Abstract
This report describes the nosocomial acquisition of Candida parapsilosis candidemia by one of the six premature newborns housed in the same room of a neonatal intensive care unit at the Ospedale Santa Chiara, Pisa, Italy. The infant had progeria, a disorder characterized by retarded physical development and progressive senile degeneration. The infant, who was not found to harbor C. parapsilosis at the time of his admission to the intensive care unit, had exhibited symptomatic conjunctivitis before the onset of a severe bloodstream infection. In order to evaluate the source of infection and the route of transmission, two independent molecular typing methods were used to determine the genetic relatedness among the isolates recovered from the newborn, the inanimate hospital environment, hospital personnel, topically and intravenously administered medicaments, and indwelling catheters. Among the isolates collected, only those recovered from the hands of two nurses attending the newborns and from both the conjunctiva and the blood of the infected infant were genetically indistinguishable. Since C. parapsilosis was never recovered from indwelling catheters or from any of the drugs administered to the newborn, we concluded that (i) horizontal transmission of C. parapsilosis occurred through direct interaction between nurses and the newborn and (ii) the conjunctiva was the site through which C. parapsilosis entered the bloodstream. This finding highlights the possibility that a previous C. parapsilosis colonization and/or infection of other body sites may be a predisposing condition for subsequent C. parapsilosis hematogenous dissemination in severely ill newborns.
Nosocomially acquired fungemia is increasingly recognized as an important cause of morbidity and mortality in critically ill patients. Candida species are the fourth most commonly recovered organisms from all blood cultures of hospitalized individuals, with an estimated crude mortality rate of 38 to 75% (1, 16, 18). The majority of nosocomially acquired candidemia is due to Candida albicans (18), which is often responsible for severe and rapidly progressive bloodstream infections. Many reports, however, have documented the emergence of Candida tropicalis, Candida glabrata, Candida krusei, and Candida parapsilosis as important nosocomial pathogens, with C. parapsilosis identified as the predominant species causing bloodstream infections in premature newborns in neonatal intensive care units (NICUs) (1, 8-10, 16). In contrast to the aggressive nature of nosocomially acquired C. albicans candidemia, systemic infections associated with C. parapsilosis apparently cause fewer acute lethal events in premature newborns than systemic infections with C. albicans; nevertheless, C. parapsilosis fungemia significantly increases the morbidity and mortality of severely ill infants who require care in a NICU (8).
Laboratory studies have documented that C. parapsilosis is less virulent than C. albicans in animal models of infection (26). However, several factors have been identified which give C. parapsilosis a selective advantage in the hospital environment, such as its capability to adhere tenaciously to prosthetic materials and to proliferate rapidly in high concentrations of glucose (2, 25). Moreover, the propensity of clinical isolates of C. parapsilosis to form extensive biofilm (slime) in glucose-containing solutions suggests that this trait may contribute to its ability to adhere to plastic catheters and cause systemic infections in premature newborns receiving total parenteral nutrition, blood pressure transducers, or other invasive devices (8, 14, 21). Such a route of transmission may account for the occurrence of epidemic outbreaks of C. parapsilosis bloodstream infections when multidose medications are used for more than one patient (27). Moreover, repeated observations have documented that C. parapsilosis candidemia can occur in the absence of prior detectable colonization and/or symptomatic infection in other body sites of the same infant (5, 25), thus suggesting further that C. parapsilosis may gain access to the bloodstream directly from exogenous sources.
C. parapsilosis is a ubiquitous microorganism in the natural environment and is frequently isolated from soil, water, and plants (3). The primary reservoir of C. parapsilosis in the hospital setting is unknown, but C. parapsilosis carriage on the skin of healthy individuals, particularly on the hands of health care workers, has long been recognized (4, 12, 18, 7). In several instances, indirect transmission via the hands of nurses has been identified as responsible for outbreaks of catheter-related disseminated C. parapsilosis infections in NICUs (8, 20). However, the possibility that a prior colonization of premature newborns at other body sites could be the source of nosocomial C. parapsilosis candidemia cannot be excluded. While several studies have excluded vertical transmission from mother to newborn, infant colonization with C. parapsilosis has been shown to occur either by horizontal transmission from nurses (8, 22) or by cross-infection between infants through the hands of health care workers (24). Nevertheless, direct evidence demonstrating that nosocomial disseminated C. parapsilosis infection may be linked to prior infant colonization is still lacking. Indeed, while the use of molecular techniques has firmly established that exogenous sources should be clinically suspected and epidemiologically investigated when C. parapsilosis is isolated from blood samples of infants housed in NICUs, the role of previous C. parapsilosis colonization in the development of subsequent fungemia is still under question.
