Abstract
The nucleotide sequences of the partial rpoB gene were determined from 38 Legionella species, including 15 serogroups of Legionella pneumophila. These sequences were then used to infer the phylogenetic relationships among the Legionella species in order to establish a molecular differentiation method appropriate for them. The sequences (300 bp) and the phylogenetic tree of rpoB were compared to those from analyses using 16S rRNA gene and mip sequences. The trees inferred from these three gene sequences revealed significant differences. This sequence incongruence between the rpoB tree and the other trees might have originated from the high frequency of synonymous base substitutions and/or from horizontal gene transfer among the Legionella species. The nucleotide variation of rpoB enabled more evident differentiation among the Legionella species than was achievable by the 16S rRNA gene and even by mip in some cases. Two subspecies of L. pneumophila (L. pneumophila subsp. pneumophila and subsp. fraseri) were clearly distinguished by rpoB but not by 16S rRNA gene and mip analysis. One hundred and five strains isolated from patient tissues and environments in Korea and Japan could be identified by comparison of rpoB sequence similarity and phylogenetic trees. These results suggest that the partial sequences of rpoB determined in this study might be applicable to the molecular differentiation of Legionella species.
Legionella pneumophila, the causative agent of Legionnaires' disease and the type species of Legionella, was first recognized in 1977 following an epidemic of acute pneumonia in Philadelphia, Pennsylvania. Legionellosis is usually acquired in the community and accounts for 2 to 15% of all community-acquired pneumonia that requires hospitalization in the United States (27). So far, of the more than 40 Legionella species that have been characterized and classified by various methods (8, 30), 21 have been reported to be pathogenic to humans (20, 30) A classification scheme constructed by various methods can be efficiently used for the rapid and precise identification of clinical isolates in the diagnostic laboratory. One of these methods is the determination of molecular phylogenetic relationships or typing (18). Legionella species have also been previously analyzed using 16S rRNA gene (1, 6, 8) and macrophage infectivity potentiator (mip) sequences (19, 20). The 16S rRNA gene is by far the most widely used molecular marker to determine the phylogenetic relationships of bacteria (31). However, its utility has been questioned because of its heterogeneity (4). The mip gene encodes Mip, which has been reported to play a significant role in virulence (2). It has been shown that mip is useful for the discrimination of Legionella species (19, 20). However, incongruences have been found between the phylogenies elucidated from the 16S rRNA gene and mip sequences (8, 20). Relatively low branch-supporting bootstrap values have restricted the comprehensive elucidation of the phylogenetic relationships within the Legionella species. The use of only a single gene for phylogenetic study makes it difficult to distinguish the phylogenetic relationships among species from the viewpoint of gene history (18). Therefore, the importance of comparing the sequences of several genes to evaluate a comprehensive bacterial phylogeny has been stressed (18). Consequently, in the present study we investigated the value of another gene, rpoB, to supplement the classification and identification scheme for Legionella species.
The rpoB gene encodes the β subunit of DNA-dependent RNA polymerase (24), and rifampin resistance is related to mutations in a particular region of rpoB (10). Recently, rpoB sequences were used as an alternative tool either for determining the phylogeny of or for identifying enteric bacteria (16), Mycobacterium (10), spirochetes including Borrelia (14, 21), and Bartonella (22). In this study, partial rpoB sequences (300 bp), containing a region that though highly conserved still has a remarkable ability for species differentiation (10, 12, 14, 22), were determined for genotypic classification of Legionella species and the results were compared with those results from the 16S rRNA gene and mip. In addition, culture isolates of Legionella species were identified based on rpoB sequences.
