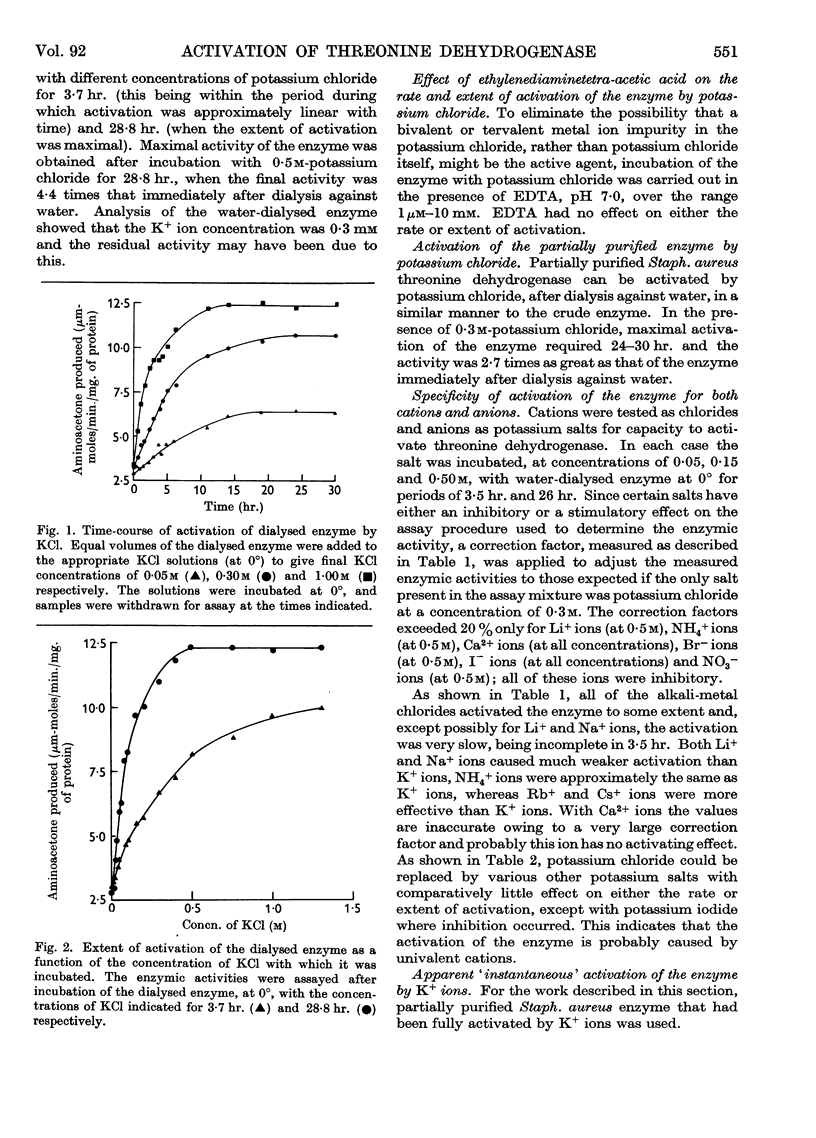
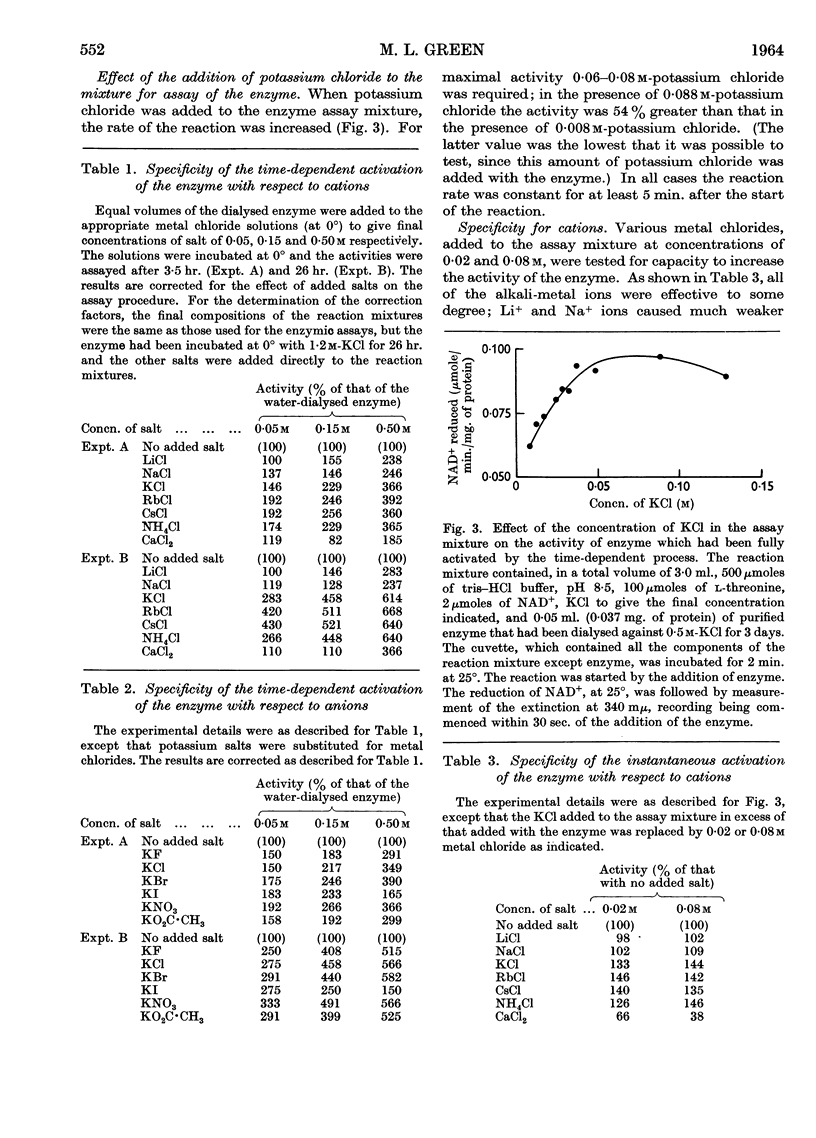
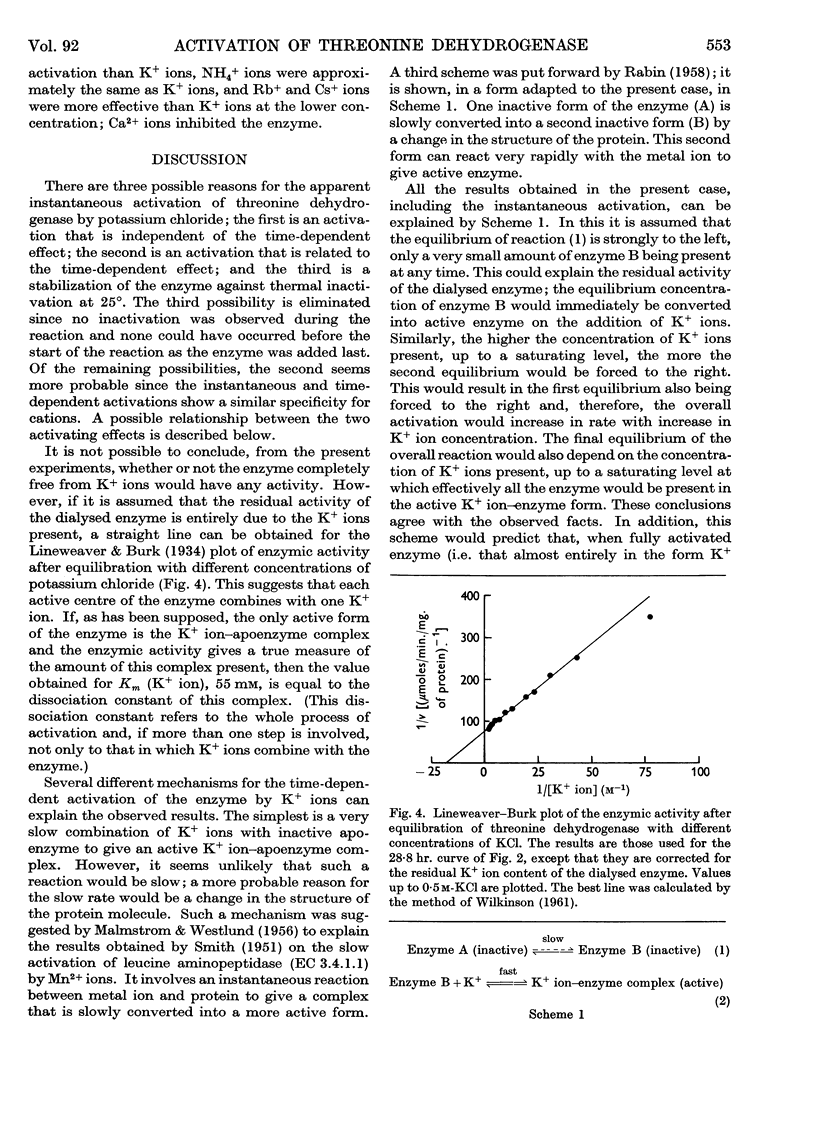
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GREEN M. L. A time-dependent effect of K on L-threonine dehydrogenase. Biochim Biophys Acta. 1963 Apr 9;67:682–685. doi: 10.1016/0006-3002(63)91882-1. [DOI] [PubMed] [Google Scholar]
- Green M. L., Elliott W. H. The enzymic formation of aminoacetone from threonine and its further metabolism. Biochem J. 1964 Sep;92(3):537–549. doi: 10.1042/bj0920537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAPPOLD F. C., STRUYVENBERG A. The activation of tryptophanase apo-enzyme by potassium, ammonium and rubidium ions. Biochem J. 1954 Nov;58(3):379–382. doi: 10.1042/bj0580379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- KORKES