This study was undertaken to evaluate the source of infection and the route of transmission of nosocomial C. parapsilosis candidemia acquired by one of the six premature newborns housed in the same room of a NICU. The infant had progeria, a disorder characterized by retarded physical development and progressive senile degeneration (6, 11). This baby exhibited symptomatic conjunctivitis before the onset of bloodstream infection could be demonstrated. Molecular typing methods, used to determine genetic relatedness among environmental and clinical isolates, led to the demonstration that (i) symptomatic conjunctivitis was acquired by horizontal transmission of C. parapsilosis through direct interactions between health care workers and the newborn and (ii) the conjunctiva was the site through which C. parapsilosis entered the bloodstream. C. parapsilosis colonization and/or infection of other body sites, therefore, may be regarded as a predisposing condition for subsequent development of C. parapsilosis candidemia in severely ill newborns.
MATERIALS AND METHODS
C. parapsilosis sources.
Oropharyngeal, nasal, and conjunctival swabs, as well as urine and stool samples, of six newborns (NB1 to NB6) housed in the same room of a NICU at the Ospedale Santa Chiara, Pisa, Italy, were collected from each infant on the day of admission and at the time of symptomatic infection; blood samples from peripheral venous access were collected only when clinically indicated. Samples were also taken from the hands of the medical personnel (25 nurses, 5 physicians, and 5 technicians) working in the NICU by use of the broth bag method (15). Indwelling catheters were enriched in Sabouraud broth and incubated for 24 h at 37°C before being plated on Sabouraud agar. Enriched samples as well as topically and intravenously administered medicaments (100 μl), including ophthalmic and hyperalimentation solutions, were plated on Sabouraud agar supplemented with gentamicin and chloramphenicol (Difco, Detroit, Mich.) and incubated for 24 h at 37°C. Samples from environmental surfaces of the patient room, including ventilator tubing, nebulizers, humidifiers, the floor, sink areas, and other surfaces exposed to hand contact, were collected by agar plate contact by using plate count agar (PCA; PBI International, Milan, Italy). Yeast colonies were identified on the basis of the pattern of sugar utilization (ID32C; bioMérieux, Marcy l'Etoile, France), and C. parapsilosis strains showed the ID32C biogram number 5547350213. C. parapsilosis isolates (three colonies for each isolate) were stored on agar slants at room temperature, and their identification was confirmed as previously described (17). Unrelated C. parapsilosis isolates used were an environmental isolate (Cp:e2) from the infectious-disease unit of a different hospital and a clinical isolate (Cp:op1) from the respiratory tract of an outpatient suffering from symptomatic candidiasis. C. parapsilosis strains ATCC 96140 and ATCC 96144 were included in this study as reference strains.
Determination of electrophoretic karyotype.
Yeast cells were grown in Sabouraud broth until early stationary phase, suspended in 50 mM sodium EDTA (pH 8.0) (Sigma Chemical Co., Milan, Italy) to a final concentration of 109 CFU/ml, and embedded in plugs of 1% low-melting-point agarose (Nalgene, Rochester, N.Y.) as described elsewhere (13). Embedded yeast cells were treated with lyticase (Sigma), proteinase K (Gibco, Life Technologies, Milan, Italy), and β-mercaptoethanol (Sigma) to digest the cell wall and cell membrane without affecting the integrity of DNA. The karyotype of each isolate was determined by pulsed-field gel electrophoresis (PFGE) on 1% agarose gels in 0.5× TBE (Tris-borate [pH 8.0]-1 mM sodium EDTA [pH 8.0]) with a CHEF-DR II system (Bio-Rad, Richmond, Calif.). The reliability of this method was confirmed by running three separate preparations of agarose plugs containing DNA of the same C. parapsilosis isolate; moreover, at least three colonies of the same isolate were treated and karyotyped as independently collected strains. Resolution of chromosome-sized DNA molecules was performed at a voltage gradient of 4.5 V/cm and an angle of 120°; block 1 consisted of a 24-h run time with a pulse time of 120 s, and block 2 consisted of a 36-h run time with a pulse time of 240 s. Gels were stained with ethidium bromide (0.5 μg/ml; Merck, Darmstadt, Germany), and DNA bands were visualized with the Image Master VDS apparatus (Pharmacia Biotech, Uppsala, Sweden). Chromosomes of Saccharomyces cerevisiae YNN295 (Bio-Rad) were used as reference DNA markers for determination of the molecular size of C. parapsilosis DNA bands.