Fifty-five reference strains of 38 Legionella species were used in this study (Table 1). Among the 15 serogroups of L. pneumophila, three strains of serogroups, i.e., 4, 5, and 15, were L. pneumophila subsp. fraseri, while the others were L. pneumophila subsp. pneumophila. One hundred and five culture isolates, of which 10 strains were isolated from pneumonia patients and the others from cooling water, were identified by rpoB sequence analysis. These isolates were grown on buffered charcoal yeast extract agar and identified on the basis of cysteine requirement, autofluorescence, and biochemical tests such as those for gelatinase, urease, catalase, oxidase, peroxidase, and β-lactamase activities; hippurate hydrolysis; nitrate reduction; and browning of tyrosine-supplemented agar (8). They were provided by Mi-Yeoun Park at the Korean National Institute of Health and H. Miyamoto at the University of Occupational and Environmental Health for blinded identification by rpoB sequence analysis.
TABLE 1.
Legionella strains used to determine the rpoB sequences in this study
| Species (serogroup) | Strain no.b | Accession no.
|
||
|---|---|---|---|---|
| rpoB | 16S rRNAc | mipd | ||
| L. pneumophila (1)a | ATCC 33152 | AF367748 | M36023 | S42595 |
| L. pneumophila (1)a | ATCC 33153 | AY036036 | ||
| L. pneumophila (1)a | SF9 | AY036037 | ||
| L. pneumophila (1)a | ATCC 43109 | AY036038 | ||
| L. pneumophila (2)a | ATCC 33154 | AY036039 | ||
| L. pneumophila (3)a | ATCC 33155 | AY036040 | ||
| L. pneumophila (4)a | ATCC 33156 | AY036041 | ||
| L. pneumophila (5)a | ATCC 33216 | AY036042 | ||
| L. pneumophila (6)a | ATCC 33215 | AY036043 | ||
| L. pneumophila (7)a | ATCC 33823 | AY036044 | ||
| L. pneumophila (8)a | ATCC 35096 | AY036045 | ||
| L. pneumophila (9)a | ATCC 35289 | AY036046 | ||
| L. pneumophila (10)a | ATCC 43283 | AY036047 | ||
| L. pneumophila (11)a | ATCC 43130 | AY036048 | ||
| L. pneumophila (12)a | ATCC 43290 | AY036049 | ||
| L. pneumophila (13)a | ATCC 43736 | AY036050 | ||
| L. pneumophila (14)a | ATCC 43709 | AY036051 | ||
| L. pneumophila (15)a | ATCC 35351 | AY036052 | ||
| L. adelaidensis | UOEH 13562 | AF367721 | Z49716 | U91606 |
| L. anisaa | ATCC 35292 | AF367722 | Z32635 | AF022312 |
| L. birminghamensisa | UOEH 11749 | AF367723 | Z49717 | AF047743 |
| L. bozemanii (1)a | ATCC 33217 | AF367724 | Z49719 | U91609 |
| L. brunensis | UOEH 12655 | AF367725 | Z32636 | AF022311 |
| L. cherriia | UOEH 10742 | AF367726 | Z49720 | U91635 |
| L. cincinnatiensisa | UOEH 12201 | AF367727 | Z49721 | AF022358 |
| L. dumoffiia | ATCC 33279 | AF367728 | Z32637 | AF022313 |
| L. erythra (1) | ATCC 35303 | AF367729 | M36027 | U92203 |
| L. fairfieldensis | UOEH 13563 | AF367730 | Z49722 | U92214 |
| L. feeleii (1)a | ATCC 35072 | AF367731 | Z49740 | U92205 |
| L. geestiana | ATCC 49504 | AF367732 | Z49723 | NDe |
| L. gormaniia | ATCC 33297 | AF367733 | Z42639 | AF047747 |
| L. gratiana | ATCC 49413 | AF367734 | Z49725 | U92206 |
| L. hackeliae (1)a | ATCC 35250 | AF367735 | M36028 | U92207 |
| L. israelensisa | ATCC 43119 | AF367736 | Z32640 | U92208 |
| L. jamestowniensis | ATCC 35298 | AF367737 | Z49726 | AF022339 |
| L. jordanisa | HM 7000 | AF367738 | Z32667 | U92209 |
| L. lansingensisa | ATCC 49751 | AF367739 | Z49727 | U92210 |
| L. londiniensis | ATCC 49505 | AF367740 | Z49728 | AF022346 |