S., DEL CAMPILLO A., GUNSALAS I. C., OCHOA S. Enzymatic synthesis of citric acid. IV. Pyruvate as acetyl donor. J Biol Chem. 1951 Dec;193(2):721–735. [PubMed] [Google Scholar]
- MALMSTROM B. G., WESTLUND L. E. The effect of pH on the interaction of enolase with activating metal ions. Arch Biochem Biophys. 1956 Mar;61(1):186–196. doi: 10.1016/0003-9861(56)90331-9. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- PARKS R. E., Jr, BEN-GERSHOM E., LARDY H. A. Liver fructokinase. J Biol Chem. 1957 Jul;227(1):231–242. [PubMed] [Google Scholar]
- RABIN B. R. The nature of metal-peptide complexes in aqueous solution and the relationship these have to proteolytic activity. Biochem Soc Symp. 1958;(15):21–47. [PubMed] [Google Scholar]
- RAMASASTRI B. V., BLAKLEY R. L. 5,10-Methylenetetrahydrofolic dehydrogenase from bakers' yeast. I. Partial purification and some properties. J Biol Chem. 1962 Jun;237:1982–1988. [PubMed] [Google Scholar]
- SPACKMAN D. H., SMITH E. L., BROWN D. M. Leucine aminopeptidase. IV. Isolation and properties of the enzyme from swine kidney. J Biol Chem. 1955 Jan;212(1):255–269. [PubMed] [Google Scholar]
- TABOR H., WYNGARDEN L. The enzymatic formation of formiminotetrahydrofolic acid, 5,10-methenyltetrahydrofolic acid, and 10-formyltetrahydrofolic acid in the metabolism of formiminoglutamic acid. J Biol Chem. 1959 Jul;234(7):1830–1846. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORONICK C. L., JOHNSON M. J. Carbon dioxide fixation by cell-free extracts of Aspergillus niger. J Biol Chem. 1960 Jan;235:9–15. [PubMed] [Google Scholar]