RAPD fingerprinting.
Yeasts were grown overnight in Sabouraud broth (Difco) at 37°C. Cells were harvested in early stationary phase and washed with 1 M sorbitol buffered with 4 mM potassium phosphate (pH 7.0). The pellet was suspended in 1.25 ml of the same solution containing 5 mg of lyticase/ml and was incubated at 37°C for 30 to 60 min until an average of 80% spheroplasts was observed. Spheroplasts were spun down and lysed by incubation at 60°C for 30 min in lysis buffer containing 0.2 M NaCl, 0.1 M EDTA, 5% sodium dodecyl sulfate, 50 mM Tris-HCl (pH 8.5), and 20 μg of proteinase K/ml. Nucleic acids were purified with phenol, precipitated with ethanol, and further treated with RNase. Randomly amplified polymorphic DNA (RAPD) fingerprinting of 100 ng of DNA was performed with primer RPO2 (5′-GCGATCCCCA-3′). Amplification reactions were performed in a DNA 9600 thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.) set at the conditions described by Sullivan et al. (23). Amplified DNA products were separated by electrophoresis in a 1% agarose gel containing ethidium bromide (0.5 μg/ml). Marker λ DNA-EcoRI-HindIII (Roche Diagnostic SpA, Monza, Italy) was used as a molecular size standard.
Dendrogram generation.
RAPD polymorphism was analyzed by using Image Master 1D Elite Software (Pharmacia Biotech), and dendrograms based on SAB values (similarity index between the patterns for every pair of strains A and B) were generated by the unweighted pair group method with arithmetic mean (UPGMA) (Image Master 1D Database Software; Pharmacia Biotech). The SAB was calculated as 2E/(2E + a + b), where E is the number of bands in the patterns for strains A and B sharing the same position, a is the number of bands in the pattern for strain A with no positional correlates in the pattern for strain B, and b is the number of bands in the pattern for strain B with no positional correlates in the pattern for strain A. While an SAB value of 0.00 indicates that the patterns for strains A and B share no common bands, an SAB of 1.00 indicates that all the bands in the pattern for strain A are indistinguishable from those in the pattern for strain B. SAB values ranging between 0.01 and 0.99 represent increasing levels of similarity.
RESULTS
Timing of C. parapsilosis isolation from clinical and environmental samples.
Only one (NB1) of the six preterm infants (NB1 to NB6) housed in the same room of the NICU developed C. parapsilosis candidemia during a 2-month period. This infant, who had progeria (6, 11), exhibited signs of severe conjunctivitis and bloodstream infection at days 10 and 18 posthospitalization, respectively. C. parapsilosis was isolated from the conjunctival swab (Cp:c1) and blood sample (Cp:b1) of the infant (Table 1). All the infants were screened for Candida carriage when they entered the NICU (day of admittance) by culturing oropharyngeal, nasal, and conjunctival samples as well as urine and stool specimens. C. parapsilosis was not detected in any of the samples analyzed, while C. albicans was identified in the stools of four neonates (NB3 to NB6) whose mothers had suffered from recurrent C. albicans vaginitis during pregnancy (data not shown). Because C. parapsilosis was found in the bloodstream of the infant with progeria (NB1), medical personnel and environmental surfaces of the infant room were investigated for the presence of C. parapsilosis. Monitoring for yeast was carried out weekly for 1 month. An environmental strain of C. parapsilosis (Cp:e1) was isolated only once from the sink surface of the room where the infant was hospitalized, at day 21 after his admittance to the NICU (Table 1). Hospital personnel were analyzed to detect hand carriage. Three of the nurses attending the newborns in the NICU were found to harbor C. parapsilosis on their hands, two on day 21 (Cp:hcw1 and Cp:hcw2) and one more on day 28 (Cp:hcw3) after the hospitalization of infant NB1 (Table 1). C. parapsilosis was never isolated from the topical and intravenous medicaments administered to the infant, including glucose-containing hyperalimentation and ophthalmic solutions, or from any of the indwelling catheters analyzed through routine surveillance cultures. The infant with progeria was treated with 6 mg of fluconazole (Diflucan; Pfizer Inc., New York, N.Y.)/kg of body weight once daily for 20 days and was discharged from the NICU after completion of antimycotic therapy.