| L. longbeachae (1)a | ATCC 33462 | AF367741 | M36029 | X83036 |
| L. maceacherniia | ATCC 35300 | AF367742 | Z32641 | AF022315 |
| L. micdadeia | ATCC 33218 | AF367743 | M36032 | AF023175 |
| L. moravica | ATCC 43877 | AF367744 | Z49729 | U92212 |
| L. nautarum | ATCC 49506 | AF367745 | Z49730 | U92213 |
| L. oakridgensisa | HM 7002 | AF367746 | Z32643 | U92214 |
| L. parisiensisa | UOEH 11745 | AF347747 | Z49731 | U92215 |
| L. quinlivanii (1) | ATCC 43830 | AF367749 | Z49733 | AF022347 |
| L. rubrilucens | ATCC 35304 | AF367750 | Z32643 | AF022357 |
| L. sainthelensi (1)a | ATCC 35248 | AF367751 | Z49734 | U92219 |
| L. santicrucis | UOEH 11746 | AF367752 | Z49735 | U92220 |
| L. shakespearei | ATCC 49655 | AF367753 | Z49736 | U92221 |
| L. spiritensis (1) | UOEH 11199 | AF367754 | M36030 | AF047751 |
| L. steigerwaltii | UOEH 11747 | AF367755 | Z49737 | U92223 |
| L. tucsonensisa | ATCC 49180 | AF367756 | Z32644 | U92224 |
| L. wadsworthiia | ATCC 33877 | AF367757 | Z49738 | U92225 |
| L. worsleiensis | ATCC 49508 | AF367758 | Z49739 | U9222 |
DNAs were extracted using the bead beater-phenol extraction method (10) and were used as a template for PCR. A primer pair, RL1 (5′-GAT GAT ATC GAT CAY CTD GG-3′) and RL2 (5′-TTC VGG CGT TTC AAT NGG AC-3′), designed from the consensus sequences of Escherichia coli (GenBank accession no. V00339), Coxiella burnetii (U86688), and L. pneumophila (AF087812), was used to amplify a portion of rpoB DNA (369 bp) containing the Rifr region (10, 17). Template DNA (ca. 50 ng) and 20 pmol of each primer were added to a PCR mixture tube (AccuPower PCR PreMix; Bioneer, Daejeon, Korea) containing 1 U of Taq DNA polymerase, each deoxynucleoside triphosphate at a concentration of 250 μM, 10 mM Tris-HCl (pH 8.3), 10 mM KCl, 1.5 mM MgCl2, and gel loading dye (14). The final volume was adjusted to 20 μl with distilled water. The reaction mixture was then subjected to 30 cycles for amplification. Each cycle consisted of 30 s at 95°C for denaturation, 30 s at 55°C for annealing, and 1 min at 72°C for extension, followed by final extension at 72°C for 5 min (model 9700 Thermocycler; Perkin-Elmer Cetus). Amplified PCR products were purified for sequencing using a QIAEX II gel extraction kit (Qiagen, Hilden, Germany).
Sequences of the purified PCR products were directly determined with forward and reverse primers using an Applied Biosystems automated sequencer (model 377) and a BigDye Terminator Cycle Sequencing kit (Perkin-Elmer Applied Biosystems, Warrington, United Kingdom). For the sequencing reaction, 30 ng of purified PCR products, 2.5 pmol of each primer, and 4 μl of BigDye Terminator RR mix (part no. 4303153; Perkin-Elmer Applied Biosystems) were mixed and adjusted with distilled water to a final volume of 10 μl. The reaction was run with 5% (vol/vol) dimethyl sulfoxide for 30 cycles of 15 s at 95°C, 5 s at 50°C, and 4 min at 60°C. To confirm the identity of the reference strains used in this study, we also amplified and sequenced about 400 bp from the 3′ end of the 16S rRNA gene (8). In addition, mip sequence analysis (402 bp) for 79 Korean isolates was performed to compare with the result of rpoB sequence analysis. For the amplification and sequencing of mip DNA, a new primer set, ML1 (5′-GAT AAG TTG TCT TAT AGC ATT GG-3′) and ML2 (5′-TCT GTC CAT CCT GGG ATA ACT TG-3′), was used.
