. J. Surg. Pathol. May 2020;28(3):252–258. doi: 10.1177/1066896919883679. [DOI] [PubMed] [Google Scholar]
- 12.Lakmal C., Chakrabarty B., Tan C., et al. The risk of developing pseudomyxoma peritonei from a non-perforated low grade appendiceal mucinous neoplasm found at appendicectomy. Eur. J. Surg. Oncol. Oct 2024;50(10) doi: 10.1016/j.ejso.2024.108600. [DOI] [PubMed] [Google Scholar]
- 13.Dhage-Ivatury Shubhada M.D*., Sugarbaker Paul H.M.D., .F.A.C.S,.F.R.C.S†,*. Update on the surgical approach to mucocele of the appendix. J. Am. Coll. Surg. April 2006;202(4):680–684. doi: 10.1016/j.jamcollsurg.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Sugarbaker P.H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7(1):69–76.781. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 15.Carr N.J., Cecil T.D., Mohamed F., Sobin L.H., Sugarbaker P.H., González-Moreno S., Taflampas P., Chapman S., Moran B.J. Peritoneal surface oncology group international. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi process. Am. J. Surg. Pathol. 2016 Jan;40(1):14–26. doi: 10.1097/PAS.0000000000000535. (PMID: 26492181) [DOI] [PubMed] [Google Scholar]
- 16.Yanai Y., Saito T., Hayashi T., et al. Molecular and clinicopathological features of appendiceal mucinous neoplasms. Virchows Arch. Mar 2021;478(3):413–426. doi: 10.1007/s00428-020-02906-5. [DOI] [PubMed] [Google Scholar]
- 17.Ahuja M., Mandal S., Mallya V., Singh M., Khurana N., Lal P. Low-grade appendiceal mucinous neoplasms: histomorphological spectrum in a tertiary care hospital. J. Cancer Res. Ther. Apr 1 2024;20(3):840–843. doi: 10.4103/jcrt.jcrt_149_22. [DOI] [PubMed] [Google Scholar]
- 18.Cusumano C., Carrere S., Bouillin A., et al. Laparoscopic cytoreductive surgery and HIPEC in LAMN with small volume of peritoneal disease: a valuable option of treatment for good patient-related experience measures (PREMs) Surg. Endosc. Jul 2022;36(7):4757–4763. doi: 10.1007/s00464-021-08816-0. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A.R., Brajcich B.C., Merkow R.P. Postoperative LAMN surveillance recommendations. J. Surg. Oncol. Mar 2022;125(3):546–547. doi: 10.1002/jso.26724. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A.R., Brajcich B.C., Yang A.D., Bentrem D.J., Merkow R.P. Necessity of posttreatment surveillance for low-grade appendiceal mucinous neoplasms. J. Surg. Oncol. Dec 2021;124(7):1115–1120. doi: 10.1002/jso.26621. [DOI] [PubMed] [Google Scholar]
- 21.Bowles M., Ng J.Y., Nabi H. Delivery of an incidental appendiceal mucinous neoplasm. Cureus. Jun 2022;14(6) doi: 10.7759/cureus.26214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gok M., Topal U., Akyüz M., Öz A.B., Sozuer E., Deniz K. Long-term results of low grade appendiceal mucinous neoplasm (LAMN): a retrospective analysis of 24 patients. Arch. Iran. Med. Aug 1 2021;24(8):615–621. doi: 10.34172/aim.2021.87. [DOI] [PubMed] [Google Scholar]
- 23.Istl A.C., Gage M.M., Esquivel J., Ahuja N., Greer J.B., Johnston F.M. Management of low-grade appendiceal mucinous neoplasms (LAMN): an international survey of surgeons performing CRS and HIPEC. Ann. Surg. Oncol. Jul 2021;28(7):3831–3837. doi: 10.1245/s10434-020-09312-w. [DOI] [PubMed] [Google Scholar]
- 24.Takeyama H., Murata K., Takeda T., et al. Clinical significance of lymph node dissection and lymph node metastasis in primary appendiceal tumor patients after curative resection: a retrospective multicenter cohort study. J. Gastrointest. Surg. Jan 2022;26(1):128–140. doi: 10.1007/s11605-021-05070-6. [DOI] [PubMed] [Google Scholar]
- 25.Li X., Zhou J., Dong M., Yang L. Management and prognosis of low-grade appendiceal mucinous neoplasms: a clinicopathologic analysis of 50 cases. Eur. J. Surg. Oncol. Oct 2018;44(10):1640–1645. doi: 10.1016/j.ejso.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Ballentine S.J., Carr J., Bekhor E.Y., Sarpel U., Polydorides A.D. Correction: updated staging and patient outcomes in low-grade appendiceal mucinous neoplasms. Mod. Pathol. Jul 2021;34(7):1440. doi: 10.1038/s41379-021-00811-z. [DOI] [PubMed] [Google Scholar]
- 27.Overman M.J., Fournier K., Hu C.Y., et al. Improving the AJCC/TNM staging for adenocarcinomas of the appendix: the prognostic impact of histological grade. Ann. Surg. Jun 2013;257(6):1072–1078. doi: 10.1097/SLA.0b013e318269d680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathew G., Agha R.A., Sohrabi C., Franchi T., Nicola M., Kerwan A., Agha R for the PROCESS Group Preferred reporting of case series in surgery (PROCESS) 2023 guidelines. Int. J. Surg. 2023 doi: 10.1097/JS9.0000000000000940. (article in press) [DOI] [PMC free article] [PubMed] [Google Scholar]