TABLE 1.
Description of C. parapsilosis collection
| Isolate | Sourcea | Site of isolation | Time of isolation (day) | PFGE type | RAPD type |
|---|---|---|---|---|---|
| Cp:c1 | NICU NB1 | Conjunctiva | 10 | 1 | A |
| Cp:b1 | NICU NB1 | Blood | 18 | 1 | A |
| Cp:e1 | NICU | Sink surface | 21 | 2 | A |
| Cp:hcw1 | NICU HCW | Hand | 21 | 1 | A |
| Cp:hcw2 | NICU HCW | Hand | 21 | 1 | A |
| Cp:hcw3 | NICU HCW | Hand | 28 | 3 | D |
| Cp:op1 | IDU | Respiratory tract | 4 | B | |
| Cp:e2 | OU | Sink surface | 5 | C | |
| 96144 | ATCC | 6 | F | ||
| 96140 | ATCC | 7 | E |
HCW, health care worker; IDU, infectious-disease unit; OU, ophthalmology unit; ATCC, American Type Culture Collection.
Electrophoretic karyotyping.
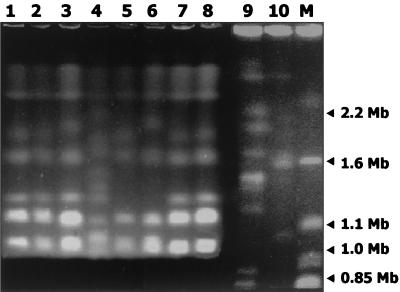
Electrophoretic karyotypes of C. parapsilosis isolates Cp:c1, Cp:b1, Cp:e1, and Cp:hcw1 to Cp:hcw3 (Fig. 1, lanes 1 to 3, 5, 7, and 8) showed three major chromosome mobility groups, as schematically represented in Fig. 2. The first mobility group (starting from the top of the gel) was shared by all the isolates and consisted of two bands of 3.0 and 2.2 Mb. The second mobility group consisted of a lower band of 1.65 Mb, common to all isolates, and an upper band which ranged from 1.92 to 1.95 Mb. The third mobility group consisted of two thicker bands of 1.02 and 1.1 Mb, common to all the isolates, and an upper band of about 1.3 Mb, which was not exhibited by Cp:hcw3 (Fig. 1, lane 5). These results revealed that Cp:c1, Cp:b1, Cp:hcw1, and Cp:hcw2 (Fig. 1, lanes 1, 2, 7, and 8) shared the same profile, designated karyotype 1, while Cp:e1 (Fig. 1, lane 3), isolated from the sink, exhibited a minor difference in the disposition of the upper band of the second mobility group (at 1.95 Mb instead of 1.92 Mb) and was designated karyotype 2 (Fig. 2). The karyotype of Cp:hcw3 (Fig. 1, lane 5), lacking the DNA band of 1.3 Mb, was designated type 3 (Fig. 2). In order to evaluate whether the karyotype similarities registered for the isolates reflected a close relation among strains endemic in the NICU or were due to a low discriminatory power of PFGE when applied to C. parapsilosis, two unrelated strains, Cp:op1 and Cp:e2 (Fig. 1, lanes 4 and 6), as well as C. parapsilosis strains ATCC 96144 and ATCC 96140 (Fig. 1, lanes 9 and 10), were included in the same runs. The four strains showed distinct karyotypes, which were different from those obtained for the C. parapsilosis isolates in the NICU, as schematically represented in Fig. 2. Cp:op1 (Fig. 1, lane 4) was the only isolate which exhibited 11 bands (at 3, 2.2, 2.1, 1.85, 1.7, 1.5, 1.35, 1.25, 1.1, 1.05, and 1.0 Mb); this isolate, assigned to karyotype 4 (Fig. 2), showed no apparent subdivision into the second and third mobility groups, while sharing the band disposition of the first mobility group with the other isolates. Cp:e2 (Fig. 1, lane 6) was characterized by an upper band of the second mobility group at 2.0 Mb instead of 1.92 Mb and by the absence of the upper band of the third mobility group; it was assigned to karyotype 5 (Fig. 2). The two ATCC strains showed the first mobility group characterized by a couple of bands migrating more slowly than the corresponding bands shared by all the other strains (Fig. 1, lanes 9 and 10). The second mobility groups of strains ATCC 96144 and ATCC 96140 showed three bands of 2.1, 1.95, and 1.65 Mb and of 2.05, 1.9, and 1.55 Mb, respectively. Strain ATCC 96144 showed five bands of 1.42, 1.4, 1.2, 0.88, and 0.8 Mb in the third mobility group, while strain ATCC 96140 showed two bands of 1.05 and 0.75 Mb. The karyotypes exhibited by ATCC strains 96144 and 96140 were designated types 6 and 7, respectively (Fig. 2). Therefore, the data presented in this study confirm that karyotyping may be regarded as a suitable molecular tool enabling efficient intraspecific discrimination of C. parapsilosis strains (21, 27); in fact, among the 10 strains analyzed, seven distinct karyotypes could be reproducibly distinguished.