The partial rpoB sequences (300 bp) were aligned using the multiple alignment program CLUSTAL X (29) and the sequences determined were submitted to GenBank (Table 1). Phylogenetic trees of Legionella were constructed by the neighbor-joining (NJ) method and the parsimony method in the PAUP program (28). In the NJ method, pairwise distances were calculated using the maximum likelihood option, and in parsimony analysis, heuristic searches were conducted with the option of tree bisection reconstruction branch swapping. C. burnetii, the etiologic agent of Q fever, was used as an outgroup. Branch supporting values were evaluated with 1,000 bootstrap replications. The 16S rRNA gene (1,396 bp) and mip (525 bp) sequences of Legionella were retrieved from the GenBank database and used to infer the phylogenetic relationships by the same method. Incongruence length difference (ILD) tests (partition homogeneity test in the PAUP program) (3) were conducted to determine whether rpoB, the 16S rRNA gene, and mip data sets were coalescent together or not. The degree of incongruence between trees was assessed by comparing the log likelihood values (11) of three NJ trees with those of the phylogenetic topologies obtained from the other genes (7). All analyses were performed using the PAUP program (28).
Similarities between the partial rpoB DNA sequences (300 bp) of Legionella species were lower than 95% in all cases except for L. jamestownensis-L. londiniensis (98.7%). The sequence divergence in rpoB was 3.5 times greater than that for the 16S rRNA gene, indicating the greater variance of rpoB. The mip sequences were more divergent than those of rpoB by a factor of 1.5. However, the relationship between the pairwise dissimilarities of rpoB and other genes was not linear, which suggested that the degree of sequence divergences was not proportional to the time needed for speciation. In contrast, the pairwise distances between the mip and 16S rRNA genes exhibited a close linear correlation (data not shown). The homoplasy index of the rpoB sequences (0.779), calculated by parsimony analysis using the PAUP program, was higher than those of the 16S rRNA gene and mip sequences (0.558 and 0.693, respectively).
The phylogeny inferred from rpoB sequences has long terminal branches with the exception of L. jamestownensis and L. londiniensis. However, bootstrap values supporting each branch in the rpoB phylogeny were relatively lower (Fig. 1) than those of the 16S rRNA gene and mip phylogenies (Fig. 2). Although rpoB, 16S rRNA gene, and mip phylogenies demonstrated partially similar relationships, they represented different topologies in many respects (Fig. 1 and 2). According to the results of ILD tests (3) of PAUP (28), three of the gene data sets were incongruent with each other (P < 0.001), indicating that three gene sequences could not be combined. Log likelihood tests (11) also indicated that the tree topology of one gene differed significantly from those of the other two genes (P < 0.0001).
FIG. 1.
Phylogenetic relationships of Legionella species inferred from partial rpoB DNA sequences. This tree was constructed by the NJ method in PAUP (28). The species C. burnetii was used as an outgroup. The bootstrap values presented at corresponding branches were evaluated from 1,000 replications. Values below 50% are not indicated. The autofluorescence (BW, blue-white; YG, yellow-green; R, red), ubiquinone (A to F), and fatty acid (I to IV) groups of each Legionella species (8, 9, 13, 20, 30) are indicated at the right. Biochemical traits identical to phylogenetic grouping are represented in boxes. The scale bar represents 5 substitutions per 100 nucleotides.
FIG. 2.
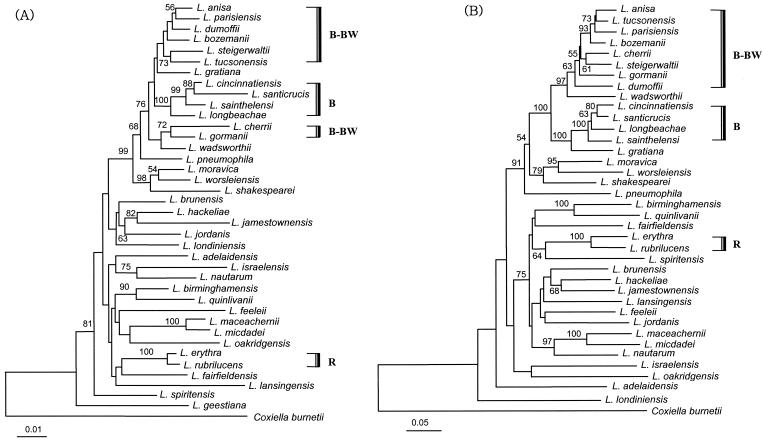
Gene trees based on 16S rRNA gene sequences (A) and mip sequences (B). These were constructed using the same method as described above for Fig. 1 for the rpoB sequences. The mip sequence of L. geestiana was not available in GenBank. B, ubiquinone group B; R, red autofluorescence group; and B-BW, ubiquinone group B and blue-white autofluorescence group. The scale bars represent the number of inferred substitutions per site.
The rpoB phylogeny indicated that L. pneumophila was closely related to L. gormanii (Fig. 1), a blue-white autofluorescent species that differs from L. pneumophila in many respects. In the mip tree (Fig. 2B), however, L. pneumophila was placed at a basal position of the B-BW and B groups (see Fig. 2 legend) and the clade of L. moravica, L. worsleiensis, and L. shakespearei. The bootstrap value supporting their relationships was relatively high, 91%. Sequence dissimilarities of rpoB between two subspecies of L. pneumophila (subsp. pneumophila and subsp. fraseri) ranged from 13.2 to 12.4%. These values exceeded those observed in other species, such as L. steigerwaltii and L. cherrii (10.4%) and L. anisa and L. parisiensis (9.2%).
There are eight blue-white autofluorescent species in the genus Legionella: L. anisa, L. parisiensis, L. bozemanii, L. cherrii, L. steigerwaltii, L. gormanii, L. dumoffii, and L. tucsonensis (8, 20). All of these species belong to ubiquinone group B (13, 20). In the mip tree (Fig. 2B), the clade formation of these eight blue-white autofluorescent species with L. wadsworthii was robustly supported by bootstrap analysis (97%), but there was no equivalent single clade in the rpoB (Fig. 1) and 16S rRNA gene phylogenies (Fig. 2A).
The other species belonging to ubiquinone group B, L. cincinnatiensis, L. santicrucis, L. longbeachae, L. sainthelensi, and L. gratiana (13, 20), all grouped well into a single clade in both the rpoB (Fig. 1) and mip (Fig. 2B) phylogenies. While their relationships were supported only moderately by the rpoB phylogeny (bootstrap value of 73%), they were fully supported by the mip phylogeny. In the 16S rRNA gene tree (Fig. 2A), L. gratiana displayed a close relationship with one of the subgroups in the blue-white autofluorescence group, though with very low bootstrap support. The other four species formed a clade that was fully supported (bootstrap value of 100%) in the 16S rRNA gene tree (Fig. 2A).
In the rpoB tree, L. maceachernii, L. micdadei, and L. nautarum formed one clade (Fig. 1), which was identical in the mip phylogeny (Fig. 2B). These three species belong to the same ubiquinone group (D) and fatty acid group III (9, 13, 20). While their relationships were poorly supported by a very low bootstrap value in the rpoB, the support was much stronger as determined by the mip phylogeny. However, in the 16S rRNA gene phylogeny, rather than clustering with L. maceachernii and L. micdadei, L. nautarum formed a distinct group along with L. israelensis (Fig. 2A).
The red autofluorescent species, L. erythra and L. rubrilucens, formed a distinct clade in all analyses (R group in Fig. 2), showing a particularly high similarity in terms of 16S rRNA gene sequences (99.4%), though the sequence similarities in rpoB and mip were not as high (88.7 and 89.0%, respectively). The yellow-green autofluorescent species, L. birminghamensis and L. wadsworthii (30), were clustered into one group in the rpoB phylogeny (Fig. 1) but not in the other two trees. These two species with yellow-green autofluorescence showed a sequence similarity of 80.7%.