FIG. 1.
Electrophoretic separation of chromosome-sized DNA bands of C. parapsilosis strains. Lanes: M, S. cerevisiae used as a marker; 1, 2, 3, 7, and 8, C. parapsilosis isolates recovered, respectively, from the conjunctiva (Cp:c1) and blood (Cp:b1) of the newborn with progeria, from the sink area of the NICU (Cp:e1), and from two health care workers of the NICU (Cp:hcw1 and Cp:hcw2); 4, 5, and 6, genetically unrelated strains of C. parapsilosis isolated, respectively, from an outpatient (Cp:op1), from one health care worker of the NICU (Cp:hcw3), and from the environment of a different ward (Cp:e2); 9 and 10, ATCC 96144 and ATCC 96140, respectively, used as reference strains.
FIG. 2.
Schematic representation of the C. parapsilosis karyotypes (numbered 1 through 7) obtained for the isolates and reference strains (listed below the corresponding karyotype) by PFGE, showing the chromosome mobility groups (I, II, and III). Molecular sizes are given on the left. Line thickness corresponds to band density and intensity of ethidium bromide staining.
RAPD fingerprinting.
RAPD fingerprinting was performed for the 10 C. parapsilosis strains for which seven distinct electrophoretic karyotypes had been determined. Six different profiles were obtained (Fig. 3A) by taking into consideration only the number and disposition of DNA bands. The six different RAPD profiles we obtained confirmed the strain discrimination already achieved by determining the electrophoretic karyotypes of the 10 C. parapsilosis strains analyzed. Indeed, as shown in Fig. 3A, the C. parapsilosis isolates recovered from the conjunctiva (Cp:c1, lane 1) and blood (Cp:b1, lane 2) of the infected infant and from the hands of two health care workers (Cp:hcw1 and Cp:hcw2, lanes 7 and 8), which showed karyotype 1 (Fig. 2), also gave the same RAPD profile, designated A, with an SAB of 1.0 (Fig. 3B). Interestingly, environmental isolate Cp:e1 (Fig. 3A, lane 3), which was recovered from the sink area at day 21 after infant NB1's candidemia episode, gave a RAPD profile indistinguishable (SAB = 1) from that obtained for Cp:c1, Cp:b1, Cp:hcw1, and Cp:hcw2 (Fig. 3A, lanes 1, 2, 7, and 8), indicating that the scant differences between karyotypes 1 and 2 were not evidenced by RAPD-PCR whole-genome fingerprinting. Nevertheless, significantly different RAPD profiles were obtained (Fig. 3A) for Cp:op1 (profile B), Cp:e2 (profile C), Cp:hcw3 (profile D), ATCC 96140 (profile E), and ATCC 96144 (profile F); their similarity values were lower than 0.75 (Fig. 3B).
FIG. 3.
Electrophoretic separation of DNA products obtained by RAPD-PCR from C. parapsilosis strains with RPO2 as a primer. (A) Lanes: M, λ DNA-EcoRI/HindIII used as a marker; 1, 2, 3, 7, and 8, C. parapsilosis isolates recovered, respectively, from the conjunctiva (Cp:c1) and blood (Cp:b1) of the newborn with progeria, from the sink area of the NICU (Cp:e1), and from two health care workers of the NICU (Cp:hcw1 and Cp:hcw2); 4, 5, and 6, genetically unrelated strains of C. parapsilosis isolated, respectively, from an outpatient (Cp:op1), from the environment of a different ward (Cp:e2), and from one health care worker of the NICU (Cp:hcw3); 9 and 10, reference strains ATCC 96140 and ATCC 96144, respectively. Molecular sizes (in kilobases) are shown to the left of the gel. (B) Dendrogram showing the genetic similarity of the strain collection.