L. geestiana, which contains unique isoprenoid quinone (Q-14) (8, 13) and fatty acid compositions (5), was placed at a basal position with respect to all Legionella species in both rpoB and 16S rRNA gene phylogenies (Fig. 1 and 2A). For rpoB sequences, the sequence similarities between L. geestiana and all other species were below 80%.
Species identification of the 105 strains was accomplished using rpoB sequence analysis of reference strains to measure the similarities and thereby infer the phylogenetic reconstruction (data not shown). Of these, 98 isolates belonged to L. pneumophila and exhibited 99.3 to 100% rpoB sequence similarities with the reference strains. The others were identified as other Legionella species (two L. bozemanii, two L. dumoffii, two L. feeleii, and one L. micdadei species), which showed 98.3 to 100% sequence similarities with each reference strain. It was interesting that 87 strains could be identified as L. pneumophila subsp. pneumophila and 11 as L. pneumophila subsp. fraseri. These strains of L. pneumophila subsp. pneumophila and subsp. fraseri showed 97.0 to 100% and 98.3 to 100% sequence similarities within each subspecies, respectively. The identified strains that belong to each subspecies showed 87.3 to 89.7% sequence similarity. However, no correlation was observed between the rpoB sequence and those of serogroups. For example, rpoB sequences of four reference strains belonging to serogroup 1 of L. pneumophila were not identical. On the other hand, mip sequences of 97 L. pneumophila strains, including 18 reference strains and 79 isolates, showed 93.4 to 100% similarities. However, unlike in rpoB analysis, no clear differentiation between two subspecies of L. pneumophila was observed in mip sequence analysis.
Recently, it was demonstrated that the rpoB-based approach to microbial community analysis or identification could practically overcome the inherent limitations of 16S rRNA gene intraspecies heterogeneity (4). In this study, we have shown that rpoB sequences are as useful as 16S rRNA gene and mip sequences in accessing and evaluating the relationships among the Legionella species. Such a simple genetic analysis promises to provide a practical scheme for the classification of Legionella species and the identification of culture isolates in the diagnostic or reference clinical laboratory.
In contrast to analysis of other genes, rpoB analysis has several advantages. In spite of the sequence's shortness (300 bp), rpoB sequence analysis was able to clearly differentiate among the Legionella species. One such example was the clear differentiation of L. erythra and L. rubrilucens by rpoB analysis. Because of their high similarity in 16S rRNA gene sequences (99.4%) and their DNA hybridization test results, the latter representing a value of just below 70% (23), they had long been considered subspecies of the same species (8). Another example was the differentiation of blue-white autofluorescent species. While their 16S rRNA gene sequences indicated similarities of at least 97.8% (8), their rpoB sequences represented 81.7 to 95.3% similarities. A further example, which might be the most significant one, was the differentiation of subspecies. L. pneumophila includes two subspecies (L. pneumophila subsp. pneumophila and subsp. fraseri), which featured 99.2% similarity (8) with 16S rRNA gene sequence and were not differentiated by mip analysis (19). However, rpoB analysis was able to clearly distinguish them (12). Coupled with the high nucleotide similarity within each subspecies (97.0 to 100% and 98.3 to 100%), the significant difference of nucleotides between the two subspecies (87.3 to 89.7%) indicates that rpoB can be a better marker in differentiation of L. pneumophila than can mip, which could not discriminate two subspecies of L. pneumophila. These results confirm the usefulness of rpoB sequence analysis in population genetic and epidemiological studies of L. pneumophila, including molecular typing.
The rpoB tree showed quite a different topology from those of the 16S rRNA gene and mip sequences (Fig. 1 and 2). The ILD and log likelihood tests suggested that phylogenetic relationships inferred from the three genes were statistically significantly different. The lack of congruence between the rpoB, 16S rRNA gene, and mip trees did not result only from frequent synonymous base substitutions. Otherwise, the observed incongruence among the three gene trees is most likely the result of horizontal gene transfer between Legionella species (7, 15, 25, 26). It is known that such horizontal gene transfer disrupts the treelike branching pattern, thus complicating the phylogenetic relationships of species (7).
Due to these discrepancies among the three gene trees, none was able to exactly explain the phylogenetic relationships within the Legionella species and the evolution of this species. This suggests that the use of several markers, such as rpoB in combination with 16S rRNA gene or mip, may be necessary for the reliable identification and phylogenetic study of Legionella. Such an approach will reduce the risk of error in molecular typing or identification.
Acknowledgments
K. S. Ko and H. K. Lee equally contributed as joint first authors.
We thank H. S. Jung (School of Biological Sciences, Seoul National University, Seoul, Korea) for his help with the phylogenetic analysis and critical reading of the manuscript. We also thank B. S. Field, R. F. Benson, and E. Brown (National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Ga.) and M. J. Kim (College of Medicine, Korea University, Seoul, Korea) for providing reference strains and clinical isolates.
This work was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Seoul, Republic of Korea (01-PJ10-PG6-01GM03-0002), and in part by the BK21 project for Medicine, Dentistry, and Pharmacy.
REFERENCES
- 1.Birtles, R. J., T. J. Rowbotham, D. Raoult, and T. G. Harrison. 1996. Phylogenetic diversity of intra-amoebal legionellae as revealed by 16S rRNA gene sequence comparison. Microbiology 142:3525-3530. [DOI] [PubMed] [Google Scholar]
- 2.Cianciotto, N. P., B. I. Eisenstein, C. H. Mody, G. B. Toews, and N. C. Engleberg. 1989. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect. Immun. 57:1255-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham, C. W. 1997. Can three incongruence tests predict when data should be combined? Mol. Biol. Evol. 14:733-740. [DOI] [PubMed] [Google Scholar]
- 4.Dahllöf, I., H. Baillie, and S. Kjelleberg. 2000. rpoB-based microbial community analysis avoids limitations inherent in 16S rRNA gene intraspecies heterogeneity. Appl. Environ. Microbiol. 66:3376-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diogo, A., A. Verissimo, M. F. Nobre, and M. S. Da Costa. 1999. Usefulness of fatty acid composition for differentiation of Legionella species. J. Clin. Microbiol. 37:2248-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fry, N. K., S. Warwick, N. A. Saunders, and T. M. Embley. 1991. The use of 16S ribosomal RNA analyses to investigate the phylogeny of the family Legionellaceae. J. Gen. Microbiol. 137:215-1222. [DOI] [PubMed] [Google Scholar]
- 7.Holmes, E. C., R. Urwin, and M. C. J. Maiden. 1999. The influence of recombination on the population structure and evolution of the human pathogen Neisseria meningitidis. Mol. Biol. Evol. 16:741-749. [DOI] [PubMed] [Google Scholar]
- 8.Hookey, J. V., N. A. Sauders, N. K. Fry, R. J. Birtles, and T. G. Harrison. 1996. Phylogeny of Legionellaceae based on small-subunit ribosomal DNA sequences and proposal of Legionella lytica comb. nov. for Legionella-like amoebal pathogens. Int. J. Syst. Bacteriol. 46:526-531. [Google Scholar]
- 9.Jantzen, E., A. Sonesson, T. Tangen, and J. Eng. 1993. Hydroxy-fatty acid profiles of Legionella species: diagnostic usefulness assessed by principal component analysis. J. Clin. Microbiol. 31:1413-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, B.-J., S.-H. Lee, M.-A. Lyu, S.-J. Kim, G.-H. Bai, S.-S. Kim, G.-T. Chae, E.-C. Kim, C.-Y. Cha, and Y.-H. Kook. 1999. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 37:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishino, H., and M. Hasegawa. 1989. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. J. Mol. Evol. 29:170-179. [DOI] [PubMed] [Google Scholar]