DISCUSSION
The present study describes a case of nosocomial acquisition of disseminated C. parapsilosis infection in one of six premature newborns housed in the same room of a NICU at the Ospedale Santa Chiara, Pisa, Italy. The infant was a severely ill and immunocompromised preterm baby, since he suffered from progeria, a disorder characterized by retarded physical development and progressive senile degeneration (6, 11). When admitted to the NICU (first day of hospitalization), the newborn did not appear to harbor C. parapsilosis, as evidenced by the absence of yeast isolation from all the specimens taken from the baby (data not shown). Moreover, C. parapsilosis carriage was not detected for any of the other five newborns on the day of their admittance to the same room of the NICU.
At day 10 of hospitalization, the infant with progeria developed symptomatic conjunctivitis that was proven to be due to C. parapsilosis, and about 1 week later (at day 18), he suffered from a severe C. parapsilosis bloodstream infection. The strains isolated from the conjunctiva (Cp:c1) and the blood (Cp:b1) were indistinguishable when genetically typed by two independent methods: they shared karyotype 1 and RAPD profile A. This finding suggested that the C. parapsilosis strain responsible for symptomatic conjunctivitis shed into the bloodstream, causing development of candidemia 1 week later. A search for C. parapsilosis was immediately performed to evaluate whether yeast contamination had occurred in ophthalmic and hyperalimentation solutions or in indwelling catheters; none of these samples ever yielded this microorganism in routine surveillance cultures. Therefore, the conjunctiva was regarded as the possible site through which C. parapsilosis entered the bloodstream of the infant with progeria. This newborn suffered from serious abnormalities of ectodermal and mesodermal tissues, characterized by atrophy of epidermis and dermis (6, 11); since corneal tissue is of mesodermal origin and conjunctiva is derived from the ectodermic leaflet, it is likely that C. parapsilosis could gain access to the bloodstream directly through defective ocular barriers. The observation that ocular infections due to C. parapsilosis, though infrequent, have never been reported in association with systemic yeast dissemination (19, 28) convincingly supports the notion that the integrity of ocular barriers efficaciously prevents the hematogenous dissemination of the yeast.
In order to identify the source of infection and the route of transmission, the search for C. parapsilosis was extended to the medical personnel as well as to environmental surfaces of the patient room. Only one environmental isolate (Cp:e1) was collected from the sink area of the newborn room. Interestingly, isolate Cp:e1 shared the RAPD profile of Cp:c1, Cp:b1, Cp:hcw1, and Cp:hcw2, suggesting strain identity for this strain collection; however, the Cp:e1 karyotype showed a minor but reproducible difference in the upper band of the second mobility group, with a band disposition at 1.95 Mb for Cp:e1 and at 1.92 Mb for Cp:c1, Cp:b1, Cp:hcw1, and Cp:hcw2. This finding was thought to indicate that isolate Cp:e1 could belong to a genotype endemic in the NICU, possibly originating from a strain that entered the NICU through nurse hand carriage. Among the nurses found to be positive for C. parapsilosis hand carriage, one harbored a C. parapsilosis strain (Cp:hcw3) exhibiting both a karyotype and a RAPD profile which were unique in the collection, while the strains isolated from the other nurses (Cp:hcw1 and Cp:hcw2) were genetically indistinguishable and shared both the karyotype and the RAPD profile of the isolates recovered from the infant with progeria. The strain identity registered for Cp:hcw1 and Cp:hcw2 suggested that cross-infection could occur between nurses.
The overall results obtained suggest that the nosocomial acquisition of C. parapsilosis fungemia by the newborn with progeria may be attributed to horizontal transmission of the yeast via direct interaction between nurses and the infant, who suffered first from symptomatic conjunctivitis and later on from systemic candidiasis. Infant colonization by vertical transmission from the mother was excluded, because C. parapsilosis was never isolated from the screening cultures performed at the time of the baby's admittance to the NICU. However, although C. parapsilosis was not isolated from the infant at admission, the method we used for yeast recovery (a swab plated on agar) may not have been sufficiently sensitive for diagnostic purposes if a low number of cells was present at the colonization site.