- 12.Ko, K. S., H. K. Lee, M.-Y. Park, M.-S. Park, K.-H. Lee, S.-Y. Woo, Y.-J. Yun, and Y.-H. Kook. 2002. Population genetic structure of Legionella pneumophila inferred from RNA polymerase gene (rpoB) and DotA gene (dotA) sequences. J. Bacteriol. 184:2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert, M. A., and C. W. Moss. 1989. Cellular fatty acid composition and isoprenoid quinone contents of 23 Legionella species. J. Clin. Microbiol. 27:465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, S.-H., B.-J. Kim, J.-H. Kim, K.-H. Park, S.-J. Kim, and Y.-Y. Kook. 2000. Differentiation of Borrelia burgdorferi sensu lato on the basis of RNA polymerase gene (rpoB) sequences. J. Clin. Microbiol. 38:2557-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mintz, C. S. 1999. Gene transfer in Legionella pneumophila. Microbes Infect. 1:1203-1209. [DOI] [PubMed] [Google Scholar]
- 16.Mollet, C. M. Drancourt, and D. Raoult. 1997. rpoB sequence analysis as a novel basis for bacterial identification. Mol. Microbiol. 26:1005-1011. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen, K., P. Hindersson, N. Høiby, and J. M. Bangsborg. 2000. Sequencing of the rpoB gene in Legionella pneumophila and characterization of mutations associated with rifampin resistance in the Legionellaceae. Antimicrob. Agents Chemother. 44:2679-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palys, T., L. K. Nakamura, and F. M. Cohan. 1997. Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int. J. Syst. Bacteriol. 47:1145-1156. [DOI] [PubMed] [Google Scholar]
- 19.Ratcliff, R. M., J. A. Lanser, P. A. Manning, and M. W. Heuzenroeder. 1998. Sequence-based classification scheme for the genus Legionella targeting the mip gene. J. Clin. Microbiol. 36:1560-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratcliff, R. M., S. C. Donnelan, J. A. Lanser, P. A. Manning, and M. W. Heuzenroeder. 1997. Interspecies sequence differences in the Mip protein from the genus Legionella: implication for function and evolutionary relatedness. Mol. Microbiol. 25:1149-1158. [DOI] [PubMed] [Google Scholar]
- 21.Renesto, P., K. Lorvellec-Guillon, M. Drancourt, and D. Raoult. 2000. rpoB gene analysis as a novel strategy for identification of spirochetes from the genera Borrelia, Treponema, and Leptospira. J. Clin. Microbiol. 38:2200-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renesto, P., J. Gouvernet, M. Drancourt, V. Roux, and D. Raoult. 2001. Use of rpoB gene analysis for detection and identification of Bartonella species. J. Clin. Microbiol. 39:430-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders, N. A., N. Doshi, and T. G. Harrison. 1992. A second serogroup of Legionella erythra serologically indistinguishable from Legionella rubrilucens. J. Appl. Bacteriol. 72:262-265. [DOI] [PubMed] [Google Scholar]
- 24.Severinov, K., A. Mustaev, A. Kukarin, O. Muzzin, I. Bass, S. A. Darst, and A. Goldfarb. 1996. Structural modules of the large subunits of RNA polymerase. J. Biol. Chem. 271:27969-27974. [DOI] [PubMed] [Google Scholar]
- 25.Smith, N. H., E. C. Homles, G. M. Donovan, G. A. Carpenter, and B. G. Spratt. 1999. Networks and groups within the genus Neisseria: analysis of argF, recA, rho, and 16S rRNA sequences from human Neisseria species. Mol. Biol. Evol. 16:773-783. [DOI] [PubMed] [Google Scholar]
- 26.Stone, B. J., and Y. A. Kwaik. 1999. Natural competence for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J. Bacteriol. 181:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson, M. S., and B. K. Hammer. 2000. Legionella pneumophila pathogenesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 54:567-613. [DOI] [PubMed] [Google Scholar]
- 28.Swofford, D. L. 1999. PAUP∗: phylogenetic analysis using parsimony (∗ and other methods), version 4. Sinauer Associates, Sunderland, Mass.
- 29.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winn, W. C. 1999. Legionella, p. 572-582. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 31.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]