As in other investigations (12, 14, 18), this report stresses the importance of impaired host defense mechanisms in the acquisition of bloodstream infection from nosocomial sources; indeed, although all the infants housed in the same room were preterm newborns and similarly exposed to cross-infection through the hands of the nurses, horizontal transmission of fungemia occurred only in the newborn with a serious defect in the integrity of cutaneous barriers. This observation, therefore, is in agreement with the general view that, in NICUs, C. parapsilosis outbreaks are mainly due to deliberate disruption of the cutaneous barrier for administration of invasive therapies and use of monitoring equipment (8, 27). Systematic identification of C. parapsilosis carriers for the purposes of isolation and preventive treatment does not appear to be warranted. In contrast, meticulous attention to hand-washing procedures, scrupulous care of medical devices, and routine glove wearing even when urgent situations arise in NICUs should be imperative, particularly when seriously ill preterm babies are hospitalized.
Acknowledgments
This work was supported by a research grant from the Italian “Ministero dell'Università e della Ricerca Scientifica e Tecnologica,” contracts MM06248147 and 2001064775.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1228. [DOI] [PubMed] [Google Scholar]
- 2.Critchley, I. A., and L. J. Douglas. 1985. Differential adhesion of pathogenic Candida species to epithelial and inert surfaces. FEMS Microbiol. Lett. 28:199-203. [Google Scholar]
- 3.Deresinski, S. C., K. V. Clemons, C. A. Kemper, K. Roesch, B. Walton, and D. A. Stevens. 1995. Genotypic analysis of pseudoepidemic due to contamination of Hanks' balanced salt solution with Candida parapsilosis. J. Clin. Microbiol. 33:2224-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diekema, D. J., S. A. Messer, R. J. Hollis, R. P. Wenzel, and M. A. Pfaller. 1997. An outbreak of Candida parapsilosis prosthetic valve endocarditis. Diagn. Microbiol. Infect. Dis. 29:147-153. [DOI] [PubMed] [Google Scholar]
- 5.Gagneur, A., J. Sizun, E. Vernotte, L. de Parscau, D. Quinio, A. M. Le Flohic, and R. Baron. 2001. Low rate of Candida parapsilosis-related colonization and infection in hospitalized preterm infants: a one-year prospective study. J. Hosp. Infect. 48:193-197. [DOI] [PubMed] [Google Scholar]
- 6.Hoppen, T., I. Hausser, U. Theile, R. Ferrari, W. Muller, and M. Rister. 2000. Neonatal progeroid syndrome (Wiedemann-Rautenstrauch syndrome): case report and review of the literature. Klin. Padiatr. 212:71-76. (In German.) [DOI] [PubMed]
- 7.Huang, Y. C., T. Y. Lin, H. S. Leu, J. L. Wu, and J. H. Wu. 1998. Yeast carriage on hands of hospital personnel working in intensive care units. J. Hosp. Infect. 39:47-51. [DOI] [PubMed] [Google Scholar]
- 8.Huang, Y. C., T. Y. Lin, H. S. Leu, H. L. Peng, J. H. Wu, and H. Y. Chang. 1999. Outbreak of Candida parapsilosis fungemia in neonatal intensive care units: clinical implication and genotyping analysis. Infection 27:97-102. [DOI] [PubMed] [Google Scholar]
- 9.Huang, Y. C., T. Y. Lin, R. I. Lien, Y. H. Chou, C. Y. Kuo, P. H. Yang, and W. S. Hsieh. 2000. Candidemia in special care nurseries: comparison of albicans and parapsilosis infection. J. Infect. 40:171-175. [DOI] [PubMed] [Google Scholar]
- 10.Kao, A. S., M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stephens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjeh. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis. 29:1164-1170. [DOI] [PubMed] [Google Scholar]
- 11.Korniszewski, L., R. Nowak, E. Okninska-Hoffmann, A. Skorka, D. Gieruszczak-Bialek, and M. Sawadro-Rochowska. 2001. Wiedemann-Rautenstrauch (neonatal progeroid) syndrome: new case with normal telomere length in skin fibroblasts. Am. J. Med. Genet. 103:144-148. [DOI] [PubMed] [Google Scholar]
- 12.Levin, A. S., S. F. Costa, N. S. Mussi, M. Basso, S. I. Sinto, C. Machado, D. C. Geiger, M. C. Villares, A. Z. Schreiber, A. A. Barone, and M. L. Branchini. 1998. Candida parapsilosis fungemia associated with implantable and semi-implantable central venous catheters and the hands of healthcare workers. Diagn. Microbiol. Infect. Dis. 30:243-249. [DOI] [PubMed] [Google Scholar]
- 13.Lupetti, A., G. Guzzi, A. Paladini, K. Swart, M. Campa, and S. Senesi. 1995. Molecular typing of Candida albicans in oral candidiasis: karyotype epidemiology with human immunodeficiency virus-seropositive patients in comparison with that with healthy carriers. J. Clin. Microbiol. 33:1238-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martino, P., C. Girmenia, A. Micozzi, R. Raccah, G. Gentile, M. Venditti, and F. Mandelli. 1993. Fungemia in patients with leukemia. Am. J. Med. Sci. 306:225-232. [DOI] [PubMed] [Google Scholar]
- 15.Pfaller, M. A. 1994. Epidemiology and control of fungal infections. Clin. Infect. Dis. 19(Suppl. 1):S8-S13. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22:S89-S94. [DOI] [PubMed] [Google Scholar]
- 17.Pontieri, E., C. Caracciolo, S. Bianchini, D. Dantonio, G. Novelli, B. Dallapiccola, and G. Carruba. 2001. Single primer pair for PCR identification of Candida parapsilosis group I isolates. J. Med. Microbiol. 50:441-448. [DOI] [PubMed] [Google Scholar]
- 18.Rangel-Frausto, M. S., T. Wiblin, H. M. Blumberg, L. Saiman, J. Patterson, M. Rinaldi, M. Pfaller, G. E. Edwards, Jr., W. Jarvis, J. Dawson, and R. P. Wenzel. 1999. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin. Infect. Dis. 29:253-258. [DOI] [PubMed] [Google Scholar]
- 19.Rhem, M. N., K. R. Wilhelmus, and R. L. Font. 1996. Infectious crystalline keratopathy caused by Candida parapsilosis. Cornea 15:543-545. [PubMed] [Google Scholar]
- 20.Saxen, H., M. Virtanen, P. Carlson, K. Hoppu, M. Pohjavuori, M. Vaara, J. Vuopio-Varkila, and H. Peltola. 1995. Neonatal Candida parapsilosis outbreak with a high case fatality rate. Pediatr. Infect. Dis. J. 14:776-781. [DOI] [PubMed] [Google Scholar]
- 21.Shin, J. H., D. H. Shin, J. W. Song, S. J. Kee, S. P. Suh, and D. W. Ryang. 2001. Electrophoretic karyotype analysis of sequential Candida parapsilosis isolates from patients with persistent or recurrent fungemia. J. Clin. Microbiol. 39:1258-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strausbaugh, L. J., D. L. Sewell, T. T. Ward, M. A. Pfaller, T. Heitzman, and R. Tjoelker. 1994. High frequency of yeast carriage on hands of hospital personnel. J. Clin. Microbiol. 32:2299-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan, D., D. Bennett, M. Henman, P. Harwood, S. Flint, F. Mulcahy, D. Shanley, and D. Coleman. 1993. Oligonucleotide fingerprinting of isolates of Candida species other than Candida albicans and of atypical Candida species from human immunodeficiency virus-positive and AIDS patients. J. Clin. Microbiol. 31:2124-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waggoner-Fountain, L. A., M. W. Walker, R. J. Hollis, M. A. Pfaller, J. E. Ferguson II, R. P. Wenzel, and L. G. Donowitz. 1996. Vertical and horizontal transmission of unique Candida species to premature newborns. Clin. Infect. Dis. 22:803-808. [DOI] [PubMed] [Google Scholar]
- 25.Weems, J. J., Jr., M. E. Chamberland, J. Ward, M. Willy, A. A. Padhye, and S. L. Solomon. 1987. Candida parapsilosis fungemia associated with parenteral nutrition and contaminated blood pressure transducers. J. Clin. Microbiol. 25:1029-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weems, J. J., Jr. 1992. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations and antimicrobial susceptibility. Clin. Infect. Dis. 14:756-766. [DOI] [PubMed] [Google Scholar]
- 27.Welbel, S. F., M. M. McNeil, R. J. Kuykendall, T. J. Lott, A. Pramanik, R. Silberman, A. D. Oberle, L. A. Bland, S. Aguero, M. Arduino, S. Crow, and W. R. Jarvis. 1996. Candida parapsilosis bloodstream infections in neonatal intensive care unit patients: epidemiologic and laboratory confirmation of a common source outbreak. Pediatr. Infect. Dis. J. 15:998-1002. [DOI] [PubMed] [Google Scholar]
- 28.Wong, V. K., W. Tasman, R. C. Eagle, Jr., and A. Rodriguez. 1997. Bilateral Candida parapsilosis endophthalmitis. Arch. Ophthalmol. 115:670-672. [DOI] [PubMed] [Google Scholar]